Abstract
There is no safe and effective standard method for glans penis augmentation. Furthermore, there has been scant research on glans penis augmentation due to a poor understanding of glans anatomy, technical difficulty, and a lack of suitable substances for augmentation. Cross-linked dextran gel is a newly developed filler for soft-tissue augmentation. We evaluated the efficacy and safety of using a novel technique to inject cross-linked dextran gel for glans penis augmentation during a 24-week follow-up study. This prospective, single-arm, multicenter study enrolled twenty healthy adult men who underwent glans penis augmentation between June and August 2013. Cross-linked dextran gel was injected into the glans penis using a simple and easy technique. The sizes of the glans penis and individual satisfaction were assessed. Any adverse event was also reported. A total of 18 individuals were analyzed; two of them were lost to follow-up. The mean procedure time and injected volume were about 30 min and 6.6 ± 0.9 ml, respectively. The mean surface areas of the glans at baseline and 24 weeks were 20.0 ± 3.5 cm2 and 33.6 ± 5.4 cm2, respectively, representing a mean increase of 68.7% ± 14.0% (P < 0.001). Sixteen individuals (88.9%) were satisfied with the outcomes, and none were dissatisfied. There were no serious adverse events during the study. Cross-linked dextran gel injection for glans penis augmentation was easy and showed a significant augmentative effect on the glans penis, good durability, and was well tolerated without serious adverse events. Therefore, cross-linked dextran gel injection may be an effective, new technique for glans penis augmentation.
Keywords: cosmetic technique, dermal fillers, dextran, glans penis
INTRODUCTION
Various penile augmentations have been performed to correct medical problems, such as micropenis, or to improve self-esteem. With the increasing need for effective, safe, and less invasive procedures, penile augmentations using injectable soft-tissue substitutes are currently in high demand.1,2 Several penile injection materials have been introduced and used for penile shaft augmentation.2,3,4,5
To the unaided eye, the penis is divided into the glans penis and shaft. Notably, the penile shaft is currently the only focus of penile augmentation. Consequently, successful penile shaft augmentation is considered to be only half-successful.6,7 Furthermore, the glans is relatively smaller than the penile shaft after augmentation, causing new imbalances in penile shape that may result in lower self-esteem. In real-world practice, most patients who seek penile augmentation desire both penile shaft and glans penis augmentation (GPA), if possible.7 However, there is no safe and effective standard method for GPA, and there has been scant research on GPA due to a poor understanding of glans anatomy, technical inexperience, and a lack of suitable substances for augmentation.6,7
Cross-linked dextran (CD) is biocompatible and enhances angiogenic responses.8,9 Moreover, CD is neocollagenetic and is replaced by the body's own tissue after dermal injection.3,10 In animal models, CD-based materials showed good efficacy and biocompatibility for soft-tissue augmentation.8,11,12,13,14 Recently, it has been used as a bulking substance for the treatment of vesicoureteral reflux and urinary incontinence.15,16,17 Based on these characteristics, CD may be an appropriate substance for GPA.
CD gel is a newly developed dermal filler for soft-tissue augmentation and is a commercially available product. The present study described in detail a novel technique for human GPA and assessed the efficacy and safety of CD gel during a follow-up period of 24 weeks after injection.
MATERIALS AND METHODS
Study design
This study used a prospective, single-arm, multicenter design and evaluated the efficacy and safety of CD gel for human GPA during a follow-up period of 24 weeks after injection. Approvals for the study were obtained from the respective Institutional Ethics Committees of the two participating institutions. Individuals were recruited by advertisement from two institutions between June and August 2013.
The size of the glans penis was measured with each individual in the supine position in a stable environment, with the penis in a flaccid state. Glandular size was defined as the surface area of the glans, which was calculated as the width of the glans multiplied by its length. The width and length were measured with a measuring tape using the center of the urethral meatus as the reference point (Figure 1).
Figure 1.
Measurement of glandular size. Glandular size was defined as the surface area of the glans, which was calculated as the width multiplied by the length of the glans penis. The width and length were measured with a measuring tape using the center of the urethral meatus as the reference point.
