Abstract
Epithelial barriers of the skin, gastrointestinal tract and airway serve common critical functions such as maintaining a physical barrier against environmental insults and allergens, as well as providing a tissue interface balancing the communication between the internal and external environments. We now understand that in allergic disease, regardless of tissue location, the homeostatic balance of the epithelial barrier is skewed towards loss of differentiation, reduced junctional integrity and impaired innate defense. Importantly, epithelial dysfunction characterized by these traits appears to pre-date atopy and development of allergic disease. Despite our growing appreciation of the centrality of barrier dysfunction in the initiation of allergic disease, many important questions remain to be answered regarding the mechanisms disrupting the normal barrier function. Although our external environment (proteases, allergens, injury) is classically thought of as a principal contributor to barrier disruption associated with allergic sensitization, there is a need to better understand contributions of the internal environment (hormones, diet, circadian clock). Systemic drivers of disease, such as alterations of the endocrine system, metabolism and aberrant control of developmental signaling, are emerging as new players in driving epithelial dysfunction and allergic predisposition at various barrier sites. Identifying such central mediators of epithelial dysfunction, using both systems biology tools and causality-driven laboratory experimentation, will be essential in building new strategic interventions to prevent or reverse the process of barrier loss in allergy.
Keywords: epithelial barrier, differentiation, mesenchyme, extracellular matrix, tight junctions, hormones, metabolism, allergic predisposition, allergic sensitization
I. Anatomy and physiology of normal barrier - the Immune Barrier
The epithelial barrier is diverse, occurring, inter alia, in the gastrointestinal tract, the urogenital tract, the respiratory system, the eyes and the skin. Epithelial cells are specialized in each of these organ systems and in the various geographic regions of each system. The main functions of epithelia are to present a physical and immune barrier, maintain a surface that accommodates commensal organisms but not pathogens, remove, degrade or neutralize environmental toxins and particulates, and maintain the balance of water and electrolytes. Specialized functions of the physical barrier include maintenance of the mucociliary escalator in airways, production of surfactants in the alveoli and small airways and production of a protective mucus blanket in the gastrointestinal and urogenital tracts. Immune Barrier refers to epithelial elements that are essential for innate immune resistance to potential pathogens, including constitutive and inducible expression of enzymes, peptides, proteins, lipids, ions and pathogen recognition receptor (PRR) systems designed to protect the host. Combined with epithelial tight junctions, these systems present a vigilant and effective barrier to microbial invasion. Many allergic diseases are now known to manifest clear disorders of the immune barrier. The purpose of this review is to discuss the presence of dysfunction of barrier, its role in allergic disease, and the molecular and endocrinological mechanisms that are underlying causes.
II. Barrier defects in type 2 diseases
Introduction
When the immune barrier is predisposed to disruption, microorganisms and antigens can gain access between epithelial cells through the basement membrane to the underlying lamina propria and connective tissue. Penetration of microbes triggers strong innate immune responses by PRR on epithelial cells and immune cells such as macrophages, ILCs and mast cells, residing among epithelial cells or on the basolateral side of the lamina reticularis. Subsequent sensitization and activation of adaptive immune responses can result, including type 2 immune responses of relevance to the allergic diathesis. Barrier loss can result from defects in several essential components, including tight junction proteins, protective antiproteases, structural elements such as filaggrin in the skin, expression of antimicrobial products, transport of ions, protons, water or antimicrobial materials and other mechanisms. Associated with loss of barrier and sensitization is also the profound activation of sensory nerves important in manifestation of disease experienced by the patient. In this section we briefly discuss some of the salient mechanisms and features of barrier loss commonly shared by all allergic diseases (summarized in Figure 1).
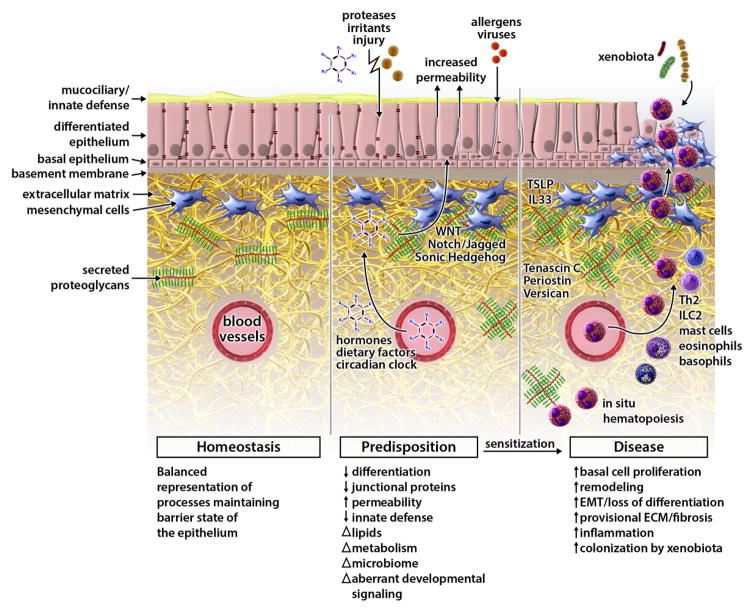
Figure 1. Key features of epithelial dysfunction common to all allergic disease.
Epithelial barriers predisposed to Type 2 allergic disease are characterized by increased permeability and aberrant behavior of morphogenetic programs that maintain epithelial homeostasis. Both exogenous (inhaled allergens, respiratory viruses, chemical sensitizers, air pollutants) and endogenous (hormones, dietary factors, altered circadian clock) disruptors of epithelial homeostasis may drive predisposition to allergic sensitization by altering homeostatic activity of developmental pathways (WNT, Notch, Hedgehog) that maintain proper epithelial-to-mesenchymal communication, epithelial differentiation, and barrier integrity. Transition to a remodeling state in disease typically follows allergic sensitization and inflammatory responses. This transition features further loss of differentiation signals, downregulation of innate defense molecules, pro-fibrotic processes, deposition of extracellular matrix, and potentiated activity of the mesenchymal unit.
Atopic Dermatitis
Atopic dermatitis (AD) is characterized by loss of barrier function of the skin culminating in a clinical phenotype characterized by formation of skin lesions. Skin barrier dysfunction is induced by disruption of the stratum corneum, a dense protein–lipid matrix, which functions as a barrier to water loss, environmental insults and allergens. Filaggrin (FLG), a filament-associated “epidermal differentiation complex” (EDC) protein essential for the regulation of epidermal homeostasis, is highly deficient in skin of many subjects with AD1. Similarly, filaggrin-like proteins hornerin and filaggrin-2 are detected at significantly lower levels in the skin of many patients with AD, irrespective of FLG genotype2. Dysregulated filaggrin is frequently discussed as central to the origins of disease3; however, barrier defects in atopic dermatitis go far beyond filaggrin deficiency. Defects in claudins 1, 4 and 8 have also been associated with the development of atopic dermatitis4–7. Disruption of the skin barrier involves defects in the entire keratinocyte terminal differentiation program via dysregulation of multiple EDC genes on human chromosome region 1q218, reduced expression of epithelial tight junction proteins9, increased transepidermal water loss (TEWL)10, 11, and reduction in epidermal natural moisturizing factors12. The impaired skin barrier function in AD also involves altered lipid profiles, including shortening of carbon chain length of stratum corneum lipids (ceramides and free fatty acids), an event that exhibits strong correlations with TEWL and occurs independently of filaggrin mutations13–16. Intriguingly, clinically unaffected, non-lesional skin in subjects with AD similarly exhibits defects in terminal keratinocyte differentiation17, as well as reduced filaggrin and lipids14, 15, suggesting a global cutaneous predisposition to barrier dysfunction in AD patients.
