The process of making antibodies is costly and time-consuming. Commercial antibodies offer a convenient solution. However, recent concerns have resulted in a National Institutes of Health (NIH) mandate to vigorously test the specificity of antibodies used in publications (http://grants.nih.gov/reproducibility). Currently, there is no standard for validation and reference data that must be provided in publications [1, 2], and crucial specificity data are often unavailable. Multiple studies have focused on issues of antibody specificity towards proteins such as G-protein-coupled receptors, kinase receptors, and integrins [3–5]. To determine whether an antibody is suitable, the following three issues must be considered: ability to detect the target (specificity), detection of the target above background (sensitivity), and generation of consistent results (reproducibility). Sensitivity is especially problematic with low-abundance proteins, for which the antibody in question can only detect high levels of target.
Problems with reproducibility often arise due to lot-to-lot variability and affect both polyclonal (pAbs) and monoclonal antibodies (mAbs). pAbs are a heterogeneous mixture of antibodies that recognize multiple epitopes of the same target protein but can also include nonspecific antibodies. Each lot, even when prepared from the same donor animal, contains diverse antibody clones and concentrations [6]. However, it is possible to reduce nonspecific binding of a pAb via immunoaffinity enrichment [7]. mAbs, although generally more consistent, are not exempt from variation. Hybridomas maintained in ascites can be contaminated with endogenous immunoglobulins and other proteins, especially if the mAb is not purified [8]. A hybridoma might also lose its antibody gene through continued passaging [7]. Additionally, epitopes that mAbs target are generally short sequences of amino acids that might exist on other proteins [8]. In one report, an mAb targeting the Met tyrosine kinase receptor—a marker of breast cancer diagnosis—revealed the target protein in the nucleus, while another lot showed membrane and cytoplasmic staining [5].
Our laboratory recently published a study focused on β6 integrin (β6), a small heterodimeric molecule involved in cell signaling [9]. The specificity of antibodies to receptors and proteins implicated in cell signaling is not well defined in the literature [6]. It is especially difficult to generate antibodies against specific integrins due to their similar structures [9], and many specification sheets report a small degree (10%–20%) of cross-reactivity with other integrins and proteins [3]. In our work, we purchased several commercial antibodies from different companies to evaluate specificity.
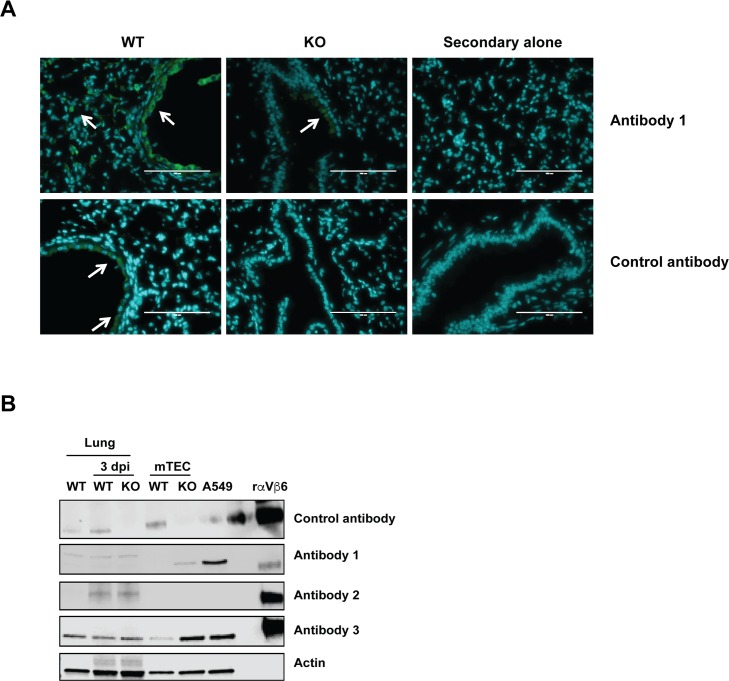
We used antibody 1 for immunofluorescence (IFA) staining of mouse pulmonary tissues to detect β6. Initially, we detected a strong signal by IFA. However, we also detected a weaker yet concerning signal in confirmed β6 knockout (KO) mice (Fig 1A). Slides stained with only secondary antibody were negative, suggesting that the signal was not due to nonspecific binding of the secondary antibody. Fortunately, we received a highly specific antibody from a collaborator (control antibody) and stained tissues successfully without detecting signal in sections from KO mice.
