Abstract
A bioassay-guided fractionation and chemical investigation of Amaryllis belladonna Steud. bulbs resulted in the isolation and identification of the new crinane alkaloid 1,4-dihydroxy-3-methoxy powellan (1), along with the 3 known crinane alkaloids 2 – 4 and the two lycorane alkaloids 5–6. The structures were elucidated by interpretation of combined HR-ESIMS, CD and 2D NMR spectroscopic data. Among these isolated compounds the lycorane-type alkaloid acetylcaranine (5) exhibited strong antiplasmodial activity, while compounds 3 and 4 were moderately active, and compounds 1 and 6 were inactive.
Keywords: Amaryllis belladonna, crinane, lycorine, antiplasmodial, bioassay-guided
Graphical Abstract
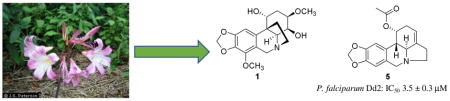
Malaria is a life-threatening blood disease caused by parasites of the Plasmodium genus that are transmitted by the bite of the female Anopheles mosquito.1 Malaria causes symptoms including fever, fatigue, vomiting, and headaches, and it can also cause yellow skin, seizures, coma, and death in severe cases.1 More than 212 million people were estimated to have been infected with malaria in 2015, leading to 429,000 deaths.2 The disease is widespread in low-income and tropical regions, with the majority of deaths occurring in Africa.2 Widespread antimalarial drug resistance is also a growing problem, since P. falciparum has developed resistance to all current treatments including the newer antimalarial drug artemisinin, and this is an increasing problem in some parts of Southeast Asia.2 Therefore, it is important to develop new antimalarial agents as rapidly as possible. Since natural products have been the source of the two major drugs quinine3 and artemisinin,4 our search focused on finding new antiplasmodial agents from medicinal and other plants from the superb Natural Products Discovery Institute (NPDI) Repository.5
During this search, we observed that a MeOH extract of Amaryllis belladonna Steud. (Amaryllidaceae) bulbs exhibited significant antiplasmodial activity against the chloroquine-resistant Dd2 strain of P. falciparum. Previous studies have reported the isolation of antineoplastic and antifungal constituents from this plant,6–8 and its alkaloid constituents have recently been reviewed,9 but there have been no reports on the isolation of any antimalarial agents. We thus selected this extract for bioassay-guided fractionation and isolation, and this yielded one new alkaloid along with five known alkaloids, two of which had moderate antiplasmodial activity (Fig. 1)