Asthma
An intact functional mucosal barrier is crucial for the maintenance of airway homeostasis. Despite the existence of multiple clinical endotypes, most forms of asthma exhibit a dysregulated epithelial barrier. Asthmatic epithelium is characterized by an increase in basal and goblet cells18 and a decrease in terminally differentiated ciliated cells, frequently accompanied by basement membrane thickening19, 20 and epithelial shedding with the formation of Creola bodies consisting of clusters of shed epithelium (even in mild forms of disease)21–23. Disruption of epithelial tight and adherens junctions is typical for asthma, with marked loss of E-cadherin24 and claudin-1825. The underlying extracellular matrix, which supports homeostasis and repair of the epithelium, also undergoes significant remodeling, characterized by increased deposition of provisional matrix components, such as the glycoproteins fibronectin26, periostin27, tenascin-C28, 29, hyaluronan and versican30. Epithelial-to-mesenchymal transition (EMT) was proposed as a mechanism underlying epithelial dedifferentiation and perpetuated remodeling31; however, this remains a topic of debate, due to the lack of markers accurately defining this process in asthma32. Mucociliary clearance, which depends on the cooperation between submucosal glands, goblet cells and ciliated cells, and normal chemical barrier function of the asthmatic epithelium are also compromised. There are profound changes in relative proportions and viscosity of mucins MUC5AC and MUC5B that in turn contribute to airway obstruction33, 34, and downregulation of lipoxins35. Perturbations in sphingolipid balance with increased levels of ceramides have also been reported in asthmatic airway epithelium 36.
Allergic Rhinitis
Mucosal epithelial barrier disruption is observed in models of allergic rhinitis and in patients37. Allergens can contain proteases and have been shown to disrupt epithelial tight junctions38, 39. Nasal challenge with histamine, or with antigen in a sensitized subject, can cause significant plasma exudation across the epithelial barrier. This is a non-destructive process and may be a first line defense of respiratory mucosa40. A proteomic study detected changes reflecting altered epithelial permeability in patients with allergic rhinitis, including increased α2 macroglobulin, a measure of vascular and epithelial leak, and decreased levels of the protective antileukoproteinase SLPI41. Reduced levels of barrier proteins E-cadherin and zonula occludens-1 (ZO-1) were observed in nasal mucosal tissue from allergic rhinitis patients compared to healthy controls using real time PCR, Western blot and immunohistochemistry42. In vitro, IL-4 and TNF induced loss of these markers in epithelium. Steelant et al. observed decreased barrier function measured in vitro using primary epithelial cells collected in patients with allergic rhinitis compared to healthy controls and found decreased expression of the tight junction proteins occludin and ZO-143.
Chronic rhinosinusitis
Early studies provided evidence for defective barrier in patients with chronic rhinosinusitis (CRS) using immunohistochemistry, bioelectric evaluation and ion transport function as indicators44, 45. Using air-liquid interface epithelial cultures, Soyka et al. showed reduced barrier function using tissues collected from patients with or without CRS46. A number of studies have focused on the loss of epithelial tight junctions in CRS patients by assessing levels of individual tight junction proteins37, 47. Alterations of expression of ZO-1 and E-cadherin were reported by Jang et al.48. Epithelial damage, including shortening of desmosomes, was reported by Shahana et al. in a study using electron microscopy in polyp tissue derived from asthmatic patients49. Reductions of the tight junction proteins claudin-1 and occludin in CRS tissues were reported in another study50. The group of Bachert reported that ZO-1, occludin and E-cadherin were all reduced in mature polyps removed from CRSwNP patients51. Moreover, aquaporin 5, a marker of epithelial differentiation, was significantly reduced in sinonasal samples of CRSwNP subjects compared to CRSsNP or controls52. Physical evidence of disruption of epithelial barrier in CRS includes prominent acanthosis and acantholysis47. The loss of differentiation and acanthosis may result from a cycle of ongoing injury and repair associated with epithelial to mesenchymal transition (EMT). Factors that have been identified in tissues from CRS patients that might stimulate barrier loss and acanthosis include TGFα, oncostatin M, epiregulin, hypoxic activation of HIF1α and endocrine deficiency (see below)47, 53–55.
Eosinophilic Esophagitis (EoE)
The esophagus is composed of stratified squamous epithelium remarkably similar to the epithelium of the skin, only differing in its lack of a cornified layer and its possession of a mucous layer56. As occurs in other Type 2 diseases, the normal structure of the epithelium is disrupted in EoE, including basal cell hyperplasia, dilated intracellular spaces and impaired barrier function and cell junctions57, 58. These changes are thought to be associated with dysregulated epithelial differentiation and impaired epithelial barrier formation. As in the skin, epithelium in EoE is characterized by dysregulation of genes of the epidermal differentiation complex on human chromosome 1q21, including filaggrin, involucrin and several small proline-rich repeat family members59. In esophageal epithelial cells, filaggrin was shown to be negatively regulated in response to IL-13 ex vivo59. Desmosomal and tight junctional proteins desmoglein-1, E-cadherin and claudin-1 are reduced in active EoE60, 61. Epithelial barriers in eosinophilic esophagitis also exhibit characteristic features of EMT, which correlate with subepithelial fibrosis and eosinophil counts in human biopsies62.
III. Mechanisms of barrier defects
Introduction
Barrier defects can be induced by loss or defects in key proteins that comprise tight or adherens junctions, disruption of barrier by environmental exposures (including proteases, chemical injury or trauma), inflammatory responses that induce barrier disrupting Th2 cytokines and endogenous mechanisms such as altered central metabolism and imbalance of hormones that regulate epithelial homeostasis. The purpose of this section is to briefly consider some of the external and internal factors that are thought to be of particular importance in the disruption of barriers in allergic diseases.
Genetic and epigenetic barrier defects
Genome-wide association studies (GWAS) and positional cloning have successfully identified several risk alleles and loci reproducibly associated with atopic dermatitis, asthma and eosinophilic esophagitis. Interestingly, the majority of allergic susceptibility candidate genes control epithelial barrier homeostasis. Null gene mutations of filaggrin (FLG) (as mentioned previously, an important component of terminal keratinocyte differentiation) are the most significant known risk factor for atopic dermatitis and eosinophilic esophagitis, along with mutations of other epidermal differentiation cluster (EDC) genes59. Polymorphisms in asthma susceptibility genes ORMDL3, GSDMB, PCDH1, CDHR3, ADAM33, SMAD3, IL1RL1 and IL18R1 are now thought to be linked to aberrant epithelial remodeling, the unfolded protein response and lipid biosynthesis63–65. A recent pathway-based association study by Barreto-Luis et al.66 revealed novel asthma susceptibility loci near WNT pathway genes that regulate barrier morphogenesis. Even though genetic associations are stronger within clinically well-defined subgroups of disease67, overall allergic disease-associated alleles typically have small effect sizes and cannot account for the rapidly rising prevalence of allergy68, reinforcing the significance of environmental factors in development of allergic diseases. Significantly, environmental inputs can affect gene transcription via heritable epigenetic regulation that does not require alterations in gene sequence, including DNA and histone modifications, as well as changes in noncoding RNAs69. One of the notable epigenetic changes in asthmatic epithelium involves hypo-methylation of KRT569, which increases keratin 5 expression in basal epithelium and may therefore lead to dysregulated epithelial differentiation70–72. The potential for epigenetic “reprogramming” is evident when asthmatic epithelium cultured in normal media still retains persistent indicators of defects in junctional maintenance, “immaturity” and repair ex vivo73. The topic of epigenetic mechanisms of epithelial remodeling is a still-emerging area of investigation of epithelial barrier dysfunction, and ongoing work is likely to produce novel insights to aberrant regulation of epithelial differentiation programs in allergic disease.
