Abstract
Galectins are an evolutionarily ancient family of glycan-binding proteins (GBPs) and are found in all animals. Although they were discovered over 30 years ago, ideas about their biological functions continue to evolve. Current evidence indicates that galectins, which are the only known GBPs that occur free in the cytoplasm and extracellularly, are involved in a variety of intracellular and extracellular pathways contributing to homeostasis, cellular turnover, cell adhesion, and immunity. Here we review evolving insights into galectin biology from a historical perspective and explore current evidence regarding biological roles of galectins.
Keywords: Galectin, Glycan binging protein (GBP), Homeostasis, Cellular turnover, Cell adhesion, Immunity, Neoplasia, Carbohydrates
1 Introduction
Although the importance of cell surface carbohydrates in normal cellular physiology remained elusive for many years, recent studies demonstrate that these highly complex macromolecules possess diverse roles in many cellular processes [1]. In addition to directly impacting glycoprotein function, complex carbohydrate structures serve as ligands for glycan binding proteins (GBPs), which enable cell surface glycan-dependent signaling [2–4]. Recent studies demonstrate that GBP–cell surface carbohydrate interactions play key roles in a variety of processes ranging from cellular turnover to innate immunity. Given the plasticity of immune cells, which not only enables differentiation of cellular function but also results in distinct alterations in cell surface glycosylation [5], GBP–glycan interactions appear to be especially important in the regulation of immunity.
Among GBPs with immunoregulatory activities, galectin family members appear to regulate a wide variety of immunological processes in addition to serving as factors directly involved in microbial killing [6–9]. In this review, we attempt to link original descriptions concerning the immunological activities of galectins to new mechanistic insights into their regulatory activities within immunity and beyond. In doing so, we do not seek to provide an exhaustive review regarding the biological activities of the entire galectin family, especially given the rapidly expanding nature of this field. However, we seek to highlight salient features of galectins that illustrate the broad regulatory capacity of likely one of the most pleiotropic protein families described.
2 Discovery of Galectins
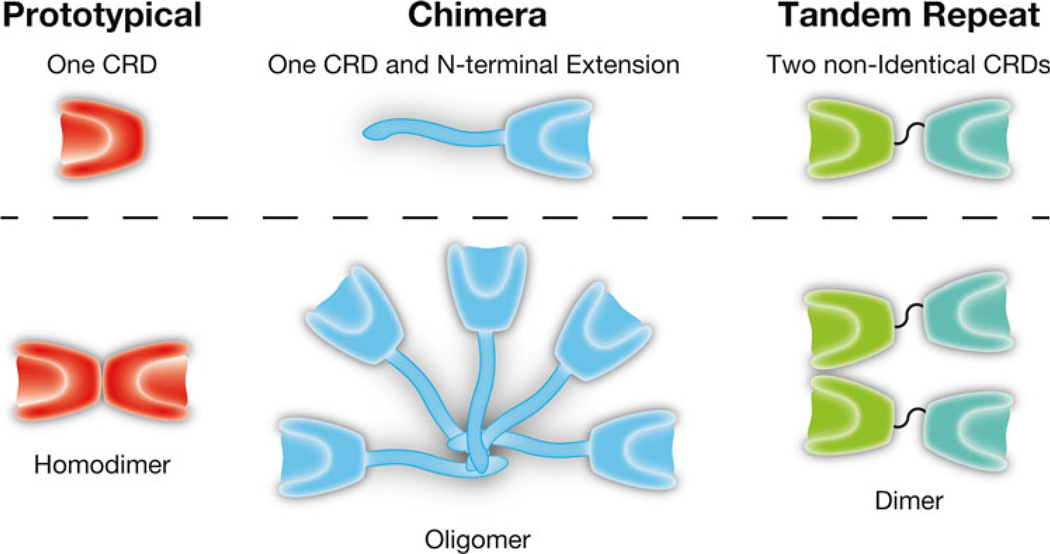
Galectins were identified within a few years after the pioneering work in the 1960s of Ashwell and Morrell who discovered the asialoglycoprotein receptor, the first GBP described in vertebrates [10, 11]. Although some of the physiological functions of that receptor have only recently been elucidated [2], these early studies suggested the potential existence of other mammalian GBPs. In 1975, the first vertebrate galectin (an ortholog of mammalian galectin-1) was discovered in the electric organ of the electric eel Electrophorus electricus by Teichberg and colleagues [12]. This was followed shortly thereafter by isolation of galectins in the laboratories of Barondes and Kornfeld, who reported similar proteins in avian and mammalian sources, respectively [12–14]. There are now 15 described members of the galectin family (Gal-1 through −15) encoded in mammals (11 are known in humans), and all range in subunit size from 14 to 39 kDa [15] (Fig. 1). They are found in all metazoans and are the only known GBPs in animals that occur both in the cytoplasm, where they are synthesized on free polyribosomes, and on the plasma membrane and extracellular matrix [15].
Fig. 1.
The galectin family of β-galactoside binding proteins. Galectins are classified into three distinct groups based on their quaternary structure: prototypical, chimeric, and tandem repeat. Prototypical: Gal-1, Gal-2, Gal-7, Gal-10, Gal-13, and Gal-14. Chimeric: Gal-3. Tandem repeat: Gal-4, Gal-8, Gal-9, and Gal-12
3 Galectin-1: Regulator of Adaptive Immunity
Although the discovery of this ancient galectin family of GBPs was important in confirming the existence of multiple mammalian GBPs, the physiological functions of galectins were more difficult to define. Early studies suggested that Gal-1 might regulate the development of muscle, the first mammalian organ from which the protein was isolated [14], including maintenance of the neuromuscular junction [16–18]. Teichberg and colleagues examined whether administration of Gal-1 might affect the pathological sequelae associated with neuromuscular junction pathology. They employed an animal model of myasthenia gravis induced by autoantibody formation against the acetylcholine receptor. Consistent with the potential involvement of Gal-1 in the neuromuscular junction, exogenously added Gal-1 appeared to cause a significant enhancement of muscle function. However, the apparent amelioration of disease actually resulted from the ability of Gal-1 to suppress the autoimmunity needed to generate a myasthenia gravis model [16–18]. Thus, an indirect result of these experiments was the first evidence for what continues to be one of the more intriguing properties of Gal-1, namely, its ability to significantly suppress immune function [7, 12].
4 Gal-1 Regulation of T Cells
While several studies suggested that Gal-1 might regulate adaptive immunity [6, 7, 19–25], it was not until nearly a decade later that studies began to provide a mechanistic insight into Gal-1-mediated immunosuppression. Early studies suggested that Gal-1 may actually induce lymphocyte proliferation, which suggested to the authors that Gal-1 may enhance the development of suppressor T cells [7]. Gal-1 also appeared to mediate adhesion of thymocytes to epithelial cells, which supported a possible role for Gal-1 in the regulation of T cell development [26, 27]. However, it was a seminal paper by Baum and colleagues that suggested that Gal-1 might directly impact T cell viability by inducing apoptosis, that provided the most substantial mechanistic insight into the immunomodulatory activities of Gal-1 [28]. That study first reported that Gal-1 could induce apoptosis of primary activated T cells and several T cell lines, including MOLT-4 and ARR cells. Thus, this study suggested that Gal-1 might regulate adaptive immunity through directly inducing apoptotic death of effector T cells [28].
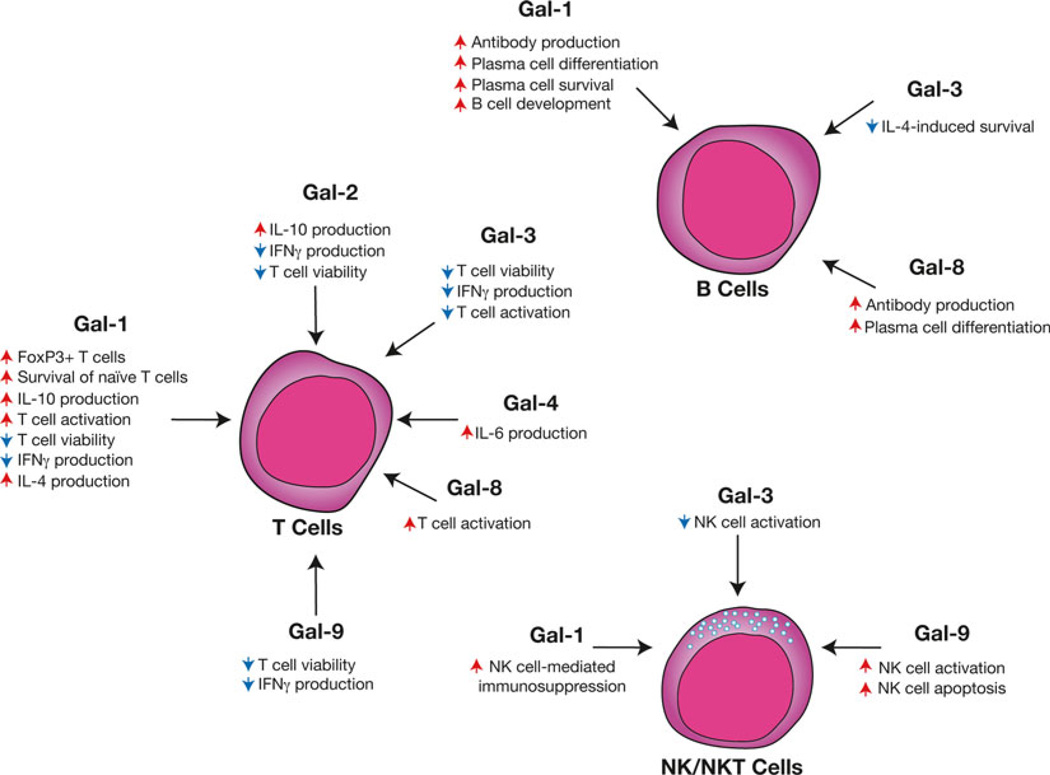
In addition to directly inducing cell death in activated T cells, subsequent studies suggested that Gal-1 might also serve as a key regulator of a variety of other T cell functions (Fig. 2). For example, Gal-1 induces robust IL-10 production in both CD4+ and CD8+ T cells while inhibiting IFN-γ formation, which suggests that Gal-1 may reduce adaptive immune responses by altering T cell cytokine production [29, 30]. Consistent with this, adoptive transfer of CD4+ T cells from Gal-1 treated mice, which displayed similar cytokine profiles observed following in vitro incubation with Gal-1, protected mice from uveitis with the same efficiency as injection of Gal-1 alone [23]. In addition, injection of Gal-1 into IL-10 null mice fails to convey the immunoprotective properties of Gal-1, strongly suggesting a role for IL-10 and possibly other cytokines, in mediating the immunosuppressive activities of this protein [25]. Similarly, Gal-1-Ig chimera constructs can induce significant IL-10 by T cells, providing a useful therapeutic approach to enhancing Gal-1-mediated immunosuppression [31, 32]. Regulatory T cells (Tregs) may also utilize Gal-1 to induce tolerance, as Tregs from Gal-1 null mice exhibit an impaired capacity to suppress T cell activation [33]. Furthermore, Gal-1 itself appears to facilitate the formation of induced Tregs (iTregs) by upregulating FOXP3 expression in peripheral activated T cells, strongly suggesting a key role for Gal-1 in Treg effector function and development [34]. Perturbation of the cytokine milieu by Gal-1 may indirectly impact T cell viability, as distinct T cell populations often rely on specific cytokines to maintain viability in vivo [35, 36].
