Abstract
One of the most important advances in the treatment of non-small cell lung cancer (NSCLC) has been the identification of molecular alterations vulnerable to targeted inhibition, such as mutations in the epidermal growth factor receptor (EGFR) gene. EGFR tyrosine kinase inhibitors (EGFR-TKIs) are targeted agents used to treat EGFR mutation-positive advanced NSCLC showing significant improvements in terms of response rate (RR) and progression-free survival (PFS) compared to conventional chemotherapy. However, all patients eventually develop resistance to first-line EGFR-TKIs. The most common mechanism of acquired resistance is the secondary acquisition of a single missense mutation within exon 20 in the EGFR gene, known as the T790M mutation (49–60%). New agents targeting the T790M mutation have undergone clinical development, and among these, osimertinib has shown significant activity in relapsing EGFR mutation positive patients harbouring the T790M mutation. Although precision medicine is a reality for NSCLC, obtaining relevant tissue for repeated molecular analysis from these patients remains a challenge. In this article, a group of experts from the Spanish Society of Medical Oncology (SEOM) and the Spanish Lung Cancer Group (GECP) evaluated the role of rebiopsy and the potential application of plasma-testing methodologies in advanced EGFR mutation patients progressing after EGFR-TKI.
Keywords: Epidermal growth factor receptor (EGFR), tyrosine kinase inhibitor (TKI), osimertinib, liquid biopsy, rebiopsy
Introduction
Lung cancer is one of the leading causes of cancer death worldwide (1). Non-small cell lung cancer (NSCLC) accounts for 85–90% of lung cancers. The 5-year survival rate for all stages is around 17%, while for stage IV NSCLC it is approximately 2%. In recent years, one of the most important advances has been the identification of molecular alterations vulnerable to targeted inhibition. The majority of these alterations occur in adenocarcinomas, although potential targets in squamous cell carcinomas (SCC) are also emerging (2) (Figure 1). The Lung Cancer Mutation Consortium (LCMC) evaluated actionable oncogenic drivers in 10 genes from 1,102 patients with NSCLC from 14 American centers. An oncogenic driver alteration was detected in 64% of cases (3). Molecular profiling has been used to choose therapies or enroll patients into clinical trials. Those patients with oncogenic driver alterations who received a targeted therapy had a significant improvement in overall survival (OS) compared with those with genetic alterations but not treated with targeted agents, or those with no druggable target.
Figure 1.
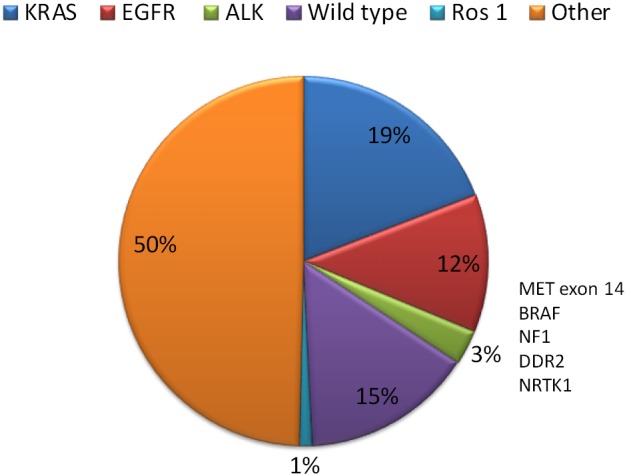
Mutations found in NSCLC patients. EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; NSCLC, non-small cell lung cancer. Modified from Rosell and Karachaliou (2).
Activating mutations of the epidermal growth factor receptor (EGFR) gene have been the first molecular event that could be targeted with specific drugs in NSCLC. EGFR mutations are found in 10–12% of Caucasians with adenocarcinoma and are more frequent in never smokers, females, and in patients of East Asian ethnicity. The frequency of EGFR mutations in the Spanish population is around 10–16% of patients (4,5). The most common EGFR mutations are a deletion in exon 19 (Del19) and the exon 21 L858R point mutation (85–90%). ALK rearrangements, mainly translocations, occur in around 4% of NSCLC (6). Drugs targeting EGFR, ALK and ROS1 genes, respectively, are currently approved. The prevalence of other molecular alterations with potentially actionable drugs, such as MET amplification, HER2 mutations, RET fusions, and BRAF mutation, is low (<2%), and early clinical trials have shown the activity of targeting drugs in these small subgroups of genetically defined patient population.
However, and despite initial responses to targeted therapies, all patients will eventually show progression of disease due to both primary and secondarily acquired resistance mechanisms to targeted agents. For those EGFR mutation-positive patients receiving EGFR-tyrosine kinase inhibitors (EGFR-TKIs), the most common mechanism of acquired resistance is the secondary acquisition of a single missense mutation within exon 20 in the EGFR gene, known as the T790M mutation (49–60%) (7). New agents targeting the T790M mutation have undergone clinical development, and among these, osimertinib has shown significant activity in relapsing EGFR mutation positive patients harbouring the T790M mutation (8). Very recently, osimertinib has been approved for use in patients who develop this specific resistance. Although precision medicine is a reality for NSCLC, obtaining relevant tissue for repeated molecular analysis from these patients remains a challenge. In this article, a group of experts from the Spanish Society of Medical Oncology (SEOM) and the Spanish Lung Cancer Group (GECP) evaluated the role of rebiopsy and the potential application of plasma-testing methodologies in advanced EGFR mutation patients progressing after EGFR-TKI.
Clinical management of EGFR mutation-positive NSCLC patients
Studies comparing EGFR-TKIs with chemotherapy
There have been nine phases III studies comparing a first-generation reversible EGFR-TKI (either gefitinib or erlotinib), or a second-generation irreversible EGFR-TKI (afatinib), with platinum doublets as first-li86tt8rt8ne treatment in EGFR mutation-positive NSCLC patients (Table 1).
