Abstract
Water retaining agent (WRA) is widely used for soil erosion control and agricultural water saving. Here, we evaluated the effects of the combination of beneficial soil bacterium Bacillus amyloliquefaciens strain GB03 and WRA (the compound is super absorbent hydrogels) on drought tolerance of perennial ryegrass (Lolium perenne L.). Seedlings were subjected to natural drought for maximum 20 days by stopping watering and then rewatered for seven days. Plant survival rate, biomass, photosynthesis, water status and leaf cell membrane integrity were measured. The results showed that under severe drought stress (20-day natural drought), compared to control, GB03, WRA and GB03+WRA all significantly improved shoot fresh weight, dry weight, relative water content (RWC) and chlorophyll content and decreased leaf relative electric conductivity (REC) and leaf malondialdehyde (MDA) content; GB03+WRA significantly enhanced chlorophyll content compared to control and other two treatments. Seven days after rewatering, GB03, WRA and GB03+WRA all significantly enhanced plant survival rate, biomass, RWC and maintained chlorophyll content compared to control; GB03+WRA significantly enhanced plant survival rate, biomass and chlorophyll content compared to control and other two treatments. The results established that GB03 together with water retaining agent promotes ryegrass growth under drought conditions by improving survival rate and maintaining chlorophyll content.
Keywords: Bacillus amyloliquefaciens, perennial ryegrass, water retaining agent, synergistic effects, drought tolerance
1. Introduction
Drought as one of the major abiotic stresses has been weighing heavily against the agricultural productivity worldwide [1,2], since most of crops and forage plants grown to feed the global population are highly sensitive to drought [3]. Drought also induces severe desertification, with a progressive reduction of the vegetation cover coupled with rapid soil erosion in arid and semi-arid climatic regions [4,5]. Drought affects water potential and turgor in plants, resulting in the changes of physiological and morphological traits. Fresh weight and relative water content are two parameters commonly adopted to measure the impact of drought stress on plant growth [6]. Drought decreases plant chlorophyll content, which is directly related to photosynthesis rate [7]. Drought is also known to increase the reactive oxygen species (ROS) in plant cells, which are well recognized for lipid peroxidation and cell membrane deterioration, resulting in secondary oxidative stress [8,9]. Among various impacts of drought stress on plant growth, nutrient and water availability are mainly discussed [10,11].
Plant growth promoting rhizobacteria (PGPRs) are microorganisms associated with plant roots and can confer beneficial effects on the host plants [12]. Early research reported that the PGPR Paenibacillus polymyxa enhanced drought tolerance of Arabidopsis thaliana [13]. Bacillus amyloliquefaciens had been applied to several commercial crops and shown the remarkable effects on the increase in plant growth, disease resistance as well as salt and drought tolerance [14,15,16]. B. amyloliquefaciens strain GB03 enhanced growth and abiotic stress tolerance in Arabidopsis by emitting a complex blend of volatile organic compounds (VOCs) [17,18,19,20,21]. These VOCs activated differential expression of approximately 600 transcripts including genes related to cell wall modifications, primary and secondary metabolism, hormone regulation and stress response [19]. Recent studies reported that GB03 also promoted growth and salt tolerance in wheat (Triticum aestivum) [22], white clover (Trifolium repens L.) [23] and a halophytic grass Puccinellia tenuiflora [24].
Super absorbent hydrogels used as water retaining agents (WRA) in agriculture were formed from highly hydrophilic cross-linked polymers, which possess high water absorption capacity [25]. It was found that the hydrogels can mitigate soil erosion by reducing sediment and nutrient losses [26,27,28]. The hydrogels can also absorb water and nutrients and subsequently release them gradually [29,30,31]. In addition, Sojka et al. found that hydrogel promoted soil colonization of microorganism, including bacteria and mycorrhiza [32]. It increases the plant available water in the soil, which prolongs plant survival time under drought stress [33,34,35,36]. Hayat et al. proved that the administration of WRA induced substantial changes in soil physical properties by increasing saturation percentage while decreasing particle density and bulk density, so as to promote crop productivity [37].
Perennial ryegrass (Lolium perenne L.) is an important grass species for pasture, forage and turf in the world [38]. This cool-season grass species is native to Northern Europe, Asia and Africa and widely distributed in many temperate regions all over the world [39]. It has good turf quality with quick establishment [40]. Like many other turf grasses, however, it is also a drought sensitive species [41].
Although either B. amyloliquefaciens GB03 or WRA can enhance the plant performance under drought conditions, the combination effects of both of them have not been reported to date. The objective of this research was to investigate the synergistic effects of the beneficial soil bacterium strain Bacillus amyloliquefaciens GB03 and water retaining agent on drought tolerance of perennial ryegrass. Plants were subjected to drought treatments by stopping watering for 20 days. Plant survival rate and parameters related to plant biomass, photosynthesis, water status and leaf cell membrane integrity were assessed.
2. Results
2.1. B. amyloliquefaciens GB03 and WRA Promoted Ryegrass Growth under Drought Condition
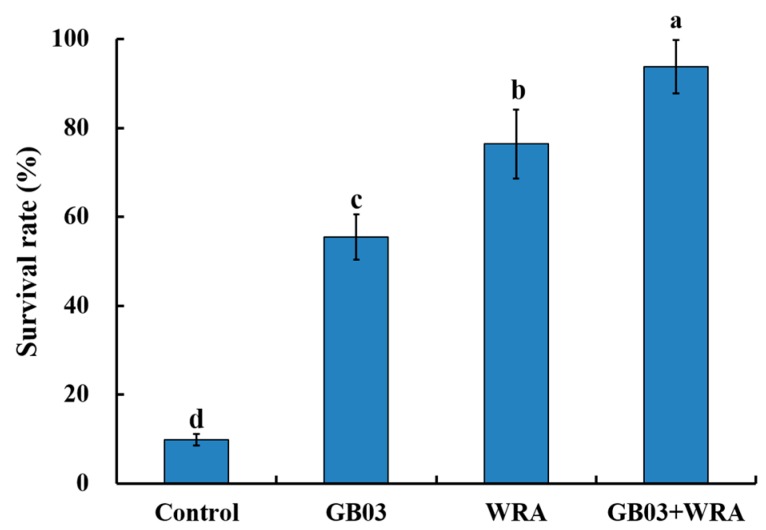
Table 1 showed that the soil water contents of WRA and GB03+WRA treatments were higher than those of control and GB03 treatment. GB03 and WRA significantly increased leaf growth and plant density of ryegrass compared to control during all phases (p < 0.05) (Figure 1). No significant difference was observed among all treatments for 20-day-old (before drought treatment, BT) and 10-day natural drought seedlings. However, after 20 days natural drought and then seven days after rewatering, seedlings treated with GB03+WRA grew well and exhibited the highest survival rate (approximately 94%), compared with those treated with WRA (76%) that was significantly higher than that of seedlings treated with GB03 (55%) or control (10%) (Figure 2).