Fig. 2.
Distinct galectin family members possess the capacity to differentially regulate lymphocytes. Some of the earliest studies suggested that galectins appear to possess immunomodulatory activity by directly impacting T cell viability. Subsequent studies suggested that galectin might not only regulate T cell viability but also regulate cytokine secretion and activation. Several galectins might also play a variety of roles in the development, activation and differentiation of B cells. Similar galectin-mediated regulation may play a role in NK and NKT cell activation and viability. Representative galectin-regulated activities are shown. Red arrows indicate an activity that the respective galectin increases, while blue arrows signify galectin-induced decreases in the accompanying activity
In addition to directly altering T cell cytokine production, several studies suggest that Gal-1 might also inhibit T cell activation, both in vitro and following injection during T cell activation in vivo, providing an additional mechanism whereby Gal-1 might inhibit adaptive immunity [6, 19–24, 37]. Recent studies also demonstrate an in vivo role for Gal-1 in T cell development. TCR transgenic Gal-1 null mice appear to possess altered central selection, which favors the generation of CD8αα T cells, among other alterations in T cell behavior, providing further evidence that Gal-1 affects multiple aspects of T cell biology [38]. In contrast to the ability of Gal-1 to inhibit activation and induce apoptosis of activated T cells, Gal-1 might actually sustain naive T cell survival in peripheral tissue [39]. In addition, recent studies suggest that under certain circumstances Gal-1 may actually facilitate T cell activation in the absence or presence of cognate ligand on antigen presenting cells [40] (Fig. 2).
5 T Cell Regulation: A General Theme of the Galectin Family
In the wake of early studies demonstrating that Gal-1 possessed potent immunoregulatory activities, many studies began to examine the potential role of other galectin family members in the regulation of T cell viability and function (Fig. 2). For example, subsequent studies demonstrated that Gal-3, Gal-8, and Gal-9 regulate thymocyte and T cell viability [41–45]. While different galectin family members appear to signal apoptosis in T cells, individual galectin-mediated regulation of T cell viability likely occurs through engagement of distinct counter receptors. For example, Gal-1 fails to inhibit Gal-3 induced Ca 2+ flux or apoptosis in primary activated T cells, although both Gal-1 and Gal-3 recognize their respective receptors with similar affinity [30], strongly suggesting engagement of distinct ligands [46]. Similarly, recent work demonstrated that Gal-9, but not Gal-1 or Gal-3, specifically induces apoptosis in CD4+ TH1 cells through engagement of Tim-3 [42], although Tim-3 fails to relay Gal-9 signaling in several T leukemic cell lines [47].
Similar to Gal-1, other galectin family members appear to regulate other aspects of T cell biology in addition to T cell viability. For example, Gal-3 appears to regulate T cell activation and the induction of T cell anergy by restricting lateral mobilization of the TCR into the immunological synapse or by dissociating CD8 from the TCR, respectively [48, 49]. Similar to Gal-1, Gal-2 induces IL-10 production, along with IL-5 and TGF-β, while also decreasing IFN-γ and IL-2, which can significantly impact T cell activation and differentiation [50]. Gal-10, originally known as Charcot–Leyden protein, appears to also be a key player in proper Treg function [51]. In contrast, several studies suggest that Gal-4 may actually exhibit pro-inflammatory activity, inducing IL-6 production and expansion of CD4+ T cells [52], likely following specific colitis-associated changes in CD4+ T cell glycosylation [53]. Gal-3 may also regulate CD4+ T cell differentiation by reducing IL-5 secretion [54]. Interestingly, in contrast to Gal-8-induced apoptosis in activated T cells [55], similar to Gal-1, recent studies suggest that Gal-8 may likewise enhance T cell proliferation [40] (Fig. 2).
6 Galectin Regulation of B Cells
Galectins also appear to serve as key regulators of B cells (Fig. 2). Similar to the ability of Gal-1-mediated thymocyte engagement of thymic epithelial cells, work by Schiff and colleagues demonstrated that Gal-1 can likewise facilitate developing B cell interactions with key stromal constituents within the bone marrow [56]. Subsequent studies demonstrated that Gal-1 mediates B cell stromal contacts by facilitating interactions between the surrogate light chain, α5β1, α4β1, and α4β7 integrins, and ADAM15/fibronectin [57, 58]. These interactions ultimately result in relocalization of the B cell receptor to the B cell-stromal interface to form an immunological synapse that induces critical signals necessary for B cell development [57, 59]. Stromal cells expressing Gal-1 appear to reflect a subset distinct from those expressing IL-7 [60], suggesting a unique role for Gal-1 in B cell maturation. Consistent with this, Gal-1 KO mice experience impaired pre-BII-cell development following marrow challenge [59].
In addition to facilitating B cell development, galectins may also play an important role in B cell activation, antibody secretion and differentiation into plasma cells. Gal-1 null mice generate reduced antibody levels following immunization with T cell dependent antigens [61]. Although this could in part reflect Gal-1-mediated regulation of T cell activation and differentiation, Gal-1 KO mice also display reduced antibody formation following challenge with T cell-independent antigens [61], strongly suggesting a direct role for Gal-1 in B cell activation and antibody secretion. Consistent with this, ectopic expression of Gal-1 within B cells facilitates B cell activation and antibody secretion in vitro, likely through engagement of cell surface glycans [62]. Endogenous Gal-1 also appears to be significantly upregulated during B cell differentiation into plasma cells, likely through a BLIMP1-dependent mechanism [61, 62], strongly suggesting a role for B cell-derived Gal-1 in plasma cell biology. Indeed, Gal-1 KO plasma cells display enhanced sensitivity to apoptosis [61], suggesting that Gal-1 may in part maintain antibody secretion by sustaining plasma cell survival. In addition to Gal-1, recent studies suggest that Gal-8 may also play a key role in B cell differentiation [63]. Knockdown of Gal-8 in Gal-1 KO B cells results in further inhibition of plasma cell differentiation and antibody production [63]. Additional studies suggest that Gal-3 may also impact B cell differentiation and survival [64].
7 Galectins and Natural Killer (NK)/Natural Killer T (NKT) Cells
Following embryo implantation, unique NK cell subsets within the decidua may utilize Gal-1 as an effector molecule in the maintenance of fetal–maternal tolerance [65, 66]. NK cells in this setting secrete Gal-1 that, in turn, appears to engage infiltrating T cells, inducing their apoptosis [67]. In contrast, Gal-3 appears to serve as a neoplastic-derived inhibitor of NK function by inhibiting NKG2D-mediated NK cell activation [68, 69], providing a potential mechanism of inhibiting antitumor immunity. In contrast to the inhibitory activity of Gal-3 on NK cells, Gal-9 induces NK cell activation through ligation of Tim3, possibly enhancing antiviral immunity in the setting of HIV infection [70]. However, Gal-9 appears to exert the opposite effect on NKT cells [71], by directly inducing apoptotic cell death [72]. Gal-9 may also facilitate NKT cell activation indirectly by inducing IL-15-dependent NKT cell activation by neighboring macrophages [72]. Similar to Gal-1, alterations in Gal-9-mediated NK and NKT cell function may contribute to the pathogenesis of preeclampsia [73] (Fig. 2).
