Abstract
Type 2 diabetes mellitus is becoming increasingly prevalent worldwide, and has become one of the greatest threats to global health. Bariatric surgery was initially designed to achieve weight loss, and subsequently was noted to induce improvements or remission of type 2 diabetes. Currently, these bariatric operations, such as Roux‐en‐Y gastric bypass and sleeve gastrectomy, are the most effective procedures for the treatment of obesity and type 2 diabetes mellitus worldwide. However, the specific mechanism mediating the beneficial effects of metabolic surgery has remained largely unknown. Those mechanical explanations, such as restriction and malabsorption, are challenged by accumulating evidence from human and animal models of these procedures, which points to the weight‐independent factors, such as hormones, bile acids, gut microbiota, nervous system and other potential underlying mechanisms. A growing body of evidence suggests that gut microbiota are associated with the development of several metabolic disorders, and bile acids and FXR signaling are important for the metabolic benefits of bariatric surgery. Given the close relationship between bacteria and bile acids, it is reasonable to propose that microbiota–bile acid interactions play a role in the mechanisms underlying the effects of metabolic surgery.
Keywords: Bariatric surgery, Bile acids, Gut microbiota
Introduction
Obesity is becoming increasingly prevalent worldwide, and has become the most common metabolic disease1. In 2014, nearly 2 billion people were overweight, and more than 600 million of these individuals were obese2. The prevalence of obesity among USA adults was >33% from 2011 to 20143. Being overweight is a major risk factor for public health, as it is related to the incidence of several comorbidities, including type 2 diabetes mellitus, heart disease, stroke and certain types of cancer4. Additionally, obesity is generally considered to be a strong risk factor for diabetes5. Thus, the growing worldwide prevalence of type 2 diabetes mellitus, which is the most common type of diabetes, is associated with an increasing number of overweight and obese individuals6. In 2015, an estimated 415 million individuals had diabetes; this is predicted to increase to 642 million by 20406. A systematic literature review showed that the obesity prevalence of adult patients with type 2 diabetes mellitus was >30% in most observational studies from different regions in the world; it even exceeded 50% in some studies. Furthermore, in Asia, the prevalence rate was reported to reach 56.1%7. Diabetes has become one of the greatest threats to global health, and imposes a considerable economic burden on both individuals and countries6.
Bariatric surgery was initially applied to the treatment of morbid obesity >60 years ago8. The procedure was originally designed to achieve and sustain weight loss, and it was subsequently noted to induce improvements in glucose regulation9, 10. In both short‐ and long‐term trials, clinical studies have shown that bariatric surgery results in substantial weight loss and either improvement or remission of type 2 diabetes mellitus11, 12, 13. As clinical data were accumulated, some versions of these procedures were ultimately abandoned because of complications related to the surgery. Currently, Roux‐en‐Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are two of the most popular types of bariatric surgeries applied in the treatment of obesity and type 2 diabetes worldwide14.
The surgery itself has led to the mechanical hypothesis that bariatric operations generally induce weight loss by restricting the stomach size and/or bypassing some of the intestine to cause nutrient malabsorption. However, remission of type 2 diabetes mellitus has been observed after gastric bypass before the occurrence of significant weight reduction, showing that the glucose improvement resulting from metabolic operations occurs not only because of weight loss, but also due to weight‐independent factors, such as gut hormones, bile acids, gut microbiota, nervous system and other potential underlying mechanisms15, 16, 17, 18. To date, the specific mechanism mediating the beneficial effects of metabolic surgery have remained largely unknown. Recent findings have suggested that gut microbiota could regulate host energy metabolism, and that altered intestinal flora contribute to the development of several metabolic disorders19. Furthermore, it is known that bacteria also play an important role in the synthesis and metabolism of bile acids, and one study has shown that bile acids are an important contributor to glucose improvement20. Thus, the question arises whether the gut microbiota, bile acids or their interactions play a role in the beneficial effects induced by bariatric surgery on host energy metabolism.
The present review aims to summarize the present knowledge of the impacts of bariatric surgery on the host intestinal microbiota, bile acids and the cross‐talk between them, and to discuss further potential contributions to the metabolic effects of weight‐loss surgery on obesity and type 2 diabetes mellitus.
