Abstract
The high human immunodeficiency virus (HIV) prevalence in sub-Saharan Africa has markedly changed the epidemiology and presentation of adult meningitis. We conducted a systematic review using PubMed, Embase, Ovid, CENTRAL, and African Index Medicus to identify studies in Africa with data on neurological outcomes in adults after meningitis. We found 22 articles meeting inclusion criteria. From 4 studies with predominately pneumococcal meningitis, a median of 19% of survivors experienced hearing loss up to 40 days. Two studies of cryptococcal meningitis evaluated 6- to 12-month outcomes; in one, 41% of survivors had global neurocognitive impairment and 20% severe impairment at 1 year, and in a second 30% of survivors had intermediate disability and 10% severe disability at 6 months. A single small study of patients with tuberculosis/HIV found marked disability in 20% (6 of 30) at 9 months. Despite the high burden of meningitis in sub-Saharan Africa, little is known about neurological outcomes of patients with HIV-associated meningitides.
Keywords: Africa, HIV, meningitis, neurological complications, neurological sequelae
Meningitis is associated with high morbidity and mortality in resource-rich and resource-limited settings [1]. The human immunodeficiency virus (HIV) epidemic in sub-Saharan Africa (SSA) led to an increase in incidence of meningitis, especially in adults with advanced HIV disease [2, 3]. Case-fatality estimates for 3 of the main causes of adult meningitis in SSA—cryptoccoccal, pneumococcal, and tuberculous meningitis—range from 41% to 70%, and meningitis is the cause of up to 20% of deaths in HIV-infected cohorts [4–6]. In addition to the high mortality, ongoing disability from neurological sequelae in survivors further increases the burden of disease due to meningitis [7].
Multiple studies from Africa have reported neurological sequelae in pediatric survivors of meningitis [8]. Pediatric studies show an increased risk of mortality from bacterial meningitis in HIV-infected versus uninfected children and greater risk of recurrence, suggesting that these patients are more susceptible to poor outcomes [9, 10]. However, the epidemiology and etiology of meningitis differs markedly between adults and children, and little is known about long-term outcomes of adult meningitis in Africa. From population-based estimates in rural Kenya, meningitis was one of the leading causes of disability-adjusted life years lost due to high case-fatality rates and long-term disability in HIV-uninfected survivors [7]. A clear understanding of long-term disability of adult meningitis survivors in the context of the high regional burden of HIV-associated meningitis has important public health and economic implications for low- and middle-income countries [6, 11]. The aim of this systematic review is to describe our current understanding of neurologic sequelae in adult survivors of meningitis in Africa.
METHODS
Literature Search Strategy
We conducted a systematic review of articles published on neurological sequelae of adult meningitis in Africa with no lower limit on start date until June 30, 2017. A search strategy was developed with 3 components: one identifying articles on “meningitis” or “meningoencephalitis,” a second limiting the search to articles from Africa, and a third identifying articles reporting on neurological sequelae of disease. We searched 5 databases, including PubMed, Embase, Ovid, CENTRAL, and African Index Medicus. The full search strategy is shown in Table 1. Three reviewers (D. W. G., H. K. M., and M. W. T.) independently screened the databases using these search terms. We identified relevant supplemental articles through review of reference lists of relevant articles (published reviews and included studies) in the primary search. Citations were uploaded into an EndNote Library and duplicate articles were removed.
Table 1.
PubMed Search Terms
| Search component 1 | [meningitis OR meningoencephalitis] |
| Search component 2 | [Africa OR African OR Algeria OR Algerian OR Angola OR Angolan OR Benin OR Beninese OR Beninoise OR Botswana OR Botswanan OR Motswana OR Batswana OR Burkina Faso OR Burkinabe OR Burundi OR Burundian OR Cameroon OR Cameroonian OR cape Verde OR cape Verdean OR cab Verdeans OR central African republic OR central African OR chad OR Chadian OR Comoros OR Comorian OR democratic republic of the Congo OR Congolese OR republic of the Congo OR congo-brazzaville OR cote d’ivoire OR ivory coast OR Ivorian OR Ivoirian OR Djibouti OR Djiboutian OR Egypt OR Egyptian OR equatorial guinea OR equatorial guinean OR Equatoguinean OR Eritrea OR Eritrean OR Ethiopia OR Ethiopian OR Gabon OR Gabonese OR Gambia OR Gambian OR Ghana OR Ghanaian OR guinea OR Guinean OR Guinea- Bissau OR Bissau OR Kenya OR Kenyan OR Lesotho OR Basotho OR Mosotho OR Libya OR Libyan OR Madagascar OR Malagasy OR Malawi OR Malawian OR Mali OR Malian OR Mauritania OR Mauritanian OR Mauritius OR Mauritian OR Mayotte OR Mahuran OR morocco OR Moroccan OR Mozambique OR Mozambican OR Namibia OR Namibian OR Niger OR nigerien OR Nigeria OR Nigerian OR Rwanda OR Rwandan OR Rwandese OR Senegal OR Senegalese OR Seychelles OR Seychellois OR seychelloise OR sierra Leone OR sierra Leonean OR Somalia OR Somali OR south Africa OR south African OR Sudan OR Sudanese OR Swaziland OR Swazi OR Tanzania OR Tanzanian OR Togo OR Togolese OR Tunisia OR Tunisian OR Uganda OR Ugandan OR western Sahara OR western Saharan OR Zambia OR Zambian OR Zimbabwe OR Zimbabwean] |
| Search component 3 | [cognitive OR cognition OR neurocognitive OR neurocognition OR complications OR attention OR behavior OR behavioral OR behaviors OR sequelae OR impairment OR retardation OR epilepsy OR disorder OR learning OR memory OR function OR functions OR functional OR dysfunction OR dysfunctional OR dysfunctions OR deficit OR neuropsychological OR psychological OR psychomotor OR motor OR hyperactivity OR disability OR disabilities OR iq OR intelligence OR hearing OR sensorineural OR deaf OR deafness OR “rankin scale”] |
Citation Screening (Inclusion/Exclusion Criteria)
We screened citations against standardized eligibility criteria with initial title and abstract search followed by full text review of potentially eligible articles. Inclusion criteria included the following: (1) involving patients ≥12 years of age; (2) conducted at health centers in Africa; (3) reporting on prevalence or incidence of any neurologic sequelae in patients with meningitis; and (4) using a prospective or retrospective observational cohort methodology or randomized controlled trials. We excluded case studies and case series with less than 20 patients, because of concern for heterogeneity of reported outcomes and high likelihood of publication of nonrepresentative cases in case reports or case series, as well as published conference abstracts. For studies with pediatric and adult outcomes data separately reported, we excluded pediatric patients in our analysis. Adult and pediatric meningitis outcome data were intermixed in several included studies, primarily studies of Neisseria meningitidis meningitis. Inclusion of patients ≥12 years of age was decided as a developmentally meaningful cutoff (following rapid neurodevelopmental change, particularly in the first decade of life). We included studies reporting outcomes from microbiologically confirmed meningitis or meningitis diagnosed using molecular methods, and we also included studies with cases diagnosed using a combination of microbiological and/or molecular methods and cerebrospinal fluid (CSF) profiles suggestive of bacterial meningitis (CSF with polymorphonuclear cell pleocytosis), as well as N meningitidis diagnosed clinically during meningococcal meningitis epidemics. No language restrictions were placed on the search. Although no formal quality criteria were used to determine inclusion or exclusion of articles, we undertook a subjective assessment of trial quality based on established guidelines [12].