Table 1. Randomised trials evaluating EGFR-TKIs in EGFR-mutation positive patients with NSCLC.
| Study/phase | Treatment arms | No. patients; region | RR (%); P | PFS (months) | HR; P | OS (months) | HR; P |
|---|---|---|---|---|---|---|---|
| IPASS/III (9,10) | Gefitinib vs. carboplatin-taxol | 261; Asia | 71.2 vs. 47.3; <0.001 | 9.5 vs. 6.3 | 0.48; <0.001 | 21.6 vs. 21.9 | 1; 0.990 |
| First-SIGNAL/III (11) | Gefitinib vs. cisplatin-gemcitabine | 96; Korea | 84.6 vs. 37.5; 0.002 | 8.5 vs. 6.7 | 0.54; 0.086 | 27.2 vs. 25.6 | 1.043; 0.428 |
| WJTOC 3405/III (12,13) | Gefitinib vs. cisplatin-docetaxel | 177; Japan | 62.1 vs. 32.2; <0.0001 | 9.2 vs. 6.3 | 0.488; <0.0001 | 36.0 vs. 39.0 | 1.19; 0.443 |
| NEJ002/III (14,15) | Gefitinib vs. carboplatin-taxol | 230; Japan | 73.7 vs. 30.7; <0.001 | 10.4 vs. 5.4 | 0.30; <0.001 | 27.7 vs. 26.6 | 0.887; 0.483 |
| OPTIMAL/III (16,17) | Erlotinib vs. carboplatin-gemcitabine | 154; China | 83.0 vs. 36.0; <0.0001 | 13.1 vs. 4.6 | 0.16; <0.0001 | 22.8 vs. 27.2 | 1.19; 0.2663 |
| EURTAC/III (18) | Erlotinib vs. cisplatin-docetaxel | 174; Europe | 58.0 vs. 15.0; <0.0001 | 9.7 vs. 5.2 | 0.37; <0.0001 | 19.3 vs. 19.5 | 1.04; 0.87 |
| ENSURE/III (19) | Erlotinib vs. cisplatin-gemcitabine | 148; Asia | 62.7 vs. 33.6; <0.0001 | 11.0 vs. 5.3 | 0.34; <0.0001 | 26.3 vs. 25.5 | 0.91; 0.607 |
| LUX-LUNG 3/III (20,21) | Afatinib vs. cisplatin-pemetrexed | 345; Global | 56.0 vs s. 23.0; 0.001 | 11.1 vs. 6.9 | 0.58; 0.001 | 28.2 vs. 28.2 | 0.88; 0.39 |
| LUX-LUNG 6/III (21,22) | Afatinib vs. cisplatin-gemcitabine | 364; China | 67.0 vs. 23.0; <0.0001 | 11.0 vs. 5.6 | 0.28; <0.001 | 23.1 vs. 23.5 | 0.93; 0.61 |
| LUX-LUNG 7/II (23,24) | Afatinib vs. gefitinib | 319; Global | 70.0 vs. 56.0; 0.0083 | 11.0 vs. 10.9 | 0.73; <0.017 | 27.9 vs. 24.5 | 0.86; 0.258 |
| JO25567/II (25) | Erlotinib + bevacizumab vs. erlotinib | 154; Japan | 69.3 vs. 63.6; 0.4951 | 16.0 vs. 9.7 | 0.54; 0.0015 | NR | NR |
EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; HR, hazard ratio; NR, not reached; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; RR, response rate.
The first two studies, IPASS and First-SIGNAL, were conducted in a population with clinical features associated with a higher EGFR mutation rate. Subsequent studies were conducted exclusively in patients with EGFR mutations. The primary objective in these studies was progression-free survival (PFS), except in First-SIGNAL where the primary objective was overall survival (OS). All the studies showed significant differences in PFS (except First-SIGNAL, which showed a trend towards better PFS) and response rate (RR) in favour of EGFR-TKI therapy. Even so, no significant differences in OS were seen in any of the studies, probably because of treatment crossover after progression. All the studies showed a better toxicity profile with EGFR-TKIs, although this treatment was associated with higher rates of skin rash and diarrhoea. The studies also showed improved improvement in the quality of life in for EGFR-TKI-treated patients.
Gefitinib
The IPASS study was conducted in Asian adenocarcinoma patients who were non-smokers or former smokers who had smoked less than 10 pack-years. Patients were randomised to receive gefitinib or carboplatin combined with paclitaxel (9). The study met its primary objective of non-inferior PFS (5.7 vs. 5.8 months; P<0.0001). Regarding retrospective EGFR mutation analysis, histological specimens were only available in 36% of patients, and a significant benefit in PFS (9.5 vs. 6.3 months; P<0.001) and RR (71.2% vs. 47.3%, P=0.0001) was seen in favour of gefitinib in the EGFR mutation-positive subgroup. In terms of OS, there were no significant differences either in the overall study population (P=0.10) or in the EGFR mutation-positive subgroup (21.6 vs. 21.9 months; P=0.990) (10).
The First-SIGNAL study, conducted in Korean non-smokers with adenocarcinomas, compared gefitinib with combination cisplatin and gemcitabine (11). The general population did not meet either the primary objective of OS (22.3 vs. 22.9 months; P=0.604) or the PFS objective (5.8 vs. 6.4 months; P=0.128). About EGFR mutation analysis, material was only available from 31% of patients. A favourable trend was seen in PFS (8.5 vs. 6.7 months; P=0.086), with a significantly higher RR for gefitinib (84.6% vs. 37.5%; P=0.002) but no significant differences in OS (27.2 vs. 25.6 months; P=0.428), in the EGFR mutation-positive patient subgroup.
Study WJTOG3405 compared gefitinib with combined cisplatin and docetaxel in Japanese patients harbouring EGFR mutations (12,13). The study showed greater PFS (9.2 vs. 6.3 months; P<0.0001) and RR (62.1% vs. 32.2%; P=0.0001) for gefitinib, with no differences in OS (36 vs. 39 months; P=0.443).
Study NEJ002 also evaluated the efficacy of gefitinib versus combination carboplatin and paclitaxel, in Japanese patients (14,15). A significant increase was observed in PFS (10.8 vs. 5.4 months; P<0.001) and RR (73.7% vs. 30.7%; P<0.001) in favour of gefitinib. No significant differences were found in OS (27.7 vs. 26.6 months; P=0.483).