Individuals visited each institution 4 weeks after injection to check the penile condition and to determine of any adverse events (AEs) had occurred. At 24 weeks after injection, glandular size was measured. Individual satisfaction was also assessed from grade 0 to 4 (G0–4), in order of increasing satisfaction, with G0 indicating dissatisfaction; G1, about equally satisfied and dissatisfied; G2, somewhat satisfied; G3, moderately satisfied; and G4, very satisfied.6
Subjects
Healthy males of 20 years age and older who felt that they had a small penis (small penis syndrome) and wanted to receive GPA were enrolled in our study. Before augmentation, individuals provided their clinical history and detailed physical examinations were performed, focusing on the anatomical features of the penis and on psychological details, in order to exclude those who were unsuitable for inclusion in the current study. They also underwent preliminary assessments, including vital signs, urine analysis, complete blood count, hepatic function, and blood clotting function using serum biochemical analysis. Exclusion criteria included any history of psychological or psychiatric illness, congenital or acquired penile malformation, previous plastic surgery on the glans penis, and any chronic major systemic disease, including diabetes and coagulopathy. All individuals had been circumcised before participating in the study although previous circumcision was not required for inclusion.
After the study design and possible complications after injection were explained, the individuals signed an informed consent form. A total of twenty individuals from two medical institutions (ten from each) were finally included in the study.
Injection material
CD, which is derived from dextran used as volume expander, is a microsphere with a molecular weight and diameter of ~510 kDa and 65–125 μm, respectively. Its positive surface charges attract macrophages. In turn, the macrophages release transforming growth factor (TGF)-beta and interleukins, which stimulate fibroblasts to produce collagen fibers. After being reabsorbed, dextran is replaced by the body's own tissue.18
CD gel (Lipen-D®, CheongHwa Medipower Corporation, Jangseoung, Korea) is a novel dermal filler for soft-tissue augmentation and is a commercially available product that was approved by the Korean Food and Drug Administration in 2012. CD gel consists of 3.84% CD, 0.17% hydroxylpropyl methylcellulose, 0.05% sodium hydroxide, and 95.94% water. The CD microspheres were 63–125 μm in diameter with a pH of 5.8–7.6. As a result, 1 ml of CD gel contained approximately 37.2 mg dextranomer microspheres.14
Injection method
An experienced surgeon performed the procedure at each institute. The procedure was carried out in an office setting with the individual in the supine position. A penile block was induced by injecting 2 ml of 0.2% lidocaine into the penis root. In addition, 0.2% lidocaine was injected underneath the coronal sulcus (Supplementary Video Clip 1).
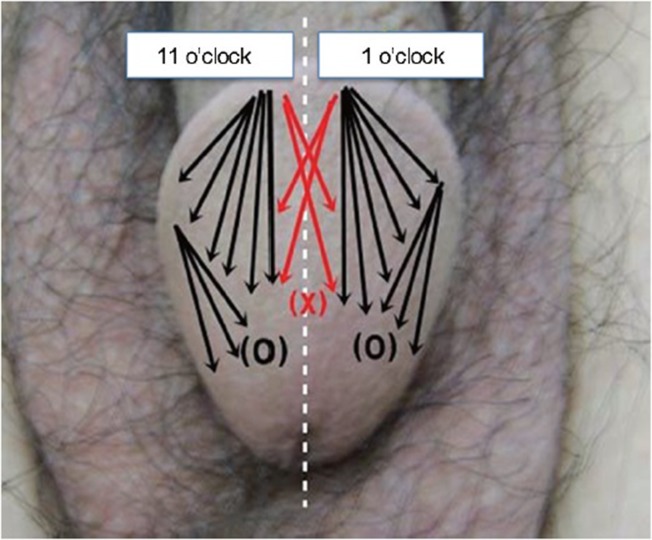
After the anesthesia had taken effect, CD gel was injected into the subcutaneous tissue (lamina propria layer) of the glans penis by the fanning technique through a 24-gauge needle (Figure 2). For this technique, the injection needle was indwelled at the 1 and 11 o’clock positions on the corona of the glans to avoid dorsal pedicle injury. Thereafter, the needle was directed posteriorly and laterally, parallel, or tangential to the glans penis, distributing the material as uniformly as possible by a continuous back and forth movement, while constantly pressing the syringe plunger. If necessary, the injection needle was indwelled between the 1 and 5 o’clock positions or between the 7 and 11 o’clock positions (multiple puncture technique).19 To avoid urethral compression or injury, the injected material did not cover the ventral part of the glans penis or around the urethral meatus. The mean procedure time and injected volume were about 30 min and 6.6 ± 0.9 ml (range: 4.9–8.8 ml), respectively. After the procedure, the injected surface was thoroughly massaged, to redistribute the gel as uniformly as possible (Supplementary Video Clip 1).