Role of Gut Microbiota in Bariatric Surgery
Numerous micro‐organisms exist on the surface of the skin and mucosal linings in the human body. The human intestinal tract is colonized by a unique collection of microbes, termed the human gut microbiota, which include more than 3 million non‐redundant microbial genes, exceeding the number of genes in the human genome by approximately 150‐fold21. Gut microbiota can affect various aspects of host metabolism, such as energy biogenesis, biosynthesis of steroid hormones and bile salt metabolism. Given the large number and varied functions of intestinal microbes, it should not be surprising that they contribute to the incidence of several diseases, including metabolic diseases22. Accumulating data suggest that alterations in gut microbiota are associated with obesity and diabetes, which has been attributed to an increased energy‐harvesting capacity from the diet23. Accordingly, modified gut microbes can improve such metabolic disorders. This is supported by a study that showed that an oral infusion of intestinal microbiota from lean donors to mice with metabolic syndrome can result in a temporary improvement of insulin sensitivity, showing a correlation between gut microbiota and host glucose metabolism24.
Bariatric surgery has been found to induce weight loss and improve glucose metabolism, but whether it causes specific alterations in the intestinal microbiota profile that contribute to improvement in metabolic disorders remains unknown. Based on a direct comparison of patients pre‐ and post‐surgery, a recent study showed that in addition to weight reduction and glucose improvement, alterations in gut microbiota, including increased diversity and altered composition, were observed within 3 months of RYGB in morbidly obese patients. Furthermore, more than half of the altered species were maintained in relative abundance over a long‐term follow‐up period, indicating that bariatric surgery could result in rapid and sustained shifts in the gut microbiome of an individual25. Furthermore, compared with individuals of normal weight or with morbid obesity, obese patients who had undergone RYGB at least 6 months prior also had a substantially restructured gut microbial makeup26. These findings clearly show that bariatric surgery alters the intestinal community. It has been reported that bodyweight reduction caused by lifestyle interventions, such as caloric restriction, are also correlated with alterations in gut microbiota27. However, Damms‐Machado et al.28 found that bariatric surgery, rather than a low‐calorie diet, could reverse the gut microbe profile in obese individuals towards a phenotype similar to that found in normal individuals.
Phylogenetic analysis showed that gut microbiota from human stool samples primarily contained six bacterial phyla: Bacteroidetes, Firmicutes, Proteobacteia, Actinobacteria, Fusobacteria and Verrucomicrobia26. Another study29 using metagenomic sequencing showed they were chiefly assigned to seven phyla, adding Cyanobacteria to the aforementioned six phyla. At the phylum level, compared with those at baseline or in non‐operated controls, human intestinal bacterial alterations in obese individuals after RYGB included a significantly increased abundance of Proteobacteria and Bacteroidetes, and decreased Firmicutes25, 26, 28, 29, 30, 31. These findings were largely in accord with studies in rodents, but there were similar changes in the relative abundance of Firmicutes among groups of individuals with dietary restriction or RYGB32. Furthermore, the Bacteroidetes‐to‐Firmicutes ratio was reported to be increased after weight‐loss surgery28. By contrast, another study showed that the abundance of Bacteroidetes was decreased after RYGB29. At the species level, Palleja et al.25 reported that compared with samples obtained pre‐surgery, up to 19 species showed a changed relative abundance at 1 year post‐RYGB surgery, which included dramatically increased Escherichia coli, Klebsiella pneumoniae, Veillonella dispar and Veillonella parvula. In that study, the authors also observed that there was no significant difference in species abundance between the first 3 months and 1 year post‐procedure, providing the evidence that the restructuring of the intestinal microbial community occurred as early as 3 months after the procedure25. In contrast to RYGB, laparoscopic sleeve gastrectomy decreased the relative abundance of Eubacterium rectale, Bacteroides vulgatus, Bacteroides sp.3_1_40A, Coprococcus comes, Ruminococcus obeum, Dorea longicatena, Lachnospiraceae bact.5_1_63FAA and Clostridium sp. L2_50 in obese individuals compared with the effects of dietary intervention28.
