Abstract
Clinical translation of intravenous therapies to treat disseminated or metastatic cancer is imperative. Comparative oncology, the evaluation of novel cancer therapies in animals with spontaneous cancer, can be utilized to inform and accelerate clinical translation. Preclinical murine studies demonstrate that single shot systemic therapy with a VSV-IFNβ-NIS, a novel recombinant oncolytic Vesicular stomatitis virus (VSV), can induce curative remission in tumor bearing mice. Clinical translation of VSV-IFNβ-NIS therapy is dependent on comprehensive assessment of clinical toxicities, virus shedding, pharmacokinetics, and efficacy in clinically relevant models. Dogs spontaneously develop cancer with comparable etiology, clinical progression and response to therapy as human malignancies. A comparative oncology study was carried out to investigate feasibility and tolerability of intravenous oncolytic VSV-IFNβ-NIS therapy in pet dogs with spontaneous cancer. Nine dogs with various malignancies were treated with a single intravenous dose of VSV-IFNβ-NIS. Two dogs with high-grade peripheral T-cell lymphoma had rapid but transient remission of disseminated disease and transient hepatotoxicity that resolved spontaneously. There was no shedding of infectious virus. Correlative pharmacokinetic studies revealed elevated levels of VSV RNA in blood in dogs with measurable disease remission. This is the first evaluation of intravenous oncolytic virus therapy for spontaneous canine cancer, demonstrating that VSV-IFNβ-NIS is well-tolerated and safe in dogs with advanced or metastatic disease. This approach has informed clinical translation, including dose and target indication selection, leading to a clinical investigation of intravenous VSV-IFNβ-NIS therapy, and provided preliminary evidence of clinical efficacy, and potential biomarkers that correlate with therapeutic response.
Keywords: oncolytic, virotherapy, canine, comparative oncology
Introduction
Effective treatment of advanced, metastatic and disseminated cancers requires therapeutic agents that can be administered intravenously to reach diffuse sites of malignancy. Current intravenous chemotherapy regimens are arduous, protracted, and associated with significant side effects. Oncolytic Viruses (OVs) describes the use of viruses engineered to destroy cancer. OV therapies can be administered intravenously to selectively amplify in and kill malignant cells, and potently stimulate the immune system to recognize tumor antigens that can potentially control or eradicate disseminated tumor (1–3). The oncolytic virotherapy approach is a burgeoning field, with recent completion of a landmark Phase III clinical trial and FDA approval of the first oncolytic therapy, Talimogene Laherparepvec (an engineered oncolytic Herpesvirus), for the intratumoral treatment of relapsed melanoma (4). Clinical trials evaluating intravenously administered OV therapies to date, however, have demonstrated limited efficacy in cancer patients (5).
Vesicular stomatitis virus (VSV) is rapidly replicating RNA virus of the Rhabdoviridae family with a natural tropism for malignant cells (6). VSV is ideally suited for development as an intravenous cancer therapy due to (i) the low occurrence of pre-existing immunity; (ii) weak pathogenicity in humans; (iii) potent immunogenicity to stimulate antitumor immunity; and (iv) the ability to manufacture high titer clinical grade virus stocks for intravenous administration (7). VSV has been engineered to express Interferon-beta (IFNβ) and the sodium iodide symporter (NIS). IFNβ enhances VSV specificity by activating antiviral innate immune responses in normal cells, resulting in selective amplification of malignant cells with defective Type I IFN responses (1, 8). The NIS gene is normally expressed in thyroid follicular cells and is responsible for iodide accumulation needed for thyroid hormone synthesis. Engineering OVs to express NIS allows the use of NIS specific radiotracers to noninvasively image virus bio-distribution both in preclinical and clinical settings. In addition, NIS expressing OVs present the opportunity to utilize radio-virotherapy to enhance tumor killing by combination with therapeutic radioisotopes (9, 10). Previous evaluation of VSV-IFNβ-NIS in an immune competent, syngeneic murine myeloma model demonstrating that single shot systemic therapy induces rapid and durable remission of myeloma tumors. Detailed analysis of the mechanism of action revealed that intravenously administered VSV-IFNβ-NIS replicates selectively in and rapidly kill tumor cells, and stimulate a T-cell mediated antitumor immune response to induce durable tumor remission (11).
Clinical translation of novel cancer therapies minimally requires preclinical assessment of drug toxicity, safety, and pharmacologic effect in disease bearing animals (12). Food and Drug administration (FDA) guidance recommends additional preclinical assessment of virus shedding and viremia for clinical development of OV therapies(13). Rodent models, while valuable to establish therapeutic utility and define mechanisms of action, limit evaluation of novel cancer therapies due to dissimilarity in size, physiology and disease compared to humans (14). Comparative oncology, the study of naturally occurring cancer in animals, allows evaluation of novel cancer treatments in pet dogs, that are similar in size, genetic make-up and physiology, diagnosed with spontaneous cancers, which has highly comparable etiology, progression, immune landscape and heterogeneity as human cancer (15–19). The comparative oncology approach allows collection of biological specimens in sufficient quantities to serially monitor virus shedding, pharmacokinetics and pharmacodynamics; making it a particularly powerful method to facilitate and inform clinical development and dosing regimens of novel intravenously administered OV therapies, while facilitating the development of novel treatments for canine cancer. Correlative studies carried out during the evaluation of novel cancer therapies in spontaneous, heterogeneous canine cancer can potentially yield insights into determinants of therapeutic response and facilitate identification and testing of clinically relevant biomarkers of exposure and response to intravenously administered OV therapies.
Previously, a preclinical rapid dose-escalation study of intravenous VSV-hIFNβ-NIS administration in purpose-bred healthy, immune-competent dogs indicated that an intravenous dose of 1010 TCID50 was well-tolerated in dogs with dose limiting toxicities (DLTs) observed at a ten-fold higher dose included hepatotoxicity and coagulopathy (20). This manuscript describes a clinical feasibility study to test the tolerability of intravenous VSV-IFNβ-NIS in client-owned dogs with spontaneous cancer. The main objectives were to evaluate clinical response to and tolerability of a single intravenous dose of VSV-IFNβ-NIS, expressing either human or canine IFNβ thus allowing a comparison of VSV therapy with species specific, biologically active IFNβ versus nonspecific, but partially active IFNβ. Additionally, we establish the shedding profile in biologic samples (urine, feces and buccal swab samples) while also monitoring of viremia and development of neutralizing antibodies against VSV. Correlative pharmacokinetic analyses were carried out to investigate relationships between clinical disease responses and quantitation of VSV RNA copies in blood and sustained expression of human IFNβ. Pharmacokinetic monitoring was utilized to evaluate the impact of dose modification of intravenous VSV-IFNβ-NIS administration, in order to explore whether repeated intravenous dose administration could extend persistence of infectious virus in blood. These data were expressly collected to directly inform clinical dose selection, clinical trial design and monitoring and initiation of a Phase I study evaluating intravenous VSV-IFNβ-NIS therapy in patients with relapsed or refractory cancer.
