Abstract
In this work, asymmetrical flow field-flow fractionation (AF4) coupled with UV/Vis, multi-angle light scattering (MALS), and differential refractive index (dRI) detectors (AF4-UV-MALS-dRI) was employed for analysis of glutamate decarboxylase (LbGadB) from Lactobacillus brevis (L. brevis). AF4 provided molecular weight (MW) (or size)-based separation of dimer, hexamer, and aggregates of LbGadB. The effect of pH on oligomerization of LbGadB was investigated, and then AF4 results were compared to those from molecular modeling. The MWs measured by AF4-UV-MALS-dRI for dimeric and hexameric forms of LbGadB were 110 and 350 kDa, respectively, which are in good agreements with those theoretically calculated (110 and 330 kDa). The molecular sizes determined by AF4-UV-MALS-dRI were also in good agreement with those obtained from molecular modeling (6 and 10 nm, respectively, for dimeric and hexameric from AF4-UV-MALS-dRI and 6.4 × 7.6 and 7.6 × 13.1 nm from molecular modeling). The effects of temperature, salt type, and salt concentration on oligomerization of LbGadB were also investigated using dynamic light scattering (DLS). It was found that the hexameric form of LbGadB was most stable at pH 6 and in presence of NaCl or KCl. The results indicate that AF4, in combination of various online detectors mentioned above, provides an effective tool for monitoring of oligomerization of LbGadB under different conditions, such as temperature, pH, type of salts, and salt concentrations.
Electronic supplementary material
The online version of this article (10.1007/s00216-017-0735-6) contains supplementary material, which is available to authorized users.
Keywords: Glutamate decarboxylase (GAD), Oligomerization, Asymmetrical flow field-flow fractionation (AF4), Multi-angle light scattering (MALS), Lactobacillus brevis (L. brevis), Probiotic
Introduction
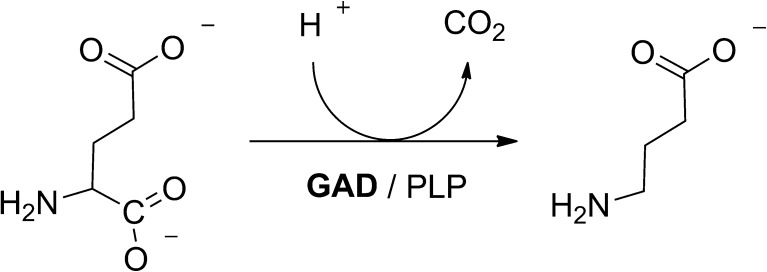
Glutamate decarboxylase (GAD) catalyzes decarboxylation of glutamic acid giving γ-aminobutyric acid (GABA) (Fig. 1). GAD uses pyridoxal phosphate (PLP) as the co-factor and H+ as the co-substrate. This enzyme is present in a variety of organisms, from bacteria to humans. GABA is one of the main neurotransmission inhibitors in the central nervous system. In addition, evidence exist that GABA can lower blood pressure in patients with mild hypertension and that it has other potential beneficial health effects, although mechanisms are not known yet [1, 2]. While GABA is an attractive potential functional ingredient for food, chemically synthesized GABA is not accepted for use in food [3].
Fig. 1.
Reaction catalyzed by the glutamate decarboxylase (LbGadB) in the presence of pyridoxal phosphate (PLP) as the co-factor
GABA is found in some fermented food, as for instance in kimchi [4]. Some strains of Lactobacillus brevis (L. brevis) were identified as GABA producers [3]. Due to its potential health effects in humans, L. brevis is recognized as a putative probiotic [5]. Thus, the potential production of GABA at industrial levels using L. brevis as whole cell or its glutamate decarboxylase (LbGadB; notice that “GAD” represents the general name of glutamate decarboxylases present in a variety of organisms, while “LbGadB” represent specifically the glutamate decarboxylase from L. brevis) enzyme is very attractive. Therefore, there is great interest to understand the structure and function of LbGadB. It was previously reported that the highest level of activity is reached when GAD is in the hexameric form, while the lowest was observed when GAD was present as a dimer [6, 7]. Thus, oligomerization plays an important role in the GAD mechanism.
In general, size-exclusion chromatography (SEC), gel electrophoresis, and field-flow fractionation (FFF) are used to observe oligomerization of proteins.
SEC is commonly used for measurement of hydrodynamic size (or more commonly molecular weight, MW) based on size-based separation of analytes and their size distribution from calibration curve of standard samples or the multi-angle light scattering (MALS) [8, 9]. However, difficulties are frequently encountered when SEC is applied to high MW analytes as they may undergo degradation by shear or they may be trapped in the SEC columns [10, 11]. Furthermore, analytes may reach the column exclusion limit or the permeation limit leading to underestimation or overestimation of MW, in addition to blockage of the column [11, 12].