Table 1.
Soil water content after 20 days of natural drought. Values are means with SEs (n = 12). Different letters indicate significant differences among treatments at p < 0.05 (ANOVA and Duncan’s multiple range test).
| Treatments | Control | GB03 | WRA | GB03+WRA |
|---|---|---|---|---|
| Soil water content (mg/g·DW) | 37.7 ± 3.9 b | 33.3 ± 04.7 b | 61.8 ± 2.0 a | 62.4 ± 5.9 a |
Figure 1.
Ryegrass growth status of different treatments in four vegetation phases. From left to right: control, GB03, WRA and GB03+WRA treatments; from top to bottom: 20-day-old seedlings (before treatment, BT), 10-day natural drought (10DD), 20-day natural drought (20DD) and 7 days after rewatering (7DR).
Figure 2.
The survival rate of ryegrass seedlings with 20 days natural drought and then seven days after rewatering. Values are means and bars indicate standard errors (SEs) (n = 12). Columns with different letters indicate significant differences among treatments at p < 0.05 (ANOVA and Duncan’s post-hoc multiple comparison test).
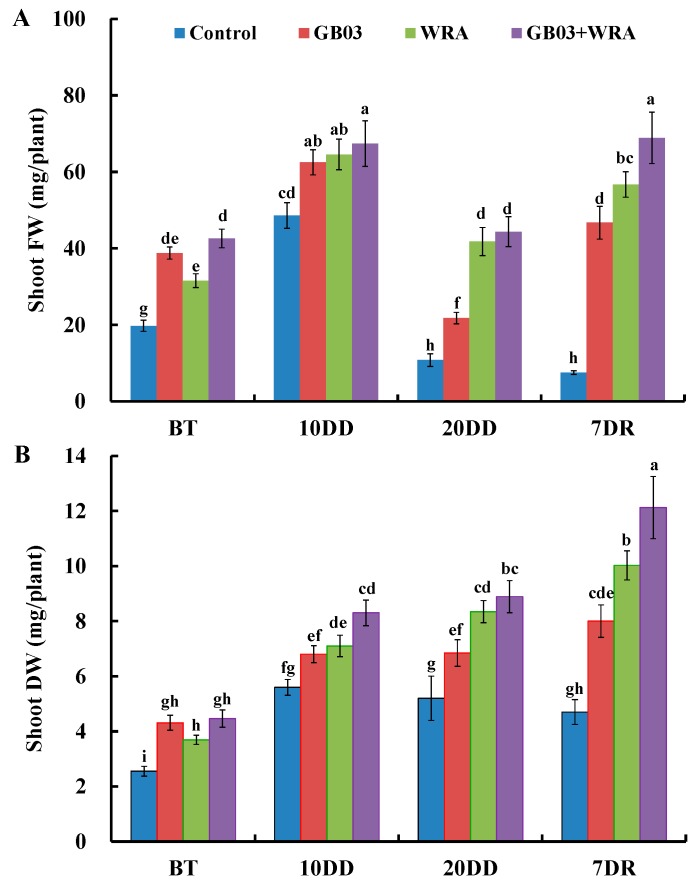
After growing for 20 days (BT), GB03 and WRA significantly enhanced shoot fresh weight by 96.3% and 59.7% compared to control, respectively; moreover, the shoot fresh weight of GB03+WRA treatment was 116% and 35.0% significantly higher than that of control and WRA treatment, respectively (Figure 3A). After 10-days of drought treatment, three treatments (GB03, WRA and GB03+WRA) had significantly 28.5%, 32.8%, and 38.6% higher fresh weight than control, respectively. Drought stress triggered significant decreases in shoot fresh weight when seedlings were withheld water for 20 days: all treatments (control, GB03, WRA and GB03+WRA, respectively) decreased by 77.8%, 65.1%, 35.3% and 34.2%. However, in this phase, the fresh weights of GB03 and WRA treatments were significantly 1.02-fold and 2.87-fold higher than that of control, respectively; GB03+WRA treatment was 1.04-fold and 3.11-fold significantly higher in shoot fresh weight than GB03 treatment and control, respectively. Seven days after rewatering, shoot fresh weights for all treatments (except for control) increased significantly than that in 20-day drought. In this phase, three treatments (GB03, WRA and GB03+WRA) were significantly 5.20-fold, 6.52-fold and 8.14-fold higher in fresh weight than control, respectively; the shoot fresh weight in GB03+WRA and WRA treatments were 47.4% and 21.3% significantly higher than that in GB03 treatment, respectively, and GB03+WRA increased shoot fresh weight significantly compared to control and other two treatments (Figure 3A).
Figure 3.
Effects of GB03, water retaining agent (WRA), or the combination of GB03+WRA on shoot fresh weight (FW) (A) and dry weight (DW) (B) of ryegrass. BT, before treatment (20-day-old seedling); 10DD, 10-day natural drought; 20DD, 20-day natural drought; 7DR, 7 days after rewatering. Values are means and bars indicate SEs (n = 12). Columns with different letters indicate significant differences among treatments at p < 0.05 (ANOVA and Duncan’s post-hoc multiple comparison test).