Environmental contributions to barrier loss
Barriers of the skin, airways and gastrointestinal tract constantly experience biological and chemical insults from the surrounding environment. Allergens, such as house dust mite or pollens, are capable of disrupting physical integrity of the barrier via their protease activity, which degrades adhesion proteins and triggers epithelial alarmin cytokine response74–78. Respiratory viruses promote airway epithelial barrier dysfunction by disrupting epithelial junctions, which may represent an inciting or sustaining event linking viral infections and allergic inflammation79, 80. Interestingly, asthma susceptibility barrier gene CDHR3 is a receptor for rhinovirus C65. Many chemicals and irritants prevalent in industrial environments act as adjuvants and sensitizers, disrupting normal functions of the epithelial barrier, setting off alarmin responses and promoting allergic sensitization81, 82. Air pollution associates with the development of atopic dermatitis in both children and adults83, 84. Less obvious, but equally important, environmental contributors to barrier loss do not disrupt barrier externally, but act as endogenous factors in dysregulation of epithelial homeostatic processes. Stimulation of the aryl hydrocarbon receptor (AHR) by ingested or inhaled xenobiotics, such as polycyclic aromatic hydrocarbons, is now known to affect epidermal differentiation and skin barrier formation85. Xenoestrogens linked to development of allergy, such as bisphenols and phthalates, mimic the natural action of nuclear hormonal receptor ligands (estrogens, androgens) and thus disrupt normal epithelial homeostatic processes86–88. A multitude of dietary factors have the capacity to alter epithelial behavior via direct receptor action or indirect regulation of tissue metabolism. For example, Fischer et al. demonstrated that vitamin D supplementation, acting via the vitamin D receptor pathway, reduces EMT processes and improves barrier function in several clinically relevant murine models of asthma89. Fatty acid-deficient diets spontaneously induce skin barrier disruption via alteration of skin metabolism90, 91. Moreover, the intestinal microbiome is now emerging as an important regulator of metabolism with consequences for epithelial and immune homeostasis92.
Defects in hormonal signaling as early events
Contributions of biological systems other than the immune system to disruption of epithelial homeostasis and priming of allergic response are currently poorly understood. Several studies report insulin resistance in children and adults with asthma93, 94, as well as association of asthma and atopic eczema with pre-diabetes and metabolic syndrome95. We found profound changes in serum hormonal profiles of pre-pubertal non-obese allergic children compared to non-allergic controls, as well as chronic rhinosinusitis patients compared to healthy controls, including decreased levels in serum insulin and increased output of thyroid and growth hormones96. Hormones play a critical role in maintenance of epithelial barrier homeostasis and integrity via their integration with epithelial morphogenetic programs88, 97, evidenced by deficiencies in wound healing and epithelial dysfunction in patients with thyroid disease and diabetes98, 99. Insulin and IGF-1 are essential drivers of epithelial differentiation and regulators of energy metabolism. Double transgenic mice lacking both insulin and IGF-1 receptors are characterized by severe defects in epithelial differentiation, severely impaired stratification of the epidermis, spontaneous lesions and overall loss of skin barrier function100. Sex steroids, and estrogen in particular, are recently receiving more attention for their potential to explain sex bias in prevalence of allergic disease101. Glucocorticoids have profound effects on epithelial differentiation102, 103 and exemplify the therapeutic potential of manipulating the endocrine system in treatment of allergic disease.
Disruption of normal epithelial development
The significance of early life events in initiation and propagation of allergic disease is now widely recognized. The first two years of life represent a window of susceptibility for development of asthma. Importantly, epithelial barriers in childhood are shaped and regulated by active on-going developmental programs. In particular, morphogenesis of lung epithelial barriers continues throughout normal postnatal development, characterized by ongoing septation and epithelialization of the lung104. Consequently, perturbation of barrier morphogenesis in childhood may have a lasting effect on adult epithelium via alteration of developmental programs and epigenetic reprogramming at developmental checkpoints105–107. Multiple lines of evidence now implicate active re-engagement of morphogenetic programs in adult disease, typically not seen in adult homeostatic tissue108–110. Alterations of the Wnt66, Hippo111, Notch/Jagged112, 113 and Hedgehog114 developmental pathways all exhibit strong association with epithelial remodeling and allergy. Early disruption of normal barrier development frequently pre-dates atopy11, 44 and is emerging as central to initiation of the “atopic march”. Mouse models of the atopic march demonstrate conclusively that sensitization to allergens via disrupted skin barriers is sufficient to elicit an immune response at other barrier sites115, 116. Causes of disruption of epithelial morphogenesis early in life are not well understood. Early life viral exposures117, 118 and changes in microbiota119 are some of the better understood factors driving aberrant epithelial responses and a predisposition to allergic sensitization.
The Injury-Repair cycle and EMT
Exposure to environmental factors causes injury or disruption of normal homeostasis of the epithelium followed by engagement of robust repair processes to minimize further damage from microbes and environment. Mature differentiated epithelial cells undergo an epithelial to mesenchymal transition (EMT) in which the cells lose their attachments to basement membrane and each other, lose their polarity, begin to divide and become migratory in order to rapidly cover the injured area. During this process, epithelial cells lose expression of tight junction proteins ZO-1, occludin, E-cadherin and other markers of mature differentiated epithelium. The mesenchymal cells derived from the basal epithelial cells begin to produce desmin, fibronectin, tenascin, laminin, collagens and others proteins that form a provisional matrix to protect the exposed basement membrane or lamina propria and express differentiation markers such as α-smooth muscle actin. Evidence for EMT usually consists of loss of tight junction proteins, gain of provisional matrix proteins or biomarkers, or both. Early studies by Davies, Holgate, Hackett, Knight and others found evidence for persistence of EMT in asthma120–123, although it is more often described as a mild, “partial EMT” phenotype, which could contribute to poor expression of conventional EMT markers in asthmatic airways32. More recent studies found similar evidence for increased EMT in allergic rhinitis42, 43. Several studies have demonstrated ongoing EMT in CRS37, 47, 49–51. Two studies have demonstrated ongoing EMT in eosinophilic esophagitis62, 124. However, to date, the authors have not found any reports of EMT (as classically defined by developmental biologists) in atopic dermatitis, despite abundant evidence for loss of epithelial differentiation programs in the AD skin. Most chronic type 2 inflammatory diseases thus often appear to have a chronic EMT-based ongoing injury-repair cycle.