Bariatric surgery can change the intestinal micro‐organism pattern in response to gastric restriction or rearrangement of the intestinal tract induced by SG or RYGB. For example, reduced gastric acid secretion can produce an increase in pH in the downstream digestive tract, particularly in the colon. Consequently, altered nutrient presentation resulting from an incompletely digested diet that enters the downstream gut after both procedures could alter the gut environment and affect the composition of the intestinal bacteria. Many differences exist between these two types of procedures. So, are the alterations in the gut microbiota after the two procedures similar or different? Murphy et al.33 investigated changes in intestinal microbes after laparoscopic‐RYGB or ‐SG by metagenomic sequencing in diabetes patients with obesity, and they observed that RYGB induced decreased Bacteroidetes, but increased Firmicutes and Actinobacteria. By contrast, SG produced increased Bacteroidetes. At the species level, only increased Roseburia intestinalis, a species belonging to the Firmicutes phylum, was common to these two types of surgery in patients with diabetes who were in remission. These results were surprising. First, after RYGB, alterations in gut microbiota were different from the findings mentioned above. Second, sleeve gastrectomy had fewer effects on the intestinal microbiota than RYGB, consistent with another study carried out in rodents34. One possible reason for this difference is that, compared with RYGB, SG induces relatively mild physical manipulations of the intestinal tract.
Taken together, these data show that bariatric surgery, especially RYGB, could induce rapid and sustained alterations of the individual gut microbiome in obese individuals. These observations raise the question of whether these alterations in intestinal flora contribute to the metabolic improvements that occur after metabolic surgery. In rodents, Liou et al.32 showed that germ‐free mice that were administered fecal preparations from RYGB‐treated mice exhibited lower bodyweight than germ‐free mice that received bacterial transplants from sham‐operated mice. This shows that the changed profile in the distal gut microbiota is a driver of weight loss after RYGB surgery. Furthermore, transplantation of fecal preparations from RYGB‐operated obese patients to germ‐free mice induced fat mass loss, indicating that an altered gut microbiome after RYGB might play a role in reducing adiposity after bariatric surgery31. Based on these data, it is difficult to conclude that gut bacteria are essential for the effects of RYGB, but we can conclude that changes in gut microbiota induced by RYGB are sufficient to produce weight loss.
Role of Bile Acids in Bariatric Surgery
Bile acids are a host of steroid molecules that can be synthesized from cholesterol in the liver, conjugated to taurine or glycine to enhance water solubility, secreted into the bile and discharged into the duodenum after a meal where they act as surfactants to facilitate lipid ingestion35. These molecules are then efficiently (i.e., >95%) reabsorbed by both active transport in the terminal ileum and passive absorption in the colon. The molecules are then further recirculated to the liver through portal vein blood. This process is known as enterohepatic circulation (Figure 1).
Figure 1.
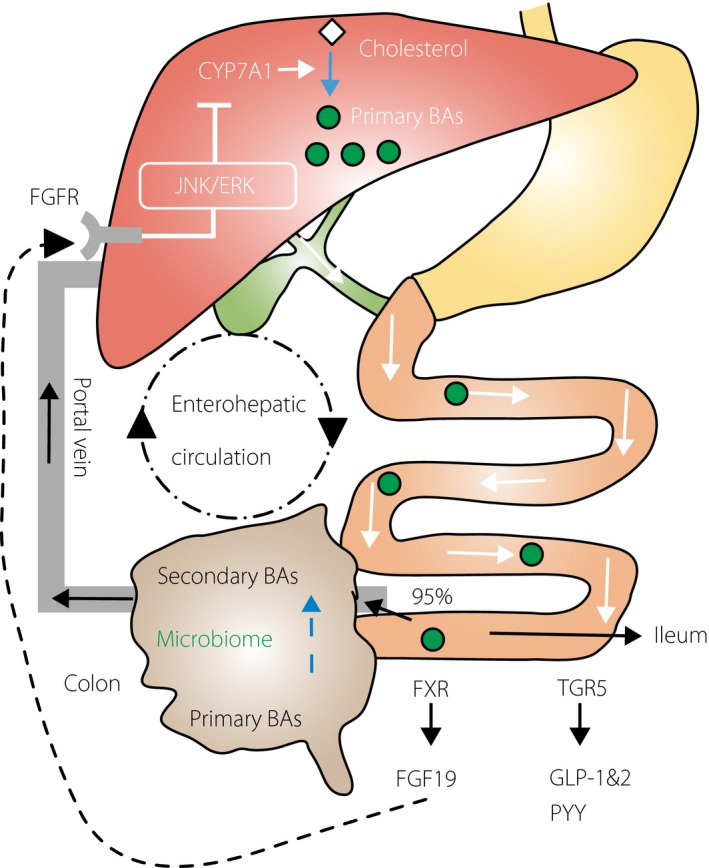