Materials and Methods
Generation and characterization of recombinant Oncolytic Vesicular stomatitis virus
Recombinant oncolytic VSV-IFNβ-NIS was generated as previously described (11). For the purpose of this study we also generated VSV expressing canine IFNβ and NIS. Briefly, restriction site flanked cDNA coding for canine IFNβ was incorporated into a single NotI site at the M/G junction in the pVSV-XN2 plasmid containing the VSV antigenome. VSV-cIFNβ-NIS virus was rescued and amplified in BHK-21 cells as previously described (21). Growth kinetics of recombinant VSVs was tested by infection of BHK-21 cells (MOI 3.0, 1h at 37°C). Viral titer was measured in supernatant by overlay on BHK cells of serially diluted supernatant to measure Tissue culture infective dose (TCID50) determined using the Spearman and Karber equation. Radio-iodide uptake was confirmed in vitro as previously described by incubation of BHK-21 cells following infection with recombinant VSVs, in the presence of radio-labeled NaI (I125 at 1×105 cpm) +/− 100uM potassium perchlorate (KClO4) (9). Functional IFNβ expression was confirmed by ultracentrifugation of supernatant (Beckman Coulter Life Sciences) following infection of BHK-21 cells with recombinant VSVs to remove virus. Diluted supernatant was added to human melanoma Mel-624 cells or canine Madine Darby Canine Kidney (MDCK) cells for 12h, followed by infection with VSV-GFP at an MOI of 1.0. Cell viability was assessed by MTT (3-(4,5-dimethylthiazlyl-2)-2,-5-diphenyltetrozolium bromide) assay (ATCC 30–1010K).
Cell culture
BHK-21 cells were purchased in 2012 from American Type Cell Culture (ATCC), Manassas VA, and MDCK cells were generously provided by P. Harris (Mayo Clinic) in 2013. Both lines were cultured in Dulbecco modified Eagles medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin in 5% CO2. Mel-624 melanoma cells were generously provided in 2011 by R. Vile (Mayo Clinic) and were grown in DMEM supplemented as described above. Cell lines are routinely tested for mycoplasma contamination after cells are thawed and cultured immediately before use but were not otherwise tested or authenticated.
Study enrollment and clinical care of client-owned dogs
Client owned dogs receive medical care at the University of Tennessee College Of Veterinary Medicine (UTCVM). The University of Tennessee-Knoxville (UTK) animal facilities are registered with the United States Department of Agriculture and have an assurance on file with the Office of Laboratory Animal Welfare. The UTK animal care and use program (IACUC) is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC-I). Animal care was provided in adherence to the AAALAC-I guidelines with oversight provided by the UTK Office of Laboratory Animal Care (OLAC). Following a cancer diagnosis, pet-owners give written informed consent to enroll their pet dog in this study. All study procedures were approved by the University of Tennessee-Knoxville Institutional Animal Care and Use Committee (UTK-IACUC). Consented dogs were confirmed to meet study eligibility criteria as indicated in Supplemental Table 1. Prior to entry into the study, dogs were housed in USDA-compliant and IACUC-approved housing units within the UTCVM laboratory animal facilities. Dogs were fed once daily in the morning a commercially-available dry dog food (Hill’s Science Diet Canine Maintenance) and had tap water to drink ad libitum. Following treatment, study dogs were moved into a USDA/IACUC/UTK-Biosafety office-approved housing unit within the UTCVM for 7 days.
Table 1.
Summary of toxicity, biosafety and therapeutic response
Dogs diagnosed with various types of cancer were enrolled and treated with the indicated dose of VSV-hIFNβ-NIS or VSV-cIFNβ-NIS. Heptatotoxicity, the primary dose limiting toxicity is described. Safety data indicate samples tested for IVR are negative (−). Summary of therapeutic response during the 28 day study period is indicated (PR: Partial response).
| STUDY ID | BREED, SEX & AGE | DISEASE TYPE | TX (& TX TIME) | TOXICITY | SAFETY (IVR) | OUTCOME | |
|---|---|---|---|---|---|---|---|
| 1 | UTK01 | Mix, Female (spayed) 10 yrs | Anal Adeno-carcinoma |
1.6×1010 TCID50 VSV-hIFN-NIS (At Diagnosis) |
No notable adverse events | Urine (−) Buccal (−) |
STABLE DISEASE 30 days; followed by tumor resection |
| 2 | UTK02 | Mix, Male (Cast.) 10yrs | Multiple Myeloma (IgA) |
1×1010 TCID50 VSV-cIFN-NIS (At Diagnosis) |
Mild/transient ALT elev. | Urine (−) Buccal (−) |
STABLE DISEASE |
| 3 | UTK03 | German Shepherd, Female, (spayed); 8 yrs | B-cell lymphoma (peripheral LN) |
2.4×1010 TCID50 VSV-cIFN-NIS (At Diagnosis) |
Mild/transient ALT increase | Urine (−) Buccal (−) |
STABLE DISEASE |
| 4 | UTK04 | Boxer, Male (Cast.) 8yrs | T-cell lymphoma |
2.2×1010 TCID50 VSV-hIFN-NIS (At Diagnosis) |
2X ULN ALT elevation, transient, self-limiting | Urine (−) Buccal (−) |
PARTIAL RESPONSE Rapid disease remission, relapse PR: ~21 days |
| 5 | UTK05 | Mix, Male (Cast.) 6yrs | B-cell lymphoma (peripheral LN) |
1.7×1010 TCID50 VSV-hIFN-NIS (At Diagnosis) |
Mild/transient ALT increase | Urine (−) Buccal (−) |
PROGRESSIVE DISEASE |
| 6 | UTK06 | Schnauzer Female (spayed) 10yrs | Multifocal cutaneous melanoma |
1.02×1010 TCID50 VSV-cIFN-NIS (At Diagnosis) |
Mild/transient ALP increase | Urine (−) Buccal (−) |
STABLE DISEASE |
| 7 | UTK07 | Hound Mix, Female (spayed) 12 yrs | B-cell lymphoma (peripheral LN) |
1.7×1010 TCID50 VSV-cIFN-NIS (Refractory disease) |
No notable adverse events | Urine (−) Buccal (−) |
PROGRESSIVE DISEASE (rapid progression) |
| 8 | UTK08 | Boxer, Female (spayed) 10yrs | T-cell lymphoma |
2.2×1010 TCID50 VSV-hIFN-NIS (At Diagnosis) |
10X ULN ALT elevation, transient, self-limiting | Urine (−) Buccal (−) |
PARTIAL RESPONSE Rapid disease remission, relapse PR: ~36 days |
| 9 | UTK09 | Pembroke Welsh Corgi Female (spayed) 11 yrs | B-cell lymphoma |
1.2×1010 TCID50 VSV-cIFN-NIS (Refractory disease) |
No notable adverse events | Urine (−) Buccal (−) |
PROGRESSIVE DISEASE Brief, transient response and disease progression |
| 10 | UTK10 | Metastatic OS, maxillary lesion and lung mets |
2 doses of 2.2×1010 TCID50 VSV-hIFN-NIS @ 0 and 48h (At Diagnosis) |
No notable adverse events | Urine (−) Buccal(−) |
STABLE DISEASE SD 6 months; maxillary lesion progression @ 6m |
Test article and Intravenous administration
Preclinical grade VSV-hIFNβ-NIS or VSV-cIFNβ-NIS was manufactured at the Mayo Clinic Viral Vector Production labs. Virus lots were confirmed negative benzonase and endotoxin contaminations. Virus was shipped on dry ice and received at an approved BSL2 laboratory at the University of Tennessee Medical Center for storage. Virus stocks were thawed and formulated by dilution in sterile saline based on calculated dose immediately prior to administration to dogs in a final volume of 10ml. The test article was administered as a 2–5 minute intravenous bolus via a sterilely placed intravenous cephalic catheter.
