Abstract
Ketamine is a non-competitive antagonist at the N-methyl-d-aspartate receptor. It has recently been found to have antidepressant effects and is a drug of abuse, suggesting it may have dopaminergic effects. To examine the effect of ketamine on the dopamine systems, we carried out a systematic review and meta-analysis of dopamine measures in the rodent, human and primate brain following acute and chronic ketamine administration relative to a drug-free baseline or control condition. Systematic search of PubMed and PsychInfo electronic databases yielded 40 original peer-reviewed studies. There were sufficient rodent studies of the acute effects of ketamine at sub-anaesthetic doses for meta-analysis. Acute ketamine administration in rodents is associated with significantly increased dopamine levels in the cortex (Hedge’s g= 1.33, P<0.01), striatum (Hedge’s g=0.57, P<0.05) and the nucleus accumbens (Hedge’s g=1.30, P<0.05) compared to control conditions, and 62–180% increases in dopamine neuron population activity. Sub-analysis indicated elevations were more marked in in vivo (g=1.93) than ex vivo (g=0.50) studies. There were not enough studies for meta-analysis in other brain regions studied (hippocampus, ventral pallidum and cerebellum), or of the effects of chronic ketamine administration, although consistent increases in cortical dopamine levels (from 88 to 180%) were reported in the latter studies. In contrast, no study showed an effect of anaesthetic doses (>100 mg kg−1) of ketamine on dopamine levels ex vivo, although this remains to be tested in vivo. Findings in non-human primates and in human studies using positron emission tomography were not consistent. The studies reviewed here provide evidence that acute ketamine administration leads to dopamine release in the rodent brain. We discuss the inter-species variation in the ketamine induced dopamine release as well as the implications for understanding psychiatric disorders, in particular substance abuse, schizophrenia, and the potential antidepressant properties of ketamine, and comparisons with stimulants and other NMDA antagonists. Finally we identify future research needs.
Introduction
Ketamine, a phencyclidine (PCP) hydrochloride derivative, is used as a common dissociative anaesthetic and in pain treatment. It is a non-competitive antagonist at the N-methyl-d-aspartate (NMDA) excitatory ligand-gated ion channel and binds to the PCP-binding site of the receptor to prevent the influx of Ca+2 ions following binding by glycine and glutamate co-agonists.1
Ketamine is a ‘club drug’ of abuse. The annual prevalence of ketamine use in young adults ranges from 0.8 to 1.8%.2 At sub-anaesthetic doses ketamine induces behavioural and neurochemical alterations associated with symptoms of schizophrenia in humans.3, 4, 5 Interestingly, ketamine has recently emerged as a potential treatment for major depressive disorder.6 A single dose of 0.5 mg kg−1 of ketamine has been shown to have a rapid and relatively potent antidepressant effects.7, 8 However the dose of ketamine used in the treatment of resistant major depression is similar to that shown to have psychotomimetic and cognitive effects.9
The abuse potential and psychotomimetic effects of ketamine have been linked to the dopaminergic system. Moreover, its dopaminergic effects may also contribute to its antidepressant effects. In view of this we aimed to systematically review and meta-analyse the evidence that sub-anaesthetic doses of ketamine affect dopamine levels. In addition we summarise findings at anaesthetic doses to enable comparison.
Methods
Search strategy and study selection
A PubMed and Psych INFO electronic database search was performed using the search terms “ketamine” AND “dopamine” from July 1972 to mid-July 2016 (see Supplementary Figure 1 for study selection details). Inclusion criteria were: (1) racemic ketamine administered at sub-anaesthetic doses (⩽100 mg kg−1 i.p. in rodents), (2) measures of dopamine levels in brain. Exclusion criteria were (1) studies which used ketamine at anaesthetic doses (>100 mg kg−1 i.p. in rodents), (2) studies which lacked a baseline condition or control group, (3) studies that were in non-English language, (4) studies that did not report original data, (5) not reporting the s.d.’s or s.e.m, (6) in vitro studies, and (7) studies that did not report dopamine levels although they may report other dopaminergic outcome measures (for example, metabolite levels). We used the same search criteria to identify non-human primate and human studies but did not apply a dose cut-off as this may vary by species and route of administration. We used 100 mg kg−1 as the upper dose limit in rodents as ketamine is used at greater than this as a starting dose for injectable anaesthesia in rodents and we aimed to focus on sub-anaesthetic doses associated with behavioural and psychological effects.10 However, to enable comparison with the effects of ketamine at anaesthetic doses, we also performed a separate search using the search terms ‘ketamine’ AND (‘high dose’ OR ‘anaesthesia’ OR ‘anaesthetic’) AND ‘dopamine’ and summarise the results of studies investigating the effect of anaesthetic doses of ketamine administration on dopaminergic outcomes relative to control condition.
Data extraction and statistical analyses
The following variables were extracted from all the studies: authors, year of publication, subject characteristics of the control and ketamine group (species, strain, sex, age and weight), dopaminergic measure characteristics (method, duration of ketamine treatment, the dose of ketamine used, route of administration of the drug, the dopaminergic outcome measure under investigation, and time the outcome was investigated in relation to the injection of the drug).
The main outcome measure was the effect size for the difference in dopamine levels following ketamine administration relative to a drug-free baseline or control condition. Where a mean and variance was not reported in the text, data were extracted from graphs and percentage increase and decrease were calculated. Fold change was converted to a percentage value for consistency. Plot digitizer software was used to examine reliability for the data from studies where data were available only in a plot format (http://plotdigitizer.sourceforge.net/). When data are provided for more than one time point, the time point which showed the largest percentage change was chosen for all studies for consistency.
A meta-analysis and sub-analyses for specific methods (that is, measurement of dopamine level by (1) in vivo= microdialysis and fast-scan voltammetry; or (2) ex vivo= following decapitation) were performed when there were at least five studies investigating dopamine levels in a specific region of interest. Where studies investigated dopamine effects following two or more doses of ketamine administration, the dose which documented the highest percentage change was used for the meta-analysis. For these reasons the effect sizes in the meta-analyses should be considered as the largest potential effect of ketamine on the dopamine measures.
The statistical analyses of the extracted data were conducted using the R statistical programming language version 3.2.2 with the ‘metafor’ package. The main outcome measure was the effect size for the dopaminergic index in the cortex, striatum and nucleus accumbens following acute ketamine administration using a random effects model. Publication bias was assessed using funnel plots as well as regression tests. Heterogeneity was estimated using the I2 value (I2 values <50% indicate low to moderate heterogeneity, whereas I2 >50% indicate moderate to high heterogeneity). Leave-one-out sensitivity analyses were conducted. A significance level of P<0.05 (two-tailed) was taken as significant. Meta-analysis was conducted where, there were more than 5 studies in a brain area as results with <5 studies might be unstable. In regions where there were not enough data for a meta-analysis, we have summarised the findings in the table. Publication bias and sensitivity analyses were conducted for meta-analyses including at least 8 original studies.