Inflammation and barrier - a vicious cycle
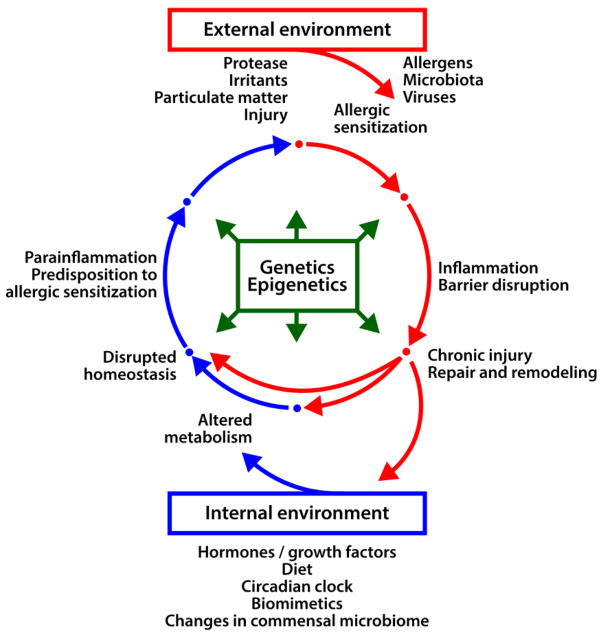
Whether inflammation is the primary cause of barrier disruption or whether it is barrier dysfunction that leads to sensitization and aberrant inflammatory response poses a long-standing “chicken-egg” dilemma (Figure 2). Aberrant innate immune activity at barrier sites favors sensitization to innocuous antigens and if there is an ensuing adaptive immune response, it can lead to a full-scale inflammatory attack on epithelial cells. During chronic inflammation associated with repeated or persistent antigen exposure, inflammation can lead to persistent disruption of epithelial junctions, as well as epithelial remodeling and sub-epithelial fibrosis driven by chronic perpetuated repair. Conversely, an accumulating body of evidence points to a causal role for barrier dysfunction as a primary driver of the allergic response and a vicious cycle of barrier leak125–127. Strikingly, only 30% to 40% of cases of allergic disease (asthma, eczema, allergic rhinitis) in early childhood are attributable to atopy and 60% to 70% of cases result from yet unknown factors128. In most “atopic march” cases, increased skin permeability at birth and non-atopic eczema frequently predate development of atopy11, 127, regardless of filaggrin mutations10. Moreover, although the atopic march assumes that one allergic disease brings risk of acquisition of another allergic disease later in life, it may involve sensitization to antigens different from those that triggered the initial disease129, 130. From this perspective, an alternative view might be that atopy and allergic inflammation are both secondary to a yet-unknown systemic process progressively afflicting epithelial barriers of the skin, gut and airway. Based on our observations of hormonal imbalance in allergic children (described above), we hypothesize that dysregulation of the endocrine system could represent a missing systemic link underlying susceptibility to initial barrier dysfunction. Remarkable similarities in the biology of barrier dysfunction across different allergic diseases (Figure 1), including upper and lower “unified” airway responses and non-lesional defects in clinically unaffected skin of atopic dermatitis patients, all support the existence of such a systemic trigger of disease131–133. Transgenic mouse models with defects in proteins maintaining epithelial homeostasis, such as filaggrin, are also characterized by spontaneous allergic sensitization and enhanced allergic inflammation134–137. This notion of causality of a pre-existing barrier problem fits well with an emerging view of Type 2 immunity as a “restorative” response evolved to maintain tissue homeostasis and assist with repair and remodeling138, 139. For example, Huang et al.140 provide supporting evidence suggesting that Th2 immune responses and homeostatic eosinophil activity141 may have evolved not to expel parasites, but rather to remodel tissue that contains parasitic infestation and minimize potential damage to the host.
Figure 2. Changing states of the epithelial barrier on a homeostasis-allergic disease continuum.
Dysregulation of normal epithelial barrier function is a gradual process involving multiple causal factors. This includes genetics, epigenetics and influences of the external and internal epithelial environments.
IV. Identifying key drivers of defects and the basis for predisposition to barrier disease
The existence of a dilemma about whether it is barrier loss or sensitization that is the primary cause of this vicious cycle illustrates well the complexity of allergic pathogenesis (Figure 2). In health, multiple biological systems and processes must work in unison to maintain tissue homeostasis at barrier sites. This includes morphogenetic/developmental programs, metabolism, the endocrine and the immune systems. There is a growing number of molecules that we now believe are of critical importance in regulating barrier biology; however, despite exhibiting strong phenotypes in models of disease, they may not be central drivers of disease pathogenesis. Filaggrin, for example, is only one of many molecules in epidermal differentiation complex (EDC)142. Knock-down of filaggrin alone was not sufficient to affect lipid composition and permeability in an ex vivo skin model143. Several other EDC molecules play an equally critical role in epithelial homeostasis and are frequently downregulated with filaggrin in AD, independently of filaggrin genotype or atopic status8. Such changes in differentiation status of cells are intricately intertwined with changes in tissue metabolic demands144, aberrant immune responses145 and epigenetic rewiring146. The question then becomes which non-genetic factors have capacity to dysregulate the entire EDC cluster, affecting the balance of the whole system? Identifying such central mediators is the key to finding strategies to restore the homeostatic state of the epithelial barrier. The endocrine system and changes in central metabolism have the potential to be central drivers of the predisposition to barrier defects, given the systemic regulatory effects of hormones (insulin, EGF-1) on epithelial morphogenetic programs of all barrier sites147–149, and their regulatory roles for metabolism and immunity. Our understanding of central mediators of allergic disease is currently impeded by use of reductionist approaches and limited focus on processes of the immune system, insufficient understanding of complexity of biological interactions that control normal epithelial homeostasis, the dynamic nature of biological systems and the extreme clinical heterogeneity of asthma and other allergic diseases. Studies of systems and integrative biology address exactly these challenges, and are now emerging as critical in advancing all branches of biomedical science. Next generation sequencing technology, shared availability of “big data” and advances in computational biology are new promising tools for discovery of novel drivers of barrier dysfunction150, 151.
V. Targets for therapeutic intervention
There are severe forms of the diseases discussed in this review, asthma, allergic rhinitis, chronic rhinosinusitis, eosinophilic esophagitis and atopic dermatitis, that are difficult to manage and for which there is a serious unmet medical need for new and more effective medications. At present, one of the most highly effective classes of drugs for these diseases is topical glucocorticosteroids. These drugs improve elements of innate immunity as well as barrier function, as measured by a number of criteria, including markers of tight junction presence and function. Although the benefit of corticosteroids derives from suppressing the inflammatory response, e.g. expression of IL-13 and other cytokines, as well as direct effects on epithelial cells to promote barrier integrity, the relative contribution of these two effects is not clear. New classes of drugs are rapidly emerging to help manage disease in patients for whom glucocorticosteroids are inadequate to control disease. This includes small molecule drugs that block signaling pathways (CRTH2, GATA3, Jak and other kinases, etc.) and monoclonal antibodies that block cytokine signaling or eliminate key allergic effector cells such as eosinophils and mast cells (IL-5, IL-13, IL-5Rα, IL-4/IL-13Rα, Siglec-8 etc.). The pursuit of barrier restorative therapies is another emerging field152. Based on the hypothesis advanced here that endocrine defects are important in disease pathogenesis, it will be worthwhile to test manipulation of signaling of other hormones (insulin, growth hormone, thyroid hormone etc.), seeking benefit for patients.