Clinical monitoring protocol
Dogs were fasted overnight prior to virus administration and monitored continuously on the day of virus administration (Day 0). Rectal temperature, heart and respiratory rates, and general attitude/demeanor were recorded every 1–2 hours or as the dogs’ condition dictated. Appetite and presence of any gastrointestinal upset (e.g. vomiting, diarrhea) was noted. On every day of study, the dogs’ vital signs (rectal temperature, heart and respiratory rates), overall attitude and appetite, and physical exam findings were recorded. Clinical pathology was monitored by collection of blood drawn via Vacutainer for a Complete Blood Count (CBC), serum biochemistry and electrolyte panel (CHEM), and coagulation panel (COAG). All analyses were performed at the University of Tennessee College of Veterinary Medicine Clinical Pathology and Microbiology Laboratories. Tumor burden was monitored as indicated in Supplemental Table 2. Disease burden in dogs with peripheral nodal lymphoma in dogs was assessed and monitored using criteria previously established by the Veterinary Cooperative Oncology Group (VCOG) (22).
Viremia, pharmacokinetic and virus shedding studies
Virus pharmacokinetics and viremia were assessed in whole blood collected in RNAprotect animal blood tubes (Qiagen). RNA was isolated using Qiagen RNeasy animal blood system and analyzed quantitative real-time polymerase chain reaction (qRT-PCR) to detect VSV-N gene copies as previously described (19). Blood was also collected in a heparinized CPT tube to avoid clotting and centrifuged to separate peripheral blood mononuclear cells (PBMCs) and plasma. PBMCs and plasma were assessed to detect infectious virus and VSV-N gene copies by qRT-PCR in isolated RNA. Briefly, PBMCs were subject to freeze-thaw, centrifuged and supernatant was collected and tested for infectious virus by titration on BHK cells. Biologic samples, specifically urine, buccal swabs, and fecal samples, were collected and tested to monitor virus shedding as previously described (20). Briefly, urine was collected by cystocentesis or urinary tract catheterization in a sterile falcon tube, centrifuged, and urine cell pellet was collected. Buccal swabs were collected using a sterile Omni swab (Whatman). Urine cell pellet and buccal swab samples were tested to detect infectious virus, using an infectious virus recovery (IVR) assay, and virus genome, by qRT-PCR detection of VSV-N gene copies. Feces was collected fresh and processed. RNA was isolated from fecal supernatant using Qiagen Viral RNA mini kit and qRT-PCR was utilized to detect VSV-N gene copies.
Antibody neutralization assay
Serum samples were collected using Vacutainer serum separator tubes (BD Life Sciences) and tested to detect neutralizing antibodies against VSV as previously described (20). Briefly, complement inactivation was carried out by incubation of serum 56°C. Dilutions of serum were pre-incubated with 500 TCID50 VSV-GFP and the virus/serum mix were added to previously plated Vero cells in triplicate. Cytotoxicity was assessed 48h later by measurement of CPE positive wells at each serum dilution, and the minimum virus dilution that did protect cells from VSV induced CPE was recorded.
Measurement of human IFNβ in plasma
Plasma samples were collected at indicated time points and frozen at −80°C. Plasma samples were diluted and assayed for detection of human IFNβ using the Verikine high-sensitivity human IFNβ ELISA kit (PBL assay science, Piscataway, NJ). The limits of detection for this assay were 60–7500pg/mL human IFNβ.
Surgical resection and histopathology analysis
UTK01, a 12 year old spayed female mixed breed research dog weighing at UTCVM was diagnosed with anal adenocarcinoma and received a single dose of VSV-hIFNβ-NIS. Disease stabilization occurred, thus 45 days post VSV treatment, UTK01 underwent surgical resection of the adenocarcinoma. Resected tumor was assessed by standard hematoxylin and eosin (H&E) staining and immunohistochemistry to detect canine CD3+ lymphocytes.
Results
Test article characterization
Recombinant VSV expressing IFNβ and NIS, VSV-IFNβ-NIS, was previously generated and characterized demonstrating rapid cytotoxicity against myeloma and lymphoma cell lines in vitro, and potent therapeutic efficacy following single shot intravenous treatment in an immune competent syngeneic murine myeloma model (11, 12). For the purpose of this study, we generated a VSV expressing canine IFNβ and NIS, VSV-cIFNβ-NIS. Preclinical virus stocks of VSV-hIFNβ-NIS and VSV-cIFNβ-NIS were prepared and tested to ensure test article integrity, sterility, and absence of endotoxins. Virus viability, activity and functional gene expression of purified stocks of VSV-hIFNβ-NIS and VSV-cIFNβ-NIS was confirmed in vitro following infection of susceptible BHK cells, indicating both test viruses replicated rapidly and comparably, albeit with a slightly reduced maximal titer compared to rVSV-GFP in BHK cells (Figure 1a). Functional NIS expression was confirmed by perchlorate sensitive radio-iodine uptake in infected BHK cells (Figure 1b). Functional human and canine IFNβ expression was tested in human Mel-624 (melanoma) cells and canine MDCK (madin-darby canine kidney) cells respectively. Virally expressed IFNβ derived from supernatant of infected BHK-21 cells induced protective antiviral innate immune responses. Human IFNβ from VSV-hIFNβ-NIS infected cells was protective in human Mel-624 cells (Figure 1c), while canine and human IFNβ (from VSV-cIFNβ-NIS and VSV-hIFNβ-NIS infected cells) were protective in MDCK cells indicating human IFNβ is partially cross-reactive in canine tissues. These assays confirm the replicative capacity and functional gene expression of the recombinant oncolytic VSV test articles under investigation in this study.
Figure 1. Characterization of recombinant oncolytic Vesicular stomatitis viruses expressing human or canine IFNβ and NIS.