Three treatments (GB03, WRA and GB03+WRA) significantly increased shoot dry weight by 68.7%, 44.5% and 74.7% compared to control, respectively, after growing for 20 days (Figure 3B). After 10-day drought, WRA and GB03+WRA treatments increased shoot dry weight significantly by 26.8% and 48.2%, respectively, compared to control. After 10-day drought, three treatments (GB03, WRA and GB03+WRA) were significantly 31.5%, 60.5% and 70.9% higher in dry weight than control, respectively. Seven days after rewatering, WRA and GB03+WRA treatments increased shoot dry weight significantly by 20.1% and 36.4%, respectively, compared to those in 20-day drought treatment. In this phase, three treatments (GB03, WRA and GB03+WRA) were significantly 0.72-fold, 1.13-fold and 1.57-fold higher in shoot dry weight, respectively, compared to control, and GB03+WRA increased shoot dry weight significantly compared to control and other two treatments (Figure 3B).
2.2. GB03 and WRA Maintained the Relative Water Content (RWC) under Drought Condition
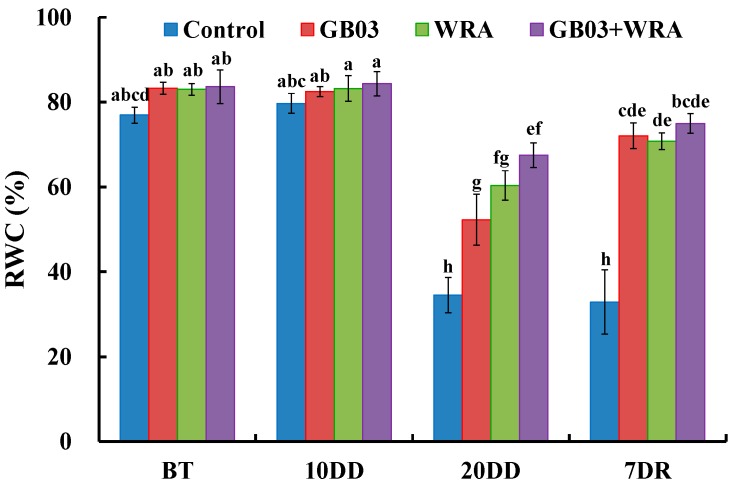
To probe the plant water status, RWC was assayed in ryegrass leaves. As shown in plant biomass (shoot fresh weight and dry weight), 10-day drought treatment was tolerable for ryegrass with all treatments no significant difference in RWC. After stopping watering for 20 days, all treatments decreased RWC significantly by 56.7%, 36.6%, 27.4% and 20.0%, respectively, compared to those in 10-day drought; the three treatments (GB03, WRA and GB03+WRA) were 51.4%, 74.8% and 95.5% significantly higher in RWC, respectively, compared to control; GB03+WRA treatment was also 29.1% significantly higher than GB03 treatment, indicating that GB03 and WRA together could effectively maintain the RWC in ryegrass. Seven days after rewatering, three treatments (GB03, WRA and GB03+WRA) were significantly 1.19-, 1.15- and 1.27-folds higher than control in RWC, respectively (Figure 4).
Figure 4.
Effects of GB03, water retaining agent (WRA), or the combination of GB03+WRA on leaf relative water content (RWC) of ryegrass. BT, before treatment (20-day-old seedling); 10DD, 10-day natural drought; 20DD, 20-day natural drought; 7DR, 7 days after rewatering. Values are means and bars indicate SEs (n = 12). Columns with different letters indicate significant differences among treatments at p < 0.05 (ANOVA and Duncan’s post-hoc multiple comparison test).
2.3. GB03 and WRA Maintained Chlorophyll Content
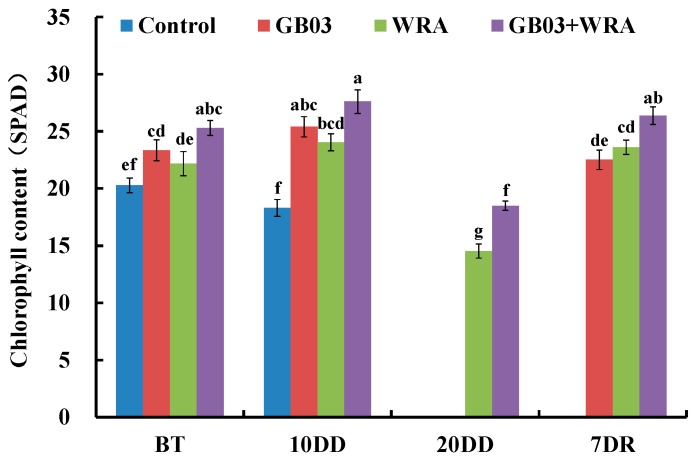
After growing for 20 days (BT), chlorophyll content for GB03 and GB03+WRA treatments was 15.1% and 24.8% significantly higher than that in control, respectively; chlorophyll content in GB03+WRA was significantly 14.1% higher than that in WRA. After 10-day drought treatment, chlorophyll content was 38.8%, 31.4% and 50.8% significantly higher in GB03, WRA and GB03+WRA treatments than in control, respectively (Figure 5). After 20-day drought treatment, the chlorophyll content in WRA was 21.4% significantly lower than that in GB03+WRA, however, seedlings with GB03 treatment and control became too wilt and chlorophyll content was unmeasurable. Seven days after rewatering, the chlorophyll content in all three treatments returned to the BT level, while that in control was still beyond measurement. The chlorophyll content in GB03+WRA treatment was 17.1% and 11.7% significantly higher than those in GB03 and WRA treatments, respectively. Therefore, WRA together with GB03 effectively maintained ryegrass chlorophyll content, especially when seedlings were under severe drought stress (20-day drought) (Figure 5).
Figure 5.