8 Galectins and DCs/Macrophages
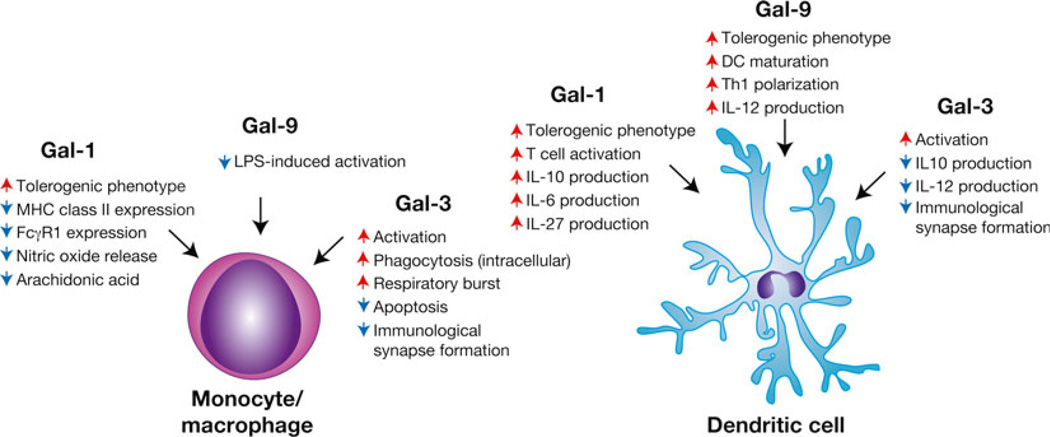
Given the regulatory network responsible for lymphocyte activation and the intimate connection between innate immunity and the development of an adaptive immune response [74], it is not surprising that galectin family members also appear to regulate a variety of antigen presenting cells (APCs) (Fig. 3). Early studies suggested that individual galectin family members might induce macrophage chemotaxis, macrophage activation, and monocyte to macrophage differentiation [75–78]. In addition, galectins appear to directly enhance macrophage phagocytosis [79, 80]. While all of these features would be predicted to enhance overall antigen uptake and presentation, more recent studies suggest that several galectin family members may also directly impact the ability of APCs to activate antigen specific T cells. For example, Gal-3 appears to regulate immunological synapse formation by engaging beta1,6 N-acetylglucosaminyltransferase V (Mgat5)-dependent N glycans on various glycoproteins within the immunological synapse [48]. Gal-3-mediated interactions appear to reduce synapse formation and therefore inhibit T cell activation [48]. Consistent with this, Mgat5 KO recipients display heightened T cell activation and an increased propensity to develop autoimmunity [48]. In contrast, Gal-9 appears to enhance T cell activation in the setting of autoimmunity [81]. Similar results suggest that Gal-1 and Gal-8 may enhance T cell activation by facilitating T cell interactions with APCs in the presence of cognate antigen [40].
Fig. 3.
Galectins differentially regulate monocytes/macrophages and dendritic cells. Different galectin family members appear to possess the ability to directly impact the activity of monocytes/macrophages and dendritic cells with significant implications not only on generalized inflammation but also on the ability of these cells to engage cells involved in adaptive immunity. Several galectins also appear to directly regulate T cell-antigen presenting cell interactions with implications on subsequent T cell activation and differentiation. Representative galectin-regulated activities are shown. Red arrows indicate an activity that the respective galectin increases, while blue arrows signify galectin-induced decreases in the accompanying activity
In addition to potentially regulating the formation and stability of the immunological synapse, individual galectins may also differentially impact the immunological outcome of APC-T cell interactions by altering APC cytokine profiles following activation. Incubation of DCs in the presence of Gal-1 results in the formation of tolerogenic DCs that subsequently induce IL-10-expressing T cells following TCR ligation [82]. Transfer of Gal-1-treated DCs similarly results in tolerance induction in a variety of settings. For example, transfer of Gal-1-treated DCs reduces IFNγ and increases IL-10, ultimately resulting in enhanced maintenance of fetal–maternal tolerance following maternal stress [82]. Similarly, Gal-1 reduces cell surface MHC class II levels following IFNγ treatment, yet differentially regulates FcγR1 expression depending on the presence or absence of other cytokines [83]. Gal-9 appears to also possess the capacity to induce tolerogenic APC function. Gal-9 suppresses LPS-induced TNF secretion by macrophages and transfer of DCs from Gal-9-treated mice attenuates acute lung injury [84]. These findings do not appear to be limited to murine models as Gal-1 inhibits DC production of IFNγ in patients with psoriasis [85] (Fig. 3).
In contrast to the ability of Gal-1 and Gal-9 to induce tolerogenic activity in APCs, Gal-3 appears to engage pro-inflammatory programs in various APC populations (Fig. 3). For example, in vitro studies demonstrate that Gal-3 induces macrophage activation and significant TNFα secretion [86]. Consistent with this, Gal-3 KO mice display reduced macrophage activation and inflammatory sequelae associated with liver injury [87]. Similarly, DCs isolated from Gal-3 KO mice immunized with MOG in the setting of experimental autoimmune encephalomyelitis likewise exhibit increased IL-10 production and decreased IFNγ [88]. Importantly, similar changes in DC activation and cytokine secretion can be observed in Gal-3 KO mice in the setting of reperfusion injury, stroke and Con-A-induced hepatitis [20, 89, 90], strongly suggesting that Gal-3 enhances APC activation and pro-inflammatory cytokine secretion in a variety of settings [91]. Although incubation of recombinant Gal-3 can regulate macrophage function, transfer of macrophages isolated from Gal-3 KO mice into WT recipients results in a similar outcome [91], strongly suggesting that Gal-3-mediated regulation can be a cell intrinsic phenomenon. Similar to Gal-3, recent results suggest that in certain settings, Gal-9 may also promote DC maturation with subsequent enhancement of NK cell activation [92].
9 Galectin Regulation of Granulocytes/Mast Cells
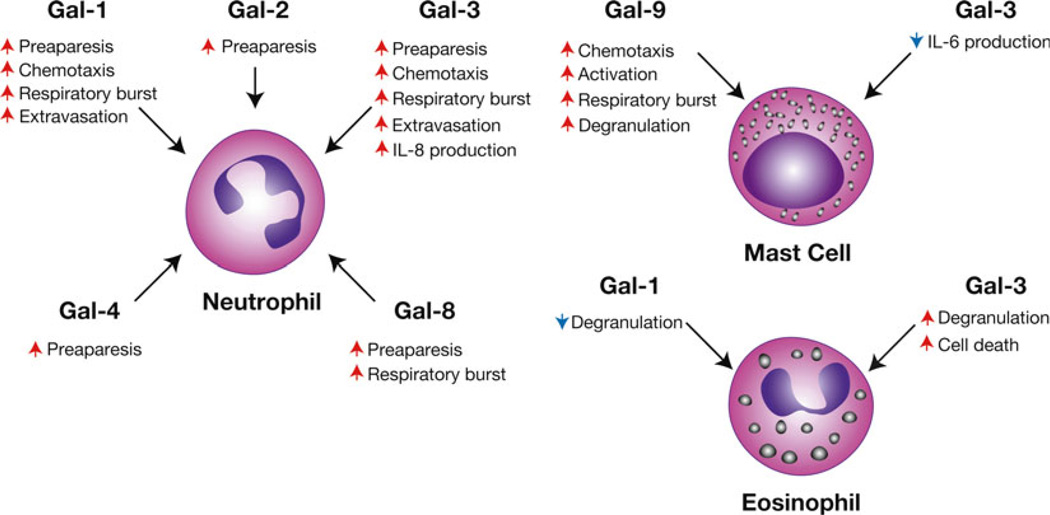
The apparent ability of Gal-1 to induce apoptotic cell death in T cells, coupled with growing evidence that Gal-1 may be involved in many aspects of immune regulation, strongly suggested that Gal-1 might be a general regulator of leukocyte activation, trafficking, and viability (Fig. 4). Consistent with this, early studies demonstrated that Gal-1 can induce neutrophil NADPH activation and enhance superoxide production, possibly through interactions with CD66a and CD66b [93, 94]. In addition to facilitating neutrophil activation, different galectin family members also appear to regulate neutrophil extravasation and induce differential effects on chemotaxis, depending on the activation state of the cells [131–134]. Similar to what had been observed in activated T cells [28], several studies suggest that galectins may also aid in neutrophil turnover. For example, recent results suggested that Gal-1 induces exposure of PS, a common ligand responsible for triggering cellular removal in cells undergoing apoptosis, in activated neutrophils and the promyelocytic cell line, HL60 [30, 95, 96]. However, in contrast to the effects of Gal-1 on T cells, induction of PS exposure in activated neutrophils by Gal-1 appeared to occur in the conspicuous absence of cell death [30, 95–97]. Despite the inability of Gal-1 to induce apoptosis in activated neutrophils, Gal-1-induced PS exposure sensitized cells to phagocytic removal [95, 97]. This form of removal may be unique to neutrophils [98] as it prepares cells for removal without inducing cell death, a process recently termed “preaparesis” [30]. As late apoptosis is often accompanied by loss of membrane integrity, this form of cellular turnover may facilitate the maintenance of membrane integrity in the setting of acute inflammation until appropriate phagocytic removal occurs. The ability to induce PS exposure in the absence of apoptosis in neutrophils does not appear to be limited to Gal-1, as subsequent studies demonstrated that Gal-2, Gal-3, Gal-4, and Gal-8 also possess a similar ability to induce apoptosis-independent expression of PS [96, 99, 100], although distinct signaling pathways appear to be engaged [96].
Fig. 4.