Data Abstraction and Analysis
Study information was entered into Microsoft Excel spreadsheets, and accuracy was verified through consensus from all authors. Study year(s), country and clinical setting, study design, meningitis pathogen(s), patient clinical characteristics (age distribution and HIV status), follow-up period, outcomes measured, and outcomes data including both death and neurological sequelae were entered into spreadsheets (Table 2 and Table 3). We categorized outcomes according to common sequelae, eg, hearing loss, as performed in previous meningitis reviews [8, 13]. Tables were organized by main causative pathogen and further sorted by year of publication.
Table 2.
Articles Included in Review
| Author, Publication Year [Reference] | Country, Setting, Year(s) | Study Type | Meningitis Type | Patient Assessed for Sequelae ±Death | Sex Distribution, %Male (n/N) | Age in Years, Mean (SD)* | HIV Status, % (n/N) | Treatment Regimen | Follow-Up | Outcome Measures Recorded |
|---|---|---|---|---|---|---|---|---|---|---|
| Neisseria meningitidis Meningitis | ||||||||||
| Coldiron, 2016 [45] | Niger, home visits, 2015 | Retrospective cohort | Of 194 CSF samples with positive PCR testing, identified N meningitis serogroup C (74%), serogroup W (19%) Streptococcus pneumoniae (6%), N meningitidis serogroup unspecified (1%) | 369 | 60% (220 of 369) | Median 5–14‡ | NS | Reported standard treatment ceftriaxone ×5 days | Single evaluation 3.5–9 months after meningitis | Paralysis, anosmia, convulsions, hearing loss, loss of developmental milestones, persistent mental incapacity |
| Jusot, 2013 [46] | Niger, health facilities from 4 of 8 regions, 2010–2011 | Prospective cohort | N meningitidis serogroup W (87%), serogroup A (6%), and serogroup C (1%), S pneumoniae (6%) | 67 | 53% (44 of 83)† | 13‡ | NS | NS | Range 50–141 days | Functional symptoms, hearing loss, motor impairment, psychological troubles (using Conners’ questionnaire) |
| Seydi, 2002 [20] | Senegal, referral hospital, 1999 | Prospective cohort | N meningitidis serogroup A (100%) | 70 | 60% (42 of 70) | Median 20 (range 1–68)‡ | NS | Chloramphenicol 50 mg/kg IM daily in 3 doses ×8 days average (84%); cefotaxime 50 mg/kg IV daily ×5 days (9%); ceftriaxone 50 mg/ kg IM/IV daily ×5 days (7%) | In-hospital | Hearing loss |
| Hodgson, 2001 [47] | Ghana, district hospital, 1999 | Prospective cohort | N meningitidis serogroup A | 505 | 44% (225 of 505) | 24 (15)‡ | NS | NS | Surviving patients evaluated 2 years after outbreak | Cerebellar disorder, cranial nerve palsy, hydrocephalus, motor deficit |
| Heyman, 1998 [25] | Zaire (Democratic Republic of Congo), refugee field hospital, 1994 | Retrospective cohort | Bacterial meningitis during N meningitis epidemic (included cases confirmed by CSF positive for Gram-negative diplococci, culture, or soluble antigen test OR based on clinical picture and turbid CSF) | 45 | 48% (25 of 52)† | 13 (standard error of the mean 1)‡ | 12% (2 of 17) | Penicillin 4 million units IV 6 times/day + chloramphenicol 1 gram 4 times/ day (29 of 37 [78%] patients with records); chloramphenicol (6 of 37 [16%]); penicillin (1 of 37) [3%]; ciprofloxacin (1 of 37 [3%]) | In-hospital | Clinically obvious neurological damage |
| Fekade, 1992 [48] | Ethiopia, referral hospital, 1988 | Prospective cohort | Bacterial meningitis (CSF with polymorphonuclear cell pleocytosis, low glucose, and high protein OR positive Gram stain OR bacterial culture OR clinical presentation consistent with N meningitis); all presumed N meningitidis (serogroup A) | 204 | 64% (179 of 278)† | Range 15–49; 57% in age group 15–19† | NS | Penicillin-based therapy (80%); Combination penicillin and chloramphenicol (20%); treatment duration 7–10 days | In-hospital | Cranial nerve palsy, deafness, hemiplegia |
| Girgis, 1989 [18] | Egypt, referral hospital and US Navy research unit, 1983-NS | Prospective unblinded RCT | N meningitidis (62%), S pneumoniae (25%), Haemophilus influenzae (13%) | 429 | 65% (278 of 429) | 14 (9) in group treated with dexamethasone; 13 (9) in control group‡ | NS | Ampicillin 160 mg/kg IV daily in 4 doses + chloramphenicol 100 mg/ kg IV daily in 4 doses ×8 days ±dexamethasone 12 mg IV every 12 hours ×3 days | Monthly ×6 months | Hearing loss, hemiparesis |
| Smith, 1988 [49] | Gambia, outpatient therapy, 1982–1983 | Prospective cohort | Presumed N meningitidis serogroup A during epidemic; 15.3% (25 of 157) bacteriologically confirmed by CSF or blood culture with most cases diagnosed clinically | 157 | 50% (78 of 157) | Median 10–14‡ | NS | Chloramphenicol 3 grams IM once | 6–12 months | Generalized sequelae (irritability/poor cooperation, slow response, mental retardation, severe brain damage), coordination impairment, cranial nerve palsy, hearing loss, motor deficit, visual loss |
| Habib, 1979 [16] | Egypt, referral hospital and US Navy research unit, 1966–1973 | Prospective cohort | N meningitidis (100%) | 375 (≥10 years) | 56% (438 of 775)† | ≥10 years; 73% in age group 10–19‡ | N/A | NS | Monthly ×6 months | Hearing loss |
| Streptococcus pneumoniae Meningitis | ||||||||||
| Ajdukiewicz, 2011 [22] | Malawi, referral hospital, 2006–2008 | Prospective RCT | Bacterial meningitis (CSF with >100 white cells/μL with polymorphonuclear cell predominance or cloudy CSF); 41% confirmed S pneumoniae and 42% overall confirmed bacterial | 125§ | 48% (61 of 128)† | Median 32 (IQR, 27–38)† | 84% (104 of 124) | Ceftriaxone 2 grams IV twice daily ×minimum 10 days | 40 days | Hearing loss, neurological disability by Glasgow Outcome Score |
| Manga, 2008 [21] | Senegal, referral hospital, 1995–2004 | Retrospective cohort | S pneumoniae (100%) | 73 | 67% (49 of 73) | 44 (19.5) | 12% (9 of 73) | Ampicillin or amoxicillin 200 mg/kg IV daily in 3 doses; cefotaxime 100–200 mg/kg IV 3 times daily; ceftriaxone 50–75 mg/kg IV daily; gentamicin 3 mg/kg IV daily with a β-lactam; chloramphenicol 100 mg/ kg IV daily | In-hospital | Deafness, facial palsy, hemiparesis, oculomotor paralysis |
| Scarborough, 2007 [23] | Malawi, referral hospital, 2002–2005 | Prospective RCT | Bacterial meningitis (CSF with >100 white cells/μL or cloudy CSF); S pneumoniae (56%), N meningitidis (4%), other Gram-negative organism (6%), other (1%) | 465 | 49% (230 of 465) | 32 (11) | 90% (389 of 434) | Ceftriaxone 2 grams IV/IM twice daily ×10 days ±dexamethasone 16 mg IV twice daily ×4 days | 40 days | Blindness, debility, hearing loss, intellectual impairment, paresis, seizure disorder |
| Okome-Nkoumou, 1999 [26] | Gabon, referral hospital, 1991–1995 | Retrospective cohort | S pneumoniae (65%), N meningitidis (29%), Escherichia coli (4%), Pseudomonas aeruginosa (2%) | 85 | 71% (60 of 85) | Median 33 (range 16–60) | 18% (15 of 85) | Amoxicillin/clavulanate 12 grams IV daily ×15 days (19%); cefotaxime 6 grams IV daily ×10 days (56%); cefotaxime 6 grams IV daily + dexamethasone 0.5 mg IV twice daily ×10 days (25%) | In-hospital | Deafness |