Figure 2.
Cross-linked dextran gel was injected into the subcutaneous tissue (lamina propria layer) of the glans penis using the fanning technique. To avoid urethral compression or injury, the injected material did not cover the ventral part of the glans penis or around the urethral meatus
Main outcome measures
The primary endpoint was an increase in glans surface area of 20% or more at 24 weeks after injection. Well-designed research on GPA is lacking. In our preliminary results, the mean glandular size increase after injection was 30%, with a cut-off point of marked increase to the unaided eye of 20%. The secondary endpoint was G2 or greater on the satisfaction scale, which indicated that the individual was at least somewhat satisfied with the results of the CD gel injection. Differences in outcomes between institutes were also assessed.
Safety assessments
Vital signs and penile conditions were recorded at baseline and 4 and 24 weeks after injection. AEs were also reported to investigators, immediately or when the individuals visited each institution for follow-up assessment.
Statistical analyses
Our study was designed with 80% power and a 5% significance level to detect a 20% increase in mean surface area of the glans penis, assuming a standard deviation of 10%, based on our preliminary results and those of a previous study.6 Thus, at least 17 individuals were required for statistical analysis.
The increase in glans surface area at 24 weeks after injection was determined using one-sample t-tests. The mean differences in glans penis surface area between baseline and 24 weeks after injection were determined using paired t-tests. Frequency and percentages were used to represent individual satisfaction and AEs. Differences in outcomes between institutes were determined using Mann–Whitney U-tests for continuous variables and Fisher's exact tests for categorical variables.
All tests were two-sided, and P < 0.05 was considered statistically significant. IBM SPSS Statistics for Windows, version 21.0 (IBM Corporation, Armonk, NY, USA) was used for all statistical assessments.
RESULTS
Subjects
Of twenty individuals who received injections, two were lost to follow-up due to subject choice. Finally, 18 individuals were analyzed. Their mean age was 52.0 ± 7.1 (range: 33.0–65.0) years.
Efficacy
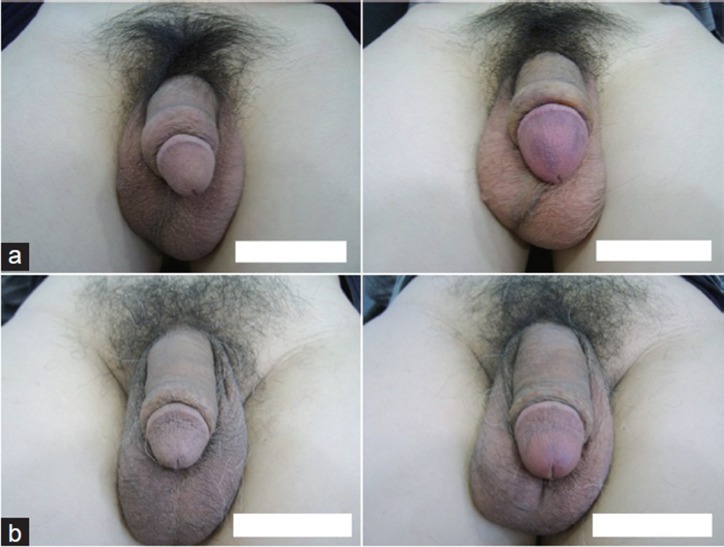
The CD gels appeared to be uniformly distributed over the glandular surface 24 weeks after injection, with no evidence of migration from the injection site (Figure 3).
