Abstract
Metabolic glycoengineering is a specialization of metabolic engineering that focuses on using small molecule metabolites to manipulate biosynthetic pathways responsible for oligosaccharide and glycoconjugate production. As outlined in this article, this technique has blossomed in mammalian systems over the past three decades but has made only modest progress in prokaryotes. Nevertheless, a sufficient foundation now exists to support several important applications of metabolic glycoengineering in bacteria based on methods to preferentially direct metabolic intermediates into pathways involved in lipopolysaccharide, peptidoglycan, teichoic acid, or capsule polysaccharide production. An overview of current applications and future prospects for this technology are provided in this report.
Keywords: Metabolic oligosaccharide engineering, Carbohydrate engineering, Lipopolysaccharides, Petidoglycans, Nucleotide sugar production, Recombinant glycoprotein production, Oligosaccharide synthesis
Introduction to metabolic engineering and glycoengineering
In the broadest sense, metabolic engineering aims to direct changes in cellular properties by modifying existing cellular pathways or by introducing new ones and stresses the importance of metabolic pathway integration and the use of metabolic fluxes as determinants of cell physiology and a means of metabolic control. More specifically, this article complements metabolic engineering approaches designed to expand the substrate ranges of certain organisms and produce metabolites that are either new to just the host cell [1–3] or to all of biology [4, 5]. Attempts to manipulate metabolic pathways in order to improve properties and productivity of microorganisms began with the use of chemical mutagenesis and creative selection techniques. The discovery of molecular biological techniques for DNA recombination further ignited the field as the new-found ability to use genetic engineering tools to make precise changes to specific enzymes along a metabolic pathway spurred a wide range of metabolic engineering applications. For example, metabolic engineering has been used to boost the productivity and yield of native products produced by microbes [6–9], improve general cellular properties such as the ability to withstand hypoxic fermentation conditions [10], and prevent overflow metabolism [11].
This article focuses on a sub-specialty of metabolic engineering typically referred to a “metabolic oligosaccharide engineering” (MOE [12–14]) or “metabolic glycoengineering” (MG [15, 16] or MGE; the latter term will be used throughout this review)). As outlined in Fig. 1, MGE efforts most often seek to install non-natural monosaccharides into the glycans of living organisms but can also control the rates of flux of natural metabolites through biosynthetic pathways. This technology was first demonstrated in mammals over 20 years ago [19, 20] when comparable metabolic engineering endeavors in microbes were not focused on sugars but rather largely explored the biosynthetic incorporation of non-natural amino acids to expand protein structure and function [21–23]. While a limited number of non-natural amino acid analogs were tolerated and incorporated by the existing natural t-RNA synthetases (e.g., azidohomoalanine [21]), the metabolic incorporation of most non-natural amino acids required complementary mutations to the corresponding t-RNA synthetase [24–27].
Fig. 1.
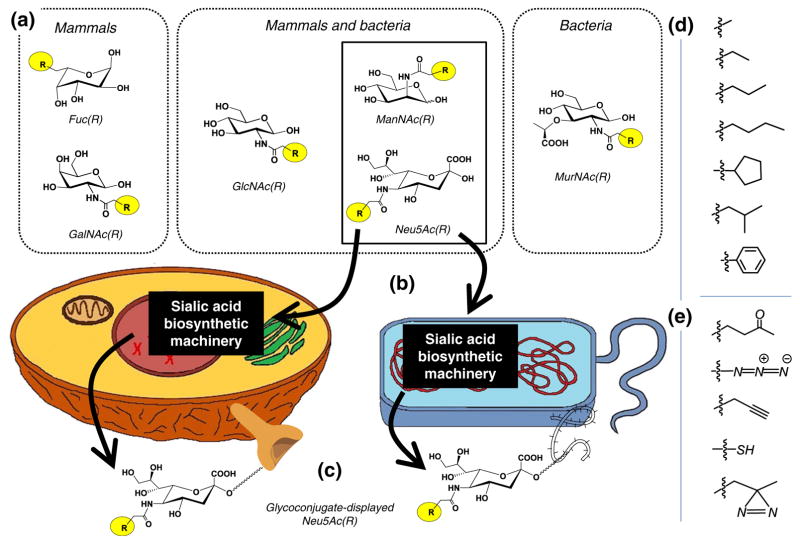
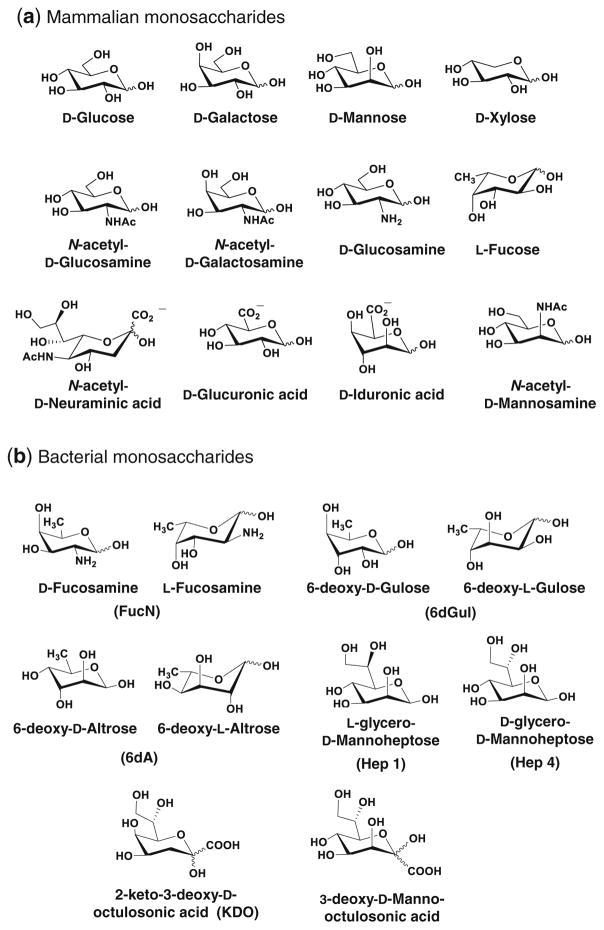
Comparative overview of MGE in mammals and bacteria. (a) Different – but overlapping – sets of non-natural (i.e., “R” -modified) monosaccharides have been exploited for MGE in mammals and bacteria. For example, fucose (Fuc) and N-acetyl-D-galactosamine (GalNAc) have only been reported in mammalian MGE experiments while N-acetyl-D-glucosamine (GlcNAc), N-acetyl-D-mannosamine (ManNAc), and N-acetyl-D-neuraminic acid (Neu5Ac, also known as sialic acid, Sia) have been used in both systems although with sometimes dramatically different efficiencies as discussed further in the main text and Fig. 6. Other monosaccharides (e.g., N-acetyl-D-muramic acid, MurNAc as well additional candidates shown in Fig. 9) are used exclusively by prokaryotes. (b) The non-natural sugars – illustrated by ManNAc(R) and Neu5Ac(R) –are taken up by cells and enter the targeted biosynthetic pathway and (c) displayed on the cell surface as the corresponding R-modified glycoconjugate. A sampling of R groups include those with (d) extended alkyl groups and even ring structures and (e) those bearing unique chemical functionalities not normally found in glycans such as ketones, azides, alkynes, thiols, and diazarine, as shown
Conceptually, MGE is very similar to efforts to metabolically install non-natural amino acids into proteins insofar as it involves incorporation of non-natural small molecule building blocks (i.e., monosaccharides instead of amino acids) into larger biopolymers (i.e., oligo- and polysaccharides instead of peptides and proteins). The complexity of the dual tasks of simultaneously manipulating the enzymes involved in protein biosynthesis and altering metabolic flux with small molecules rendered non-natural amino acid-based protein engineering in bacterial systems as a rather arduous task. Subsequently, the sugar-based MGE technology evolved along a considerably different path largely bypassing bacteria during its early stage development but instead flourished in eukaryotes where incorporation of non-natural sugar analogs into biomacromolecules occurred without the need to “tweak” the biosynthetic machinery [13, 15, 19, 28, 29].
The next section of this paper (in Section 2) gives an overview of MGE in mammals and provides a synopsis of various factors that have caused this technology to lag in bacteria. Section 3 then describes recent and ongoing developments that are opening new doors for the application of MGE methods in microbes; these include recombinant glycoprotein production and new ways to synthesize oligosaccharides, which are discussed in Sections 4 and 5, respectively. The chapter will then conclude with future prospects in Section 6.
Challenges for MGE in bacteria
MGE was pioneered in mammals
In the early 1980s, observations that changes in sialic acid metabolism played a role in carcinogenesis led to attempts to inhibit sialic acid production [30]. Based on consolidating biochemical knowledge that N-acetyl-D-mannosamine (ManNAc) is the first committed metabolic intermediate in the sialic acid pathway [31], fluoro analogs of ManNAc were designed to inhibit the biosynthesis of this sugar but instead, metabolic incorporation of the corresponding glycosides into cell surface components was observed [32, 33]. Efforts to inhibit glycosylation using MGE-inspired approaches eventually met with a degree of success—for example, disaccharide analogs inhibit glycosylation pathways as well as specific sialylated glycan motifs such as sialyl lewis X [34, 35] and ManNAc analogs inhibited polysialic acid production [36, 37]. More significantly, however, the very early experiments that showed metabolic utilization of the non-natural analogs instead of enzyme inhibition ultimately led to the realization that ManNAc analogs designed to block the sialic acid pathway instead opened the door to what has now become the robust field of MGE. At the start of the 1980s ManNAc analogs were already under investigation by Reutter, Cerný, and colleagues [38] and by the late 1980s enzyme-based assays performed by Brossmer and others showed that sialyltransferases had substantial permissivity for installation of non-natural sialic acids into glycans [39–41]. Reutter and colleagues built on these results and proved that ManNAc analogs with extended N-acyl groups could be used to deliberately modify the cell surfaces or mammalian cells both in vitro and in living animals [19, 20, 42].
The permissivity of the sialic acid pathway evident in both living cells and in rodents in the early experiments from ~25 years ago allowed MGE to progress rapidly in mammalian systems. This technology ultimately resulted in ManNAc analogs bearing a diverse repertoire of chemical functional groups including hydroxyls [43], thiols [44], azides [45], ketones [46], phenyl aryl azides [47], diazarines [48] and even highly fluorinated anti-adhesive alkyl chains [49, 50] to be introduced onto the cell surface via non-natural forms of sialic acid. In addition, pathways that install GlcNAc, GalNAc, and fucose into glycoconjugates have been exploited for MGE in mammalian cells [15]. By contrast, MGE in prokaryotes progressed slowly and remains rather paltry by comparison in part because this approach provided a remarkably easy method to incorporate non-natural sugar analogs into biomacromolecules of almost any mammalian cell line and in part because development of this technology in bacteria was considered to be of lower priority compared to perceived high value human systems—for example, for the diagnosis or treatment of cancer [46, 51, 52]. As a result, the usual “develop-the-basic-technology-in-easy-to-manipulate-prokaryotes-before-moving-on-to-more-complex-eukaryote-and-mammalian-systems” progression of biological research was skipped over during the evolution of MGE. However, as will be discussed below, there also are legitimate underlying biological as well as practical and technical issues that have slowed and sometimes completely thwarted the use of MGE in bacteria.