Study sample and methodological characteristics
The literature search identified 1263 potentially relevant articles for initial screening. Duplications (N=424) were identified using a function in Endnote and confirmed by manual screening of the titles. We excluded 774 studies from first assessment of titles and abstracts. Sixty-five abstracts were classified as possible for inclusion and full texts were obtained. Forty papers were excluded from further analysis. Of the 25 included studies, 21 investigated changes in dopaminergic measure following ketamine treatment in the rodent brain and four in the primate brain. A total of fifteen studies were included in meta-analyses (see Supplementary Figure 1 for a PRISMA diagram of literature search).
Supplementary Table 1 lists the subject characteristics for the included studies identified from our main and separate searches. From the studies reporting animal sex, all studies were done in male animals. Twenty rodent studies investigated the acute effects of ketamine, whereas only six studies investigated the chronic effects (four of which investigated both acute and chronic effects of ketamine on dopaminergic systems; Supplementary Tables 2 and 4). Out of the four primate studies, two used a constant infusion of ketamine while the other two studies investigated the effects of a single ketamine injection (Supplementary Table 6).
Results
Meta-analysis of dopamine levels in the frontal cortex following acute ketamine administration in rodents
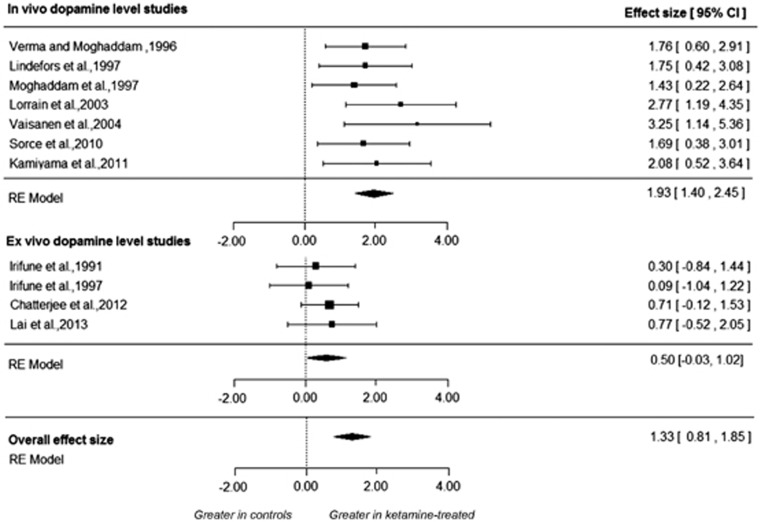
Meta-analysis of 11 studies involving 72 ketamine-treated and 70 vehicle-treated rodents, showed a significant increase in dopamine levels in the cortex after ketamine administration (range of ketamine dose 18–100 mg kg−1) compared to control state with an effect size of 1.33 ((95% confidence interval (CI), 0.81–1.85), P<0.001; Figure 1).11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 The sub-analysis of the in vivo studies showed a significant increase in dopamine levels in the cortex with an effect size of 1.93 ((95% CI 1.40, 2.45), P<0.001). The sub-analysis of the ex vivo dopamine level studies did not show a difference in dopamine levels in the ketamine relative to the control condition (effect size=0.50 (CI,−0.03–1.02), P=0.064).
Figure 1.
The effect of acute ketamine on frontal cortical dopamine levels. Meta analysis of dopamine levels in the frontal cortex following acute ketamine administration. There was a large significant overall effect of ketamine on dopamine measures (summary effect size=1.33, p<0.001). Sub-analysis pooled effect size shown for in vivo microdialysis studies in the top panel, ex vivo dopamine level studies in the bottom panel and overall effect size for both microdialysis and ex vivo studies combined.
There was evidence of significant heterogeneity among the studies (I2=45.8% (95% CI, 0–85.54%); P<0.05). The regression test for funnel plot asymmetry was significant (t=3.31, df=9 and P=0.01), suggesting publication bias is likely. Trim-fill analysis estimated three missing studies on the left side. The results remained significant after correcting for putatively missing studies (effect size: 1.02; CI, 0.46–1.59, P<0.01; Supplementary Figure 2). The summary effect size reached significance in all cases in the leave-one-out analysis, with summary effect sizes varying from 1.19 to 1.45 (all P<0.001). A study investigating change in dopaminergic levels following administration of a specific enantiomer of ketamine rather than a racemic mixture of ketamine was excluded from the meta-analysis, but the results of the study were in line with the racemic findings.22
Meta-analysis of dopamine levels in striatum following acute ketamine administration
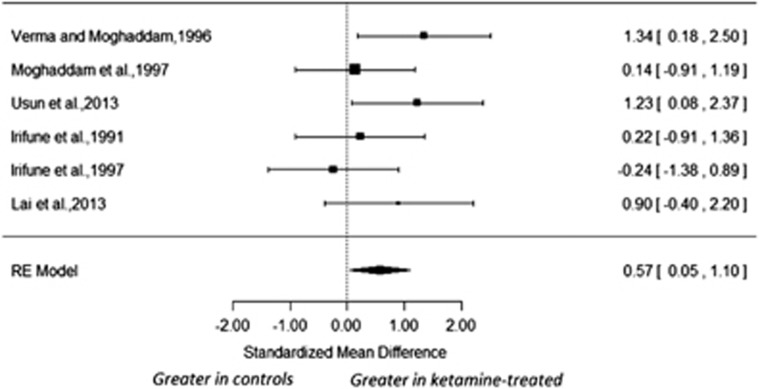
Meta-analysis of six studies involving 38 ketamine-treated and 38 vehicle-treated rodents, showed a significant increase in dopamine levels following acute ketamine (range of dose 10–50 mg kg−1) compared to the control condition (effect size: 0.57; (CI, 0.05–1.10); P=0.03; Figure 2).12, 13, 15, 18, 21, 23 The I2 value was 19.96% (95% CI, 0.00–86.38%), indicating low to moderate heterogeneity.
Figure 2.
Meta-analysis showing the effect of acute ketamine on striatal dopamine levels. There was a significant increase in dopamine measures following ketamine (effect size=0.57; P=0.03).