Erlotinib
Three phases III studies have compared erlotinib with a platinum doublet in patients with EGFR mutations. The OPTIMAL study, conducted in a Chinese population, compared erlotinib therapy with combination carboplatin and gemcitabine. A significant benefit was seen in PFS (13.1 vs. 4.6 months; P<0.0001) and RR (83% vs. 36%; P<0.0001) for erlotinib (16,17). There was no evidence of any differences in OS (22.8 vs. 27.2 months; P=0.2663).
The EURTAC study, conducted in European patients, showed a significant benefit in favour of erlotinib in PFS (9.7 vs. 5.2 months; P<0.0001) and RR (58% vs. 15%; P<0.0001) (18). No differences were found in OS (19.3 vs. 19.5 months; P=0.87).
The ENSURE study compared erlotinib therapy with combination cisplatin and gemcitabine in the Asian population. Significant differences were observed in favour of erlotinib in PFS (11.0 vs. 5.5 months; P<0.0001) and RR (62.7% vs. 33.6%; P<0.0001) (19). Again, there were no differences in OS (26.3 vs. 25.5 months; P=0.607).
Afatinib
Two studies have compared afatinib with a platinum doublet in EGFR mutation-positive patients. The LUX-Lung 3 study compared afatinib with the combination cisplatin-pemetrexed in EGFR mutation-positive patients. It showed a significant benefit in favour of afatinib in PFS (11.0 vs. 6.9 months; P=0.001) and RR (56% vs. 23%; P=0.001), with no differences in OS (28.2 vs. 28.2 months; P=0.39) (20,21). On the other hand, the LUX-Lung 6 study compared afatinib with the combination cisplatin-gemcitabine, obtaining benefits in PFS (11.0 vs. 5.6 months; P<0.001) and RR (67% vs. 23%, P<0.0001) for afatinib, with no differences in OS (23.1 vs. 23.5 months; P=0.61) (21,22).
Randomised studies comparing two EGFR-TKIs
Currently, only the results of the LUX-Lung 7 study are available (23). This randomised phase IIb trial compared afatinib with gefitinib. The study showed a small but significant benefit in favour of afatinib in PFS (11.0 vs. 10.9 months; P=0.017) and RR (70% vs. 56%; P=0.0083), but no impact on OS (27.9 vs. 24.5 months; P=0.258) (24).
Randomised studies evaluating erlotinib in combination with bevacizumab
A Japanese randomised phase II study compared the efficacy of the combination erlotinib plus bevacizumab against erlotinib monotherapy (25). Its primary objective was PFS, and a significant benefit was observed in favour of the combination (16.0 vs. 9.7 months; P=0.0015). No differences were seen in RR (69.3% vs. 63.6%; P=0.4951). At the time of publication, survival data is not yet available. As far as adverse effects are concerned, the combination showed statistically significantly higher rates of hypertension (60% vs. 10%) and proteinuria of grade 3 or above (8% vs. 0%).
Clinical and molecular features of EGFR mutation-positive NSCLC patients who progress on EGFR-TKIs
Three models of progression on EGFR-TKI therapy have been described. These may have implications for treatment management in these patients (26):
Dramatic progression: patients who, after 3 months or more of disease control on EGFR-TKIs, show rapid progression with a significant, usually symptomatic, increase in tumour burden of the disease;
Gradual progression: patients who, after 6 months or more of disease control on EGFR-TKIs, show slow disease progression, with no significant increase in tumour burden and usually few symptoms;
Local progression: patients who, after 3 months or more of disease control on EGFR-TKIs, show a solitary extracranial lesion or limited progression in the central nervous system, with few associated symptoms.
Although EGFR mutation-positive patients derive benefit from treatment with reversible and irreversible TKIs, studies indicate that most of them will nevertheless suffer disease progression within 9 to 12 months (10-22).
Several mechanisms of acquired resistance to EGFR-TKI therapy have been described. They can be divided into three main categories: the presence of a secondary mutation in EGFR; the presence of bypass track activation; and phenotypic transformation. In almost 30% of cases, the mechanism of resistance is unknown (Figure 2).
Figure 2.
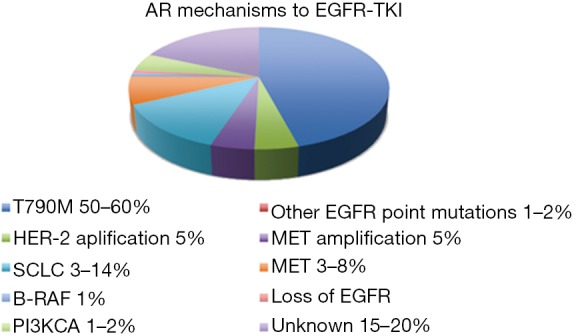
Mechanisms of AR to EGFR-TKI therapy. AR, acquired resistance; EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; SCLC, small cell lung cancer.
Second-site mutations in EGFR
Approximately 50–60% of cases of acquired EGFR-TKI resistance have the T790M mutation. It is located in the EGFR tyrosine kinase domain and coexists with the activating mutation in EGFR (27,28). The T790M mutation produces EGFR-TKI resistance by increasing the binding affinity between ATP and EGFR, causing decreased binding by EGFR-TKIs (29).
The T790M mutation can also be detected in the tumours of EGFR-TKI naïve patients, and is associated with a worse prognosis (30). However, patients with an acquired T790M mutation tend to show slower progression, with greater prevalence of pleuropulmonary and lymphatic spread, progression at existing metastatic sites, and better functional status than patients without the T790M mutation who also progress (31,32).
In patients with acquired EGFR-TKI resistance, clones with and without the T790M mutation can coexist. This might explain the “flare” phenomenon sometimes seen when this treatment is stopped, and also why patients can respond again to an EGFR-TKI after previous treatment discontinuation (33,34).
As well as the T790M mutation, other mutations associated with acquired EGFR-TKI resistance have also been described: T854A in exon 21, and L747S and D761Y in exon 19, albeit at a lower frequency than T790M (35-37).