Figure 1.
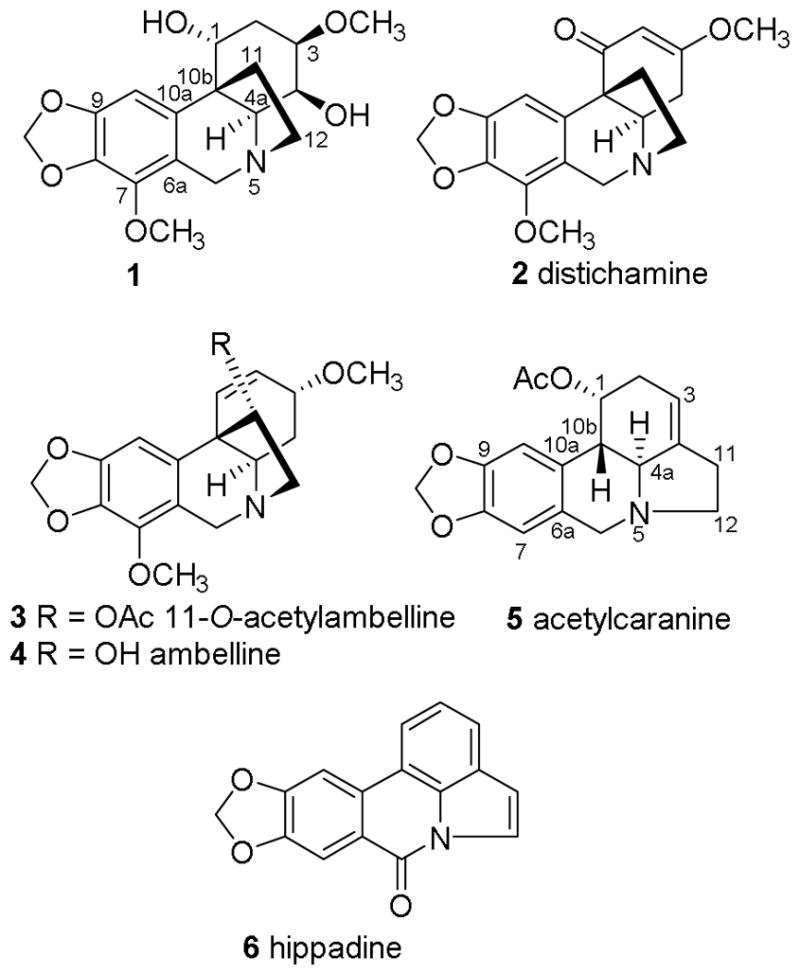
Compounds isolated from Amaryllis belladonna
Compound 1 was isolated as a white, amorphous powder and its molecular formula was determined to be C18H23NO6 by HREIMS data (observed m/z 349.1492 [M]+, calculated for C18H23NO6+ 349.1520), indicating 8 degrees of unsaturation. Its 1H NMR spectrum (Table 1) had one signal for a singlet aromatic proton at H-10 (δH 6.61), signals for four methylene protons at H-2 (δH 1.75, 1.40), H-6 (δH 4.19, 3.71), H-11 (δH 2.37, 2.00), and H-12 (δH 3.16, 2.77), for three oxygenated methine protons at H-1 (δH 3.07), H-3 (δH 3.97), and H-4 (δH 3.31–3.33), and for two methylenedioxy protons (δH 5.85). The 13C NMR spectrum revealed signals for a methylenedioxy carbon (δC 100.5), a quaternary carbon (δC 41.4), six aromatic carbons (δC 96.3, 138.9, 117.8, 141.0, 147.9, and 133.3), and two methoxy (δC 59.1 and 57.5), four methylene (δC 25.2, 58.6, 39.2, and 52.5), and three oxygenated methine (δC 61.2, 74.9, and 55.1) carbons.
Table 1.
1H and 13C NMR Data of 1a
| position | δH (J in Hz) | δC |
|---|---|---|
| 1 | 3.07, dd (11.08, 3.15) | 61.2 |
| 2 | 1.75, d (13.4), 1.40, td (13.5, 2.90) | 25.2 |
| 3 | 3.97, m | 74.9 |
| 4 | 3.31–3.33, m | 55.1 |
| 4a | 3.76, d (3.4) | 53.9 |
| 6 | 4.19, d (17.4) 3.71, d (17.4) | 58.6 |
| 6a | 117.8 | |
| 7 | 141.0 | |
| 8 | 133.3 | |
| 9 | 147.9 | |
| 10 | 6.61, s | 96.3 |
| 10a | 138.9 | |
| 10b | 41.4 | |
| 11 | 2.35–2.39, m; 2.00, ddd (13.0, 10.8, 5.0) | 39.2 |
| 12 | 3.16, td (11.5, 10.5, 4.8), 2.75–2.78, m | 52.5 |
| OCH2O | 5.84–5.88, m | 100.5 |
| 3-OCH3 | 3.42, s | 57.5 |
| 7-OCH3 | 3.96, s | 59.1 |
1H (400 MHz) and 13C (100 MHz) NMR data were obtained in CDCl3
The HSQC and 1H–1H COSY spectra of 1 (Fig. 2) disclosed two spin systems of H-1 to H-4a and H-11 to H-12, which led to the establishment of the structure fragments C-1 to C-4a and C-11 to C-12. HMBC correlations were observed from the methylenedioxy protons (δH 5.85) and the single aromatic proton (δH 6.61, H-10) to two oxygenated aromatic carbons (δC 133.3, C-8 and 147.9, C-9), and from the aromatic proton (δH 6.61, H-10) and the deshielded methylene protons (δH 4.19, 3.71, H-6) to two aromatic carbons (δC 138.9, C-10a and 117.8, C-6a).
Figure 2.