| Ford, 1994 [27] | Swaziland, national report system at 4 district hospitals, 1991–1992 | Prospective cohort | Bacterial meningitis (CSF with >1000 white cells/mm3, >75% polymorphonuclear cells, glucose <1.9 mmol/L, and protein >1 g/L, or positive Gram stain OR bacterial culture); in larger cohort of 85 patients (including children), S pneumoniae (29%), N meningitidis (12%), H influenzae (9%), others (9%) | 24 adults (≥15 years) | NS | ≥15 (no further break-down) | 12% (3 of 24) with known HIV (likely significantly higher) | Benzyl-penicillin 4 million units IV every 4 hours + chloramphenicol 25 mg/kg IV every 6 hours ×7–10 days | In-hospital | Ataxia, confusion, cranial nerve palsy, deafness, developmental delay, hemiparesis, hydrocephalus, seizure disorder, confusion |
| Other/Mixed Bacterial Meningitis | ||||||||||
| El-Gindy, 2015 [28] | Egypt, fever hospital, 2013–2015 | Prospective cohort | Confirmed bacterial meningitis (positive CSF stain and/or culture); including 26% (16 of 61) tuberculous meningitis; 74% (45 of 61) other bacterial meningitis, including S pneumoniae (40%), N meningitidis (20%), H influenzae (22%), S aureus (13%), E coli (4%) | 61 adults (≥16 years | 69% (42 of 61) | 61% (37 of 61) 16–40, 24% (15 of 61) 41–60, 15% (9 of 61) ≥61 | NS | NS | In-hospital | Mini-Mental status exam (MMSE), Wechsler Memory Scale (WMS), Glasgow Outcome Scale (GOS), cranial nerve palsy, ischemic brain insult, seizures, speech disorders, hydrocephalus |
| Hammad, 2011 [17] | Egypt, referral hospital and US Navy research unit, 1993–2009 | Retrospective cohort | All gram-negative bacilli; E coli (25%), Proteus mirabilis (22%), P aeruginosa (17%), Salmonella typhi (17%), Klebsiella pneumoniae (14%), Enterobacter spp (5%) | 95 | 64% (61 of 95) | 13 (6) in cases of primary meningitis; 32 (8) in cases of secondary meningitis‡ | NS | Ceftriaxone 3 grams IV daily ×10 days + dexamethasone 0.2 mg/kg IV ×3 days | In-hospital | Cerebral damage, hearing loss, hemiplegia, hydrocephalus, optic atrophy, seizure disorder |
| TB Meningitis | ||||||||||
| Marais, 2013 [33] | South Africa, referral hospital, 2009–2010 | Prospective cohort | Tuberculous meningitis (both “definite” cases with CSF acid-fast stain positive and/or culture positive AND “probable” cases with clinical, laboratory, and radiographic features in absence of other diagnosis) | 34 | 56% (19 of 34) | Median 33 (IQR, 29–44) | 100% (34 of 34); all antiretroviral naive | Antituberculous therapy given according to national guidelines with prednisone 1.5 mg/kg PO daily × 6 weeks then prednisone 0.75 mg/ kg PO daily × 2 weeks (unless TB-IRIS); antiretroviral therapy initiated at 2 weeks | 2, 4, 6, and 12 weeks, 6 months, 9 months | Marked cognitive impairment by International HIV Dementia Scale, hemiparesis, hearing loss |
| Girgis, 1998 [19] | Egypt, referral hospital and US Navy research unit, 1976–1996 | Prospective cohort | Tuberculous meningitis (100% culture-confirmed) | 857 | 58% (497 of 857) | 17 (13)‡ | NS (all patients who received HIV testing were negative) | P-aminosalicylic acid + isoniazid + streptomycin; ethambutol + isoniazid + streptomycin; ethambutol + isoniazid + streptomycin ± dexamethasone; rifampin + isoniazid + streptomycin; all patients treated for 2 years | Eye exam weekly ×6 months during hospital stay then monthly outpatient visits up to 2 years | Cerebral atrophy, cranial nerve palsy, fundus changes, hemiparesis-paraplegia, hydrocephalus |
| Cryptococcal Meningitis | ||||||||||
| Montgomery, 2017 [32] | Uganda, referral hospital, 2010–2012 | Prospective RCT | Cryptococcal meningitis (100%) | 90 | 60% (54 of 90) | Median 36 (IQR, 30–40) | 100% (90 of 90) | Amphotericin B 0.7–1.0 mg/kg IV daily + fluconazole 800 mg PO daily ×2 weeks; then fluconazole 800 mg PO daily ×3 weeks; then fluconazole 400 mg PO daily ×8 weeks; then fluconazole 200 mg PO daily; antiretroviral therapy initiated at 5 weeks; participants randomized to early (1–2 weeks after diagnosis) or late (5 weeks after diagnosis) antiretroviral therapy | Single evaluation 1 month after meningitis diagnosis | Battery of neuropsychological tests evaluating 8 domains; calculated quantitative neurocognitive performance score (QNPZ-8) as mean of individual z-scores and compared with age- and education-adjusted scores of HIV-uninfected Ugandans; also tested Karnofsky, International HIV Dementia Scale, and Center for Epidemiologic Studies Depression (CES-D) Scale scores |
| Beardsley, 2016 [30] | Malawi, Uganda, Indonesia, Laos, Thailand, Indonesia, referral hospitals, 2013–2014 | Prospective RCT | Cryptococcal meningitis (100%) | 226 (excluding patients treated with adjunctive dexamethasone) | 58% (132 of 226) | Median 35 (IQR, 30–40) | 100% (226 of 226) | Amphotericin B 1.0 mg/kg IV daily + fluconazole 800 mg PO daily ×2 weeks; then fluconazole 800 mg PO daily ×8 weeks; then fluconazole 200 mg PO daily; antiretroviral therapy initiated at 5 weeks | 10 weeks and 6 months | Visual acuity at 10 weeks, level of disability at 10 weeks and 6 months (assessed by combination of modified Rankin scale and 2 questions [does the patient require help from anybody for everyday activities, and has the illness left the patient with any other problems?]) |
| Carlson, 2014 [29] | Uganda, referral hospital, 2010–2013 | Prospective cohort | Cryptococcal meningitis (100%) | 78 | 62% (48 of 78) | 35 (8) | 100% (78 of 78) | Amphotericin B 0.7–1.0 mg/kg IV daily + fluconazole 800 mg PO daily ×2 weeks; then fluconazole 800 mg PO daily ×3 weeks; then fluconazole 400 mg PO daily ×8 weeks; then fluconazole 200 mg PO daily; antiretroviral therapy initiated at 5 weeks | 1, 3, 6, and 12 months | Battery of neuropsychological tests evaluating 8 domains; calculated quantitative neurocognitive performance score (QNPZ-8) as mean of individual z-scores and compared with age- and education-adjusted scores of HIV-uninfected Ugandans |
| Rothe, 2013 [24] | Malawi, referral hospital, 2010–2011 | Prospective cohort | Cryptococcal meningitis (100%) | 60 | 55% (33 of 60) | Median 32 (IQ,R 29–39) | 100% (60 of 60) | Fluconazole 800 mg PO ×2 weeks; then fluconazole 400 mg PO daily ×6 weeks; then fluconazole 200 mg PO daily; antiretroviral therapy initiated at 4 weeks | 4 weeks, 10 weeks, 52 weeks | Neurological function tested at 52 weeks by modified Rankin Scale score and Abbreviated Mental Test Score |
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IM, intramuscular; IV, intravenous; IQR, interquartile range; N/A, not applicable; NS, not stated; OR, odds ratio; PCR, polymerase chain reaction; PO, per os (oral); RCT, randomized controlled trial; SD, standard deviation; TB-IRIS, tuberculosis-associated immune reconstitution inflammatory syndrome.