Figure 3.
Representative figures of cross-linked dextran gel injection for glans penis augmentation. The gels were uniformly distributed, and the glans penis 24 weeks after injection (right) was larger than that before injection (left). (a) Case number 10 (44-year-old man). The injected volume was 6.4 ml, and the surface area of the glans penis had increased by 10.20 cm2 (48.23%) at 24 weeks after injection. (b) Case number 11 (57-year-old man). The injected volume was 6.4 ml, and the surface area of the glans penis had increased by 11.69 cm2 (71.28%) at 24 weeks after injection.
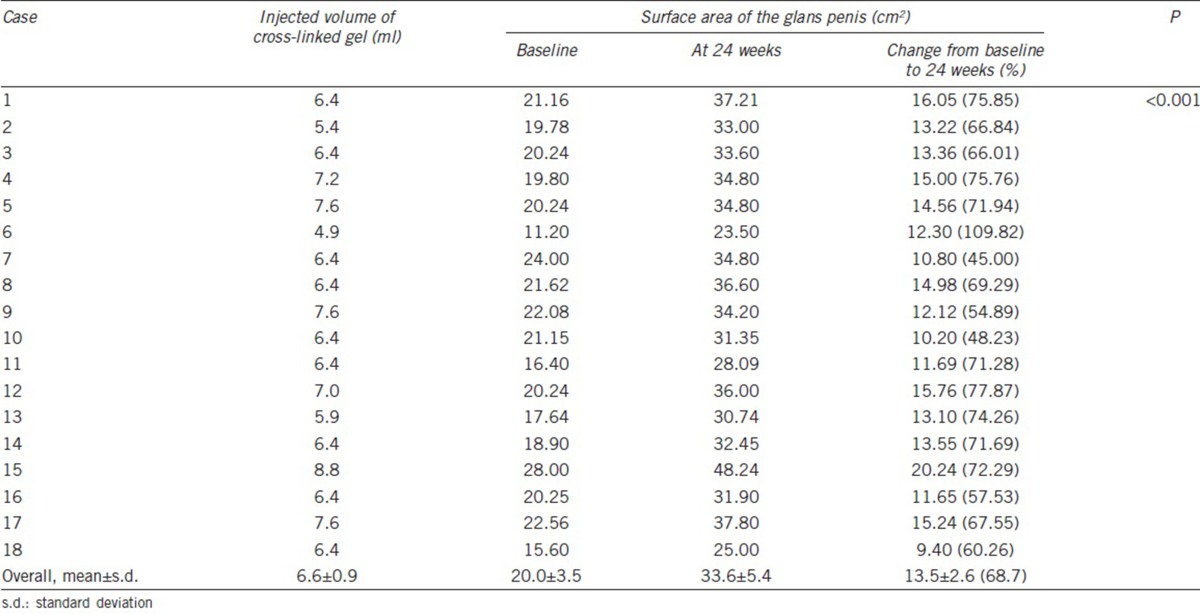
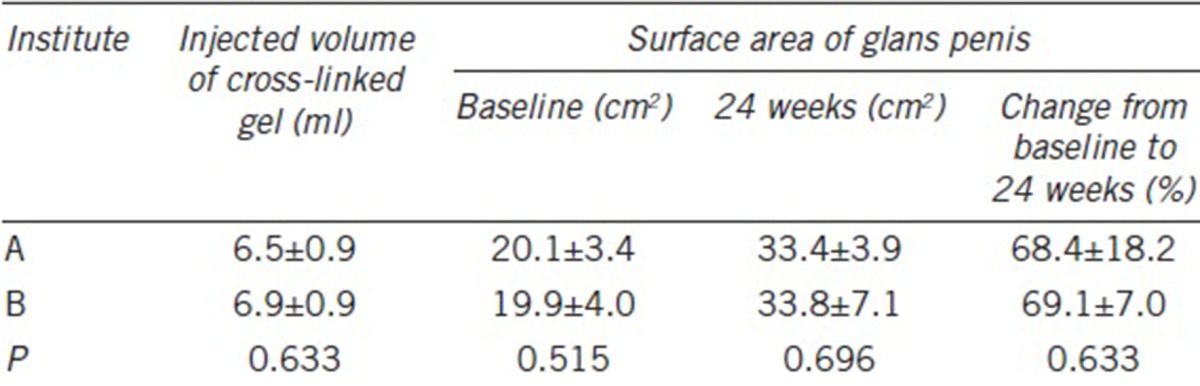
At 24 weeks after injection, the surface areas of the glans penis had increased by 20% or more in all individuals (100%; Table 1). The mean surface areas at baseline and 24 weeks after injection were 20.0 ± 3.5 cm2 and 33.6 ± 5.4 cm2, respectively, representing a mean increase of 68.7% ± 14.0% (P < 0.001). The mean surface area increased by 13.5 ± 2.6 cm2 (standard error: 0.6; 95% confidence interval: 12.2–14.8; P < 0.001). There were no significant differences in the outcomes between the two institutes (P > 0.05; Table 2).
