Abstract
Purpose of the Review
A wide array of sleep and circadian deficits have been observed in patients with Alzheimer’s Disease (AD). However, the vast majority of these studies have focused on later-stage AD, and do not shed light on the possibility that circadian dysfunction contributes to AD pathogenesis. The goal of this review it to examine the evidence supporting or refuting the hypothesis that circadian dysfunction plays an important role in early AD pathogenesis or AD risk in humans.
Recent Findings
Few studies have addressed the role of the circadian system in very early AD, or prior to AD diagnosis. AD appears to have a long presymtomatic phase during which pathology is present but cognition remains normal. Studies evaluating circadian function in cognitively-normal elderly or early-stage AD have thus far not incorporated AD biomarkers. Thus, the cause-and-effect relationship between circadian dysfunction and early-stage AD remains unclear.
Summary
Circadian dysfunction becomes apparent in AD as dementia progresses, but it is unknown at which point in the pathogenic process rhythms begin to deteriorate. Further, it is unknown if exposure to circadian disruption in middle age increases AD risk later in life. This review address gaps in current knowledge on this topic, and proposes several critical directions for future research which might help to clarify the potential pathogenic role of circadian clock dysfunction in AD.
Keywords: circadian, Alzheimer’s Disease, neurodegeneration, amyloid, clock genes
Introduction
Alzheimer’s Disease (AD) is the most common cause of dementia in the United States, currently affecting more than 5 million people and costing over $200 billion annually [1]. Clinically, AD is classically characterized by a gradual, insidious onset of memory loss which expands to multi-domain cognitive impairment. Pathologically, the hallmarks of the disease are amyloid plaques, extracellular accumulations composed primarily of aggregated amyloid-beta (Aβ) peptides, and neurofibrillary tangles, formed from the aggregation of misfolded, hyperphsophorylated tau protein. AD generally occurs after the age of 65, and becomes highly prevalent among people older than 80. While many studies examine dementia rather than AD specifically, it is worth noting that not all dementia is caused by AD, though AD is by far the most common cause of dementia in the elderly [1]. Among the many terrible symptoms of AD, disturbances of sleep and day-night rhythms are common and can be a major cause of morbidity. Any clinician who cares for AD patients is all too familiar with accounts of patients becoming confused or agitated in the evening (a phenomenon termed “sundowning”), mistaking night for day, getting dressed for work in the wee hours of the morning, or napping throughout the day [2]. AD patients frequently exhibit problems with sleep, but also with circadian rhythms, the 24-hour cycles of the body. While sleep and circadian rhythms are closely interrelated, they are distinct entities with separate neuroanatomical and molecular substrates. As sleep disturbances in AD are reviewed extensively elsewhere [3–6], I will focus here on circadian rhythms and their disruption in AD. I will examine the evidence supporting the notion that circadian dysfunction may contribute to AD pathogenesis, drawing primarily from the human literature, and discuss areas of critical need for further investigation to address this issue.
The Mammalian Circadian Clock
Circadian rhythms in behavior and physiologic functions are observed in most organisms on earth, ranging from plants to bacteria to humans [7, 8]. The circadian system in mammals serves to synchronize internal function with the external environment, particularly the light-dark cycle, though other external circadian cues exist. The mammalian circadian system is hierarchical, consisting of a central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, and peripheral clocks in most cells throughout the body [9]. The SCN is the “master clock” of the body and serves to synchronize peripheral clocks to the light:dark cycle, creating coherent organismal circadian rhythms in behavior, physiology, and cellular function. The SCN receives direct neuronal input from the retina, and exposure to light causes induction of clock gene expression and synchronizes the core circadian machinery in pacemaker neurons in the SCN [10, 11]. Circuitry within the SCN integrates this signal, resulting in robust circadian oscillations in neuronal firing. The SCN contains primarily GABAergic neurons, though the neuropeptides arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) are co-expressed in subsets of SCN neurons and are critical to SCN function [12–16]. The SCN projects to other brain nuclei, including the pineal gland to regulate melatonin, sleep-wake centers, and to regions regulating hormone secretion and autonomic function[11]. Through these connection, the SCN can signal to the entire body, synchronizing peripheral clocks and diverse behavioral, endocrine, physiologic, and transcriptional functions to the light:dark cycle.