Bypass track activation
Other mechanisms of acquired resistance to EGFR-TKIs exist, based on the activation of parallel signalling pathways, in which the EGFR pathway is activated independently. The first mechanism to be described was amplification of the MET receptor tyrosine kinase gene and over-expression of its ligand hepatocyte growth factor (HGF) (38-41). MET amplification leads to activation by ERBB3 phosphorylation, which maintains activation of the PI3K/AKT signalling cascade, providing an alternative signalling pathway even in the presence of an EGFR-TKI. MET amplification has been detected in 5–22% of samples from patients with acquired EGFR-TKI resistance (7), can be detected together with the T790M mutation in 40% of cases (38,42), and may be found in 3% of treatment-naïve EGFR mutation-positive patients (38).
Another bypass mechanism of acquired EGFR-TKI resistance, found in preclinical models, consists of MET activation by over-expression of its ligand HGF (43). Other mutations that may activate alternative pathways as mechanisms of acquired EGFR-TKI resistance are PI3KCA mutation (7), HER2 amplification (44) or BRAF mutation (45). Loss of the activating EGFR mutation has also been described as a mechanism of acquired resistance to EGFR-TKIs (46).
Phenotypic transformation
It has also been observed that histological transformation can act as a mechanism of acquired EGFR-TKI resistance. This includes tumour transformation to small-cell lung carcinoma (SCLC) (3–14%) and epithelial to mesenchymal transition (EMT) (8%) (7,47). The mechanism by which these histological transformations take place is unknown, as is the rate at which they occur.
EMT is the mechanism by which tumour cells lose their epithelial phenotype and develop mesenchymal-like morphology. This transition is accompanied by the loss of binding proteins, such as E-cadherin, and the acquisition of mesenchymal markers, such as vimentin or fibronectin (48). EMT has been linked to AXL activation (49), increased NOTCH-1 expression (50), or aberrant expression of transforming growth factor beta (TGF-β) (51,52).
Therapeutic management in EGFR mutation-positive NSCLC patients who progress on EGFR-TKI therapy
Acquired resistance to EGFR-TKIs has been widely investigated, and several therapeutic strategies to counter this have been explored.
Benefit of maintaining EGFR-TKI despite progression
The historical algorithm for cancer treatment has been to discontinue a therapy at the time of progression and switch to another drug. However, with oncogene-addicted cancers, this paradigm may require revision. Treatment selection considers type (slow vs. rapid) and location (single versus multiple sites) of progression, and the presence of cancer-related symptoms. For patients with EGFR mutation-positive who develop localised disease progression, several studies suggest that local therapy to these sites, with surgery or radiation, in combination with ongoing use of the same EGFR-TKI might also be clinically beneficial (53).
In one retrospective study of patients with EGFR-mutant NSCLC and acquired resistance, near 25% experienced a clinically significant rapid flare of disease within days of stopping an EGFR-TKI (34). One explanation for this phenomenon is that tumors are comprised of heterogeneous population of cell clones. Likely, a large proportion of such cell clones are still sensitive to the original EGFR-TKI, but latent (G0 cell-cycle arrest), and they could grow rapidly without EGFR-TKI. Repeated biopsies have documented that if chemotherapy is given instead of erlotinib after the development of erlotinib-acquired resistance, a previously documented T790M mutation can “disappear” and patients can then re-respond to erlotinib (7).
Evidence suggests that in case of slow progression, continuation of treatment with EGFR-TKIs may be an option for selected patients, in particular for those who have benefitted from EGFR-TKIs and lack of cancer-related symptoms. In case of progression in a single site, local radiotherapy or surgery may be added to continued treatment with EGFR-TKIs (54). There is no evidence that the switch to other types of EGFR-TKIs improves OS. However, switch to afatinib or dacomitinib after progression to first generation EGFR-TKIs have shown to improve PFS and RR, but not OS (55,56).
Benefit of chemotherapy with or without EGFR-TKI
Most patients are chemotherapy-naive at the time of acquired resistance. One current major clinical question is whether patients with widespread acquired resistance should stop their initial EGFR-TKI (with potential flare risk) and switch to chemotherapy, or continue EGFR-TKI beyond progression with the addition of chemotherapy to the regimen (57). Several prospective trials had focus in this question.
The results of a phase II study suggested that the addition of standard chemotherapy to 33 EGFR-mutant patients treated with gefitinib and three cycles of cisplatin plus docetaxel might prevent the development of acquired resistance to EGFR-TKIs (58). In the LUX-Lung 5 trial, 202 patients with progressive disease following clinical benefit with afatinib were randomized to afatinib plus paclitaxel, or investigator’s single-agent chemotherapy. PFS (5.6 vs. 2.8 months; P=0.003) and RR (32.1% vs. 13.2%; P=0.005) significantly improved with afatinib plus paclitaxel, but there was no difference in OS (59). However, the phase III IMPRESS study showed no significant improvement in PFS and OS with continued use of gefitinib plus pemetrexed/cisplatin doublet chemotherapy compared with chemotherapy alone in 265 EGFR mutation-positive patients who progressed on first-line treatment with EGFR-TKIs (60).
There is a lack of data regarding the efficacy of chemotherapy plus EGFR-TKIs in advanced NSCLC with intrinsic resistance due to the presence of de novo T790M mutations.
Therapeutic management of patients EGFR T790M-positive
Second-generation EGFR-TKIs, such as afatinib, dacomitinib, or neratinib, inhibit T790M in vitro, but with insufficient efficacy in clinical studies. The combination of afatinib with cetuximab may overcome T790M-mediated resistance in preclinical studies. In a phase II study with 126 EGFR mutant-positive patients who progressed to EGFR-TKI, the RR was 29% and PFS 4.7 months, without differences according T790M status (61). In the BELIEF study, the treatment of patients T790M-positive with the combination of erlotinib plus bevacizumab resulted in 1-year PFS of 72% (62).