Bacterial glycosylation is dramatically different than mammalian glycosylation
Introduction to bacterial glycans
A brief overview of glycans found in Gram-positive and Gram-negative bacteria will be given in order to orient the reader with regards to targeting prokaryotic poly- and oligosaccharide structures. Understanding the fundamental differences between Gram-positive and Gram-negative bacteria is of critical importance for developing effective MGE strategies for purposes ranging from initiating immune response against carbohydrate antigens associated with pathogenic microbes to disrupting their ability to adhere to mammalian cellular surfaces or for using bacteria as hosts for recombinant glycoprotein production. Although the categorization of bacteria into Gram-positive and Gram-negative by no means encompasses all nuances of prokaryotic diversity, this classification underscores very important and distinct structural features that distinguish these two broad categories of bacteria that—pertinent for MGE efforts – mainly stem from differences in carbohydrate structures.
Bacteria peptidoglycans
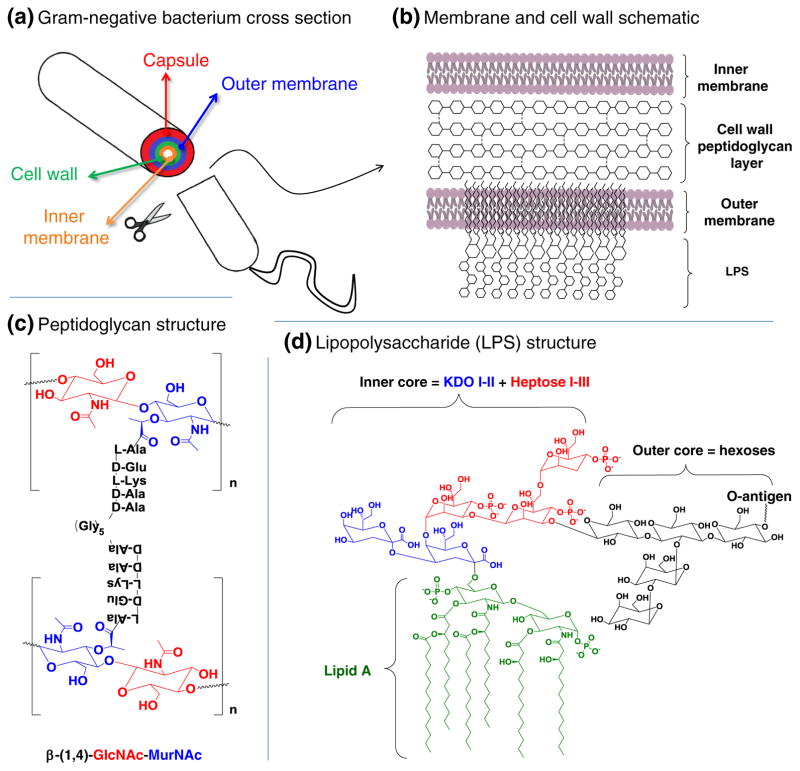
Both Gram-positive and Gram-negative bacteria contain similar peptidoglycan chemical structures– specifically, a repeating dissacharide unit consisting of (β1–4) linked N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) coupled to a peptide chain that is crosslinked with other peptide chains in order to form a 3-D mesh-like structure (Fig. 2a and b). One of the most striking differences between Gram-positive and Gram-negative bacteria, however, is the thickness of this peptidoglycan layer that can range anywhere from 30 to 100 nm for Gram-negative bacteria while this layer is much thinner, at only a few nanometers, for Gram-positive bacteria. A basic understanding of the peptidoglycan structure has been known for decades and disrupting cell wall peptidoglycan synthesis forms the basis of many important classes of antibiotics. Indeed, by inhibiting peptidoglycan crosslinking in a way, pioneering antibiotics such as penicillin could be considered to be early MGE agents. Today, there many ongoing efforts to provide new insights into the structural nuances and complexities of bacterial wall structure to maintain and improve the effectiveness of these important medicines. For example, recent efforts using atomic force microscopy have begun to unravel the spatial organization of the peptidoglycan layer revealing concentric ring like structures oriented in planes perpendicular to the long axis of the cell (Fig. 2a–c) [53] as well as nuances of covering lipopolysaccharide structures (Fig. 2d and additional discussion below).
Fig. 2.
Gram-negative bacterial membranes, cell wall, and capsular structures. (a) A cross section of a Gram-negative bacterium is shown with a more detailed schematic of each component given in (b) with further chemical-level details of peptidoglycan and LPS structures provided in panels (c) and (d), respectively
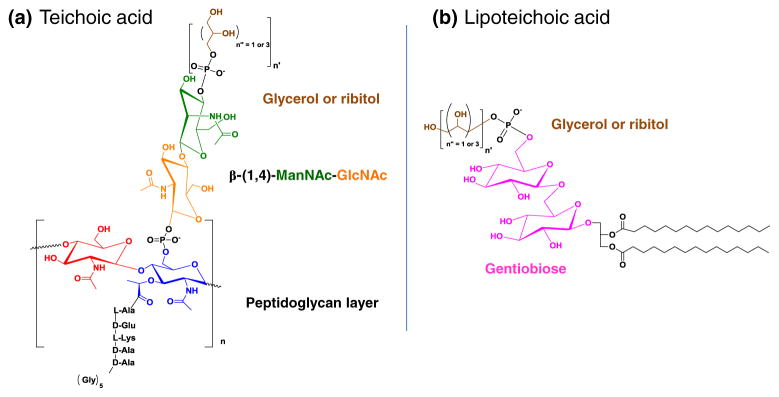
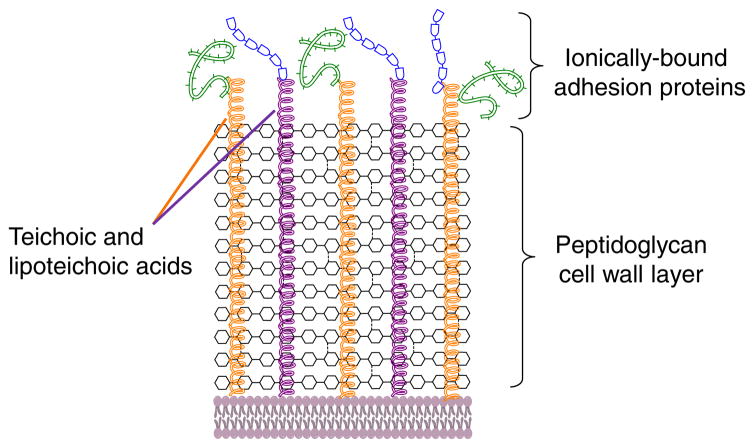
An exclusive feature found in Gram-positive bacteria is the presence of wall teichoic acids (WTAs), which are anionic glycopolymers that are covalently attached to the peptidoglycan layer via a phosphodiester bond with the C6 hydroxyl group of N-acetylmuramic acid carbohydrates (Fig. 3a). While the structures of WTAs can differ, the most common meme for WTAs consists of a ManNAc (β1→4) GlcNAc disaccharide linked to the peptidoglycan layer followed by a polymer of either glycerol or ribitol phosphodiester repeats [54]. WTAs, along with lipteichoic acids (teichoic acid linked to fatty acids via gentiobiose, Fig. 3b), can account for as much as 60 % of cell wall mass in Gram-positive bacteria. The abundance of teichoic acids suggests that they have a role in crucial functions that allow Gram-positive bacteria survive and proliferate in a wide range of potentially hostile environments. Adhesins, which are proteins on the surface of bacteria such as S. aureus that help to adhere to host tissue, are ionically linked to WTAs. In addition to providing a site for surface proteins to bind ionically, teichoic acids also play a role in maintaining cell wall rigidity and help regulate cell wall growth and degradation (Fig. 4) [54].
Fig. 3.
Structures of (a) teichoic acid and (b) lipoteichoic acid
Fig. 4.
Schematic of the cell wall and surface components of Gram-positive bacteria
Outer membrane and polysaccharide capsules
Another major architectural difference between Gram-positive and Gram-negative bacteria is found in the outer membrane (OM), a prominent feature found only in Gram-negative bacteria. The OM predominantly features lipopolysaccharide (LPS) or lipooligosaccharide (LOS) structures that have the principal function of providing a barrier of protection for the OM. Briefly, as shown earlier in Fig. 2d, LPS consists of glucosamine dissaccharides that have lipophilic ester linked acyl chains (lipid A) attached to an inner core oligosaccharide, and an extended polysaccharide from the inner core that is called the O-antigen that consists of sugar moieties common to the host (i.e., mammalian monosaccharides, Fig. 5a) as well sugars unique to the bacteria (Fig. 5b). The presence of “non-human” monosaccharides renders LPS and LOS potently immunogenic (hence the name “O-antigen”) and these molecules—which are broadly known as endotoxins – constitute the primary epitopes responsible for sepsis.
Fig. 5.
Structures of common (a) host (mammalian) and (b) bacterial monosaccharides. The “mammalian” sugars shown essentially comprise all monosaccharides that participate in human glycosylation under normal conditions but in many cases they also occur in bacteria. By comparison, only a small sampling of bacterial monosaccharides are shown here
A final carbohydrate structure, found on both Gram-positive and Gram-negative bacteria, is a polysaccharide capsule with versatile protective functions for the microbe ranging from evasion of detection from the host immune system to prevention of dessication to assisting with cellular adhesion. Polysaccharide capsules consist of a variety of different repeating acidic carbohydrate monomers and a specific type of bacteria may produce a wide array of different capsules; E. coli, for instance, produces over 70 different types of capsules [55, 56]. Although the types of capsules are diverse in structure, sugars found in mammals form the basis of common groups; for example Ia consists of hexuronic acids and neutral sugars, Ib contains hexuronic acids and N-acetylated hexosamines, and group II capsules are comprised of hexuronic acid, KDO, and sialic acid along with neutral or amino sugars [57].
Factors that hinder MGE in bacteria
Now that a brief overview of bacterial carbohydrate architectures has been provided, challenges inherent in using MGE to incorporate non-natural sugars into microbes will be discussed. Some of these features are fairly straight forward—for example, overlying dense capsular structures can bury metabolically incorporated cell wall or LOS analogs, making them inaccessible for display on the cells surface –and will not be discussed in detail. A sampling of more substantial issues that hinder MGE in microbial systems will be discussed in the following subsections.
Pathways need to be intercepted at different points/with different intermediates, e.g., sialic acid
Schilling and coworkers demonstrated that unlike mammalian cells that leverage the substrate flexibility of their sialic acid pathway to allow the use of ManNAc analogs [19], bacterial cells such as Haemophilus ducreyi were able to process and install the synthetic sialic acid analogs but not their ManNAc precursors [58]. These bacteria apparently lack the ability to synthesize sialic acids de novo but instead express permeases that allow them to scavenge sialic acid from their surroundings and subsequently install them into their glycoconjugates [59, 60]. Goon et al. carried this study further to determine that these bacterial pathways were tolerant to sialic acid analogs modified at the N-acyl position [61–63]. In fact, the bacteria were even more tolerant to large N-acyl groups than mammalian cells with measurable incorporation of alkyl chains up to 7 carbon units in length [63]; by contrast the human pathway is limited to a chain length of 5 carbons [64]. Hence while microbial cells may require a modification in the classic MGE techniques (e.g., use of sialic acid metabolic precursors rather than ManNAc, Fig. 6) these studies verified that they do possess the necessary flexibility in glycosylation machinery to make MGE feasible.
Fig. 6.