Effects of GB03, water retaining agent (WRA), or the combination of GB03+WRA on leaf chlorophyll content of ryegrass. BT, before treatment (20-day-old seedling); 10DD, 10-day natural drought; 20DD, 20-day natural drought; 7DR, 7 days after rewatering. Values are means and bars indicate SEs (n = 12). Columns with different letters indicate significant differences among treatments at p < 0.05 (ANOVA and Duncan’s post-hoc multiple comparison test).
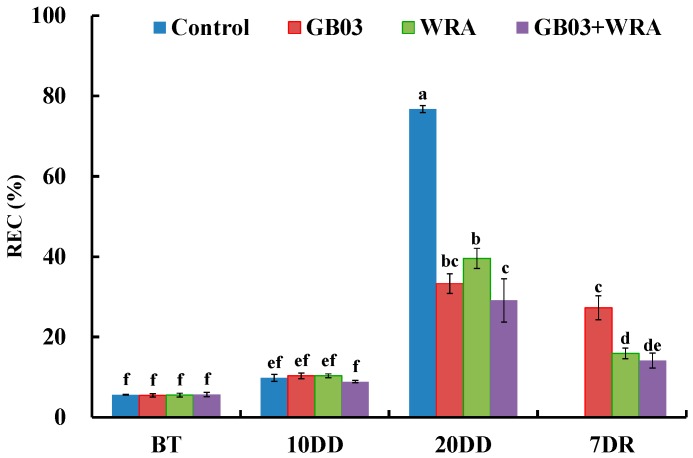
2.4. GB03 and WRA Reduced Relative Electric Conductivity (REC) and MDA Content under Drought Stress
After stopping watering for 20 days, a significant increase in REC was observed compared to 10 days of drought in control and three treatments; REC in control increased drastically by 6.80-fold. However, GB03 and WRA helped seedlings to maintain relatively lower REC and reduced REC significantly by 56.6% and 48.4% compared to control, respectively; moreover, REC in GB03+WRA treatment was 62.1% significantly lower than that in control (Figure 6). These results suggested that GB03 and WRA can ensured the relative low level of REC in ryegrass under severe drought conditions. Seven days after rewatering, REC in WRA and GB03+WRA significantly decreased by 59.8% and 51.5% compared to 20-day drought, respectively; and REC in GB03+WRA was significantly 48.2% lower than GB03. While REC in control plants was beyond measurement (Figure 6).
Figure 6.
Effects of GB03, water retaining agent (WRA), or the combination of GB03+WRA on relative electric conductivity (REC) of ryegrass. BT, before treatment (20-day-old seedling); 10DD, 10-day natural drought; 20DD, 20-day natural drought; 7DR, 7 days after rewatering Values are means and bars indicate SEs (n = 12). Columns with different letters indicate significant differences among treatments at p < 0.05 (ANOVA and Duncan’s post-hoc multiple comparison test).
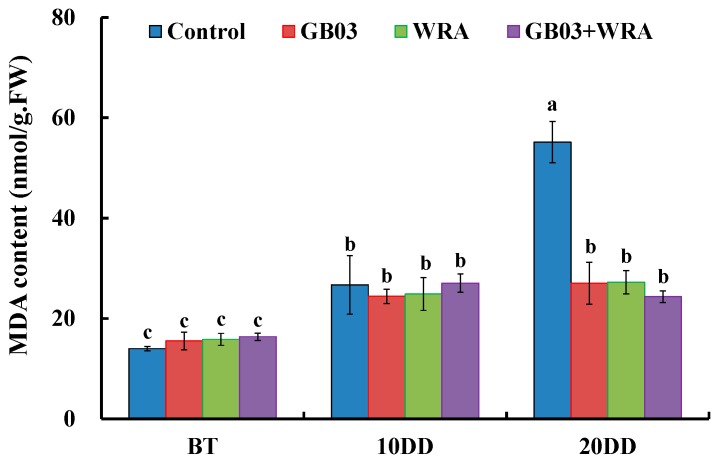
After 10-day drought, MDA contents were increased significantly by 90.9%, 57.5%, 57.0% and 65.3% compared with their corresponding BT levels. After 20-day drought, MDA content only in control plants was increased significantly by 1.07-fold compared to 10-day drought, whereas those in the three treatments were still maintained the previous phase level; GB03+WRA treatments was 55.9% significantly lower than control in MDA content (Figure 7).
Figure 7.
Effects of GB03, water retaining agent (WRA), or the combination of GB03+WRA on leaf malondialdehyde (MDA) content of ryegrass. BT, before treatment (20-day-old seedling); 10DD, 10-day natural drought; 20DD, 20-day natural drought; 7DR, 7 days after rewatering. Values are means and bars indicate SEs (n = 12). Columns with different letters indicate significant differences among treatments at p < 0.05 (ANOVA and Duncan’s post-hoc multiple comparison test).
3. Discussion
3.1. Synergistic Effects of GB03 and WRA on Plant Growth under Drought Condition
Considerable progress has been made in fathoming mechanisms underlying Bacillus-mediated plant growth promotion and crop yield increase; these mechanisms include increased nutrient availability, synthesizing plant hormones and the production of volatile organic compounds [15,16,17,18,19,20]. Plant growth promotion meditated by Bacillus amyloliquefaciens has been reported in many species including Arabidopsis [15,20,42], maize (Zea mays L.) [43], tomato (Lycopersicon esculentum) [44], wheat (Triticum aestivum) [22], white clover (Trifolium repens L. cultivar Huia) [23] and Puccinellia tenuiflora [24]. Gagné-Bourque et al. also proved that B. subtilis enhanced Brachypodium distachyon growth under drought stress [45].