Fig 1. Specificity of β6 antibodies in immunofluorescent microscopy and western blot analysis.
(A) Mouse lung sections collected at 3 dpi were stained for β6. Arrows indicate areas of β6 expression. Green = β6; blue = nuclei. Bar is 100 μm. (B) Lysates prepared from lung tissue or tracheal epithelial cells were immunoblotted to detect β6 by using a panel of antibodies. β6, β6 integrin; dpi, days post infection; KO, knockout; mTEC, murine tracheal epithelial cell; WT, wild type.
Western blots presented a similar problem. Lysates from whole lung were collected at 3 days post infection (dpi), a time when β6 is expressed in response to damage but before the infection has resulted in sloughing of β6+ epithelial cells [10]. Lysates of infected murine tracheal epithelial cells (mTECs), A549 cells (human lung adenocarcinoma cells, which highly express β6 and are often used as a positive control for antibodies that cross-react with human β6 [11]), as well as recombinant αVβ6 integrin as a positive control, were separated under reducing and nonreducing conditions with a panel of commercially available anti-β6 antibodies (Fig 1B). The control antibody detected more β6 at 3 dpi in infected wild-type (WT) lung compared with uninfected WT lung and detected β6 in WT mTECs but not in KO mTECs or KO lung. A faint band was also detectable in A549 lysate. However, antibody 1 detected bands in all 3 lung samples and, oddly, in KO but not WT mTECs. Surprisingly, antibody 2, which detects human β6, could detect bands in WT and KO lung homogenate but not in mTECs or A549. Antibody 3 detected strong signal in all samples tested. All antibodies easily detected recombinant αVβ6.
We chose antibody 3 to determine what was being detected in the KO samples. We performed sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to separate lysates from KO lung and KO mTECs and excised the protein band corresponding with the band detected by antibody 3 to identify the proteins by mass spectrometry. Nine proteins were present in both samples at a threshold of 10 spectral counts or greater (Table 1). Although these data do not confirm that antibody 3 is binding to these proteins, their presence suggests they are likely candidates. However, it is also possible that the proteins are simply of a similar molecular weight.
Table 1. Proteins present in area recognized by antibody 3.
| Gene | Protein | Mass (kDa) |
|---|---|---|
| ACTN4 | Alpha-actinin-4 | 105 |
| EEF2 | Elongation factor 2 | 95 |
| GSN | Gelsolin | 86 |
| HSP1AB | Heat shock 70 kDA protein 1B | 70 |
| HSP90AA1 | Heat shock protein HSP 90-alpha | 85 |
| HSP90AB1 | Heat shock protein HSP 90-beta | 83 |
| MSN | Moesin | 68 |
| PLS3 | Plastin-3 | 71 |
| PKM | Pyruvate kinase PKM | 58 (dimerized) |
Our experiences highlight the importance of validating reagents even if obtained from commercial sources. Not only could nonspecific antibodies compromise research, there could be serious consequences leading to misdiagnoses or to incorrect or delayed treatments when they are used for clinical screenings. Consider that β6 itself is an important biomarker in the clinical setting, often confirming disease. The presence of β6 in tissue sections is used as an indicator for many types of cancer, including cholangiocarcinoma [12], malignant epithelial ovarian cancer [13], breast cancer [5, 14], and pancreatic cancer [15]; it is also a determinant of the metastatic potential of thyroid cancer [16]. β6 is also used as a diagnostic agent for foot and mouth disease virus due to its ability to bind and identify all representative serotypes of the virus in diagnostic ELISAs [17]. Nonspecificity could have a significant impact on the conclusions drawn from clinical trials and lead to incorrect diagnosis [14, 15, 18–20]. These problems are highlighted in several publications [21–24].
The solution is stringent validation. Several groups have published their own laboratory’s antibody validation workflow process (summarized in [6]). Most agree that the first step is a western blot using multiple cell lines or tissues known to express the protein of interest [6, 25]. As we and others have discovered, it is advantageous to use a KO animal or cell line to demonstrate specificity [25]. We would not have known there was a problem with antibody 1 if we had not used KO mice as a control. However, because not all proteins have KO models, it is beneficial to test the antibody in cells in which the target protein has been silenced by RNA interference, for example [25]. Cells transfected to overexpress the target or multiple antibodies binding to different epitopes of the target protein are useful positive controls [25]. A specific antibody should also show a titration effect when the samples or the antibody itself is diluted. Ask the following questions: is the antibody only detecting protein in cell lines expected to express that protein? Are staining patterns similar when different antibodies to the same target are used? Is protein abundance between those antibodies congruent?