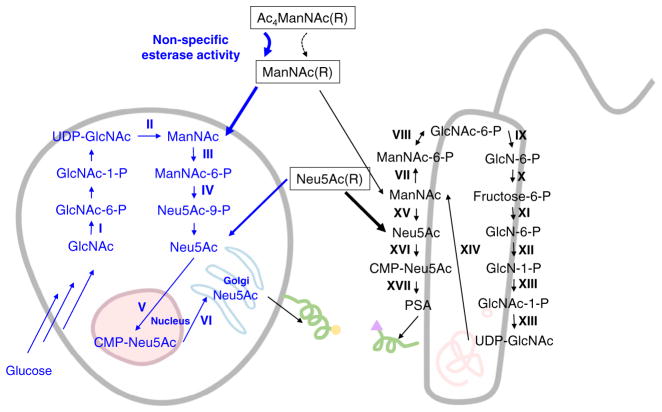
Comparison of MGE of sialic acid in mammalian cells (left [17]) and bacteria (right [18]). Exogenously-supplied, non-natural R-modified sugar analogs (with a sampling of R groups provided in Fig. 1) used to modulate sialic display in glycoconjugates include the three compounds shown in boxed form, namely Ac4ManNAc(R), ManNAc(R), and Neu5Ac(R). For mammalian systems acylated precursors such as Ac4ManNAc(R) are preferred for MGE because these compounds are used with 3 orders of magnitude or greater efficiency compared to their non-acylated counterparts (e.g., ManNAc(R)). Non-specific esterases (which are thought to be mostly intracellular but can also be extracellular as shown) convert acylated monosaccharides into their perhydroxyl form (e.g., ManNAc(R), which can then enter that targeted biosynthetic pathways. Interestingly, in mammals the use of the downstream intermediate Neu5Ac(R) is less efficient in mammalian cells than the use of the upstream ManNAc analogs. By contrast as discussed in more detail in the main text, in some prokaryotic systems Neu5Ac(R) are required because many bacteria lack the ability to convert ManNAc to Neu5Ac (step XV); furthermore, even in bacteria that have this ability, esterase activity might be too slow to use peracylated analogs. These type of nuances, which are only now being cataloged for bacteria, require careful planning when considering MGE experiments in prokaryotes. The enzymes shown are: I GlcNAc kinase, II. UDP-GlcNAc 2-epimerase, III. ManNAc Kinase, IV. Sialic acid 9-phosphate synthase, V. CMP-sialic acid synthase, VI. Steroid-sulfatase, VII. ManNAc kinase, VIII. ManNAc-6-P-2-epimerase, IX. GlcNAc-6-P deacetylase, X. Glucosamine-6-P deaminase, XI. GlcN-6-P synthase, XII. Phosphoglucosamine mutase, XIII. GlcNAc-1-P uridyltransferase, XIV. Sialic acid epimerase, XV. Sialic acid synthase, XVI. Sialic acid synthetase, XVII. Polysialyltransferase
SCFA-mediated delivery in bacteria can be limited by esterase activity
The uptake of sugar analogs into cells, which is a necessary prerequisite for a MGE experiment, is generally highly inefficient–for example, a lack of transporters for almost all modified monosaccharides leaves pinocytosis as the most likely route for MGE precursors to enter mammalian cells – and often requires millimolar concentrations. Although some bacteria have permeases that allow them to uptake sialic acid variants directly, the concentration required for these transporters is usually in the one millimolar range, which is at least an order of magnitude higher than acylated (e.g., acetylated or butanoylated) analogs now used in mammalian cells [65]. Furthermore, sialic acid permeases do not facilitate the uptake of the several other types of monosaccharides now used in MGE [15, 66]. As a general method to increase the efficiency of cellular uptake, short chain fatty acids (SCFA), originally acetates, were attached to sugar analogs to make them more lipophilic and hence improve their entry into the cell [34, 52, 67]. These acetylated analogs were metabolized almost 900 fold more efficiently, allowing for robust cellular responses in micromolar concentration ranges [68]. Attempts to further improve cellular uptake by increasing lipophilicity using longer chain SCFAs like propionate and butyrate groups resulted in 1800 fold and 2100 fold higher fluxes through the sialic acid pathway, respectively when compared to ordinary ManNAc [64].
It is generally accepted that hybrid SCFA-monosaccharide molecules now widely used in MGE are rapidly hydrolyzed within mammalian cells [69], allowing the liberated hydroxyl form of the sugar to enter the targeted biosynthetic pathway. However, although bacterial cells have similar esterases and lipases as mammalian cells, studies have shown that the levels and activities of these enzymes towards non-specific substrates are much lower than in mammals and at least some bacteria are not able to process the acetylated analogs [70]. This constraint, however, does not appear to be a universal feature of bacteria as efficient use of Ac4GlcNAz has been reported in Helicobacter pylori [71]. Nevertheless, the overall usefulness of SCFA-monosaccharides currently remains unclear because of relatively little investigation of this topic; therefore a case by case assessment is required when planning a MGE experiment with a new strain of bacteria or new type of sugar analogs. In cases where this approach is not feasible, alternative approaches to deliver non-natural monosaccharides efficiently into bacteria may include nanoparticle or liposomal delivery [72, 73].
Immunogenic carbohydrates are already displayed on bacteria
Besides technical issues that have the potential to hinder the transition of MGE technologies from mammalians systems to bacteria, in some cases underlying biological features of carbohydrate structures pose conceptual barriers. For example, major MGE initiatives in mammals simply appear to not apply to bacteria. There have been ongoing efforts to incorporate non-natural sialic acids into tumor associated carbohydrate antigens (TACAs) to increase the antigenicity of these epitopes by distinguishing them structurally from naturally-occurring sialosides [74–78]. Therefore the presence of many non-mammalian, highly immunogenic monosaccharides (Fig. 5) in bacteria can render the additional display of non-natural sugars by MGE moot if the goal is to alert the immune system to the presence on non-self antigens. However, there are critical exceptions where MGE may play a role even in bacteria in this regard; for example as discussed below in Section 3.1.4 installation of non-natural sugar residues into immune-shielding bacterial capsules could hold therapeutic value. In addition, the ability to generate antibodies to non-natural sugars–either of mammalian or bacterial origins – allows “pulse-chase” labeling experiments to probe the biosynthesis of targeted glycans following the general procedure outlined by Lemieux and coauthors [79].
Bacteria provide new opportunities for MGE
As mentioned above in Section 2, while challenges remain in extending MGE to prokaryotes, a sufficient scientific foundation is now in place to apply this technology to several aspects of bacteria that have substantial biomedical or industrial significance. Several of these categories that are currently “in play” are described below in Section 3.1 with a sampling of future directions outlined in Section 3.2.
Metabolic glycoengineering of bacterial glycans
In 2013, the Center for Disease Control (CDC) released a report stating that resistance to antibiotic regimens causes two million illnesses and 23,000 premature deaths per year [80]. In addition to “conventional” infections, the increasing prevalence of medical procedures such as organ transplantations or cancer treatments are often associated with infection [81, 82]. All in all, resistance to antibiotics now threatens to unravel over a century’s worth of medical advancements. While bacteria are often associated with infection and deleterious consequences, it has become increasingly clear that beneficial bacteria that compromise the “microbiome” have fundamental roles in both maintaining and promoting human health [83]. Many current antibiotic regimens used to fight infection kill broad classes of bacterial types indiscriminately and can damage beneficial bacteria of the microbiome that may take years to recover [84–86]. Newer paradigms for antibiotic drug discovery strive to achieve highly targeted specificities where pathogenic bacteria are exclusively killed while concomitantly avoiding deleterious side effects brought about by perturbing the beneficial microbiome. In this regard–and although largely speculative at present – MGE provides intriguing and untapped opportunities for selective targeting, imaging, elimination, and eradication of subsets of harmful and pathogenic bacteria while leaving the larger microbiome unharmed. As discussed below in turn, MGE strategies can be envisioned to selectively target LPS, peptidoglycans, and teichoic acids in species-specific ways that, if successful, could enable imaging of bacteria, disruption of their adhesive properties, or ultimately even targeted cell death.
Bacteria-specific pathway targeting into LPS
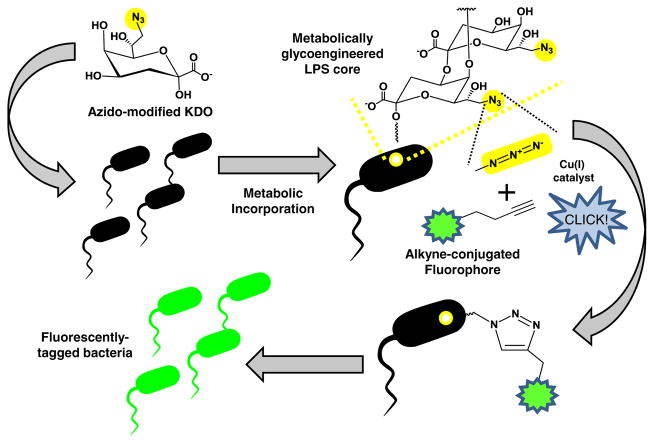
As discussed above, features of bacterial glycosylation different than human glycosylation has hindered application of MGE to microbes in the past. However, a detailed understanding of bacterial glycosylation pathways and their idiosyncrasies opens the door to MGE strategies that can potentially target bacteria with considerable specificity. For example, the early work by Goon et al. revealed that unnatural sialic acid analogs with elongated N-acyl chains up to seven carbons long were able to be incorporated into O-antigen glycan structures, specifically that of H. ducreyi [63]; by comparison, N-acyl chains of 6 or more carbons are virtually excluded from the mammalian sialic acid pathway [64, 87]. Targeting of the LPS structure of Gram-negative bacteria with such bacterial-specific carbohydrate precursors (e.g., with 6 or 7 carbon N-acyl chains not tolerated by the human enzymes) or corresponding bioorthogonal handles offers an attractive starting point to probe the role of LPS structures in virulence, adhesion, or the ability of a pathogen to evade host immune detection. Towards this objective, Dumont et al. recently reported a metabolic labeling approach in order to label E. coli LPS O-antigens through azido modification of a KDO moiety in the inner core oligosaccharide [88] which was then used to image the bacteria (Fig. 7). It is noteworthy that Gram-positive bacteria such as B. subtilis and S. aureus do not utilize KDO in their cell walls, thus the KDO engineering strategy employed by Dumont et al. may provide a way to image or deliver drugs to selective types of bacteria. Incorporation of bioorthogonal handles in LPS structures provides a potential technology platform for the in vivo imaging of bacteria to aid in early detection of clinical infections, a reactive site that could be exploited for drug delivery, or in a different context, a method to isolate and purify Gram-negative bacteria for industrial purposes.
Fig. 7.
MGE of LPS. Azido-modified KDO (natural KDO is shown in Fig. 5) can be metabolically incorporated into the LPS core structures (see Fig. 3). Once incorporated into LPS components, the azide group can be tagged with alkyne-conjugated fluorophores using the “Click” reaction resulting in fluorescently tagged bacteria
Bacteria-specific pathway targeting into peptidoglycans
Seminal work from Sadamoto and coworkers [89–92], focused on chemically engineering a set of synthetic peptidoglycan precursors that could be incorporated into bacterial cell walls in order to install a fluorescent moiety, modulate growth inhibition, and adhesion. By utilizing UDP-MurNAc penta-peptide derivatives, Sadamato and coworkers were able to metabolically incorporate the MurNAc analogs into bacteria peptidoglycans. It has been proposed that through the use of UDP-MurNAc precursors that can transverse through bacterial cellular machinery and appear on the cell surface of certain types of bacteria without the need of genetic modification, bacteria such as Lactobacillus plantarum can be designed with surface peptides that can bind to things like heavy metals, and could possibly be used for environmental cleanup while attenuating the risk that GMOs may pose once released into the environment [93]. In addition to practical applications such as environmental remediation that potentially hold great commercial and societal relevance, the biologic “messenger functions” of bacterial cell wall-derived muropeptides [94] are substantial. For example, synthetic peptidoglycan precusors have been used to promote germination of bacterial spores [95].