The molecular machinery responsible for cell-autonomous rhythmicity is present in nearly every cell in the body and orchestrates oscillations in cellular transcription [9]. Thus, cells exhibit circadian rhythms in transcription and function even in the absence of input from the SCN, and even when grown in culture outside the body [9, 17]. The core circadian clock machinery is present and rhythmic in neurons, astrocytes, and microglia in the brain [17–22], though it roles in brain health and disease are unclear. In its simplest form, the core circadian clock consists of a primary transcriptional-translational feedback loop which relies on the bHLH/PAS transcription factors BMAL1 (also known as ARNTL) and CLOCK, which heterodimerize and bind to E-Box motifs to drive transcription of many genes [9]. BMAL1/CLOCK heterodimers drive transcription of negative feedback repressors (including Period (Per1,2,3), Cryptochrome (Cry1,2), and REV-ERB (Nr1d1, Nr1d2) genes, which directly or indirectly repress BMAL1/CLOCK-mediated transcription. BMAL1 levels are also positively regulated by retinoic acid receptor (ROR) transcription factors binding to ROR response elements (RREs) [23]. Transcriptomic studies suggest that in peripheral tissues, 10–20% of all mRNA transcripts show circadian oscillation [24], while up to half of all transcripts show oscillation in at least one tissue [25]. In humans, 15% of all blood or saliva metabolites are rhythmic [26]. Thus, the circadian clock is a critical integrator of cellular metabolism and transcription in peripheral tissue.
Considering the broad influence that the circadian system has multiple aspects normal physiology, it is perhaps not surprising that disrupted circadian clock function is associated with pathology. In mice, disrupted rhythms or clock gene deletion are associated with exacerbation of many disease states, including diabetes [27], cardiovascular disease[28, 29], inflammation [30, 31], and neurodegeneration [32]. In humans, circadian rhythm disturbances, often caused by employment and lifestyle issues such as night shift work, are associated with increased risk of diseases such as diabetes, breast cancer, and coronary artery disease [33–36]. It is therefore reasonable to hypothesize that chronically disrupted circadian rhythms might play a role in age-related neurodegenerative diseases, the most common of which is AD.
Alterations in Behavioral Circadian Rhythms in AD
Alterations in the day-night activity pattern of dementia patients has been described for decades [37, 38], though these reports were primarily focused on sleep disturbances. In the late 1980s, studies of circadian function began to appear in the literature, demonstrating disrupted circadian rhythms in rest-activity behavior [39] or temperature [40]. Activity rhythms in humans are often monitored using actigraphy, a method of recording the amount of movement each minute of the day using wristwatch-like devices[41]. In the past 25 years, dozens of subsequent studies, most using actigraphy, have described abnormalities in circadian function in AD patients. Initial studies demonstrated fragmentation of activity rhythms, with increased activity at night, decreased activity during the day, decreased rhythm amplitude, and phase delay[42–44]. This phase delay means that the peak of activity is ~4 hours later in AD patients than controls, and has been used as an explanation for “sundowning”, a phenomenon of increased confusion and agitation in dementia patients in early evening[2]. Disrupted circadian rhythms in melatonin secretion [45] and temperature [40] have also been described in AD. Body temperature also exhibits circadian variation, though interestingly this rhythm does not appear to be consistently disrupted in AD[42, 46]. Some multimodal studies have compared actigraphy with other circadian markers in the same patients. A recent study examined actigraphy, melatonin levels, and clock gene mRNA levels in buccal mucosa in home-dwelling AD patients with moderate AD dementia. Daily rhythms in melatonin secretion were blunted in AD patients, and Bmal1 mRNA rhythms was slightly phase-delayed[47]. Hatfield et al examined both actigraphy and salivary cortisol oscillations in home-dwelling AD patients with either mild or moderate dementia[48]. They observed progressive behavioral circadian rhythm fragmentation and loss of amplitude as dementia worsened, though cortisol rhythms were less affected. Gehrman et al. found a complex relationship between dementia severity and circadian function, though poor activity rhythms and poor cognition were correlated, and only patients with MMSE<24 were included in the study (suggesting that even the mildest participants had dementia) [49]. Thus, circadian rhythm abnormalities, in particular fragmentation, phase delay, and loss of nocturnal melatonin surge, have been extensively described in patients with symptomatic AD dementia, though there some discrepancies regarding rhythms in temperature and cortisol secretion.