Consistent with the recent study of Galeş et al. [34], the soil moisture at the end of the drought treatment showed that the WRA’s property in retaining the water and releasing it afterward (Table 1). Because of this property, treatments amended with WRA in this study positively affected plant growth and the more severe the drought was, the better they performed in comparison to controls (Figure 1 and Figure 2). Similar results for tomato and cucumber were reported by El-Hady and Wanas [46]. The property of WRA, however, is hard to evaluate due to its depending largely on temperature, humidity, the particle size of hydrogel and the properties of the soil [47]. Interestingly, here we found that the combined effect of GB03 and WRA on promoting plant growth was greater than that of either of them.
3.2. GB03 and WRA Maintained Relatively Higher RWC Level in Ryegrass Leaves
As one of the best criteria for measuring the water status in plants, RWC indicates the water metabolic activity in tissues as drought-resistant species usually have higher RWC in their leaves [48]. Hence, RWC could also be used as an ideal parameter to probe the PGPR-mediated plant drought tolerance. Indeed, many researchers have reported that under drought stress, plants with PGPR inoculation maintained higher RWC as compared to those without, suggesting that PGPR strains could effectively prolong the plant survival under drought conditions [49,50,51]. In this work, GB03 effectively enhanced the RWC by 51.4% over control under severe drought treatment (20-day drought) (Figure 4), which was consistent with previous research in sorghum [52]. Dodd et al. claimed that the increase in RWC might be a consequence of changes of the sensitivity in stomatal closure [53]. Despite the progress in the recent decade, the mechanisms behind increased RWC with PGPR treatment remain to be elucidated. After stopping watering for 20 days, WRA also elevated the level of RWC in ryegrass by 74.8% over control. This was understandable because WRA retained soil water available for plant. GB03 together with WRA maintained relatively higher RWC level than GB03 or WRA in ryegrass leaves although not significantly.
3.3. Synergistic Effects of GB03 and WRA in Ryegrass Leaf Chlorophyll Content under Severe Drought Condition
Leaf chlorophyll content is also an important physiological parameter positively affecting plant photosynthesis rate [7]. As one of the symptoms of photo-oxidation, drought-triggered decrease in chlorophyll content has been observed in maize [54], sorghum [52] and white clover [23]. Zhang et al. found that GB03 increased photosynthetic capacity in Arabidopsis by raising photosystem II photosynthetic activity and chlorophyll content [20]. In ryegrass, the increase and maintenance in chlorophyll content by GB03 inoculation were observed under normal condition (BT) and moderate drought condition (10-day drought) (Figure 5). It was observed that WRA enhanced chlorophyll content in maize and soybean crop [33]. With the effect of WRA, the chlorophyll content in this study maintained relatively high level even under severe drought stress (20-day drought). GB03 alone failed to function under severe drought; however, the combination of t GB03 and WRA showed significantly better effect than WRA alone (Figure 5).
3.4. GB03 and WRA Alleviated Cell Membrane Damage under Drought Conditions
Drought incurs oxidative stress in plants by increasing ROS that is able to effectively degrade membrane lipids and to exacerbate lipid peroxidation [55,56]. The degradation of cell membrane results in the increase of REC. Besides, with the increase of ROS, the content of MDA follows rapidly. Thus the content of MDA has also been considered as a suitable index of oxidative damage [50,57]. Vardharajula et al. reported Bacillus spp. HYD-B17 decreased REC in maize by 26.3% under drought conditions [50]. Similar results were reported by Sandhya et al. [49] and Naveed et al. [51]. In the current study, GB03 and WRA reduced REC significantly in ryegrass under severe drought stress (20-day drought) and prolonged the seedlings’ survivability (Figure 6). As for using MDA as a marker to probe cell damage, Han et al. found GB03 significantly decreased the MDA content in white clover under stress [23]. Here we found similar result in ryegrass under severe drought stress (20-day drought); in addition, we also found that WRA together with GB03 decreased MDA content to the same statistical level as GB03 inoculation and WRA treatments (Figure 7).
4. Materials and Methods
4.1. Bacterial Suspension Culture
Bacillus amyloliquefaciens strain GB03 (presented by Professor Paul W. Paré at Texas Tech University, Lubbock, TX, USA) was streaked onto Luria broth (LB) agar plates and incubated at 28 °C in darkness for 24 h. Bacterial cells were then transferred from LB agar plates to liquid LB medium and cultured under 28 °C and 250 rpm to yield 109 colony forming units (CFU)/mL, as measured by optical density and a series of dilutions [24,58].
4.2. Plant Growth and Treatments
Perennial ryegrass (Lolium perenne L. cv. Esquire) seeds (Beijing Top Green Seed Co., Ltd., Beijing, China) were surface sterilized with 2% NaClO (sodium hypochlorite) for 1 min followed by 70% ethanol for 10 min, and rinsed by sterilized water five times. Seeds were then grown in pre-sterilized plastic pots (diameter 20 cm, depth 15 cm, 1.5 g seeds/pot with 12 replicates) filled with 1800 g of heat-sterilized (95 °C, 48 h) vermiculite, sand and field top soil mixture with their volume ratio of 1:1:1. Each pot was inoculated with 10 mL GB03 suspension culture or 10 mL liquid LB medium. Then, half of each group of above two treatments was applied WRA (5 g/pot, the compound is super absorbent hydrogels provided by Zhuhai Nongshen Biotechnology Co., Ltd., Zhuhai, China). Thus, four treatments were set: control, GB03, WRA and GB03+WRA. Each treatment contained 12 replications (12 pots) and all 48 pots were daily rearranged to avoid any border effect or light heterogeneity. Each pot was irrigated with tap water (250 mL) every 3 days until drought treatment started. Plants were grown in greenhouse under 28 °C/23 °C (day/night), the photoperiod was 16/8 h (light/dark) and the relative humidity was about 70%.
Twenty-day-old plants were subjected to drought stress by stopping watering for 20 days, and then regular watering was continued. Seedlings were harvested on the 0th (BT), 10th and 20th day after stopping watering and 7th day after rewatering for plant biomass and physiological measurements. Soil samples were taken to measure soil moisture on the 20th day after stopping watering (Table 1).