VI. Summary and conclusions
Many important questions remain to be answered regarding the mechanisms by which the epithelial barrier becomes disrupted in allergic diseases. We also need to better understand the role that barrier loss plays as both an initiator and as a consequence of the inflammatory process. We need to know more about genetic and epigenetic factors that cause barrier loss, as well as the environmental triggers and the cellular and molecular mediators and targets of the process. Identifying the central mediators of the response and the consequences of barrier dysfunction in disease will be essential in building a rationale and in strategic design of new interventions to prevent or reverse the process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792–9. doi: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Mechin MC, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013;131:1094–102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 3.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 4.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–86. e1–7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tokumasu R, Yamaga K, Yamazaki Y, Murota H, Suzuki K, Tamura A, et al. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc Natl Acad Sci U S A. 2016;113:E4061–8. doi: 10.1073/pnas.1525474113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esaki H, Ewald DA, Ungar B, Rozenblit M, Zheng X, Xu H, et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J Allergy Clin Immunol. 2015;135:153–63. doi: 10.1016/j.jaci.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu HS, Kang MJ, Kwon JW, Lee SY, Lee E, Yang SI, et al. Claudin-1 polymorphism modifies the effect of mold exposure on the development of atopic dermatitis and production of IgE. J Allergy Clin Immunol. 2015;135:827–30. e5. doi: 10.1016/j.jaci.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Guttman-Yassky E, Suarez-Farinas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009;124:1235–44. e58. doi: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Yuki T, Tobiishi M, Kusaka-Kikushima A, Ota Y, Tokura Y. Impaired Tight Junctions in Atopic Dermatitis Skin and in a Skin-Equivalent Model Treated with Interleukin-17. PLoS One. 2016;11:e0161759. doi: 10.1371/journal.pone.0161759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horimukai K, Morita K, Narita M, Kondo M, Kabashima S, Inoue E, et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol Int. 2016;65:103–8. doi: 10.1016/j.alit.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, McLean WH, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930–5. e1. doi: 10.1016/j.jaci.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Chittock J, Cooke A, Lavender T, Brown K, Wigley A, Victor S, et al. Development of stratum corneum chymotrypsin-like protease activity and natural moisturizing factors from birth to 4 weeks of age compared with adults. Br J Dermatol. 2016;175:713–20. doi: 10.1111/bjd.14568. [DOI] [PubMed] [Google Scholar]
- 13.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755–66. doi: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo KM, Hwang JH, Bae S, Nahm DH, Park HS, Ye YM, et al. Relationship of ceramide-, and free fatty acid-cholesterol ratios in the stratum corneum with skin barrier function of normal, atopic dermatitis lesional and non-lesional skins. J Dermatol Sci. 2015;77:71–4. doi: 10.1016/j.jdermsci.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 15.van Smeden J, Janssens M, Kaye EC, Caspers PJ, Lavrijsen AP, Vreeken RJ, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol. 2014;23:45–52. doi: 10.1111/exd.12293. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Villarreal M, Stewart S, Choi J, Indra G, Babineau DC, et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in Atopic Dermatitis. Br J Dermatol. 2017 doi: 10.1111/bjd.15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954–64. e1–4. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med. 2001;163:517–23. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- 19.Honkova L, Uhlik J, Berankova K, Svobodova T, Pohunek P. Epithelial basement membrane thickening is related to TGF-Beta 1 expression in children with chronic respiratory diseases. Pediatr Allergy Immunol. 2014;25:593–9. doi: 10.1111/pai.12275. [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Yin G, Liu J, Liu X, Zhao S. Epithelial apoptosis and loss in airways of children with asthma. J Asthma. 2011;48:358–65. doi: 10.3109/02770903.2011.565848. [DOI] [PubMed] [Google Scholar]
- 21.Trautmann A, Kruger K, Akdis M, Muller-Wening D, Akkaya A, Brocker EB, et al. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol. 2005;138:142–50. doi: 10.1159/000088436. [DOI] [PubMed] [Google Scholar]
- 22.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–83. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- 23.Wilson SJ, Rigden HM, Ward JA, Laviolette M, Jarjour NN, Djukanovic R. The relationship between eosinophilia and airway remodelling in mild asthma. Clin Exp Allergy. 2013;43:1342–50. doi: 10.1111/cea.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heijink IH, Kies PM, Kauffman HF, Postma DS, van Oosterhout AJ, Vellenga E. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J Immunol. 2007;178:7678–85. doi: 10.4049/jimmunol.178.12.7678. [DOI] [PubMed] [Google Scholar]
- 25.Sweerus K, Lachowicz-Scroggins M, Gordon E, LaFemina M, Huang X, Parikh M, et al. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139:72–81. e1. doi: 10.1016/j.jaci.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge Q, Zeng Q, Tjin G, Lau E, Black JL, Oliver BG, et al. Differential deposition of fibronectin by asthmatic bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1093–102. doi: 10.1152/ajplung.00019.2015. [DOI] [PubMed] [Google Scholar]
- 27.Izuhara K, Ohta S, Ono J. Using Periostin as a Biomarker in the Treatment of Asthma. Allergy Asthma Immunol Res. 2016;8:491–8. doi: 10.4168/aair.2016.8.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bains SN, Tourkina E, Atkinson C, Joseph K, Tholanikunnel B, Chu HW, et al. Loss of caveolin-1 from bronchial epithelial cells and monocytes in human subjects with asthma. Allergy. 2012;67:1601–4. doi: 10.1111/all.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsurikisawa N, Oshikata C, Tsuburai T, Saito H, Sekiya K, Tanimoto H, et al. Bronchial reactivity to histamine is correlated with airway remodeling in adults with moderate to severe asthma. J Asthma. 2010;47:841–8. doi: 10.3109/02770903.2010.504876. [DOI] [PubMed] [Google Scholar]
- 30.Wight TN. Provisional matrix: A role for versican and hyaluronan. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson JR, Roos A, Berg T, Nord M, Fuxe J. Chronic respiratory aeroallergen exposure in mice induces epithelial-mesenchymal transition in the large airways. PLoS One. 2011;6:e16175. doi: 10.1371/journal.pone.0016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–5. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 33.Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, et al. Abnormalities in MUC5AC and MUC5B Protein in Airway Mucus in Asthma. Am J Respir Crit Care Med. 2016;194:1296–9. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–30. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zehethofer N, Bermbach S, Hagner S, Garn H, Muller J, Goldmann T, et al. Lipid Analysis of Airway Epithelial Cells for Studying Respiratory Diseases. Chromatographia. 2015;78:403–13. doi: 10.1007/s10337-014-2787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang N, Van Crombruggen K, Gevaert E, Bachert C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy. 2016;71:295–307. doi: 10.1111/all.12809. [DOI] [PubMed] [Google Scholar]