Meta-analysis of dopamine levels in the nucleus accumbens following acute ketamine administration
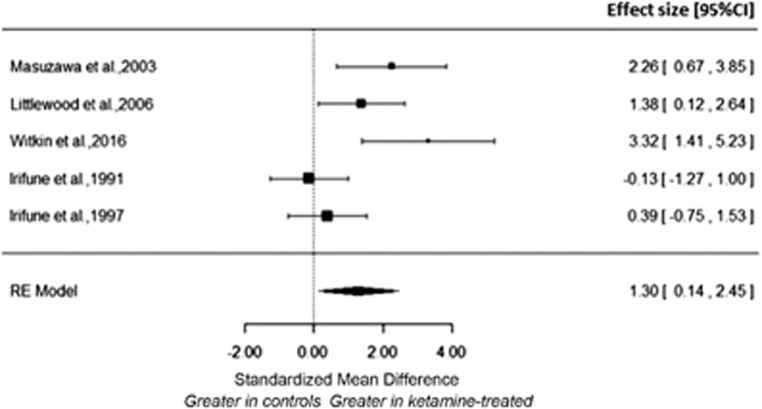
Meta-analysis of five studies involving 28 ketamine-treated and 28 vehicle-treated rodents, showed a significant increase in dopamine levels in the ketamine (range of dose 10–100 mg kg−1) relative to the control condition with an effect size of 1.30 ((CI, 0.14–2.45), P=0.028) Figure 3).12, 13, 22, 24, 25 The I2 value was 79.29% (95% CI, 18.46–97.03%), indicating high heterogeneity.
Figure 3.
Meta-analysis of the effect of acute ketamine on nucleus accumbens dopamine levels. There was a significant increase in dopamine levels in the nucleus accumbens following ketamine with a large effect size (summary effect size=1.30; P=0.028).
Dopamine levels in other brain regions in rodents
There were too few studies to permit meta-analysis in other brain regions. The majority of studies report no changes in dopamine levels in the hippocampus, brainstem and ventral pallidum following acute ketamine administration (Supplementary Table 2).12, 13, 25 Likewise, the studies retrieved report no changes in dopamine levels in the hippocampus, midbrain and cerebellum following chronic ketamine administration (Supplementary Table 4).11, 26
Dopamine neuron firing in ventral tegmental area
Four studies assessed dopamine neuron firing in the ventral tegmental area (VTA) of rats following ketamine administration.22, 27, 28, 29 Acute ketamine (in three studies) caused an increase in population activity of 62–180%, with no change in firing rate or burst activity in three out of four studies whereas chronic ketamine (1 study) had no significant effect on dopamine neuron firing (Supplementary Table 3).
Dopamine levels following chronic ketamine administration in rodents
There were too few studies to permit meta-analysis of the effect of chronic ketamine treatment on dopamine levels in any brain region. In total three studies investigated the chronic effect of ketamine administration (range of dose: 15–100 mg kg−1 per day) on the percentage difference in dopamine levels in the cortex in rodents for a range of 8 days to 3 months.11, 16, 30 All three studies reported increases in dopamine levels in the cortex ranging from 88% to over 180%. In the striatum the results are inconsistent with one out of three studies showing increase in dopamine levels, whilst the other two found no significant effect of ketamine on dopamine levels. A potential explanation of the lack of effect in one study is the relatively low dose and route of administration of ketamine, which was 15 mg kg−1 in liquid diet31 and the methodology used to assess dopamine levels in the other study30 (Supplementary Table 4).
Effect of anaesthetic doses of ketamine on dopamine levels in rodents
Four studies assessed dopamine levels in cortex, striatum, nucleus accumbens, brainstem and hippocampus of rodents following anaesthetic dose of ketamine administration (range 150–350 mg kg−1)).12, 13, 32, 33 All four studies consistently reported no change in ex vivo dopamine levels (Supplementary Table 5).
Effects of acute and chronic ketamine on the dopaminergic system in non-human primates and humans
Microdialysis studies
A majority of the non-human primate studies (three out of four) showed no effect of ketamine on dopamine levels in the cortex or striatal regions (Supplementary Table 6).34, 35, 36 One study showed a small but significant 30% increase in dopamine in the striatum compared to baseline levels following a single acute ketamine injection.37 No study investigated the effect of chronic ketamine administration on dopamine levels.
Positron emission tomography studies
Table 1 summarises studies on effect of ketamine on the dopaminergic system in non-human primates. The majority of the studies in non-human primates investigated effects of anaesthetic doses (3–10 mg kg−1) of ketamine. The one study to investigate dopamine synthesis capacity found ketamine increased dopamine synthesis, although it used an anaesthetic dose.36 Interestingly, both anaesthetic36 and sub-anaesthetic doses38 showed reductions in D2/3 receptor availability, consistent with dopamine release following ketamine. However, findings of the effects of ketamine on dopamine transporter (DAT) availability are variable with sub-anaesthetic doses showing no significant change,34 whilst studies using ketamine doses in an anaesthetic range36, 39, 40 showed increases in DAT availability. All studies were done in small samples, 3–5 non-human primates, thus findings should be interpreted with caution and more studies with sub-anaesthetic doses of ketamine are needed.
Table 1. PET studies of dopaminergic function in non-human primates after ketamine administration compared to control.
| Dopamine system studied | Study | Ketamine | Ketamine dose and route of administration | State of animal | Duration of treatment | When the outcome investigated | Ligand | Radiotracer administration | Study design/ control group | Analytical method | Region of interest | Na | Outcome measure | Change in dopamine measure after ketamine infusion compared to control condition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dopamine synthesis | Tsukada et al.36 | Racemic | 3 and 10 mg kg−1 h−1, i.v | Anaesthetised | Infusion throughout scan | 30 mins prior to scan | L-[ β -11C]DOPA | i.v | Within/ saline | Graphical analysis (L-[ β -11C]DOPA) | Str | 4 | Dopamine synthesis rate | ↑ |
| Dopamine release | Hashimoto et al., 201738 | R and S-ketamine | 0.5 mg kg−1 | Sub-anaesthetised | Infusion 40 mins | After the end of infusion | [11C]raclopride | i.v | Saline | Reference tissue model | Caudate/putamen | 4 | Δ D2/3 receptor binding potentialb | ↓29% (S-Ketamine) |
| ↔ No change (R Ketamine) | ||||||||||||||
| Tsukada et al.36 | Racemic | 3 and 10 mg kg−1 h−1, i.v | Anaesthetised | Infusion throughout scan | 30 mins prior to scan | [11C]raclopride | i.v | Within/ saline | Kinetic analysis ([11C]raclopride) | Str | 4 | Δ Binding potentialb | ↓ | |
| Dopamine transporter | Tsukada et al.36 | Racemic | 3 and 10 mg kg−1 h−1, i.v | Anaesthetised | Infusion throughout scan | 30 mins prior to scan | [11C]β-CFT | i.v | Within/ saline | Kinetic analysis ([11C]β-CFT) | Str | 4 | DAT binding potential | ↓ |
| Yamamoto et al.34 | Racemic | 0.5 & 1.5 mg kg−1 | Sub-anaesthetised | Infusion 40 mins | After the end of infusion | [11C]β-CFT | Bolus i.v | Within/ saline | Reference tissue model | Ct, Str, Midbrain, Thal | 5 | DAT binding potential | ↔ No change | |
| Harada et al.40 | Racemic | 3 mg kg−1 h−1 | Anaesthetised | Infusion throughout scan | 60 mins before tracers | [11C]β-CFT & [11C]β-CIT-FE | i.v | Within/ saline | Kinetic analysis | Str | 3 | DAT binding potential | ↓ ([11C]β-CFT) | |
| ↔ No change ([11C]β-CIT-FE) | ||||||||||||||
| Tsukada et al.39 | Racemic | 3 and 10 mg kg−1 h−1, i.v | Anaesthetised | Infusion throughout scan | 60 mins before tracers | [11C]β-CFT & [11C]β-CIT-FE | i.v | Within/ saline | Kinetic analysis | Str | 5 | DAT binding potential | ↓ (3 mg kg−1) |
Abbreviations: Ct, cortex; DA, dopamine; DAT, dopamine transporter; i.m, intra muscular; i.v, intravenous; NA, not available; PET, positron emission tomography; Str, striatum; Thal, thalamus.