The Neuropathology of Circadian Dysfunction in AD
Normal circadian function relies on an intact SCN, and several studies have demonstrated degeneration of SCN neurons in aging and AD. Neuron expressing arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) play key roles in SCN synchronization and circadian rhythm output[13, 14, 50]. Loss of VIPergic neurons in aging and AD is correlated with decline in the amplitude of behavioral circadian rhythms in humans[51]. Decreased numbers of both AVP- and VIP-expressing neurons have been described in the postmortem SCN of patients with AD, as compared to aged matched controls[52–55]. The pineal gland receives direct output from the SCN in order to generate circadian oscillations in melatonin secretion, and postmortem studies have demonstrated altered clock gene oscillation in the pineal of AD patients [56]. This is in keeping with numerous studies showing blunted circadian oscillations in melatonin secretion in AD patients [45, 57–59]. Loss of melatonin MT1 receptors on SCN neurons in AD was also reported, suggesting that melatonin may no longer influence rhythmic SCN output [54]. Thus, there is degeneration of both the central clock (the SCN) and dysregulation of pineal melatonin secretion, suggesting that clock gene rhythms should be altered throughout the brain.
Circadian oscillations in clock gene mRNA can be quantified from human post-mortem brain tissue, based on the time of day of death of each individual [60, 61]. While this has not been analyzed extensively in AD, one study demonstrated altered synchronization in rhythms of clock gene expression (including PER1, PER2, and BMAL1) in different brain regions in AD patients as compared to controls [62]. Circadian oscillations in methylation, an epigenetic means of regulating transcription, have been observed in mice [63], and are also present in human cerebral cortex and are dysregulated in AD [64]. Accordingly, altered circadian transcription of Bmal1 in the cortex is associated with abnormal Bmal1 promoter methylation in AD post-mortem tissue [65]. While informative, these post-mortem gene expression studies are somewhat limited in the detail and accuracy with which clock gene expression can be observed, as they rely on the assumption that clock oscillation stops immediately at death, and that clock gene mRNA levels are not substantially altered by the circumstances of death or the postmortem interval before autopsy and tissue extraction. While some alterations in clock gene expression appear to occur in AD brain, our understanding of this phenomenon is still incomplete.
What do we know about circadian dysfunction in early or presymptomatic AD?
While the body of evidence linking AD and circadian rhythms appears substantial, it becomes clear that the story is far from complete when we consider current concepts of AD pathogenesis and diagnosis.