Galectins regulate granulocyte activation and turnover. Galectin family members appear to possess the ability to differentially activate various granulocytes, including neutrophils, eosinophils, and mast cells. In addition, galectins appear to regulate the turnover and chemotaxis of various granulocyte populations. Representative galectin-regulated activities are shown. Red arrows indicate an activity that the respective galectin increases, while blue arrows signify galectin-induced decreases in the accompanying activity
In addition to potentially regulating neutrophils, early studies suggested that galectins may also regulate other granulocyte populations. Galectin-10, the first galectin actually described, but originally known as Charcot-Leyden protein [101, 102], accumulates as crystals in vivo under allergic or parasitic conditions associated with hypereosinophilia [102]. Although the exact stimulus and purpose of copious Gal-10 accumulation in this setting remains to be elucidated, these results suggest that Gal-10 may serve a regulatory or effector role in eosinophil function. Originally described as one of several T cell-derived factors capable of regulating eosinophils [103], Gal-9 secreted by activated T cells appears to drive eosinophil chemotaxis [104]. Subsequent studies suggested that Gal-9 may not only facilitate eosinophil migration, but may also regulate extravasation and viability [105–107].
In addition to regulating neutrophils and eosinophils, some of the earliest work examining the potential immunomodulatory activity of galectins involved mast cell biology. Pioneering work by Fu-Tong Liu’s group demonstrated that a unique protein, the epsilon binding protein, recognized IgE. Subsequent studies identified epsilon binding protein as Gal-3 [108, 109]. Gal-3 not only appeared by bind IgE [110] but also recognized the Fcε receptor (FcεR) on mast cells [111], suggesting a general regulatory role for Gal-3 in mast cell function. Indeed, Gal-3 appears to directly engage the FcεR and induce mast cell degranulation [111]. Consistent with this, Gal-3 KO mast cells display significantly impaired degranulation [112]. In addition, recent studies suggest that Gal-3 may actually regulate mast cell viability [113]. In contrast to Gal-3, Gal-1 may induce the opposite effect on mast cells, as injection of Gal-1 appears to inhibit mast cell degranulation following inflammatory challenge in vivo [114] (Fig. 4).
10 Galectins Regulate Hemostasis, Tissue Repair, and Angiogenesis
Given the breadth of activities attributed to individual galectin family members in the regulation of immunity and the intimate association of immunity with coagulation [115], it appears that galectins also evolved important roles in hemostasis (Fig. 5). Several studies demonstrated that Gal-1 directly triggers platelet activation and aggregation in vitro, likely through interactions with αIIβ3 integrins [116]. In addition, Gal-1 appears to enhance platelet sensitivity to activation by other agonists [117]. Although the addition of exogenous Gal-1 can induce platelet activation, co-incubation of platelets with a Gal-1 blocking antibody significantly inhibits ADP-induced platelet aggregation [117], strongly suggesting a role for platelet-derived Gal-1 in platelet activation. Consistent with these in vitro results, Gal-1 KO mice experience increased bleeding time [116], suggesting that Gal-1 plays an important role in hemostasis in vivo. Galectin-mediated platelet activation does not appear to be limited to Gal-1, as Gal-8 similarly activates platelets [118]. However, in contrast to Gal-1, Gal-8 signals through a GP1β-dependent pathway, although a similar downstream Erk pathway becomes activated following engagement by either galectin [116, 118]. In addition to regulating platelet function, Gal-8 appears to regulate Factor V levels by enhancing its endocytic recycling in megakaryocytes [119]. In contrast, Gal-1 and Gal-3 appear to negatively regulate von Willebrand factor (vWF) interactions with platelets through direct interactions with vWF N glycans [120].
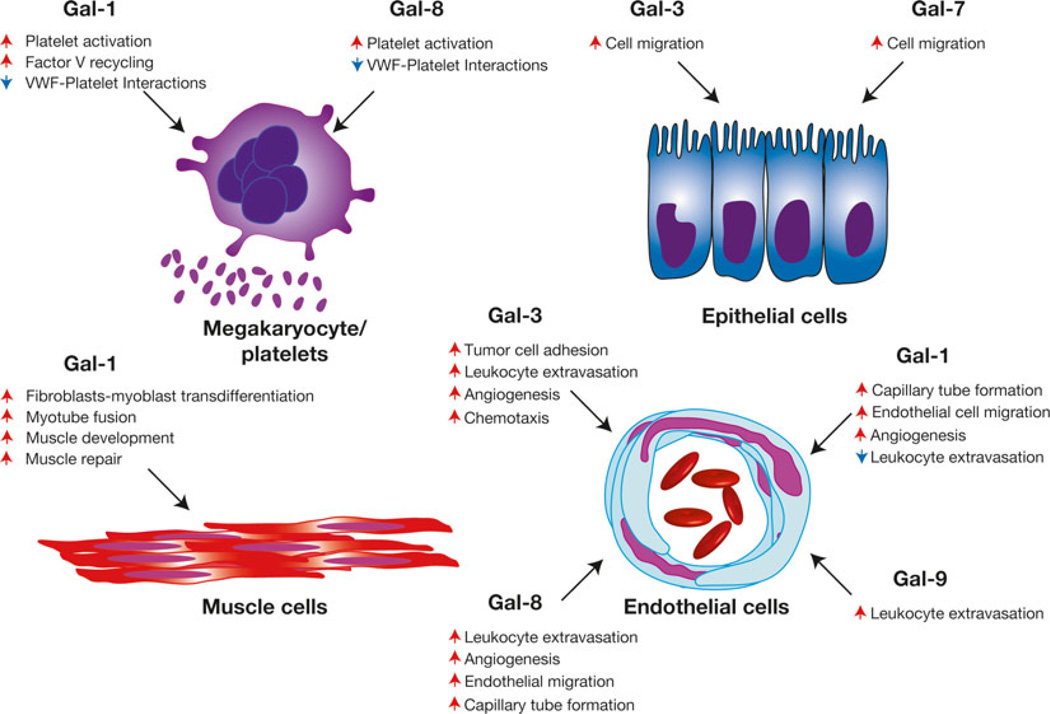
Fig. 5.
Galectins regulate hemostasis, angiogenesis and tissue repair. Various members of the galectin family regulate megakaryocyte activity, hemostasis, angiogenesis, epithelial migration, and general tissue repair following injury. Representative galectin-regulated activities are shown. Red arrows indicate an activity that the respective galectin increases, while blue arrows signify galectin-induced decreases in the accompanying activity. Plt = platelet, vWF = von Willebrand Factor
In addition to regulating hemostasis, different galectin family members appear to play a critical role in tissue repair. Work by Panjwani and colleagues demonstrated that Gal-7 in particular plays a critical role in epithelial repair, as Gal-7 induces the cell migration and proliferation necessary for wound closure in an in vitro wound healing model [121, 122] (Fig. 5). Consistent with this, Gal-7 KO mice displayed reduced epithelial repair following wounding in vivo [123]. Gal-3 likewise appears to play a critical role in this process, while Gal-1 failed to exhibit similar activity [121]. In addition to epithelial repair, early studies suggested that Gal-1 might facilitate muscle development and repair by inducing myotube fusion and fibroblast to myocyte transdifferentiation [124, 125]. Gal-1 displays a unique striated localization pattern within muscle and muscle injury results in a significant increase in Gal-1 expression [126, 127]. Gal-1 KO mice exhibit impaired muscle regeneration following BaCl2-induced muscle injury [124]. Furthermore, knockdown of Gal-1 in zebrafish resulted in impaired muscle formation, suggesting a role in embryonic muscle development [128].
An important component of injury repair is the development of blood vessels, in which endothelial cells play a critical role [129]. Endothelial cells express several galectin family members, including Gal-1, Gal-3, and Gal-9 and activation increases galectin expression [130]. While enhanced galectin expression may regulate endothelial interactions with leukocytes and subsequent leukocyte extravasation [131–134], several galectins appear to directly impact endothelial cell-mediated regulation of angiogenesis [135, 136]. Incubation of endothelial cells with Gal-3 results in microtube development [137], possibly through NG2, a driver of angiogenesis [138], by enhancing NG2 interactions with α3β1 integrins [138]. Several studies suggest that Gal-1 may also facilitate angiogenesis, as knockdown of Gal-1 reduces endothelial migration and expansion. Unlike Gal-3, Gal-1-mediated regulation of angiogenesis appears to occur through interactions with VEGFR1 and VEGFR2 [139]. Disruption of this interaction and subsequent loss of VEGFR2 signaling likely contributes to the pathogenesis of preeclampsia, as Gal-1 KO mice exhibit lower levels of desidual vascularization and an increased frequency of preeclampsia [140]. Consistent with this, individuals with preeclampsia display reduced levels of Gal-1 expression [140]. Similar studies suggested that Gal-8 might induce endothelial cell migration and angiogenesis through interactions with CD166 [141]. In contrast, a recent study suggested that Gal-1 might engage CD146 to induce endothelial cell apoptosis [142].
11 Intracellular Roles of Galectins
Although the extracellular carbohydrate binding activities of galectins became their defining feature [143], galectin synthesis occurs within the cytosol, where individual galectin family members often accumulate prior to export through a noncanonical Golgi-independent pathway [144–146]. Given their location within the cytosol, several studies have implicated various galectin family members as key players in the regulation of a variety of intracellular processes [147] (Fig. 6). For example, Gal-3 binds GSK-β and β-catenin and can facilitate GSK-β-mediated signaling and transcriptional upregulation of downstream targets, such as fascin-1 [148, 149]. Phosphorylation of Gal-3 appears to modulate Gal-3-mediated regulation of cellular signaling and survival, possibly through altering potential interactions with K-Ras-GTP [150, 151]. Additional studies suggest that similar intracellular phosphorylation events may also regulate Gal-3-mediated extracellular activities by impacting the ability of Gal-3 to recognize carbohydrate ligands [152]. Intracellular Gal-3 can also regulate signaling pathways involved in TRAIL, Fas, and TNF-induced apoptosis [153–155]. As Gal-3 contains the bcl-2 NWGR motif, several studies suggest that Gal-3-mediated regulation of cell survival may occur through a bcl-2 pathway [155, 156]. In contrast, intracellular Gal-7 appears to induce keratinocyte apoptosis following UVB-induced damage [157]. Gal-1 also appears to directly regulate cellular signaling by modulating Ras localization and signaling [158]. Gal-12 plays a critical role in adipocyte signaling [159], which likely contributes to the increased lipolysis and reduced adiposity displayed by Gal-12 KO mice [160]. While studies suggest that several members of the galectin family, such as Gal-3 and Gal-4, likely regulate membrane trafficking through carbohydrate recognition in distinct membrane microdomains outside the cell [161, 162], recent studies suggest that Gal-8 may recognize extracellular carbohydrate ligands exposed during vesicular damage following bacterial infection intracellularly [163]. Gal-8-mediated recognition of damaged vesicles appears to target these contents for autophagy, providing a unique form of intracellular antimicrobial defense [163].