N the sample size represents the total number of animals used for the comparison in question.
Greater reduction in D2/D3 receptor binding potential after ketamine administration indicates greater dopamine release; ↑significant increase, ↓significant decrease and ↔no significant change.
Effects of acute and chronic ketamine administration on the dopaminergic system in humans
Table 2 summarises the studies of the effects of ketamine challenge on dopamine release in humans. We did not find any studies which investigated the effects of ketamine on dopamine synthesis or transporter levels in human. All studies measured dopamine release following ketamine challenge using positron emission tomography (PET) imaging. Three studies reported evidence of dopamine release, as indexed by a ~14% change in D2/3 radiotracer binding, in the striatum following ketamine administration in healthy volunteers.41, 42, 43 Similar results were observed in the cingulate cortex but not in the thalamus or the frontal, temporal and parietal cortices.44 However, three studies did not detect dopamine release following ketamine infusion,45, 46, 47 although one of these showed that ketamine augmented amphetamine-induced dopamine release.46 Methodological factors, for example, the radiotracer imaging not being conducted under equilibrium conditions, may account for the discrepant finding (see refs 48 and 49 for a further discussion of these factors).
Table 2. PET studies of D2/D3 receptor availability in healthy humans after ketamine infusion compared to control condition.
| Study | Ketamine | Ketamine dose and route of administration | Duration of administration | Ligand | Radiotracer administration | Study design | Region of interest | Na | (Plasma mean±s.e.m.; ng ml−1) | Result: change in D2/D3 receptor availability after ketamine infusion compared to control conditionb |
|---|---|---|---|---|---|---|---|---|---|---|
| Aalto et al.45 | Racemic | Infusion=0.80 mg kg−1c | Infusion 15 mins prior to scan till the end of scan | [11C]raclopride | Infusion | Control group: baseline and repeat scan Ketamine group: baseline and ketamine administration | Caudate, putamen, Str | 8/8 | 293±29 | ↔ |
| Aalto et al.44 | Racemic | Infusion 325.5±57.5 ng ml−1 | Infusion 15 mins prior to scan- till the end of scan | [11C]FLB 457 | Infusion | Control group: baseline and repeat scan Ketamine group: baseline and ketamine administration | Ct regions, Thal | 8/8 | 325.5±57.5 | ↓Posterior cingulate ct ↔ (other regions) |
| Breier et al.41 | Racemic | 0.12 mg kg−1 (bolus) and 0.65 mg kg−1 (infusion)/hour=0.88 | Bolus 50 mins after tracer and 1 hour infusion | [11C]raclopride | Bolus & infusion | Control group: baseline and saline Ketamine group: baseline and ketamine administration | Str | 6/9 | NA | ↓(11%) |
| Kegeles et al.47 | Racemic | 0.12 mg kg−1 bolus and 0.65 mg kg−1 h−1=0.88 | Bolus 50 mins after start of scan and 70 mins infusion | [11C]raclopride | Bolus & infusion | Control group: baseline and saline Ketamine group: baseline and ketamine administration | Str subregions | 5/5 | 140±53 | ↔ |
| Kegeles et al.46 | Racemic | 0.2 mg kg−1 bolus and 0.4 mg kg−1 h−1=1.00 | Bolus 120 mins after tracer and 4 h infusion | [123I]IBZM | Bolus & constant infusion | Baseline scan and ketamine administration – within subject | Str | 8 | 191±38 | ↔ |
| Vernaleken et al.94 | S-ketamine | 0.097 mg kg−1 bolus and 0.25 mg/ml infusion | 35 mins before start of scan infusion was started. Infusion was continued for 30 mins | [18F]-fallypride | Bolus & constant infusion | Placebo/ketamine – within subject | Caudate nucleus, putamen, Thal, ITG, dlPFC | 10 | NA | ↑(Caudate nucleus) ↔ (other region) |
| Smith et al.42 | Racemic | 0+1.5 mg kg−1 h−1=0.50 | Infusion over 20 mins | [11C]raclopride | Infusion | Baseline scan and ketamine administration – within subject | Str | 7 | NA | ↓ (14%) |
| Vollenweider et al.43 | S-ketamine | 0.21+0.84/hour=1.47 | Bolus over 5 min | [11C]raclopride | Bolus | Placebo/ketamine – within subject | Caudate nucleus, putamen and VS | 8 | NA | ↓ (14%) |
Abbreviations: Ct, cortex; dlPFC, dorsolateral prefrontal cortex; ITG, inferior temporal gyrus; i.v, intravenous; Ket, ketamine; NA, not available; Thal, thalamus; VS, ventral striatum.
N the sample size represents the number of subjects per group.
Greater reduction in D2/D3 receptor binding potential after ketamine administration indicates greater dopamine release; ↑significant increase, ↓significant decrease,↔no significant change.
Average dose given in the study.
However, it should also be noted that striatal dopamine release with ketamine administration ranged from 30 to 60% compared to baseline while cortical regions display 150–250% changes (Supplementary Table 2). To put this in perspective, microdialysis studies in rodents show that amphetamine administration increases striatal dopamine levels to 300–400% compared to baseline,50 and this is readily detectable by PET (see review51). It has been estimated that the ratio of dopamine release to change in radiotracer binding is about 44:1.52, 53 Thus, given the relatively modest degree of dopamine release in the striatum with ketamine, this may be close to the limit of detection with PET techniques.49 Recent studies have shown that the agonist ligand [11C] PHNO is more sensitive to quantify amphetamine-induced dopamine release.54 Thus future studies with agonist ligands such as [11C] PHNO may clarify this issue (Box 1).
Suggested future research directions.