Unless otherwise stated.
Included additional patients lost to follow up, not included, or without outcomes data.
Included some pediatric patients (<12 years).
Excluded intervention arm receiving glycerol, which was associated with significantly higher mortality and disability.
Table 3.
Results Including Death and Neurological Complications From Included Articles
| Author, Publication Year [Reference] | Follow-Up Period | Pathogen(s) Studied | %Died (n/N) | %Visual Deficit (n/N) | %Hearing Deficit (n/N) | %Neurocognitive Dysfunction (n/N) | %Motor Deficit (n/N)* | %Seizure Disorder (n/N) | %Other Outcomes (n/N) |
|---|---|---|---|---|---|---|---|---|---|
| Neisseria meningitidis meningitis | |||||||||
| Coldiron, 2016 [45] | Single evaluation 3.5–9 months after meningitis | N meningitidis primary pathogen identified (94% of CSF samples with positive PCR) | 17% (64 of 369) of patient households with home visits; 12% (23 of 189) of patients with confirmed N meningitidis serogroup C | 12% (15 of 126) of patients any confirmed pathogen had any hearing loss | 3% (4 of 126) with any confirmed pathogen had persistent mental incapacity or loss of milestones | 11% (33 of 305) of surviving patients, including 15% (19 of 126) with confirmed N meningitidis serogroup C had any sequellae reported | |||
| Jusot, 2013 [46] | Range 50–141 days | N meningitidis (94%) | N/A | 31% (21 of 67) any hearing loss; 10% (7 of 67) severe hearing loss | 12% (8 of 65) walking or tonus impairment | Asthenia 37% (24 of 65); headache 31% (21 of 67); psychological troubles 16%; vertigo 22% (15 of 67) | |||
| Seydi, 2002 20 | In-hospital | N meningitidis | 3% (3 of 73) | 3% (2 of 71) deaf | |||||
| Hodgson, 2001 [47] | 2 years | N meningitidis | N/A | 6% (32 of 496) any hearing loss; 2% (8 of 496) severe hearing loss | 1% (3 of 505) decreased motor strength | Hydrocephalus 0% (0 of 505); hyperreflexia of upper limbs 15% (78 of 505) | |||
| Heyman, 1998 [25] | In-hospital | N meningitidis | 13% (6 of 45) | Residual neurological damage 5% (2 of 39) | |||||
| Fekade, 1992 [48] | In-hospital | Mostly N meningitidis | 21% (43 of 204) | 2% (3 of 161) deaf | 2% (4 of 161) hemiplegia | Cranial nerve palsy 2% (3 of 161) | |||
| Girgis, 1989 [18] | Monthly ×6 months | Majority N meningitidis (62%) | 14% (62 of 429) [lower case fatality rate in steroid group] | 3% (9 of 367) severe hearing loss | 1% (3 of 367) hemiparesis (all in patients with N meningitidis infection) | ||||
| Smith, 1988 [49] | 6–12 months | N meningitidis | N/A | 23% (23 of 102) any visual loss; 6% (6 of 102) moderate-to-severe | 6% (9 of 155) any sensorineural hearing loss; 4% (6 of 155) severe-to-profound | 17% (27 of 156) any; 3% (5 of 156) moderate-to-severe | 13% (21 of 156) at least mild motor defect; 2% (3 of 156) moderate-to-severe | Coordination impairment 11% (17 of 155); cranial nerve palsy 9% (14 of 155) | |
| Habib, 1979 [16] | Monthly ×6 months | N meningitidis | N/A | 4% (16 of 375) any hearing loss; 2% (7 of 375) deaf | |||||
| Streptococcus pneumoniae Meningitis | |||||||||
| Ajdukiewicz, 2011 [22] | 40 days | S pneumoniae (41% confirmed) | 49% (61 of 125); 39% (20 of 51) with proven S pneumoniae | 34% (14 of 41) deaf | Persistent vegetative state 22% (14 of 63) | ||||
| Manga, 2008 [21] | In-hospital | S pneumoniae (100%) | 70% (51 of 73) | 4% (1 of 22) deaf | 32% (7 of 22) motor deficit of extremity | Cranial nerve palsy 23% (5 of 22) | |||
| Scarborough, 2007 [23] | 40 days | Majority S pneumoniae (56%) | 54% (249 of 459); 51% (140 of 272) proven S pneumoniae | 3% (7 of 202) blind | 34% (66 of 195) any hearing loss; 12% (24 of 195) severe hearing loss | 6% (13 of 202) | 8% (17 of 202) | 1% (2 of 202) | Debility 5% (10 of 202) |
| Okome-Nkoumou, 1999 [26] | In-hospital | Majority S pneumoniae (65%) | 17% (15 of 85) | 3% (2 of 70) hearing loss | |||||
| Ford, 1994 [27] | In-hospital | S pneumoniae (29%) most common isolate | 62% (15 of 24) | Any neurological sequelae in 33% (3 of 9) | |||||
| Other Bacterial Meningitis | |||||||||
| El-Gindy 2015 [28] | In-hospital | Mix confirmed bacterial meningitis pathogens and tuberculous meningitis | 33% (20 of 61); 31% (5 of 16) with tuberculous meningitis and 33% (15 of 45) with bacterial meningitis | Bacterial meningitis 13% (4 of 30) severe impairment by MMSE and 30–47% impairment in WMS domains; tuberculous meningitis 27% (3 of 11) severe impairment by MMSE and 18–54% impairment in WMS domains | Bacterial meningitis 3% (1 of 30) with seizures | Bacterial meningitis 23% (7 of 30) cranial nerve palsy, 33% (10 of 30) ischemic brain insult, 3% (1 of 30) speech disorder, and 43% (13 of 30) survivors with severe disability by GOS; tuberculous meningitis 9% (1 of 11) cranial nerve palsy, 54% (6 of 11) ischemic brain insult, 9% (1 of 11) hydrocephalus, and 45% (5 of 11) survivors with severe disability by GOS score | |||