Bacteria-specific pathway targeting into teichoic acids
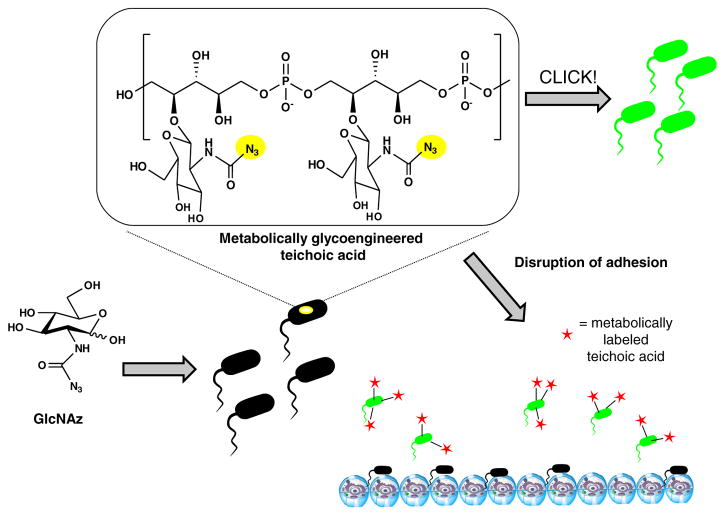
Few examples exist of MOE applied toward specific bacterial pathways that build teichoic acids, however, Memmel et al. have beautifully demonstrated an approach whereby azido modified N-acetyl glucosamine (GlcNAz) was used to metabolically engineer the surfaces of S. aureus (Fig. 8) [96]. After clicking with Alexa-488, adhesion to human bladder cancer cell line T24 was significantly reduced. This example highlights another potential strategy for using engineered carbohydrates to attack bacteria specific glycosylation of teichoic acids which, as discussed above in Section 2, are critical for ionically linking bacterial adhesive molecules. Manipulating the adhesive properties of pathogenic bacteria may in the future be specifically targeted with engineered carbohydrates for disruption to decrease virulence while simultaneously bypassing the inherent flaws that lead to antibiotic resistance that stems from the selective pressures imposed by most germicidal antibiotic treatments.
Fig. 8.
MGE of teichoic acid. Azido-modified GlcNAc (GlcNAz) can be metabolically incorporated into teichoic acids, which can be further conjugated using click reactions shown in Fig. 7 to again fluorescently label the bacteria or alternatively, to conjugate non-adhesive moieties on the surfaces of the bacteria towards endpoints such as the avoidance or destruction of biofilms
Bacteria-specific pathway targeting into capsule polysaccharides
Many bacteria that live in harsh environments surround themselves with polysaccharide capsules as a protective mechanism. An outstanding two-decade old example of this phenomenon are the polysialic acid capsules [97] that coat bacteria that live in a variety of harsh environments ranging from sewage to the human body, where the bugs are under assault from the host’s immune system. Insofar as one purpose of polysaccharide capsules is to shield pathogenic bacteria from the human immune system, the ability to chemically modify these sugars to render them immunogenic opens the door to new therapeutic strategies [98]. In the particular case of polysialic acid, it was also about 20 years ago that the Jennings’ group showed that a simple modification to the N-acyl group of sialic acid – specifically a one carbonyl extension of the N-acetyl group – provided the resulting “Sia5Prop” epitope with enhanced immunogenicity [99]. In subsequent work the ability of “Prop” (and longer chain [36]) sialoside analogs to be incorporated into mammalian polysialic acid has been extensively investigated but primarily only in mammalian cells. Therefore, it remains an intriguing possibility that MGE techniques can be exploited to install non-natural monosaccharides into polysialic acid and other polysaccharides that pathogenic bacteria use to escape detection by the human immune system.
Future directions: targeting species-specific glycosylation pathways in bacteria
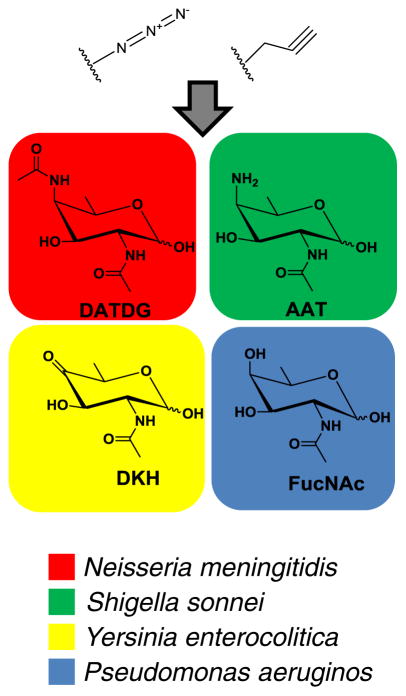
A recent review by Emmadi and Kulkarni has highlighted the chemical synthesis of particularly fascinating and extremely rare monosaccharides used by certain species of bacteria [100]. For example, 2-acetamindo-4-amino-2,4,6-trideoxy-D-galactose (AAT, Fig. 9) is expressed on the O-specific side chain of Shigella sonnei LPS, a bacterium that causes dysentery [101]. A similar sugar, 2,4-diacetamido-2,4,6-trideoxy-D-galactose (DATDG) was discovered to be directly attached to Neisseria meningitidis pili, which are essential filamentous protein structures for adhesion [102]. In another example, N--acetylfucosamine (FucNAc) is incorporated into the glycans that decorate the pili of Pseudomonas aeruginos, which is a major virulence factor [103]. P. aeruginosa often causes severe infections for patients with compromised immune systems. In a final example given here, D-xylo-6-deoxy-4-ketohexosamine (DKH) was both identified in and deemed crucial for the biological role of the LPS outer core of Yersinia enterocolitica, a bacterium that causes Yersiniosis when raw or undercooked pork products are consumed [104].
Fig. 9.
Bacterial monosaccharides that are candidates for more selective replacement using MGE. Azido or Alkyne groups, in theory, could be incorporated onto the rare sugar scaffolds shown. The engineered rare monosaccharides would then be used for species specific MGE of bacteria, as indicated by color
The sampling of rare monosaccharides just mentioned, and quite possibly additional ones yet to be discovered, provide a unique opportunity for MGE, where a similar strategy of azido or alkyne labeling of the aforementioned rare monosaccharides can be used to MGE extremely specific species of bacteria. Additionally, the metabolic pathways that synthesize and incorporate these rare sugars may also provide very attractive targets for inhibition. Such a narrow window of treatment is virtually unachievable with our current stockpile of antibiotics and therefore the targeting of extremely rare bacterial monosaccharides could, in theory, provide a therapeutic window for selective treatment of relevant infectious disease while avoiding the side effects that result from indiscriminately destroying the beneficial microbiome. A union between newer and very powerful bioanalytical techniques and modern chemistry may pave the way for both identifying other rare monosaccharides utilized on a bacterium species specific level and using identified rare monosaccharides as scaffolds for MGE. In the wake of the emerging threat of bacterial resistance and the dearth of a new generation of antibiotics in the pipeline, it is clear that newer paradigms need to be explored for both novel and very precise ways to treat infections. Ultimately, it would not be surprising if glycobiology and MGE were at the forefront towards these efforts.
Recombinant glycoprotein production
Challenges in manufacturing therapeutic proteins in bacteria
The recombinant protein industry has grown greatly in recent times and is projected to become a $200 to 300 billion market within the next 5 years. While they suffer certain limitations, bacteria have many advantages over mammalian production systems for the manufacturing of therapeutic proteins including low cost, high productivity and ease of adaptation [105]. The main limitation of bacterial systems is that bacterial cells have primitive folding and secretion machinery and in most cases don’t have the necessary glycosylation machinery to produce and secrete properly folded mammalian proteins [106]. Various strategies such as lysis and chaperone assisted folding to renature cytoplasmic proteins [107] and tight control of the E. coli cellular milieu during production [108] have ameliorated the “protein folding problem” inherent in prokaryote manufacturing of recombinant proteins.
A lack of glycosylation, however remains a major hurdle in the use of bacteria to mass produce recombinant mammalian proteins because glycosylation is known to have a profound impact on their functioning. Glycans play roles in organizing the primary amino acid sequence of a protein into secondary, tertiary and quaternary structures [109–112]; for example, large polar oligosaccharides can help direct the nearby peptide towards the surface of the protein [113–115]. Glycosylation also has an integral role in signal recognition as well as in glycoprotein clearance [116–118]. Finally, implications of deficient glycosylation in bacterial expression systems extend beyond folding and secretion insofar as glycan moieties are important determinants of in vivo activity and pharmacological properties when therapeutic proteins are introduced into the human body.
Towards mammalian-like glycosylation in prokaryotes
Encouragingly, a number of studies show evidence of protein glycosylation in bacteria that, if properly controlled and manipulated, hold promise for the production of therapeutic glycoproteins [119–124]. For example, Campylobacter jejuni provides a singular example of a bacterium with a well characterized N-glycosylation pathway [125, 126] but atypical N-glycosylation systems have also recently been reported in Haemophilus influenza [127]. The high-molecular-weight adhesin 1 of H. influenzae that is responsible for interacting with the sialylated N-glycoproteins on the host cell’s surface has been found to be glycosylated at 31 sites [128]. In contrast, O-glycosylation systems have been well characterized in a number of bacteria [129, 130]. While the function played by O-glycans is still relatively obscure, certain studies suggest that these glycans may help to protect the bacteria from immune recognition [131, 132] and it is an intriguing possibility that the O-glycan biosynthetic machinery can be exploited to improve recombinant protein production and quality.
A cautionary aspect of potentially substituting O-glycans for N-glycans, however, is that in addition to the simple presence of glycan structures on a recombinant protein, the actual composition of the carbohydrate moiety is a critical determinant of biological activity in many cases. This principle is exemplified by antibodies where the composition of the glycan attached to the constant domain of an IgG antibody has a pronounced effect on its binding affinities [133]. Furthermore, the presence or absence of fucose and sialic acid on the glycan present in the Fc region of an antibody can act as a switch between pro- and anti-inflammatory activity [134–136]. While it remains important to ensure that proteins are glycosylated, glycans maintain very specific compositions to tune biological activities in many cases. Therefore the exciting discovery of glycosylation machinery in certain bacteria that in principle can provide a means of mass producing glycosylated recombinant proteins that bear a closer resemblance to those produced in mammalian cells remains tempered by the stringent demands to very closely reproduce “humanized” glycopatterns. Towards this end, MGE strategies can be a useful tool to alter these glycans by increasing sialylation or by introducing modified sugar analogs into them, allowing us to tune them to some degree to better resemble their mammalian counterparts.