Bile acid biosynthesis, enterohepatic circulation and potential functions through receptors in the ileum. Bile acids (BAs) are synthesized from cholesterol in the liver with the rate‐limiting enzyme 7α‐hydroxylase (CYP7A1), conjugated to taurine or glycine, secreted into the bile, discharged into the duodenum then efficiently (i.e., >95%) reabsorbed in the terminal ileum and the colon. The molecules are then further recirculated to the liver through portal vein blood. This process is known as enterohepatic circulation. In the ileum, bile acids facilitate the secretion of fibroblast growth factor 19 (FGF19; known as FGF15 in mice), which circulates to the liver and reduces the expression of CYP7A1 to inhibit hepatic bile acid synthesis through the farnesoid X receptor (FXR), and stimulates the excretion of peptide YY (PYY), glucagon‐like peptide 1 (GLP‐1) and glucagon‐like peptide 2 (GLP‐2) through a G protein‐coupled receptor (TGR5). Primary bile acids are modified by intestinal microbiota, including transformation into secondary bile acids in the colon. FGF19 binds to FGFR4 to activate c‐Jun N‐terminal kinase/extracellular signal‐regulated kinase (JNK/ERK) signaling that inhibits expression of CYP7A1. FGFR4, fibroblast growth factor receptor 4.
It has been reported that serum bile acids levels are significantly increased in obese patients 2–4 years after gastric bypass surgery compared with the levels in weight‐matched individuals. Furthermore, these levels are negatively correlated with those of postprandial blood glucose, but positively correlated with the maximal secretion of glucagon‐like peptide‐1, showing that bile acids might be associated with improved glucose and lipid metabolism after metabolic surgery36. Another study showed that vertical sleeve gastrectomy (VSG) also increased the level of circulating serum bile acids as well as levels of conjugated and unconjugated BAs independent of energy restriction in obese patients with diabetes37. Therefore, these data suggest that in humans, both RYGB and VSG can result in increased circulating levels of BA, which are strongly correlated with metabolic improvements. This finding is also supported by experiments carried out in rodents38, 39. Both the RYGB and VSG procedures physically change the upper gastrointestinal tract, likely impacting bile acid enterohepatic circulation and increasing bile acid levels, which can contribute to the metabolic effects observed after operations.
Bile acids can play roles in several metabolic processes, particularly glucose and energy metabolism40. Flynn et al.41 used diet‐induced obesity mouse models and carried out biliary diversions from the gallbladder directly to the duodenum, jejunum or ileum (GB‐IL) to match the altered bile delivery in the intestine caused by the RYGB. Compared with other biliary operations, GB‐IL induced the most substantial reduction in bodyweight, and improvement in lipid and glucose metabolism, similar to RYGB. That study showed that bile acids can exert beneficial effects on metabolic disorders independently of surgical manipulation of intestinal continuity. Additionally, it was observed that serum bile acid levels were significantly elevated in GB‐IL mice, but were not notably different in the bile diversion to the duodenum or bile diversion to the jejunum cohorts compared with the levels in mice with diet‐induced obesity, showing that bile acids can primarily play a functional role in the hindgut41. The role of bile acids in glucose metabolism is also supported by the clinical use of bile acid‐binding resins, which can improve blood glucose in patients with type 2 diabetes42. Based on these data, it is difficult to conclude whether bile acids contribute to the effects of RYGB, but it is clear that bile diversion could be sufficient to result in weight loss, and improved glucose and lipid metabolism independent of surgical rearrangement of the digestive tract.