| Hammad, 2011 [17] | In-hospital | Gram-negative bacilli | 29% (28 of 95) | 9% (6 of 67) optic atrophy | 6% (4 of 67) hearing loss | 7% (5 of 67) hemiplegia | 7% (5 of 67) | Cerebral damage 1% (1 of 67); hydrocephalus 7% (5 of 67) | |
| TB Meningitis | |||||||||
| Marais, 2013 [33] | 2, 4, 6, and 12 weeks, 6 months, 9 months | Mycobacterium tuberculosis | 12% (4 of 34) | 3% (1 of 30) hearing loss | 20% (6 of 30) cognitive impairment | 7% (2 of 30) hemiparesis | |||
| Girgis, 1998 [19] | Once weekly ×6 months then monthly through 2 years | M tuberculosis | 57% (490 of 857) | 5% (20 of 367) hemiparesis/hemiplegia | Cerebral atrophy 3% (11 of 367); cranial nerve palsy 5% (20 of 367); fundus changes 8% (30 of 367); hydrocephalus 8% (30 of 367) | ||||
| Cryptococcal Meningitis | |||||||||
| Montgomery, 2017 [32] ‡ | 1 month after HIV diagnosis | Cryptococcus neoformans | N/A | 92% with International HIV Dementia Scale score ≤10 (indicative of possible HIV-associated neurocognitive disorder) compared with 66% of HIV-infected controls without meningitis; mean QNPC-8 z-score −2.22 (referenced against age- and education-adjusted, HIV-negative Ugandan population) | Median Karnofsky score 60 (IQR, 50–70); higher rates of depression than HIV-infected controls without meningitis (median CES-D score 23 [IQR, 16–30] and 9 [IQR, 5–17], respectively) | ||||
| Beardsley, 2016 [30] | 10 weeks and 6 months | C neoformans | 48% (109 of 226) at 6 months | 4% (5 of 127) decreased visual acuity at 10 weeks | 36% (46 of 127) survivors with intermediate and 20% (26 of 127) with severe disability at 10 weeks; 30% (34 of 114) with intermediate and 10% (12 of 114) with severe disability at 6 months | ||||
| Carlson, 2014 [29] ‡ | 1, 3, 6, and 12 months | C neoformans | 12% (9 of 78)† | 89% (69 of 78) with composite z-score < −1 at 1 month, 59% (41 of 69) at 3 months, 47% (32 of 68) at 6 months, and 41% (26 of 64) at 12 months | Inability to work at 12 months 11% (7 of 62) | ||||
| Rothe, 2013 [24] | 4 weeks, 10 weeks, 52 weeks | C neoformans | 72% (43 of 60) at 12 months; additional 4 patients lost to follow-up | 0% (0 of 6) tested at 52 weeks had significant disability by modified Rankin Scale score and Abbreviated Mental Test Score | |||||
Abbreviations: CSF, cerebrospinal fluid; CES-D, Center for Epidemiologic Studies Depression; GOS, Glasgow Outcome Scale; HIV, human immunodeficiency virus; IQR, interquartile range; MMSE, Mini-Mental Status Exam; N/A, not applicable; PCR, polymearse chain reaction; RCT, randomized controlled trial; TB, tuberculosis; WMS, Wechsler Memory Scale.
Included hemiparesis, hemiplegia, unspecified motor deficit in category.
Overall 6-month mortality in study 38% (67 of 177).
Included outcomes of patients from the same prospective RCT.
No formal meta-analysis was undertaken due to the small number of studies meeting inclusion criteria as well as heterogeneity in causative pathogen, follow-up period, and neurological sequelae evaluated. Descriptive statistics were used to analyze outcomes using percentage, median and interquartile range, mean and standard deviation, or other descriptive measures as indicated. We did not report pooled neurological sequelae of individual studies due to significant heterogeneity in types of sequelae evaluated, method of sequelae evaluation between studies, and limited number of studies for most major causative pathogens.
RESULTS
Literature Search and Citation Screening
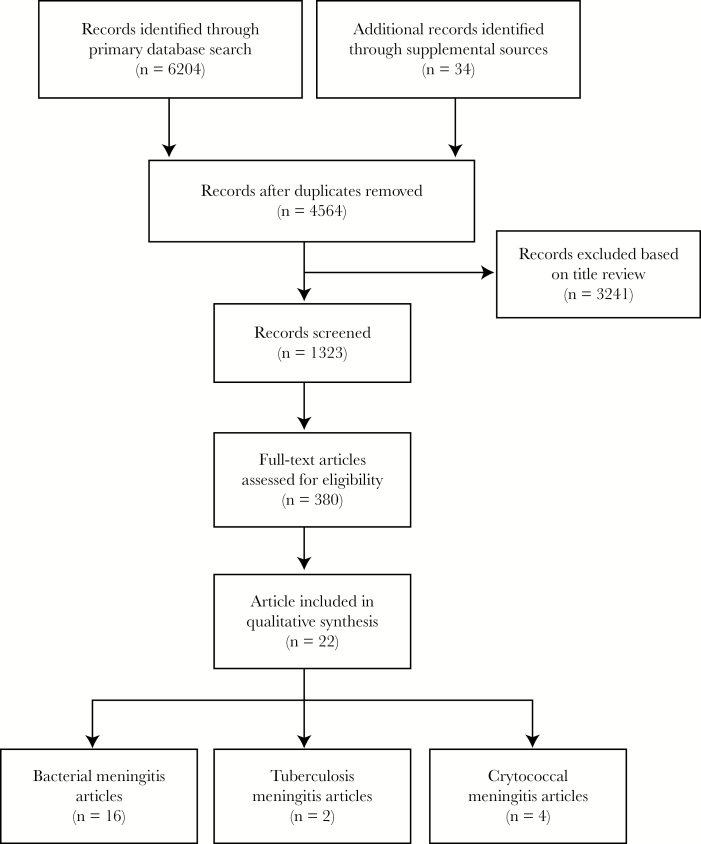
The search yielded 6204 original articles for review on June 30, 2017. The database was deduplicated, and then a primary screen by article title was executed (Figure 1); 3241 articles were excluded at this point. Most references were screened out because they contained data in neonatal and/or pediatric populations or described case studies or small case series. A secondary screening of full abstracts was then conducted in which a further 943 articles were excluded for not including data on neurological outcomes. In articles that did not include abstracts, we reviewed full-text articles. Three additional review articles were identified, and relevant references from these reviews were included in the final article list [8, 14, 15]. Full text of the remaining 380 articles was obtained and each article was reviewed. Of these, 22 met all inclusion criteria and were included in the final review.
Figure 1.
Diagram of literature search.
Articles in the Review
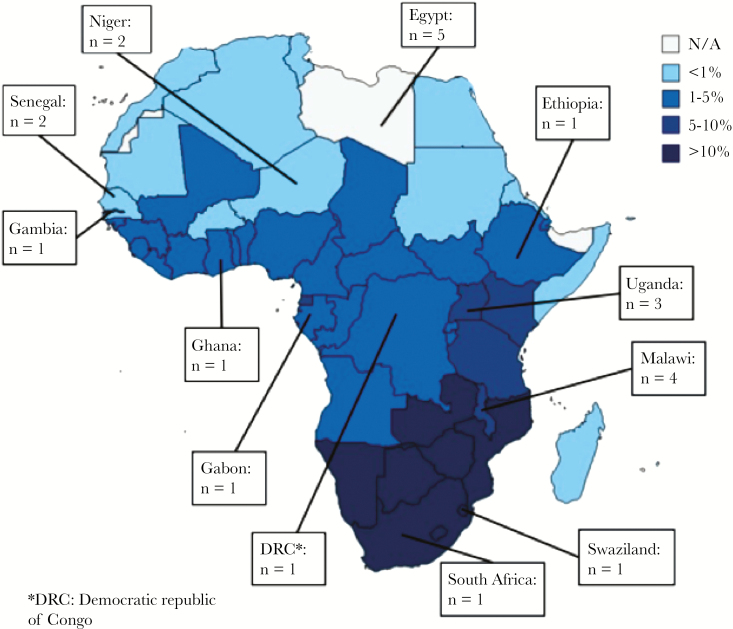
We identified 22 articles meeting inclusion criteria with 12 African countries represented (Figure 2). In all but 1 study, patients were treated for meningitis in hospital settings, with treatment provided at referral centers in 17 studies. The only or predominate cause of meningitis was nontuberculous bacterial in 16 studies, Mycobacterium tuberculosis in 2 studies, and Cryptococcus in 4 studies.