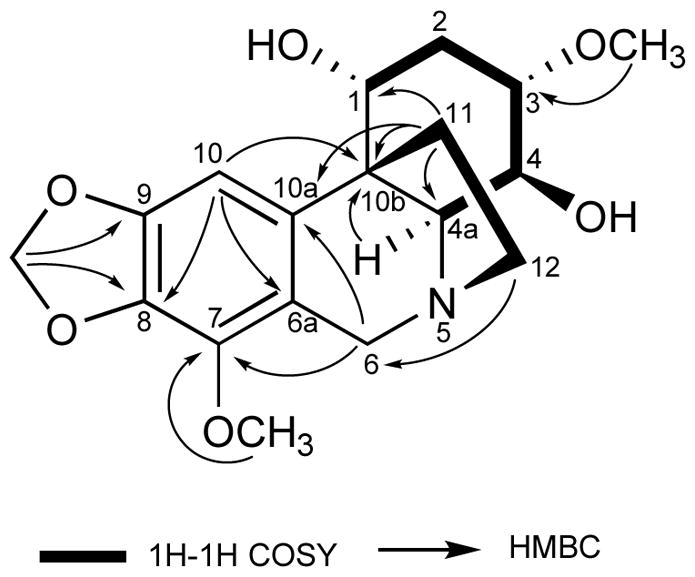
Key COSY and HMBC correlations of compound 1
Correlations were also observed from the deshielded methylene protons (δH 4.19, 3.71, H-6) and the oxygenated methine proton (δH 3.04, H-1) to the deshielded methine carbon (δC 53.9, C-4a). Taken together, these correlations indicated that 1 has an amaryllidaceae alkaloid ring system.10,11
Additional HMBC correlations from the methylene protons (δH 2.37, 2.00, H-11) to a quaternary carbon at C-10b (δC 41.4), an aromatic quaternary carbon at C-10a (δC 138.9), and an oxygenated carbon at C-1 of a cyclohexane ring (δC 61.2), as well as correlations from methylene protons (δH 3.16, 2.77, H-12) to the deshielded methylene carbon (δC 58.6, C-6) indicated that 1 was a crinane-type alkaloid with a 5,10b ethano bridge attached to a tetrahydrophenanthridine (Fig. 2).10,11 The correlations from δH 3.42 to C-3 (δC 74.9) and δH 3.96 to C-7 (δC 141.0) confirmed the locations of the two methoxy groups at C-3 and C-7, respectively.
The relative configuration of 1 was established through analysis of NOESY and 1H-1H coupling constant data. NOESY correlations from H-1 (δH 3.07) to H-2eq (δH 1.75), H-1 to 3-OCH3, H-2eq (δH 1.75) to 3-OCH3 (δH 3.42), H-3 (δH 3.97) to H-4 (δH 3.31–3.33), and H-4 (δH 3.31–3.33) to H-4a (δH 3.76) were observed, consistent with similar correlations in 3-O-demethyl-3-O-(3-hydroxybutanoyl) haemanthamin12 and 2-O-acetylbulbisine.TFA.13 The absence of a NOESY correlation from H-4 to 3-OCH3 was consistent with the assigned stereochemistry. The small vicinal coupling constant (3JH-H = 3.4 Hz) of H-4/H-4a also indicated that these protons are syn configured.11,14,15 The structure of 1 was thus assigned as 1R,4S-dihydroxy-3R-methoxy powellan.11
Compounds 2–4 were identified as distichamine, 11-O-acetylambelline, and ambelline, respectively, by comparison of spectroscopic and other physical properties with literature values.15,16 The 13C NMR spectrum of compound 2 was similar to that of compound 1, but instead of signals for two hydroxylated carbons in the cyclohexane ring, it had signals for the carbons of a double bond at C-2 (δC 102.1) and C-3 (δC 174.3), and a ketone at C-1 (δC 199.0). The large deshielding of C-3 in 2 was indicative of oxy-substitution at this olefinic carbon (Fig. 8S).