Preclinical research
1. Further studies to determine the effect of chronic administration (>10 days) of ketamine on dopaminergic function.
2. Studies needed in females to investigate whether there are gender differences.
3. Studies needed to investigate whether there are strain differences.
4. Determine the effects of acute and chronic ketamine on dopaminergic function using translational techniques such as micro-positron emission tomography.
5. Studies to investigate the effect of acute and chronic ketamine on dopamine neuron firing and how that relates with measures of dopamine release and dopamine synthesis capacity.
6. Studies to investigate correlations between dopamine release and psychotomimetic or antidepressant effects of ketamine and other NMDA receptor antagonists.
7. Studies are needed in dopamine transporter (DAT) knock-out (KO) mice to investigate whether the effects of ketamine on dopaminergic function are independent of DAT blockade.
8. Investigate effects of various sub-anaestheic doses of ketamine on dopamine synthesis, transporter and release in primates.
Clinical research
1. Investigate effects of dopamine synthesis and transporter availability following acute ketamine administration.
2. Investigate dopamine release following ketamine challenge using agonist ligand such as [11C] PHNO.
3. Studies needed to investigate dopamine synthesis, release and transporter availability in chronic users.
One study in chronic ketamine users reported an upregulation of D1 receptor availability in the frontal cortex.55 However, there are no studies investigating the effects of chronic ketamine use on dopamine synthesis, transporter availability or release. Future research should investigate the effect of ketamine on these aspects of dopamine function in humans (Box 1).
Discussion
Our meta-analysis shows that acute ketamine administration increases dopamine levels in the striatum, the nucleus accumbens and the frontal cortex in rodents compared to controls with medium to very large effect sizes (Hedge’s g: 0.57, 1.3 and 1.33 respectively). These findings are summarised in Figure 4. Specifically there was evidence for increased dopamine levels following ketamine in the frontal cortex in the majority (9 out of 11) of studies in rodents, with increases ranging from 50 to 400% (Supplementary Table 2). All three studies of the effects of acute ketamine administration on dopamine neuron firing in the VTA of rodents consistently showed an increase in firing (Supplementary Table 3).22, 27, 28 Interestingly the effect size of the dopaminergic increase following acute ketamine treatment is numerically higher in the nucleus accumbens and the frontal cortex compared to the striatum. This could be potentially attributed to the higher doses of ketamine used in the studies of these two regions (frontal cortex range of doses: 18–100 mg kg−1; nucleus accumbens range of doses: 10–100 mg kg−1) compared to the striatum (10–50 mg kg−1). Alternatively it could suggest that ketamine preferentially increases dopamine release in the nucleus accumbens and cortex relative to the striatum. Studies directly comparing dopamine release across regions at the same dose of ketamine are needed to test this hypothesis. Although there were too few studies for meta-analysis, we also found evidence for consistent elevation of dopamine levels following chronic ketamine administration in the frontal cortex.11, 16, 30 These findings extend evidence of increased dopamine metabolite levels such as Homovanillic acid (HVA), 3,4-dihydroxyphenylacetic acid (DOPAC) and 3-methoxytyramine (3-MT), following acute11, 13, 16, 56 and chronic ketamine administration in the cortex.11, 16, 30 Taken together these findings indicate that acute ketamine elicits a significant dopaminergic response in cortex, striatum and nucleus accumbens in rodents and suggest that this is also the case with chronic administration (Supplementary Table 4). Nevertheless further in vivo studies are needed to determine the effects of chronic ketamine administration on dopamine release (see Box 1 for suggested future directions).
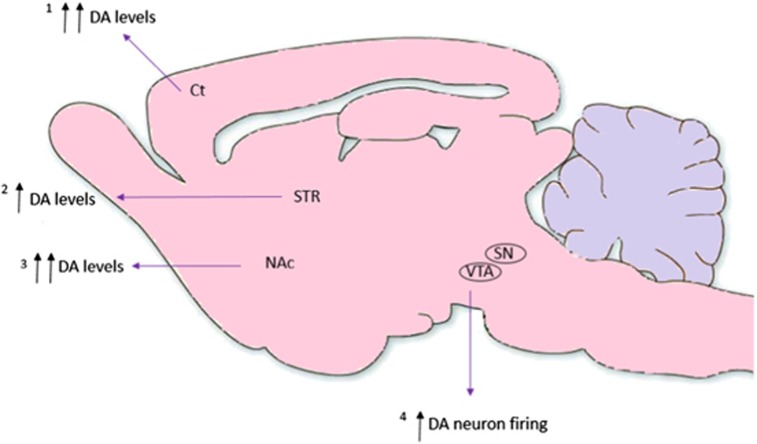
Figure 4.
Showing the location of the major dopaminergic findings following acute ketamine administration from our meta-analyses and qualitative review. 1Meta-analysis finding with effect size of 1.33 [95% CI, 0.81-1.85 p<0.001]. 2Meta-analysis finding with effect size of 0.57 [95% CI, 0.05 – 1.10 p< 0.05]. 3Meta-analysis finding with effect size of 1.30 [95% CI, 0.14 – 2.45 p< 0.05]. 4Found in the acute studies to measure this to date (Supplementary Table 3). Arrows denote the relative increases in dopamine levels in each region of interest.
General methodological considerations
It is important to note that we found heterogeneity across the analyses in the cortex and nucleus accumbens. Our sub-analysis by method for the cortex showed that the ex vivo studies, which measured total dopamine content in homogenised tissue, showed no significant effects of ketamine, unlike the in vivo studies, where consistent and large elevations were seen. Moreover, where significant differences were detected in ex vivo studies, they were more modest than those reported by in vivo studies (Table 2), suggesting that ex vivo methods may be less sensitive than in vivo methods. There were too few studies for separate sub-analyses in the striatum and in the nucleus accumbens but the same pattern of results is seen for ex vivo and in vivo studies in these regions. Thus, whilst variations between studies in terms of the range of doses of ketamine and the time lapse between the last ketamine treatment may contribute, differences between ex vivo and in vivo studies are likely to be a major contributor to heterogeneity. However, this variability might be expected to weaken effects rather than explain the elevations we report. Moreover we employed a random effects model, which is robust to heterogeneity in effects. Of note where more than one dose of ketamine was used in a study, the dose which elicited the highest difference in the dopaminergic measure was chosen for the meta-analysis. Thus the effect sizes calculated from these studies should be considered an estimate of the largest likely effect size. In addition we summarised the effects of anaesthetic dose of ketamine on dopamine levels in the rodent brain. All studies showed consistently no change in dopamine levels following anaesthetic dose of ketamine (Supplementary Table 5). However all studies used ex vivo methods of dopamine level measurement. This observation highlights the fundamental issue raised by our sub-analysis by method and further supports that in vivo studies are required to delineate the anaesthetic ketamine effect on dopaminergic function over the limitations of ex vivo methods. Moreover further studies are required to investigate the dose at which the stimulatory effects of ketamine on dopamine function decline. Finally general limitations of included studies are that only male animals were used and there was no report of ketamine brain or plasma levels. Whilst there is no sex difference in the ketamine brain levels in mice and rats,57, 58 it has been shown that there are higher numbers of dopaminergic cells in female than male rats.59 Thus extrapolations of dopaminergic modulation elicited by ketamine in females should be treated with care and studies in females are needed (Box 1). In addition strain-specific effect of acute and repeated ketamine on dopaminergic function remains to be directly tested (Box 1).