Figure 2.
Origin of studies included in review and estimated human immunodeficiency virus prevalence.
Four studies were published at a single referral hospital/US Navy research unit in Egypt [16–19] and 2 at a single referral hospital in Senegal [20, 21], both countries with a low HIV prevalence. Three studies were published from a single large referral hospital in Malawi with predominately HIV-infected patients (84%–100% HIV prevalence) [22–24]. Follow-up duration was variable: 8 of the 22 studies presented only in-hospital neurological sequelae of survivors, and follow-up for the remaining 14 studies ranged from 1 month to 2 years. Twelve articles were prospective cohort studies, 5 were retrospective cohort studies, and 5 were prospective randomized-controlled trials (RCTs) [Table 2].
Neisseria meningitidis Meningitis
Neisseria meningitidis was the principle pathogen identified in 9 bacterial studies with 2718 patients. Neisseria meningitidis was the only pathogen isolated from 6 studies. Penicillin or ampicillin and/or chloramphenicol were used to treat patients in most studies. Only 1 study [25] gave any information on HIV serostatus and reported low prevalence. Follow-up ranged from in-hospital to a 2-year follow-up of survivors. Males constituted 56% (1529 of 2718) of patients (Table 2).
Ascertainment of adult outcomes was imperfect because most (8 of 9) studies included both adult and pediatric patients without separation by age category. Mortality data were included in 5 studies and ranged from 3% to 21% (median 13%). In meningitis survivors, hearing loss (8 studies) and motor deficits (5 studies) were the most commonly reported neurological sequelae. Any hearing loss was found in 2%–31% of patients (median 5%) with severe/profound hearing loss reported in 2%–10% of patients (median 3%). Motor deficits were variably defined and ranged from 1% to 13% (median 2%) in 5 studies with moderate-to-severe disability (eg, hemiplegia/hemiparesis) in 1%–2% (median 2%) from 3 studies. One study reported 6% of patients with moderate-to-severe visual loss and 3% with moderate-to-severe neurocognitive deficit at evaluation 6 to 12 months after the meningitis episode (Table 3).
Steptococcus pneumoniae and Other Bacterial Meningitides
Five studies including 772 patients reported on neurological outcomes with Streptococcus pneumoniae as the single or most commonly confirmed pathogen. Streptococcus pneumoniae was the only pathogen in 1 study [21], was identified in 65% of cases in 1 study limited to microbiologically confirmed cases [26], and was isolated in 29%–56% of cases from 3 studies with a large percentage of cases diagnosed by CSF cellular profile [22, 23, 27]. Treatment differed between studies, and adjunctive dexamethasone was used for a subgroup of patients in only 2 studies [23, 26]. Human immunodeficiency virus prevalence was high across studies, between 12% and 90%. Follow-up ranged from in-hospital to 40 days. Males constituted 53% (400 of 751) of cases (Table 2).
Two prospective RCTs from Malawi of primarily HIV-infected adult patients were high quality with the remaining studies of relatively poor quality [22, 23]. Reported case-fatality rate ranged from 17% to 70% (median 54%). In the 2 Malawian prospective RCTs with mostly HIV-infected adults, mortality ranged from 49% to 54% overall and 39% to 51% in cases of proven pneumococcal meningitis, all managed with ceftriaxone (±dexamethasone). Hearing loss was reported to affect 3%–34% (median 19%) of patients in 4 studies, with severe/profound hearing loss reported in 4%–34% (median 12%) of patients in 3 studies. Motor deficits were reported at 8%–32% in 2 studies, with no other outcome documented in more than a single study (Table 3).
The 2 Malawian studies with predominately HIV-infected patients reported high rates of neurological sequelae in survivors [22, 23]. In Ajdukiewicz et al [22], 22% (14 of 63) survivors, with or without pneumococcal disease, remained in a persistent vegetative state (Glasgow Outcome Score 2) at 40 days. In Scarborough et al [23], 23% (47 of 202) of 40-day survivors and 29% (38 of 130) of those with proven pneumococcal meningitis had 1 or more neurological sequelae (blindness, debility, complete deafness, intellectual impairment, paresis, or recurrent seizures).
One retrospective cohort study from Egypt evaluated neurological sequelae in 95 patients with microbiologically confirmed, Gram-negative, bacillary meningitis [17]. This study included both adults and children and did not include data on HIV status. The case-fatality rate was 29% (28 of 95), and the rate of pooled in-hospital sequelae (cerebral damage, hearing loss, hemiplegia, hydrocephalus, optic atrophy, and/or seizure disorder) in survivors was 39% (26 of 67). Six percent (4 of 67) of survivors had hearing loss, 7% (5 of 67) had hemiplegia, 7% (5 of 67) had seizure disorder, and 9% (6 of 67) had optic atrophy. Another study from Egypt included patients with a mix of microbiologically confirmed bacterial meningitis and tuberculous meningitis [28]. At hospital discharge, 43% (13 of 30) of survivors with bacterial meningitis and 45% (5 of 11) with tuberculous meningitis had severe disability determined by Glasgow Outcomes Scale score.
Cryptococcal Meningitis
Four articles evaluated neurological sequelae of HIV-infected adult patients with cryptococcal meningitis [24, 29–31]. One good-quality study from Uganda reported global neurocognitive dysfunction, defined as mean composite score of multidomain neurocognitive testing at least 1 standard deviation below that of HIV-uninfected Ugandans (z-score < −1), serially over 1 year in cryptococcal meningitis survivors who received amphotericin-based induction therapy. At 1 month, 89% (69 of 78) of survivors had impaired neurocognitive function. This improved to 41% (26 of 64) at 1 year [29]. Meningitis survivors had only slightly higher disability compared with HIV-infected Ugandans without a prior history of neurological infection. The case-fatality rate for patients who lived to 1 month was 12% (9 of 78) at 1 year; 5 of those patients died before a 1-month follow up, and 3 patients died before the 6-month follow up. A second study from the same prospective RCT reported additional 1-month neurocognitive outcomes [32]. At 1 month, 92% of survivors had an International HIV Dementia Scale score ≤10, suggesting possible HIV-associated neurocognitive disorder. Six-month neurocognitive outcomes from another quality large prospective RCT found that 20% of survivors had severe disability at 10 weeks and 10% at 6 months, defined as answering yes to the question “Does the patient require help from anybody for everyday activities?” or with a Modified Rankin Scale score of ≥3 (symptoms that restrict lifestyle and prevent totally independent living) [30]. Of note, this study included patients from countries in both Africa (Malawi and Uganda) and Asian countries, but neurocognitive outcomes were not disaggregated by country. We also excluded patients receiving experimental dexamethasone therapy, which was associated with higher rates of mortality and other adverse outcomes and is not part of the usual treatment.