- 38.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–42. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 39.Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–33. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Persson CG, Erjefalt I, Alkner U, Baumgarten C, Greiff L, Gustafsson B, et al. Plasma exudation as a first line respiratory mucosal defence. Clin Exp Allergy. 1991;21:17–24. doi: 10.1111/j.1365-2222.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 41.Tomazic PV, Birner-Gruenberger R, Leitner A, Obrist B, Spoerk S, Lang-Loidolt D. Nasal mucus proteomic changes reflect altered immune responses and epithelial permeability in patients with allergic rhinitis. J Allergy Clin Immunol. 2014;133:741–50. doi: 10.1016/j.jaci.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 42.Lee HJ, Kim B, Im NR, Lee DY, Kim HK, Lee SH, et al. Decreased expression of E-cadherin and ZO-1 in the nasal mucosa of patients with allergic rhinitis: Altered regulation of E-cadherin by IL-4, IL-5, and TNF-alpha. Am J Rhinol Allergy. 2016;30:173–8. doi: 10.2500/ajra.2016.30.4295. [DOI] [PubMed] [Google Scholar]
- 43.Steelant B, Farre R, Wawrzyniak P, Belmans J, Dekimpe E, Vanheel H, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137:1043–53. e1–5. doi: 10.1016/j.jaci.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein JM, Gorfien J, Noble B, Yankaskas JR. Nasal polyposis: immunohistochemistry and bioelectrical findings (a hypothesis for the development of nasal polyps) J Allergy Clin Immunol. 1997;99:165–75. doi: 10.1016/s0091-6749(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 45.Dejima K, Randell SH, Stutts MJ, Senior BA, Boucher RC. Potential role of abnormal ion transport in the pathogenesis of chronic sinusitis. Arch Otolaryngol Head Neck Surg. 2006;132:1352–62. doi: 10.1001/archotol.132.12.1352. [DOI] [PubMed] [Google Scholar]
- 46.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012;130:1087–96. e10. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 47.Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017;12:331–57. doi: 10.1146/annurev-pathol-052016-100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang YJ, Kim HG, Koo TW, Chung PS. Localization of ZO-1 and E-cadherin in the nasal polyp epithelium. Eur Arch Otorhinolaryngol. 2002;259:465–9. doi: 10.1007/s00405-002-0500-z. [DOI] [PubMed] [Google Scholar]
- 49.Shahana S, Jaunmuktane Z, Asplund MS, Roomans GM. Ultrastructural investigation of epithelial damage in asthmatic and non-asthmatic nasal polyps. Respir Med. 2006;100:2018–28. doi: 10.1016/j.rmed.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Rogers GA, Den Beste K, Parkos CA, Nusrat A, Delgaudio JM, Wise SK. Epithelial tight junction alterations in nasal polyposis. Int Forum Allergy Rhinol. 2011;1:50–4. doi: 10.1002/alr.20014. [DOI] [PubMed] [Google Scholar]
- 51.Meng J, Zhou P, Liu Y, Liu F, Yi X, Liu S, et al. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS One. 2013;8:e82373. doi: 10.1371/journal.pone.0082373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shikani AH, Sidhaye VK, Basaraba RJ, Shikani HJ, Alqudah MA, Kirk N, et al. Mucosal expression of aquaporin 5 and epithelial barrier proteins in chronic rhinosinusitis with and without nasal polyps. Am J Otolaryngol. 2014;35:377–83. doi: 10.1016/j.amjoto.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci E, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol. 2015;136:737–46. e4. doi: 10.1016/j.jaci.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berdnikovs S. Meta-analysis of gene expression microarrays reveals novel biomarkers consistent with altered functionality of mucosal barrier in patients with chronic rhinosinusitis. 2014 AAAAI Annual Meeting: Aaaai; 2014. [Google Scholar]
- 56.Rosekrans SL, Baan B, Muncan V, van den Brink GR. Esophageal development and epithelial homeostasis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G216–28. doi: 10.1152/ajpgi.00088.2015. [DOI] [PubMed] [Google Scholar]
- 57.Odze RD. Pathology of eosinophilic esophagitis: what the clinician needs to know. Am J Gastroenterol. 2009;104:485–90. doi: 10.1038/ajg.2008.40. [DOI] [PubMed] [Google Scholar]
- 58.Ravelli AM, Villanacci V, Ruzzenenti N, Grigolato P, Tobanelli P, Klersy C, et al. Dilated intercellular spaces: a major morphological feature of esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:510–5. doi: 10.1097/01.mpg.0000215312.78664.b9. [DOI] [PubMed] [Google Scholar]
- 59.Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184:4033–41. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7:718–29. doi: 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdulnour-Nakhoul SM, Al-Tawil Y, Gyftopoulos AA, Brown KL, Hansen M, Butcher KF, et al. Alterations in junctional proteins, inflammatory mediators and extracellular matrix molecules in eosinophilic esophagitis. Clin Immunol. 2013;148:265–78. doi: 10.1016/j.clim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Kagalwalla AF, Akhtar N, Woodruff SA, Rea BA, Masterson JC, Mukkada V, et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol. 2012;129:1387–96. e7. doi: 10.1016/j.jaci.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paulenda T, Draber P. The role of ORMDL proteins, guardians of cellular sphingolipids, in asthma. Allergy. 2016;71:918–30. doi: 10.1111/all.12877. [DOI] [PubMed] [Google Scholar]
- 65.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112:5485–90. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barreto-Luis A, Corrales A, Acosta-Herrera M, Gonzalez-Colino C, Cumplido J, Martinez-Tadeo J, et al. A pathway-based association study reveals variants from Wnt signalling genes contributing to asthma susceptibility. Clin Exp Allergy. 2017 doi: 10.1111/cea.12883. [DOI] [PubMed] [Google Scholar]
- 67.Lavoie-Charland E, Berube JC, Boulet LP, Bosse Y. Asthma susceptibility variants are more strongly associated with clinically similar subgroups. J Asthma. 2016;53:907–13. doi: 10.3109/02770903.2016.1165699. [DOI] [PubMed] [Google Scholar]
- 68.Ober C. Asthma Genetics in the Post-GWAS Era. Ann Am Thorac Soc. 2016;13(Suppl 1):S85–90. doi: 10.1513/AnnalsATS.201507-459MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stefanowicz D, Hackett TL, Garmaroudi FS, Gunther OP, Neumann S, Sutanto EN, et al. DNA methylation profiles of airway epithelial cells and PBMCs from healthy, atopic and asthmatic children. PLoS One. 2012;7:e44213. doi: 10.1371/journal.pone.0044213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moheimani F, Hsu AC, Reid AT, Williams T, Kicic A, Stick SM, et al. The genetic and epigenetic landscapes of the epithelium in asthma. Respir Res. 2016;17:119. doi: 10.1186/s12931-016-0434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ji H, Zhang X, Oh S, Mayhew CN, Ulm A, Somineni HK, et al. Dynamic transcriptional and epigenomic reprogramming from pediatric nasal epithelial cells to induced pluripotent stem cells. J Allergy Clin Immunol. 2015;135:236–44. doi: 10.1016/j.jaci.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kicic A, Sutanto EN, Stevens PT, Knight DA, Stick SM. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med. 2006;174:1110–8. doi: 10.1164/rccm.200603-392OC. [DOI] [PubMed] [Google Scholar]
- 73.Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol. 2007;7:63–8. doi: 10.1097/ACI.0b013e328013d61b. [DOI] [PubMed] [Google Scholar]
