Abstract
The ability to control pattern formation is critical for the both the embryonic development of complex structures as well as for the regeneration/repair of damaged or missing tissues and organs. In addition to chemical gradients and gene regulatory networks, endogenous ion flows are key regulators of cell behavior. Not only do bioelectric cues provide information needed for the initial development of structures, they also enable the robust restoration of normal pattern after injury. In order to expand our basic understanding of morphogenetic processes responsible for the repair of complex anatomy, we need to identify the roles of endogenous voltage gradients, ion flows, and electric fields. In complement to the current focus on molecular genetics, decoding the information transduced by bioelectric cues enhances our knowledge of the dynamic control of growth and pattern formation. Recent advances in science and technology place us in an exciting time to elucidate the interplay between molecular-genetic inputs and important biophysical cues that direct the creation of tissues and organs. Moving forward, these new insights enable additional approaches to direct cell behavior and may result in profound advances in augmentation of regenerative capacity.
Keywords: bioelectricity, ion channel, resting potential, voltage, patterning
Introduction
A critical goal of regenerative biology and medicine is to understand and control the mechanisms underlying the processes directing growth and patterning. Alongside conventionally-studied transcriptional networks and chemical cues, additional inputs enable cells to cooperate and make decisions necessary for the repair and remodeling of complex anatomical structures. Endogenous ion flows serve as important regulators of cell behavior, coordinating cell activity during pattern homeostasis. Located within cell membranes, ion channels, pores, and pumps create a complex language of bioelectric signals that is tightly integrated with gene regulatory networks to direct cell behavior toward the creation and maintenance of functional tissues and organs. Here we discuss the known roles of ion-based physiological processes in directing cell behavior during pattern formation and regeneration. Specifically excluded in this review are the fast-acting action potentials associated with neurons and muscle cells, externally-applied electromagnetic fields and radiation, and ultra-weak photon emission.
What is developmental bioelectricity?
All cells drive and respond to changes in transmembrane voltage potential (Vmem). Unlike fast-spiking currents normally associated with nerve and muscle cell activity, ion pumps, channels, and pores distribute specific ion species across cellular plasma membranes to produce slowly-changing spatial patterns of resting potential (Figure 1). In addition, groups of cells can be electrically connected via the diffusion of small molecules between cells through electrical synapses known as gap junctions (Fitzharris and Baltz, 2006; Mathews and Levin, 2017). These transmembrane potentials, fluxes of individual ions, and iso-electric cell compartments established by gap junctions, convey information to target cells, their neighbors, and in some instances, to distant locations. This signaling modality is used to process and transmit information about regenerative parameters such as cell type, tissue size, positional information, axial polarity, and organ identity (Levin, 2014; Levin et al., 2017; Pitcairn and McLaughlin, 2016). Importantly, these signals (unlike the familiar mRNA and protein signals) can only be characterized in the living state. Furthermore, the ability of channels and gap junctions to open and close post-translationally means that bioelectric cell states are a complex function of a given cell's microenvironment history, impinging physiological signals, and expression levels of electrogenic machinery.
Figure 1. Bioelectrical signaling drives pattern formation at the level of the cell, tissue, and organism.
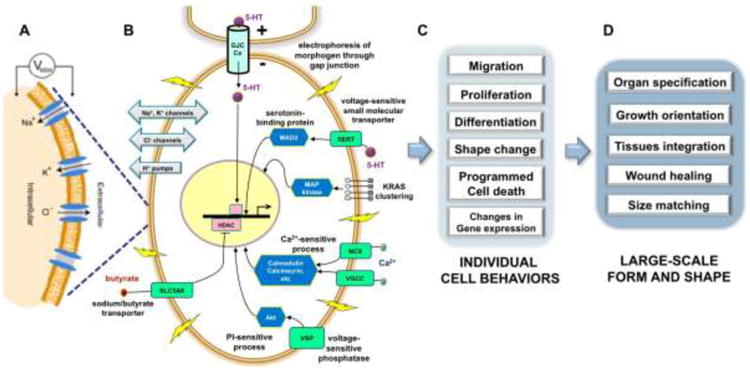
(A) Changes in transmembrane voltage are transduced (B) by a set of membrane mechanisms (voltage-powered transporters of serotonin and butyrate, voltage-gated calcium channels, voltage-regulated phosphatases, and others) into second-messenger cascades that regulate gene expression, thus directing cell behavior (C) such as, migration, proliferation, cell death, differentiation, gene expression, and shape changes. (D) In turn, these changes in cell behavior enable the creation of complex structures. Abbreviations: 5-HT, 5-hydroxytryptamine, also known as serotonin; HDAC, histone deacetylase; MAD3, Max-interacting transcriptional repressor; Akt, serine/threonine-specific protein kinase; GJC, gap junction communication; NCX, Na+/Ca2+ exchanger; VGCC, voltage-gated calcium channel; Cx, connexin; MAP kinase, mitogen-activated protein kinase. Lightning bolts represent changes in resting membrane potential. Panels A and B modified with permission (Levin, 2007b).
Over a century of observations in developmental bioelectricity - a historical perspective
The “electrical properties of living tissues” have been discussed by scientists for over a century (Mathews, 1903); prescient workers such as Burr (Burr and Northrop, 1935a) and Lund (Lund, 1947) characterized bioelectrical gradients in developing and regenerative systems, and used applied voltages to show that bioelectric signals were not merely epiphenomena of housekeeping physiology but were instructive for specific changes in growth and patterning in a range of fungal, plant, invertebrate, and vertebrate species. Marsh and Beams spearheaded some of the useful studies supporting an instructive role for bioelectric signaling during tissue patterning. By applying external electric fields to worm fragments, they demonstrated the ability to specifically alter the anterior-posterior polarity of regenerating fragments of planaria (Marsh and Beams, 1947, 1952). Subsequent instrumental work by several researchers including Lionel Jaffe, Richard Nuccitelli, Richard Borgens, Colin McCaig, and Ken Robinson, found that the electrical properties of single cells, neural tissues, epithelia, and entire appendages, were able to direct growth, morphology, and tissue polarity during regeneration of a wide range of model species (Borgens, 1982, 1983, 1986; Borgens, 1989; Jaffe, 1980, 1981, 1982; Jaffe et al., 1974; McCaig et al., 2005; Nuccitelli and Jaffe, 1974, 1976; Robinson, 1983).
With the advent of modern molecular, cellular, and genomic methodologies, more recently researchers have built upon these early studies to characterize proteins responsible for generating the bioelectric signals, transduction machinery that converts voltage change into second-messenger cascades, the gene regulatory networks downstream of bioelectric signaling, and ultimately the underlying mechanisms that direct cell behavior. The development of molecular-resolution genetic and pharmacological tools to investigate and manipulate ion flow has revealed that changes in resting potential can control individual cell behaviors including: proliferation, cell death, migration, and differentiation, in of a wide variety of cells types (Figure 1). In addition, recent data implicate endogenous spatiotemporal patterns of Vmem in regulating processes during embryonic development, regeneration, and patterning, that when altered, are responsible for a wide range of channelopathies and birth defects (Matusik, 2017; Persson and Bondke Persson, 2016).
Cell-level control of behavior by ion-mediated processes
During the creation of organized tissues, the ability to control cell behavior is critical for the formation of properly patterned structures. Large-scale morphogenesis necessitates the coordination of individual cells whose function is regulated via the integration of molecular cues and endogenous bioelectrical signals (as outlined in Figure 1). Both the creation of functional organs during development and regeneration of missing structures post-injury, require careful regulation of cell movement and positioning. Examples of cell migration events include the movement of progenitor cells towards the injury site observed in: planaria (Salo and Baguna, 1985), zebrafish (brains, hearts, fins) (Salo and Baguna, 1985; Tahara et al., 2016; Zupanc, 2006), and stem cell homing (Chute, 2006). Over half a century ago, several groups reported that electric fields could be used to direct cell behavior to orient cells either parallel or perpendicular to the field line, extend cell processes, or direct migration relative to the positioning of an anode or cathode (Anderson, 1951; Hyman and Bellamy, 1922). Although there is some debate over which cell types respond to physiologically relevant electric fields (Robinson and Cormie, 2008), subsequent work has shown numerous embryonic and somatic cell types exhibit galvanotaxis in electric fields in vivo (Pullar and Isseroff, 2005; Stump and Robinson, 1983; Yao et al., 2008; Zhao et al., 1997). It has been postulated that during embryogenesis these electric fields serve to both polarize the early vertebrate embryos as well as provide important positional cues that direct cell movements necessary for morphogenesis and pattern formation (Pitcairn et al., 2017; Shi and Borgens, 1995). This is especially relevant for guiding innervation and the movement of epithelial cells to close wounds – key components of regenerative response (Cao et al., 2013; Reid et al., 2005; Yamashita et al., 2013). Studies examining the migration of pigment cell derivatives originating from the neural crest, demonstrated altering bioelectrical events during early stages of embryogenesis caused melanocytes to inappropriately colonize tissues and organs in Xenopus tadpoles (Blackiston et al., 2011a; Morokuma et al., 2008). Similarly, a mutation in zebrafish that disrupts the pore function of an inwardly rectifying potassium channel (Kir7.1) altered the migration, but not the differentiation, of melanosomes (Iwashita et al., 2006).
Since it is a prerequisite for metastasis, much research has focused on targeting cell migration as way to hinder cancer progression. Ion transport proteins are easily accessible in cell membranes and are often overexpressed or activated in cancer (Becchetti et al., 2017; Funk, 2015; Pollak et al., 2017); thus, inhibition of the mechanisms underlying metastasis offers great therapeutic potential. Numerous studies have implicated bioelectric signaling as one of the mediators of electric guidance in migrating metastatic cancer cells (Fraser et al., 2005; Litan and Langhans, 2015; Mycielska and Djamgoz, 2004; Schwab, 2001; Schwab et al., 2012; Schwab and Stock, 2014).
Integration of cell proliferation into the overall pattern of a tissue and its size during regeneration can be controlled by membrane potential. As a general rule, proliferating cells tend to be more depolarized than non-proliferating (often differentiated) cells. This observation was further explored in a comparative analysis examining the membrane voltage of numerous cell types that revealed a striking functional connection between resting Vmem and the ability to progress through the cell cycle (Binggeli and Weinstein, 1986; Levin, 2012; Rao et al., 2015). Although the control of cell division by bioelectric signals is not always cell-autonomous (Morokuma et al., 2008; Pai et al., 2015a), in several studies proliferation appears to be controlled predominantly by a cell's own membrane potential (Arcangeli et al., 1993; Cone, 1974).
While the underlying mechanisms have not been completely elucidated, there is growing evidence that ion flow plays a role in regulating cell division (MacFarlane and Sontheimer, 2000; Ouadid-Ahidouch and Ahidouch, 2008, 2013; Ouadid-Ahidouch et al., 2016; Putney and Barber, 2003; Valenzuela et al., 2000). More specifically, numerous studies have implicated K+ currents as playing a key role in mediating cell division and cell cycle progression (Deng et al., 2007; MacFarlane and Sontheimer, 2000; Rouzaire-Dubois et al., 1993; Urrego et al., 2014). In the zebrafish, a mutation that reduces intracellular K+ concentration, and consequently hyperpolarizing cells, results in a dramatic increase in the fin and barbel size due to increased proliferation (Perathoner et al., 2014). Early observations that wide-spectrum potassium channel blockers inhibit proliferation in T-lymphocytes (DeCoursey et al., 1984) provided some of the first evidence that K+ concentration could regulate cell cycle progression in mammalian cells. More recently, numerous studies examining a diverse array of cell types have demonstrated potassium channel activity is functionally linked to cell proliferation. For example, the voltage-gated potassium channel KV1.3 (KCNA3) has been implicated in the control of cell cycle in many cell types including: proliferating oligodendrocyte progenitors during G1/S transition (Chittajallu et al., 2002), microglia cells (Kotecha and Schlichter, 1999), and macrophages (Vicente et al., 2003).
The activity of chloride channels has also been shown to modulate cell proliferation. For instance, inhibition of volume-regulated Cl- channels (VRCCs) results in p27 accumulation and G1 cell cycle arrest in T-cell leukemia cells and human embryonic kidney (HEK) cells (Renaudo et al., 2007). Similarly, blocking the function of TMEM16A (a calcium-activated Cl- channel), arrests colorectal cancer cells in G1 (Sui et al., 2014). In addition, gliomas often express higher levels and activity of voltage dependent (CLC family) chloride channels, contributing to depolarized membranes (Olsen et al., 2003). A reduction of Cl- channel activity via exposure to chemical inhibitors or siRNA-mediated knockdown both reduces Cl- currents and inhibits proliferation of gliomas (Lui et al., 2010; Yang et al., 2006). The induction of proliferative states via altering membrane potential (Sundelacruz et al., 2013b) offers an important addition to regenerative biologists seeking to induce growth after injury.