Table 1.
Changes in the surface area of the glans penis between baseline and 24 weeks after injection of cross-linked dextran gel
Table 2.
Comparison of the outcomes of cross-linked dextran gel injection between the two institutes
The satisfaction scores after infection in the 18 individuals were G1 (two individuals, 11.1%), G2 (12 individuals, 66.7%), and G3 (four individuals, 22.2%). Sixteen (88.9%) individuals were satisfied with the outcomes of the injection, and no individual was dissatisfied with the outcome.
Safety
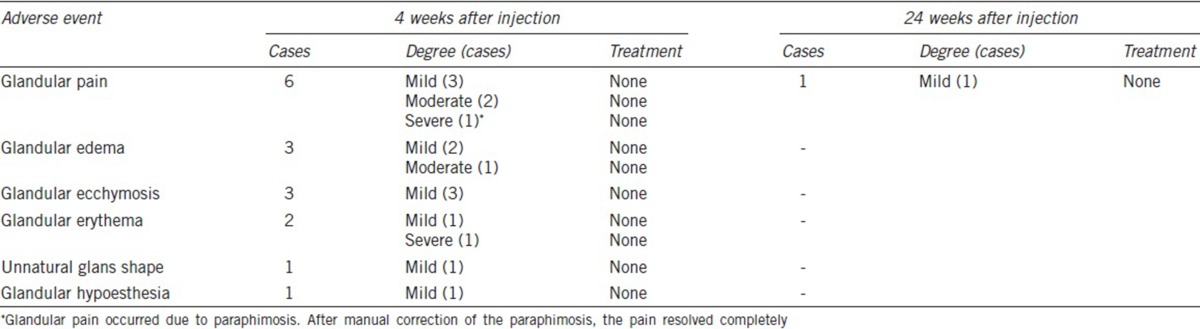
All individuals experienced varying degrees of glandular pain and edema immediately after injection. However, these symptoms spontaneously subsided within 2 weeks for most individuals. At 24 weeks after injection, only one individual presented with mild glandular pain (Table 3). At 4 weeks after injection, the reported symptoms included ecchymosis (three individuals, 16.7%), erythema (two individuals, 11.1%), unnatural glans shape (one individual, 5.6%), and glandular hypoesthesia (one individual, 5.6%). However, these symptoms resolved without treatment, and no case lasted for 24 weeks. There were no serious AEs, such as glandular necrosis or ulceration, glandular surface irregularity, and erectile dysfunction, during the study.
Table 3.
Treatment-related adverse events during the study period
DISCUSSION
Currently, the primary focus of penile augmentation is typically the penile shaft, and not the glans penis. As a result, even successful augmentation of the penile shaft is considered only half-successful.6,7 Nevertheless, there has been little to no research on GPA. In this context, our prospective study is remarkable, because our results showed that injection of a newly developed CD gel resulted in substantially increased glandular size and was well tolerated, without any serious AEs. Our results suggest that CD gel injection may be an effective new technique for GPA.