In addition to roles in intestinal fat ingestion, accumulating evidence suggests that bile acids exert important effects as hormones by activating receptors that include a G protein‐coupled receptor (TGR5) and the farnesoid X receptor (FXR) (ligand‐activated transcription factor)40. In the ileum, the activation of FXR by bile acids facilitates the secretion of fibroblast growth factor 19 (FGF19; known as FGF15 in mice), which circulates to the liver in portal blood and reduces the expression of 7α‐hydroxylase to inhibit hepatic bile acid synthesis (Figure 1)43. Thus, we can hypothesize that bile acids exert beneficial effects on lipid and glucose metabolism after bariatric surgery by changing signaling pathways through related receptors. Flynn et al.41 found that VSG was associated with increased bile acid levels in obese mice, and that the absence of FXR actually decreased the effects of VSG on bodyweight reduction and improved glucose control, showing that bile acids and FXR signaling are important for the metabolic benefits of VSG. Indeed, it has also been reported that in the absence of FXR, reduced bodyweight and improved glucose homeostasis were observed in mice43, 44. Similarly, a role for TGR5 in the metabolic effects of bariatric surgery is supported by findings that TGR5 deficiency can reduce the beneficial effects of VSG45.
As aforementioned, FGF19 is a target of FXR. Recent studies have shown a role for bile acids and FGF19 in the effects associated with bariatric surgery. One study randomly assigned patients with type 2 diabetes to either RYGB or intensive medical management and followed the patients for 12 months. The study showed that RYGB increased the concentrations of bile acids and FGF19, mediating metabolic effects compared with intensive medical management independently of weight loss46. Similarly, larger increases in serum levels of FGF19 and bile acids were observed in patients with diabetes remission after metabolic surgery compared with those in patients with diabetes non‐remission47. In contrast, another study found that circulating bile acids and FGF19 were unchanged in patients with type 2 diabetes, whereas glucose tolerance and insulin sensitivity improved significantly at early stages after bariatric surgery48. Thus, it appears that bile acids and FGF19 did not work for the beneficial effects on glucose metabolism after bariatric surgery in that study.
Furthermore, another prospective study that examined serum bile acid levels before and after surgery in morbidly obese patients undergoing bariatric surgery showed that circulating bile acids were decreased at 1 week post surgery, mildly increased at 3 months after surgery and significantly increased at 1 year post‐surgery. However, a substantial increase in peptide YY and glucagon‐like peptide 1 excretion and improved metabolic control could be observed as soon as 1 week after the operation, and was maintained in the following 12 months. This finding shows that increased plasma bile acids might not contribute to the rapid metabolic improvements observed shortly after weight‐loss surgery49.
Taken together, the contribution of the increased pool of circulating bile acids and related signaling pathways to the metabolic effects of bariatric surgery remains to be fully elucidated.
Cross‐talk between Gut Microbiota and Bile Acids and its Role in Bariatric Surgery
The formation of bile acids in the liver is a complex process. Hepatic bile acid synthesis is completed through two biosynthetic pathways (classical and alternative pathways), which are initiated by 7α‐hydroxylase and sterol‐27‐hydroxylase, respectively. 7α‐Hydroxylase stimulates the 7α‐hydroxylation of cholesterol, and is the crucial enzyme in the production of bile acids. In humans, the classical pathway yields two primary bile acids, cholic acid (CA) and chenodeoxycholic acid, whereas the alternative pathway chiefly produces CA. More than 75% of total bile acid production is generally produced by the former pathway. However, primary bile acids in rodents are CA and muricholic acids35. When they reach the gut, primary bile acids are transformed into the secondary bile acids deoxycholic acid and lithocholic acid in humans, and ω–muricholic acids in rodents35.