4.3. Plant Survival Rate, Biomass and Physiological Measurements
The numbers of survived plants and dead plants in each pot were counted after 20 days natural drought and then and survival rate (%) was calculated (n = 12).
Two plants from each of the 12 pots (12 replications) at each stage were sampled to measure biomass and physiological indexes. Shoot fresh weight was measured at once after harvest. Turgid weights of leaves were measured after they were soaked in distilled water in test tubes at 4 °C overnight in the dark. Finally, shoots and leaves were dried in an oven under 80 °C for 48 h and weighed again to get shoot and leaf dry weight. Leaf relative water content (RWC) was estimated according to the method described by Barrs and Weatherley [59] and then calculated using the following equation, where FW represents the leaf fresh weight, TW the leaf turgid weight and DW the leaf dry weight.
Total chlorophyll content was measured by using chlorophyll meter (SPAD 502, Konica Minolta Sensing, Inc., Osaka, Japan).
Leaf relative electric conductivity (REC) was measured to estimate leaf cell membrane damage using an electric conductivity meter (EC215, Hanna Corporation, Italy) as described by Peever and Higgins [60] and Niu et al. [61] with slight modifications. REC (%) was calculated using the following equation, where S1 and S2 refer to conductivity of ryegrass live leaves and boiled leaves, respectively.
To probe leaf oxidative damage, the biomarker malondialdehyde (MDA) was extracted and determined spectrophotometrically using a thiobarbituric acid (TBA) protocol [23]. Reagent kit was supplied by Suzhou Comin Biotechnology Co., Ltd. (Suzhou, China) Absorbance was determined at 532 and 600 nm using a UV spectrophotometer (UV-2102C, Unico Instrument Co., Ltd., Shanghai, China).
4.4. Data Analysis
Results of the growth and physiological parameters were presented as means with standard errors (n = 12). All the data were subjected to one-way analysis of variance (ANOVA) and Duncan’s post-hoc multiple comparison tests were used to detect significant differences among means at a significance level of p < 0.05 using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
5. Conclusions
This study demonstrated that either GB03 or WRA promotes ryegrass survival and growth under drought conditions by directly or indirectly maintaining survival rate, biomass, relative water content, leaf chlorophyll content and cell membrane integrity. Furthermore, the synergistic effect of GB03 and WRA on drought tolerance promotion of ryegrass were greater than that of either of them by improving survival rate, biomass and chlorophyll content. This work could be helpful to develop a fresh and excellent approach for turf to cope with the challenge of global fresh water insufficiency.
Acknowledgments
This research was supported by National Natural Science Foundation of China (grant No. 31222053 and 31172256), Hui-Chun Chin and Tsung-Dao Lee Chinese Undergraduate Research Endowment (JZH0089) and Science and Technology Support Program of Gansu Province, China (1604NKCA077).
Abbreviations
| BT | Before drought treatment |
| CFU | Colony Forming Units |
| LB | Luria Broth |
| MDA | Malondialdehyde |
| PGPR | Plant Growth Promoting Rhizobacteria |
| REC | Relative electric conductivity |
| RWC | Relative Water Content |
| SEs | Standard errors |
| VOCs | Volatile Organic Compounds |
| WRA | Water Retaining Agent |
Author Contributions
Jin-Lin Zhang conceived and designed the experiments. An-Yu Su, Shu-Qi Niu and Yuan-Zheng Liu performed the experiments and Ao-Lei He, Qi Zhao, Qing-Qing Han and Sardar Ali Khan gave helps. An-Yu Su and Jin-Lin Zhang analyzed the data and wrote the paper. Paul W. Paré and Meng-Fei Li revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kramer P.J., Boyer J.S. Water Relations of Plants and Soils. Academic Press; Cambridge, MA, USA: 1995. [Google Scholar]
- 2.Hassine A.B., Bouzid S., Lutts S. Does habitat of Atriplex halimus L. affect plant strategy for osmotic adjustment? Acta Physiol. Plant. 2010;32:325–331. doi: 10.1007/s11738-009-0410-4. [DOI] [Google Scholar]
- 3.Wu G.Q., Xi J.J., Wang Q., Bao A.K., Ma Q., Zhang J.L., Wang S.M. The ZxNHX gene encoding tonoplast Na+/H+ antiporter from the xerophyte Zygophyllum xanthoxylum plays important roles in response to salt and drought. J. Plant Physiol. 2011;168:758–767. doi: 10.1016/j.jplph.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Martínez J.P., Kinet J.M., Bajji M., Lutts S. NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J. Exp. Bot. 2005;56:2421–2431. doi: 10.1093/jxb/eri235. [DOI] [PubMed] [Google Scholar]
- 5.Slama I., Ghnaya T., Messedi D., Hessini K., Labidi N., Savoure A., Abdelly C. Effect of sodium chloride on the response of the halophyte species Sesuvium portulacastrum grown in mannitol-induced water stress. J. Plant Res. 2007;120:291–299. doi: 10.1007/s10265-006-0056-x. [DOI] [PubMed] [Google Scholar]
- 6.Jaleel C.A., Manivannan P., Wahid A., Farooq M., Al-Juburi H.J., Somasundaram R., Panneerselvam R. Drought Stress in Plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009;11:100–105. [Google Scholar]
- 7.Ma Q., Yue L.J., Zhang J.L., Wu G.Q., Bao A.K., Wang S.M. Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol. 2012;32:4–13. doi: 10.1093/treephys/tpr098. [DOI] [PubMed] [Google Scholar]
- 8.Hendry G.A.F. Oxygen, free radical processes and seed longevity. Seed Sci. Res. 1993;3:141–153. doi: 10.1017/S0960258500001720. [DOI] [Google Scholar]