Asymmetrical flow field-flow fractionation (AF4) also provides separation of analytes based on their hydrodynamic sizes. In AF4, an open channel without packing material is used [13] and AF4 thus has several advantages over SEC. Due to the relatively gentle separation conditions in AF4 (i.e., low pressure and shear), degradation of analytes is prevented during separation. AF4 has been successfully used for the separation and characterization of high MW analytes including proteins, DNA, viruses, and polysaccharides [14–17]. In addition, AF4 provides means to determine some physical properties, without calibration, such as MW, size, molecular density, and conformation when it is coupled online with the MALS.
The stability of the active hexameric form of GAD from L. brevis depends on several factors, including temperature, pH, salt concentration, and type of salt. Thus, the study of the effect of these factors on the GAD oligomerization is fundamental to optimize the reaction conditions. In this work, the use of diverse techniques, such as AF4 coupled online with MALS (AF4-MALS), dynamic light scattering (DLS), differential scanning fluorimetry (DSF), and molecular modeling methods, allows the integrated characterization of the main physicochemical factors that determine the stability of the oligomeric enzyme GAD.
Material and methods
Materials
Citric acid (C6H8O7), sodium hydrogen phosphate anhydrous (Na2HPO4), sodium chloride (NaCl), potassium chloride (KCl), calcium chloride (CaCl2), ammonium sulfate ((NH4)2SO4), and sodium azide (NaN3) were purchased from Sigma-Aldrich (St. Louis, USA).The carrier liquid for AF4 was prepared with water purified through a Milli-Q purification system (Millipore Co. Ltd., Billerica, USA, resistance = 18.2 MΩ/cm).
Molecular cloning
L. brevis DSM 1269 was purchased from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures. The strain was cultured in a MRS (Man, Rogosa, and Sharpe) medium at 30 °C overnight after which genomic DNA was extracted using E.Z.N.A Genomic Isolation Kit (Omega Bio-Tek, USA). PCR primers, specific for GAD gene, were designed based on the genomic sequence of strain ATCC 367: Forward 5′-ATG ACG ACT ATC ATA TGA ATA AAA ACG ATC AGG AAA C-3′ and reverse 5′-GTC AGC TGC CCC TCG AGA CTT CGA ACG GTG GTC-3′ with restriction sites (underlined in the sequences) for NdeI and XhoI, respectively. The amplified gene was inserted in the protein expression vector pET21b giving the construct pET21b::LbGadB, which was introduced in Escherichia coli (E. coli) Origami 2 (DE3) (Novagen brand, Merck KGaA, Darmstadt, Germany). Recombinant E. coli was grown in 500 mL of LB (Luria Bertani) medium supplemented with 100 μg/mL ampicillin, at 37 °C. Recombinant LbGadB production was induced when the culture optical density (OD) at λ = 600 nm reaches 0.6, with isopropyl β-D-1-thiogalactopyranoside, at 30 °C during 6 h. Finally, the cell pelleted was harvested by centrifugation at 8000×g per 10 min in a Sorvall refrigerated centrifuge (RC5C, USA) for the recombinant protein purification.
Protein purification
Recombinant LbGadB was purified by ion-metal affinity chromatography. E. coli cell pellet was washed twice with binding buffer pH 7.4 (50 mM sodium phosphate, 0.5 M NaCl, and 20 mM imidazole). Cell lysis was performed suspending 1 g of cell pellet in 5 mL BugBuster® Protein Extraction Reagent (San Diego, CA, USA) with 5 μL Lysonase™ Bioprocessing Reagent and incubated for 30 min at 25 °C. Next, the suspension was centrifuged at 14,000×g, the precipitate was discarded, and the supernatant was injected in a 5-mL HisTrap column FF (GE Healthcare, Uppsala, Sweden) previously equilibrated with binding buffer. After injection, the column was washed with binding buffer and the recombinant protein eluted with elution buffer pH 7.4 (50 mM sodium phosphate, 0.5 M NaCl, and 0.5 M imidazole). Finally, the excess of NaCl was removed by dialysis in 50 mM sodium phosphate buffer at pH 7.4. Protein purity was determined by SDS-PAGE and the concentration was quantified spectrophotometrically at 280 nm.
Molecular modeling
Hybrid homology model of LbGadB was constructed using the YASARA software [18]. Crystallographic structures of other glutamate decarboxylases deposited in the Protein Data Bank (PDB codes: 3HBX, 1XEY, 1PMM, and 3MAD, with amino acid sequence identities of 38, 39, 38, and 23%, respectively) were used as templates. The modeled structure was refined by molecular dynamic simulation using the AMBER03 force field [19]. The solvent was simulated with explicit molecules of water. Analysis of the modeled structure was done using UCSF Chimera v1.11.2 [20].