Nearly every study of circadian rhythms in living AD patients has relied upon a clinical diagnosis of AD, or in some cases simply “dementia”. We know that mixed neurodegenerative pathology is present in many if not most dementia patients, as evidence of α-synuclein, TDP-43, and vascular disesae are often observed alongside the typical Aβ plaques and tau neurofibrillary tangles[66–68]. The potential contributions of these other pathologies to circadian dysfunction are unknown. More concerning, we don’t know at what point in the pathogenic process of AD circadian dysfunction begins. The advent of amyloid PET imaging and cerebrospinal fluid biomarkers for AD pathology (including Aβ42, tau, and phospho-tau) has facilitated the detection and longitudinal monitoring of Aβ and tau pathology in living people, revolutionizing our understanding of AD pathogenesis[69–71]. A wealth of human data from numerous longitudinal studies which combine these biomarkers with detailed cognitive phenotyping has revealed that amyloid plaque pathology appears 10–20 years prior to the onset of cognitive symptoms in AD, and that increases in CSF tau levels often precede the onset of symptomatic cognitive decline by a few years[72–76]. This same timeframe is present in patients with rare autosomal dominant forms of AD caused by inherited mutations in genes related to Aβ production[77]. Thus, the pathogenic cascade which leads to clinical AD has been active for at least a decade before a clinical diagnosis of AD is possibly made. What is happening to circadian rhythms during this presymptomatic period, or at the very least during the earliest stages of the disease? Surprisingly little circadian data exists for patients with very early AD, or those with “mild cognitive impairment” (MCI), a diagnosis which is in many cases equivalent to very early AD[78]. A single small study demonstrated a mild circadian phase advance in MCI patients in several circadian parameters, as well as altered sleep indices including increased wake after sleep onset (WASO) [79]. Even less is known about “presymptomatic” or preclinical AD, defined as individuals with positive AD biomarkers but no apparent cognitive decline[71]. To our knowledge, no studies of circadian function in preclinical AD patients have yet been published. Ju et al. examined sleep parameters using actigraphy in a cohort of cognitively-normal patients, ~30% of whom had preclinical AD (as evidenced by decreased CSF Aβ42 levels), and found that amyloid pathology was significantly associated with reduced sleep efficiency and increased napping, suggesting a possible circadian effect [80]. This information begs a major question: Does circadian dysfunction cause, or at least contribute to the early pathogenesis of AD? In order to answer this question in humans, a number of research questions need to be addressed. These include the following:
Does circadian dysfunction precede cognitive decline in AD?
If we are to implicate circadian dysfunction as an important contributor to disease pathogenesis, we need to know if rhythm disturbances or clock gene repression are present early in the disease, or are simply a late consequence of neurodegeneration. Nearly all existing data on rhythms in AD is based on symptomatic dementia patients, and some studies have failed to find differences even in mild symptomatic dementia [48]. The primary evidence supporting the idea that rhythm disturbances precede dementia comes from the work of Tranah et al., who examined actigraphy data from 1282 older women (average age 83), all of whom were cognitively normal at the study start [81]. They quantified several circadian endpoints, and determined which participants went on to develop dementia in the ensuing 5-year followup. In their analyses, older women with blunted rhythm amplitude, decreased robustness, or phase delay were more likely to go on to develop dementia, with odds ratios around 1.5. These findings are compelling, but there are some shortcomings. The cognitive assessment was quite limited, as validated dementia measures such as the Clinical Dementia Rating or ADAS-COG were not available. Also, we do not know if these women developed AD, or some other dementia (though AD is by far the most likely statistically). Further, we do not know how many women in the study had “preclinical” AD pathology (amyloid and/or tau pathology in the brain without obvious cognitive impairment) during the study, though considering the average age of the participants, this number is likely to approach 40% [82]. Thus, we cannot conclude that circadian dysfunction causes AD, as an alternative explanation is that presymptomatic AD pathology was present in many of the people, causing circadian dysfunction and also increasing risk of subsequent dementia. Several other studies have addressed a similar question, but from the sleep angle. Fragmentation of sleep has been identified in cognitively normal elderly and shown to increase subsequent risk of AD [83]. While sleep fragmentation may or may not be due to circadian rhythm disruption, these studies provide additional evidence that alterations in day-night behavioral rhythms are disturbed before the onset of clinical dementia in AD, though the relationship of these changes to brain pathology is unknown. The evidence linking early sleep problems in cognitively-normal people to future AD risk is stronger [84–88], though it also remains unclear how much of this sleep phenotype could be caused by circadian clock dysfunction.