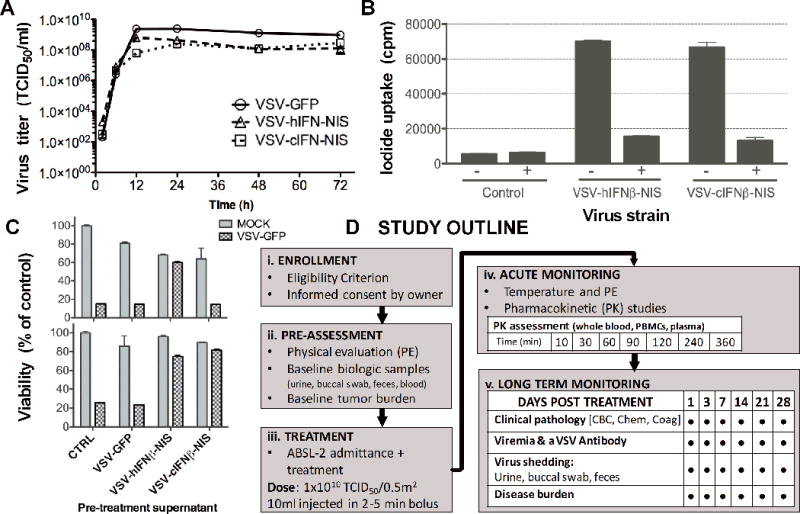
VSV expressing canine or human IFNβ and the sodium iodide symporter (NIS) were tested in vitro to evaluate (A) virus replication in BHK-21 cells by infection with VSV-hIFNβ-NIS, VSV-cIFNβ-NIS or VSV-GFP at a MOI of 3.0 and measurement of virus titer in TCID50; (B) functional NIS expression by measurement of radio-iodide (125I) uptake 24h post infection of BHK-21 cells (MOI 1.0) in the presence (+) or absence (−) of NIS specific inhibitor KCLO4; Expression of (C) functional human or canine IFNβ expression by protection of human Mel-624 or canine MDCK cells respectively following exposure to ultracentrifuged virus infection supernatant (using VSV-GFP cell supernatant as a control), followed by infection with VSV-GFP (MOI 0.1). Cell viability was assessed 48h later by MTT assay and shown as a percentage of control mock infected cells; (D) Veterinary clinical study flow diagram outlining procedures for (i) enrollment, (ii) pre-assessment, (iii) treatment, (iv) acute monitoring, and (v) long-term monitoring.
Study Design and Enrollment
A fixed dose feasibility study investigating tolerability, as a primary endpoint, and efficacy, as a secondary endpoint, of intravenously administered recombinant oncolytic VSV-hIFNβ-NIS or VSV-cIFNβ-NIS was implemented in client-owned dogs with spontaneous cancer at a starting intravenous dose of 1×1010 TCID50/0.5m2, having previously demonstrated the tolerability of this dose in purpose bred laboratory Beagle dogs (20). Owners of dogs diagnosed with cancer at the University of Tennessee Veterinary Medical Center provided written informed consent and dogs to assess compliance with study eligibility criteria (Supplemental Table 1). One research dog and eight client-owned dogs were enrolled initially into the study, each receiving a single intravenous dose of VSV-cIFNβ-NIS or VSV-hIFNβ-NIS. A tenth dog with metastatic osteosarcoma was enrolled and received two intravenous doses of VSV-hIFNβ-NIS, with the second dose administered 48 hours following the first. All procedures were carried out with approval of the University of Tennessee Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC).
Treatment and Monitoring
One research dog and nine client-owned dogs diagnosed with various spontaneous malignancies were enrolled including 4 dogs with B-cell lymphoma and 2 dogs with T-cell lymphoma (Supplemental Table 2). Enrolled dogs underwent a pre-assessment to confirm diagnosis of malignancy and establish tumor burden, clinical pathology and collect biologic samples at baseline (Figure 1d). Dogs were housed in ABSL-2 housing and fasted overnight prior to virus administration. Dose was calculated based on surface area conversion, where each dog received 1×1010 TCID50/0.5m2 (Table 1). Virus was diluted in sterile saline to a 10ml injection volume and administered to each dog via a sterilely-placed intravenous cephalic catheter in a 2–5 minute intravenous bolus. Dogs were monitored continuously on the day(s) of virus administration to record temperature, vitals and general demeanor, and blood samples were collected for pharmacokinetic monitoring. Continued monitoring included physical examinations, and collection of blood, urine, buccal swab and fecal samples to monitor clinical pathology, viremia, shedding, and antibody response as indicated (Figure 1d). Disease burden was monitored by methods specific to indication type as listed in Supplemental Table 2. Peripheral lymphoma burden and response were measured using response criteria established by the Veterinary Cooperative Oncology group (VCOG). A summary of dogs treated, signalment, indication, dose received, clinical toxicities, virus shedding and treatment outcome are provided in Table 1.
Clinical toxicity and adverse events
Dogs were monitored by physical evaluations and clinical pathology testing of blood samples, including Complete blood count (CBC), blood chemistry (CHEM) and coagulation (COAG) tests, to monitor clinical toxicities (Figure 2). Clinical toxicities were evaluated according to the Veterinary cooperative oncology group Common terminology criteria for adverse events (VCOG-CTCAE) following biological antineoplastic therapy in dogs (v1.1, July 2011) (22). Dose limiting toxicities of intravenous VSV-IFNβ-NIS administration include hepatotoxicity and coagulopathy (20). There were no observable symptoms of discomfort or toxicity during intravenous VSV-IFNβ-NIS infusion or following treatment. All treated dogs developed a mild fever starting approximately 3h post infusion that normalized by 24h. Only one dog, UTK08, a 10 year old spayed female Boxer diagnosed with high-grade peripheral T-cell lymphoma who received 2.2×1010 TCID50 VSV-hIFNβ-NIS, had a notable adverse event, specifically transient hepatotoxicity indicated by an alanine aminotransferase (ALT) elevation of approximately 10-fold of upper limit of normal (ULN) that resolved spontaneously (Figure 2a). The ALT elevation was considered Grade 3 toxicity according to VCOG criteria but was not considered to be dose limiting as it was both transient and self-limiting. UTK04, another Boxer diagnosed with high-grade T-cell lymphoma, had a transient ALT elevation of ~2-fold ULN following VSV-hIFNβ-NIS treatment. Notably, both dogs with transient ALT elevations also had peripheral disease remission and partial responses to IV VSV therapy as described below. All dogs developed transient reduction in white blood cells (WBC) in blood, indicative of mild, transient lymphopenia following intravenous VSV-IFNβ-NIS treatment (Figure 2b). There were no notable coagulopathies except as expected in one dog (UTK02) diagnosed with spontaneous myeloma induced hyperglobulinemia, wherein prolongations of prothrombin time (PT) and activated Partial Thromboplastin Time (aPTT) were documented at baseline (figure 2c). Finally, there were no adverse events related to repeated intravenous administration as noted following treatment of one dog, UTK10, who received two doses of VSV-hIFNβ-NIS, providing preliminary evidence of the feasibility of repeat dosing.
Figure 2. Clinical pathology testing of canine blood samples.