Asymmetrical field-flow fractionation
The asymmetrical flow field-flow fractionation (AF4) used in this work was an Eclipse 3+ system (Wyatt Technology, Dernbach, Germany) coupled online with a UV detector (UV-975, Jasco Corporation, Japan) set at 280 nm, a multi-angle light scattering (MALS) detector (DAWN HELEOS ΙΙ, Wyatt Technology), and a differential refractive index (dRI) detector (Optilab T-rEX, Wyatt Technology). The AF4 channel was trapezoidal with the tip-to-tip length of 26.5 cm and the width at the inlet and outlet of 2.2 and 0.6 cm, respectively, and was equipped with a 350-μm-thick Mylar spacer and a regenerated cellulose (RC) membrane (molecular weight cut-off of 10 kDa, Millipore, Bedford, USA).The AF4 carrier liquid with various pH (3 to 8) was 10 mM citrate-phosphate buffer and was pumped into the AF4 channel using an Agilent 1200 HPLC pump equipped with an auto-sampler and an in-line vacuum degasser (Agilent Technologies, Waldbronn, Germany). The channel flow rate was kept constant at 0.5 mL/min, while the cross flow rate was kept constant at 4.5 mL/min for the first 20 min; after which, it was exponentially decreased from 4.5 to 0.1 mL/min with the half-life time of 2 min and then kept constant at 0.1 mL/min for 30 min. The channel was washed with the carrier liquid for 10 min without cross flow at the end of each run. All AF4 experiments were performed at room temperature. The collection and processing of AF4 data were performed using the ASTRA software (Version 6.1.1, Wyatt Technology) with the d n/d c value of 0.185 mL/g. In all cases, the Berry method was used to fit the light scattering data [21, 22]. From AF4 retention time, the hydrodynamic diameter (d H) of a sample was calculated by the AF4 theory using the FFFHydRad 2.0 software [23, 24].
Determination of thermal stability of LbGadB
Thermal stability of LbGadB in different pH values was determined using the Prometheus NT 48 nanoDSF (NanoTemper Technologies, GmbH, Munich Germany). Protein samples were prepared in pH 4, 5, 6, 7, 8 (McIlvaine buffer system, 100 mM), and 9.6 (glycin-NaOH buffer, 100 mM). The capillaries were directly filled with 10 μL of every sample. Intrinsic fluorescence at emission wavelengths of 330 and 350 nm was monitored in a temperature gradient from 20 to 90 °C. All data analysis was performed using the PR control software (Version 2.0, Munich Germany).
Dynamic light scattering
The dynamic light scattering (DLS) analysis was performed using DynaPro Plate Reader ΙΙ (Wyatt Technology) equipped with a laser with the wavelength of 830 nm as the light source. The analysis conditions were as follows: temperature = 25, 37, 45, and 60 °C; accumulation time = 100 s; and number accumulation = 10. The LbGadB was prepared using various salt types (NaCl, KCl, CaCl2, and (NH4)2SO4) at concentrations (0, 0.3, 0.6, 0.9, and 1.2 M). Each sample of 60 μL was put in the supported 384-well plate before DLS analysis.
Results and discussion
Protein production
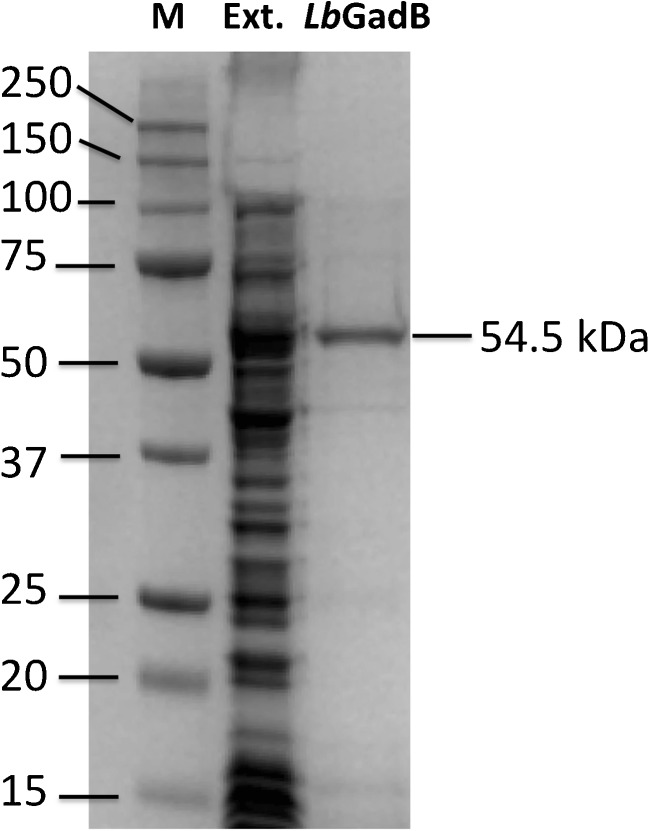
Glutamate decarboxylase gene from L. brevis DSM 1269 was cloned and the recombinant protein was successfully produced in E. coli. The gene was sequenced and deposited in the GenBank (accession code: KX417371). The protein sequence was obtained by theoretical translation of the gene. The protein sequence was identical to the LbGadB of the strain ATCC 367. The MW of the monomer was determined experimentally by SDS-PAGE (see Fig. 2) and theoretically from the protein sequence, giving the consistent value of 54.5 kDa.