How do circadian rhythm disturbances correlate with modern biomarkers of AD?
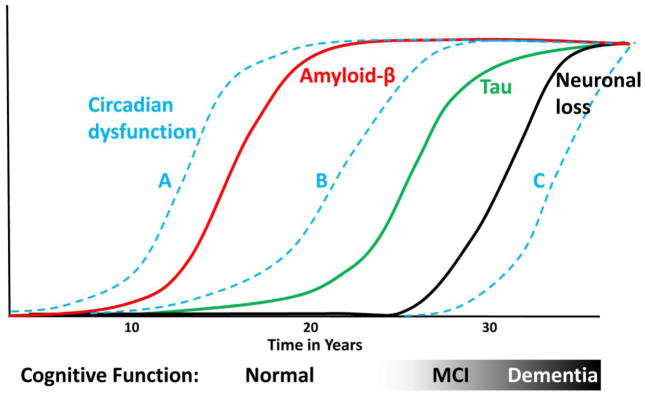
Most studies of sleep and rhythm abnormalities in AD have used patients with moderate AD. We need more information about how sleep and circadian function are impacted at earlier stages, and particularly in presymptomatic patients with biomarker evidence of AD pathology. Clinicopathologic correlations between different AD biomarkers (such as amyloid or tau PET imaging or CSF Aβ, tau, and inflammatory markers) are needed to understand the potential substrates for sleep and rhythm disruption. Ultimately, we need to add circadian rhythm dysfunction to the timecourse of biomarker changes in AD (Figure 1). Existing studies examining circadian rhythms and AD risk in cognitively-normal older people are limited by the likely high prevalence of presymptomatic amyloid pathology in these cohorts, as both circadian disruption and increased risk of AD could simply be related to having amyloid plaques[82]. Thus, AD biomarkers are needed in such studies in the future.
Figure 1. Circadian dysfunction in the timecourse of AD pathogenesis.
Theoretical curves showing the timecourse of amyloid plaque deposition (red line), tau aggregation (green line), and neuronal loss (black line) in relation to cognitive function in AD, modified from previous studies[72, 76, 77]. Blue dotted lines show possible positions for circadian dysfunction. Line A suggests a causal role, while B suggests that presymptomatic AD pathology leads to circadian disruption (which could then contribute to early disease). Line C suggests that circadian dysfunction is a late consequence of dementia.
Does shift work or other circadian disruption increase lifetime risk of AD?
Large-scale epidemiologic studies have shown that certain lifestyle-related exposures to circadian disruption (such as working nights or other shiftwork) increases risk of diseases such as diabetes, cancer, and coronary artery disease [33, 35, 36]. Two small studies suggest that flight attendants who are subjected to chronic jetlag exhibit temporal lobe atrophy and cognitive impairment, suggesting some impact of chronic circadian disruption on brain function[89, 90]. To our knowledge, no other such data exists for any neurodegenerative disease, including AD. This type of data is needed and may help to solve the chick-or-egg problem of circadian rhythm disorders in AD.
Can we tap into big data to address these questions?
In the era of wearable devices which track our every step, heartbeat, and tweet, we need to harness these new technologies for collecting rich sleep and circadian data across very large populations. Sensor-rich wearables, which can collect multiple physiologic parameters and upload the data seamlessly via the internet, should be employed across AD observational studies, and smartphone-based programs which collect all types of data could be used across large populations of older adults to understand the interaction between aging, sleep, circadian rhythms and AD risk. Unfortunately, the technology has outpaced our ability to ethically employ it to our advantage. Issues of privacy are a major stumbling block, but collaboration with large tech firms who already collect massive data from people around the world would be a first step.
Is it sleep or the clock?