In addition to inducing inflammation, gut microbiota plays a key role in modulating bile acids, including their biosynthesis and biotransformation (Figure 2). Bile acid biosynthesis consists of several enzymatic catalysis reactions. A previous study showed that the expression levels of most of those enzymes, including 7α‐hydroxylase and sterol‐27‐hydroxylase, were decreased in livers of conventionally raised mice compared with those in germ‐free mice, showing that gut microbiota can regulate the formation of bile acids through these enzymatic reactions50. Furthermore, after discharge into the gut, primary bile acids are modified by intestinal microbiota, including deconjugation and further transformation into secondary bile acids, such as CA to deoxycholic acid or chenodeoxycholic acid to lithocholic acid, which results in an increased chemical diversity of the bile acid pool40. Sayin et al.50 showed that the distal gut was predominantly colonized by secondary bile acids and deconjugated bile acids in conventionally raised mice, whereas in germ‐free mice the bile acid pool chiefly contained primary conjugated bile acids and almost no secondary bile acids. Furthermore, in the absence of bacteria, BA diversity was significantly reduced. These data showed that the BA composition is dependent on gut microbes35. Microbial deconjugation is catalyzed by bile salt hydrolase. Through metagenomic analysis, Jones et al.51 found that bile salt hydrolase activity is widespread in the human intestinal microbiota. Primary deconjugated bile acids are also transformed to secondary bile acids through 7α/β‐dehydroxylation carried out by microorganisms. It has been shown that 7α/β‐dehydroxylation activity is present in gut bacteria, such as Clostridium spp52. Conversely, bile acids can directly inhibit bacterial growth as a detergent and thereby indirectly regulate the composition of gut microbes through signaling pathways, such as hormone‐mediated signaling (Figure 2)53. This was supported by a study that showed that oral administration of CA altered the composition of gut microbiota in rats54. Thus, there is a close relationship between gut microbiota and bile acids.
Figure 2.
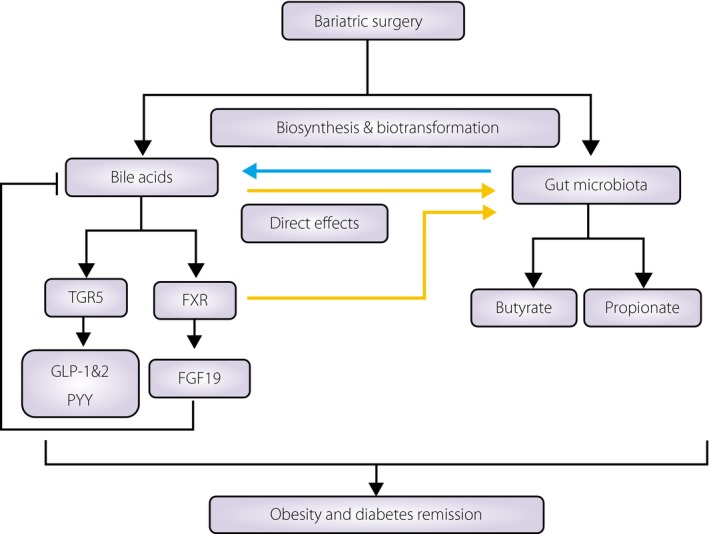
The relationship between the effects of bariatric surgery on the composition of bile acids and the gut microbiota. Bariatric surgery impacts bile acid enterohepatic circulation and increases bile acid levels, which might contribute to the metabolic effects after operations through signaling pathways. Furthermore, it also can change the pattern of intestinal microbiota, which can produce short‐chain fatty acids, such as butyrate and propionate, and improve metabolic regulation. Gut microbiota plays a key role in modulating bile acids, including their biosynthesis and biotransformation. Conversely, bile acids can directly inhibit bacterial growth as a detergent, and can indirectly regulate the composition of gut microbes through signaling pathways. FGF19 (known as FGF15 in mice), fibroblast growth factor 19; FXR, farnesoid X receptor; GLP‐1, glucagon‐like peptide 1; GLP‐2, glucagon‐like peptide 2; PYY, peptide YY; TGR5, a G protein‐coupled receptor.
The relative abundance of certain bacteria, such as Bacteroides and Roseburia, was significantly altered in wild‐type mice after VSG compared with the effect of sham‐surgery, but did not vary after surgery among FXR knockout mice, which failed to show the metabolic effects of VSG to maintain weight loss and improve glucose tolerance. These findings raise the possibility that VSG induces metabolic effects through bile acids, FXR signaling and gut microbiota20.
In addition to increased total bile acid levels and tauro‐ω–muricholic acid, GB‐IL induced markedly decreased levels of FXR compared with those observed in obese mice. The same study also reported that the relative abundance of Firmicutes was reduced and there was a dramatic increase in Bacteroidetes, in a manner similar to that observed after RYGB41. These findings suggest that bile acids can remodel the pattern of intestinal microbes, similar to that induced after bariatric surgery.