Fig. 2.
Expression and purification of LbGadB from L. brevis DSM 1269
Molecular modeling
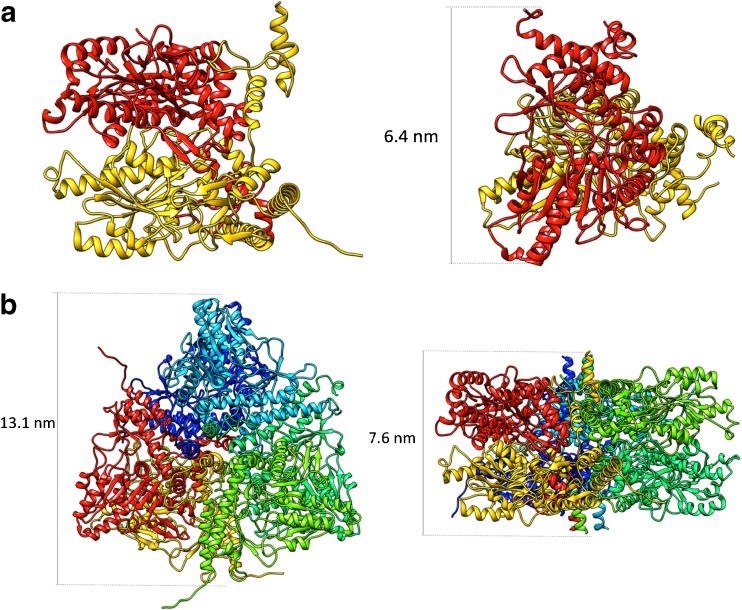
The molecular model was obtained in the hexameric form, which is a trimer of dimers. The overall structure has two layers, where every dimer contributes with one subunit to each layer. Ramachandran plot analysis showed that 94.5% of the amino acids were in the preferred regions, 5% in the allowed regions, and 0.5% were outliers; this suggests that the model is acceptable. The dimer dimension was 6.4 × 7.6 nm, while the hexamer diameter was 13.1 nm and the width was 7.6 nm (Fig. 3).
Fig. 3.
Molecular models of LbGadB: a dimer and b hexamer
Effect of the pH in the oligomerization of LbGadB: AF4 studies
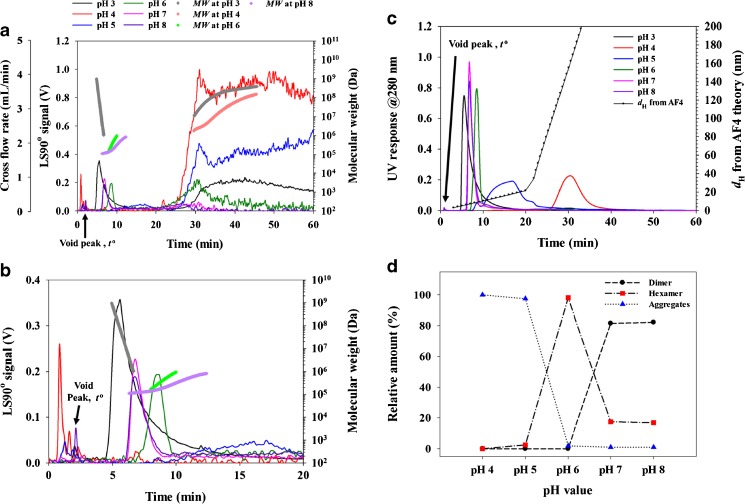
Figure 4 shows AF4-UV-MALS fractograms of the recombinant LbGadB obtained at various pH. Figure 4a shows the LS responses (measured at 90°) and the molecular weight distributions (MWD). Figure 4b shows the same fractograms as those in Fig. 4a at the retention time of 0~20 min. The UV responses are shown in Fig. 4c. As shown in Fig. 4a–c, at pH 7 and 8, the dimers of LbGadB are eluted at ~ 7 min. At pH 6, hexamers are formed, and thus the elution time was increased (~ 9 min). Also a broad band was observed at pH 6 at around 30 min, probably due to elution of LbGadB aggregates. When pH was further lowered down to 5 and then 4, the intensity of the broad band at around 30 min increases, due to more aggregation, with no distinct hexamer peak observed. It is noted, at pH 3, the intensity of the broad band was decreased, and a tailed band was observed at the elution time of around 5 min, which is due to large aggregates eluting in the steric/hyperlayer mode [25, 26]. As shown in Fig. 4b, the molecular weight decreases with increasing time for the tailed band, which confirms the aggregates are eluted by the steric/hyperlayer mode. The average MW of the tailed band at 5 min was 5.7 × 108 Da by determined by MALS, which is much higher than the average MW (1.1 × 105 Da) of the 7-min peak observed at pH 8.