Tuberculosis Meningitis
We included 2 articles with 891 patients with sequelae data for tuberculosis meningitis. One high-quality but small study with 34 antiretroviral therapy (ART)-naive HIV-infected adults with tuberculous meningitis (confirmed or suspected) evaluated patients for tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) after ART initiation. The case-fatality rate was 12% (4 of 34) at 9 months, with all deaths occurring in patients with TB-IRIS at time points of 33, 53, 60, and 118 days after tuberculous meningitis diagnosis. Of 9-month survivors, 20% (6 of 30) had marked disability: 20% (6 of 30) cognitive impairment (5 of 6 marked impairment defined as HIV dementia scale score <10), 7% (2 of 30) hemiparesis, and 3% (1 of 30) hearing loss [33]. A second study from a referral hospital in Egypt reported outcomes in a large cohort of patients treated for tuberculous meningitis over 2 decades (1976–1996) [19]. This study included adults and children together, and no patients were known to be HIV-infected. Patients received 3-drug antituberculous therapy for 2 years, during which period they were serially assessed for neurological sequelae. In total, 57% (490 of 857) of patients died and 30% (111 of 367) of patients survived with severe neurological sequelae (including cranial nerve palsy 5% [20 of 367], hemiparesis-paraplegia 5% [20 of 367], and hydrocephalus 8% [30 of 367]).
DISCUSSION
In our systematic review of neurological sequelae of adult meningitis in Africa, we identified 22 articles with neurological sequelae outcome data in meningitis survivors. This included 12 studies in counties with high HIV prevalence. These studies, published over a more than 35-year period, provide limited data showing high rates of postmeningitis disability in adult meningitis survivors from resource-limited settings, particularly in those with HIV coinfection. More importantly, they reveal the paucity of high-quality data regarding the frequency and spectrum of neurological sequelae after adult meningitis from prospective studies, and they provide insight into gaps in our current understanding of long-term disability of adult meningitis survivors. Accurate data regarding the long-term outcomes in adult meningitis survivors are essential for accurate estimates of disease burden and, given the frequency of adult meningitis in Africa, have broad public health and economic implications.
Our review highlights that little is known about long-term neurological sequelae in survivors of HIV-associated cryptococcal meningitis and tuberculous meningitis, 2 of the principle causes of adult meningitis in sub-Saharan Africa. Two articles included Ugandan patients from a single prospective RCT [29, 32]. As in prior studies in the region, acute mortality was high at 38% by 26 weeks of follow up [4, 34–38]. These studies found significant impairment in the early weeks after incident cryptococcal meningitis, with Montgomery et al [32] reporting 92% of survivors with an International AIDS Dementia Scale score suggestive of neurocognitive impairment, compared with 66% of HIV-infected controls without meningitis. With longer follow-up, Carlson et al [29] reported persistent neurocognitive deficits in approximately half of survivors at 1 year. The relationship between this long-term neurocognitive impairment and cryptococcal meningitis remains unclear. Although cryptococcal meningitis survivors also showed worse neurocognitive function compared with HIV-infected Ugandans, these differences were small by 1 year of follow-up, and the control patients generally had less advanced HIV disease (mostly stage II and III) [29]. Neurocognitive deficits may have been a consequence of meningitis and/or other contributing factors, such as HIV-associated neurocognitive disorder [39, 40]. The choice of comparator also limits inferential reasoning and could be strengthened in this and other studies evaluating neurocognitive domains with alternative comparator groups, such as HIV-infected populations started early on ART. A second multicountry prospective RCT conducted in African and Asian countries found that 20% and 10% of survivors reported requiring help with everyday activities at 10 weeks and 6 months, respectively [30]. Taken together, these data suggest significant impairment early after incident cryptococcal meningitis, a period during which adherence to ART and continuation of fluconazole maintenance therapy are critical to prevent cryptococcal meningitis relapse and for overall survival.
Five studies described neurological sequelae in adult meningitis with S pneumoniae, the most common cause of bacterial meningitis in HIV-infected adults, as the predominate pathogen [2, 41]. Two of these studies [22, 23], both from Malawi, consisted largely of HIV-infected adults and found approximately half of patients died of meningitis with one quarter of survivors left with severe disability, such as with persistent vegetative states or hemiparesis. These adverse outcomes are significantly higher than rates of death and disability reported in resource-rich countries with low HIV prevalence, but they are more reflective of African pediatric populations [8, 14]. These data also likely underestimated the impact of pneumococcal meningitis in HIV-infected African cohorts because patients were enrolled in controlled clinical trials at a large referral hospital and given highly effective antibiotic therapy with ceftriaxone. Because follow up was limited to 40 days, they may also have underestimated delayed outcomes such as epilepsy [42]. Unfortunately, in Africa, pneumococcal vaccination has demonstrated mixed efficacy for reducing invasive pneumococcal disease, and dexamethasone therapy has not been associated with lower mortality or neurological sequelae in HIV-infected adults [23, 43, 44]. The best method for reduction in risk of incident disease and associated neurological sequelae is early HIV recognition and initiation of ART coupled with timely and appropriate antibiotic therapy [41].
Our review has several important limitations. First, case definitions, outcome measures, and means of assessment were highly variable between studies. A lack of validated neurocognitive and limited neurological function assessments created very heterogeneous data and prevented formal meta-analysis. Second, follow-up periods differed considerably with only approximately half of studies reporting outcomes after hospital discharge to characterize clinical evolution of disease. Third, because there were few studies evaluating outcomes in cryptococcal and tuberculous meningitis, no firm conclusions could be drawn about neurological outcomes in survivors but rather emphasizes the need for future studies, especially prospective cohort studies, to include multidomain measures of neurological outcomes of disease. Finally, for most studies involving meningococcal meningitis, neurological sequelae of pediatric and adult cases were not reported separately, which limited our ability to make firm conclusions about adult outcomes. In this mixed population, however, death and disability was significantly lower than in cases of pneumococcal meningitis, as reported elsewhere [14].
CONCLUSIONS
In conclusion, although settings with high HIV prevalence in Africa have both a high incidence of meningitis and early mortality, long-term sequelae in adult survivors remains poorly characterized. Limited data suggest high rates of neurological disability associated with HIV-related pneumococcal and TB meningitis. Little is known about neurological sequelae in survivors of HIV-associated cryptococcal meningitis, although studies suggest extremely high prevalence of neurocognitive and functional impairment in the early weeks after incident disease. Furthermore, long-term multidomain testing of neurological outcomes for survivors of HIV-associated cryptococcal meningitis and TB meningitis is warranted. Tools need to be chosen carefully and consider challenges with repeat testing, such as risk of learner bias.
There is a need for improved understanding of neurological sequelae of adult meningitis in Africa. Pneumococcal meningitis in the setting of high HIV prevalence confers a high risk of both death and severe neurological sequelae in survivors. Little is published on sequelae from HIV-associated cryptococcal or tuberculous meningitis, highlighting an urgent need to more accurately define outcomes of survivors in future studies.