GPA with injection has been overlooked due to a poor understanding of glans anatomy, technical inexperience, and a lack of suitable substances for augmentation.6,7 However, a recent animal study suggested the potential space of the glans penis for GPA.20 The animal study showed that the implants were well maintained in the lamina propria layer of the glans penis for 1 year.6,20 In addition, it is not technically complicated to inject gels into the human glans penis, due to its elastic nature. Our technique is easy in facilitating the injection of gels (Supplementary Video Clip 1).
The choice of an adequate substance must be carefully considered in injection-based GPA. Ideal substances for soft-tissue augmentation should maintain their volume and should be safe, biocompatible, and nonmigratory. Because the glans penis is a highly vascularized structure and the safe space for injection is relatively small, the choice of an appropriate substance is of utmost importance. In this respect, CD gel was particularly well suited for GPA in our study. Dextran is a complex, branched glucan that is safe enough for use as an antithrombotic agent or volume expander.10 CD is derived from dextran and consists of microspheres. Its positive surface charges apparently attract macrophages. In turn, the macrophages release TGF-beta and interleukins, which stimulate fibroblasts to produce collagen fibers, a process called neocollagenesis. After being reabsorbed, dextran is replaced by the body's own tissue (living implant).10,18 These characteristics suggest the potential for CD to be a safe, biocompatible, and nonmigratory substance for GPA.
Previously, the effects of the same CD gel product used in our study were assessed in a 24-month rat study.14 The augmentation effects of CD gel decreased slowly but were largely maintained. In addition, the sequential volume changes from 3 weeks to 24 months after injection were not significant. CD gel induced strong collagen deposition inside and outside the gel at 3 weeks after injection. The thickness and intensity of collagen deposition increased gradually for up to 24 months after injection. The gel was well tolerated, did not cause serious AEs, and provided a significant augmentative effect in that study. Our human study was designed based on the results of that animal model study.
To our knowledge, the only three materials for GPA with injection have been assessed in literature.6,21,22 Perovic et al.21 reported the results of GPA by injection of a hydrogel that included a synthetic cross-linked polymer in 13 patients with glandular deformities. They concluded that the hydrogel was effective and tolerable at a mean of 17-month follow-up. However, seven of 13 patients received reinjections (more than two times) to maintain a lasting GPA effect. Therefore, the results were not maintained in more than half of the patients. Shaeer reported the results of GPA by injection of polyacrylamide gel in four patients with penile prosthesis implantation. However, the augmentative effects only lasted for an average of 5 months.22 Furthermore, even if serious AEs were not reported in that study, polyacrylamide can cause granuloma formation or delayed hypersensitivity reactions.23 Hyaluronic acid (HA) gel, which has been proven to be effective and safe in various augmentation fields, is also reportedly effective as an injectable material for GPA. Kim et al.6 reported a significant increase in glandular circumference at a 1-year follow-up. The same investigators also reported the results of 5-year follow-ups with only 15% decrease in glandular circumference.24 However, in almost all studies on GPA with HA injection, HA gel was associated with delayed ejaculation, thus restricting its use to patients with premature ejaculation.19,22,24,25,26
Interestingly, delayed ejaculation was not reported in our study. Only one individual complained about glandular hypoesthesia, which disappeared during the study. As mentioned, although HA gel injection is a promising tool for GPA, delayed ejaculation may restrict its use to only those patients with premature ejaculation.19,22,24,25,26 Therefore, it is notable that CD gel injection was not associated with delayed ejaculation in our study. Although the exact mechanism is unknown, one potential hypothesis is that CD gel, unlike HA gel, can induce neovascularization and be replaced by the body's own tissue (living implant).10,14,18 During this pathogenesis, glandular sensory function may be maintained or regenerated.