Biological processes such as regeneration require a careful balance between cell proliferation and the elimination of cells through programmed cell death (PCD). Thus, in addition to regulating cell division, induction of PCD must also be controlled. Although counterintuitive, research in diverse model systems revealed that dying cells can secrete mitogens that directly stimulate stem or progenitor cell proliferation (Chera et al., 2009; Fan and Bergmann, 2008a; Gauron et al., 2013; Huh et al., 2004; Li et al., 2010; Perez-Garijo et al., 2004; Sakurai et al., 2008). Coined the “phoenix rising pathway” by Li et al., wound healing, cell proliferation, and formation of blastema cells, are regulated in part by dying cells (Li et al., 2010). Since the original observations over forty years ago, the process of apoptotic-induced compensatory proliferation has been found to be an evolutionarily conserved process (Fogarty and Bergmann, 2017). Importantly, not only can dying cells induce a mitotic response in neighboring cells, but studies in Drosophila, Xenopus, Hydra, mice, and planaria have demonstrated a role for apoptotic cells in repair and regeneration (Chera et al., 2009; Fan and Bergmann, 2008a, b; Hwang et al., 2004; Tseng et al., 2007).
Irrespective of the specific route cells employ to undergo programmed cell death, typically extrinsic and intrinsic pathways lead to similar biological events including: a reduction in cell volume, caspase activation, nuclear condensation, DNA fragmentation, and apoptotic body formation. Regardless of the apoptotic stimuli, a reduction in cell volume is a universal feature of programmed cell death that is evolutionarily conserved among species ranging from worms to mammals (Bortner and Cidlowski, 1998, 2007; Lang et al., 2007). This cell shrinkage (termed apoptotic volume decrease [AVD]) results from a significant loss of intracellular potassium, sodium, and chloride ions that are essential for the activation of caspases and nucleases (Bortner and Cidlowski, 2007). Numerous studies have demonstrated prevention of AVD inhibits the execution of programmed cell death in most cell types (Bortner and Cidlowski, 2002, 2004; Heimlich et al., 2004; Yu and Choi, 2000). Hence, in order to create a permissive environment for the apoptotic machinery to function, the underlying flux of ions in dying cells must be carefully regulated. One of the regulators of apoptosis appears to be resting potential, even of distant cells, as has been shown in the patterning of the Xenopus brain (Pai et al., 2015a) and the regeneration of the planarian head (Beane et al., 2013).
In addition to cell migration, proliferation, and apoptosis, distinctive cell types must be present for functional tissues and organs to regenerate. Early studies using tissue from Rana pipiens embryos, demonstrated that ventral ectoderm explants differentiate into different cell types by modulating the ion content of the extracellular medium (Barth and Barth, 1974a; Barth and Barth, 1974b). More recently, studies demonstrated that the combination of BMP signaling and Ca2+-mediated electric activity regulates the appropriate differentiation of the spinal neurons during embryogenesis (Sundelacruz et al., 2009; Swapna and Borodinsky, 2012). Furthermore, bioelectric control of cell differentiation has recently been demonstrated in human cells, such as mesenchymal stem cells (Sundelacruz et al., 2008, 2013a). In addition to the role for bioelectric signaling during development, numerous studies have shown altering Vmem can not only direct the differentiation of tissues during embryogenesis, but can also direct the fate of a wide range of regeneration-relevant stem cells, including neural, hepatic, mesenchymal, and cancer (Biagiotti et al., 2006; Wang et al., 2005; Wenisch et al., 2006). Studies in fish have identified a role for, and transcriptional targets of, gap junction-mediated signaling in control of skeletal and joint elements in zebrafish fin regeneration (Banerji et al., 2016; Ton and Iovine, 2012, 2013).
Due to their ability to self-renew via mitotic divisions, combined with the role ion channels play during cell cycle progression, it is not surprising that multiple functional channel currents have been observed in stem cell populations (Li and Deng, 2011; Liebau et al., 2013; Moore, 2005). Another important characteristic of stem cells is their ability to differentiate into a diverse range of specialized cell types. Consequently, numerous studies have revealed a role for bioelectric signaling during the differentiation of precursor cells. For instance, not only do adipose-derived mesenchymal stem cells (AD-MSCs) express several voltage-gated ion channels (VGICs) channels (Bai et al., 2007), blocking the function of these channels inhibited the osteogenic differentiation of these stem cells (Zhang et al., 2016). Moreover, both differentiation and proliferation are controlled by changes in Vmem in numerous cell types including: human mesenchymal stem cells (HMSCs) (Sundelacruz et al., 2009; Sundelacruz et al., 2008), cardiomyocytes (Genovese et al., 2008; Lan et al., 2014a; Lan et al., 2014b; van Vliet et al., 2010), hepatocytes (Bautista et al., 2017), embryonic stem cells (Ng et al., 2010; Yamada et al., 2007), and neural stem cells (Aprea and Calegari, 2012; Lange et al., 2011). Combined, mounting evidence implicates transmembrane potential as a broadly-conserved mechanism used to direct the migration, proliferation, apoptosis, and differentiation of cells. Because of the development of new tools to manipulate membrane resting potential, Vmem control is an attractive target for manipulating cells in regenerative applications both in vitro and in vivo.
Tissue-level pre-patterns mediated by bioelectric control mechanisms
Although controlling an individual cell's behavior is important, in order to create a functional organ during development or replace a missing structure via regeneration, the behavior of groups of cells must be carefully regulated to produce properly patterned structures. Over the years, gradients of growth factors and other transcriptional activators have been implicated in mediating the differential gene activation observed during the formation of complex structures. However, gene regulatory networks must work in concert with physical sources of information to establish anatomical order on multiple scales, from organs to the entire body plan. While physical forces such as pressures and tensions (Mammoto and Ingber, 2010; Navis and Bagnat, 2015) are well-known to be important, it is becoming increasingly appreciated that spatio-temporal patterns of Vmem are also an important instructive feature of large-scale pattern regulation.
In the early 20th century, Yale University biologist Harold S. Burr and colleagues postulated that some of underlying patterning information that directed groups of cells stemmed from what he called electro-dynamic fields. Burr's early studies in 1930s and 1940s focused on carefully measuring and correlating voltage gradients with future developmental patterns in numerous organisms (Burr, 1944; Burr and Northrop, 1935b). He speculated these voltage gradients were not only quantitatively predictive of future morphology, but also contained important patterning information. Similar to the electro-dynamic fields described by Burr eighty years ago, researchers examining amphibian development discovered an increase in the resting potential in the developing neural plate, that is absent from adjacent ectodermal cells (Blackshaw and Warner, 1976). Inhibition of this increase in resting potential resulted in abnormal morphology, with craniofacial structures the most severely malformed (Messenger and Warner, 1979). These initial observations were further characterized by Shi and Borgens who measured the gradients of extracellular electric fields found in amphibian embryos and speculated that these gradients provided positional information to the developing animal (Shi and Borgens, 1995). Experiments by Vandenberg and colleagues (2011) in the frog embryo revealed the existence of a bioelectric pre-pattern in the developing face that predicted the prospective location of eyes and other craniofacial structures (Figure 2A). When these bioelectric pre-patterns were perturbed experimentally, the prospective boundaries of craniofacial gene expression, and the subsequent gross morphology of craniofacial structures, were altered (Vandenberg and Morrie, 2011). More recent work has revealed this “bioelectric face” pattern to underlie the misregulation of craniofacial patterning genes observed in human syndromes with facial dysmorphias (Adams et al., 2016). Similar bioelectric patterns, recently characterized in the Xenopus brain (Pai et al., 2015b), are responsible for the balance of proliferation and apoptosis needed to produce a brain that is size-matched to the surrounding tissues, an important aspect of regenerative response.
Figure 2. Bioelectric cues can specify pre-pattern information needed to create complex structures and direct the reprogramming of complete organs via non– cell-autonomous patterning signals.
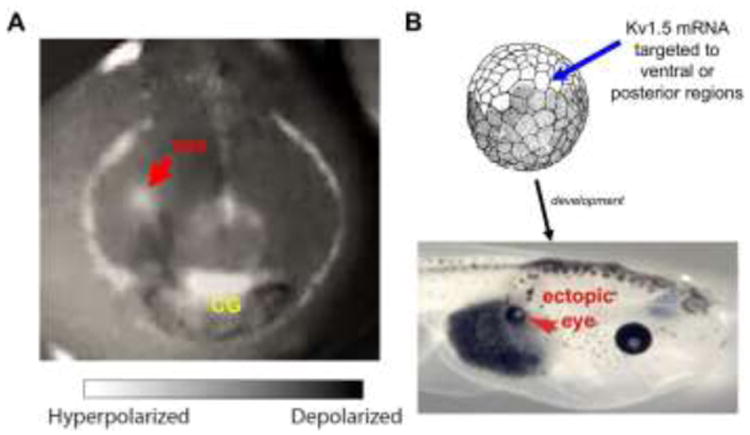
(A) Spatial distributions of resting potential gradients reveal the existence of complex prepatterns in vivo. Imaging with a voltage-sensitive fluorescent dye in the Xenopus nascent face reveals the borders of patterning compartments and organ locations prior to the induction of face-specific patterning transcripts. Anterior/face, red arrow denotes position of future right eye field. Modified with permission from (Vandenberg and Morrie, 2011). (B) Manipulation of these endogenous patterns by misexpression of ion channels can result in organ-level reprogramming. For example, targeted Vmem change, via misexpression of ion channels in the frog embryo, induces the formation of ectopic structures such as complete eyes, even in regions normally not competent to form eyes such as the gut (red arrow). Modified with permission from (Pai et al., 2012).
While there are numerous examples of the existence of endogenous bioelectric gradients across anatomical distances, in order to direct the creation of complex structures the ion-mediated information must be carefully regulated across populations of cells. In addition to receptor-mediated signal exchange, information can also be distributed via the direct cell-cell exchange of small molecules passing through gap junctions. This extremely flexible system for communication allows for the rapid synchronization amongst cells in a tissue that can be regulated at multiple levels. Gap junctions have been shown to both regulate a cell's ability to sense extracellular electric fields, but also organize cells into functional domains (Caveney, 1985; Cooper, 1984; Cooper et al., 1989; Levin, 2007a; Warner, 1985). Thus, gap junctional communication provides the ability to facilitate the rapid flow of information in a multicellular organism, or in some cases, to isolate groups of cells and thus create boundaries between compartments (Pitts et al., 1988; Rela and Szczupak, 2004; Sutor and Hagerty, 2005). Importantly, cells linked by gap junctions can react as a single unit to stimuli such as the weak electric fields commonly found in developing and regenerating tissues (Cooper, 1984). In addition, gap junctions not only shape electrical properties of populations of cells, but they themselves are sensitive to changes in pH and transmembrane potential. These characteristics provide a way for groups of cells to use both positive- and negative-feedback mechanisms to regulate level of activity and spatial organization of bioelectric properties within tissues in vivo.
Patterning and morphogenesis of tissues and organs: bioelectric inputs
Beyond the control of cell fate decisions, several studies demonstrated bioelectric cues can also alter the morphogenesis and position of whole organs, which can occur even when differentiation remains unperturbed. For example, recently research investigating the role of a cation channel during early embryogenesis demonstrated that altering channel function during embryogenesis affected the expression of important genes directing organ position and morphogenesis, resulting to mispositioned hearts with abnormal morphology, but genes directing the differentiation of the cardiac precursor cells such as, Xbra, nkx2.5, Cardiac troponin-T were unaltered (Pitcairn et al., 2017). A role for bioelectric signaling during large-scale patterning has also been observed in mammals, revealed by the numerous channelopathies that cause dysmorphic features. For example, human patients with Andersen-Tawil Syndrome that carry mutations in another inwardly rectifying potassium channel, Kir2.1, have limb and craniofacial abnormalities including, mispositioned ears, dental defects, fusion of digits (syndactyly), shortening of the digits (brachydactyly), and malformed or cleft palate (Plaster et al., 2001; Yoon et al., 2006). The Kir2.1 knockout mouse also has many of these malformed and mispositioned structures further supporting the channel function is needed for the normal development of these tissues and organs (Dahal et al., 2012; Zaritsky et al., 2000). Recently studies in Drosophila melanogaster examining the role of a potassium channel Kir2.1 homolog, Irk2, reported reduction of Irk2 ion channel resulted in the abnormal patterning of wings that was most likely caused by disrupting Dpp/BMP signaling (Dahal et al., 2012; Dahal et al., 2017).