Production of nucleotide sugars and oligosaccharides
Metabolic considerations beyond glycosyltransferases: Nucleotide sugar production
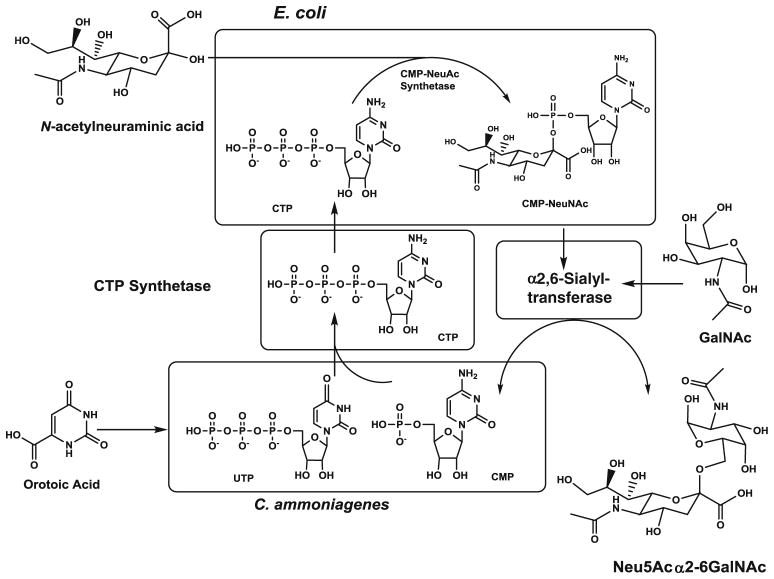
One of the key concepts of metabolic engineering is the idea that a better understanding of metabolism and cellular function can be attained by considering a set of reactions in their entirety rather than focusing on individual components in isolation. Hence, it is often critical to shift attention away from individual enzymatic reactions and towards to the entire integrated metabolic network [137]. Nascent efforts to produce recombinant glycoproteins in prokaryotes (e.g., as described in Section 4 above) illustrates this concept insofar as the ability of certain bacterial species or strains to produce different types of glycans fundamentally depends on glycosyltransferase expression. An equally critical part of glycan production, however, is the availability of nucleotide sugars that serve as essential high energy, activated co-substrates for glycosyltransferase reactions. The complex interplay between metabolic contributions – e.g., nucleotide sugars – and the canonical biosynthetic enzymes – e.g., sialyltransferases – is provided in the overview shown in Fig. 10.
Fig. 10.
Synthesis of Neu5Acα2-6GalNAc by combining CMP-Neu5Ac production with an E. coli strain that overproduces the α2,6-sialyltransferase
The supply of these nucleotide sugars are likely to be limited in host cells that have metabolic networks not optimized for over-expression of glycoproteins, which are relatively rare even in species capable of their production. As a consequence, prospects for oligosaccharide or glycoprotein biosynthesis using bacterial glycosyltransferases through expression in E. coli or other bacterial species on an industrial scale can be rate-limiting. In principle, this shortcoming can be overcome by nucleotide sugar regeneration and strategies towards achieving the efficient production of these compounds have focused on (i) in situ regeneration of the natural sugar nucleotides and (ii) preparation of unnatural sugar nucleotides, having a simpler structure and consequently a lower price [138].
Enzymatic regeneration of natural nucleotide sugars is one strategy that has been pursued to increase efficiency, lower the cost of sugar donors, and also eliminate the problem of product inhibition which is caused by the released nucleoside phosphate towards the glycosyltransferase, and thus facilitate the synthesis of oligosaccharides on a large scale [139]. In this manner, the expense of sugar nucleotides, and product inhibition of the glycosyltransferase by the resulting nucleoside di-or mono-phosphates (NDPs or NMPs) are overcome by using enzymes such as pyruvate kinase and phosphoenolpyruvate (PEP) [140]. Furthermore, new inexpensive kinase catalyst systems such as polyphosphate kinase/polyphosphate serve as an alternative to the pyruvate kinase/PEP system [141]. Recycling systems for UDP-Gal incorporating UDP-Gal 4-epimerase (UDPGE) [142] have been reported for the large-scale synthesis of N-acetyllactosamine (LacNAc), which solve the problem of β-1,4-galactosyltrasferase product inhibition and reduced the cost of expensive sugar donor.
Oligosaccharide production: coupling nucleotide sugar production with glycosyltransferases
Oligosaccharide production can be facilitated by “mix and match” combinations of biosynthetic components from different bacterial strains, which is conceptually illustrated in Fig. 10. In such synthetic schemes, coupling of bacterial genes can be achieved in cells permeabilized by treatment with surfactants or organic solvents and used as enzyme bags. High-energy phosphates such as phosphoribiosylpyrophosphate and ATP can be supplied by the metabolism of glucose in the cells. In a similar manner, CDP-choline has been produced by the combination of Corynebacterium ammoniagenes and recombinant E. coli cells expressing the genes involved in the biosynthesis of CDP-choline. One important advantage of using intact living cells is scalability because this approach avoids expensive starting materials and enzyme isolation; essentially, once an appropriate strain has been engineered, it can be easily scaled-up in a fermentor to produce large quantities of product. In one example, genetic manipulation of sugar nucleotide biosynthetic pathways in microorganisms enabled preparative scale synthesis of UDP-Gal [143]. However, because of the high cost of nucleotide sugars, they essentially comprise a bottleneck in chemoenzymatic or automated synthesis of carbohydrates [144, 145]. Consequently, innovative methods to generate these valuable compounds in bacterial systems hold substantial medical importance [146].
Bacterial production systems have enabled the synthesis of several notable carbohydrate structures. One example is the synthesis of the globotriose trisaccharide without side-products from simple and inexpensive starting materials [147] by using three bacterial strains: C. ammoniagenes for production of UTP from orotic acid (again shown in Fig. 10), recombinant E. coli containing genes for UDP-galactose synthesis, and another recombinant E. coli strain possessing α-1, 4-galactosyltransferase. Coupling of these three strains enabled production of 266 mM (134 g/L) of globotriose with lactose supplied as the acceptor. Similar strategies were also used to produce the disaccharide, N-acetyllactosamine, sialylated oligosaccharides, and a Lewis x trisaccharide [147–150]. Product concentrations were also impressive, ranging from 20 to 140 g/L. Using this approach, sugar nucleotides including UDP-Gal, CMP-Neu5Ac, UDP-GlcNAc and GDP-Fuc have been successfully produced on a large scale [151].
The use of innovative bacterial production systems for production of important carbohydrate structures has had several addition notable successes. P. G. Wang’s group has been at the forefront of these efforts with pioneering efforts such as the 2002 report of “superbugs” capable of producing the α-Gal carbohydrate epiptope [152]. To give a brief perspective on the medical importance of the α-Gal carbohydrate structure, this trisaccharide is involved in immuno-rejection of xenotransplanted porcine organs [153, 154] and also has been implicated in severe anaphylactic shock in therapeutic antibodies used to treat cancer patients [155, 156]. However, these systems are not generally utilized as extensively, due to some of the enzymes required for the regeneration schemes being difficult to obtain. Recent advances in sugar nucleotide recycling systems include efforts in engineering and chemical synthesis. Unnatural azide-containing sugar analogs of sialic acid [45], N-acetylgalactosamine (GalNAc) [157], N-acetylglucosamine (GlcNAc) [158] and fucose (Fuc) [159, 160] have been incorporated into several classes of eukaryotic cellular glycans without the disruption of further glycan elaboration of the glycoproteins. Based on the observation that GlcNAc is a common metabolic precursor to bacterial monosaccharides, Goon et al. exploited a peracetylated version of GlcNAz, Ac4GlcNAz, to metabolically label and profile H. pylori’s glycoproteins [63]. Peracetylation of this non-natural sugar facilitates its cellular entry, where it is converted to GlcNAz, processed by cellular machinery, and ultimately incorporated into glycoproteins. Treatment of H. pylori with Ac4GlcNAz resulted in robust azide-labeling of a large number of glycoproteins [71].
Summary and conclusions
Glycans of Gram-positive and Gram-negative bacteria are key structural features that are omnipresent and can be potentially exploited through glycometabolic and glycoengineering approaches in order to achieve anything from targeted drug delivery to engineering bacteria with specialized surface features for industrial settings. Through use of various MGE approaches, characteristics of bacteria can be manipulated without requiring exogenous genetic manipulations that come with their own concerns. Many challenges still lie ahead for targeting bacterial glycans with unnatural carbohydrates or designing glycomimetics based off of bacterial carbohydrate based precursors, but the glyco approach provides an enormous new venue through which new therapies can be developed in order to tackle the growing problem of drug resistant pathogens and, conversely, could be used to beneficially engineer bacteria for industrialized purposes, such as for environmental remediation and energy production.
Acknowledgments
Funding was obtained from the National Institutes of Health, NCI grant R01CA112314.