In our study, AEs were mild and transient and resolved without treatment. There were no serious AEs, such as glandular necrosis or ulceration, during the study. However, the use of CD may involve the risk of the appearance of serious and persistent AEs, such as tissue necrosis, especially in patients with vasculopathy.27
Our study had several limitations. First, the duration of follow-up was insufficient to confirm the long-term efficacy and safety of the CD gel. However, our study is ongoing for a long-term follow-up. Despite this limitation, we believe that CD gel is a promising tool for GPA, based on the results of our study and those of animal model testing.14 Second, the measurement method of glandular size used in our study was not validated although it was tested and reliable in our preliminary study. However, it must also be noted that there is no known validated measurement method, and it is impossible in practice to measure the glandular size precisely. Third, our satisfaction scale was skewed in favor of satisfaction, which might have created some bias, although it was made based on a previous study and our preliminary study in which dissatisfaction was rarely reported.6 Fourth, there was no objective comparison of erectile glans size or objective report of satisfaction of erectile glans size before and after CD injection. Fifth, our study had no control group although it was reasonably designed. Finally, histologic examination was not performed although histology was previously assessed in an animal model.14
CONCLUSIONS
CD gel injection for GPA was easy and showed a significant augmentative effect on the glans penis, good durability at 24 weeks after injection, and was well tolerated, without serious AEs. Thus, CD gel injection may be an effective new tool for GPA.
AUTHOR CONTRIBUTIONS
DYY and DGM planned and conducted the study. DYY, KK, DGM, JWK, and WKL coordinated the study. SHL and WKL analyzed and interpreted data. WKL drafted the manuscript. DYY, DGM, and WKL supervised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors were investigators on a clinical trial sponsored by Cheong-Hwa Medipower Corporation, Jangseoung, Korea.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Vardi Y, Gruenwald I. The status of penile enhancement procedures. Curr Opin Urol. 2009;19:601–5. doi: 10.1097/MOU.0b013e3283318f31. [DOI] [PubMed] [Google Scholar]
- 2.Yang DY, Lee WK, Kim SC. Tolerability and efficacy of newly developed penile injection of cross-linked dextran and polymethylmethacrylate mixture on penile enhancement: 6 months follow-up. Int J Impot Res. 2013;25:99–103. doi: 10.1038/ijir.2012.41. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J Cosmet Laser Ther. 2009;11:240–7. doi: 10.3109/14764170903341731. [DOI] [PubMed] [Google Scholar]
- 4.Panfilov DE. Augmentative phalloplasty. Aesthetic Plast Surg. 2006;30:183–97. doi: 10.1007/s00266-004-0153-y. [DOI] [PubMed] [Google Scholar]
- 5.Vardi Y, Harshai Y, Gil T, Gruenwald I. A critical analysis of penile enhancement procedures for patients with normal penile size: surgical techniques, success, and complications. Eur Urol. 2008;54:1042–50. doi: 10.1016/j.eururo.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ, Kwak TI, Jeon BG, Cheon J, Moon DG. Human glans penis augmentation using injectable hyaluronic acid gel. Int J Impot Res. 2003;15:439–43. doi: 10.1038/sj.ijir.3901044. [DOI] [PubMed] [Google Scholar]
- 7.Moon DG, Kwak TI, Kim JJ. Glans penis augmentation using hyaluronic acid gel as an injectable filler. World J Mens Health. 2015;33:50–61. doi: 10.5534/wjmh.2015.33.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadee JA, Brouwer LA, den Otter W, Hennink WE, van Luyn JA. A comparative biocompatibility study of microspheres based on crosslinked dextran or poly (lactic-co-glycolic) acid after subcutaneous injection in rats. J Biomed Mater Res A. 2001;56:600–9. doi: 10.1002/1097-4636(20010915)56:4<600::aid-jbm1133>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Sun G, Zhang X, Shen YI, Sebastian R, Dickinson LE, et al. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci U S A. 2011;108:20976–81. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354–66. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]