- 9.Sgherri C.L.M., Maffei M., Navari-Izzo F. Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J. Plant Physiol. 2000;157:273–279. doi: 10.1016/S0176-1617(00)80048-6. [DOI] [Google Scholar]
- 10.Gholamhoseini M., Ghalavand A., Dolatabadian A., Jamshidi E., Khodaei-Joghan A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013;117:106–114. doi: 10.1016/j.agwat.2012.11.007. [DOI] [Google Scholar]
- 11.Agnew C., Warren A. A framework for tackling drought and land degradation. J. Arid Environ. 1996;33:309–320. doi: 10.1006/jare.1996.0067. [DOI] [Google Scholar]
- 12.Egamberdieva D., Kucharova Z. Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fertil. Soils. 2009;45:563–571. doi: 10.1007/s00374-009-0366-y. [DOI] [Google Scholar]
- 13.Timmusk S., Wagner E.G. The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: A possible connection between biotic and abiotic stress responses. Mol. Plant Microbe Interact. 1999;12:951–959. doi: 10.1094/MPMI.1999.12.11.951. [DOI] [PubMed] [Google Scholar]
- 14.Kloepper J.W. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- 15.Paré P.W., Zhang H., Aziz M., Xie X., Kim M.-S., Shen X., Zhang J. Biocommunication in Soil Microorganisms. Springer; Berlin, Germany: 2011. Beneficial rhizobacteria induce plant growth: Mapping signaling networks in Arabidopsis; pp. 403–412. [Google Scholar]
- 16.Gao S., Wu H., Wang W., Yang Y., Xie S., Xie Y., Gao X. Efficient colonization and harpins mediated enhancement in growth and biocontrol of wilt disease in tomato by Bacillus subtilis. Lett. Appl. Microbiol. 2013;57:526–533. doi: 10.1111/lam.12144. [DOI] [PubMed] [Google Scholar]
- 17.Ryu C.-M., Farag M.A., Hu C., Reddy M.S., Wei H., Paré P.W., Kloepper J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu C.-M., Farag M.A., Hu C.-H., Reddy M.S., Kloepper J.W., Paré P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134:1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H., Kim M.S., Krishnamachari V., Payton P., Sun Y., Grimson M., Farag M.A., Ryu C.M., Allen R., Melo I.S., et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta. 2007;226:839–851. doi: 10.1007/s00425-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H., Xie X., Kim M.S., Kornyeyev D.A., Holaday S., Paré P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008;56:264–273. doi: 10.1111/j.1365-313X.2008.03593.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Sun Y., Xie X., Kim M.S., Dowd S.E., Paré P.W. A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 2009;58:568–577. doi: 10.1111/j.1365-313X.2009.03803.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J.L., Aziz M., Qiao Y., Han Q.Q., Li J., Wang Y.Q., Shen X., Wang S.M., Paré P.W. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci. 2014;65:423–427. doi: 10.1071/CP13456. [DOI] [Google Scholar]
- 23.Han Q.-Q., Lü X.-P., Bai J.-P., Qiao Y., Paré P.W., Wang S.-M., Zhang J.-L., Wu Y.-N., Pang X.-P., Xu W.-B., et al. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014;5:525. doi: 10.3389/fpls.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu S.-Q., Li H.-R., Paré P.W., Aziz M., Wang S.-M., Shi H., Li J., Han Q.-Q., Guo S.-Q., Li J., et al. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil. 2015;407:217–230. doi: 10.1007/s11104-015-2767-z. [DOI] [Google Scholar]
- 25.Karadağ E., Uzum O.B., Saraydin D. Swelling equilibria and dye adsorption studies of chemically crosslinked superabsorbent acrylamide/maleic acid hydrogels. Eur. Polym. J. 2002;38:2133–2141. doi: 10.1016/S0014-3057(02)00117-9. [DOI] [Google Scholar]
- 26.Sojka R.E., Bjorneberg D.L., Entry J.A., Lentz R.D., Orts W.J. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007;92:75–162. [Google Scholar]
- 27.Szögi A.A., Leib B.G., Redulla C.A., Stevens R.G., Mathews G.R., Strausz D.A. Erosion control practices integrated with polyacrylamide for nutrient reduction in rill irrigation runoff. Agric. Water Manag. 2007;91:43–50. doi: 10.1016/j.agwat.2007.04.003. [DOI] [Google Scholar]
- 28.Sepaskhah A.R., Shahabizad V. Effects of water quality and PAM application rate on the control of soil erosion, water infiltration and runoff for different soil textures measured in a rainfall simulator. Biosyst. Eng. 2010;106:513–520. doi: 10.1016/j.biosystemseng.2010.05.019. [DOI] [Google Scholar]
- 29.Sepaskhah A.R., Bazrafshan-Jahromi A.R. Controlling runoff and erosion in sloping land with polyacrylamide under a rainfall simulator. Biosyst. Eng. 2006;93:469–474. doi: 10.1016/j.biosystemseng.2006.01.003. [DOI] [Google Scholar]
- 30.El-Hamshary H. Synthesis and water sorption studies of pH sensitive poly (acrylamide-co-itaconic acid) hydrogels. Eur. Polym. J. 2007;43:4830–4838. doi: 10.1016/j.eurpolymj.2007.08.018. [DOI] [Google Scholar]
- 31.Farrell C., Ang X.Q., Rayner J.P. Water-retention additives increase plant available water in green roof substrates. Ecol. Eng. 2013;52:112–118. doi: 10.1016/j.ecoleng.2012.12.098. [DOI] [Google Scholar]
- 32.Sojka R.E., Entry J.A., Fuhrmann J.J. The influence of high application rates of polyacrylamide on microbial metabolic potential in an agricultural soil. Appl. Soil Ecol. 2006;32:243–252. doi: 10.1016/j.apsoil.2005.06.007. [DOI] [Google Scholar]
- 33.Galeş D.C., Trincă L.C., Cazacu A., Peptu C.A., Jităreanu G. Effects of a hydrogel on the cambic chernozem soil’s hydrophysic indicators and plant morphophysiological parameters. Geoderma. 2016;267:102–111. doi: 10.1016/j.geoderma.2015.12.008. [DOI] [Google Scholar]