References
- 1.Zhang M, Eddy C, Deanda K, Finkelstein M, Picataggio S. Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis. Science. 1995;267:240–243. doi: 10.1126/science.267.5195.240. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Ramakrishnan S, Teeri TT, Knowles JKC, Hartley BS. Saccharomyces cerevisiae cells secreting an Aspergillus niger β-galactosidase grow on whey permeate. Nat Biotechnol. 1992;10:82–85. doi: 10.1038/nbt0192-82. [DOI] [PubMed] [Google Scholar]
- 3.Brabetz W, Liebl W, Schleifer KH. Studies on the utilization of lactose by Corynebacterium glutamicum, bearing the lactose operon of Escherichia coli. Arch Microbiol. 1991;155:607–612. doi: 10.1007/BF00245357. [DOI] [PubMed] [Google Scholar]
- 4.Slater SC, Voige W, Dennis D. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-b-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1998;170:4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert P, Steinbuchel A, Schlegel HG. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-b-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988;170:4837–4847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda M, Katsumata R. Metabolic engineering to produce tyrosine or phenylalanine in a tryptophan-producing Corynebacterium glutamicum strain. Appl Environ Microbiol. 1992;58:781. doi: 10.1128/aem.58.3.781-785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eikmanns BJ, Kleinertz E, Liebl W, Sahm H. A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene. 1991;102:93–98. doi: 10.1016/0378-1119(91)90545-m. [DOI] [PubMed] [Google Scholar]
- 8.Colon G, Nguyen T, Jetten MSM, Sinskey A, Stephanopoulos G. Production of isoleucine by overexpression of ilvA in a Corynebacterium lactofermentum threonine producer. Appl Microbiol Biotechnol. 1995;43:482–488. doi: 10.1007/BF00218453. [DOI] [PubMed] [Google Scholar]
- 9.Tong IT, Liao HH, Cameron D. 1,3-Propanediol production by Escherichia coli expressing genes from the Klebsiella pneumoniae dha regulon. Appl Environ Microbiol. 1991;57:3541–3546. doi: 10.1128/aem.57.12.3541-3546.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khosla C, Bailey JE. Heterologous expression of a bacterial haemoglobin improves the growth properties of recombinant Escherichia coli. Nature. 1988;331:633–635. doi: 10.1038/331633a0. [DOI] [PubMed] [Google Scholar]
- 11.Aristidou AA, San KY, Bennett GN. Modification of central metabolic pathway in Escherichia coli to reduce acetate accumulation by heterologous expression of the Bacillus subtilis acetolactate synthase gene. Biotechnol Bioeng. 1994;44:944–951. doi: 10.1002/bit.260440810. [DOI] [PubMed] [Google Scholar]
- 12.Dube DH, Bertozzi CR. Metabolic oligosaccharide engineering as a tool for glycobiology. Curr Opin Chem Biol. 2003;7:616–625. doi: 10.1016/j.cbpa.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Campbell CT, Sampathkumar SG, Weier C, Yarema KJ. Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Mol Biosyst. 2007;3:187–194. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- 14.Aich U, Yarema KJ. Non-natural sugar analogues: chemical probes for metabolic oligosaccharide engineering. In: FraserReid BO, Tatsuta K, Thiem J, editors. Glycoscience. Springer-Verlag; Berlin Heidelberg: 2008. pp. 2133–2190. [Google Scholar]
- 15.Du J, Meledeo MA, Wang Z, Khanna HS, Paruchuri VDP, Yarema KJ. Metabolic glycoengineering: sialic acid and beyond. Glycobiology. 2009;19:1382–1401. doi: 10.1093/glycob/cwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Du J, Che PL, Meledeo MA, Yarema KJ. Hexosamine analogs: from metabolic glycoengineering to drug discovery. Curr Opin Chem Biol. 2009;13:565–572. doi: 10.1016/j.cbpa.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viswanathan K, Lawrence S, Hinderlich S, Yarema KJ, Lee YC, Betenbaugh M. Engineering sialic acid synthetic ability into insect cells: identifying metabolic bottlenecks and devising strategies to overcome them. Biochemistry. 2003;42:15215–15225. doi: 10.1021/bi034994s. [DOI] [PubMed] [Google Scholar]
- 18.Ringenberg MA, Steenbergen SM, Vimr ER. The first committed step in the biosynthesis of sialic acid by Escherichia coli K1 does not involve a phosphorylated N-acetylmannosamine intermediate. Mol Microbiol. 2003;50:961–975. doi: 10.1046/j.1365-2958.2003.03741.x. [DOI] [PubMed] [Google Scholar]
- 19.Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J Biol Chem. 1992;267:16934–16938. [PubMed] [Google Scholar]
- 20.Kayser H, Geilen CC, Paul C, Zeitler R, Reutter W. Incorporation of N-acyl-2-amino-2-deoxy-hexoses into glycosphingolipids of the pheochromocytoma cell line PC 12. FEBS Lett. 1992;301:137–140. doi: 10.1016/0014-5793(92)81233-c. [DOI] [PubMed] [Google Scholar]
- 21.Kiick KL, Tirrell DA. Protein engineering by in vivo incorporation of non-natural amino acids: control of incorporation of methionine analogues by methionyl-tRNA synthetase. Tetrahedron. 2000;56:9487–9493. [Google Scholar]
- 22.Link AJ, Mock ML, Tirrell DA. Non-canonical amino acids in protein engineering. Curr Opin Biotechnol. 2003;14:603–609. doi: 10.1016/j.copbio.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 24.Hohsaka T, Sisido M. Incorporation of non-natural amino acids into proteins. Curr Opin Chem Biol. 2002;6:809–815. doi: 10.1016/s1367-5931(02)00376-9. [DOI] [PubMed] [Google Scholar]
- 25.Chin JW, Martin AB, King DS, Wang L, Schultz PG. Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:11020–11024. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin JW, Santoro SW, Martin AB, King DS, Wang L, Schultz PG. Addition of p-azido-l-phenylalanine to the genetic code of Escherichia coli. J Am Chem Soc. 2002;124:9026–9027. doi: 10.1021/ja027007w. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Xie J, Schultz PG. Expanding the genetic code. Annu Rev Biophys Biomol Struct. 2006;35:225–249. doi: 10.1146/annurev.biophys.35.101105.121507. [DOI] [PubMed] [Google Scholar]
- 28.Keppler OT, Horstkorte R, Pawlita M, Schmidt C, Reutter W. Biochemical engineering of the N-acyl side chain of sialic acid: biological implications. Glycobiology. 2001;11:11R–18R. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]
- 29.Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. Metabolic delivery of ketone groups to sialic acid residues. Application to cell surface glycoform engineering. J Biol Chem. 1998;273:31168–31179. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- 30.Roth J. Cellular sialoglyconjugates: a histochemical perspective. Histochem J. 1993;25:687–710. doi: 10.1007/BF00211765. [DOI] [PubMed] [Google Scholar]
- 31.Tanner ME. The enzymes of sialic acid biosynthesis. Bioorg Chem. 2005;33:216–228. doi: 10.1016/j.bioorg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz EL, Hadfield AF, Brown AE, Sartorelli AC. Modification of sialic acid metabolism of murine erythroleukemia cells by analogs of N-acetylmannosamine. Biochim Biophys Acta. 1983;762:489–497. doi: 10.1016/0167-4889(83)90051-4. [DOI] [PubMed] [Google Scholar]
- 33.Hadfield AF, Mella SL, Sartorelli AC. N-Acetyl-D-mannosamine analogues as potential inhibitors of sialic acid biosynthesis. J Pharm Sci. 1983;72:748–751. doi: 10.1002/jps.2600720709. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar AK, Fritz TA, Taylor WH, Esko JD. Disaccharide uptake and priming in animal cells: inhibition of sialyl Lewis X by acetylated Gal b1,4GalcNAc bonaphthalenemethanol. Proc Natl Acad Sci U S A. 1995;92:3323–3327. doi: 10.1073/pnas.92.8.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuster MM, Brown JR, Wang L, Esko JD. A disaccharide precursor of sialyl Lewis X inhibits metastatic potential of tumor cells. Cancer Res. 2003;63:2775–2781. [PubMed] [Google Scholar]
- 36.Mahal LK, Charter NW, Angata K, Fukuda M, Koshland DE, Jr, Bertozzi CR. A small-molecule modulator of poly-a2,8-sialic acid expression on cultured neurons and tumor cells. Science. 2001;294:380–382. doi: 10.1126/science.1062192. [DOI] [PubMed] [Google Scholar]
- 37.Horstkorte R, Mühlenhoff M, Reutter W, Nöhring S, Zimmermann-Kordmann M, GerardySchahn R. Selective inhibition of polysialyltransferase ST8SiaII by unnatural sialic acids. Exp Cell Res. 2004;298:268–274. doi: 10.1016/j.yexcr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Grünholz HJ, Harms E, Opetz M, Reutter W, Cerný M. Inhibition of in vitro biosynthesis of N-acetylneuraminic acid by N-acyl- and N-alkyl-2-amino-2-deoxyhexoses. Carbohydr Res. 1981;96:259–270. doi: 10.1016/s0008-6215(00)81876-5. [DOI] [PubMed] [Google Scholar]
- 39.Gross HJ, Brossmer R. Enzymatic introduction of a fluorescent sialic acid into oligosaccharide chains of glycoproteins. Eur J Biochem. 1988;177:583–589. doi: 10.1111/j.1432-1033.1988.tb14410.x. [DOI] [PubMed] [Google Scholar]
- 40.Gross HJ, Rose U, Krause JM, Paulson JC, Schmid K, Feeny RE, Brossmer R. Transfer of synthetic sialic acid analogues to N- and O-linked glycoprotein glycans using four different mammalian sialyltransferases. Biochemistry. 1989;28:7386–7392. doi: 10.1021/bi00444a036. [DOI] [PubMed] [Google Scholar]
- 41.Brossmer R, Gross HJ. Sialic acid analogs and application for preparation of neoglycoconjugates. Methods Enzymol. 1994;247:153–176. doi: 10.1016/s0076-6879(94)47013-8. [DOI] [PubMed] [Google Scholar]
- 42.Kayser H, Geilen CC, Paul C, Zeitler R, Reutter W. New amino sugar analogues are incorporated at different rates into glycoproteins of mouse organs. Experientia. 1993;49:885–887. doi: 10.1007/BF01952603. [DOI] [PubMed] [Google Scholar]
- 43.Collins BE, Fralich TJ, Itonori S, Ichikawa Y, Schnaar RL. Conversion of cellular sialic acid expression from N-acetyl- to N-glycolylneuraminic acid using a synthetic precursor, N-glycolylmannosamine pentaacetate: inhibition of myelin-associated glycoprotein binding to neural cells. Glycobiology. 2000;10:11–20. doi: 10.1093/glycob/10.1.11. [DOI] [PubMed] [Google Scholar]
- 44.Sampathkumar SG, Li AV, Jones MB, Sun Z, Yarema KJ. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat Chem Biol. 2006;2:149–152. doi: 10.1038/nchembio770. [DOI] [PubMed] [Google Scholar]
- 45.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 46.Mahal LK, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 47.Han S, Collins BE, Bengtson P, Paulson JC. Homo-multimeric complexes of CD22 revealed by in situ photoaffinity protein-glycan crosslinking. Nat Chem Biol. 2005;1:93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka Y, Kohler JJ. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J Am Chem Soc. 2008;130:3278–3279. doi: 10.1021/ja7109772. [DOI] [PubMed] [Google Scholar]
- 49.Dafik L, d’Alarcao M, Kumar K. Fluorination of mammalian cell surfaces via the sialic acid biosynthetic pathway Bioorg. Med Chem Lett. 2008;18:5945–5947. doi: 10.1016/j.bmcl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dafik L, d’Alarcao M, Kumar K. Modulation of cellular adhesion by glycoengineering. J Med Chem. 2010;53:4277–4284. doi: 10.1021/jm100374g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahal LK, Yarema KJ, Lemieux GA, Bertozzi CR. Chemical approaches to glycobiology: engineering cell surface sialic acids for tumor targeting. In: Inoue Y, Lee YC, Troy FA II, editors. Sialobiology and other novel forms of glycosylation. Gakushin Publishing Company; Osaka: 1999. pp. 273–280. [Google Scholar]
- 52.Lemieux GA, Yarema KJ, Jacobs CL, Bertozzi CR. Exploiting differences in sialoside expression for selective targeting of MRI contrast reagents. J Am Chem Soc. 1999;121:4278–4279. [Google Scholar]