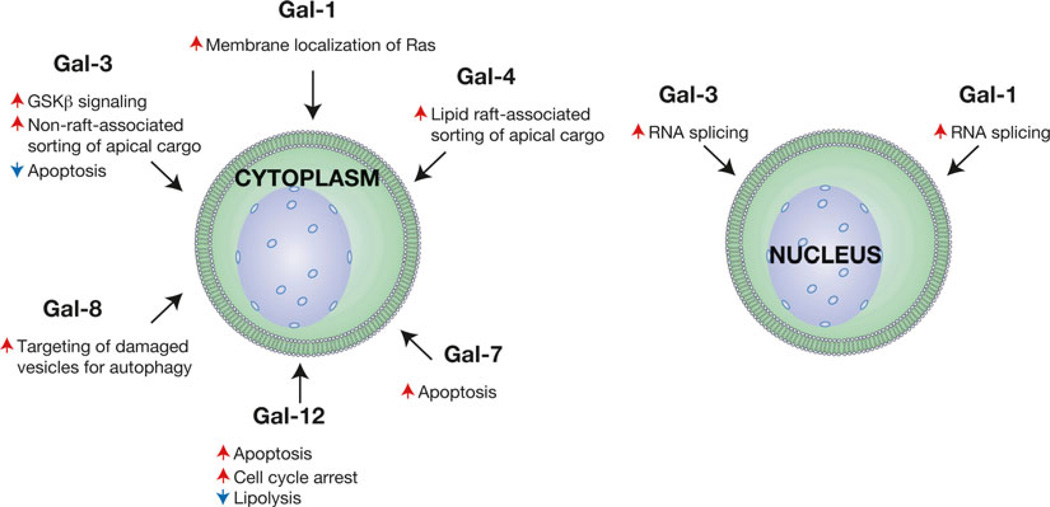
Fig. 6.
Galectin family members regulate various intracellular processes. While the earliest studies on galectins demonstrated that these proteins recognize extracellular carbohydrate ligands, subsequent studies have demonstrated that galectins possess significant roles in the regulation of a variety of fundamental intracellular activities, both in the cytoplasm and the nucleus. Representative galectin-regulated activities are shown. Red arrows indicate an activity that the respective galectin increases, while blue arrows signify galectin-induced decreases in the accompanying activity
In addition to regulating signaling pathways in the cytosol, galectins appear to modulate nuclear activities within the cell (Fig. 6). For example, some of the earliest work suggesting an intracellular role for galectins demonstrated that Gal-1 and Gal-3 might regulate a process as fundamental as RNA splicing [164]. Using nuclear extracts isolated from HeLa cells to examine RNA processing, depletion of Gal-1 or Gal-3 significantly inhibited RNA splicing, while reconstitution of only one of these galectins would largely restore this activity, strongly suggesting potential compensatory activity [164]. Subsequent studies demonstrated that Gal-1 and Gal-3 localize not only in the nucleus but also co-localize and directly interact with known factors involved in RNA splicing [165–167]. Consistent with their potential role in nuclear activities, including transcriptional regulation [168], nuclear import of galectin family members appears to be a highly regulated process [169, 170]. Inhibition of Gal-3 nuclear trafficking, by preventing serine phosphorylation via site directed mutagenesis, appears to significantly impact its ability to regulate cell survival [171].
12 Galectins and Neoplastic Disease
As galectins appear to play key regulatory roles in general immunity, hemostasis, vascular biology, cell signaling and viability, it is not surprising that altered galectin expression may impact neoplastic transformation and progression. Given the fundamental contribution of cell growth dysregulation in neoplastic transformation, it is fitting that some of the earliest studies suggesting that galectins may facilitate neoplastic transformation examined the potential impact of intracellular galectins on neoplastic cell survival. For example, seminal studies by Liu, Raz and others demonstrated that upregulation of Gal-3 can significantly reduce cellular sensitivity to apoptotic stimuli [150, 151, 153–155]. In contrast, neoplastic transformation of keratinized epithelial cells appears to result in downregulation of Gal-7 [157], consistent with its ability to induce apoptosis following cellular injury [172]. Furthermore, dysregulation of other galectin pathways also appears to likewise impact cellular signaling, with a significant effect on cell growth and neoplastic progression [173]. In addition to directly regulating neoplastic transformation at the cellular level, galectin family members may also facilitate overall tumor growth and dissemination by enhancing tumor angiogenesis [174, 175]. Several galectins, in particular Gal-3, appear to engage circulating neoplastic cells and enhance their extravasation, directly facilitating metastasis [176].
Given the role of immunosurveillance in the inhibition of neoplastic disease [177], transformed cells may also utilize galectins to inhibit immunological rejection of neoplastic lesions. Early studies demonstrated that overexpression of Gal-1 by neoplastic cells likely enhances neoplastic cell survival by directly inhibiting antitumor immunity. For example, overexpression of Gal-1 in a variety of settings, including neuroblastoma and breast cancer, appears to impair CD4+ T cells, CD8+ T cells and DC function, strongly suggesting a role for Gal-1 in the suppression of antitumor immunity [178, 179]. Consistent with this, knockdown of Gal-1 in neoplastic cells prior to transfer in vivo results in considerable expansion and enhanced function of CD4+ and CD8+ T cells, decreased levels of Tregs and enhanced DC function [178, 179]. Gal-1-mediated regulation of antitumor immunity may similarly impact neoplastic hematological disease. Increased expression of Gal-1 in patients with Hodgkin’s lymphoma correlates with a poorer prognosis and may likewise reflect significant modulation of antitumor immunity [34, 180]. In contrast, other galectins may also actually facilitate antitumor immunity. For example, Gal-9-mediated alterations in macrophage activation appear to result in significant NK cell activation, thereby enhancing NK cell-mediated antitumor immunity [92].
13 The Biochemistry of Galectins
Sensitivity to oxidative inactivation
In addition to the ability of galectins to recognize carbohydrates, the unique sensitivity of several galectin family members to oxidative inactivation represents one of their earliest recognized features. Indeed, the recognition of galectin sensitivity to redox environments provided the first name of this class of GBPs: the S-type, or thiol-dependent, lectins [181–185]. Some of the first studies on Gal-1 demonstrated that in the absence of reducing conditions Gal-1 gradually oxidizes [182], which results in profound conformational changes that preclude dimerization and recognition of carbohydrate ligands [186–188] (Fig. 7). However, redox regulation is not limited to Gal-1, as several other galectins, including Gal-2 and Gal-7, appeared to also be sensitive to oxidative inactivation.
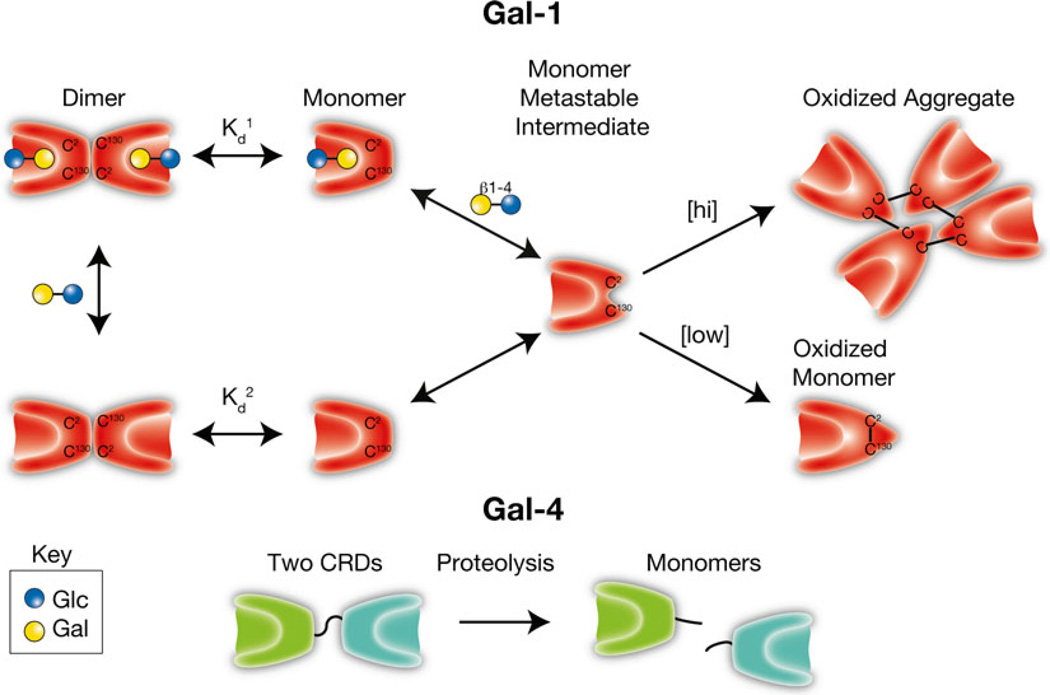
Fig. 7.