- 34.Jobin P., Caron J., Bernier P.-Y., Dansereau B. Impact of Two hydrophilic acrylic-based polymers on the physical properties of three substrates and the growth of Petunia ×hybrida ‘Brilliant Pink’. J. Am. Soc. Hortic. Sci. 2004;129:449–457. [Google Scholar]
- 35.Hüttermann A., Orikiriza L.J.B., Agaba H. Application of superabsorbent polymers for improving the ecological chemistry of degraded or polluted lands. Clean Soil Air Water. 2009;37:517–526. doi: 10.1002/clen.200900048. [DOI] [Google Scholar]
- 36.Agaba H., Orikiriza L.J.B., Obua J., Kabasa J.D., Worbes M., Hüttermann A. Hydrogel amendment to sandy soil reduces irrigation frequency and improves the biomass of Agrostis stolonifera. Agric. Sci. 2011;2:544–550. doi: 10.4236/as.2011.24071. [DOI] [Google Scholar]
- 37.Hayat R., Ali S. Water absorption by synthetic polymer (Aquasorb) and its effect on soil properties and tomato yield. Int. J. Agric. Biol. 2004;6:998–1200. [Google Scholar]
- 38.Kane K.H. Effects of endophyte infection on drought stress tolerance of Lolium perenne accessions from the Mediterranean region. Environ. Exp. Bot. 2011;71:337–344. doi: 10.1016/j.envexpbot.2011.01.002. [DOI] [Google Scholar]
- 39.Buxton D.R., Mertens D.R., Fisher D.S. Forage Quality and Ruminant Utilization. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; Madison, WI, USA: 1996. [Google Scholar]
- 40.Wang J.P., Bughrara S.S. Evaluation of drought tolerance for Atlas fescue, perennial ryegrass, and their progeny. Euphytica. 2008;164:113–122. doi: 10.1007/s10681-008-9669-6. [DOI] [Google Scholar]
- 41.Norris I.B. Relationships between growth and measured weather factors among contrasting varieties of Lolium, Dactylis and Festuca species. Grass Forage Sci. 1985;40:151–159. doi: 10.1111/j.1365-2494.1985.tb01732.x. [DOI] [Google Scholar]
- 42.Xie X., Zhang H., Paré P.W. Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03) Plant Signal. Behav. 2009;4:948–953. doi: 10.4161/psb.4.10.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavaglieri L., Orlando J., Rodríguez M.I., Chulze S., Etcheverry M. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 2005;156:748–754. doi: 10.1016/j.resmic.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Chowdappa P., Mohan Kumar S.P., Jyothi Lakshmi M., Upreti K.K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control. 2013;65:109–117. doi: 10.1016/j.biocontrol.2012.11.009. [DOI] [Google Scholar]
- 45.Gagné-Bourque F., Mayer B.F., Charron J.B., Vali H., Bertrand A., Jabaji S. Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS ONE. 2015;10:1–23. doi: 10.1371/journal.pone.0130456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Hady O.A., Wanas S.A. Water and fertilizer use efficiency by cucumber grown under stress on sandy soil treated with acrylamide hydrogels. J. Appl. Sci. Res. 2006;2:1293–1297. [Google Scholar]
- 47.Kim S., Iyer G., Nadarajah A., Frantz J.M., Spongberg A.L. Polyacrylamide hydrogel properties for horticultural applications. Int. J. Polym. Anal. Charact. 2010;15:307–318. doi: 10.1080/1023666X.2010.493271. [DOI] [Google Scholar]
- 48.Jarvis P.G., Jarvis M.S. The water relations of tree seedlings. IV. Some aspects of the tissue water relations and drought resistance. Physiol. Plant. 1963;16:215–235. doi: 10.1111/j.1399-3054.1963.tb08327.x. [DOI] [Google Scholar]
- 49.Sandhya V., Ali S.Z., Grover M., Reddy G., Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010;62:21–30. doi: 10.1007/s10725-010-9479-4. [DOI] [Google Scholar]
- 50.Vardharajula S., Zulfikar Ali S., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011;6:1–14. doi: 10.1080/17429145.2010.535178. [DOI] [Google Scholar]
- 51.Naveed M., Mitter B., Reichenauer T.G., Wieczorek K., Sessitsch A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014;97:30–39. doi: 10.1016/j.envexpbot.2013.09.014. [DOI] [Google Scholar]
- 52.Grover M., Madhubala R., Ali S.Z., Yadav S.K., Venkateswarlu B. Influence of Bacillus spp. strains on seedling growth and physiological parameters of sorghum under moisture stress conditions. J. Basic Microbiol. 2014;54:951–961. doi: 10.1002/jobm.201300250. [DOI] [PubMed] [Google Scholar]
- 53.Dodd I.C., Zinovkina N.Y., Safronova V.I., Belimov A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010;157:361–379. doi: 10.1111/j.1744-7348.2010.00439.x. [DOI] [Google Scholar]
- 54.Nelson D.E., Repetti P.P., Adams T.R., Creelman R.A., Wu J., Warner D.C., Anstrom D.C., Bensen R.J., Castiglioni P.P., Donnarummo M.G., et al. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. USA. 2007;104:16450–16455. doi: 10.1073/pnas.0707193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 56.Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- 57.Møller I.M., Jensen P.E., Hansson A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Kim M.-S., Sun Y., Dowd S.E., Shi H., Paré P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interact. 2008;21:737–744. doi: 10.1094/MPMI-21-6-0737. [DOI] [PubMed] [Google Scholar]
- 59.Barrs H.D., Weatherley P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- 60.Peever T.L., Higgins V.J. Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 1989;90:867–875. doi: 10.1104/pp.90.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niu M., Huang Y., Sun S., Sun J., Cao H., Shabala S., Bie Z. Root respiratory burst oxidase homologue-dependent H2O2 production confers salt tolerance on a grafted cucumber by controlling Na+ exclusion and stomatal closure. J. Exp Bot. 2017 doi: 10.1093/jxb/erx386. [DOI] [PMC free article] [PubMed] [Google Scholar]