As an additional resource, www.antibodypedia.com provides a repository of validation data that is searchable by target. Similar databases are available (summarized in [7]), but the generation of one standardized repository of information would be useful. Do not rely on the literature. Many journals have strict space limitations, and the methods section is often truncated, omitting validation controls. Journals have inconsistent requirements for information supplied, such as clone number, lot number, or dilution used. For example, knowing the exact lot used in the publication could help with reproducibility issues in other labs. Western blots are commonly cropped to show only the band of interest, omitting any nonspecific bands; however, seeing the full data can help researchers troubleshoot antibody-based assays and avoid wasting research funding on repeating unreported data [7]. In fact, many journals are pushing towards standard requirements to report details of antibodies used [1]. Because new research depends upon previously published data, the onus is on every researcher to properly validate tools.
Acknowledgments
The authors thank Virginia Hargest for assistance with tissue staining and Vishwajeeth Pagala of the St. Jude Children’s Research Hospital Proteomics and Mass Spectrometry Core for assistance with proteomics.
Funding Statement
This work was supported by ALSAC and NIAID contract HHSN272201400006C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gore AC. Editorial: antibody validation requirements for articles published in endocrinology. Endocrinology. 2013;154(2):579–80. doi: 10.1210/en.2012-2222 . [DOI] [PubMed] [Google Scholar]
- 2.Pradidarcheep W, Labruyere WT, Dabhoiwala NF, Lamers WH. Lack of specificity of commercially available antisera: better specifications needed. J Histochem Cytochem. 2008;56(12):1099–111. doi: 10.1369/jhc.2008.952101 ; PubMed Central PMCID: PMCPMC2583905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klotz SA, Pendrak ML, Hein RC. Antibodies to alpha5beta1 and alpha(v)beta3 integrins react with Candida albicans alcohol dehydrogenase. Microbiology. 2001;147(Pt 11):3159–64. doi: 10.1099/00221287-147-11-3159 . [DOI] [PubMed] [Google Scholar]
- 4.Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):385–8. doi: 10.1007/s00210-009-0395-y . [DOI] [PubMed] [Google Scholar]
- 5.Pozner-Moulis S, Cregger M, Camp RL, Rimm DL. Antibody validation by quantitative analysis of protein expression using expression of Met in breast cancer as a model. Lab Invest. 2007;87(3):251–60. doi: 10.1038/labinvest.3700515 . [DOI] [PubMed] [Google Scholar]
- 6.Schonbrunn A. Editorial: Antibody can get it right: confronting problems of antibody specificity and irreproducibility. Mol Endocrinol. 2014;28(9):1403–7. doi: 10.1210/me.2014-1230 ; PubMed Central PMCID: PMCPMC4154242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauly D, Hanack K. How to avoid pitfalls in antibody use. F1000Res. 2015;4:691 doi: 10.12688/f1000research.6894.1 ; PubMed Central PMCID: PMCPMC4722690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos-Vara JA. Technical aspects of immunohistochemistry. Veterinary pathology. 2005;42(4):405–26. doi: 10.1354/vp.42-4-405 . [DOI] [PubMed] [Google Scholar]
- 9.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3(3). doi: 10.1101/cshperspect.a004994 ; PubMed Central PMCID: PMCPMC3039929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Wu J, Zhu W, Pytela R, Sheppard D. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. American journal of respiratory cell and molecular biology. 1998;19(4):636–42. doi: 10.1165/ajrcmb.19.4.3293 . [DOI] [PubMed] [Google Scholar]
- 11.Heikkila O, Susi P, Stanway G, Hyypia T. Integrin alphaVbeta6 is a high-affinity receptor for coxsackievirus A9. The Journal of general virology. 2009;90(Pt 1):197–204. doi: 10.1099/vir.0.004838-0 . [DOI] [PubMed] [Google Scholar]