The role of currents during appendage regeneration provides a more complex example of morphogenetic control of tissues and organs directed by bioelectric cues. Over half a century ago, Becker postulated that the bioelectric state of cells in regenerating organisms contained information that directed limb regeneration (Becker, 1961). Years later this theory was further probed by several investigators including Borgens and Lassalle who measured the bioelectric state in regenerating and non-regenerating organisms post limb amputation (Borgens, 1982, 1983; Lassalle, 1979, 1980). In both regenerating and non-regenerating animals examined immediately after injury, the wound surface was strongly negatively charged relative to surrounding tissue. Once the epithelium covered the wound, a switch to a positive charge was observed. However, only in regenerating organisms does the potential reverse a second time to return to a negative state. Since these currents persist over a prolonged period of time and can be detected weeks after the initial injury, it is unlikely that the resulting changes in electric fields are simply a byproduct of passive ion leaking from damaged cells. Importantly, functional experiments where these bioelectric gradients were altered via shunting, ion channel blockers, electrical isolation, or exogenous reversal of the gradient, inhibited regeneration and serve to provide further evidence that biophysical events are necessary components for regeneration (Borgens, 1982; Hotary and Robinson, 1992; Jenkins et al., 1996; Novak and Sironval, 1975). Combined, these experiments provided the groundwork that links bioelectric state during limb regeneration to critical information needed to direct normal anatomy of structures.
Going beyond the endogenous role for ion channel activity during regeneration, numerous studies have examined the sufficiency of bioelectric cues in inducing or augmenting regeneration (Sisken, 1992; Sisken et al., 1993). More recent experiments in the rodent limb have used marker analysis to confirm the augmentation of regenerative response induced via bioelectric stimulation (Leppik et al., 2015). To date, several labs have demonstrated the application of exogenous fields can induce the regeneration of appendages in less-regenerative organisms, including anuran amphibians (Borgens et al., 1977; Sharma and Niazi, 1990; Smith, 1967), chick (Sisken and Fowler, 1981), and even mammals (Becker, 1972; Sisken et al., 1984; Smith, 1981). For example, the use of ionophores to induce depolarization has been shown to trigger leg regeneration in post-metamorphic (non-regenerative) frogs (Tseng and Levin, 2013) (Figure 3A-B).
Figure 3. Large-scale bioelectric patterns are instructive for shape.
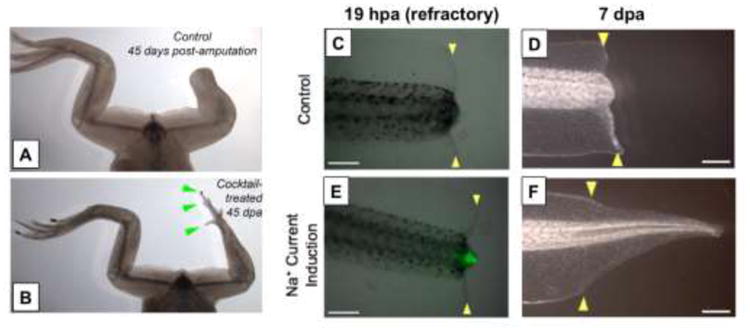
(A-B) Limb regeneration does not normally occur in post-metamorphic froglets. After only 24 hours of exposure, an ionophore cocktail designed to specifically alter the bioelectric state of the blastema triggers growth of an entire limb (green arrowheads indicate the appearance of distal elements such as toes and toenails). Used with permission from (Tseng and Levin, 2013). (C-D) During the refractory period in Xenopus, tail regeneration does not occur. (E-F) A one-hour exposure of the animal to an ionophore cocktail induces sodium influx into the bud, which triggers the regeneration of an entire new tail. This example illustrates how a simple signal can trigger a complex, self-limiting downstream morphogenetic cascade appropriate in orientation, scaling, and location within the host organism. Exploiting such endogenous “master-regulator” triggers may be a powerful strategy for regenerative medicine, to restore complex organs long before we have the knowledge to micromanage its creation from specific cell types. Yellow arrows indicate location of amputation. Abbreviations: hpa, hours post amputation; dpa, days post amputation. Modified with permission from (Tseng et al., 2010a).
The tadpole tail of the frog Xenopus laevis is able to regenerate a complete tail (including muscle, spinal cord, epidermis, vasculature, and notochord), making it an excellent model system for investigating tissue repair and regeneration and potential therapeutics at post-regenerative life stages (Tseng and Levin, 2008) (Figure 3C-F). Over the last decade researchers have identified multiple molecular mechanisms that regulate tail regeneration such as: Wnt-FGF, TGF-β, Notch, and BMP (Beck et al., 2003; Ho and Whitman, 2008; Lin and Slack, 2008; Slack et al., 2004). As with limb regeneration, in addition to the signaling molecules that mediate the regrowth of the amputated structure, tadpole tail regeneration is also regulated by biophysical factors. For example, the amount of current detected in amputated tadpole tails correlated with regenerative ability and the restoration of missing tissue was reduced manipulation when bioelectric cues were altered (Reid et al., 2009). Additionally, a combination of pharmacological and molecular-genetic approaches implicated the continuous pumping of H+ at wound site mediated by the V-ATPase hydrogen pump as an instructive factor mediating proliferation and pattern formation during tail regeneration (Adams et al., 2007a). Not only is this current required during normal regeneration, but during stages when tadpoles cannot regenerate ablated tail tissue, regeneration can also be artificially induced via expressing a yeast H+-ATPase (PMA-1) to manipulate H+ efflux (Adams et al., 2007a; Masuda and Montero-Lomeli, 2000). The expression and function of bioelectric machinery such as NaV1.2 channel revealed an important molecular marker that distinguishes true regeneration from wound healing. These initial observations were extended to demonstrate that a small molecule cocktail targeting sodium flux was sufficient to trigger complete tail regeneration even after a nonpermissive wound epithelium had formed (Tseng et al., 2010b) (Figure 3C-F). Furthermore, recent advances in optogenetics demonstrate this approach can be used to trigger regenerative response of a complex multi-tissue appendage (the Xenopus tail, including spinal cord) via light stimulation of appropriate bioelectric states (Adams et al., 2013).
Changes in membrane potential have also been implicated in eye development and patterning. For example, experiments conducted in X. laevis demonstrated that the cell fields that will eventually contribute to eye formation are demarcated by hyperpolarization long before any of the eye transcription factors are expressed. Altering the pattern of these hyperpolarized regions induced changes in eye-specific gene expression and defects in eye morphogenesis; more importantly, when other cells were artificially hyperpolarized in early Xenopus embryos, ectopic eye tissue was induced (Pai et al., 2012) (Figure 2B); this could even occur far from the anterior neural field (such as the gut), suggesting revision of standard views of competence restrictions in various tissues.
Axial patterning
Axis determination is a critical component of creating complex structures during development and regeneration. Seminal experiments conducted by Marsh and Beams showed that simply applying external electric fields to the regenerating planarian fragments caused specific reversals of normal anterior-posterior polarity (Marsh and Beams, 1947, 1952). In these experiments, when the anterior cut faced the negative cathode, regeneration proceeded normally. In sharp contrast, when the anterior cut was exposed to the positive anode, double-headed worms were created. This work drove the formulation of models of electrophoretic redistribution of morphogen signals (Lange and Steele, 1978). Further evidence that membrane voltage controls head-versus-tail identity during planarian regeneration was reported in more recent studies that took advantage of a chemical genetics approach to examine the effects of ion flux on axial patterning. In this study, researchers revealed H+/K+-ATPase-mediated depolarization of the regenerating worm blastema drives head formation, even at posterior-facing wounds (Beane et al., 2011) (Figure 4A). Subsequent work showed that modulation of bioelectric network connectivity can switch genetically wild-type planaria to a permanent 2-headed state (regenerating as bi-axial forms after repeated, additional cuts in pure water) (Oviedo et al., 2010). Recent evidence reveals that the endogenous body-wide bioelectric gradient is the determinant of head-tail identity in animals that can regenerate fragments with 2 heads despite the original worm's normal anatomy, histology, molecular marker expression, and stem cell distribution (Durant et al., 2017) (Figure 4B). Remarkably, planaria can be converted between 1-head (normal phenotype) and 2-head (mispatterned) forms by transient, brief modulations of a bioelectric circuit that dictates radically different body plans in future rounds of regeneration in plain water; this model system this provides one of the best examples of the instructive role of bioelectrics in setting, and executing, the pattern memory that guides large-scale regeneration.
Figure 4. Large-scale bioelectric patterns direct the morphology of regenerated structures.
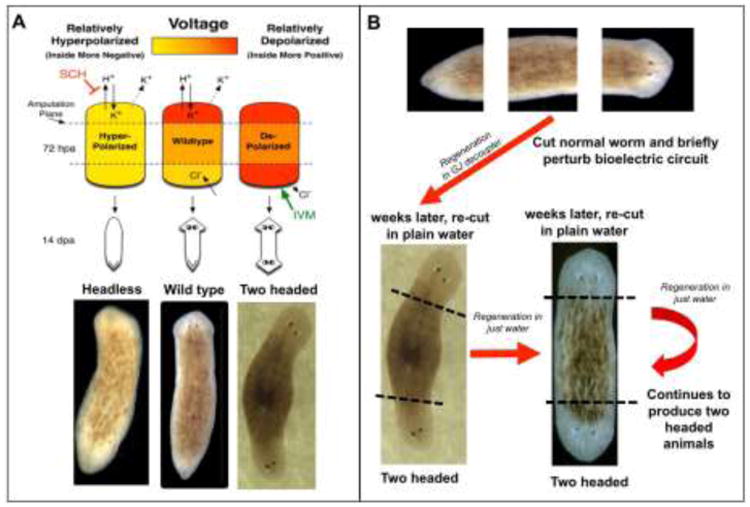
(A) Understanding of the bioelectric circuit that controls anterior–posterior specification in a fragment of regenerating planaria can be used to design drug cocktails that alter the regenerating anatomical structures produced by adult stem cells. Using this information, the desired target morphology can be created including inducing the posterior-facing blastema to build a secondary head in planaria. Modified with permission from (Beane et al., 2011). (B) Pattern memory encoded in bioelectric circuits can be altered by manipulating bioelectric cues. Planarian head-tail polarity is regulated in part by an endogenous voltage gradient. When cut fragments are briefly exposed to reagents to alter the topology of bioelectric cues (e.g., gap junction targeting drugs or RNAi targeting innexins), their regeneration results in the creation of two headed animals. Remarkably, weeks later, when these same animals are re-cut in plain water over multiple rounds of regeneration, the two-headed worm phenotype persists. Recent work (Durant et al., 2017) shows that these two-headed forms can be re-set back to a permanent one-head state by a manipulation of the H+/K+-ATPase component of the circuit, and reveals how epigenetic long-term pattern memory that can be stored in bioelectric circuits.
In addition to a role in anterior-posterior axis determination, bioelectric cues are also essential for patterning the left-right (L-R) axis in a wide range of organisms (Levin, 2005; Levin, 2012; Vandenberg and Levin, 2010, 2013). For example, in Xenopus the asymmetric localization of 4 electrogenic proteins, including the H+/K+-ATPase exchanger and V-ATPase H+ pump, results in differential L-R voltage and pH gradients that help establish normal L-R patterning during embryogenesis (Adams et al., 2006a; Aw et al., 2010; Levin et al., 2002). Although the timing of activity varies, the role of bioelectric cues in L-R axis establishment has also been observed in invertebrates such as the sea squirt and sea urchin as well as in other vertebrates including zebrafish, chick, mouse, and even human patients (Fukumoto et al., 2005; Hibino et al., 2006; Kawakami et al., 2005; Levin et al., 2002; Miyachi, 2017; Shimeld and Levin, 2006; Vandenberg and Levin, 2010).