Third-generation EGFR-TKIs target EGFR-activating mutations and the T790M resistance mutation. On the other hand, they less effectively inhibit wild-type EGFR. Thus, these EGFR-TKIs should have greater efficacy and less toxicity in comparison to first- and second-generation EGFR-TKIs. Third-generation EGFR-TKIs in clinical development include osimertinib, rociletinib, HM61713, and others (63).
Osimertinib is the only drug approved through several clinical trials (64). In the phase I part of the AURA trial (NCT01802632), patients received osimertinib 20, 40, 80, 160 or 240 mg/day (n=31), or five expansion cohorts at different doses (n=222). The EGFR T790M mutation was detected in the tumor samples from 138 of the 222 patients (62%) of the expansion cohorts, not detected in 62 patients (28%), and unknown in 22 patients (10%). Of 239 patients evaluated for response, 123 (51%) had a confirmed response. The disease control rate (DCR) was 84%. Of the 138 patients with confirmed EGFR T790M mutation, 127 could be evaluated for response. The RR was observed in 78 patients (61%), and DCR in 121 patients (95%). Regarding duration of response (DR) and PFS, in the subgroup of patients with EGFR T790M mutation, 88% of patients had DR of ≥6 months, with a median PFS of 9.6 months (8).
The AURA 2 trial (NCT02094261) was a single-arm phase II study of AZD9291 80 mg/day in patients with T790M-positive NSCLC after failure of first-line EGFR-TKI. A total of 210 patients were included. The RR was 71% and the DCR was 92%. The median DR was 7.8 months and the median PFS was 8.6 months. Rate of patients who are alive without progression at 6 months were 70% (65).
In the pooled data from the two AURA studies (the AURA phase II extension study of cohorts and the AURA 2 included a total of 411 patients, of which 14 patients had no measurable disease and patients with negative T790M were excluded), the RR was 66% (263/398) and the DCR was 91% (360/397). The median DR was not reached and median PFS was 9.7 months (Table 2) (66).
Table 2. Efficacy data of osimertinib in EGFR mutant patients with NSCLC after progression on EGFR-TKI.
| Efficacy (95% CI) | AURA (expansion phase I) (n=63) (8) | AURA (expansion phase II) (n=201) (8) | AURA 2 phase II (n=210) (65) | Pooled AURA I–II (n=411) (66) | AURA 3 (n=279) (67) |
|---|---|---|---|---|---|
| RR (%) | 61 [48–74] | 61 [54–68] | 71 [64–67] | 66 [61–71] | 71 [65–76] |
| DR (months) | 9.7 [8.3–NR] | NR | 7.8 [7.1–NR] | NR [8.3–NR] | 9.7 [8.3–11.6] |
| DR up to 6 months (%) | 72 [54–84] | 83 [74–89] | 75 [65–82] | 78 [72–84] | 49 |
| DCR (%) | 95 [86–99] | 90 [85–94] | 91 [87–95] | 91 [88–94] | 93 [90–96] |
| PFS (months) | 11 [7–15] | NR [8.1–NR] | 8.6 [8.2–9.7] | 9.7 [8.3–NR] | 10.1 [8.3–12.3] |
DCR, disease control rate; DR, duration of response; EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; NR, not reached; NSCLC, non-small cell lung cancer; PFS, progression-free survival; RR, response rate.
The AURA 3 trial (NCT02151981) is a phase 3 trial including 419 advanced NSCLC patients with T790M-positive who had disease progression after first-line EGFR-TKI therapy, and were randomized to receive oral osimertinib versus chemotherapy based on platinum and pemetrexed. The RR and PFS were significantly superior with osimertinib in comparison with chemotherapy (71% vs. 31% and 10.1 vs. 4.4 months, respectively; HR: 0.30; 95% CI: 0.23–0.41; P<0.001). In 144 patients with metastases to the central nervous system (CNS), PFS was 8.5 vs. 4.2 months, respectively (HR: 0.32; 95% CI: 0.21–0.49). The proportion of patients with adverse events of grade 3 or higher was lower with osimertinib (23%) than with chemotherapy (47%) (67).
Additionally, the FLAURA trial (NCT02296125) is an ongoing phase III trial that compare osimertinib versus gefitinib or erlotinib as first-line therapy in advanced EGFR mutation-positive NSCLC.
Therapeutic management of patients with other molecular alterations
Intrinsic resistance to EGFR-TKIs can be developed through upregulation or amplification of MET. In a phase II trial investigating the MET inhibitor INC280 plus gefitinib in EGFR-mutated and MET-positive NSCLC patients who progressed after prior EGFR-TKI treatment, 6 of 41 patients (15%) reached a response (68). The dual MET-VEGF inhibitor cabozantinib plus erlotinib showed a RR of 11% in EGFR-mutated NSCLC patients following progression on EGFR-TKI therapy (69). Targeting the PI3K pathway could be a novel strategy to overcome TKI resistance. The dual inhibitor of PI3K/mTOR, NVP-BEZ235, was found to inhibit the growth of gefitinib-resistant NSCLC cells in vivo as well as in vitro (70). A phase II study of the AKT inhibitor MK-2206 plus erlotinib showed a RR of 9% in advanced mutant EGFR-positive NSCLC previously treated with erlotinib, with a PFS of 4.4 months (71). Therefore, specific inhibitors targeting MET, PI3K, or other pathways may be promising treatments for NSCLC patients with mutations associated with intrinsic resistance to EGFR-TKIs.
Indications for rebiopsy in EGFR mutation-positive NSCLC patients who progress on EGFR-TKIs
Considering the efficacy of drugs such as osimertinib for treating patients with the T790M resistance mutation (8,65), it is important to determine whether this mutation is present at the time of progression (72-75).
Whenever possible, tissue should be obtained from the most accessible part of a lesion that has progressed, be it a new lesion, a metastasis of the primary tumour, or lymphadenopathies. In a retrospective study led by Kawamura, in which the role of rebiopsy was analyzed in 120 patients, no differences in mutation rate were found between rebiopsies of the primary lesion or of metastases (76). In another study analysing 88 tumour specimens collected synchronously or metachronously from the same or different sites, Quéré et al. found no discordance in the detection of EGFR mutations between the various biopsy sites (77). Likewise, virtually no differences were found between specimens of primary tumour and metastases using next-generation sequencing techniques (78).