- 53.Andre G, Kulakauskas S, Chapot-Chartier MP, Navet B, Deghorain M, Bernard E, Hols P, Dufrêne YF. Imaging the nanoscale organization of peptidoglycan in living Lactococcus lactis cells. Nat Commun. 2010;1:1–8. doi: 10.1038/ncomms1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swoboda JG, Campbell J, Meredith TC, Walker S. Wall teichoic acid function, biosynthesis, and inhibition. ChemBioChem. 2010;11:35–45. doi: 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulnois GJ, Jann K. Bacterial polysaccharide capsule synthesis, export and evolution of structural diversity. Mol Microbiol. 1989;3:1819–1823. doi: 10.1111/j.1365-2958.1989.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 56.Jann B, Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- 57.Nizet V, Esko JD. Essentials of Glycobiology. 2. Chapter 39. Cold Spring Harbor Laboratory; 2009. Bacterial and Viral Infections. http://www.ncbi.nlm.nih.gov/books/NBK1952/ [PubMed] [Google Scholar]
- 58.Schilling B, Goon S, Samuels NM, Gaucher SP, Leary JA, Bertozzi CR, Gibson BW. Biosynthesis of sialylated lipooligosaccharides in Haemophilus ducreyi is dependent on exogenous sialic acid and not mannosamine. Incorporation studies using N-acylmannosamine analogues, N-Glycolylneuraminic acid, and 13C-labeled N-acetylneuraminic acid. Biochemistry. 2001;40:12666–12677. doi: 10.1021/bi0107849. [DOI] [PubMed] [Google Scholar]
- 59.Plumbridge J, Vimr E. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J Bacteriol. 1999;181:47–54. doi: 10.1128/jb.181.1.47-54.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vimr E, Lichtensteiger C, Steenbergen S. Sialic acid metabolism’s dual function in Haemophilus influenzae. Mol Microbiol. 2000;36:1113–1123. doi: 10.1046/j.1365-2958.2000.01925.x. [DOI] [PubMed] [Google Scholar]
- 61.Goon S, Bertozzi CR. Metabolic substrate engineering as a tool for glycobiology. In: Wang PG, Bertozzi CR, editors. Glycochemistry. Principles, synthesis, and applications. Marcel Dekker, Inc; New York: 2001. pp. 641–674. [Google Scholar]
- 62.Goon S, Bertozzi CR. Metabolic substrate engineering as a tool for glycobiology. J Carbohydr Chem. 2002;21:943–977. [Google Scholar]
- 63.Goon S, Schilling B, Tullius MV, Gibson BW, Bertozzi CR. Metabolic incorporation of unnatural sialic acids into Haemophilus ducreyi lipooligosaccharides. Proc Natl Acad Sci U S A. 2003;18:3089–3094. doi: 10.1073/pnas.0437851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim EJ, Sampathkumar SG, Jones MB, Rhee JK, Baskaran G, Yarema KJ. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acetylmannosamine (ManNAc) analogs in Jurkat (human T-lymphoma-derived) cells. J Biol Chem. 2004;279:18342–18352. doi: 10.1074/jbc.M400205200. [DOI] [PubMed] [Google Scholar]
- 65.Almaraz RT, Aich U, Khanna HS, Tan E, Bhattacharya R, Shah S, Yarema KJ. Metabolic oligosaccharide engineering with N-acyl functionalized ManNAc analogues: cytotoxicity, metabolic flux, and glycan-display considerations. Biotechnol Bioeng. 2012;109:992–1006. doi: 10.1002/bit.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almaraz RT, Mathew MP, Tan E, Yarema KJ. Metabolic oligosaccharide engineering: implications for selectin-mediated adhesion and leukocyte extravasation. Ann Biomed Eng. 2012;40:806–815. doi: 10.1007/s10439-011-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarkar AK, Rostand KS, Jain RK, Matta KL, Esko JD. Fucosylation of disaccharide precursors of sialyl Lewis X inhibit selectin-mediated cell adhesion. J Biol Chem. 1997;272:25608–25616. doi: 10.1074/jbc.272.41.25608. [DOI] [PubMed] [Google Scholar]
- 68.Jones MB, Teng H, Rhee JK, Baskaran G, Lahar N, Yarema KJ. Characterization of the cellular uptake and metabolic conversion of acetylated N-acetylmannosamine (ManNAc) analogues to sialic acids. Biotechnol Bioeng. 2004;85:394–405. doi: 10.1002/bit.10901. [DOI] [PubMed] [Google Scholar]
- 69.Mathew MP, Tan E, Shah S, Bhattacharya R, Meledeo MA, Huang J, Espinoza FA, Yarema KJ. Extracellular and intracellular esterase processing of SCFA-hexosamine analogs: implications for metabolic glycoengineering and drug delivery. Bioorg Med Chem Lett. 2012;22:6929–6933. doi: 10.1016/j.bmcl.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonczak AK, Simova Z, Tippmann EM. A critical examination of Escherichia coli esterase activity. J Biol Chem. 2010;284:28795–28800. doi: 10.1074/jbc.M109.027409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koenigs MB, Richardson EA, Dube DH. Metabolic profiling of Helicobacter pylori glycosylation. Mol Biosyst. 2009;5:909–912. doi: 10.1039/b902178g. [DOI] [PubMed] [Google Scholar]
- 72.Jones MN. Use of liposomes to deliver bactericides to bacterial biofilms. Methods Enzymol. 2005;391:211–228. doi: 10.1016/S0076-6879(05)91013-6. [DOI] [PubMed] [Google Scholar]
- 73.Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–623. doi: 10.1016/j.jconrel.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 74.Liu T, Guo Z, Yang Q, Sad S, Jennings HJ. Biochemical engineering of surface a2,8 polysialic acid for immunotargeting tumor cells. J Biol Chem. 2000;275:32832–32836. doi: 10.1074/jbc.C000573200. [DOI] [PubMed] [Google Scholar]
- 75.Pan Y, Chefalo P, Nagy N, Harding C, Guo Z. Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. J Med Chem. 2005;48:875–883. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chefalo P, Pan YB, Nagy N, Harding C, Guo ZW. Preparation and immunological studies of protein conjugates of N-acylneuraminic acids. Glycoconj J. 2004;20:407–414. doi: 10.1023/B:GLYC.0000033997.01760.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chefalo P, Pan Y, Nagy N, Guo Z, Harding CV. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-D-mannosamine. Biochemistry. 2006;45:3733–3739. doi: 10.1021/bi052161r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu L, Gong X, Wang Q, Li J, Hu H, Wu Q, Zhang J, Guo Z. Combining synthetic carbohydrate vaccines with cancer cell glycoengineering for effective cancer immunotherapy. Cancer Immunol Immunother. 2012;61:2045–2054. doi: 10.1007/s00262-012-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lemieux GA, Bertozzi CR. Modulating cell surface immuno-reactivity by metabolic induction of unnatural carbohydrate antigens. Chem Biol. 2001;8:265–275. doi: 10.1016/s1074-5521(01)00008-4. [DOI] [PubMed] [Google Scholar]
- 80.Sugerman DT. JAMA patient page. Antibiotic resistance. J Am Med Assoc. 2013;310:2212. doi: 10.1001/jama.2013.282120. [DOI] [PubMed] [Google Scholar]
- 81.Aisenberg G, Rolston KV, Safdar A. Bacteremia caused by Achromobacter and Alcaligenes species in 46 patients with cancer (1989–2003) Cancer. 2004;101:2134–2140. doi: 10.1002/cncr.20604. [DOI] [PubMed] [Google Scholar]
- 82.Fishman JA, Greenwald MA, Kuehnert MJ. Enhancing transplant safety: a new era in the microbiologic evaluation of organ donors? Am J Transplant. 2007;7:2652–2654. doi: 10.1111/j.1600-6143.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- 83.Cho I, Blaser MJ. The Human Microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Relman DA. Restoration of the gut microbial habitat as a disease therapy. Nat Biotechnol. 2013;31:35–37. doi: 10.1038/nbt.2475. [DOI] [PubMed] [Google Scholar]
- 86.Blaser M. Antibiotic overuse: stop the killing of beneficial bacteria. Nature. 2011;476:393–394. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 87.Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR. Substrate specificity of the sialic acid biosynthetic pathway. Biochemistry. 2001;40:12864–12874. doi: 10.1021/bi010862s. [DOI] [PubMed] [Google Scholar]
- 88.Dumont A, Malleron A, Awwad M, Dukan S, Vauzeilles B. Click-mediated labeling of bacterial membranes through metabolic modification of the lipopolysaccharide inner core. Angew Chem Int Ed. 2012;51:3143–3146. doi: 10.1002/anie.201108127. [DOI] [PubMed] [Google Scholar]
- 89.Sadamoto R, Niikura K, Sears PS, Liu H, Wong CH, Suksomcheep A, Tomita F, Monde K, Nishimura SI. Cell-wall engineering of living bacteria. J Am Chem Soc. 2002;124:9018–9019. doi: 10.1021/ja026133x. [DOI] [PubMed] [Google Scholar]
- 90.Sadamoto R, Niikura K, Ueda T, Monde K, Fukuhara N, Nishimura SI. Control of bacteria adhesion by cell-wall engineering. J Am Chem Soc. 2004;126:3755–3761. doi: 10.1021/ja039391i. [DOI] [PubMed] [Google Scholar]
- 91.Sadamoto R, Nishimura S-I. Chemo-biological approach to modification of the bacterial cell wall. In: Yarema K, editor. Handbook of carbohydrate engineering. CRC Press/Taylor & Francis; Boca Raton: 2005. pp. 495–506. [Google Scholar]
- 92.Ueda T, Feng F, Sadamoto R, Niikura K, Monde K, Nishimura SI. Synthesis of 4-fluorinated UDP-MurNAc penta-peptide as an inhibitor of bacterial growth. Org Lett. 2004;6:1753–1756. doi: 10.1021/ol049598w. [DOI] [PubMed] [Google Scholar]
- 93.Wu CH, Mulchandani A, Chen W. Versatile microbial surface-display for environmental remediation and biofuels production. Trends Microbiol. 2008;16:181–188. doi: 10.1016/j.tim.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Boudreau MA, Fisher JF, Mobashery S. Messenger functions of the bacterial cell wall-derived muropeptides. Biochemistry. 2012;51:2974–2990. doi: 10.1021/bi300174x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee M, Hesek D, Shah IM, Oliver AG, Dworkin J, Mobashery S. Synthetic peptidoglycan motifs for germination of bacterial spores. ChemBioChem. 2010;11:2525–2529. doi: 10.1002/cbic.201000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Memmel E, Homann A, Oelschlaeger TA, Seibel J. Metabolic glycoengineering of Staphylococcus aureus reduces its adherence to human T24 bladder carcinoma cells. Chem Commun (Camb) 2013;49:7301–7303. doi: 10.1039/c3cc43424a. [DOI] [PubMed] [Google Scholar]
- 97.Vimr E, Steenbergen S, Cieslewicz M. Biosynthesis of the polysialic acid capsule in Escherichia coli K1. J Ind Microbiol. 1995:352–360. doi: 10.1007/BF01569991. [DOI] [PubMed] [Google Scholar]
- 98.Jennings HJ. Chemically modified capsular polysaccharides as vaccines. Adv Exp Med Biol. 1988;228:495–550. doi: 10.1007/978-1-4613-1663-3_18. [DOI] [PubMed] [Google Scholar]
- 99.Hayrinen J, Jennings H, Raff HV, Rougon G, Hanai N, Gerardy-Schahn R, Finne J. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J Infect Dis. 1995;171:1481–1490. doi: 10.1093/infdis/171.6.1481. [DOI] [PubMed] [Google Scholar]
- 100.Emmadi M, Kulkarni SS. Recent advances in synthesis of bacterial rare sugar building blocks and their applications. Nat Prod Rep. 2014;31:870–879. doi: 10.1039/c4np00003j. [DOI] [PubMed] [Google Scholar]
- 101.Xu DQ, Cisar JO, Ambulos N, Jr, Burr DH, Kopecko DJ. Molecular cloning and characterization of genes for Shigella sonnei form I O polysaccharide: Proposed biosynthetic pathway and stable expression in a live salmonella vaccine vector. Infect Immun. 2002;70:4414–4423. doi: 10.1128/IAI.70.8.4414-4423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stimson E, Virji M, Makepeace K, Dell A, Morris HR, Payne G, Saunders JR, Jennings MP, Barker S, Panico M, Blench I, Moxon ER. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 103.Castric P, Cassels FJ, Carlson RW. Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J Biol Chem. 2001;276:26479–26485. doi: 10.1074/jbc.M102685200. [DOI] [PubMed] [Google Scholar]