These data show that weight‐loss surgery can affect the interplay between bile acids and gut microbiota, which can contribute to the metabolic effects observed after metabolic surgery.
Conclusions
The potent metabolic effects of bariatric surgery raise the possibility that patients with type 2 diabetes could be cured: the operation not only improves obesity, glucose tolerance and insulin sensitivity, but also opens a new avenue to gain insights into the etiology of metabolic disorders. Studies show that bile acid levels and gut microbiota are altered after weight‐loss surgery, and might be associated with metabolic improvements. Given the close relationship and mutual effect, it is reasonable to propose that microbiota–bile acid interactions play a role in the mechanisms underlying the effects of metabolic surgery. Thus, further studies are warranted to identify which interactions contribute to the observed beneficial effects and our understanding of how they work.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Key Project of Shanghai Health and Family Planning Commission (grant no. 201440026), and the Key Medical Subject Construction Project of Shanghai Health and Family Planning Commission (grant no. ZK2015A02).
J Diabetes Investig 2018; 9: 13–20
Contributor Information
Xueli Zhang, Email: lejing1996@aliyun.com.
Weiping Jia, Email: wpjia@sjtu.edu.cn.
References
- 1. Ng M, Fleming T, Robinson M, et al Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Obesity and overweight fact sheet. World Health Organization Last Updated: June 2016. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed January 6, 2017.
- 3. CDC . Overweight and obesity, Adult Obesity Facts. CDC Last Updated: September 1, 2016. Available from: https://www.cdc.gov/obesity/data/adult.html. Accessed January 6, 2017.
- 4. Field AE, Coakley EH, Must A, et al Impact of overweight on the risk of developing common chronic diseases during a 10‐year period. Arch Intern Med 2001; 161: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 5. Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab 2005; 19: 649–663. [DOI] [PubMed] [Google Scholar]
- 6. International Diabetes Federation . IDF diabetes Atlas, 7th edn Brussels: International Diabetes Federation, 2015. Available from: http://www.diabetesatlas.org/. Accessed January 6, 2017. [Google Scholar]
- 7. Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes 2013; 6: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kremen AJ, Linner JH, Nelson CH. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann Surg 1954; 140: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mason EE. History of obesity surgery. Surg Obes Relat Dis 2005; 1: 123–125. [DOI] [PubMed] [Google Scholar]
- 10. Pories WJ, Caro JF, Flickinger EG, et al The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg 1987; 206: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mingrone G, Panunzi S, De Gaetano A, et al Bariatric‐metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow‐up of an open‐label, single‐centre, randomised controlled trial. Lancet 2015; 386: 964–973. [DOI] [PubMed] [Google Scholar]
- 12. Mehaffey JH, LaPar DJ, Clement KC, et al 10‐Year Outcomes After Roux‐en‐Y Gastric Bypass. Ann Surg 2016; 264: 121–126. [DOI] [PubMed] [Google Scholar]
- 13. Schauer PR, Bhatt DL, Kirwan JP, et al Bariatric surgery versus intensive medical therapy for diabetes–3‐year outcomes. N Engl J Med 2014; 370: 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017; 14: 160–169. [DOI] [PubMed] [Google Scholar]
- 15. Shah SS, Todkar JS, Shah PS, et al Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35 kg/m(2). Surg Obes Relat Dis 2010; 6: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 2009; 150: 2518–2525. [DOI] [PubMed] [Google Scholar]
- 17. Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 2013; 10: 575–584. [DOI] [PubMed] [Google Scholar]
- 18. Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab 2015; 21: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hartstra AV, Bouter KE, Backhed F, et al Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015; 38: 159–165. [DOI] [PubMed] [Google Scholar]
- 20. Ryan KK, Tremaroli V, Clemmensen C, et al FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin J, Li R, Raes J, et al A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med 2016; 375: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 23. Turnbaugh PJ, Ley RE, Mahowald MA, et al An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 24. Vrieze A, Van Nood E, Holleman F, et al Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913–916. [DOI] [PubMed] [Google Scholar]
- 25. Palleja A, Kashani A, Allin KH, et al Roux‐en‐Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med 2016; 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang H, DiBaise JK, Zuccolo A, et al Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 2009; 106: 2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nadal I, Santacruz A, Marcos A, et al Shifts in clostridia, bacteroides and immunoglobulin‐coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes 2009; 33: 758–767. [DOI] [PubMed] [Google Scholar]