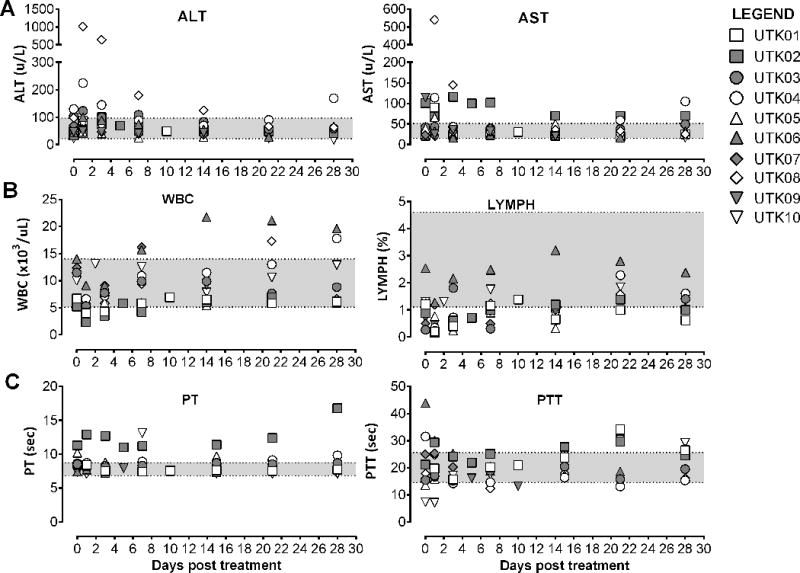
Blood was collected from enrolled dogs at baseline and following systemic VSV therapy. Selected analyses are shown indicating (A) liver function tests (ALT and AST) (B) Complete blood count (CBC) including WBC and LYMPH and (C) Coagulation testing (PT and PTT). Normal range is delineated by the shaded region. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: alkaline phosphatase; WBC: white blood cell count; LYMPH: percentage lymphocytes; PT: prothrombin time; PTT: partial thromboplastin time.
Therapeutic response
Therapeutic response was monitored by measuring disease burden as indicated (Supplemental Table 2) for 28 days following VSV-IFNβ-NIS therapy depending on the cancer type and was observed to be variable (Table 1). Disease burden in dogs with peripheral lymphoma was measured using established guidelines (23). Two dogs with advanced peripheral T-cell lymphoma (UTK04 and UTK08), each treated with a single intravenous dose of 2.2×1010 TCID50 VSV-hIFNβ-NIS, had rapid disease remission of disseminated peripheral lymphoma indicated by reduction in diameters of all peripheral lesions (figure 3a–b) resulting in measurable partial responses of 40% and 60% respectively (figure 3c). In both cases, remission was transient, lasting approximately ~21 days and ~36 days before disease relapsed. UTK08, who had rapid remission of peripheral lesions, received an additional intratumoral injection of 3×1010 TCID50 VSV-hIFNβ-NIS on Day 19 in the right mandibular lymph node lesion that was relapsing (Figure 3b, depicted by arrow), with no measurable impact on lesion size. Two dogs with spontaneous anal adenocarcinoma and advanced peripheral B-cell lymphoma had stable and progressive disease respectively. Three of five dogs treated with VSV-cIFNβ-NIS, including dogs with myeloma, peripheral B-cell lymphoma and multifocal cutaneous melanoma, had stable disease, while two dogs with advanced peripheral B-cell lymphoma had disease progression. UTK01, a dog with anal adenocarcinoma, had surgical resection of the tumor 45 days after receiving intravenous VSV-hIFNβ-NIS treatment. Histopathological analysis of the resected tumor indicated a high infiltration of T-cells compared to historical controls of the same tumor type (Supplemental Figure 1) indicative of tumor specific immune infiltration following intravenous VSV treatment.
Figure 3. Remission of peripheral T-cell lymphoma following intravenous VSV-hIFNβ-NIS treatment.
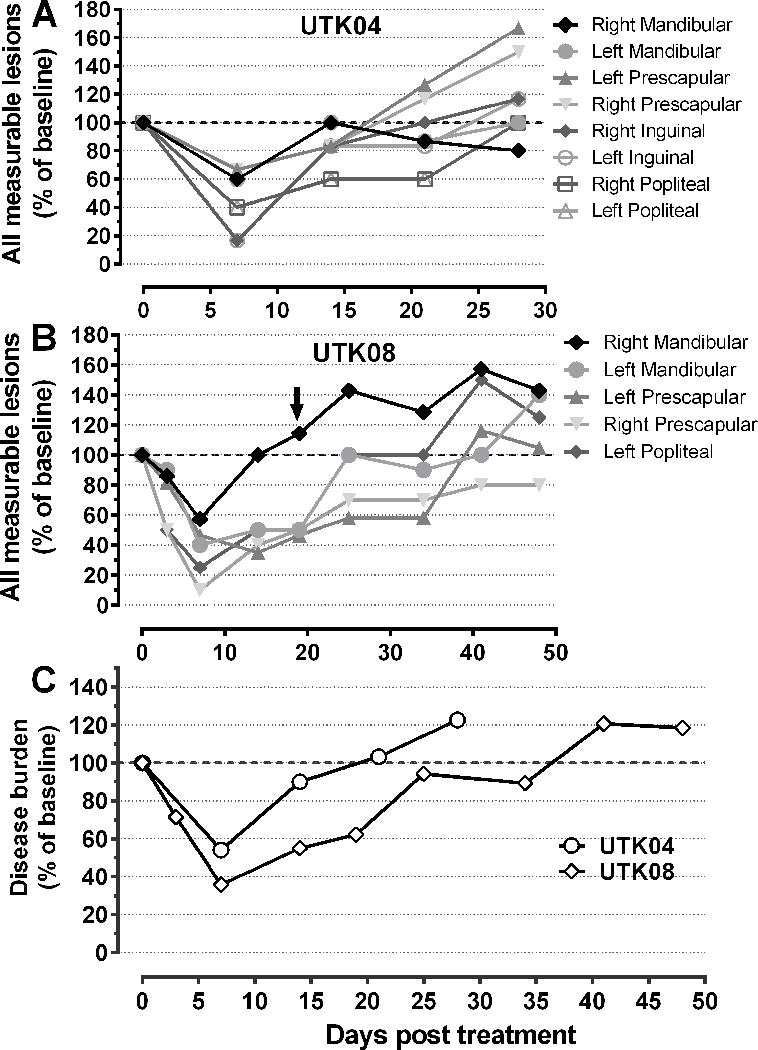
Disease burden was monitoring in two responding dogs, (A) UTK04 and (B) UTK08, both with peripheral T-cell lymphoma, who received a single intravenous dose of 2.2×1010 TCID50. Disease remission was monitored by serial caliper measurements and shown as percentage change in individual lesions compared to baseline measurement, including mandibular, prescapular, popliteal, and inguinal nodal lesions; (C) Therapeutic response in responding dogs was assessed based on response criterion established by the Veterinary Cooperative Oncology Group (VCOG) with disease burden shown as a percentage of baseline based on mean sum LD (Longest diameter) of five target lesions.