Mechanism of ketamine’s action on the dopamine system
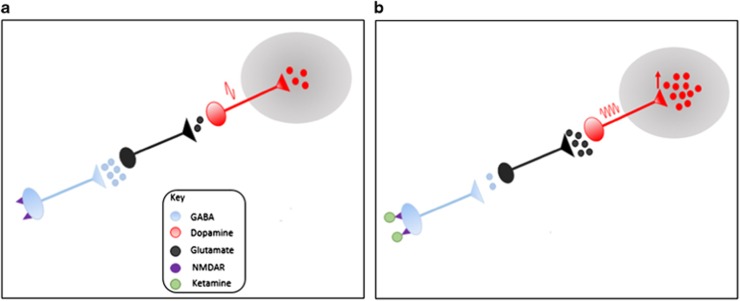
The mechanism underlying ketamine’s action on dopaminergic neurons remains to be fully established. However, several lines of evidence indicate that this involves N-methyl-d-aspartate receptor (NMDAR) blockade on GABAergic interneurons that regulate excitatory projections to the midbrain dopamine neuron cell bodies. Specifically NMDAR antagonists were shown to decrease GABAergic interneuron function60 and this in turn leads to an increase in pyramidal cell firing which is thought to lead to increased excitation of dopamine neurons.61 In line with this, there is evidence that NMDAR antagonists induce excessive glutamate release.62, 63, 64, 65 All three studies of the effects of acute ketamine administration on dopamine neurons in the VTA showed an increase in firing (Supplementary Table 3).22, 27, 28 Taken with our meta-analytic findings, this is consistent with the hypothesis that the disinhibition of glutamatergic projections onto dopamine neurons increases activation of dopaminergic neurons (Figure 5), although it remains to be directly shown that this is solely due to glutamate release.
Figure 5.
Theorised mechanism of action of ketamine in mediating dopaminergic change. Ketamine (green) blocks NMDA receptors (purple) on GABAergic interneurons (blue) disinhibiting glutamate neurons (black) projecting to dopamine neurons in the midbrain, increasing glutamate release and subsequently increasing dopamine neuron firing (red) and thus increasing dopamine levels in projection targets such as the striatum and cortex.
Comparison with stimulants
In the rodent brain the magnitude of dopamine changes is lower with ketamine relative to those seen with amphetamine and cocaine. Unlike ketamine, amphetamine at doses of 0.25 mg kg−1 and 1 mg kg−1 increased dopamine output with a maximal percentage increase of 550% and 1000% in nucleus accumbens and 250% and 520% in the caudate, respectively.66 The median dose of ketamine used in rodent studies was 30 mg kg−1 and the lowest dose was 5 mg kg−1. Thus, whilst ketamine acts on the dopamine system, it is probably not as potent as these stimulants, although direct comparisons are needed to test this.
In addition it should be noted that in vitro studies show ketamine is a DAT antagonist, and thus blockade of dopamine reuptake could contribute to ketamine’s dopaminergic effects. However, its affinity for the DAT (Ki=66.8±25.9 μm) is over an order of magnitude lower than its affinity for the NMDA receptor (Ki=3.1 μm).67, 68 It has been observed that the density of dopamine transporter (DAT) in the prefrontal cortex is much lower than in the striatum,69 and DAT blockade is not particularly effective in increasing dopamine levels in the prefrontal cortex.70 Our meta-analyses indicate there is greater dopamine increase with ketamine in the prefrontal cortex relative to the striatum. This suggests that ketamine’s effects on dopaminergic function are unlikely to be attributable to blockade of dopamine uptake at sub-anaesthetic doses as if that were the case effects would be much larger in the striatum than the prefrontal cortex. However, studies are needed in DAT knock-out mice to investigate whether the effects of ketamine on dopaminergic function are independent of DAT blockade (Box 1).
Comparison with other NMDA receptor antagonists
In the rodent brain the magnitude of dopamine changes is lower with ketamine relative to those seen with other NMDA receptor antagonists such as PCP and dizocilpine (MK-801), but not memantine. For example, a 5 mg kg−1 acute dose of PCP produces a 380–500% increase in prefontal cortical dopamine levels and a 120–190% in nucleus accumbens,71, 72 whilst 5–10 mg kg−1 doses of ketamine result in increases ~50% in these regions (Table 2). Similarly MK-801 at a dose of 0.1 mg kg−1 increased dopamine levels by 190% in PFC and 75% in NAc, respectively.73 Moreover, acute PCP at 1 mg kg−1 i.v produced a maximal increase of 500% in VTA A10 dopamine neuronal firing rate.74 In contrast, acute administration of 20 mg kg−1 of the moderate-affinity NMDA receptor antagonist memantine did not change dopamine levels in prefrontal cortex.75 Thus the rank order of dopaminergic effects of these NMDA antagonists appears to correspond to the rank order of their NMDA receptor affinities, but there have yet to be direct comparisons. Interestingly, it was shown that dopamine transmission in corticolimbic system was temporally dissociated from PCP-induced locomotor effects.76 However, the relationship between ketamine’s effects on dopamine release and the antidepressant, addictive and psychotomimetic effects of ketamine have not been investigated. This is an important future direction to aid understanding of the contribution of dopaminergic mechanisms to these effects (Box 1).
Comparison between rodent and primate studies
In contrast to the rodent studies, only one of the four primate studies we identified reported a significant change in dopamine release following ketamine administration. Human PET imaging studies, where change in radiotracer binding is used to index dopamine release,49 also show inconsistency with some but not all studies showing changes in radiotracer binding with ketamine.47, 77, 78 One potential explanation for the discrepancy between rodent and primate studies could be the difference in the timings in which dopaminergic outcome was measured following ketamine injection. In the majority of rodent studies dopaminergic outcome was measured 10–60 min post ketamine injection, whereas in three out of four studies in non-human primates dopaminergic outcome was measured 2 h following ketamine administration. Interestingly, the study which documented 30% increase from baseline measured dopamine levels 45 min post ketamine administration.37 Thus the timing of measures in some of the primate studies may have missed the peak dopamine effects, and doses were lower than many of the rodent studies. Further studies in this time range and with higher doses are needed to fully determine the effects of ketamine on the dopamine system in primates.