While the details of how voltage gradients couple to early events are somewhat divergent among phyla with different body plans, work in amphibians has provided the most detailed picture of one evolutionary strategy. In Xenopus, the bioelectric gradients formed in early embryogenesis provide the motive force for a charged molecule, serotonin, to pass through gap junctions and become localized on the right side of the embryo where it functions as an electrophoretic morphogen (Fukumoto et al., 2005; Vandenberg et al., 2014; Zhang and Levin, 2009). Altering serotonin signaling or channel function results in the randomization of canonical L-R positioning proteins such as Nodal, Lefty, Pitx2 and situs ambiguous (heterotaxy). The L-R pathway provides a good illustration of the molecular mechanisms by which bioelectrics regulates global pattern, as all of the steps are known – from cytoskeletal machinery that regulates channel positioning (Lobikin et al., 2012), to the transduction of voltage gradient information from single cell chirality into body-wide asymmetric transcriptional cascades of canonical genes (McDowell et al., 2016a; McDowell et al., 2016b). The latter process is executed by lateralized epigenetic silencing of left-sided genes such as Nodal by electrophoretic movement of morphogens (Carneiro et al., 2011), revealing a tight integration of bioelectrics and biochemical signaling to link the dynamics of single cells to the creation of complex structures. Given the importance of epigenetic transcriptional control during regeneration in amphibia, fish, and planaria (Anderson et al., 2009a; Hamada et al., 2015; Robb and Sanchez Alvarado, 2014; Tseng et al., 2011a), it is likely that the interplay of bioelectrics and chromatin modification machinery will be increasingly characterized in regeneration, as it has been in development (Carneiro et al., 2011) and tumorigenesis (Chernet and Levin, 2014).
Ion flux and control of the size of structures
During the regeneration of missing structures, in addition to proper patterning, the ability to regulate the final size of newly created structures is equally important. This is accomplished via the coordination of the number of each cell type required to make a tissue with the final dimensions of a regenerating structure. In the late 1940s Moment conducted a systematic study of earthworm regeneration to understand the mechanisms utilized to control growth and cell division (Moment, 1947; Moment, 1949a, b). He presented evidence that electrical events were correlated with posterior regeneration and postulated regenerating worms generated a critical inhibitory voltage which functioned as a limiting factory to regulate growth (Moment, 1949a). A few years later Kurtz and Schrank conducted additional experiments that served to further confirm the initial findings demonstrating changes in voltage accompany cessation of regenerative growth in earthworms (Kurtz and Schrank, 1955). In zebrafish, a gain-of-function mutation in a potassium channel, K2P (encoded by the kcnk5b gene) resulted in allometric overgrowth of the fins (Perathoner et al., 2014), identifying and characterizing a new electrogenic control of appendage size.
Bioelectric cues in plants
The involvement of bioelectric signaling in regenerative processes is not just restricted to animals; it is also a mechanism used to direct cell behavior in plants. Some of the earliest studies in this field involved plants, addressing both the endogenous bioelectric correlates of plant morphogenesis (Burr and Sinnott, 1944; Rehm, 1938) and the effects of applied fields on plant growth (Berry et al., 1947; Rehm, 1939). Some of the pioneering studies by Lionel Jaffee and colleagues over forty years ago recognized that pollen tubules behave as electrical dipoles, with inward currents leaking the apical parts of the tube, and outward (positive) currents arising from the grain (Weisenseel and Jaffe, 1976; Weisenseel et al., 1975); this work gave rise to one of the first models of bioelectrical self-organization and polarity (Bentrup and Jaffe, 1968; Jaffe, 1968). Similar to pollen tubules, the outgrowth of root hairs occurs in a polar fashion and is also guided by an external electrical field (Michard et al., 2009b). The role of ion flux is not restricted to the normal growth of pollen tubules and root hairs but is also important for regenerative processes. For example, studies have shown that both alternating (Cogalniceanu et al., 1998) and continuous (Rathore and Goldsworthy, 1985) weak electric current can increase the proficiency of in vitro regeneration in tobacco tissue cultures. Molecular studies have revealed the details of physiological gradients driving pollen tube extension and patterning (Certal et al., 2008; Michard et al., 2009a; Michard et al., 2008; Robinson and Messerli, 2002), including bi-directional feedback between the activity of ion transporters and the distribution of small signaling molecules such as auxin and GABA (Goldsworthy and Rathore, 1985; Ramesh et al., 2015; Schrank, 1951). More recently, studies using examining Arabidopsis root regeneration in vivo, demonstrated that the regeneration process of the root tip apical meristem could be perturbed by a brief exposure of the stump to an external electric field (Kral et al., 2016). Thus, electric fields are capable of directing cellular processes in a wide spectrum of tissue types as well as across diverse taxa.
Molecular mechanisms
Although initial observations that bioelectric cues play a role in the formation and patterning of complex structures span decades of research, it is only more recently that molecular mechanisms responsible for sensing, interpreting, and translating weak bioelectric signaling into changes and gene expression have been elucidated. Not surprisingly, bioelectric cues are found both upstream and downstream of traditionally studied genetic and biochemical elements (Figure 1). Though still only examined in a small handful of contexts, the study of bioelectrics is revealing a robust conservation of mechanisms. For example, galvanotactic guidance of cell migration occurs in fungi (Gow and Morris, 1995) as it does in mammalian cells during wound healing (Nakajima et al., 2015) and retinal patterning (Yamashita, 2013). Bioelectric gradients establish polarity in single yeast cells (Haupt et al., 2014; Minc and Chang, 2010) as in whole metazoan axes (Aw and Levin, 2009). The same voltage regulators, like the plasma membrane V-ATPase proton pump, are involved broadly from stem cell regulation in eye patterning (Nuckels et al., 2009) to the determination of axial polarity in L-R asymmetry (Adams et al., 2006b) and the induction of tail regeneration (Adams et al., 2007b). Even the downstream targets of voltage change appear to be well-conserved from amphibia to man, as revealed by recent microarray studies comparing transcriptional responses to Vmem change in frog, axolotl regeneration, and human mesenchymal stem cells (Pai et al., 2016).
How do changes in the resting potential at the plasma membrane regulate downstream gene expression? To date many studies have described mechanisms for transduction of ion flows and voltage gradients into second-messenger cascades that mediate changes in gene expression including: activation of calcium influx via voltage gated calcium channels (Deisseroth et al., 2004; Sasaki et al., 2000), conformational changes in integrin signaling (Arcangeli and Becchetti, 2006; Olivotto et al., 1996), voltage regulated phosphatase activity (Murata et al., 2005; Okamura and Dixon, 2011), clustering of RAS proteins (Zhou et al., 2015), and regulation of small morphogens' movement in and out of cells via voltage-regulated transporters (Blackiston et al., 2011a; Fukumoto et al., 2005).
Since calcium is one of the most widely used second messengers in cell biology and participates in numerous cell functions, it is not surprising that Ca2+ influx (via voltage-sensitive Ca2+ channels) has been linked to the regulation of downstream events modulating pattern formation (Barbado et al., 2009; Calabrese, 2014). Of particular interest is calcium's ability to control gene function, providing a link between membrane potential and gene expression. Unlike many other second messengers, it cannot be metabolized; while present in relatively high concentrations in the extracellular space, its presence inside cells is tightly regulated. Thus, the ability to control intracellular Ca2+ concentration and location is critical for regulating a wide variety of cell functions. Not only can Ca2+ control rapid-signaling processes like electrical excitability and neurotransmitter release, but it can also control cellular events that occur more gradually. Ca2+ often recruits intracellular signaling pathways (e.g., calmodulin, calcineurin, calpains) that persist longer in cells than the Ca2+, to control cellular events that develop over a large time scale (Jaffe, 1995, 1999; Slusarski and Pelegri, 2007). Many studies have examined the role of voltage-dependent calcium channels in mediating gene expression in response to membrane depolarization. Some of the initial evidence that activity of voltage-gated calcium channels can direct gene regulation comes from studies demonstrating chronic depolarization of PC12 cells induced an increase in c-fos expression levels (Morgan and Curran, 1986). More recently, in experiments where Ca2+ signaling is inhibited via the addition of ryanodine, a reduction in the number of muscle progenitor cells and activated satellite cells was observed in the regenerating tadpole muscle tissue (Tu and Borodinsky, 2014). Another study examining the specification of commissural interneurons in the tadpole spinal cord, provided evidence for an interaction Ca2+-dependent electrical activity and of Smad-mediated BMP signaling (Swapna and Borodinsky, 2012).
Other Vmem-transducing processes include adhesive receptor signaling mechanisms (Arcangeli and Becchetti, 2006; Liu et al., 2005; Meyers et al., 2004; Nesti et al., 2002). In fact, research dating back to the early nineties provided evidence that integrin-mediated cell adhesion to the extracellular matrix is often coupled with ion activation via a physical association between integrin receptors and channel proteins. This interaction can subsequently regulate cell behavior such as: cell migration, proliferation, apoptosis and differentiation (Becchetti et al., 1992; Schwartz, 1993). A good example of this interaction is observed in PC12 cells whose shift to a neuronal phenotype is induced by the physical interaction of voltage-gated Ca2+ channels and neural cell adhesion molecules/N-cadherins (Doherty et al., 1991). Over the last several decades, numerous studies have demonstrated adhesive receptors (especially integrins) physically and functionally associated with several classes of ion channels (Arcangeli and Becchetti, 2006).
The recent discovery that voltage-sensitive phosphatases (VSPs) can hydrolyze phosphoinositides upon depolarization of membrane potential provides a novel mechanism of how electrical activity can directly alter biochemical signaling. VSPs contain two functional modules: a voltage sensitive domain (VSD) and a phosphatase enzyme region (Murata et al., 2005; Murata and Okamura, 2007). The voltage sensitive domain regulates the enzymatic activity of VSP to dephosphorylate target molecules (either phosphatidylinositol 3,4,5-trisphosphate or phosphatidylinositol 4,5-bisphosphate). Interestingly, the cytoplasmic region of VSP has considerable sequence homology to a tumor suppressor enzyme, PTEN (phosphatase and tensin homolog), suggesting VSP has enzyme activity similar to PTEN, a protein that regulates the activity of many genes via canonical mechanisms (Iwasaki et al., 2008). Thus, although additional research is needed better understand this class of proteins, VSPs constitute a novel mechanism for coupling intracellular gene regulatory networks to electrical activity at the plasma membrane. A comprehensive and elegant study implicated bioelectric control, mediated by PTEN machinery, of wound healing in the mammalian eye (Zhao et al., 2006).
Over the years, numerous studies have demonstrated the function of intracellular transporters of signaling molecules (e.g., serotonin) can be altered by changes in bioelectrical activity. This voltage-controlled movement of second messengers, such as neurotransmitters, facilities the binding of intracellular receptors and transcription of target genes. For example, in early frog embryos bioelectrical gradients drive serotonin movement through gap junction-connected cell paths (by simple electrophoresis) and by modulating the SERT voltage-gated transporter (Fukumoto et al., 2005; Fukumoto and Levin, 2003; Levin et al., 2006). A very similar scheme involving electrogenic regulation of serotonin movement is observed in determining the amount of innervation from tissue transplants (Blackiston et al., 2011b; Blackiston et al., 2017), a finding that could be leveraged for advances in regenerative medicine.
There are several lines of evidence that suggest the epigenetic regulation of chromatin state is critical for pattern formation and regeneration. For example, in contrast to young tadpole limbs, the froglet limb no longer expresses important patterning genes like Sonic Hedgehog (shh) and reduced ability regeneration missing structures (Yakushiji et al., 2007). Sequence analysis of the limb enhancer region in young and old animals revealed that tadpole limbs were hypomethylated whereas froglet limbs were hypermethylated (Yakushiji et al., 2007). In addition, in zebrafish mutants carrying a loss-of-function allele of Dnmt-1 (DNA methyltransferase 1) are less able to regenerate ablated pancreatic cells (Anderson et al., 2009b). Because butyrate is an inhibitor of HDAC1, the movement of butyrate through an ion-dependent transporter SLC5A8 can also trigger epigenetic responses leading to tumorigenesis and altered regenerative ability (Chernet and Levin, 2013; Chernet et al., 2014; Davie, 2003; Gupta et al., 2006; Tong et al., 2004; Tseng et al., 2011b; Tseng and Levin, 2012). Hence, changes in membrane voltage can result in the acetylation of chromatin and modified rates of transcription.
Recent transcriptomic and proteomic analyses of regeneration have revealed prominent bioelectric components within the regenerative response (Chang et al., 2017; Rabinowitz et al., 2017), and linked them functionally to other physiological signals such as reactive oxygen species (ROS) (Ferreira et al., 2016). Likewise, modern optical imaging of in vivo physiology is beginning to provide a comprehensive multi-dimensional picture of the biophysics of amphibian tail regeneration (Ozkucur et al., 2010).