Virus Shedding
Shedding studies were carried out to monitor release of infectious materials following intravenous administration of replication competent oncolytic VSV. Buccal swabs, urine (separated into urine cell pellet and supernatant), and fecal samples were collected. All samples were assessed using highly sensitive quantitative real-time polymerase chain reaction (qRT-PCR) to detect VSV-N gene copies. Quantitative RT-PCR analyses indicated some buccal swab, urine or fecal samples had detectable VSV-N gene copies, though all samples were close to or below Limit of Detection (LOD, Supplemental Figure 2). Detection of viral RNA by qRT-PCR is not indicative of infectious virus, and in accordance with FDA recommendations (13) buccal swabs and urine samples were also assayed to detect presence of infectious virus using an infectious virus recovery (IVR) assay by overlay on susceptible BHK-21 cells, indicating no detectable infectious virus. Some buccal swab samples were toxic to cells in culture due to the predictable presence of nonviral contaminants, and absence of infectious virus was confirmed by filtration and repeat of overlay of cell supernatant and confirming absence of VSV RNA by qRT-PCR. These data indicate the absence of virus shedding in biological excreta following intravenous VSV-IFNβ-NIS administration in tumor bearing dogs.
Virus Pharmacokinetics in blood
The comparative oncology approach is amenable to pharmacokinetic monitoring. Serial blood sample collection was carried out starting at 10 minutes and up to 6 hours following intravenous VSV-IFNβ-NIS administration to detect VSV RNA in whole blood, and both VSV RNA and infectious virus in isolated peripheral blood mononuclear cells (PBMCs) and plasma. Pharmacokinetic studies indicate an immediate transient decline (in the first 60min), then stabilization of VSV RNA over 6 hours (Figure 4a). Infectious virus was detected only in PBMC samples (Figure 4b) and not in plasma (Figure 4d). Measurement of VSV RNA bio-distribution in blood indicates that intravenously administered VSV localizes to PBMCs and does not remain in plasma (Figure 4c–d). These data also indicate that VSV RNA is detectable at higher quantities in PBMCs compared to whole blood. Notably, the two dogs with measurable disease remission, UTK04 and UTK08, had ~10-fold higher VSV-N gene copy number in blood compared to dogs with no measurable disease remission, peaking at ~2h post IV VSV-IFNβ-NIS administration. The highest quantity of infectious virus was recovered in PBMCs isolated from UTK08, the dog with the best response to intravenous VSV-IFNβ-NIS therapy. These data indicate that assessment of isolated PBMCs is the most sensitive method to measure both VSV RNA and infectious virus. Furthermore, these data provide preliminary indication that virus pharmacokinetics may correlate with therapeutic response to intravenously administered oncolytic VSV therapy.
Figure 4. Assessment of virus pharmacokinetics, bio-distribution, viremia and antibody neutralization following intravenous VSV-IFNβ-NIS treatment.
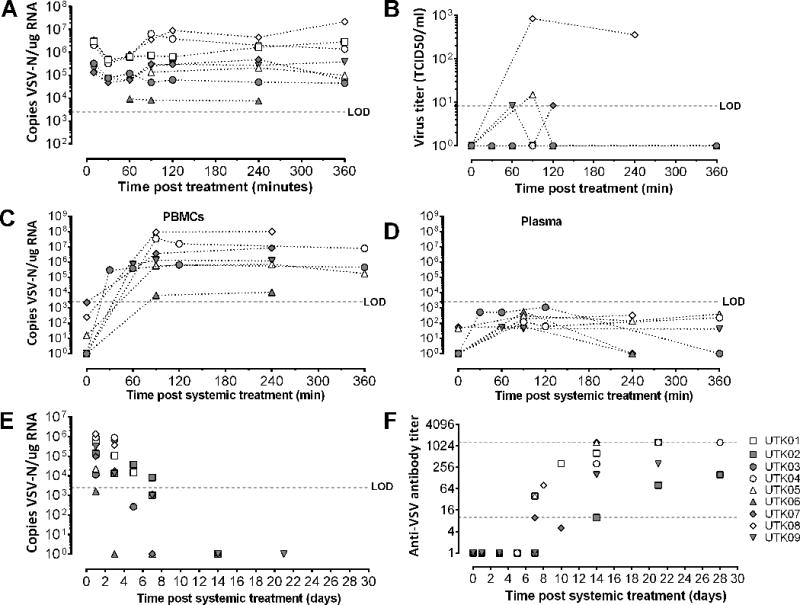
Blood samples were collected immediately following intravenous VSV-IFNβ-NIS treatment in tumor bearing dogs for pharmacokinetic studies. RNA was isolated from (A) whole blood to detect VSV-N gene copies by qRT-PCR; (B) PBMCs were isolated to detect infectious virus by overlay on susceptible BHK cells to measure TCID50. Virus bio-distribution was assessed in (c) PBMCs or (d) plasma indicating VSV-N genes were detectable primarily in PBMCs and below limit of detection (LOD) in plasma. Whole blood and serum were collected to monitor (e) viremia and (f) anti-VSV neutralizing antibodies at indicated time points following intravenous VSV-IFNβ-NIS treatment.
Viremia and Neutralizing antibodies
Blood samples were analyzed to monitor viremia and presence of neutralizing antibodies following intravenous VSV-IFNβ-NIS therapy. Virus genomes were detectable in whole blood at 24h post infusion and decayed rapidly between days 3 to 7, with genomes undetectable (or below limit of detection) by day 14 (Figure 4e). Anti-VSV neutralizing antibodies were detectable in serum by day 7 in most treated dogs (Figure 4f), coinciding with clearance of virus from blood. Analysis of antibody response in dogs indicated there was a delay in generation of neutralizing antibodies against VSV in a myeloma bearing dog, UTK02, and lower antibody titers in dogs with B-cell malignancies (e.g. UTK07, UTK09). The immune suppressed state of patients with B-cell malignancies may impact generation of antiviral antibodies, but this observation would need to be confirmed in a larger cohort of tumor-bearing dogs.
Monitoring virus transgene expression
Virus activity can be monitored by measurement of expression or biological activity of encoded transgenes (10). Here we measured the human IFNβ levels in plasma from 3 dogs treated with VSV-hIFNβ-NIS, using this as a surrogate soluble marker of the total burden virus infected cells, One dog treated with VSV-cIFNβ-NIS and a non tumor bearing (NTB) dog administered with a single dose of VSV-hIFNβ-NIS were included as controls. These data indicate that human IFNβ is measurable in plasma and rapidly increases following VSV-hIFNβ-NIS administration in tumor bearing or non tumor bearing dogs. Human IFNβ expression peaked at approximately ~6h post intravenous VSV-hIFNβ-NIS administration (Supplemental figure 3). Plasma levels of human IFNβ in responding dogs (UTK04 and UTK08) were only slightly higher at 90min post treatment but were detectable for a longer time (up to 7 days post treatment) compared to nonresponsive (UTK05) or NTB control. These data demonstrate facile monitoring of both quantity and duration of transgene expression that may provide convenient biomarkers of therapeutic response following treatment of intravenously administered recombinant oncolytic therapies.