- 11.Alkan M, Ciftci AO, Talim B, Senocak ME, Caglar M, et al. Histologcial response to injected dextranomer-based implant in a rat model. Pediatr Surg Int. 2007;23:183–7. doi: 10.1007/s00383-006-1818-1. [DOI] [PubMed] [Google Scholar]
- 12.De Jong WH, Dormans JA, Van Steenbergen MJ, Verharen HW, Hennink WE. Tissue response in the rat and the mouse to degradable dextran hydrogels. J Biomed Mater Res A. 2007;83:538–45. doi: 10.1002/jbm.a.31302. [DOI] [PubMed] [Google Scholar]
- 13.Elzayat EA, Karsenty G, Bismar TA, Corcos J. Volume changes and histological response to injected dextranomer/hyaluronic acid copolymer (ZuidexTM) and collagen (ContigenVR) in rats. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:247–52. doi: 10.1007/s00192-007-0414-9. [DOI] [PubMed] [Google Scholar]
- 14.Cena RB, Park JG, Kim HJ, Son KY, Kim DS, et al. Effects of crosslinked dextran in hydroxylpropyl methylcellulose on soft tissue augmentation in rats. J Biomed Mater Res B Appl Biomater. 2014;102:131–40. doi: 10.1002/jbm.b.32989. [DOI] [PubMed] [Google Scholar]
- 15.Stenberg A, Lackgren G. A new bioimplant for the endoscopic treatment of vesicoureteral reflux: experimental and short-term clinical results. J Urol. 1995;154:800–3. doi: 10.1097/00005392-199508000-00127. [DOI] [PubMed] [Google Scholar]
- 16.Lottmann HB, Margaryan M, Bernuy M, Grosz A, Aigrain Y, et al. Long-term effects of dextranomer endoscopic injections for treatment of urinary incontinence: an update of a prospective study of 31 patients. J Urol. 2006;175:1485–9. doi: 10.1016/S0022-5347(05)00669-5. [DOI] [PubMed] [Google Scholar]
- 17.Jung HJ, Im YJ, Lee YS, Kim MJ, Han SW. Is a secondary procedure necessary in every case of failed endoscopic treatment for vesicoureteral reflux? Korean J Urol. 2015;56:398–404. doi: 10.4111/kju.2015.56.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eppley BL, Summerlin DJ, Prevel CD, Sadove AM. Effects of positively charged biomaterial for dermal and subcutaneous augmentation. Aesthetic Plast Surg. 1994;18:413–6. doi: 10.1007/BF00451350. [DOI] [PubMed] [Google Scholar]
- 19.Abdallah H, Abdelnasser T, Hosny H, Selim O, Al-Ahwany A, et al. Treatment of premature ejaculation by glans penis augmentation using hyaluronic acid gel: a pilot study. Andrologia. 2012;44:650–3. doi: 10.1111/j.1439-0272.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 20.Moon DG, Kwak TI, Cho HY, Bae JH, Park HS, et al. Augmentation of glans penis using injectable hyaluronic acid gel. Int J Impot Res. 2003;15:456–60. doi: 10.1038/sj.ijir.3901058. [DOI] [PubMed] [Google Scholar]
- 21.Perovic S, Radojicic ZI, Djordjevic ML, Vukadinovic VV. Enlargement and sculpturing of a small and deformed glans. J Urol. 2003;170:1686–90. doi: 10.1097/01.ju.0000084431.99013.13. [DOI] [PubMed] [Google Scholar]
- 22.Shaeer O. Supersizing the penis following penile prosthesis implantation. J Sex Med. 2010;7:2608–16. doi: 10.1111/j.1743-6109.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi PS. Emerging permanent filler technologies: focus on Aquamid. Clin Cosmet Investig Dermatol. 2014;7:261–6. doi: 10.2147/CCID.S46650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak TI, Jin MH, Kim JJ, Moon DG. Long-term effects of glans penis augmentation using injectable hyaluronic acid gel for premature ejaculation. Int J Impot Res. 2008;4:425–8. doi: 10.1038/ijir.2008.26. [DOI] [PubMed] [Google Scholar]
- 25.Kim JJ, Kwak TI, Jeon BG, Cheon J, Moon DG. Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation. Int J Impot Res. 2004;16:547–51. doi: 10.1038/sj.ijir.3901226. [DOI] [PubMed] [Google Scholar]
- 26.Seftel AD. Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation. J Urol. 2005;173:2077. [PubMed] [Google Scholar]
- 27.Zyczkowski M, Prokopowicz G, Zajęcki W, Paradysz A. Complications following endoscopic treatment of vesicoureteric reflux with Deflux® – two case studies. Cent European J Urol. 2012;65:230–1. doi: 10.5173/ceju.2012.04.art12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.