There will be times when obtaining a tissue biopsy is difficult or impossible. In these cases, other techniques can be used, such as liquid biopsy. Studies indicate that peripheral blood contains circulating free DNA (cfDNA), including from circulating tumour cells (CTCs), as well as small amounts of circulating tumour DNA (ctDNA). Such DNA can be detected by various techniques (cobas®, therascreen®, BEAMing, ddPCR). A retrospective study that conducted BEAMing analysis on over 200 samples of cfDNA from patients treated with osimertinib from the AURA study found 70% sensitivity for detecting the T790M mutation (79). Outcomes in patients whose plasma proved positive for T790M were equivalent to those seen in patients with positive tissue tests (PFS: 9.7 months). A recent study compared T790M mutation detection rates in cfDNA and CTCs against biopsies in 40 patients. The T790M mutation was found in 75% of biopsies, 70% of CTC samples and 80% of ctDNA samples. It was concluded that the various ways of detecting cfDNA are similar (80).
Plasma samples were also analyzed by BEAMing and cobas® and compared against tissue in the Phase I rociletinib study. Positive percent agreement was found to be 73% for BEAMing and 64% for cobas® (81). Thress et al. compared the EGFR mutations present in 38 ctDNA samples using two non-digital platforms (cobas® EGFR mutation test and therascreen® EGFR ARMS-PCR) and two digital platforms (Droplet DigitalTM and BEAMing dPCR), using tissue for test comparisons (82). They found that both cobas® and BEAMing possessed greater sensitivity for detecting the T790M mutation. They also found that 30% of patients whose biopsies were previously negative or inconclusive tested positive for the T790M mutation with cobas® and BEAMing (Table 3). In another study, reported at ASCO 2016, samples from over 400 patients treated with rociletinib were analyzed. The T790M mutation was detected in tissue (therascreen®), plasma (BEAMing) and urine (Trovagene), in 417, 189 and 136 patients, respectively. Good correlation of RR and duration of response was also observed between the different assay procedures (83). It can therefore be concluded that the gold standard is to detect the mutation in rebiopsy tissue, whereas liquid biopsy is useful in cases in which rebiopsy would be difficult or impossible (Figures 3,4).
Table 3. Comparison of the different techniques used to detect T790M in circulating DNA (adapted from Thress et al.) (82).
| Technique | cobas® (%) | therascreen® (%) | ddPCR (%) | BEAMing (%) |
|---|---|---|---|---|
| Sensitivity | 41 | 29 | 71 | 71 |
| Specificity | 100 | 100 | 83 | 67 |
| Concordance | 57 | 48 | 74 | 79 |
It can therefore be concluded that the gold standard is to detect the mutation in rebiopsy tissue, whereas liquid biopsy is useful in cases in which rebiopsy would be difficult or impossible (Figures 3,4).
Figure 3.

Treatment algorithm for managing EGFR mutation-positive NSCLC patients. EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; NSCLC, non-small cell lung cancer. Modified from Novello et al. (84).
Figure 4.

Diagnostic protocol for tissue and liquid biopsies for the T790M mutation. EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase inhibitors; NSCLC, non-small cell lung cancer. Modified from Oxnard et al. (79).
Conclusions
The identification of actionable oncogenic driver mutations in NSCLC patients has changed their treatment, greatly affecting the OS of patients administered targeted agents compared with those administered other therapies or whose tumours have no known genetic dependency to date.
EGFR mutations (deletions in exon 19 or mutations in exon 21) are present in 10–12% of the Caucasian population with NSCLC. Several reversible and irreversible EGFR-TKIs exist, however, that are effective against these tumours, as confirmed by nine superiority trials versus platinum doublet chemotherapy as first-line treatment. Unfortunately, EGFR-TKI activity lasts for 9 to 12 months, after which resistance develops by various mechanisms (acquired resistance). The most common resistance mechanism (50–60%) is acquisition of the missense mutation in codon 790 of EGFR exon 20 (T790M). Other mechanisms of TKI resistance, such as MET amplification, PI3KCA mutations, HER2 amplification, BRAF mutation, loss of the activating EGFR mutation, phenotypic transformation of the tumour to SCLC and EMT, are less common.
The development of several agents active against the T790M mutation, such as osimertinib, has changed the approach to progression after initial EGFR-TKIs, not just in terms of treatment but also in respect of determining the mechanisms by which the tumour escaped from the first EGFR-TKI, and the various forms of NSCLC progression.
Clinical progression on the first EGFR-TKI may be manifested locally, gradually or dramatically, entailing a different approach to treatment in each case. In cases of slow progression, where symptoms are mild or absent, treatment with the same initial EGFR-TKI can be maintained, together with surgery or radiotherapy for the progressing lesion. In other cases, it will be necessary to identify which resistance mechanism has arisen, discontinue EGFR-TKIs, and administer a specific anti-T790M agent if tests for this mutation prove positive.
Osimertinib is the only approved EGFR-TKI that is active against the EGFR T790M mutation. It has shown a RR of over 70% in progression after an initial EGFR-TKI, at least 90% disease control rate, and PFS of at least 10 months. These results make it essential to identify the molecular alteration causing resistance to initial EGFR-TKIs, both for the therapeutic consequences and for the patient’s benefit. If accessible and feasible, relapsed tissue should be taken from the primary lesion or lymphadenopathies, resorting to analogue or digital analyses of liquid biopsies when specimen collection is difficult or impossible. Several recent studies have confirmed the similarity of results obtained from tissue or cfDNA, in terms of sensitivity, specificity and concordance. Not only do these methods provide similar treatment benefits, but they also enable the mutation to be monitored and quantified when the patient progresses on EGFR-TKIs.