- 28. Damms‐Machado A, Mitra S, Schollenberger AE, et al Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int 2015; 2015: 806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Graessler J, Qin Y, Zhong H, et al Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 2013; 13: 514–522. [DOI] [PubMed] [Google Scholar]
- 30. Kong LC, Tap J, Aron‐Wisnewsky J, et al Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutri 2013; 98: 16–24. [DOI] [PubMed] [Google Scholar]
- 31. Tremaroli V, Karlsson F, Werling M, et al Roux‐en‐Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long‐Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab 2015; 22: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liou AP, Paziuk M, Luevano JM Jr, et al Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013; 5: 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy R, Tsai P, Jullig M, et al Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg 2017; 27: 917–925. [DOI] [PubMed] [Google Scholar]
- 34. Guo Y, Liu CQ, Shan CX, et al Gut microbiota after Roux‐en‐Y gastric bypass and sleeve gastrectomy in a diabetic rat model: increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev 2017; 33: e2857. [DOI] [PubMed] [Google Scholar]
- 35. Wahlstrom A, Sayin SI, Marschall HU, et al Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 2016; 24: 41–50. [DOI] [PubMed] [Google Scholar]
- 36. Patti ME, Houten SM, Bianco AC, et al Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 2009; 17: 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jahansouz C, Xu H, Hertzel AV, et al Bile Acids Increase Independently From Hypocaloric Restriction After Bariatric Surgery. Ann Surg 2016; 264: 1022–1028. [DOI] [PubMed] [Google Scholar]
- 38. Schreck CE, Kline DL. Repellency of two controlled‐release formulations of deet against Anopheles quadrimaculatus and Aedes taeniorhynchus mosquitoes. J Am Mosq Control Assoc 1989; 5: 91–94. [PubMed] [Google Scholar]
- 39. Myronovych A, Kirby M, Ryan KK, et al Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight‐loss‐independent manner. Obesity 2014; 22: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap–bile acids in metabolic control. Nat Rev Endocrinol 2014; 10: 488–498. [DOI] [PubMed] [Google Scholar]
- 41. Flynn CR, Albaugh VL, Cai S, et al Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun 2015; 6: 7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smushkin G, Sathananthan M, Piccinini F, et al The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes 2013; 62: 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mazidi M, de Caravatto PP, Speakman JR, et al Mechanisms of Action of Surgical Interventions on Weight‐Related Diseases: the Potential Role of Bile Acids. Obes Surg 2017; 27: 826–836. [DOI] [PubMed] [Google Scholar]
- 44. Prawitt J, Abdelkarim M, Stroeve JH, et al Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 2011; 60: 1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ding L, Sousa KM, Jin L, et al Vertical sleeve gastrectomy activates GPBAR‐1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology 2016; 64: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sachdev S, Wang Q, Billington C, et al FGF 19 and Bile Acids Increase Following Roux‐en‐Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes Surg 2016; 26: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gerhard GS, Styer AM, Wood GC, et al A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux‐en‐Y gastric bypass. Diabetes Care 2013; 36: 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jorgensen NB, Dirksen C, Bojsen‐Moller KN, et al Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J Clin Endocrinol Metab 2015; 100: E396–E406. [DOI] [PubMed] [Google Scholar]
- 49. Steinert RE, Peterli R, Keller S, et al Bile acids and gut peptide secretion after bariatric surgery: a 1‐year prospective randomized pilot trial. Obesity 2013; 21: E660–E668. [DOI] [PubMed] [Google Scholar]
- 50. Sayin SI, Wahlstrom A, Felin J, et al Gut microbiota regulates bile acid metabolism by reducing the levels of tauro‐beta‐muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013; 17: 225–235. [DOI] [PubMed] [Google Scholar]
- 51. Jones BV, Begley M, Hill C, et al Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA 2008; 105: 13580–13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res 2015; 56: 1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Inagaki T, Moschetta A, Lee YK, et al Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 2006; 103: 3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Islam KB, Fukiya S, Hagio M, et al Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141: 1773–1781. [DOI] [PubMed] [Google Scholar]