- 74.Stremnitzer C, Manzano-Szalai K, Starkl P, Willensdorfer A, Schrom S, Singer J, et al. Epicutaneously applied Der p 2 induces a strong TH 2-biased antibody response in C57BL/6 mice, independent of functional TLR4. Allergy. 2014;69:741–51. doi: 10.1111/all.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vinhas R, Cortes L, Cardoso I, Mendes VM, Manadas B, Todo-Bom A, et al. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy. 2011;66:1088–98. doi: 10.1111/j.1398-9995.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 76.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kale SL, Agrawal K, Gaur SN, Arora N. Cockroach protease allergen induces allergic airway inflammation via epithelial cell activation. Sci Rep. 2017;7:42341. doi: 10.1038/srep42341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stentzel S, Teufelberger A, Nordengrun M, Kolata J, Schmidt F, van Crombruggen K, et al. Staphylococcal serine protease-like proteins are pacemakers of allergic airway reactions to Staphylococcus aureus. J Allergy Clin Immunol. 2017;139:492–500. e8. doi: 10.1016/j.jaci.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 79.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178:1271–81. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rezaee F, DeSando SA, Ivanov AI, Chapman TJ, Knowlden SA, Beck LA, et al. Sustained protein kinase D activation mediates respiratory syncytial virus-induced airway barrier disruption. J Virol. 2013;87:11088–95. doi: 10.1128/JVI.01573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirano M, Tanaka S, Asami O. Classification of polycyclic aromatic hydrocarbons based on mutagenicity in lung tissue through DNA microarray. Environ Toxicol. 2013;28:652–9. doi: 10.1002/tox.20761. [DOI] [PubMed] [Google Scholar]
- 82.Friedmann PS, Pickard C. Contact hypersensitivity: quantitative aspects, susceptibility and risk factors. EXS. 2014;104:51–71. doi: 10.1007/978-3-0348-0726-5_5. [DOI] [PubMed] [Google Scholar]
- 83.Tang KT, Ku KC, Chen DY, Lin CH, Tsuang BJ, Chen YH. Adult atopic dermatitis and exposure to air pollutants-a nationwide population-based study. Ann Allergy Asthma Immunol. 2017;118:351–5. doi: 10.1016/j.anai.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Huang CC, Wen HJ, Chen PC, Chiang TL, Lin SJ, Guo YL. Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol. 2015;173:981–8. doi: 10.1111/bjd.14039. [DOI] [PubMed] [Google Scholar]
- 85.van den Bogaard EH, Podolsky MA, Smits JP, Cui X, John C, Gowda K, et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J Invest Dermatol. 2015;135:1320–8. doi: 10.1038/jid.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol. 2013;131:736–42. doi: 10.1016/j.jaci.2012.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paulose T, Speroni L, Sonnenschein C, Soto AM. Estrogens in the wrong place at the wrong time: Fetal BPA exposure and mammary cancer. Reprod Toxicol. 2015;54:58–65. doi: 10.1016/j.reprotox.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berdnikovs S, Loffredo L, Abdala-Valencia H. Disruption of the endocrine system leads to local mucosal responses favoring allergic sensitization (MUC2P.919) The Journal of Immunology. 2015;194:65.2-.2. [Google Scholar]
- 89.Fischer KD, Hall SC, Agrawal DK. Vitamin D Supplementation Reduces Induction of Epithelial-Mesenchymal Transition in Allergen Sensitized and Challenged Mice. PLoS One. 2016;11:e0149180. doi: 10.1371/journal.pone.0149180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahrens K, Schunck M, Podda GF, Meingassner J, Stuetz A, Schroder JM, et al. Mechanical and metabolic injury to the skin barrier leads to increased expression of murine beta-defensin-1, -3, and -14. J Invest Dermatol. 2011;131:443–52. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- 91.Hansmann B, Ahrens K, Wu Z, Proksch E, Meyer-Hoffert U, Schroder JM. Murine filaggrin-2 is involved in epithelial barrier function and down-regulated in metabolically induced skin barrier dysfunction. Exp Dermatol. 2012;21:271–6. doi: 10.1111/j.1600-0625.2012.01449.x. [DOI] [PubMed] [Google Scholar]
- 92.Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852–60. doi: 10.1016/j.jaci.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 93.Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39:700–7. doi: 10.1111/j.1365-2222.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 94.Morishita R, do Franco MC, Suano-Souza FI, Sole D, Puccini RF, Strufaldi MW. Body mass index, adipokines and insulin resistance in asthmatic children and adolescents. J Asthma. 2016;53:478–84. doi: 10.3109/02770903.2015.1113544. [DOI] [PubMed] [Google Scholar]
- 95.Garmendia JV, Moreno D, Garcia AH, De Sanctis JB. Metabolic syndrome and asthma. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:60–6. doi: 10.2174/1872214807666140107151023. [DOI] [PubMed] [Google Scholar]
- 96.Berdnikovs S, Abdala-Valencia H, Loffredo LF, Erickson K, Browning M, Saber R, et al. Systemic imbalance in hormone levels associates with epithelial barrier dysfunction in allergic disease. Journal of Allergy and Clinical Immunology. 2017;139:AB263. [Google Scholar]
- 97.Roarty K, Rosen JM. Wnt and mammary stem cells: hormones cannot fly wingless. Curr Opin Pharmacol. 2010;10:643–9. doi: 10.1016/j.coph.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mullin GE, Eastern JS. Cutaneous signs of thyroid disease. Am Fam Physician. 1986;34:93–8. [PubMed] [Google Scholar]
- 99.Salazar JJ, Ennis WJ, Koh TJ. Diabetes medications: Impact on inflammation and wound healing. J Diabetes Complications. 2016;30:746–52. doi: 10.1016/j.jdiacomp.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gunschmann C, Stachelscheid H, Akyuz MD, Schmitz A, Missero C, Bruning JC, et al. Insulin/IGF-1 controls epidermal morphogenesis via regulation of FoxO-mediated p63 inhibition. Dev Cell. 2013;26:176–87. doi: 10.1016/j.devcel.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Draijer C, Hylkema MN, Boorsma CE, Klok PA, Robbe P, Timens W, et al. Sexual maturation protects against development of lung inflammation through estrogen. Am J Physiol Lung Cell Mol Physiol. 2016;310:L166–74. doi: 10.1152/ajplung.00119.2015. [DOI] [PubMed] [Google Scholar]
- 102.Chen H, Sun X, Chi R, Li X, Feng J, Wu J, et al. Glucocorticoid dexamethasone regulates the differentiation of mouse conducting airway epithelial progenitor cells. Steroids. 2014;80:44–50. doi: 10.1016/j.steroids.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 103.Sevilla LM, Latorre V, Sanchis A, Perez P. Epidermal inactivation of the glucocorticoid receptor triggers skin barrier defects and cutaneous inflammation. J Invest Dermatol. 2013;133:361–70. doi: 10.1038/jid.2012.281. [DOI] [PubMed] [Google Scholar]
- 104.Shi W, Xu J, Warburton D. Development, repair and fibrosis: what is common and why it matters. Respirology. 2009;14:656–65. doi: 10.1111/j.1440-1843.2009.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elliott EN, Kaestner KH. Epigenetic regulation of the intestinal epithelium. Cell Mol Life Sci. 2015;72:4139–56. doi: 10.1007/s00018-015-1997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adli M, Parlak M, Li Y, El-Dahr SS. Epigenetic States of nephron progenitors and epithelial differentiation. J Cell Biochem. 2015;116:893–902. doi: 10.1002/jcb.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cavazza A, Miccio A, Romano O, Petiti L, Malagoli Tagliazucchi G, Peano C, et al. Dynamic Transcriptional and Epigenetic Regulation of Human Epidermal Keratinocyte Differentiation. Stem Cell Reports. 2016;6:618–32. doi: 10.1016/j.stemcr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chanda D, Kurundkar A, Rangarajan S, Locy M, Bernard K, Sharma NS, et al. Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis. Sci Rep. 2016;6:37445. doi: 10.1038/srep37445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Batra H, Antony VB. The pleural mesothelium in development and disease. Front Physiol. 2014;5:284. doi: 10.3389/fphys.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou J, Xu F, Yu JJ, Zhang W. YAP is up-regulated in the bronchial airway smooth muscle of the chronic asthma mouse model. Int J Clin Exp Pathol. 2015;8:11132–9. [PMC free article] [PubMed] [Google Scholar]