Acknowledgements
The authors wish to thank Fernando Sánchez-Barbero and HealthCo S.L. (Madrid, Spain) for his editorial help in preparing the first draft of this manuscript. The necessary scientific meetings along with medical writing services were funded by AstraZeneca Spain.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends - An update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. 10.1158/1055-9965.EPI-15-0578 [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354-6. 10.1016/S0140-6736(15)01125-3 [DOI] [PubMed] [Google Scholar]
- 3.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. 10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. 10.1056/NEJMoa0904554 [DOI] [PubMed] [Google Scholar]
- 5.Esteban E, Majem M, Martínez Aguillo M, et al. Prevalence of EGFR mutations in newly diagnosed locally advanced or metastatic non-small cell lung cancer Spanish patients and its association with histological subtypes and clinical features: The Spanish REASON study. Cancer Epidemiol 2015;39:291-7. 10.1016/j.canep.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Vidal J, Clavé S, de Muga S, et al. Assessment of ALK status by FISH on 1000 Spanish non-small cell lung cancer patients. J Thorac Oncol 2014;9:1816-20. 10.1097/JTO.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 7.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. 10.1056/NEJMoa1411817 [DOI] [PubMed] [Google Scholar]
- 9.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. 10.1200/JCO.2010.33.4235 [DOI] [PubMed] [Google Scholar]
- 11.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 12.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T, Morita S, Yatabe Y, et al. Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2012;30:abstr 7521.
- 14.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 15.Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. 10.1093/annonc/mds214 [DOI] [PubMed] [Google Scholar]
- 16.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. 10.1093/annonc/mdv276 [DOI] [PubMed] [Google Scholar]
- 18.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 19.Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. 10.1093/annonc/mdv270 [DOI] [PubMed] [Google Scholar]
- 20.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 21.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 22.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 23.Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 24.Paz-Ares L, Tan EH, Zhang L, et al. editors. Afatinib (A) vs gefitinib (G) in patients (pts) with EGFR mutation-positive (EGFRm+) non-small-cell lung cancer (NSCLC): overall survival (OS) data from the phase IIb trial LUX-Lung 7 (LL7) abstr LBA43). European Society for Medical Oncology (ESMO); Copenhagen, Denmark, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. 10.1016/S1470-2045(14)70381-X [DOI] [PubMed] [Google Scholar]
- 26.Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33-9. 10.1016/j.lungcan.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92. 10.1056/NEJMoa044238 [DOI] [PubMed] [Google Scholar]
- 28.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. 10.1371/journal.pmed.0020073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. 10.1073/pnas.0709662105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res 2011;17:1160-8. 10.1158/1078-0432.CCR-10-2158 [DOI] [PubMed] [Google Scholar]
- 31.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 2011;3:90ra59. 10.1126/scitranslmed.3002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. 10.1158/1078-0432.CCR-10-2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res 2007;13:5150-5. 10.1158/1078-0432.CCR-07-0560 [DOI] [PubMed] [Google Scholar]
- 34.Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298-303. 10.1158/1078-0432.CCR-11-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bean J, Riely GJ, Balak M, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res 2008;14:7519-25. 10.1158/1078-0432.CCR-08-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa DB, Schumer ST, Tenen DG, et al. Differential responses to erlotinib in epidermal growth factor receptor (EGFR)-mutated lung cancers with acquired resistance to gefitinib carrying the L747S or T790M secondary mutations. J Clin Oncol 2008;26:1182-4; author reply 1184-6. 10.1200/JCO.2007.14.9039 [DOI] [PubMed] [Google Scholar]
- 37.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 2006;12:6494-501. 10.1158/1078-0432.CCR-06-1570 [DOI] [PubMed] [Google Scholar]
- 38.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7. 10.1073/pnas.0710370104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 40.Yano S, Takeuchi S, Nakagawa T, et al. Ligand-triggered resistance to molecular targeted drugs in lung cancer: roles of hepatocyte growth factor and epidermal growth factor receptor ligands. Cancer Sci 2012;103:1189-94. 10.1111/j.1349-7006.2012.02279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson KW, Sandler AB. The role of MET receptor tyrosine kinase in non-small cell lung cancer and clinical development of targeted anti-MET agents. Oncologist 2013;18:115-22. 10.1634/theoncologist.2012-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suda K, Murakami I, Katayama T, et al. Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res 2010;16:5489-98. 10.1158/1078-0432.CCR-10-1371 [DOI] [PubMed] [Google Scholar]
- 43.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 2008;68:9479-87. 10.1158/0008-5472.CAN-08-1643 [DOI] [PubMed] [Google Scholar]
- 44.Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2012;2:922-33. 10.1158/2159-8290.CD-12-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohashi K, Sequist LV, Arcila ME, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A 2012;109:E2127-33. 10.1073/pnas.1203530109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabara K, Kanda R, Sonoda K, et al. Loss of activating EGFR mutant gene contributes to acquired resistance to EGFR tyrosine kinase inhibitors in lung cancer cells. PLoS One 2012;7:e41017. 10.1371/journal.pone.0041017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suda K, Tomizawa K, Fujii M, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol 2011;6:1152-61. 10.1097/JTO.0b013e318216ee52 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012;44:852-60. 10.1038/ng.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie M, Zhang L, He CS, et al. Activation of Notch-1 enhances epithelial-mesenchymal transition in gefitinib-acquired resistant lung cancer cells. J Cell Biochem 2012;113:1501-13. [DOI] [PubMed] [Google Scholar]
- 51.Shan B, Yao TP, Nguyen HT, et al. Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem 2008;283:21065-73. 10.1074/jbc.M802786200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serizawa M, Takahashi T, Yamamoto N, et al. Combined treatment with erlotinib and a transforming growth factor-beta type I receptor inhibitor effectively suppresses the enhanced motility of erlotinib-resistant non-small-cell lung cancer cells. J Thorac Oncol 2013;8:259-69. 10.1097/JTO.0b013e318279e942 [DOI] [PubMed] [Google Scholar]
- 53.Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346-51. 10.1097/JTO.0b013e31827e1f83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.García-Campelo R, Bernabé R, Cobo M, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (NSCLC) 2015. Clin Transl Oncol 2015;17:1020-9. 10.1007/s12094-015-1455-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. 10.1016/S1470-2045(12)70087-6 [DOI] [PubMed] [Google Scholar]
- 56.Ellis PM, Liu G, Millward M, et al. NCIC CTG BR.26: A phase III randomized, double blind, placebo controlled trial of dacomitinib versus placebo in patients with advanced/metastatic non-small cell lung cancer (NSCLC) who received prior chemotherapy and an EGFR TKI. J Clin Oncol 2014;32:abstr 8036.