Galectin activity is regulated by oxidation and proteolysis. Galectins, first called S-type lectins secondary to the requirement of several galectins for reduced thiols to maintain carbohydrate recognition activity, can form intramolecular and intermolecular disulfide bridges that often result in significant conformational changes that preclude carbohydrate recognition. As monomers appear to be a key intermediate in oxidative inactivation and carbohydrates can drive dimerization, ligand appears to reduce oxidative inactivation by facilitating dimer formation (Kd1 < Kd2). [hi] = higher concentrations of Gal-1. [low] = lower concentrations of Gal-1. In contrast, several galectins, especially tandem repeat and chimeric galectins, rely on linker peptide bound carbohydrate recognition domains or N-terminal collagen-like domains to facilitate dimerization/oligomerization. Cleavage of intervening peptides that connect oligomerization domains to functional carbohydrate recognition domains can render carbohydrate recognition domains monomeric and therefore incapable of generating molecular lattices typically thought to be required for optimal galectin-mediated signaling
While Gal-1 oxidation does occur following removal of reducing agents, several factors appear to regulate this process. Gal-1 engagement of ligand stabilizes dimer formation and reduces Gal-1 sensitivity to oxidative inactivation [12, 182, 188] (Fig. 7). As a result, the unique sensitivity of Gal-1 to oxidative inactivation may be critical in regulating the spatial and temporal activity of this and related family members. Rapid oxidation of Gal-1 following tissue damage likely reduces the ability of Gal-1 and other galectins to inhibit neutrophils and other leukocytes immediately following injury, allowing these cells to neutralize potential pathogens and remove necrotic debris. However, as neutrophils and other leukocytes encroach on viable tissue surrounding an area of inflammation, leukocyte mediated-damage may release reduced, and therefore active, Gal-1, allowing engagement of leukocyte ligands and signaling of leukocyte turnover [127, 188]. Furthermore, while the redox environment can directly impact Gal-1 function, several studies suggest that the redox status of the cells themselves may also alter cellular sensitivity to Gal-1 signaling. For example, while Gal-1 typically induces apoptosis in activated T cells in the presence of reducing conditions [28], similar alterations in viability may be less apparent in the absence of reducing agents [30, 96, 97]. As a result, the outcome of Gal-1 engagement of cellular ligands appears to reflect the integration of a variety of signals that determine whether cellular death occurs. It remains possible that reducing conditions in vitro may also serve as a surrogate for other unknown factors in vivo that similarly regulate cellular fate following Gal-1 engagement. While Gal-1 oxidation results in loss of carbohydrate binding activity, oxidized Gal-1 itself appears to possess distinct biological activity. Several studies demonstrate that oxidized Gal-1 can facilitate neuronal regeneration [189, 190], suggesting that oxidation may not only limit Gal-1 carbohydrate binding activity, but also result in acquisition of alternative biological properties. In this way, Gal-1, and perhaps other galectins behave as morpheeins, proteins that possess the unique ability to regulate diverse biological processes depending on the conformational state of the protein [191].
Although oxidative inactivation likely plays a critical role in the regulation of many prototypical galectins, additional regulatory mechanisms likely govern the activity of other galectin family members. For example, chimeric Gal-3 and tandem repeat galectins, unlike prototypical galectins, possess unique domains or linker peptides that facilitate oligomerization or functional bivalency. Sequences between the collagen-like “oligomerization” domain and the carbohydrate recognition domain of Gal-3, can be cleaved by several proteases, including matrix metalloproteinases 2 and 9 and collagenase 3 [192, 193]. As Gal-3-mediated signaling and galectin signaling in general often requires clustering and lattice formation of counter receptors, collagen domain removal-induced loss of Gal-3 oligomerization likely contributes to the inability of the CRD of Gal-3 alone to signal cells [194]. While recent studies suggest that the individual domains of tandem repeat galectins may retain unique biological activity, many of the putative functions of tandem repeat galectins also require intact full length proteins for optimal signaling [100, 195], suggesting that proteolytic cleavage of the linker peptide responsible for tethering carbohydrate recognition domains would likewise inhibit this subgroup’s function [196] (Fig. 7).
Carbohydrate recognition
Perhaps the most unique and defining feature of galectins lies in their ability to regulate cell behavior through the recognition of highly modifiable carbohydrate structures [5]. Although it should be noted that subtle, but fundamental differences in carbohydrate binding preferences were noted in elegant studies utilizing only a few defined carbohydrates [197, 198], these early studies were limited by the number of available test glycans [197, 198]. Using frontal affinity chromatography coupled with a large library of target compounds, Hirabayashi and colleagues demonstrated that individual galectin family members possess unique specificity for many different carbohydrates [199]. Additional studies, using a wide variety of different modalities in addition to frontal affinity chromatography, including isothermal calorimetry, fluorescence polarization, and more recently, glycan microarrays [99, 199–201], strongly suggest that individual galectin family members exhibit unique and overlapping carbohydrate binding specificity that in addition to differences in quaternary structure [99, 100, 202], likely contributes to the distinct and overlapping biological activities displayed by this protein family.
Although galectins recognize the common disaccharide motif Galβ1–4GlcNAc, numerous studies suggest that many galectin family members prefer polymers of this structure in the form of poly-N-acetyllactosamine (polyLacNAc), when expressed on the cell surface. However, the mode and mechanisms of polyLacNAc interactions appear to vary among different galectin family members, resulting in a differential impact of polyLacNAc modification on galectin recognition [100, 203]. For example, while Gal-1 and Gal-2 recognize the terminal N-acetyllactosamine (LacNAc) determinant [204], these prototypical galectins prefer the motif expressed on extended glycans, either as N-glycans or polyLacNAc glycans bearing this terminal LacNAc structure [203–205]. As Gal-1 and Gal-2 exists as homodimers, yet only recognize the terminal LacNAc motif, this preference likely only occurs following glycan immobilization, where extended glycans may make cross-linking interactions more favorable. Consistent with this, in solution-based assays, Gal-1 and Gal-2 do not display the same preference for extended ligands [203, 205]. Not only may homodimerization influence carbohydrate binding specificity, but binding of ligand itself can actually enhance Gal-1 dimerization [188]. Similar results demonstrate that ligand may also influence the quaternary state of other galectins, such as Gal-3 [202]. In contrast to Gal-1 and Gal-2, Gal-3 and Gal-8, which do not exist as rigid homodimers, recognize internal LacNAc motifs within extended polyLacNAc [100, 203]. As these two galectin family members possess oligomeric carbohydrate recognition domains organized through flexible linker peptides, conformational constraints that provide the specificity of Gal-1 and Gal-2 toward extended glycans terminating in LacNAc following immobilization may not be relevant. Consistent with this, Gal-3 displays a preference for polyLacNAc repeats in solid phase and solution-based assays [203].
Distinct recognition of polyLacNAc results in a differential impact of polyLacNAc sialylation, a highly regulatable glycan modification, on the binding and signaling of these galectin family members [22]. Sialylation of the terminal galactose of LacNAc commonly occurs in two distinct linkages. The α2–3 sialylation linkage reflects addition of the sialic acid to the 3-OH of galactose, while α2–6 sialylation refers to the attachment of sialic acid to the 6-OH of galactose. As Gal-1 and Gal-2 recognize the terminal LacNAc, sialylation can significantly and differentially impact glycan recognition [206]. For example, although Gal-1 recognizes α2–3 sialylated glycans, it fails to recognize glycans following α2–6 sialylation [22, 203]. In contrast, Gal-2 fails to recognize glycans following sialylation with either linkage [203]. Gal-3 binds internal LacNAc within polyLacNAc, and sialylation of polyLacNAc with either linkage fails to significantly alter Gal-3 binding and signaling [22, 203], although in different cellular contexts sialylation may also impact Gal-3 binding and signaling [206]. Unlike Gal-1, Gal-2, and Gal-3, Gal-4, Gal-8, and Gal-9 possesses two unique carbohydrate recognition domains. Intriguingly, the N-terminal domain of Gal-8 recognizes sialic acid, while the C-terminal domain prefers non-sialylated glycans [207]. In contrast, the individual domains of Gal-4 and Gal-9 do not appear to possess drastic differences in glycan binding. Recent studies suggest that Gal-8 may form dimers and signal leukocytes entirely through the C-terminal domain [100], suggesting that tandem repeat galectins may in general form higher order quaternary structures that may be important in signaling. Additional studies suggest that Gal-9 can also associate with Gal-3 and Gal-8, suggesting not only that galectins may form higher order homotypic oligomers, but that complex hetero-oligomers may also occur [208]. Given the ability of galectins to form highly ordered lattices on cell surfaces and the potential impact of galectin-induced lattice formation in galectin signaling [194], these results suggest that in addition to distinct carbohydrate recognition preferences, differences in quaternary structure also likely impact the potential outcome of galectin engagement of a cell. Alterations in galectin binding to glycoconjugate ligands may not only regulate cellular signaling, but could also significantly impact galectin-mediated regulation of the sorting of cell surface glycoproteins [209–211]. In addition to common glycoprotein modifications, galectins appear to also possess affinity for glycolipids, including GM1 [212]. Given the proclivity of GM1 for unique lipid microdomains [213], galectin–glycolipid interactions may directly impact cellular signaling [214].