Conclusions and next steps
Endogenous bioelectrical states serve as instructive signals in patterning at multiple levels of organization, from single cells to the whole body plan. Vmem gradients specify information such as: initiating modules for complex self-limiting organogenesis (Adams et al., 2007b; Pai et al., 2015b), setting axial polarity (Beane et al., 2011; Durant et al., 2017; Levin, 2006; Oviedo et al., 2010; Stern and MacKenzie, 1983), serving as prepatterns for the layout of large regions (Adams et al., 2016; Pai et al., 2015a; Vandenberg et al., 2011), and even determining the shape and size of structures (Emmons-Bell et al., 2015; Perathoner et al., 2014). Despite the progress that has been made thus far, the field still faces a number of major questions. These include a more in depth understanding of the mechanisms by which cells compare bioelectric state across distances, elucidation of how bioelectric cues interface with chemical gradients and physical forces, and development of quantitative models of bioelectric circuits that are able to store patterning information needed to create complex structures. The ability of bioelectric signaling to direct cell behavior has been described in the literature for over a century, yet only recently are we gaining sufficient insight about mechanisms and global dynamics to enable biomedicine to unlock this valuable information. It is crucial to point out that continued advances in the control of regenerative patterning will require not only increase reductive detail on subcellular molecular pathways, but also integrative work to understand how large-scale pattern is established (and how growth is limited once appropriate anatomy has been restored) by large-scale bioelectrical circuits. Moving forward, researchers need to extend our knowledge about gene regulatory networks and signaling cascades to include information generated at the level of bioelectricity.
Highlights.
Endogenous ion flows are important regulators of cell behavior.
Creating complex structures requires the integration of molecular inputs and biophysical cues.
Bioelectrical states play a key role in the robust restoration of normal pattern after injury.
Bioelectric cues serve as instructive signals in patterning at multiple levels of organization.
Acknowledgments
We appreciate the numerous helpful discussions with members of the McLaughlin, Levin, and Adams labs, as well as many others in the developmental bioelectricity field. We apologize to the many workers in this field whose work could not be discussed in detail due to length constraints. This paper is dedicated to R. O. Becker, a tireless investigator of bioelectricity in regeneration. We gratefully acknowledge support by an Allen Discovery Center award from the Paul G. Allen Frontiers Group (No. 12171), the G. Harold and Leila Y. Mathers Charitable Foundation (No. TFU141), the Templeton World Charity Foundation (No. TWCF0089/AB55), and the W. M. Keck Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams D, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007a;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Adams D, Robinson K, Fukumoto T, Yuan S, Albertson R, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006a;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007b;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006b;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Tseng AS, Levin M. Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo. Biology open. 2013;2:306–313. doi: 10.1242/bio.20133665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Uzel SG, Akagi J, Wlodkowic D, Andreeva V, Yelick PC, Devitt-Lee A, Pare JF, Levin M. Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J Physiol. 2016;594:3245–3270. doi: 10.1113/JP271930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JD. Galvanotaxis of slime mold. J Gen Physiol. 1951;35:1–16. doi: 10.1085/jgp.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Bosch JA, Goll MG, Hesselson D, Dong PD, Shin D, Chi NC, Shin CH, Schlegel A, Halpern M, Stainier DY. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev Biol. 2009a;334:213–223. doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Bosch JA, Goll MG, Hesselson D, Dong PDS, Shin DH, Chi NC, Shin CH, Schlegel A, Halpern M, Stainier DYR. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Developmental Biology. 2009b;334:213–223. doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprea J, Calegari F. Bioelectric state and cell cycle control of Mammalian neural stem cells. Stem Cells Int. 2012;2012:816049. doi: 10.1155/2012/816049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A, Becchetti A. Complex functional interaction between integrin receptors and ion channels. Trends in Cell Biology. 2006;16:631–639. doi: 10.1016/j.tcb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Arcangeli A, Carla M, Del Bene MR, Becchetti A, Wanke E, Olivotto M. Polar/apolar compounds induce leukemia cell differentiation by modulating cell-surface potential. Proc Natl Acad Sci U S A. 1993;90:5858–5862. doi: 10.1073/pnas.90.12.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster J, Pearson W, Nichols C, Shi NQ, Carneiro K, Levin M. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Developmental Biology. 2010;346:39–53. doi: 10.1016/j.ydbio.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development. 2009;136:355–366. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Ma J, Pan Z, Song YH, Freyberg S, Yan Y, Vykoukal D, Alt E. Electrophysiological properties of human adipose tissue-derived stem cells. Am J Physiol Cell Physiol. 2007;293:C1539–1550. doi: 10.1152/ajpcell.00089.2007. [DOI] [PubMed] [Google Scholar]
- Banerji R, Eble DM, Iovine MK, Skibbens RV. Esco2 Regulates cx43 Expression During Skeletal Regeneration in the Zebrafish Fin. Developmental Dynamics. 2016;245:7–21. doi: 10.1002/dvdy.24354. [DOI] [PubMed] [Google Scholar]
- Barbado M, Fablet K, Ronjat M, De Waard M. Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta. 2009;1793:1096–1104. doi: 10.1016/j.bbamcr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Barth LG, Barth LJ. Ionic regulation of embryonic induction and cell differentiation in Rana Pipiens. Developmental Biology. 1974a doi: 10.1016/s0012-1606(74)80004-7. [DOI] [PubMed] [Google Scholar]
- Barth LJ, Barth LG. Effect of the potassium ion on induction of notochord from gastrula ectoderm of Rana pipiens. Biol Bull. 1974b;146:313–325. doi: 10.2307/1540407. [DOI] [PubMed] [Google Scholar]
- Bautista W, Lipschitz J, McKay A, Minuk GY. Cancer Stem Cells are Depolarized Relative to Normal Stem Cells Derived from Human Livers. Ann Hepatol. 2017;16:297–303. doi: 10.5604/16652681.1231590. [DOI] [PubMed] [Google Scholar]
- Beane WS, Morokuma J, Adams DS, Levin M. A Chemical Genetics Approach Reveals H,K-ATPase-Mediated Membrane Voltage Is Required for Planarian Head Regeneration. Chem Biol. 2011;18:77–89. doi: 10.1016/j.chembiol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane WS, Morokuma J, Lemire JM, Levin M. Bioelectric signaling regulates head and organ size during planarian regeneration. Development. 2013;140:313–322. doi: 10.1242/dev.086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti A, Arcangeli A, Delbene MR, Olivotto M, Wanke E. Response to Fibronectin-Integrin Interaction in Leukemia-Cells - Delayed Enhancing of a K+ Current. P Roy Soc B-Biol Sci. 1992;248:235–240. doi: 10.1098/rspb.1992.0067. [DOI] [PubMed] [Google Scholar]
- Becchetti A, Crescioli S, Zanieri F, Petroni G, Mercatelli R, Coppola S, Gasparoli L, D'Amico M, Pillozzi S, Crociani O, Stefanini M, Fiore A, Carraresi L, Morello V, Manoli S, Brizzi MF, Ricci D, Rinaldi M, Masi A, Schmidt T, Quercioli F, Defilippi P, Arcangeli A. The conformational state of hERG1 channels determines integrin association, downstream signaling, and cancer progression. Sci Signal. 2017;10 doi: 10.1126/scisignal.aaf3236. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JM. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Becker RO. The Bioelectric Factors in Amphibian-Limb Regeneration. J Bone Joint Surg Am. 1961;43:643–656. [PubMed] [Google Scholar]
- Becker RO. Stimulation of partial limb regeneration in rats [9] Nature. 1972;235:109–111. doi: 10.1038/235109a0. [DOI] [PubMed] [Google Scholar]
- Bentrup FW, Jaffe LF. Analyzing the “group effect”: rheotropic responses of developing fucus eggs. Protoplasma. 1968;65:25–35. doi: 10.1007/BF01666369. [DOI] [PubMed] [Google Scholar]
- Berry LJ, Gardiner MS, Gilmartin RT. Preliminary studies of atypical growth in onion roots subjected to continuous applied electric currents of low intensities. Growth. 1947;11:155–175. [Google Scholar]
- Biagiotti T, D'Amico M, Marzi I, Di Gennaro P, Arcangeli A, Wanke E, Olivotto M. Cell renewing in neuroblastoma: electrophysiological and immunocytochemical characterization of stem cells and derivatives. Stem Cells. 2006;24:443–453. doi: 10.1634/stemcells.2004-0264. [DOI] [PubMed] [Google Scholar]
- Binggeli R, Weinstein RC. Membrane-Potentials and Sodium-Channels -Hypotheses for Growth-Regulation and Cancer Formation Based on Changes in Sodium-Channels and Gap-Junctions. Journal of Theoretical Biology. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Blackiston D, Adams D, Lemire J, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Disease Models & Mechanisms. 2011a;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Disease models & mechanisms. 2011b;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, Vien K, Levin M. Serotonergic stimulation induces nerve growth and promotes visual learning via posterior eye grafts in a vertebrate model of induced sensory plasticity. npj Regenerative Medicine. 2017;2:8. doi: 10.1038/s41536-017-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw SE, Warner AE. Alterations in resting membrane properties during neural plate stages of development of the nervous system. J Physiol. 1976;255:231–247. doi: 10.1113/jphysiol.1976.sp011277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB. What is the role of naturally produced electric current in vertebrate regeneration and healing. Int Rev Cytol. 1982;76:245–298. doi: 10.1016/s0074-7696(08)61793-3. [DOI] [PubMed] [Google Scholar]
- Borgens RB. The role of ionic current in the regeneration and development of the amphibian limb. Prog Clin Biol Res. 1983;110 Pt A:597–608. [PubMed] [Google Scholar]
- Borgens RB. The role of natural and applied electric fields in neuronal regeneration and development. Prog Clin Biol Res. 1986;210:239–250. [PubMed] [Google Scholar]
- Borgens RB. Electric fields in vertebrate repair : natural and applied voltages in vertebrate regeneration and healing. A.R. Liss; New York: 1989. [Google Scholar]
- Borgens RB, Vanable JW, Jr, Jaffe LF. Bioelectricity and regeneration. I. Initiation of frog limb regeneration by minute currents. Journal of Experimental Zoology. 1977;200:403–416. doi: 10.1002/jez.1402000310. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. A necessary role for cell shrinkage in apoptosis. Biochem Pharmacol. 1998;56:1549–1559. doi: 10.1016/s0006-2952(98)00225-1. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002;9:1307–1310. doi: 10.1038/sj.cdd.4401126. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Arch. 2004;448:313–318. doi: 10.1007/s00424-004-1266-5. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys. 2007;462:176–188. doi: 10.1016/j.abb.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr HS. The Meaning of Bio-Electric Potentials. The Yale journal of biology and medicine. 1944;16:353–360. [PMC free article] [PubMed] [Google Scholar]
- Burr HS, Northrop F. The electrodynamic theory of life. Quarterly Review of Biology. 1935a;10:322–333. [Google Scholar]
- Burr HS, Northrop FSC. The electro-dynamic theory of life. Q Rev Biol. 1935b;10:322–333. [Google Scholar]
- Burr HS, Sinnott EW. Electrical correlates of form in cucurbit fruits. American Journal of Botany. 1944;31:249–253. [Google Scholar]
- Calabrese RL. Channeling the Central Dogma. Neuron. 2014;82:725–727. doi: 10.1016/j.neuron.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, Yamoah E, Zhao M. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO reports. 2013;14:184–190. doi: 10.1038/embor.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, Diaz E, Kortagere S, Lemire JM, Levin M. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol. 2011;11:29. doi: 10.1186/1471-213X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caveney S. The Role of Gap-Junctions in Development. Annual review of physiology. 1985;47:319–335. doi: 10.1146/annurev.ph.47.030185.001535. [DOI] [PubMed] [Google Scholar]
- Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguez-Leon J, Wu HM, Cheung AY, Feijo JA. Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell. 2008;20:614–634. doi: 10.1105/tpc.106.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Baker J, Wills A. Transcriptional dynamics of tail regeneration in Xenopus tropicalis. Genesis. 2017 doi: 10.1002/dvg.23015. [DOI] [PubMed] [Google Scholar]
- Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, Martinou JC, Galliot B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Chernet B, Levin M. Endogenous Voltage Potentials and the Microenvironment: Bioelectric Signals that Reveal, Induce and Normalize Cancer. Journal of Clinical and Experimental Oncology. 2013;(1):S1–002. doi: 10.4172/2324-9110.S1-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet BT, Fields C, Levin M. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front Physiol. 2014;5:519. doi: 10.3389/fphys.2014.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet BT, Levin M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget. 2014;5:3287–3306. doi: 10.18632/oncotarget.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, McBain CJ, Gallo V. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G(1)/S phase progression of the cell cycle. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute JP. Stem cell homing. Curr Opin Hematol. 2006;13:399–406. doi: 10.1097/01.moh.0000245698.62511.3d. [DOI] [PubMed] [Google Scholar]
- Cogalniceanu G, Radu M, Fologea D, Moisoi N, Brezeanu A. Stimulation of tobacco shoot regeneration by alternating weak electric field. Bioelectrochemistry and Bioenergetics. 1998;44:257–260. [Google Scholar]
- Cone CD., Jr The role of the surface electrical transmembrane potential in normal and malignant mitogenesis. Ann N Y Acad Sci. 1974;238:420–435. doi: 10.1111/j.1749-6632.1974.tb26808.x. [DOI] [PubMed] [Google Scholar]
- Cooper MS. Gap-Junctions Increase the Sensitivity of Tissue-Cells to Exogenous Electric-Fields. Journal of Theoretical Biology. 1984;111:123–130. doi: 10.1016/s0022-5193(84)80200-3. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Miller JP, Fraser SE. Electrophoretic Repatterning of Charged Cytoplasmic Molecules within Tissues Coupled by Gap-Junctions by Externally Applied Electric-Fields. Developmental Biology. 1989;132:179–188. doi: 10.1016/0012-1606(89)90216-9. [DOI] [PubMed] [Google Scholar]
- Dahal GJ, Gassaway B, Kwok B, Tong Y, Ptáček LJ, Bates E. An inwardly rectifying K+ channel is required for patterning. Development. 2012;139:3653–3664. doi: 10.1242/dev.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal GR, Pradhan SJ, Bates EA. Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development. 2017;144:2771–2783. doi: 10.1242/dev.146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485s–2493s. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Deng XL, Lau CP, Lai K, Cheung KF, Lau GK, Li GR. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif. 2007;40:656–670. doi: 10.1111/j.1365-2184.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Ashton SV, Moore SE, Walsh FS. Morphoregulatory Activities of Ncam and N-Cadherin Can Be Accounted for by G Protein-Dependent Activation of L-Type and N-Type Neuronal Ca2+ Channels. Cell. 1991;67:21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- Durant F, Morokuma J, Fields C, Williams K, Adams DS, Levin M. Long-Term, Stochastic Editing of Regenerative Anatomy via Targeting Endogenous Bioelectric Gradients. Biophys J. 2017;112:2231–2243. doi: 10.1016/j.bpj.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons-Bell M, Durant F, Hammelman J, Bessonov N, Volpert V, Morokuma J, Pinet K, Adams DS, Pietak A, Lobo D, Levin M. Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms. International Journal of Molecular Sciences. 2015;16:27865–27896. doi: 10.3390/ijms161126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation The Cell is dead Long live the Cell! Trends in Cell Biology. 2008a;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008b;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F, Luxardi G, Reid B, Zhao M. Early bioelectric activities mediate redox-modulated regeneration. Development. 2016 doi: 10.1242/dev.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzharris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development. 2006;133:591–599. doi: 10.1242/dev.02246. [DOI] [PubMed] [Google Scholar]
- Fogarty CE, Bergmann A. Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017 doi: 10.1038/cdd.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyuturk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema I, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Current Biology. 2005;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Levin M. Serotonin is a novel very early signaling mechanism in left-right asymmetry. Developmental Biology. 2003;259:490–490. [Google Scholar]
- Funk RH. Endogenous electric fields as guiding cue for cell migration. Front Physiol. 2015;6:143. doi: 10.3389/fphys.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vriz S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep-Uk. 2013:3. doi: 10.1038/srep02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese JA, Spadaccio C, Langer J, Habe J, Jackson J, Patel AN. Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts. Biochem Biophys Res Commun. 2008;370:450–455. doi: 10.1016/j.bbrc.2008.03.115. [DOI] [PubMed] [Google Scholar]
- Goldsworthy A, Rathore KS. The Electrical Control of Growth in Plant-Tissue Cultures - the Polar Transport of Auxin. J Exp Bot. 1985;36:1134–1141. [Google Scholar]
- Gow NA, Morris BM. The electric fungus. Botanical Journal of Scotland. 1995;47:263–277. [Google Scholar]
- Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Bando T, Nakamura T, Ishimaru Y, Mito T, Noji S, Tomioka K, Ohuchi H. Leg regeneration is epigenetically regulated by histone H3K27 methylation in the cricket Gryllus bimaculatus. Development. 2015;142:2916–2927. doi: 10.1242/dev.122598. [DOI] [PubMed] [Google Scholar]
- Haupt A, Campetelli A, Bonazzi D, Piel M, Chang F, Minc N. Electrochemical regulation of budding yeast polarity. PLoS biology. 2014;12:e1002029. doi: 10.1371/journal.pbio.1002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimlich G, Bortner CD, Cidlowski JA. Apoptosis and cell volume regulation: the importance of ions and ion channels. Adv Exp Med Biol. 2004;559:189–203. [PubMed] [Google Scholar]
- Hibino T, Ishii Y, Levin M, Nishino A. Ion flow regulates left–right asymmetry in sea urchin development. Development Genes and Evolution. 2006;216:265–276. doi: 10.1007/s00427-005-0051-6. [DOI] [PubMed] [Google Scholar]
- Ho DM, Whitman M. TGF-beta signaling is required for multiple processes during Xenopus tail regeneration. Dev Biol. 2008;315:203–216. doi: 10.1016/j.ydbio.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Hwang JS, Kobayashi C, Agata K, Ikeo K, Gojobori T. Detection of apoptosis during planarian regeneration by the expression of apoptosis-related genes and TUNEL assay. Gene. 2004;333:15–25. doi: 10.1016/j.gene.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Hyman LH, Bellamy AW. Studies on the correlation between metabolic gradients electrical gradients and galvanotaxis I. Biol Bull-Us. 1922;43:313–347. [Google Scholar]
- Iwasaki H, Murata Y, Kim YJ, Hossain MI, Worby CA, Dixon JE, McCormack T, Sasaki T, Okamura Y. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7970–7975. doi: 10.1073/pnas.0803936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita M, Watanabe M, Ishii M, Chen T, Johnson SL. Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7. 1 mutation: implications for the regulation of melanosome movement. PLOS Genetics. 2006;2:e197. doi: 10.1371/journal.pgen.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LF. Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. The Role of Ion Currents in Establishing Developmental Gradients. Eur J Cell Biol. 1980;22:454–454. [Google Scholar]
- Jaffe LF. The Role of Ionic Currents in Establishing Developmental Pattern. Philos T Roy Soc B. 1981;295:553–566. doi: 10.1098/rstb.1981.0160. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. Developmental order, its origin and regulation. A.R. Liss; New York: 1982. Developmental currents, voltages and gradients. [Google Scholar]
- Jaffe LF. Calcium Waves and Development. Ciba F Symp. 1995;188:4–12. doi: 10.1002/9780470514696.ch2. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. Organization of early development by calcium patterns. Bioessays. 1999;21:657–667. doi: 10.1002/(SICI)1521-1878(199908)21:8<657::AID-BIES5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Robinson KR, Picologlou BF. Letter: A uniform current model of the endogenous current in an egg. J Theor Biol. 1974;45:593–595. doi: 10.1016/0022-5193(74)90134-9. [DOI] [PubMed] [Google Scholar]
- Jenkins LS, Duerstock BS, Borgens RB. Reduction of the Current of Injury Leaving the Amputation Inhibits Limb Regeneration in the Red Spotted Newt. Developmental Biology. 1996;178:251–262. doi: 10.1006/dbio.1996.0216. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Raya Á, Raya RM, Rodríguez-Esteban C. Retinoic acid signalling links left–right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature. 2005;435:165–171. doi: 10.1038/nature03512. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Schlichter LC. A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10680–10693. doi: 10.1523/JNEUROSCI.19-24-10680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral N, Ougolnikova AH, Sena G. Externally imposed electric field enhances plant root tip regeneration. Regeneration. 2016;3:156–167. doi: 10.1002/reg2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz I, Schrank AR. Bioelectrical Properties of Intact and Regenerating Earthworms, Eisenia foetida. Physiological Zoology. 1955;28:322–330. [Google Scholar]
- Lan JY, Williams C, Levin M, Black L., III Depolarization of Cellular Resting Membrane Potential Promotes Neonatal Cardiomyocyte Proliferation In Vitro. Cel Mol Bioeng. 2014a:1–14. doi: 10.1007/s12195-014-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan JY, Williams C, Levin M, Black LD., 3rd Depolarization of Cellular Resting Membrane Potential Promotes Neonatal Cardiomyocyte Proliferation In Vitro. Cell Mol Bioeng. 2014b;7:432–445. doi: 10.1007/s12195-014-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Foller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007;428:209–225. doi: 10.1016/S0076-6879(07)28011-5. [DOI] [PubMed] [Google Scholar]
- Lange C, Prenninger S, Knuckles P, Taylor V, Levin M, Calegari F. The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex. Stem Cells Dev. 2011;20:843–850. doi: 10.1089/scd.2010.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CS, Steele VE. The mechanism of anterior-posterior polarity control in planarians. Differentiation. 1978;11:1–12. doi: 10.1111/j.1432-0436.1978.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Lassalle B. Surface-Potentials and the Control of Amphibian Limb Regeneration. J Embryol Exp Morph. 1979;53:213–223. [PubMed] [Google Scholar]
- Lassalle B. Are Surface-Potentials Necessary for Amphibian Limb Regeneration. Developmental Biology. 1980;75:460–466. doi: 10.1016/0012-1606(80)90177-3. [DOI] [PubMed] [Google Scholar]
- Leppik LP, Froemel D, Slavici A, Ovadia ZN, Hudak L, Henrich D, Marzi I, Barker JH. Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci Rep. 2015;5:18353. doi: 10.1038/srep18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mechanisms of Development. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Levin M. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- Levin M. Gap junctional communication in morphogenesis. Prog Biophys Mol Biol. 2007a;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends in Cell Biology. 2007b;17:261–270. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. BioEssays. 2012;34:205–217. doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. The Journal of Physiology. 2014;592:2295–2305. doi: 10.1113/jphysiol.2014.271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: Left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci-Basel. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Levin M, Pezzulo G, Finkelstein JM. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu Rev Biomed Eng. 2017;19:353–387. doi: 10.1146/annurev-bioeng-071114-040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson K, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Li F, Huang Q, Chen J, Peng YL, Roop DR, Bedford JS, Li CY. Apoptotic Cells Activate the “Phoenix Rising” Pathway to Promote Wound Healing and Tissue Regeneration. Science Signaling. 2010;3 doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GR, Deng XL. Functional ion channels in stem cells. World J Stem Cells. 2011;3:19–24. doi: 10.4252/wjsc.v3.i3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebau S, Kleger A, Levin M, Yu SP. Stem cells and ion channels. Stem Cells Int. 2013;2013:238635. doi: 10.1155/2013/238635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Slack JM. Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Dev Biol. 2008;316:323–335. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Litan A, Langhans SA. Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015;9:86. doi: 10.3389/fncel.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A, Saltiel AR. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2005;2:165–177. doi: 10.1016/j.cmet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Lobikin M, Wang G, Xu J, Hsieh YW, Chuang CF, Lemire JM, Levin M. Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12586–12591. doi: 10.1073/pnas.1202659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui VCH, Lung SSS, Pu JKS, Hung KN, Leung GKK. Invasion of Human Glioma Cells Is Regulated by Multiple Chloride Channels Including ClC-3. Anticancer Res. 2010;30:4515–4524. [PubMed] [Google Scholar]