Feasibility of repeated intravenous VSV-IFNβ-NIS administration
Pharmacokinetic studies provide a preliminary indication that higher levels of viral RNA in blood may correlate with therapeutic response to intravenous VSV-IFNβ-NIS therapy. Modification of dose regimen, including repeated dosing, may be utilized to improve therapeutic response by optimizing pharmacokinetics and improving bio-availability of cancer therapies (24,25), particularly following intravenous administration. To test the feasibility of repeated intravenous VSV-IFNβ-NIS dosing, we enrolled one dog, UTK10, with maxillary osteosarcoma (OSA) and metastatic lung nodules who received 2 doses 2.2×1010 TCID50. Treatment was well tolerated with no notable adverse events associated with repeated VSV administration (figure 2), and no detectable shedding of infectious virus (Supplemental figure 2). As previously observed, VSV genomes were detected primarily in PBMCs (figure 5a). Infectious virus (denoted by asterisks) was detectable in PBMCs following the first and second dose of VSV-hIFNβ-NIS, indicating that repeated dosing can extend the persistence of infectious virus in blood. Correlative monitoring of human IFNβ levels in plasma indicated that human IFNβ was not extended or increased due to repeated VSV-hIFNβ-NIS administration (figure 5b). Dogs with metastatic OSA generally have a median life expectancy of 2–3 months, but this is highly variable depending on site of metastasis (bone vs. soft tissues) and type of treatment sought by pet owners (26). UTK10 had stable disease for 6 months before progression of the maxillary lesion led to humane euthanasia. Chest radiographs taken at the time of euthanasia indicated no significant progression of the pulmonary metastatic nodules.
Figure 5. Assessment of virus pharmacokinetics and transgene expression following two-dose intravenous VSV-IFNβ-NIS treatment in a dog with spontaneous osteosarcoma.
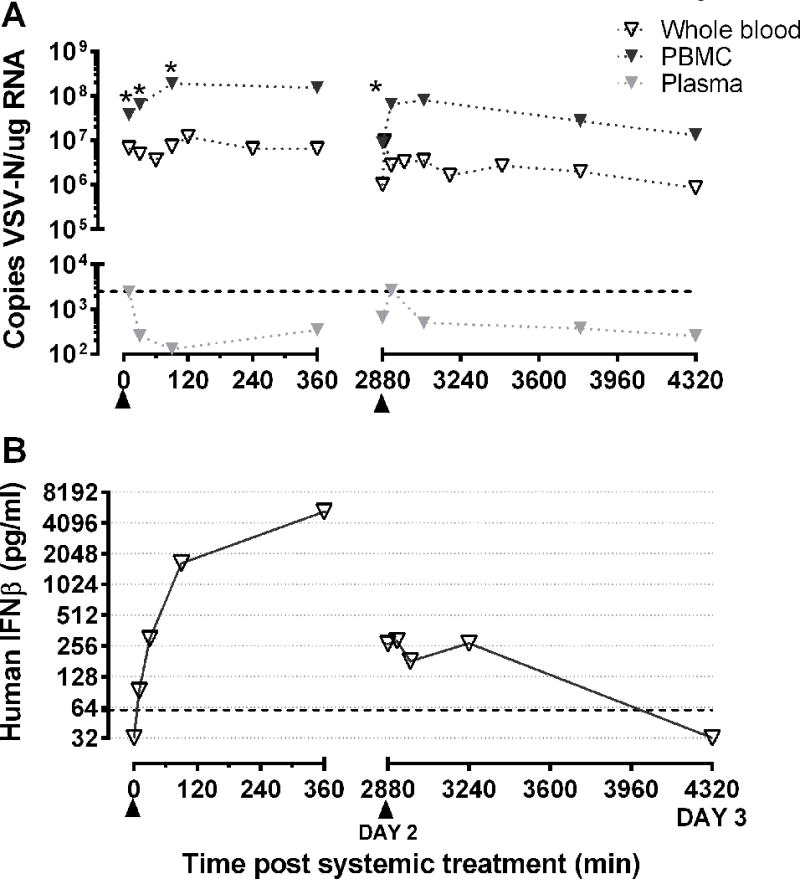
(a) RNA was isolated from whole blood, PBMCs and plasma collected immediately following VSV-IFNβ-NIS treatment to detect VSV-N gene copies by qRT-PCR. Infectious virus was detected in PBMCs and samples positive for recovery of infectious VSV are denoted using an asterisk (*). (B) Human IFNβ was monitored in plasma collected at indicated time points following administration of two IV doses of VSV-IFNβ-NIS (denoted by ▲)
Discussion
This study provides the first evaluation of intravenous oncolytic virus therapy in dogs with spontaneous cancer. Oncolytic virus therapy uses engineered viruses to induce durable tumor remission through a combination of virus replication, lytic destruction of tumor cells, and activation of immune mediated tumor recognition and elimination (3). Oncolytic virus products are generally attenuated and have reduced virulence and pathogenicity compared to the wild type strains (1). Clinical development of novel cancer therapies requires comprehensive preclinical assessment of clinical toxicities and therapeutic efficacy (12). In addition, clinical translation of replication-competent OV therapies requires assessment of virus shedding and biosafety (13). While useful to perform a preliminary assessment of toxicity, efficacy, and mechanisms of action of novel anticancer agents (27), preclinical rodent models limit clinically relevant translational assessment due to disparities in body size, physiology, genetic and immunologic attributes between rodents and humans. Comparative oncology allows clinically relevant evaluation of novel oncolytic virus therapies, as naturally occurring canine cancers recapitulate the genetic and molecular heterogeneity and tumor immune microenvironment of human cancer (15). The ability to collect serially biological samples from the same animal, including blood and biological excreta, enables clinical and correlative monitoring. Clinical monitoring of clinical toxicities and biosafety including viremia and virus shedding is critical to clinical translation. Correlative monitoring including virus pharmacokinetics and pharmacodynamics, transgene expression, immune responses allow us to investigate determinants and identify biomarkers that are predictive of or correlate with clinical toxicity or efficacy.