14 Galectins in Innate Immunity
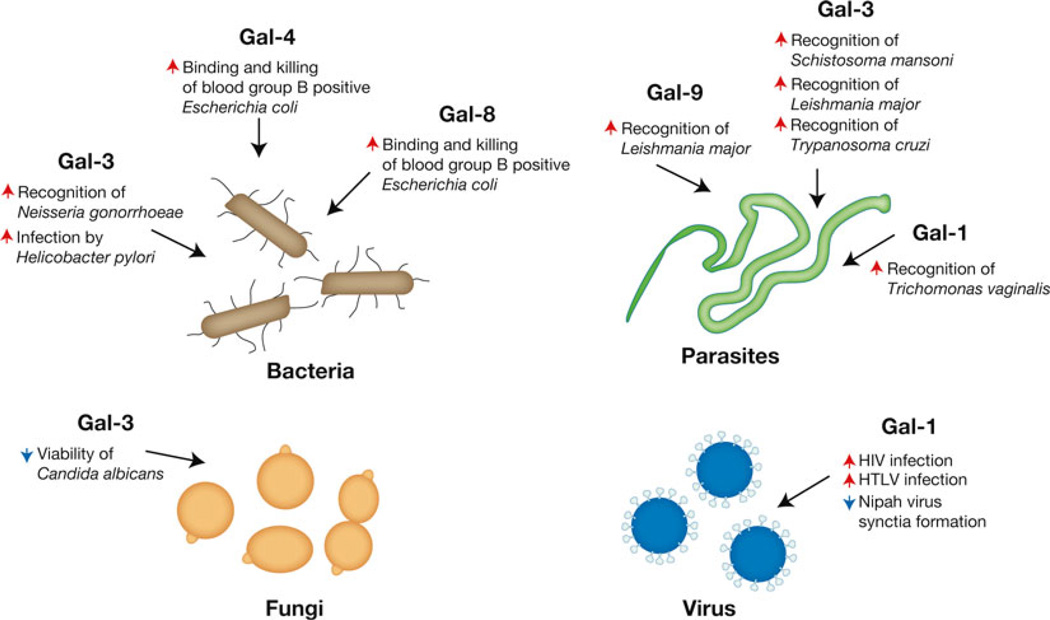
As galectin family members recognize a wide variety of highly modifiable carbohydrate structures, it is not surprising that individual galectin family members appear to be implicated in nearly every biological process described. However, recent studies provide insight into what may be one of the first biological roles of galectin family members—their ability to recognize pathogens [215]. Similar to the ability of mannose-binding protein, complement and other soluble immune factors [216, 217], Gal-3 recognizes a variety of microbes, including Neisseria gonorrhoeae, Candida Albicans, Toxoplasma gondii, Leishmania major, Schistosoma mansoni, and Trypanosoma cruzi [8, 215, 218–222], while Gal-9 also appears to recognize Leishmania major [223, 224]. Similarly, several studies suggest that Gal-1 binds Nipah, Influenza, and HIV viruses [225, 223] [226, 227] and that other galectins may specifically recognize unique microbial species [9].
Galectin-mediated interactions with pathogens appear to result in a wide variety of consequences, from enhanced phagocytosis to direct killing (Fig. 8). For example, Gal-3 recognition of Candida albicans induces direct microbial killing [8]. In contrast, Gal-3 binding of Toxoplasma gondii glycosylphosphatidylinositols facilitates parasite recognition and TNF production by macrophages [218]. In addition to recognizing glycans unique to pathogens, recent results suggest that several galectins may be able to protect individuals from microbes that express self-like antigens. For example, Gal-4 and Gal-8 recognize and kill microbes expressing the blood group B antigen, providing a potential mechanism of protection against blood group B expressing pathogens in blood group B positive individuals [9]. Recent studies utilizing microbial glycan microarrays suggest that Gal-4 and Gal-8 recognition of self-like microbial determinants may not be limited to blood group antigens, but that galectins may recognize a variety of microbes, both gram negative and gram positive, that share the unique feature of generating mammalian-like carbohydrate determinants [228, 229]. These results suggest that galectins may provide a broad form of innate immunity against molecular mimicry. Several studies also suggest that Gal-1 binds and inhibits entry of Nipah and Influenza viruses [225, 223]. Thus, galectin recognition of pathogens appears to provide innate immunity through a variety of mechanisms.
Fig. 8.
Galectins recognize a diverse range of pathogens. While the immunoregulatory roles of galectins likely represent some of their most well known functions, the ability of galectins to recognize a diverse range of pathogens may reflect some of their earliest evolutionary activities. Galectin recognition of pathogens can result in opsonization or direct microbial killing. In contrast, pathogens may utilize galectins to facilitate attachment and invasion. Representative galectin-regulated activities are shown. Red arrows indicate an activity that the respective galectin increases, while blue arrows signify galectin-induced decreases in the accompanying activity
In contrast to innate immune activity of galectins, several pathogens appear to have subverted galectin function and use these proteins as a mechanism of host attachment and invasion (Fig. 8). For example, key work by Sato and colleagues demonstrated that Gal-1 may actually facilitate HIV entry during infection [226, 227], likely through direct glycan interactions between gp120 and CD4 [230]. Gal-1 also appears to facilitate Trichomonas vaginalis infection by enhancing attachment to the vaginal epithelium [231], while Gal-3 appears to similarly aid in the colonization of Neisseria meningitidis [222]. Galectin facilitation of pathogen survival does not appear to be limited to mammals, as a Gal-9 orthologue, PpGalec, found in the midgut of the sandfly Phlebotomus papatasi, mediates attachment and facilitates survival of Leishmania major in this key vector [232].
15 Summary
Although the discovery of galectins over 30 years ago stemmed from interest in understanding the roles of carbohydrates in fundamental biological processes, this finding ultimately uncovered an entire family of potent regulatory proteins [12–14]. Given their nearly ubiquitous expression and ability to bind highly modifiable carbohydrate ligands expressed on nearly every cell, in addition to a variety of other intracellular and extracellular partners, these proteins appear to be uniquely poised to regulate a wide variety of biological activities. Consistent with this, individual members of the galectin family have been implicated in various aspects of nearly every biological process described. As a result, these proteins may not only be some of the most ancient lectins known, but given their history throughout evolution, also appear to have been a unique evolutionary substrate in many different biological systems. Future studies will undoubtedly continue to unravel additional biological functions of this complex and intriguing protein family.
Acknowledgments
This work was supported in part by grants from the National Blood Foundation, American Society of Hematology and Hemophilia of Georgia to S.R.S.
Abbreviations
- APC
Antigen presenting cell
- DC
Dendritic cell
- DTT
Dithiothreitol
- εBP
Epsilon binding protein
- ER
Endoplasmic reticulum
- FITC
Fluorescein isothiocyanate
- Gal-1
Galectin-1
- Gal-2
Galectin-2
- Gal-3
Galectin-3
- Gal-4
Galectin-4
- Gal-7
Galectin-7
- Gal-8
Galectin-8
- Gal-9
Galectin-9
- Gal-10
Galectin-10
- Gal-12
Galectin-12
- GBP
Glycan binding protein
- IFN-γ
Interferon gamma
- IgE
Immunoglobulin E
- IL-10
Interleukin-10
- IL-5
Interleukin-5
- IL-6
Interleukin 6
- KO
Knockout
- LacNAc
N-acetyllactosamine
- LPS
Lipopolysaccharide
- MOG
Myelin oligodendrocyte protein
- NK cell
Natural Killer Cell
- NKT cell
Natural Killer T cell
- PKC
Protein kinase C
- PLC-γ
Phospholipase C-gamma
- polyLacNAc
Poly-N-acetyllactosamine
- PS
Phosphatidylserine
- TCR
T cell receptor
- TGF-β
Transforming growth factor beta
- TH1
T helper cells type 1
- TH2
T helper cells type 2
- TNF
Tumor necrosis actor
- Tregs
Regulatory T cells
- vWF
von Willebrand Factor
References
- 1.Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, Etzler M. Essentials of Glycobiology. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 2.Grewal PK, Uchiyama S, Ditto D, Varki N, Le DT, Nizet V, Marth JD. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8(11):874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5(10):1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- 5.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9(6):593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 6.Offner H, Celnik B, Bringman TS, Casentini-Borocz D, Nedwin GE, Vandenbark AA. Recombinant human beta-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1990;28(2):177–184. doi: 10.1016/0165-5728(90)90032-i. [DOI] [PubMed] [Google Scholar]
- 7.Levi G, Tarrab-Hazdai R, Teichberg VI. Prevention and therapy with electrolectin of experimental autoimmune myasthenia gravis in rabbits. Eur J Immunol. 1983;13(6):500–507. doi: 10.1002/eji.1830130613. [DOI] [PubMed] [Google Scholar]
- 8.Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177(7):4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- 9.Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, Ju T, Molinaro RJ, Rivera-Marrero C, Xia B, Smith DF, Cummings RD. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med. 2010;16(3):295–301. doi: 10.1038/nm.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971;246(5):1461–1467. [PubMed] [Google Scholar]
- 11.Van Den Hamer CJ, Morell AG, Scheinberg IH, Hickman J, Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970;245(17):4397–4402. [PubMed] [Google Scholar]
- 12.Teichberg VI, Silman I, Beitsch DD, Resheff G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci U S A. 1975;72(4):1383–1387. doi: 10.1073/pnas.72.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak TP, Haywood PL, Barondes SH. Developmentally regulated lectin in embryonic chick muscle and a myogenic cell line. Biochem Biophys Res Commun. 1976;68(3):650–657. doi: 10.1016/0006-291x(76)91195-5. 0006-291X(76)91195-5 [pii] [DOI] [PubMed] [Google Scholar]
- 14.de Waard A, Hickman S, Kornfeld S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J Biol Chem. 1976;251(23):7581–7587. [PubMed] [Google Scholar]
- 15.Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19(7–9):433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- 16.Simpson DL, Thorne DR, Loh HH. Developmentally regulated lectin in neonatal rat brain. Nature. 1977;266(5600):367–369. doi: 10.1038/266367a0. [DOI] [PubMed] [Google Scholar]
- 17.Kobiler D, Barondes SH. Lectin activity from embryonic chick brain, heart, and liver: changes with development. Dev Biol. 1977;60(1):326–330. doi: 10.1016/0012-1606(77)90130-0. 0012-1606(77)90130-0 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Kaufman SJ, Lawless ML. Thiodigalactoside binding lectin and skeletal myogenesis. Differentiation. 1980;16(1):41–48. doi: 10.1111/j.1432-0436.1980.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiyama Y, Wada J, Zhang H, Morita Y, Hiragushi K, Hida K, Shikata K, Yamamura M, Kanwar YS, Makino H. Efficacy of galectins in the amelioration of nephrotoxic serum nephritis in Wistar Kyoto rats. Kidney Int. 2000;58(5):1941–1952. doi: 10.1111/j.1523-1755.2000.00366.x. [DOI] [PubMed] [Google Scholar]