- 12.Patsenker E, Wilkens L, Banz V, Osterreicher CH, Weimann R, Eisele S, et al. The alphavbeta6 integrin is a highly specific immunohistochemical marker for cholangiocarcinoma. J Hepatol. 2010;52(3):362–9. doi: 10.1016/j.jhep.2009.12.006 . [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N, Riley C, Rice GE, Quinn MA, Baker MS. Alpha(v)beta(6) integrin-A marker for the malignant potential of epithelial ovarian cancer. J Histochem Cytochem. 2002;50(10):1371–80. doi: 10.1177/002215540205001010 . [DOI] [PubMed] [Google Scholar]
- 14.Desai K, Nair MG, Prabhu JS, Vinod A, Korlimarla A, Rajarajan S, et al. High expression of integrin beta6 in association with the Rho-Rac pathway identifies a poor prognostic subgroup within HER2 amplified breast cancers. Cancer medicine. 2016;5(8):2000–11. doi: 10.1002/cam4.756 ; PubMed Central PMCID: PMCPMC4873607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Lin P, Gao C, Peng C, Liu S, Gao H, et al. Integrin beta6 acts as an unfavorable prognostic indicator and promotes cellular malignant behaviors via ERK-ETS1 pathway in pancreatic ductal adenocarcinoma (PDAC). Tumour Biol. 2016;37(4):5117–31. doi: 10.1007/s13277-015-4353-7 . [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Liang B, Gao H, Zhang F, Wang B, Dong X, et al. Integrin aVb6 as a novel marker for diagnosis and metastatic potential of thyroid carcinoma. Head & Neck Oncology. 2013;5(1). [Google Scholar]
- 17.Shimmon G, Wood BA, Morris A, Mioulet V, Grazioli S, Brocchi E, et al. Truncated Bovine Integrin Alpha-v/Beta-6 as a Universal Capture Ligand for FMD Diagnosis. PLoS ONE. 2016;11(8):e0160696 doi: 10.1371/journal.pone.0160696 ; PubMed Central PMCID: PMCPMC4975482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enyu L, Na W, Chuanzong Z, Ben W, Xiaojuan W, Yan W, et al. The clinical significance and underlying correlation of pStat-3 and integrin alphavbeta6 expression in gallbladder cancer. Oncotarget. 2017;8(12):19467–77. doi: 10.18632/oncotarget.14444 ; PubMed Central PMCID: PMCPMC5386698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song L, Fan Z, Jun N, Benjia L, Zequn L, Xilong W, et al. Tumor specific delivery and therapy mediate by integrin beta6-target immunoliposomes for beta6-siRNA in colon carcinoma. Oncotarget. 2016;7(51):85163–75. doi: 10.18632/oncotarget.13209 ; PubMed Central PMCID: PMCPMC5356726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Wei L, Yu J, Li G, Zhang X, Wang A, et al. Targeting of the beta6 gene to suppress degradation of ECM via inactivation of the MAPK pathway in breast adenocarcinoma cells. Oncol Rep. 2014;32(5):1787–95. doi: 10.3892/or.2014.3419 ; PubMed Central PMCID: PMCPMC4203328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucur O, Pennarun B, Stancu AL, Nadler M, Muraru MS, Bertomeu T, et al. Poor antibody validation is a challenge in biomedical research: a case study for detection of c-FLIP. Apoptosis. 2013;18(10):1154–62. doi: 10.1007/s10495-013-0880-0 . [DOI] [PubMed] [Google Scholar]
- 22.Herber DL, Severance EG, Cuevas J, Morgan D, Gordon MN. Biochemical and histochemical evidence of nonspecific binding of alpha7nAChR antibodies to mouse brain tissue. J Histochem Cytochem. 2004;52(10):1367–76. doi: 10.1177/002215540405201013 . [DOI] [PubMed] [Google Scholar]
- 23.Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):409–12. doi: 10.1007/s00210-008-0368-6 ; PubMed Central PMCID: PMCPMC2653258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nature reviews Drug discovery. 2011;10(9):712 doi: 10.1038/nrd3439-c1 . [DOI] [PubMed] [Google Scholar]
- 25.Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, et al. Antibody validation. Biotechniques. 2010;48(3):197–209. doi: 10.2144/000113382 ; PubMed Central PMCID: PMCPMC3891910. [DOI] [PMC free article] [PubMed] [Google Scholar]