- Lund E. Bioelectric fields and growth. Univ of Texas Press; Austin: 1947. [Google Scholar]
- MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh G, Beams HW. Electrical control of growth polarity in regenerating Dugesia tigrina. Fed Proc. 1947;6:163. [PubMed] [Google Scholar]
- Marsh G, Beams HW. Electrical control of morphogenesis in regenerating Dugesia tigrina I. Relation of axial polarity to field strength. J Cell Comp Physiol. 1952;39:191–213. doi: 10.1002/jcp.1030390203. [DOI] [PubMed] [Google Scholar]
- Masuda CA, Montero-Lomeli M. An NH2-terminal deleted plasma membrane H+-ATPase is a dominant negative mutant and is sequestered in endoplasmic reticulum derived structures. Biochem Cell Biol. 2000;78:51–58. [PubMed] [Google Scholar]
- Mathews AP. ELECTRICAL POLARITY IN THE HYDROIDS. American Journal of Physiology -- Legacy Content. 1903;8:294–299. [Google Scholar]
- Mathews J, Levin M. Gap junctional signaling in pattern regulation: Physiological network connectivity instructs growth and form. Developmental neurobiology. 2017;77:643–673. doi: 10.1002/dneu.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusik PT. J Electrocardiol. 2017. Insights into channelopathies: progress in clinical practice and research. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McDowell G, Rajadurai S, Levin M. From cytoskeletal dynamics to organ asymmetry: a nonlinear, regulative pathway underlies left-right patterning. Philos Trans R Soc Lond B Biol Sci. 2016a doi: 10.1098/rstb.2015.0409. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell GS, Lemire JM, Pare JF, Cammarata G, Lowery LA, Levin M. Conserved roles for cytoskeletal components in determining laterality. Integr Biol (Camb) 2016b;8:267–286. doi: 10.1039/c5ib00281h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger EA, Warner AE. The function of the sodium pump during differentiation of amphibian embryonic neurones. J Physiol. 1979;292:85–105. doi: 10.1113/jphysiol.1979.sp012840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers VE, Zayzafoon M, Gonda SR, Gathings WE, McDonald JM. Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. Journal of Cellular Biochemistry. 2004;93:697–707. doi: 10.1002/jcb.20229. [DOI] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijo JA. The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int J Dev Biol. 2009a;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijo JA. The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. International Journal of Developmental Biology. 2009b;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- Michard E, Dias P, Feijó JA. Tobacco pollen tubes as cellular models for ion dynamics: improved spatial and temporal resolution of extracellular flux and free cytosolic concentration of calcium and protons using pHluorin and YC3.1 CaMeleon. Sex Plant Reprod. 2008;21:169–181. [Google Scholar]
- Minc N, Chang F. Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol. 2010;20:710–716. doi: 10.1016/j.cub.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi Y. H+/K+-ATPase-Inhibition Causes Left-Right Aortic Arch Inversion in Mouse Development. Reprod Sci. 2017:1933719116687654. doi: 10.1177/1933719116687654. [DOI] [PubMed] [Google Scholar]
- Moment G. On the Anatomical Basis of Growth Limitation in the Earthworm, Eisenia Foetida. Anat Rec. 1947;99:609–609. [PubMed] [Google Scholar]
- Moment GB. On the Relation between Growth in Length, the Formation of New Segments, and Electric Potential in an Earthworm. Journal of Experimental Zoology. 1949a;112:1–12. doi: 10.1002/jez.1401120102. [DOI] [PubMed] [Google Scholar]
- Moment GB. Segment Frequencies in Anterior Regeneration in the Earthworm, Eisenia Foetida. Journal of Experimental Zoology. 1949b;111:449–456. doi: 10.1002/jez.1401110307. [DOI] [PubMed] [Google Scholar]
- Moore P. Cell biology: ion channels and stem cells. Nature. 2005;438:699–704. doi: 10.1038/438699a. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Role of Ion Flux in the Control of C-Fos Expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Adams D, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol. 2007;583:875–889. doi: 10.1113/jphysiol.2007.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycielska ME, Djamgoz MB. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci. 2004;117:1631–1639. doi: 10.1242/jcs.01125. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Zhu K, Sun YH, Hegyi B, Zeng Q, Murphy CJ, Small JV, Chen-Izu Y, Izumiya Y, Penninger JM, Zhao M. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nature Communications. 2015;6:8532. doi: 10.1038/ncomms9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navis A, Bagnat M. Developing pressures: fluid forces driving morphogenesis. Curr Opin Genet Dev. 2015;32:24–30. doi: 10.1016/j.gde.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesti LJ, Caterson EJ, Wang M, Chang R, Chapovsky F, Hoek JB, Tuan RS. TGF-beta1 calcium signaling increases alpha5 integrin expression in osteoblasts. J Orthop Res. 2002;20:1042–1049. doi: 10.1016/S0736-0266(02)00020-7. [DOI] [PubMed] [Google Scholar]
- Ng SY, Chin CH, Lau YT, Luo J, Wong CK, Bian ZX, Tsang SY. Role of voltage-gated potassium channels in the fate determination of embryonic stem cells. J Cell Physiol. 2010;224:165–177. doi: 10.1002/jcp.22113. [DOI] [PubMed] [Google Scholar]
- Novak B, Sironval C. Inhibition of regeneration of Acetabularia mediterranea enucleated posterior stalk segments by electrical isolation. Plant Science Letters. 1975;5:183–188. [Google Scholar]
- Nuccitelli R, Jaffe LF. Spontaneous current pulses through developing fucoid eggs. Proc Natl Acad Sci U S A. 1974;71:4855–4859. doi: 10.1073/pnas.71.12.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R, Jaffe LF. The ionic components of the current pulses generated by developing fucoid eggs. Dev Biol. 1976;49:518–531. doi: 10.1016/0012-1606(76)90193-7. [DOI] [PubMed] [Google Scholar]
- Nuckels RJ, Ng A, Darland T, Gross JM. The vacuolar-ATPase complex regulates retinoblast proliferation and survival, photoreceptor morphogenesis, and pigmentation in the zebrafish eye. Invest Ophthalmol Vis Sci. 2009;50:893–905. doi: 10.1167/iovs.08-2743. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Dixon JE. Voltage-sensing phosphatase: its molecular relationship with PTEN. Physiology (Bethesda) 2011;26:6–13. doi: 10.1152/physiol.00035.2010. [DOI] [PubMed] [Google Scholar]
- Olivotto M, Arcangeli A, Carla M, Wanke E. Electric fields at the plasma membrane level: A neglected element in the mechanisms of cell signalling. Bioessays. 1996;18:495–504. doi: 10.1002/bies.950180612. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Schade S, Lyons SA, Amaral MD, Sontheimer H. Expression of voltage-gated chloride channels in human glioma cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5572–5582. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Ahidouch A. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol. 2008;221:1–6. doi: 10.1007/s00232-007-9080-6. [DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Ahidouch A. K(+) channels and cell cycle progression in tumor cells. Front Physiol. 2013;4:220. doi: 10.3389/fphys.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Ahidouch A, Pardo LA. Kv10.1 K(+) channel: from physiology to cancer. Pflugers Arch. 2016;468:751–762. doi: 10.1007/s00424-015-1784-3. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, Levin M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339:188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkucur N, Epperlein HH, Funk RHW. Ion Imaging During Axolotl Tail Regeneration In Vivo. Developmental Dynamics. 2010;239:2048–2057. doi: 10.1002/dvdy.22323. [DOI] [PubMed] [Google Scholar]
- Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139:313–323. doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Lemire JM, Chen Y, Lin G, Levin M. Local and long-range endogenous resting potential gradients antagonistically regulate apoptosis and proliferation in the embryonic CNS. The International Journal of Developmental Biology. 2015a;59:327–340. doi: 10.1387/ijdb.150197ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Lemire JM, Pare JF, Lin G, Chen Y, Levin M. Endogenous Gradients of Resting Potential Instructively Pattern Embryonic Neural Tissue via Notch Signaling and Regulation of Proliferation. The Journal of Neuroscience. 2015b;35:4366–4385. doi: 10.1523/JNEUROSCI.1877-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Martyniuk CJ, Echeverri K, Sundelacruz S, Kaplan DL, Levin M. Genome-wide analysis reveals conserved transcriptional responses downstream of resting potential change in Xenopus embryos, axolotl regeneration, and human mesenchymal cell differentiation. Regeneration (Oxf) 2016;3:3–25. doi: 10.1002/reg2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perathoner S, Daane JM, Henrion U, Seebohm G, Higdon CW, Johnson SL, Nusslein-Volhard C, Harris MP. Bioelectric signaling regulates size in zebrafish fins. PLoS genetics. 2014;10:e1004080. doi: 10.1371/journal.pgen.1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- Persson PB, Bondke Persson A. Channels and channelopathies. Acta Physiol (Oxf) 2016;218:149–151. doi: 10.1111/apha.12796. [DOI] [PubMed] [Google Scholar]
- Pitcairn E, Harris H, Epiney J, Pai VP, Lemire JM, Ye B, Shi NQ, Levin M, McLaughlin KA. Coordinating heart morphogenesis: A novel role for Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels during cardiogenesis in Xenopus laevis. Communicative & Integrative Biology. 2017;10:e1309488. doi: 10.1080/19420889.2017.1309488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcairn E, McLaughlin KA. Bioelectric signaling coordinates patterning decisions during embryogenesis. Trends in Developmental Biology. 2016;9:1–9. [Google Scholar]
- Pitts JD, Finbow ME, Kam E. Junctional communication and cellular differentiation. Br J Cancer. 1988;(9):52–57. [PMC free article] [PubMed] [Google Scholar]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Pollak J, Rai KG, Funk CC, Arora S, Lee E, Zhu J, Price ND, Paddison PJ, Ramirez JM, Rostomily RC. Ion channel expression patterns in glioblastoma stem cells with functional and therapeutic implications for malignancy. PLoS One. 2017;12:e0172884. doi: 10.1371/journal.pone.0172884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci. 2005;118:2023–2034. doi: 10.1242/jcs.02330. [DOI] [PubMed] [Google Scholar]
- Putney LK, Barber DL. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem. 2003;278:44645–44649. doi: 10.1074/jbc.M308099200. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JS, Robitaille AM, Wang Y, Ray CA, Thummel R, Gu H, Djukovic D, Raftery D, Berndt JD, Moon RT. Transcriptomic, proteomic, and metabolomic landscape of positional memory in the caudal fin of zebrafish. Proc Natl Acad Sci U S A. 2017;114:E717–E726. doi: 10.1073/pnas.1620755114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, Feijo JA, Ryan PR, Gilliham M. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun. 2015;6:7879. doi: 10.1038/ncomms8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Perez-Neut M, Kaja S, Gentile S. Voltage-gated ion channels in cancer cell proliferation. Cancers (Basel) 2015;7:849–875. doi: 10.3390/cancers7020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore KS, Goldsworthy A. Electrical Control of Shoot Regeneration in Plant-Tissue Cultures. Bio-Technol. 1985;3:1107–1109. [Google Scholar]
- Rehm WS. Bud regeneration and electric polarities in Phaseolus multiflorus. Plant Physiol. 1938;13:81–101. doi: 10.1104/pp.13.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm WS. Electrical responses of Phaseolus multiflorus to electrical currents. 14 Plant physiology. 1939;14:359–363. doi: 10.1104/pp.14.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. FASEB J. 2005;19:379–386. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Song B, Zhao M. Electric currents in Xenopus tadpole tail regeneration. Dev Biol. 2009;335:198–207. doi: 10.1016/j.ydbio.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Rela L, Szczupak L. Gap junctions: their importance for the dynamics of neural circuits. Mol Neurobiol. 2004;30:341–357. doi: 10.1385/MN:30:3:341. [DOI] [PubMed] [Google Scholar]
- Renaudo A, L'Hoste S, Guizouarn H, Borgese F, Soriani O. Cancer cell cycle modulated by a functional coupling between sigma-1 receptors and Cl- channels. J Biol Chem. 2007;282:2259–2267. doi: 10.1074/jbc.M607915200. [DOI] [PubMed] [Google Scholar]
- Robb SM, Sanchez Alvarado A. Histone modifications and regeneration in the planarian Schmidtea mediterranea. Current topics in developmental biology. 2014;108:71–93. doi: 10.1016/B978-0-12-391498-9.00004-8. [DOI] [PubMed] [Google Scholar]
- Robinson KR. Endogenous electrical current leaves the limb and prelimb region of the Xenopus embryo. Dev Biol. 1983;97:203–211. doi: 10.1016/0012-1606(83)90077-5. [DOI] [PubMed] [Google Scholar]
- Robinson KR, Cormie P. Electric field effects on human spinal injury: Is there a basis in the in vitro studies? Dev Neurobiol. 2008;68:274–280. doi: 10.1002/dneu.20570. [DOI] [PubMed] [Google Scholar]