Fig. 4.
AF4-MALS-UV fractograms of LbGadB obtained at various pH. The injection volume was 200 μL at pH 3 and 4 and 150 μL at pH 5, 6, 7, and 8. a LS fractogram at 90° and MW. b Enlargement of fractogram shown in a. c UV fractogram at 280 nm and d H from AF4 theory. d Relative amount of dimer, hexamer, and aggregates of LbGadB measured from peak area of deconvoluted UV fractograms
The MW of the dimer and the hexamer of recombinant LbGadB were determined to be 110 and 350 kDa at 10 mM citrate-phosphate buffer of pH 8 and 6, respectively, by AF4-MALS. These MW values are in agreement with those measured for the LbGadB monomer, 55 kDa by SDS-PAGE and molecular modeling.
The radius of gyration (r g) of the dimer and the hexamer of the LbGadB could not be measured by MALS as the analytes behaved as isotropic scatterers, and hence, no angular dependence in the scattered light is observed. The d H was determined using AF4 theory [23, 24] and were compared with results obtained from molecular modeling for the dimer and the hexamer of LbGadB. From the AF4 theory, the d H of the dimer and the hexamer of LbGadB were determined to be 6 and 10 nm, respectively, and were in reasonable agreement with those from molecular modeling, which were 6.4 × 7.6 nm for the dimer and 7.6 × 13.1 nm for the hexamer. The sizes determined by AF4 theory and modeling are not expected to be identical, as they have different physical meanings, i.e., the d H determined by AF4 is the molecular size obtained from the diffusion coefficient (through the Stokes-Einstein equation) [27]. While, the sizes calculated from homology model are based on its atomic three-dimensional structure built using as templates crystallographic structures of homologous proteins [19].
Table 1 shows the MW and size determined by various methods for recombinant LbGadB. In Table 1, the MW and r g were determined by MALS, d H were determined by AF4 theory, and molecular dimensions by modeling, respectively.
Table 1.
Molecular weight (MW) and size of recombinant LbGadB determined from peak maximum point of pH 4, 6, and 8 by various methods
| Form of LbGadB | Molecular weight (Da) | Radius of gyration, r g (nm) | Hydrodynamic diameter, d H (nm) | Molecular dimensiona (nm) |
|---|---|---|---|---|
| Dimer | 1.1 × 105 | n.d | 6 | 6.4 × 7.6 |
| Hexamer | 3.5 × 105 | n.d | 10 | 7.6 × 13.1 |
| Aggregates | 1.8 × 106~2.2 × 108 | 70~78 | 80~292 | n.d |
n.d = no data
aMolecular modeling result
In general, proteins show UV absorption at 280 nm due to UV absorbing amino acid, such as tryptophan, tyrosine, and phenylalanine. However, the large aggregates cause scattering effects, so correction of the results is necessary such as deconvolution for quantitative analysis. Thus, all results of UV detector were deconvoluted to dimer, hexamer, and aggregates for semi-quantitative analysis of oligomerization of LbGadB. Figure 4d and Table 2 show the relative percent concentration of the dimer, hexamer, and aggregates of recombinant LbGadB obtained by deconvoluting the UV fractograms in Fig. 4c using the PeakFit software (ver. 4.0, Systat Software Inc., San Jose, USA) with the Savitsky-Golay smoothing.
Table 2.
Relative concentration of monomer, hexamer, and aggregate of LbGadB obtained by deconvolution of UV fractograms at various pH
| pH | Concentration (%) | ||
|---|---|---|---|
| Dimer | Hexamer | Aggregates | |
| 4 | – | – | 100 |
| 5 | – | 3 | 97 |
| 6 | – | 98 | 2 |
| 7 | 81 | 18 | 1.0 |
| 8 | 82 | 17 | 1.0 |
Consistent with previous studies of plant (Arabidopsis thaliana) glucatamate decarboxylase (AtGAD1) [6], it seems that LbGadB from L. brevis is stable in a dimer form at pH 7 or higher. In Fig. 4d and Table 2, only 18% of LbGadB are present in a hexamer form at pH 7. The LbGadB is present mostly as hexamer at pH 6. Then at pH 5 or lower, most of LbGadB are present as aggregates (98% at pH 5 and 100% at pH 4), indicating the hexamer of LbGadB is not stable at pH below about 5. The isoelectric point (pI) of LbGadB calculated by the molecular modeling was 5.15 in the present study and aggregation of LbGadB will be promoted at pH below pI. The hexamer concentration changes drastically from 18% at pH 7 to 98% at pH 6.