It can be very difficult at first glance to determine if time of day oscillations in biological processes are directly due to sleep, or are regulated by the circadian system. However, the implications are very different. Sleep-regulated processes may respond to one set of therapeutics, circadian clock-regulated processes to another. The two systems are interconnected, but can function independently. As an example, it remains unclear if fragmentation of behavioral rhythms and sundowning in AD are due to degeneration of sleep nuclei, or due to dysfunction of the circadian system, either at the level of SCN or peripheral clocks. Pharmacologically targeting sleep may not necessarily synchronize your circadian system. Conversely, if sleep nuclei are damaged, a robust circadian clock may still not trigger sleep. Differentiating these processes and their relative roles in AD is an important but difficult task.
What are the Mechanisms?
Does the circadian clock influence Aβ clearance, production, or aggregation? What are the effects on tau? The circadian clock has been implicated in regulation of inflammation, glial activation, oxidative stress, and autophagy, all important processes in AD [22, 31, 32, 91]. Starting with animal models, some understanding of the impact of circadian dysfunction on these neurodegenerative pathways in AD is needed. Glymphatic flow, a phenomenon of astrocyte-mediated fluxes in extracellular fluid in the brain, has been linked to sleep and is thought to mediate removal of toxin proteins, such as Aβ [92, 93]. The interplay between circadian systems and glymphatic flow is another potential area of investigation. MRI methods to quantify glymphatic flow in humans have been developed and are currently being employed in the study of AD[94].
Conclusions
The concept that dysfunction of the circadian clock may set the stage for neurodegeneration has gained traction in recent years, buoyed by data implicating sleep disturbances in AD pathogenesis. While circadian dysfunction has been extensively studied in symptomatic AD, we know very little about how very early or presymptomatic AD pathology impacts the circadian system. Furthermore, we do not know if exposure to chronic circadian disturbances, such as night shift work, during middle age may increase AD risk. Finally, the molecular mechanisms linking the circadian system to AD pathogenesis are poorly understood. By addressing some of the research questions posed herein, the role of the circadian clock in AD pathogenesis can hopefully be revealed. As our understanding of the interplay between the circadian clock and AD pathogenesis evolves, so might our ability to specifically target the circadian system therapeutically for the prevention of AD.
Acknowledgments
Erik S. Musiek is funded by NINDS K08 award K08NS079405 and an award from the Coins for Alzheimer’s Trust (CART).
Footnotes
Conflicts of Interest:
Erik S. Musiek reports grants from NIH, Alzheimer’s Association, Coins for Alzheimer’s Trust, during the conduct of the study; personal fees from Eisai Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Alzheimer’s Association. Alzhiemer’s Disease Facts and Figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry. 2001;158:704–11. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- 3.Holth JK, Patel TK, Holtzman DM. Sleep in Alzheimer’s Disease - Beyond Amyloid. Neurobiol Sleep Circad Rhythym. 2016 doi: 10.1016/j.nbscr.2016.08.002. http://dx.doi.org/10.1016/j.nbscr.2016.08.002. [DOI] [PMC free article] [PubMed]
- 4.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2014 doi: 10.1016/j.smrv.2014.03.007. S1087-0792(14)00040-9. [DOI] [PubMed] [Google Scholar]
- 7.McClung CR. Plant circadian rhythms. Plant Cell. 2006;18:792–803. doi: 10.1105/tpc.106.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–64. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohawk JA, Green CB, Takahashi JS. Central and Peripheral Circadian Clocks in Mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog ED, Hermanstyne T, Smyllie NJ, Hastings MH. Regulating the Suprachiasmatic Nucleus (SCN) Circadian Clockwork: Interplay between Cell-Autonomous and Circuit-Level Mechanisms. Cold Spring Harb Perspect Biol. 2017:9. doi: 10.1101/cshperspect.a027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–69. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol. 2010;22:362–72. doi: 10.1111/j.1365-2826.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90. doi: 10.1126/science.1238599. [DOI] [PubMed] [Google Scholar]