- Robinson KR, Messerli MA. Pulsating ion fluxes and growth at the pollen tube tip. Sci STKE. 2002;2002:PE51. doi: 10.1126/stke.2002.162.pe51. [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B, Gerard V, Dubois JM. Involvement of K+ channels in the quercetin-induced inhibition of neuroblastoma cell growth. Pflugers Arch. 1993;423:202–205. doi: 10.1007/BF00374395. [DOI] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo E, Baguna J. Cell movement in intact and regenerating planarians. Quantitation using chromosomal, nuclear and cytoplasmic markers. J Embryol Exp Morphol. 1985;89:57–70. [PubMed] [Google Scholar]
- Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn SY, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8617–8622. doi: 10.1073/pnas.97.15.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank AR. Electrical polarity and auxins. In: Skoog F, editor. Plant Growth Substances. University of Wisconsin Press; Madison: 1951. pp. 123–140. [Google Scholar]
- Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol. 2001;280:F739–747. doi: 10.1152/ajprenal.2001.280.5.F739. [DOI] [PubMed] [Google Scholar]
- Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- Schwab A, Stock C. Ion channels and transporters in tumour cell migration and invasion. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130102. doi: 10.1098/rstb.2013.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Spreading of Human Endothelial-Cells on Fibronectin or Vitronectin Triggers Elevation of Intracellular Free Calcium. Journal of Cell Biology. 1993;120:1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Niazi IA. Restoration of limb regeneration ability in frog tadpoles by electrical stimulation. Indian Journal of Experimental Biology. 1990;28:733–738. [PubMed] [Google Scholar]
- Shi R, Borgens RB. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Developmental Dynamics. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Levin M. Evidence for the regulation of left-right asymmetry in Ciona intestinalis by ion flux. Developmental Dynamics. 2006;235:1543–1553. doi: 10.1002/dvdy.20792. [DOI] [PubMed] [Google Scholar]
- Sisken BF. Electrical stimulation of nerves and their regeneration. Bioelectrochemistry and Bioenergetics. 1992;29:121–126. [Google Scholar]
- Sisken BF, Fowler I. Induction of Limb Regeneration in the Chick-Embryo. Anat Rec. 1981;199:A238–A239. [Google Scholar]
- Sisken BF, Fowler I, Romm S. Response of amputated rat limbs to fetal nerve tissue implants and direct current. Journal of Orthopaedic Research. 1984;2:177–189. doi: 10.1002/jor.1100020209. [DOI] [PubMed] [Google Scholar]
- Sisken BF, Walker J, Orgel M. Prospects on clinical applications of electrical stimulation for nerve regeneration. Journal of Cellular Biochemistry. 1993;51:404–409. doi: 10.1002/jcb.2400510404. [DOI] [PubMed] [Google Scholar]
- Slack JMW, Beck CW, Gargioli C, Christen B. Cellular and molecular mechanisms of regeneration in Xenopus. Philos T Roy Soc B. 2004;359:745–751. doi: 10.1098/rstb.2004.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Pelegri F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Developmental Biology. 2007;307:1–13. doi: 10.1016/j.ydbio.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD. Induction of partial limb regeneration in Rana pipiens by galvanic stimulation. The Anatomical Record. 1967;158:89–97. doi: 10.1002/ar.1091580110. [DOI] [PubMed] [Google Scholar]
- Smith SD. The role of electrode position in the electrical induction of limb regeneration in subadult rats. Bioelectrochemistry and Bioenergetics. 1981;8:661–670. [Google Scholar]
- Stern C, MacKenzie D. Sodium transport and the control of epiblast polarity in the early chick embryo. Journal of Embryology and Experimental Morphology. 1983;77:73–98. [PubMed] [Google Scholar]
- Stump RF, Robinson KR. Xenopus neural crest cell migration in an applied electrical field. J Cell Biol. 1983;97:1226–1233. doi: 10.1083/jcb.97.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G, Zhong L, Ma Z, Zheng J, Fang X, Ma T. Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS One. 2014;9:e115443. doi: 10.1371/journal.pone.0115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan D. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Reviews. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue engineering Part A. 2013a;19:1889–1908. doi: 10.1089/ten.tea.2012.0425.rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Li C, Choi YJ, Levin M, Kaplan DL. Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone. Biomaterials. 2013b;34:6695–6705. doi: 10.1016/j.biomaterials.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta. 2005;1719:59–68. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Swapna I, Borodinsky LN. Interplay between electrical activity and bone morphogenetic protein signaling regulates spinal neuron differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16336–16341. doi: 10.1073/pnas.1202818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara N, Brush M, Kawakami Y. Cell migration during heart regeneration in zebrafish. Dev Dyn. 2016;245:774–787. doi: 10.1002/dvdy.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton QV, Iovine MK. Semaphorin3d mediates Cx43-dependent phenotypes during fin regeneration. Dev Biol. 2012;366:195–203. doi: 10.1016/j.ydbio.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton QV, Iovine MK. Identification of an evx1-dependent joint-formation pathway during FIN regeneration. PLoS One. 2013;8:e81240. doi: 10.1371/journal.pone.0081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Bioph Res Co. 2004;317:463–471. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- Tseng A, Levin M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Communicative & Integrative Biology. 2013;6:1–8. doi: 10.4161/cib.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. Induction of vertebrate regeneration by a transient sodium current. Journal of Neuroscience. 2010a;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Adams DS, Qiu DY, Koustublian P, Levin M. Apoptosis is required during early stages of tail regeneration in Xenopus laevis. Developmental Biology. 2007;301:62–69. doi: 10.1016/j.ydbio.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. Induction of vertebrate regeneration by a transient sodium current. J Neurosci. 2010b;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Carneiro K, Lemire JM, Levin M. HDAC activity is required during Xenopus tail regeneration. PloS one. 2011a;6:e26382. doi: 10.1371/journal.pone.0026382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Carneiro K, Lemire JM, Levin M. HDAC Activity Is Required during Xenopus Tail Regeneration. Plos One. 2011b;6 doi: 10.1371/journal.pone.0026382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Levin M. Tail regeneration in Xenopus laevis as a model for understanding tissue repair. J Dent Res. 2008;87:806–816. doi: 10.1177/154405910808700909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Levin M. Transducing Bioelectric Signals into Epigenetic Pathways During Tadpole Tail Regeneration. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology. 2012;295:1541–1551. doi: 10.1002/ar.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu MK, Borodinsky LN. Spontaneous calcium transients manifest in the regenerating muscle and are necessary for skeletal muscle replenishment. Cell Calcium. 2014;56:34–41. doi: 10.1016/j.ceca.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA. Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130094. doi: 10.1098/rstb.2013.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA, Campbell TJ, Breit SN. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529 Pt 3:541–552. doi: 10.1111/j.1469-7793.2000.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet P, de Boer TP, van der Heyden MA, El Tamer MK, Sluijter JP, Doevendans PA, Goumans MJ. Hyperpolarization induces differentiation in human cardiomyocyte progenitor cells. Stem Cell Rev. 2010;6:178–185. doi: 10.1007/s12015-010-9142-5. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Blackiston DJ, Rea AC, Dore TM, Levin M. Left-right patterning in Xenopus conjoined twin embryos requires serotonin signaling and gap junctions. The International journal of developmental biology. 2014;58:799–809. doi: 10.1387/ijdb.140215ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry. Dev Dyn. 2010;239:3131–3146. doi: 10.1002/dvdy.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. A unified model for left–right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Developmental Biology. 2013;379:1–15. doi: 10.1016/j.ydbio.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Morrie RD. V-ATPase-dependent ectodermal voltage and ph regionalization are required for craniofacial morphogenesis. Developmental Dynamics. 2011;240:1889–1904. doi: 10.1002/dvdy.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Morrie RD, Adams DS. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev Dyn. 2011;240:1889–1904. doi: 10.1002/dvdy.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, Soler C, Solsona C, Celada A, Felipe A. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem. 2003;278:46307–46320. doi: 10.1074/jbc.M304388200. [DOI] [PubMed] [Google Scholar]
- Wang K, Xue T, Tsang SY, Van Huizen R, Wong CW, Lai KW, Ye Z, Cheng L, Au KW, Zhang J, Li GR, Lau CP, Tse HF, Li RA. Electrophysiological properties of pluripotent human and mouse embryonic stem cells. Stem Cells. 2005;23:1526–1534. doi: 10.1634/stemcells.2004-0299. [DOI] [PubMed] [Google Scholar]
- Warner AE. The Role of Gap-Junctions in Amphibian Development. J Embryol Exp Morph. 1985;89:365–380. [PubMed] [Google Scholar]
- Weisenseel MH, Jaffe LF. Major Growth Current through Lily Pollen Tubes Enters as K+ and Leaves as H+ Planta. 1976;133:1–7. doi: 10.1007/BF00385998. [DOI] [PubMed] [Google Scholar]
- Weisenseel MH, Nuccitelli R, Jaffe LF. Large Electrical Currents Traverse Growing Pollen Tubes. Journal of Cell Biology. 1975;66:556–567. doi: 10.1083/jcb.66.3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenisch S, Trinkaus K, Hild A, Hose D, Heiss C, Alt V, Klisch C, Meissl H, Schnettler R. Immunochemical, ultrastructural and electrophysiological investigations of bone-derived stem cells in the course of neuronal differentiation. Bone. 2006;38:911–921. doi: 10.1016/j.bone.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Yakushiji N, Suzuki M, Satoh A, Sagai T, Shiroishi T, Kobayashi H, Sasaki H, Ide H, Tamura K. Correlation between Shh expression and DNA methylation status of the limb-specific Shh enhancer region during limb regeneration in amphibians. Developmental Biology. 2007;312:171–182. doi: 10.1016/j.ydbio.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tanemura K, Okada S, Iwanami A, Nakamura M, Mizuno H, Ozawa M, Ohyama-Goto R, Kitamura N, Kawano M, Tan-Takeuchi K, Ohtsuka C, Miyawaki A, Takashima A, Ogawa M, Toyama Y, Okano H, Kondo T. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells. 2007;25:562–570. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- Yamashita M. Electric axon guidance in embryonic retina: galvanotropism revisited. Biochem Biophys Res Commun. 2013;431:280–283. doi: 10.1016/j.bbrc.2012.12.115. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Pala A, Pedrido L, Kremer Y, Welker E, Petersen CC. Membrane potential dynamics of neocortical projection neurons driving target-specific signals. Neuron. 2013;80:1477–1490. doi: 10.1016/j.neuron.2013.10.059. [DOI] [PubMed] [Google Scholar]
- Yang XY, Lai XG, Zhang Y, Pei JM, Yang AG, Zhou SS. siRNA-mediated silencing of ClC-2 gene inhibits proliferation of human U-87 glioma cells. Ai Zheng. 2006;25:805–810. [PubMed] [Google Scholar]
- Yao L, Shanley L, McCaig C, Zhao M. Small applied electric fields guide migration of hippocampal neurons. J Cell Physiol. 2008;216:527–535. doi: 10.1002/jcp.21431. [DOI] [PubMed] [Google Scholar]
- Yoon G, Quitania L, Kramer JH, Fu YH, Miller BL, Ptacek LJ. Andersen-Tawil syndrome - Definition of a neurocognitive phenotype. Neurology. 2006;66:1703–1710. doi: 10.1212/01.wnl.0000218214.64942.64. [DOI] [PubMed] [Google Scholar]
- Yu SP, Choi DW. Ions, cell volume, and apoptosis. Proc Natl Acad Sci U S A. 2000;97:9360–9362. doi: 10.1073/pnas.97.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky JJ, Eckman DM, Wellman GC. Targeted disruption of Kir2. 1 and Kir2. 2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circulation Research. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li M, Kang ET, Neoh KG. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016;32:46–56. doi: 10.1016/j.actbio.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Levin M. Particle tracking model of electrophoretic morphogen movement reveals stochastic dynamics of embryonic gradient. Developmental Dynamics. 2009;238:1923–1935. doi: 10.1002/dvdy.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, McCaig CD, Agius-Fernandez A, Forrester JV, Araki-Sasaki K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wong CO, Cho KJ, van der Hoeven D, Liang H, Thakur DP, Luo J, Babic M, Zinsmaier KE, Zhu MX, Hu H, Venkatachalam K, Hancock JF. SIGNAL TRANSDUCTION Membrane potential modulates plasma membrane phospholipid dynamics and K-Ras signaling. Science. 2015;349:873–876. doi: 10.1126/science.aaa5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc GK. Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:649–670. doi: 10.1007/s00359-006-0104-y. [DOI] [PubMed] [Google Scholar]