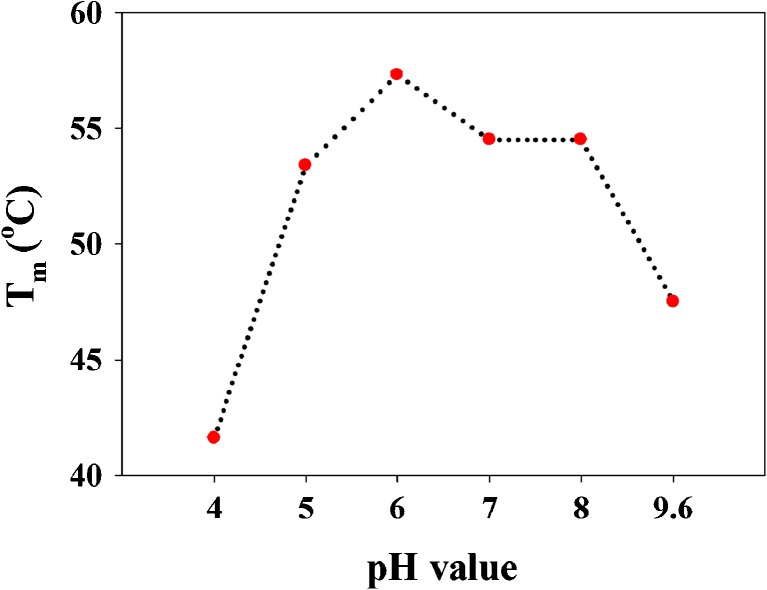
It has been reported that AtGAD1 is present mostly as hexamer at pH below 7 [6]; in this line, our results show that the hexameric form of LbGadB is more stable at pH 6 than pH 7. Both enzymes, AtGAD1 and LbGadB, share 38% of amino acid sequence identity (query cover of 94%, BLAST search). The thermal stability of LbGadB in different pHs shows the maximum stability at pH 6 (T m 57 °C) and the lowest at pH 4 (T m 46 °C) (Fig. 5). At pH 6, the main form is the hexamer, which means that this oligomeric form is the most stable form, while at pH 4 almost all of the protein aggregate (Fig. 4d). At pHs 7 and 8, the predominant form is the dimer, which is less stable than the hexamer since the thermal unfolding transition midpoint (T m 54 °C) is lower in both pHs (Fig. 5). The dimer and hexameric forms are stabilized by hydrogen-bonding interactions; therefore, the thermal stability is increased [28, 29]. While, in the case of the aggregates, it is expected that is not thermally stable because it is mostly formed physically with low or without hydrogen-bonding interactions. Thus, the results of AF4 and thermal stability mean LbGadB has most stable form occurs at pH 6. It is new discovery for oligomerization of LbGadB.
Fig. 5.
Thermal stability of LbGadB described by the thermal unfolding transition midpoint (T m) in different pH values
Effect of type and concentration of salt and temperature on oligomerization of LbGadB
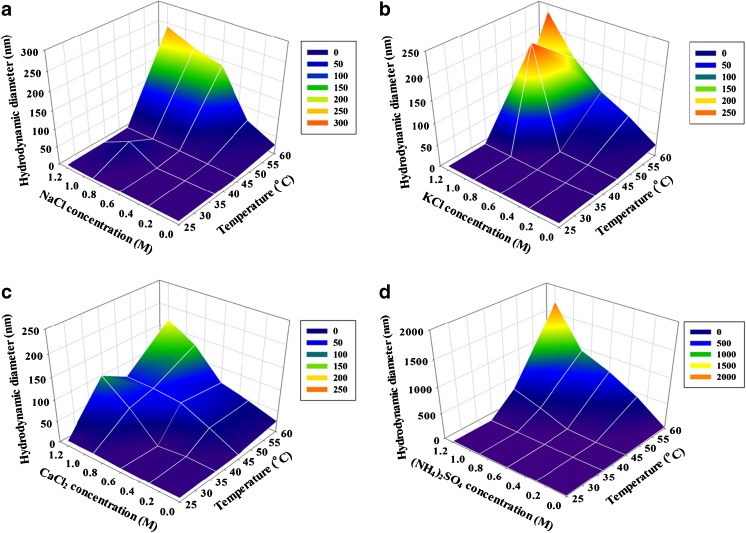
Figure 6 shows the d H of LbGadB determined by DLS with four different types of salts (NaCl, KCl, CaCl2, and (NH4)2SO4) added at five different concentrations (0, 0.3, 0.6, 0.9, and 1.2 M) at four different temperatures (25, 37, 45, and 60 °C). These salts are generally recognized as safe (GRAS) according to Food and Drug Administration (FDA). All results show a similar trend of an increase in d H with an increase of salt concentration or temperature. At the lower temperatures (25 and 37 °C), however, no significant changes in d H were observed by a change in the salt concentration. Similarly, at the lower salt concentrations (0 and 0.3 M), no significant changes in d H were observed by a change in the temperature. The effect by the salt type was not clear. Based on the results at 37 °C (see Electronic Supplementary Material (ESM) Fig. S1 for more details), the hexameric form of LbGadB is most stable in NaCl or KCl and is not affected by the salt concentration in the investigated range.