Implications for human use of ketamine
Whilst there is a clear need for more studies in primates addressing the issues discussed above, our findings in rodents that acute ketamine causes increases in dopamine levels suggests that ketamine has similar dopaminergic effects as stimulants and a number of other recreational drugs.79, 80, 81, 82, 83 This implies that its dopaminergic effects may contribute to ketamine’s abuse potential and the development of dependence.
This also has implications for the use of ketamine in the treatment of depression. Major depression is associated with blunted dopaminergic function.84, 85 It has been theorised that depressed individuals cannot obtain reward from normal social interaction because of dopamine deficiency.86 Thus, one potential mechanism by which ketamine may be effective in the treatment of depression is by increasing dopamine neuron firing, and consequently dopamine release to enable the appropriate association between social interactions and reward. Supporting this, in a stress-induced rat model of depression, ketamine was shown to restore the decreased dopamine neuron population activity and synaptic plasticity.28 However, it should be noted that other mechanisms may also play a role in ketamine’s antidepressant actions, including an action of a metabolite of ketamine on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.58
Sub-anaesthetic doses of ketamine in healthy human subjects acutely induce symptoms comparable to symptoms of schizophrenia3, 87 and similar low level symptoms are seen in chronic ketamine abusers.87, 88 Moreover, ketamine worsens psychotic symptoms in patients with schizophrenia.5, 89 Our findings suggest that ketamine’s psychotomimetic effects may involve the dopaminergic system, consistent with evidence that elevated dopamine synthesis and release capacity are seen in people at risk of developing schizophrenia.90, 91, 92, 93 Finally, our findings highlight that it would be useful for future preclinical studies to use methods that can be applied in human studies as well to test these potential implications and aid translation of findings (Box 1).
Conclusion
Acute ketamine administration leads to increased dopamine levels in the frontal cortex, in the striatum and in the nucleus accumbens in rodents. These findings suggest dopaminergic effects potentially contribute to its acute antidepressant and psychotomimetic effects. Effects are not clear-cut in the primate brain, potentially due to the timing of measures and lower doses used, and there have been few studies of chronic administration in any species. Further studies are required in primates at higher doses and to explore whether the same dopaminergic effects are seen following chronic ketamine administration.
Acknowledgments
The study was funded by Medical Research Council (MRC) UK grant to Professor Howes.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
MK conducts research funded by Medical Research Council. AHA conducts research funded by Medical Research Council and King’s College London. ODH conducts research funded by the Medical Research Council (UK), the National Institute of Health Research (UK) and the Maudsley Charity. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Astra-Zeneca, BMS, Eli Lilly, Jansenn, Lundbeck, Lyden-Delta, Servier and Roche.
Supplementary Material
References
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 1999; 51: 7–61. [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report, 2013; https://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf.
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51: 199–214. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 1997; 17: 141–150. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995; 13: 9–19. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr., Machado-Vieira R. Ketamine: translating mechanistic discoveries into the next generation of glutamate modulators for mood disorders. Mol Psychiatry 2017; 22: 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 2016; 46: 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Nagele P, Mennerick S, Conway CR. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry 2015; 6: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Ming Z, Dart AM, Du XJ. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin Exp Pharmacol Physiol 2007; 34: 499–507. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Verma R, Ganguly S, Palit G. Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology 2012;.63: 1161–1171. [DOI] [PubMed] [Google Scholar]
- Irifune M, Fukuda T, Nomoto M, Sato T, Kamata Y, Nishikawa T et al. Effects of ketamine on dopamine metabolism during anesthesia in discrete brain regions in mice: comparison with the effects during the recovery and subanesthetic phases. Brain Res 1997; 763: 281–284. [DOI] [PubMed] [Google Scholar]
- Irifune M, Shimizu T, Nomoto M. Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav 1991; 40: 399–407. [DOI] [PubMed] [Google Scholar]
- Kamiyama H, Matsumoto M, Otani S, Kimura SI, Shimamura KI, Ishikawa S et al. Mechanisms underlying ketamine-induced synaptic depression in rat hippocampus-medial prefrontal cortex pathway. Neuroscience 2011; 177: 159–169. [DOI] [PubMed] [Google Scholar]
- Lai CC, Lee LJ, Yin HS. Combinational effects of ketamine and amphetamine on behaviors and neurotransmitter systems of mice. Neurotoxicology 2013; 37: 136–143. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Barati S, OConnor WT. Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res 1997; 759: 205–212. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 2003; 117: 697–706. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V et al. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci 2010; 30: 11317–11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisanen J, Ihalainen J, Tanila H, Castren E. Effects of NMDA-receptor antagonist treatment on c-fos expression in rat brain areas implicated in schizophrenia. Cell Mol Neurobiol 2004; 24: 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: Modulation by dopamine. J Neurosci 1996;.16: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN et al. The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 2016; 358: 71–82. [DOI] [PubMed] [Google Scholar]
- Usun Y, Eybrard S, Meyer F, Louilot A. Ketamine increases striatal dopamine release and hyperlocomotion in adult rats after postnatal functional blockade of the prefrontal cortex. Behav Brain Res 2013; 256: 229–237. [DOI] [PubMed] [Google Scholar]
- Masuzawa M, Nakao S, Miyamoto E, Yamada M, Murao K, Nishi K et al. Pentobarbital inhibits ketamine-induced dopamine release in the rat nucleus accumbens: a microdialysis study. Anesth Analg 2003; 96: 148–152, table of contents. [DOI] [PubMed] [Google Scholar]
- Littlewood CL, Jones N, O'Neill MJ, Mitchell SN, Tricklebank M, Williams SC. Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology 2006; 186: 64–81. [DOI] [PubMed] [Google Scholar]
- Tan S, Lam WP, Wai MS, Yu WH, Yew DT. Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS ONE 2012; 7: e43947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Iskandrani KS, Oosterhof CA, El Mansari M, Blier P. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: an in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol 2015;.29: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 2014;.76: 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED, Ceci A. Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons. Neurosci Lett 1990; 119: 159–162. [DOI] [PubMed] [Google Scholar]
- Tan S, Lam WP, Wai MSM, Yu WHA, Yew DT. Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS ONE 2012; 7: e43947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheletti G, Lannes B, Haby C, Borrelli E, Kempf E, Warter JM et al. Chronic administration of NMDA antagonists induces D2 receptor synthesis in rat striatum. Mol Brain Res 1992; 14: 363–368. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Mueller RA, Breese GR. Effects of anesthetics and electrical stimulation on nigrostriatal dopaminergic neurons. J Pharmacol Exp Ther 1983; 224: 489–493. [PubMed] [Google Scholar]