The 1H and 13C NMR spectra of compounds 3 and 4 both had signals for oxygenated methine carbons at C-11 (δC 85.9 and 87.6) and the carbons of an olefinic double bond at C-1 and C-2 (Fig. 9S–12S). The methylene protons at H-11 in compounds 1 and 2 were replaced by a doublet of doublets at δH 5.02 and 4.23 in 3 and 4, consistent with the presence of hydroxy and acetoxy groups, respectively. We confirmed the relative configuration of 3 and 4 by observing the large coupling between H-4a and H-4β (J = 13.7 Hz, trans diaxial interaction) and H-4α and H-4β (J = 13.9 Hz, geminal) and smaller vicinal couplings of both H-4β (J = 3.9 Hz) and H-4α (J = 1.8 Hz) with H-3 (J = 3.9, 1.8 Hz).14,15 These crinane-type alkaloids have previously been reported from different Amaryllidaceae species, but are reported for the first time from Amaryllis belladonna.13,15,16
Circular dichroism experiments (Fig. 17S) confirmed that the crinane-type alkaloids 1 – 4 contain a common tetrahydro-phenanthridine moiety with a β-5,10b ethano bridge, since they all displayed a positive Cotton effect (CE) around 240–250 nm and a negative CE around 280–290nm.13,14
Compound 5 was determined to be acetylcaranine.11 Its NMR spectra were similar to those of 1 – 4, but the HMBC correlations from a methylene proton (δH 2.59, H-11) to olefinic carbons at C-3 (δC 114.1) and C-4 (δC 139.3) implied that 5 was a lycorine-type alkaloid (Fig. 15S). Compound 6 was identified as hippadine, a well-known lycorine-type alkaloid.11
The antiplasmodial activities of compounds 1 – 6 were determined against the chloroquine-resistant Dd2 strain of P. falciparum (Table 2 and Fig. S18).
Table 2.
Antiplasmodial and antiproliferative activities of compounds 1–6.
| P. falciparum Dd2 (μM) | Mammalian A2780 (μM) | |
|---|---|---|
| 1,4-dihydroxy-3-methoxy powellan (1) | 37 ± 3 | >60.0 |
| distichamine (2) | >50.0 | >60.0 |
| 11-O-acetylambelline (3) | 35 ± 1 | >60.0 |
| ambelline (4) | 7.3 ± 0.3 | >60.0 |
| acetylcaranine (5) | 3.5 ± 0.3 | 56 ± 1 |
| hippadine (6) | NA a | NAa |
Each value represents the mean ± SD of three experiments.
Not active
The lycorine-type alkaloid 5 showed the most potent inhibitory activity, with an IC50 value of 3.3 ± 0.3 μM, but compound 6 was inactive despite its structural similarity, so slight structural differences of conjugation and substituents in the tetrahydrophenanthridine moiety seem to greatly affect the inhibitory activity. The crinane-type alkaloid 3 exhibited weak inhibitory activity (IC50, 35 ± 1 μM), whereas compound 4 showed stronger activity with an IC50 7.3 ± 0.3 μM. The difference in the antiplasmodial activity of these two compounds could be explained by the placement of oxy-substitution at C-11, since acetylation of the oxygenated C-11 of the ethano bridge slightly decreased the inhibitory activity. The new crinane-type alkaloid 1 showed weak inhibitory activity (IC50, 37 ± 3 μM). Compound 2 had little inhibitory activity, in spite of its similarity to compounds 3 and 4. These results are supported by previous reports that the double bond at C1–C2 is responsible for the inhibitory activity in crinane-type alkaloids.9,13,14
We also investigated the antiproliferative activity of alkaloids 1 – 5 against A2780 ovarian cells. Only compound 5 showed any inhibitory effect, and it was very weak with an IC50 value of 56 ± 1 μM. The only reported antimalarial activity of lycorine-type alkaloids was for (+)-5,6-dehydrolycorine,17 and this is the first report of antiproliferative activity of a lycorine-type alkaloid against the A2780 cell line.17 In related work, both acetylcaranine (5) and 11-O-acetylambelline (5) were evaluated for cytotoxicity against Caco-2 and HT-29 cancer cell lines. Acetylcaranine had IC50 values of 29.5 and 19.2 μM, respectively, while 11-O-acetylambelline was inactive.18
The moderate to good antiplasmodial activity of the amaryllidaceae alkaloids 4 and 5, combined with their lack of activity against human A2780 cells, suggests that these alkaloids could be investigated further for development of novel antimalarial agents.