- 14.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–83. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An S, Harang R, Meeker K, Granados-Fuentes D, Tsai CA, Mazuski C, et al. A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc Natl Acad Sci U S A. 2013;110:E4355–61. doi: 10.1073/pnas.1307088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol. 2013;110:1097–106. doi: 10.1152/jn.00114.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–8. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci U S A. 2009;106:16493–8. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snider KH, Dziema H, Aten S, Loeser J, Norona FE, Hoyt K, et al. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav Brain Res. 2016;308:222–35. doi: 10.1016/j.bbr.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, De Pietri Tonelli D. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun. 2017;8:14336. doi: 10.1038/ncomms14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakazato R, Hotta S, Yamada D, Kou M, Nakamura S, Takahata Y, et al. The intrinsic microglial clock system regulates interleukin-6 expression. Glia. 2017;65:198–208. doi: 10.1002/glia.23087. [DOI] [PubMed] [Google Scholar]
- 22.Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171–9. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, et al. Cross-talk among ROR alpha 1 and the Rev-erb family of orphan nuclear receptors. Mol Endocrinol. 1994;8:1253–61. doi: 10.1210/mend.8.9.7838158. [DOI] [PubMed] [Google Scholar]
- 24.Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM. Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol. 2006;2:e16. doi: 10.1371/journal.pcbi.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–24. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109:2625–9. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–7. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huo M, Huang Y, Qu D, Zhang H, Wong WT, Chawla A, et al. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2016 doi: 10.1096/fj.201601030R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–8. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A. 2015;112:7231–6. doi: 10.1073/pnas.1501327112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knutsson A, Kempe A. Shift work and diabetes--a systematic review. Chronobiol Int. 2014;31:1146–51. doi: 10.3109/07420528.2014.957308. [DOI] [PubMed] [Google Scholar]
- 34.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 35.Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, et al. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA. 2016;315:1726–34. doi: 10.1001/jama.2016.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi: 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- 37.Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, Mendelson WB. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging. 1982;3:371–7. doi: 10.1016/0197-4580(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 38.Vitiello MV, Prinz PN. Alzheimer’s disease. Sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5:289–99. [PubMed] [Google Scholar]
- 39.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–72. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 40.Okawa M, Mishima K, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep. 1991;14:478–85. doi: 10.1093/sleep/14.6.478. [DOI] [PubMed] [Google Scholar]
- 41.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 42.Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging. 1995;16:765–71. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- 43.Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- 44.Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:359–68. doi: 10.1176/appi.ajgp.13.5.359. [DOI] [PubMed] [Google Scholar]
- 45.Uchida K, Okamoto N, Ohara K, Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996;717:154–9. doi: 10.1016/0006-8993(96)00086-8. [DOI] [PubMed] [Google Scholar]
- 46.Prinz PN, Christie C, Smallwood R, Vitaliano P, Bokan J, Vitiello MV, et al. Circadian temperature variation in healthy aged and in Alzheimer’s disease. J Gerontol. 1984;39:30–5. doi: 10.1093/geronj/39.1.30. [DOI] [PubMed] [Google Scholar]
- 47.Weissova K, Bartos A, Sladek M, Novakova M, Sumova A. Moderate Changes in the Circadian System of Alzheimer’s Disease Patients Detected in Their Home Environment. PLoS One. 2016;11:e0146200. doi: 10.1371/journal.pone.0146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain. 2004;127:1061–74. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 49.Gehrman P, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The relationship between dementia severity and rest/activity circadian rhythms. Neuropsychiatr Dis Treat. 2005;1:155–63. doi: 10.2147/nedt.1.2.155.61043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mieda M, Okamoto H, Sakurai T. Manipulating the Cellular Circadian Period of Arginine Vasopressin Neurons Alters the Behavioral Circadian Period. Curr Biol. 2016;26:2535–42. doi: 10.1016/j.cub.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 51*.Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78:317–22. doi: 10.1002/ana.24432. Provides correlation between circadian rhythms and SCN VIP neuron count. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 53.