- 104.Pinta E, Duda KA, Hanuszkiewicz A, Kaczyński Z, Lindner B, Miller WL, Hyytiäinen H, Vogel C, Borowski S, Kasperkiewicz K, Lam JS, Radziejewska-Lebrecht J, Skurnik M, Holst O. Identification and role of a 6-deoxy-4-keto-hexosamine in the lipopolysaccharide outer core of Yersinia enterocolitica serotype O:3. Chemistry. 2009;15:9747–9754. doi: 10.1002/chem.200901255. [DOI] [PubMed] [Google Scholar]
- 105.Terpe K. Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2006;72:211–222. doi: 10.1007/s00253-006-0465-8. [DOI] [PubMed] [Google Scholar]
- 106.Yin J, Li G, Ren X, Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol. 2007;127:335–347. doi: 10.1016/j.jbiotec.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 107.Cole P. Chaperone-assisted protein expression. Structure. 1996;4:239–242. doi: 10.1016/s0969-2126(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 108.Khow O, Suntrarachun S. Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac J Trop Biomed. 2012;2:159–162. doi: 10.1016/S2221-1691(11)60213-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wormald MR, Dwek RA. Glycoproteins: glycan presentation and protein-fold stability. Structure. 1999;7:R155–R160. doi: 10.1016/s0969-2126(99)80095-1. [DOI] [PubMed] [Google Scholar]
- 110.O’Connor SE, Imperiali B. Modulation of protein structure and function by asparagine-linked glycosylation. Chem Biol. 1996;3:803–812. doi: 10.1016/s1074-5521(96)90064-2. [DOI] [PubMed] [Google Scholar]
- 111.Otvos L, Krivulka GR, Urge L, Szendrei GI, Nagy L, Xiang ZQ, Ertl HCJ. Comparison of the effects of amino acid substitutions and b-N-vs. a-O-glycosylation on the T-cell stimulatory activity and conformation of an epitope on the rabies virus glycoprotein. Biochim Biophys Acta. 1995;1267:55–64. doi: 10.1016/0167-4889(95)00030-v. [DOI] [PubMed] [Google Scholar]
- 112.Steen PV, Rudd PM, Dwek RA, Opdenakker G. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 113.Imperiali B, Rickert KW. Conformational implications of asparagine-linked glycosylation. Proc Natl Acad Sci U S A. 1995;92:97–101. doi: 10.1073/pnas.92.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Imperiali B, O’Connor SE. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol. 1999;3:643–649. doi: 10.1016/s1367-5931(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 115.Kern G, Kern D, Jaenicke R, Seckler R. Kinetics of folding and association of differently glycosylated variants of invertase from Saccharomyces cerevisiae. Protein Sci. 1993;2:1862–1868. doi: 10.1002/pro.5560021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 117.Hooper LV, Manzella S, Baenziger J. From legumes to leukocytes: biological roles for sulfated carbohydrates. FASEB J. 1996;10:1137–1146. doi: 10.1096/fasebj.10.10.8751716. [DOI] [PubMed] [Google Scholar]
- 118.Drickamer K. Clearing up glycoprotein hormones. Cell. 1991;67:1029–1032. doi: 10.1016/0092-8674(91)90278-7. [DOI] [PubMed] [Google Scholar]
- 119.Schäffer C, Graninger M, Messner P. Prokaryotic glycosylation. Proteomics. 2001;1:248–261. doi: 10.1002/1615-9861(200102)1:2<248::AID-PROT248>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 120.Benz I, Schmidt MA. Never say never again: protein glycosylation in pathogenic bacteria. Mol Microbiol. 2002;45:267–276. doi: 10.1046/j.1365-2958.2002.03030.x. [DOI] [PubMed] [Google Scholar]
- 121.Power P, Jennings M. The genetics of glycosylation in Gram-negative bacteria. FEMS Microbiol Lett. 2003;218:211–222. doi: 10.1111/j.1574-6968.2003.tb11520.x. [DOI] [PubMed] [Google Scholar]
- 122.Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16:91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- 123.Abu-Qarn M, Eichler J, Sharon N. Not just for eukarya any-more: protein glycosylation in bacteria and archaea. Curr Opin Struct Biol. 2008;18:544–550. doi: 10.1016/j.sbi.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 124.Nothaft H, Szymanski CM. Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol. 2010;8:765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- 125.Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, Aebi M. N-Linked glycosylation in Campylobacter jejuni and it functional transfer into E. coli. Science. 2002;298:1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 126.Linton D, Dorrell N, Hitchen PG, Amber S, Karlyshev AV, Morris HR, Dell A, Valvano MA, Aebi M, Wren BW. Functional analysis of the Campylobacter jejuni N-linked protein glycosylation pathway. Mol Microbiol. 2005;55:1695–1703. doi: 10.1111/j.1365-2958.2005.04519.x. [DOI] [PubMed] [Google Scholar]
- 127.Gross J, Grass S, Davis AE, Gilmore-Erdmann P, Townsend RR, Geme JWS., III The Haemophilus influenzae HMW1 adhesin is a glycoprotein with an unusual N-linked carbohydrate modification. J Biol Chem. 2008;283:26010–26015. doi: 10.1074/jbc.M801819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.St Gene JW., III The HMW1 adhesin of nontypeable Haemophilus influenzae recognizes sialylated glycoprotein receptors on cultured human epithelial cells. Infect Immun. 1994;62:3881–3889. doi: 10.1128/iai.62.9.3881-3889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Logan SM. Flagellar glycosylation—a new component of the motility repertoire? Microbiology. 2006;152:1249–1262. doi: 10.1099/mic.0.28735-0. [DOI] [PubMed] [Google Scholar]
- 130.Ng SYM, Chaban B, Jarrell KF. Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications. J Mol Microbiol Biotechnol. 2006;11:167–191. doi: 10.1159/000094053. [DOI] [PubMed] [Google Scholar]
- 131.Cardinale J, Clark V. Expression of AniA, the major anaerobically induced outer membrane protein of Neisseria gonorrhoeae, provides protection against killing by normal human sera. Infect Immun. 2000;68:4368–4369. doi: 10.1128/iai.68.7.4368-4369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ku S, Schulz B, Power P, Jennings M. The pilin O-glycosylation pathway of pathogenic neisseria is a general system that glycosylates AniA, an outer membrane nitrite reductase. Biochem Biophys Res Commun. 2009;378:84–89. doi: 10.1016/j.bbrc.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 133.Li H, Sethurama N, Stadheim TA, Zha D, Prinz B, Ballew N, Bobrowicz P, Choi BK, Cook WJ, Cukan M. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat Biotechnol. 2006;24:210–215. doi: 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- 134.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 135.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cosgrave EF, Struwe WB, Hayes JM, Harvey DJ, Wormald MR, Rudd PM. N-linked glycan structures of the human Fcγ receptors produced in NS0 cells. J Proteome Res. 2013;12:3721–3737. doi: 10.1021/pr400344h. [DOI] [PubMed] [Google Scholar]
- 137.Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metabol Eng. 1999;1:1–11. doi: 10.1006/mben.1998.0101. [DOI] [PubMed] [Google Scholar]
- 138.Weijers CAGM, Franssen MCR, Visser GM. Glycosyltransferase-catalyzed synthesis of bioactive oligosaccharides. Biotechnol Adv. 2008;26:436–456. doi: 10.1016/j.biotechadv.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 139.Hanson S, Best M, Bryan MC, Wong CH. Chemoenzymatic synthesis of oligosaccharides and glycoproteins. Trends Biochem Sci. 2004;29:656–663. doi: 10.1016/j.tibs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 140.Ichikawa Y, Shen GJ, Wong CH. Enzyme-catalyzed synthesis of sialyl oligosaccharide with in situ regeneration of CMP-sialic acid. J Am Chem Soc. 1991;113:4698–4700. [Google Scholar]
- 141.Nishimura SI, Yamada K. Transfer of ganglioside GM3 oligosaccharide from a water soluble polymer to ceramide by ceramide glycanase. A novel approach for the chemical-enzymatic synthesis of glycosphingolipids. J Am Chem Soc. 1997;119:10555–10556. [Google Scholar]
- 142.Wong CH, Haynie SL, Whitesides GM. Enzyme-catalyzed synthesis of N-acetyllactosamine with in situ regeneration of uridine 5-diphosphate glucose and uridine 5′-diphosphate galactose. J Org Chem. 1982;47:5416–5418. [Google Scholar]
- 143.Koeller KM, Wong CH. Complex carbohydrate synthesis tools for glycobiologists: enzyme-based approach and programmable one-pot strategies. Glycobiology. 2000;10:1157–1169. doi: 10.1093/glycob/10.11.1157. [DOI] [PubMed] [Google Scholar]
- 144.Plante OJ, Palmacci ER, Seeberger PH. Automated synthesis of polysaccharides. Methods Enzymol. 2003;369:235–248. doi: 10.1016/S0076-6879(03)69013-0. [DOI] [PubMed] [Google Scholar]
- 145.Seeberger PH, Werz DB. Automated synthesis of oligosaccharides as a basis for drug discovery. Nat Rev Drug Discov. 2005;4:751–763. doi: 10.1038/nrd1823. [DOI] [PubMed] [Google Scholar]
- 146.Seeberger PH, Werz DB. Synthesis and medical applications of oligosaccharides. Nature. 2007;446:1046–1051. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- 147.Koizumi S, Endo T, Tabata K, Ozaki A. Large-scale production of UDP-galactose and globotriose by coupling metabolically engineered bacteria. Nat Biotechnol. 1998;16:847–850. doi: 10.1038/nbt0998-847. [DOI] [PubMed] [Google Scholar]
- 148.Endo T, Koizumi S, Tabata K, Kakita S, Ozaki A. Large-scale production of N-acetyllactosamine through bacterial coupling. Carbohydr Res. 1999;316:179–183. doi: 10.1016/s0008-6215(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 149.Koizumi S, Tabata K, Kakita S, Ozaki A. Large-scale production of CMP-NeuAc and sialylated oligosaccharides through bacterial coupling. Appl Microbiol Biotechnol. 2000;53:257–261. doi: 10.1007/s002530050017. [DOI] [PubMed] [Google Scholar]
- 150.Koizumi S, Endo T, Tabata K, Nagano H, Ohnishi J, Ozaki A. Large-scale production of GDP-Lewis X by bacterial coupling. J Ind Microbiol Biotechnol. 2000;25:213–217. [Google Scholar]
- 151.Woodyer RD, Johannes TW, Zhao H. Regeneration of cofactors for enzyme biocatalysis. In: Pandey A, Webb C, Soccol CR, Larroche CR, editors. Enzyme technology. Springer; Germany: 2006. pp. 83–101. [Google Scholar]
- 152.Chen X, Liu Z, Zhang J, Zhang W, Kowal P, Wang PG. Reassembled biosynthetic pathway for large-scale carbohydrate synthesis: a-Gal epitope producing “superbug”. Chembiochem. 2002;3:47–53. doi: 10.1002/1439-7633(20020104)3:1<47::AID-CBIC47>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 153.Yarema KJ, Bertozzi CR. Chemical approaches to glycobiology and emerging carbohydrate-based therapeutic agents. Curr Opin Chem Biol. 1998;2:49–61. doi: 10.1016/s1367-5931(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 154.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, Cowell-Lucero JL, Wells KD, Colman A, Polejaeva IA, Ayares DL. Targeted disruption of the a1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 155.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TAE. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. New Eng J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cabezas-Cruz A, Valdés J, de la Fuenteemail J. Cancer research meets tick vectors for infectious diseases. Lancet. 2014;14:916–917. doi: 10.1016/S1473-3099(14)70902-8. [DOI] [PubMed] [Google Scholar]
- 157.Hang HC, Yu C, Kato DL, Bertozzi CR. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc Natl Acad Sci U S A. 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, Bertozzi CR. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci U S A. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rabuka D, Hubbard SC, Laughlin ST, Argade SP, Bertozzi CR. A chemical reporter strategy to probe glycoprotein fucosylation. J Am Chem Soc. 2006;128:12078–12079. doi: 10.1021/ja064619y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci U S A. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]