Acknowledgments
Financial suppport. This work was funded by University of Pennsylvania Center for AIDS Research Grant P30 AI 045008 (to J. N. J.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jarvis JN, Meintjes G, Williams A et al. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thwaites GE, Duc Bang N, Huy Dung N et al. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with Tuberculous meningitis. J Infect Dis 2005; 192:2134–41. [DOI] [PubMed] [Google Scholar]
- 4. Jarvis JN, Bicanic T, Loyse A et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wall EC, Cartwright K, Scarborough M et al. High mortality amongst adolescents and adults with bacterial meningitis in sub-Saharan Africa: an analysis of 715 cases from Malawi. PLoS One 2013; 8:e69783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajasingham R, Smith RM, Park BJ et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Etyang AO, Munge K, Bunyasi EW et al. Burden of disease in adults admitted to hospital in a rural region of coastal Kenya: an analysis of data from linked clinical and demographic surveillance systems. Lancet Glob Health 2014; 2:e216–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramakrishnan M, Ulland AJ, Steinhardt LC et al. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med 2009; 7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molyneux EM, Tembo M, Kayira K et al. The effect of HIV infection on paediatric bacterial meningitis in Blantyre, Malawi. Arch Dis Child 2003; 88:1112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Madhi SA, Madhi A, Petersen K et al. Impact of human immunodeficiency virus type 1 infection on the epidemiology and outcome of bacterial meningitis in South African children. Int J Infect Dis 2001; 5:119–25. [DOI] [PubMed] [Google Scholar]
- 11. Murray CJ, Ortblad KF, Guinovart C et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucas MJ, Brouwer MC, van de Beek D. Neurological sequelae of bacterial meningitis. J Infect 2016; 73:18–27. [DOI] [PubMed] [Google Scholar]
- 14. Edmond K, Clark A, Korczak VS et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:317–28. [DOI] [PubMed] [Google Scholar]
- 15. Woldeamanuel YW, Girma B. A 43-year systematic review and meta-analysis: case-fatality and risk of death among adults with tuberculous meningitis in Africa. J Neurol 2014; 261:851–65. [DOI] [PubMed] [Google Scholar]
- 16. Habib RG, Girgis NI, Yassin MW et al. Hearing impairment in meningococcal meningitis. Scand J Infect Dis 1979; 11:121–3. [DOI] [PubMed] [Google Scholar]
- 17. Hammad OM, Hifnawy TM, Omran DA et al. Gram-negative bacillary meningitis in Egypt. J Egypt Public Health Assoc 2011; 86:16–20. [DOI] [PubMed] [Google Scholar]
- 18. Girgis NI, Farid Z, Mikhail IA et al. Dexamethasone treatment for bacterial meningitis in children and adults. Pediatr Infect Dis J 1989; 8:848–51. [DOI] [PubMed] [Google Scholar]
- 19. Girgis NI, Sultan Y, Farid Z et al. Tuberculosis meningitis, Abbassia Fever Hospital-Naval Medical Research Unit No. 3-Cairo, Egypt, from 1976 to 1996. Am J Trop Med Hyg 1998; 58:28–34. [DOI] [PubMed] [Google Scholar]
- 20. Seydi M, Soumare M, Sow AI et al. [Clinical, bacteriological and therapeutic aspects of meningococcal meningitis in Dakar in 1999]. Med Trop (Mars) 2002; 62:137–40. [PubMed] [Google Scholar]
- 21. Manga NM, Ndour CT, Diop SA et al. [Adult purulent meningitis caused by Streptococcus pneumoniae in Dakar, Senegal]. Med Trop (Mars) 2008; 68:625–8. [PubMed] [Google Scholar]
- 22. Ajdukiewicz KM, Cartwright KE, Scarborough M et al. Glycerol adjuvant therapy in adults with bacterial meningitis in a high HIV seroprevalence setting in Malawi: a double-blind, randomised controlled trial. Lancet Infect Dis 2011; 11:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scarborough M, Gordon SB, Whitty CJ et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med 2007; 357:2441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rothe C, Sloan DJ, Goodson P et al. A prospective longitudinal study of the clinical outcomes from cryptococcal meningitis following treatment induction with 800 mg oral fluconazole in Blantyre, Malawi. PLoS One 2013; 8:e67311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heyman SN, Ginosar Y, Niel L et al. Meningococcal meningitis among Rwandan refugees: diagnosis, management, and outcome in a field hospital. Int J Infect Dis 1998; 2:137–42. [DOI] [PubMed] [Google Scholar]
- 26. Okome-Nkoumou M, Loembe PM. Bacterial meningitis in the adult. Study of 85 cases observed in the infectious disease unit of the Fondation Jeanne Ebori (F.J.E.), Libreville, Gabon. Bull Soc Pathol Exot 1999; 92:288–91. [PubMed] [Google Scholar]
- 27. Ford H, Wright J. Bacterial meningitis in Swaziland: an 18 month prospective study of its impact. J Epidemiol Community Health 1994; 48:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Gindy EM, Ali-Eldin FA, Bayoumy I et al. Cognutuve and neurological complications of bacterial meningitis in adult patients: a hospital based study. J Egypt Soc Parasitol 2015; 45:477–84. [DOI] [PubMed] [Google Scholar]
- 29. Carlson RD, Rolfes MA, Birkenkamp KE et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis 2014; 29:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beardsley J, Wolbers M, Kibengo FM et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montgomery MP, Nakasujja N, Morawski BM et al. Neurocognitive function in HIV-infected persons with asymptomatic cryptococcal antigenemia: a comparison of three prospective cohorts. BMC Neurol 2017; 17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montgomery MP, Nakasujja N, Morawski BM et al. Neurocognitive function in HIV-infected persons with asymptomatic cryptococcal antigenemia: a comparison of three prospective cohorts. BMC Neurol 2017; 17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marais S, Meintjes G, Pepper DJ et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2013; 56:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boulware DR, Meya DB, Muzoora C et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bicanic T, Meintjes G, Wood R et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007; 45:76–80. [DOI] [PubMed] [Google Scholar]
- 36. Loyse A, Wilson D, Meintjes G et al. Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis 2012; 54:121–8. [DOI] [PubMed] [Google Scholar]
- 37. Muzoora CK, Kabanda T, Ortu G et al. Short course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitis. J Infect 2012; 64:76–81. [DOI] [PubMed] [Google Scholar]
- 38. Jackson AT, Nussbaum JC, Phulusa J et al. A phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitis. AIDS 2012; 26:1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCombe JA, Vivithanaporn P, Gill MJ, Power C. Predictors of symptomatic HIV-associated neurocognitive disorders in universal health care. HIV Med 2013; 14:99–107. [DOI] [PubMed] [Google Scholar]
- 40. Abassi M, Morawski BM, Nakigozi G et al. Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J Neurovirol 2017; 23:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Domingo P, Suarez-Lozano I, Torres F et al. Bacterial meningitis in HIV-1-infected patients in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2009; 51:582–7. [DOI] [PubMed] [Google Scholar]
- 42. Annegers JF, Hauser WA, Beghi E et al. The risk of unprovoked seizures after encephalitis and meningitis. Neurology 1988; 38:1407–10. [DOI] [PubMed] [Google Scholar]
- 43. French N, Nakiyingi J, Carpenter LM et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 2000; 355:2106–11. [DOI] [PubMed] [Google Scholar]
- 44. French N, Gordon SB, Mwalukomo T et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coldiron ME, Salou H, Sidikou F et al. Case-fatality rates and sequelae resulting from Neisseria meningitidis serogroup C epidemic, Niger, 2015. Emerg Infect Dis 2016; 22:1827–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jusot JF, Tohon Z, Yazi AA, Collard JM. Significant sequelae after bacterial meningitis in Niger: a cohort study. BMC Infect Dis 2013; 13:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hodgson A, Smith T, Gagneux S et al. Survival and sequelae of meningococcal meningitis in Ghana. Int J Epidemiol 2001; 30:1440–6. [DOI] [PubMed] [Google Scholar]
- 48. Fekade D, Zawde D. Epidemic meningococcal meningitis in adult Ethiopians in Addis Abeba, Ethiopia, 1988. Ethiop Med J 1992; 30:135–42. [PubMed] [Google Scholar]
- 49. Smith AW, Bradley AK, Wall RA et al. Sequelae of epidemic meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg 1988; 82:312–20. [DOI] [PubMed] [Google Scholar]