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–6. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 54.Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging. 2007;28:1239–47. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, et al. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131:1609–17. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20:1874–6. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- 57.Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45:417–21. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- 58.Skene DJ, Vivien-Roels B, Sparks DL, Hunsaker JC, Pevet P, Ravid D, et al. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Res. 1990;528:170–4. doi: 10.1016/0006-8993(90)90214-v. [DOI] [PubMed] [Google Scholar]
- 59.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol. 2003;38:199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 60.Lim AS, Myers AJ, Yu L, Buchman AS, Duffy JF, De Jager PL, et al. Sex difference in daily rhythms of clock gene expression in the aged human cerebral cortex. J Biol Rhythms. 2013;28:117–29. doi: 10.1177/0748730413478552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2016;113:206–11. doi: 10.1073/pnas.1508249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cermakian N, Lamont EW, Boudreau P, Boivin DB. Circadian clock gene expression in brain regions of Alzheimer ‘s disease patients and control subjects. J Biol Rhythms. 2011;26:160–70. doi: 10.1177/0748730410395732. [DOI] [PubMed] [Google Scholar]
- 63.Azzi A, Dallmann R, Casserly A, Rehrauer H, Patrignani A, Maier B, et al. Circadian behavior is light-reprogrammed by plastic DNA methylation. Nat Neurosci. 2014;17:377–82. doi: 10.1038/nn.3651. [DOI] [PubMed] [Google Scholar]
- 64.Lim AS, Srivastava GP, Yu L, Chibnik LB, Xu J, Buchman AS, et al. 24-hour rhythms of DNA methylation and their relation with rhythms of RNA expression in the human dorsolateral prefrontal cortex. PLoS Genet. 2014;10:e1004792. doi: 10.1371/journal.pgen.1004792. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65*.Cronin P, McCarthy MJ, Lim AS, Salmon DP, Galasko D, Masliah E, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.10.003. First demonstration of altered Bmal1 promoter methylation in AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–42. doi: 10.1212/wnl.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. 2013;1:65. doi: 10.1186/2051-5960-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. 2016 doi: 10.1111/bpa.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 71.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/s1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–65. doi: 10.1016/s1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–91. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/s1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 76.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–22. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Naismith SL, Hickie IB, Terpening Z, Rajaratnam SM, Hodges JR, Bolitho S, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38:857–66. doi: 10.3233/jad-131217. This is one of the only papers examining sleep changes in mild cognitive impairment. [DOI] [PubMed] [Google Scholar]
- 80**.Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–93. doi: 10.1001/jamaneurol.2013.2334. This is the only paper to date examining the relationship between CSF Aβ biomarker status and sleep parameters in cognitively-normal people. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–32. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hahn EA, Wang HX, Andel R, Fratiglioni L. A Change in Sleep Pattern May Predict Alzheimer Disease. Am J Geriatr Psychiatry. 2014;22:1262–71. doi: 10.1016/j.jagp.2013.04.015. S1064-7481(13)00233-9. [DOI] [PubMed] [Google Scholar]
- 85.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–43. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–75. doi: 10.2174/15672050113109990134. CAR-EPUB-53828 [pii] [DOI] [PubMed] [Google Scholar]
- 87*.Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, et al. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–7. doi: 10.1038/nn.4035. Explores a unique potential mechanism linking Aβ pathology to SWS disruption. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Potvin O, Lorrain D, Forget H, Dube M, Grenier S, Preville M, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–8. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 90.Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:RC66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J. 2011;30:4642–51. doi: 10.1038/emboj.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93*.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. First description of a role for sleep in the regulation of the glymphatic clearance system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]