- 112.Lafkas D, Shelton A, Chiu C, de Leon Boenig G, Chen Y, Stawicki SS, et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature. 2015;528:127–31. doi: 10.1038/nature15715. [DOI] [PubMed] [Google Scholar]
- 113.Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Furmanski AL, Saldana JI, Ono M, Sahni H, Paschalidis N, D’Acquisto F, et al. Tissue-derived hedgehog proteins modulate Th differentiation and disease. J Immunol. 2013;190:2641–9. doi: 10.4049/jimmunol.1202541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014;133:1390–9. 9e1–6. doi: 10.1016/j.jaci.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie AN, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016;138:1356–66. doi: 10.1016/j.jaci.2016.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Larkin EK, Gebretsadik T, Moore ML, Anderson LJ, Dupont WD, Chappell JD, et al. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE) BMC Pulm Med. 2015;15:45. doi: 10.1186/s12890-015-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375:411–21. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davies DE, Holgate ST. Asthma: the importance of epithelial mesenchymal communication in pathogenesis. Inflammation and the airway epithelium in asthma. Int J Biochem Cell Biol. 2002;34:1520–6. doi: 10.1016/s1357-2725(02)00048-1. [DOI] [PubMed] [Google Scholar]
- 121.Hackett TL. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol. 2012;12:53–9. doi: 10.1097/ACI.0b013e32834ec6eb. [DOI] [PubMed] [Google Scholar]
- 122.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med. 2009;180:122–33. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 123.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–56. e1–12. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 124.Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1175–87. doi: 10.1152/ajpgi.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–20. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Egawa G, Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J Allergy Clin Immunol. 2016;138:350–8. e1. doi: 10.1016/j.jaci.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 127.Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. J Allergy Clin Immunol. 2016;137:1111–6. e1–8. doi: 10.1016/j.jaci.2015.12.1312. [DOI] [PubMed] [Google Scholar]
- 128.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 129.Alduraywish SA, Standl M, Lodge CJ, Abramson MJ, Allen KJ, Erbas B, et al. Is there a march from early food sensitization to later childhood allergic airway disease? Results from two prospective birth cohort studies. Pediatr Allergy Immunol. 2017;28:30–7. doi: 10.1111/pai.12651. [DOI] [PubMed] [Google Scholar]
- 130.Alduraywish SA, Lodge CJ, Campbell B, Allen KJ, Erbas B, Lowe AJ, et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71:77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 131.Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J Invest Dermatol. 2017;137:18–25. doi: 10.1016/j.jid.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 132.Liu W, Huang C, Wang X, Cai J, Hu Y, Zou Z, et al. Multimorbidities of asthma, allergies, and airway illnesses in childhood: Chance or not chance? J Asthma. 2016:1–12. doi: 10.1080/02770903.2016.1263648. [DOI] [PubMed] [Google Scholar]
- 133.Williamson PA, Vaidyanathan S, Clearie K, Barnes M, Lipworth BJ. Airway dysfunction in nasal polyposis: a spectrum of asthmatic disease? Clin Exp Allergy. 2011;41:1379–85. doi: 10.1111/j.1365-2222.2011.03793.x. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z, Zhang LJ, Guha G, Li S, Kyrylkova K, Kioussi C, et al. Selective ablation of Ctip2/Bcl11b in epidermal keratinocytes triggers atopic dermatitis-like skin inflammatory responses in adult mice. PLoS One. 2012;7:e51262. doi: 10.1371/journal.pone.0051262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–8. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001;117:977–83. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 137.Weber S, Niessen MT, Prox J, Lullmann-Rauch R, Schmitz A, Schwanbeck R, et al. The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development. 2011;138:495–505. doi: 10.1242/dev.055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–14. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang L, Beiting DP, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, et al. Eosinophils and IL-4 Support Nematode Growth Coincident with an Innate Response to Tissue Injury. PLoS Pathog. 2015;11:e1005347. doi: 10.1371/journal.ppat.1005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–75. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kypriotou M, Huber M, Hohl D. The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp Dermatol. 2012;21:643–9. doi: 10.1111/j.1600-0625.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 143.van Drongelen V, Alloul-Ramdhani M, Danso MO, Mieremet A, Mulder A, van Smeden J, et al. Knock-down of filaggrin does not affect lipid organization and composition in stratum corneum of reconstructed human skin equivalents. Exp Dermatol. 2013;22:807–12. doi: 10.1111/exd.12271. [DOI] [PubMed] [Google Scholar]
- 144.Folmes CD, Terzic A. Energy metabolism in the acquisition and maintenance of stemness. Semin Cell Dev Biol. 2016;52:68–75. doi: 10.1016/j.semcdb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Naquet P, Giessner C, Galland F. Metabolic adaptation of tissues to stress releases metabolites influencing innate immunity. Curr Opin Immunol. 2016;38:30–8. doi: 10.1016/j.coi.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 146.Ryall JG, Cliff T, Dalton S, Sartorelli V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell. 2015;17:651–62. doi: 10.1016/j.stem.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Galvis LA, Holik AZ, Short KM, Pasquet J, Lun AT, Blewitt ME, et al. Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development. 2015;142:1458–69. doi: 10.1242/dev.122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Klok E, Lubsen NH, Chamberlain CG, McAvoy JW. Induction and maintenance of differentiation of rat lens epithelium by FGF-2, insulin and IGF-1. Exp Eye Res. 1998;67:425–31. doi: 10.1006/exer.1998.0534. [DOI] [PubMed] [Google Scholar]
- 149.Maki RG. Small is beautiful: insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28:4985–95. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ijaz T, Pazdrak K, Kalita M, Konig R, Choudhary S, Tian B, et al. Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ J. 2014;7:13. doi: 10.1186/1939-4551-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kalita M, Tian B, Gao B, Choudhary S, Wood TG, Carmical JR, et al. Systems approaches to modeling chronic mucosal inflammation. Biomed Res Int. 2013;2013:505864. doi: 10.1155/2013/505864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Ruckert B, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139:93–103. doi: 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]