Here we demonstrate the utility of the comparative oncology approach and establish the safety, both in terms of toxicity and shedding, of intravenous oncolytic VSV-IFNβ-NIS administration in dogs with spontaneous cancer. Clinical monitoring indicates that hepatotoxicity is the primary dose-limiting toxicity (DLT) following intravenous VSV-IFNβ-NIS therapy, notably observed only in dogs that had disease remission, suggesting this DLT may be associated with virus bio-availability and/or tumor lysis. Virus shedding studies confirm the absence of infectious virus shedding in biological excreta. These data support clinical translation and inform the design of adverse event monitoring for human clinical trials. This approach also allowed testing of novel oncolytic therapies in dogs with various, spontaneously occurring and heterogeneous malignancies. Results demonstrate that single shot intravenous VSV therapy can induce remission of disseminated cancer. The rapid speed of remissions of peripheral lesions observed in dogs with advanced T-cell lymphoma suggests it is due to virus mediated tumor destruction. While the results of this study are limited due to the small sample size, the observed responses led to inclusion of T-cell lymphoma as a target indication in a Phase 1 human clinical trial evaluating intravenous VSV-hIFNβ-NIS therapy (NCT03017820, clinicaltrials.gov).
Correlative monitoring including pharmacokinetics studies and measurement of transgene expression in blood provide with preliminary data indicating ~10X higher VSV RNA detectable and sustained IFNβ expression in blood from dogs with measurable disease remission (compared to dogs with stable or progressive disease). These studies suggest methods to enhance virus pharmacokinetics may improve virus bioavailability and therapeutic response. Soluble serum marker measurement, however, does not indicate location of virus infection and early IFNβ detection may be due to infection of PBMCs rather than tumor cells. Single-Photon Emission Computed Tomography or Positron Emission Tomography/Computed Tomography (SPECT/CT or PET/CT) imaging to monitor viral NIS expression would be a more effective method to serially and noninvasively monitor virus bio-distribution and confirm tumor specific virus replication (10).
Findings from this study have contributed to clinical translation of oncolytic VSV-IFNβ-NIS therapy including selection of a safe clinical starting dose to avoid protracted dose escalation, and design of clinical monitoring protocols and correlative assays to assess toxicities and virus shedding. These findings supported approval and initiation of a Phase I clinical trial to evaluate intravenous VSV-IFNβ-NIS therapy in patients with relapsed or refractory multiple myeloma and T-cell lymphoma, the latter indication included due to observed disease remission in T-cell lymphoma bearing dogs. These studies provide preliminary indication of factors that correlate with, and may be predictive biomarkers of therapeutic response including virus pharmacokinetics in blood and sustained transgene expression, and support the development of oncolytic VSV as canine cancer therapy to benefit pets and pet-owners.
Traditional pathways of cancer drug development are long and expensive. While introduction of accelerated pathways of drug approval has expedited cancer drug development, costs are high and accessibility is limited. The utilization of comparative oncology will inform new product development and refine selection of products to advance to clinical development, potentially reducing the time, cost and risk of drug development.
Supplementary Material
Acknowledgments
We wish to acknowledge Christina Mazcko for assistance in manuscript preparation
Additional information: Financial support for this clinical trial provided by grants from the Morris Animal Foundation (#D14CA-002 – A. LeBlanc); a Discovery Research Program and Career Enhancement Program award, supported in part by the Public Health Service grant number P50 CA097274 from the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (UI/MC Lymphoma SPORE) and the National Cancer Institute (S. Naik); Mayo Clinic Discovery Translational Program grant (DTP2012 – S. J. Russell and S. Naik); Intramural Research Program of the National Institutes of Health (NIH), NCI, Center for Cancer Research. The results and interpretations do not reflect the views of the US Government.
References
- 1.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert opinion on biological therapy. 2009;9:1163–76. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 2.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends in pharmacological sciences. 2007;28:326–33. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nature biotechnology. 30:658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:805629. doi: 10.1155/2012/805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4477–81. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB life. 2000;50:135–8. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 8.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77:8843–56. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–6. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 10.Miller A, Russell SJ. The use of the NIS reporter gene for optimizing oncolytic virotherapy. Expert opinion on biological therapy. 2016;16:15–32. doi: 10.1517/14712598.2016.1100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naik S, Nace R, Federspiel MJ, Barber GN, Peng KW, Russell SJ. Curative one-shot systemic virotherapy in murine myeloma. Leukemia. 2012;26:1870–8. doi: 10.1038/leu.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Center for Biologics Evaluation and Research. S9 Nonclinical Evaluation for Anticancer Pharmaceuticals. Food and Drug Administration. 2010 [Google Scholar]
- 13.Center for Biologics Evaluation and Research. Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products. Food and Drug Administration. 2015 [Google Scholar]
- 14.Cheon DJ, Orsulic S. Mouse models of cancer. Annu Rev Pathol. 2011;6:95–119. doi: 10.1146/annurev.pathol.3.121806.154244. [DOI] [PubMed] [Google Scholar]
- 15.Anderson KL, Modiano JF. Progress in Adaptive Immunotherapy for Cancer in Companion Animals: Success on the Path to a Cure. Vet Sci. 2015;2:363–87. doi: 10.3390/vetsci2040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnett RC, Vernau W, Modiano JF, Olver CS, Moore PF, Avery AC. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Veterinary Pathology. 2003;40:32–41. doi: 10.1354/vp.40-1-32. [DOI] [PubMed] [Google Scholar]
- 17.Modiano JF, Breen M. Shared pathogenesis of human and canine tumors - an inextricable link between cancer and evolution. Cancer Therapy. 2008;6:239–46. [Google Scholar]
- 18.Scott MC, Sarver AL, Gavin KJ, Thayanithy V, Getzy DM, Newman RA, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone. 2011;49:356–67. doi: 10.1016/j.bone.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc AK, Naik S, Galyon GD, Jenks N, Steele M, Peng KW, et al. Safety studies on intravenous administration of oncolytic recombinant vesicular stomatitis virus in purpose-bred beagle dogs. Human gene therapy. 24:174–81. doi: 10.1089/humc.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik S, Nace R, Barber GN, Russell SJ. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-beta. Cancer gene therapy. 2012;19:443–50. doi: 10.1038/cgt.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veterinary cooperative oncology group - common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2011 doi: 10.1111/vco.283. [DOI] [PubMed] [Google Scholar]
- 23.Vail DM, Michels GM, Khanna C, Selting KA, London CA. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)–a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2010;8:28–37. doi: 10.1111/j.1476-5829.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 24.Reece DE, Sullivan D, Lonial S, Mohrbacher AF, Chatta G, Shustik C, et al. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother Pharmacol. 2011;67:57–67. doi: 10.1007/s00280-010-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng KW, Hadac EM, Anderson BD, Myers R, Harvey M, Greiner SM, et al. Pharmacokinetics of oncolytic measles virotherapy: eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer gene therapy. 2006;13:732–8. doi: 10.1038/sj.cgt.7700948. [DOI] [PubMed] [Google Scholar]
- 26.Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985–2004) J Am Vet Med Assoc. 2006;228:1905–8. doi: 10.2460/javma.228.12.1905. [DOI] [PubMed] [Google Scholar]
- 27.Bailey K, Kirk A, Naik S, Nace R, Steele MB, Suksanpaisan L, et al. Mathematical model for radial expansion and conflation of intratumoral infectious centers predicts curative oncolytic virotherapy parameters. PLoS One. 2013;8:e73759. doi: 10.1371/journal.pone.0073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