Supplementary Material
Acknowledgments
This project was supported by the National Center for Complementary and Integrative Health under award 1 R01 AT008088, and this support is gratefully acknowledged. This work was also supported by the National Science Foundation under Grant No. CHE-0619382 for purchase of the Bruker Avance 500 NMR spectrometer and Grant No. CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer. We thank Cori Morenberg for the plant collection, Mr. B. Bebout for obtaining the mass spectra, and Dr. Tijana Grove for the use of her ECD spectrometer.
Footnotes
Supporting information, including details of plant collection and extraction and the isolation of compounds and 1 – 6, together with 1H NMR, 13C NMR, HSQC, HMBC, COSY, and NOESY spectra for compound 1, 1H and 13C NMR spectra for compounds 2 – 5, ECD spectra for compounds 1 – 6, and dose-response curves for the antiplasmodial evaluations of compounds 1 and 3 – 5 can be found in the online version at http://dx.doi.org/10.1016/j.bmc.2017.xx.xxx. MOL files of compounds 1 – 6 and raw NMR data files have also been provided.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Anonymous. About Malaria. Centers for Disease Control and Prevention; 2017. 2016. [Google Scholar]
- 2.Anonymous. World Malaria Report 2016. World Health Organization; Geneva: 2016. [Google Scholar]
- 3.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D’Alessandro U. Malaria J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu Y. Angew Chem Int Ed. 2016;55:10210. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis L. Chem Eng News. 2012;90(8):30. [Google Scholar]
- 6.Evidente A, Andolfi A, Abou-Donia AH, Touema SM, Hammoda HM, Shawky E, Motta A. Phytochemistry. 2004;65:2113. doi: 10.1016/j.phytochem.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Pettit GR, Gaddamidi V, Goswami A, Cragg GM. J Nat Prod. 1984;47:796. doi: 10.1021/np50035a007. [DOI] [PubMed] [Google Scholar]
- 8.Charlson AJ. J Ethnopharmacol. 1980;2:323. doi: 10.1016/s0378-8741(80)81014-2. [DOI] [PubMed] [Google Scholar]
- 9.Tallini LR, Andrade JP, Kaiser M, Viladomat F, Nair JJ, Zuanazzi JAS, Bastida J. Molecules. 2017:22. doi: 10.3390/molecules22091437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkov S, Romani S, Herrera M, Viladomat F, Codina C, Momekov G, Ionkova I, Bastida J. Phytother Res. 2011;25:1686. doi: 10.1002/ptr.3468. [DOI] [PubMed] [Google Scholar]
- 11.Koorbanally N, Mulholland DA, Crouch N. Phytochemistry. 2000;54:93. doi: 10.1016/s0031-9422(00)00039-x. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, de Andrade JP, Pigni NB, Torras-Claveria L, Tallini LR, de Borges SW, Viladomat F, Nair JJ, Zuanazzi JAS, Bastida J. Helv Chim Acta. 2016;99:143. [Google Scholar]
- 13.Chen CK, Lin FH, Tseng LH, Jiang CL, Lee SS. J Nat Prod. 2011;74:411. doi: 10.1021/np100819n. [DOI] [PubMed] [Google Scholar]
- 14.Nair JJ, Campbell WE, Brun R, Viladomat F, Codina C, Bastida J. Phytochemistry. 2005;66:373. doi: 10.1016/j.phytochem.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Viladomat F, Codina C, Bastida J, Mathee S, Campbell WE. Phytochemistry. 1995;40:961. [Google Scholar]
- 16.Cheesman L, Nair JJ, van Staden J. J Ethnopharmacol. 2012;140:405. doi: 10.1016/j.jep.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Hao B, Shen SF, Zhao QJ. Molecules. 2013;18:2458. doi: 10.3390/molecules18032458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaněčková N, Hošt’álková A, Šafratová M, Kuneš J, Hulcová D, Hrabinová M, Doskočil I, Štěpánková Š, Opletal L, Nováková L, Jun D, Chlebek J, Cahlíková L. RSC Adv. 2016;6:80114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