- Ke JJ, Chen HI, Jen CJ, Kuo YM, Cherng CG, Tsai YPN et al. Mutual enhancement of central neurotoxicity induced by ketamine followed by methamphetamine. Toxicology and applied pharmacology 2008; 227: 239–247. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Ohba H, Nishiyama S, Harada N, Kakiuchi T, Tsukada H et al. Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: a PET study in conscious monkeys. Neuropsychopharmacology 2013; 38: 2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe H, Inoue O, Suzuki K, Tsukada H, Itoh T, Mataga N et al. Ketamine increases the striatal N-[C-11]Methylspiperone binding in-vivo - positron emission tomography study using conscious rhesus-monkey. Brain Res 1994; 663: 191–198. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Harada N, Nishiyama S, Ohba H, Sato K, Fukumoto D et al. Ketamine decreased striatal [(11)C]raclopride binding with no alterations in static dopamine concentrations in the striatal extracellular fluid in the monkey brain: multiparametric PET studies combined with microdialysis analysis. Synapse 2000; 37: 95–103. [DOI] [PubMed] [Google Scholar]
- Adams BW, Bradberry CW, Moghaddam B. NMDA antagonist effects on striatal dopamine release: microdialysis studies in awake monkeys. Synapse 2002; 43: 12–18. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 2017; 267: 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N. Ketamine alters the availability of striatal dopamine transporter as measured by [C-11]beta-CFT and [C-11]beta-CIT-FE in the monkey brain. Synapse 2001; 42: 273–280. [DOI] [PubMed] [Google Scholar]
- Harada N, Ohba H, Fukumoto D, Kakiuchi T, Tsukada H. Potential of [(18)F]beta-CFT-FE (2beta-carbomethoxy-3beta-(4-fluorophenyl)-8-(2-[(18)F]fluoroethyl)nortropane) as a dopamine transporter ligand: A PET study in the conscious monkey brain. Synapse 2004; 54: 37–45. [DOI] [PubMed] [Google Scholar]
- Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L et al. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse 1998; 29: 142–147. [DOI] [PubMed] [Google Scholar]
- Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA et al. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology 1998; 18: 18–25. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL. Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 2000; 34: 35–43. [DOI] [PubMed] [Google Scholar]
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H et al. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology 2005; 182: 375–383. [DOI] [PubMed] [Google Scholar]
- Aalto S, Hirvonen J, Kajander J, Scheinin H, Nagren K, Vilkman H et al. Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology 2002; 164: 401–406. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry 2000; 48: 627–640. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Martinez D, Kochan LD, Hwang DR, Huang Y, Mawlawi O et al. NMDA antagonist effects on striatal dopamine release: positron emission tomography studies in humans. Synapse 2002; 43: 19–29. [DOI] [PubMed] [Google Scholar]
- Rabiner EA. Imaging of striatal dopamine release elicited with NMDA antagonists: there anything there to be seen? J Psychopharmacol 2007; 21: 253–258. [DOI] [PubMed] [Google Scholar]
- Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev 2009; 33: 1109–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem 1988; 50: 346–355. [DOI] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 2017; 74: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000; 20: 423–451. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94: 2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S et al. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 2012; 32: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M et al. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry 2005; 162: 2352–2359. [DOI] [PubMed] [Google Scholar]
- Rao TS, Kim HS, Lehmann J, Martin LL, Wood PL. Differential effects of phencyclidine (PCP) and ketamine on mesocortical and mesostriatal dopamine release in vivo. Life Sci 1989; 45: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 2017; 361: 9–16. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Pilgrim C, Reisert I. Dopamine content and metabolism in mesencephalic and diencephalic cell cultures: sex differences and effects of sex steroids. J Neurosci 1991; 11: 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun L, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol 2005; 93: 1989–2001. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 1999; 33: 523–533. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 2005; 162: 394–396. [DOI] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 2012; 17: 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee H, Kim HJ, Bang E, Lee SH, Lee DW et al. In vivo and ex vivo evidence for ketamine-induced hyperglutamatergic activity in the cerebral cortex of the rat: potential relevance to schizophrenia. NMR Biomed 2011; 24: 1235–1242. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 2015; 66: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 1989; 28: 653–661. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S et al. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology 1998; 88: 768–774. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, Tallerico T. Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol Psychiatry 2005;.10: 877–883. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 1998; 18: 2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse 1989; 4: 156–161. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 1998; 18: 5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 1998; 281: 1349–1352. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutarnate 5 (rnGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry 2007; 62: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED. Competitive Nmda receptor antagonists attenuate phencyclidine-induced excitations of A10 dopamine neurons. Eur J Pharmacol 1992; 217: 1–7. [DOI] [PubMed] [Google Scholar]
- Hesselink MB, De Boer AG, Breimer DD, Danysz W. Dopamine release in the prefrontal cortex in response to memantine following sub-chronic NMDA receptor blockade with memantine: a microdialysis study in rats. J Neural Transm (Vienna) 1999; 106: 803–818. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 1998; 18: 5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H et al. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005; 182: 375–383. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Zea-Ponce Y, Abi-Dargham A, Schneider D, Van Heertum R, Mann JJ et al. Ketamine modulation of amphetamine-induced striatal dopamine release in humans measured by [I-123 I]IBZM SPECT. J Nucl Med 1999; 40: 30p–30p. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993; 18: 247–291. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry 2006; 63: 1386–1395. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology 2006; 31: 1016–1026. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, Ashok AH, Volkow ND, Howes OD. The effects of Delta9-tetrahydrocannabinol on the dopamine system. Nature 2016; 539: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow N, Howes OD. Association of stimulants with dopaminergic alterations in users of cocaine, amphetamine, and methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry, In press 2017; 74: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry 1992; 32: 1–17. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64: 327–337. [DOI] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, Barch DM. Reward processing and risk for depression across development. Trends Cogn Sci 2016; 20: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Krystal JH, Ning Y, Chen da C, He H, Wang D et al. Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J Psychiatr Res 2015; 61: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Pepper F, Fam J, Furby H, Hughes E, Morgan C et al. Glutamate, N-acetyl aspartate and psychotic symptoms in chronic ketamine users. Psychopharmacology 2014; 231: 2107–2116. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Ketamine use: a review. Addiction (Abingdon, England) 2012; 107: 27–38. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Suridjan I, Kenk M, George TP, Wilson A, Houle S et al. Dopamine response to psychosocial stress in chronic cannabis users: a PET study with [11C]-+-PHNO. Neuropsychopharmacology 2013; 38: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry 2009; 66: 13–20. [DOI] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry 2011; 168: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry 2011; 16: 885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernaleken I, Klomp M, Moeller O, Raptis M, Nagels A, Rosch F et al. Vulnerability to psychotogenic effects of ketamine is associated with elevated D-2/3-receptor availability. Int J Neuropsychopharmacol 2013; 16: 745–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.