- 57.Moran T, Sequist LV. Timing of epidermal growth factor receptor tyrosine kinase inhibitor therapy in patients with lung cancer with EGFR mutations. J Clin Oncol 2012;30:3330-6. 10.1200/JCO.2012.43.1858 [DOI] [PubMed] [Google Scholar]
- 58.Kanda S, Horinouchi H, Fujiwara Y, et al. Cytotoxic chemotherapy may overcome the development of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) therapy. Lung Cancer 2015;89:287-93. 10.1016/j.lungcan.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 59.Schuler M, Yang JC, Park K, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-Lung 5 trial. Ann Oncol 2016;27:417-23. 10.1093/annonc/mdv597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990-8. 10.1016/S1470-2045(15)00121-7 [DOI] [PubMed] [Google Scholar]
- 61.Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036-45. 10.1158/2159-8290.CD-14-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stahel RA, Dafni U, Gautschi O, et al., editors. A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced non-small-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T790M mutation.The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial abstr 3BA). European Cancer Congress; Vienna, Austria, 2015. [Google Scholar]
- 63.Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov 2013;3:1404-15. 10.1158/2159-8290.CD-13-0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greig SL. Osimertinib: First Global Approval. Drugs 2016;76:263-73. 10.1007/s40265-015-0533-4 [DOI] [PubMed] [Google Scholar]
- 65.Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. 10.1016/S1470-2045(16)30508-3 [DOI] [PubMed] [Google Scholar]
- 66.Goss G, Yang JC, Ahn MJ, et al. AZD9291 in pre-treated patients with T790M positive advanced non-small cell lung cancer (NSCLC): Pooled analysis from two Phase II studies. Eur J Cancer 2015;51:S640:abstr 3113.
- 67.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu YL, Yang JC, Kim DW, et al. Safety and efficacy of INC280 in combination with gefitinib (gef) in patients with EGFR-mutated (mut), MET-positive NSCLC: A single-arm phase lb/ll study. J Clin Oncol 2014;32:abstr 8017.
- 69.Reckamp KL, Frankel PH, Mack PC, et al. Phase II trial of XL184 (cabozantinib) plus erlotinib in patients (pts) with advanced EGFR-mutant non-small cell lung cancer (NSCLC) with progressive disease (PD) on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy: A California Cancer Consortium phase II trial (NCI 9303). J Clin Oncol 2014;32:abstr 8014. [DOI] [PMC free article] [PubMed]
- 70.Sun Z, Li Q, Zhang S, et al. NVP-BEZ235 overcomes gefitinib-acquired resistance by down-regulating PI3K/AKT/mTOR phosphorylation. Onco Targets Ther 2015;8:269-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu L, Kikuchi E, Xu C, et al. Combined EGFR/MET or EGFR/HSP90 inhibition is effective in the treatment of lung cancers codriven by mutant EGFR containing T790M and MET. Cancer Res 2012;72:3302-11. 10.1158/0008-5472.CAN-11-3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao X, Le X, Costa DB. The safety and efficacy of osimertinib for the treatment of EGFR T790M mutation positive non-small-cell lung cancer. Expert Rev Anticancer Ther 2016;16:383-90. 10.1586/14737140.2016.1162103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jekunen AP. Role of rebiopsy in relapsed non-small cell lung cancer for directing oncology treatments. J Oncol 2015;2015:809835. [DOI] [PMC free article] [PubMed]
- 74.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-9. 10.3816/CLC.2009.n.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan DS, Yom SS, Tsao MS, et al. The International Association for the Study of Lung Cancer Consensus Statement on Optimizing Management of EGFR Mutation-Positive Non-Small Cell Lung Cancer: Status in 2016. J Thorac Oncol 2016;11:946-63. 10.1016/j.jtho.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 76.Kawamura T, Kenmotsu H, Taira T, et al. Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor-tyrosine kinase inhibitor failure. Cancer Sci 2016;107:1001-5. 10.1111/cas.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quéré G, Descourt R, Robinet G, et al. Mutational status of synchronous and metachronous tumor samples in patients with metastatic non-small-cell lung cancer. BMC Cancer 2016;16:210. 10.1186/s12885-016-2249-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vignot S, Frampton GM, Soria JC, et al. Next-generation sequencing reveals high concordance of recurrent somatic alterations between primary tumor and metastases from patients with non-small-cell lung cancer. J Clin Oncol 2013;31:2167-72. 10.1200/JCO.2012.47.7737 [DOI] [PubMed] [Google Scholar]
- 79.Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016;34:3375-82. 10.1200/JCO.2016.66.7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sundaresan TK, Sequist LV, Heymach JV, et al. Detection of T790M, the Acquired Resistance EGFR Mutation, by Tumor Biopsy versus Noninvasive Blood-Based Analyses. Clin Cancer Res 2016;22:1103-10. 10.1158/1078-0432.CCR-15-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karlovich C, Goldman JW, Sun JM, et al. Assessment of EGFR mutation status in matched plasma and tumor tissue of NSCLC patients from a phase I study of rociletinib (CO-1686). Clin Cancer Res 2016;22:2386-95. 10.1158/1078-0432.CCR-15-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015;90:509-15. 10.1016/j.lungcan.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 83.Wakelee H, Gadgeel S, Goldman JW, et al. Epidermal growth factor receptor (EGFR) genotyping of matched urine, plasma and tumor tissue from non-small cell lung cancer (NSCLC) patients (pts) treated with rociletinib. J Clin Oncol 2016;34:abstr 9001.
- 84.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. 10.1093/annonc/mdw326 [DOI] [PubMed] [Google Scholar]