Fig. 6.
Effect of temperature, salt type, and concentration on oligomerization of LbGadB determined by DLS. a Sodium chloride (NaCl). b Potassium chloride (KCl). c Calcium chloride (CaCl2). d Ammonium sulfate ((NH4)2SO4) at different temperatures and salt concentrations at pH 7
Conclusion
The MWs and sizes of dimer and hexamer of LbGadB determined by AF4-UV-MALS-dRI were in good agreements with those from molecular modeling. The effects of temperature, salt type, and salt concentration on oligomerization of LbGadB were investigated using DLS. The hexamer content of LbGadB determined by deconvolution of UV/Vis detector response was 98%, indicating the hexamer form is most stable at pH 6. The hexamer also showed high thermal stability (up to about 57 °C). This seems to be a new finding on oligomerization of LbGadB. Results prove that AF4-UV-MALS-dRI is a powerful tool for separation of dimer, hexamer, and aggregates of LbGadB and also for monitoring of oligomerization of LbGadB.
Electronic supplementary material
(PDF 675 kb)
Acknowledgements
The authors would like to thank Dr. Teresia Hallström for her assistance in using the instrument Prometheus NT.48 nanoDSF in a Demo and Prometheus NT.48 nanoDSF, Munich, Germany, for allowing us to use their instrument. The authors acknowledge the support provided by the National Research Foundation (NRF) of Korea (NRF-2013K2A3A1000086 and NRF-2016R1A2B4012105) and the Swedish Foundation for International Cooperation in Research and Higher Education (STINT).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest. There are no competing interests present and there are no patents, products in development, or marketed products to declare. Furthermore, we agree with the policies on sharing data and materials, as guide for authors.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00216-017-0735-6) contains supplementary material, which is available to authorized users.
Contributor Information
Seungho Lee, Email: slee@hnu.kr.
Javier A. Linares-Pastén, Email: javier.linares_pasten@biotek.lu.se
Lars Nilsson, Email: lars.nilsson@food.lth.se.
References
- 1.Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H. Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 2003;57(3):490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Cao Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids. 2010;39(5):1107–1116. doi: 10.1007/s00726-010-0582-7. [DOI] [PubMed] [Google Scholar]
- 3.Wu Q, Shah NP. High γ-aminobutyric acid production from lactic acid bacteria: emphasis on Lactobacillus brevis as a functional dairy starter. Crit Rev Food Sci Nutr. 2016;57(17):3661–3672. doi: 10.1080/10408398.2016.1147418. [DOI] [PubMed] [Google Scholar]
- 4.Cho YR, Chang JY, Chang HC. Production of γ-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol. 2007;17(1):104–109. [PubMed] [Google Scholar]
- 5.Yakabe T, Moore EL, Yokota S, Sui H, Nobuta Y, Fukao M, Palmer H, Yajima N. Safety assessment of Lactobacillus brevis KB290 as a probiotic strain. Food Chem Toxicol. 2009;47(10):2450–2453. doi: 10.1016/j.fct.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Astegno A, Capitani G, Dominici P. Functional roles of the hexamer organization of plant glutamate decarboxylase. Biochim Biophys Acta Proteins Proteomics. 2015;1854(9):1229–1237. doi: 10.1016/j.bbapap.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Hiraga K, Ueno Y, Oda K. Glutamate decarboxylase from Lactobacillus brevis: activation by ammonium sulfate. Biosci Biotechnol Biochem. 2008;72(5):1299–1306. doi: 10.1271/bbb.70782. [DOI] [PubMed] [Google Scholar]
- 8.Runyon JR, Williams SKR. Composition and molecular weight analysis of styrene-acrylic copolymers using thermal field-flow fractionation. J Chromatogr A. 2011;1218(38):6774–6779. doi: 10.1016/j.chroma.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 9.Striegel A, Yau WW, Kirkland JJ, Bly DD. Modern size-exclusion liquid chromatography: practice of gel permeation and gel filtration chromatography. 2. New York: Wiley; 2009. [Google Scholar]
- 10.Håkansson A, Ulmius M, Nilsson L. Asymmetrical flow field-flow fractionation enables the characterization of molecular and supramolecular properties of cereal β-glucan dispersions. Carbohydr Polym. 2012;87(1):518–523. doi: 10.1016/j.carbpol.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Cave RA, Seabrook SA, Gidley MJ, GiLbert RG. Characterization of starch by size-exclusion chromatography: the limitations imposed by shear scission. Biomacromolecules. 2009;10(8):2245–2253. doi: 10.1021/bm900426n. [DOI] [PubMed] [Google Scholar]
- 12.Lee D, Williams SKR. Thermal field-flow fractionation and multiangle light scattering of polyvinyl acetate with broad polydispersity and ultrahigh molecular weight microgel components. J Chromatogr A. 2010;1217(10):1667–1673. doi: 10.1016/j.chroma.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Schimpf ME, Caldwell K, Giddings JC. Field-flow fractionation handbook. In: Wahlund KG, editor. Chapter 18. Asymmetrical flow field-flow fractionation. New York: Wiley-Interscience; 2000. pp. 279–294. [Google Scholar]
- 14.Litzen A, Wahlund KG. Improved separation speed and efficiency for proteins, nucleic acids and viruses in asymmetrical flow field flow fractionation. J Chromatogr. 1989;476:413–421. doi: 10.1016/S0021-9673(01)93885-3. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson L. Separation and characterization of food macromolecules using field-flow fractionation: a review. Food Hydrocoll. 2013;30(1):1–11. doi: 10.1016/j.foodhyd.2012.04.007. [DOI] [Google Scholar]
- 16.Reschiglian P, Zattoni A, Roda B, Michelini E, Roda A. Field-flow fractionation and biotechnology. Trends Biotechnol. 2005;23(9):475–483. doi: 10.1016/j.tibtech.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Yohannes G, Jussila M, Hartonen K, Riekkola ML. Asymmetrical flow field-flow fractionation technique for separation and characterization of biopolymers and bioparticles. J Chromatogr A. 2011;1218(27):4104–4116. doi: 10.1016/j.chroma.2010.12.110. [DOI] [PubMed] [Google Scholar]
- 18.Krieger E, Vriend G. YASARA view—molecular graphics for all devices—from smartphones to workstations. Bioinformatics. 2014;30(20):2981–2982. doi: 10.1093/bioinformatics/btu426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger E, Darden T, Nabuurs SB, Finkelstein A, Vriend G. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins Struct Funct Genet. 2004;57(4):678–683. doi: 10.1002/prot.20251. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 21.Andersson M, Wittgren B, Wahlund K-G. Accuracy in multiangle light scattering measurements for molar mass and radius estimations. Model calculations and experiments. Anal Chem. 2003;75(16):4279–4291. doi: 10.1021/ac030128+. [DOI] [PubMed] [Google Scholar]
- 22.Berry GC. Thermodynamic and conformational properties of polystyrene. I. Light-scattering studies on dilute solutions of linear polystyrenes. J Chem Phys. 1966;44(12):4550–4564. doi: 10.1063/1.1726673. [DOI] [Google Scholar]
- 23.Håkansson A, Magnusson E, Bergenståhl B, Nilsson L. Hydrodynamic radius determination with asymmetrical flow field-flow fractionation using decaying cross-flows. Part I. A theoretical approach. J Chromatogr A. 2012;1253:120–126. doi: 10.1016/j.chroma.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson E, Håkansson A, Janiak J, Bergenståhl B, Nilsson L. Hydrodynamic radius determination with asymmetrical flow field-flow fractionation using decaying cross-flows. Part II. Experimental evaluation. J Chromatogr A. 2012;1253:127–133. doi: 10.1016/j.chroma.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Schimpf ME, Caldwell K, Giddings JC. Field-flow fractionation handbook. In: Caldwell KD, editor. Chapter 5. Steric field-flow fractionation and the steric transition. New York: Wiley-Interscience; 2000. pp. 79–94. [Google Scholar]
- 26.Schimpf ME, Caldwell K, Giddings JC. Field-flow fractionation handbook. In: Schure MR, Schimpf ME, Schettler PD, editors. Chapter 2. Retention-normal mode. New York: Wiley-Interscience; 2000. pp. 31–48. [Google Scholar]
- 27.Einstein A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann Phys. 1905;322(8):549–560. doi: 10.1002/andp.19053220806. [DOI] [Google Scholar]
- 28.Tanakai Y, Tsumoto K, Yasutake Y, Umetsu M, Yao M, Fukada H, Tanaka I, Kumagai I. How oligomerization contributes to the thermostability of an archaeon protein: protein L-isoaspartyl-O-methyltransferase from Sulfolobus tokodaii. J Biol Chem. 2004;279(31):32957–32967. doi: 10.1074/jbc.M404405200. [DOI] [PubMed] [Google Scholar]
- 29.Vogt G, Argos P. Protein thermal stability: hydrogen bonds or internal packing? Fold Des. 1997;2:S40–S46. doi: 10.1016/S1359-0278(97)00062-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 675 kb)