- 20.Santucci L, Fiorucci S, Cammilleri F, Servillo G, Federici B, Morelli A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology. 2000;31(2):399–406. doi: 10.1002/hep.510310220. [DOI] [PubMed] [Google Scholar]
- 21.Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, Rabinovich GA, Morelli A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124(5):1381–1394. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- 22.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8(8):825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 23.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2-and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176(10):6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 24.Baum LG, Blackall DP, Arias-Magallano S, Nanigian D, Uh SY, Browne JM, Hoffmann D, Emmanouilides CE, Territo MC, Baldwin GC. Amelioration of graft versus host disease by galectin-1. Clin Immunol. 2003;109(3):295–307. doi: 10.1016/j.clim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 26.Levi G, Teichberg VI. Selective interactions of electrolectins from eel electric organ and mouse thymus with mouse immature thymocytes. Immunol Lett. 1983;7(1):35–39. doi: 10.1016/0165-2478(83)90052-4. [DOI] [PubMed] [Google Scholar]
- 27.Baum LG, Pang M, Perillo NL, Wu T, Delegeane A, Uittenbogaart CH, Fukuda M, Seilhamer JJ. Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core 2 O-glycans on thymocytes and T lymphoblastoid cells. J Exp Med. 1995;181(3):877–887. doi: 10.1084/jem.181.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378(6558):736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 29.van der Leij J, van den Berg A, Blokzijl T, Harms G, van Goor H, Zwiers P, van Weeghel R, Poppema S, Visser L. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J Pathol. 2004;204(5):511–518. doi: 10.1002/path.1671. [DOI] [PubMed] [Google Scholar]
- 30.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180(5):3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 31.Cedeno-Laurent F, Opperman M, Barthel SR, Kuchroo VK, Dimitroff CJ. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J Immunol. 2012;188(7):3127–3137. doi: 10.4049/jimmunol.1103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cedeno-Laurent F, Barthel SR, Opperman MJ, Lee DM, Clark RA, Dimitroff CJ. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J Immunol. 2010;185(8):4659–4672. doi: 10.4049/jimmunol.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4 + CD25+ T cells. Blood. 2006;109(5):2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 34.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci U S A. 2007;104(32):13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181(9):5948–5955. doi: 10.4049/jimmunol.181.9.5948. 181/9/5948 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J Exp Med. 2008;205(10):2281–2294. doi: 10.1084/jem.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung CD, Patel VP, Moran M, Lewis LA, Miceli MC. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J Immunol. 2000;165(7):3722–3729. doi: 10.4049/jimmunol.165.7.3722. [DOI] [PubMed] [Google Scholar]
- 38.Liu SD, Whiting CC, Tomassian T, Pang M, Bissel SJ, Baum LG, Mossine VV, Poirier F, Huflejt ME, Miceli MC. Endogenous galectin-1 enforces class I-restricted TCR functional fate decisions in thymocytes. Blood. 2008;112(1):120–130. doi: 10.1182/blood-2007-09-114181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endharti AT, Zhou YW, Nakashima I, Suzuki H. Galectin-1 supports survival of naive T cells without promoting cell proliferation. Eur J Immunol. 2005;35(1):86–97. doi: 10.1002/eji.200425340. [DOI] [PubMed] [Google Scholar]
- 40.Tribulatti MV, Figini MG, Carabelli J, Cattaneo V, Campetella O. Redundant and antagonistic functions of galectin-1, −3, and −8 in the elicitation of T cell responses. J Immunol. 2012;188(7):2991–2999. doi: 10.4049/jimmunol.1102182. [DOI] [PubMed] [Google Scholar]
- 41.Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63(23):8302–8311. [PubMed] [Google Scholar]
- 42.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 43.Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003;170(7):3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 44.Tribulatti MV, Mucci J, Cattaneo V, Aguero F, Gilmartin T, Head SR, Campetella O. Galectin-8 induces apoptosis in the CD4(high)CD8(high) thymocyte subpopulation. Glycobiology. 2007;17(12):1404–1412. doi: 10.1093/glycob/cwm104. [DOI] [PubMed] [Google Scholar]
- 45.Eshkar Sebban L, Ronen D, Levartovsky D, Elkayam O, Caspi D, Aamar S, Amital H, Rubinow A, Golan I, Naor D, Zick Y, Golan I. The involvement of CD44 and its novel ligand galectin-8 in apoptotic regulation of autoimmune inflammation. J Immunol. 2007;179(2):1225–1235. doi: 10.4049/jimmunol.179.2.1225. [DOI] [PubMed] [Google Scholar]
- 46.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176(2):778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 47.Bi S, Earl LA, Jacobs L, Baum LG. Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J Biol Chem. 2008;283(18):12248–12258. doi: 10.1074/jbc.M800523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409(6821):733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 49.Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, Hivroz C, Nicaise J, Squifflet JL, Mourad M, Godelaine D, Boon T, van der Bruggen P. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28(3):414–424. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Sturm A, Lensch M, Andre S, Kaltner H, Wiedenmann B, Rosewicz S, Dignass AU, Gabius HJ. Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol. 2004;173(6):3825–3837. doi: 10.4049/jimmunol.173.6.3825. [DOI] [PubMed] [Google Scholar]
- 51.Kubach J, Lutter P, Bopp T, Stoll S, Becker C, Huter E, Richter C, Weingarten P, Warger T, Knop J, Mullner S, Wijdenes J, Schild H, Schmitt E, Jonuleit H. Human CD4 + CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110(5):1550–1558. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 52.Hokama A, Mizoguchi E, Sugimoto K, Shimomura Y, Tanaka Y, Yoshida M, Rietdijk ST, de Jong YP, Snapper SB, Terhorst C, Blumberg RS, Mizoguchi A. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 2004;20(6):681–693. doi: 10.1016/j.immuni.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Nishida A, Nagahama K, Imaeda H, Ogawa A, Lau CW, Kobayashi T, Hisamatsu T, Preffer FI, Mizoguchi E, Ikeuchi H, Hibi T, Fukuda M, Andoh A, Blumberg RS, Mizoguchi A. Inducible colitis-associated glycome capable of stimulating the proliferation of memory CD4+ T cells. J Exp Med. 2012;209(13):2383–2394. doi: 10.1084/jem.20112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortegano I, del Pozo V, Cardaba B, de Andres B, Gallardo S, del Amo A, Arrieta I, Jurado A, Palomino P, Liu FT, Lahoz C. Galectin-3 down-regulates IL-5 gene expression on different cell types. J Immunol. 1998;161(1):385–389. [PubMed] [Google Scholar]
- 55.Norambuena A, Metz C, Vicuna L, Silva A, Pardo E, Oyanadel C, Massardo L, Gonzalez A, Soza A. Galectin-8 induces apoptosis in Jurkat T cells by phosphatidic acid-mediated ERK1/2 activation supported by protein kinase A down-regulation. J Biol Chem. 2009;284(19):12670–12679. doi: 10.1074/jbc.M808949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99(20):13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossi B, Espeli M, Schiff C, Gauthier L. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J Immunol. 2006;177(2):796–803. doi: 10.4049/jimmunol.177.2.796. [DOI] [PubMed] [Google Scholar]
- 58.Elantak L, Espeli M, Boned A, Bornet O, Bonzi J, Gauthier L, Feracci M, Roche P, Guerlesquin F, Schiff C. Structural basis for galectin-1-dependent pre-B cell receptor (pre-BCR) activation. J Biol Chem. 2012;287(53):44703–44713. doi: 10.1074/jbc.M112.395152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Espeli M, Mancini SJ, Breton C, Poirier F, Schiff C. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood. 2009;113(23):5878–5886. doi: 10.1182/blood-2009-01-198465. [DOI] [PubMed] [Google Scholar]
- 60.Mourcin F, Breton C, Tellier J, Narang P, Chasson L, Jorquera A, Coles M, Schiff C, Mancini SJ. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117(24):6552–6561. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- 61.Anginot A, Espeli M, Chasson L, Mancini SJ, Schiff C. Galectin 1 modulates plasma cell homeostasis and regulates the humoral immune response. J Immunol. 2013;190(11):5526–5533. doi: 10.4049/jimmunol.1201885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai CM, Chiu YK, Hsu TL, Lin IY, Hsieh SL, Lin KI. Galectin-1 promotes immunoglobulin production during plasma cell differentiation. J Immunol. 2008;181(7):4570–4579. doi: 10.4049/jimmunol.181.7.4570. [DOI] [PubMed] [Google Scholar]
- 63.Tsai CM, Guan CH, Hsieh HW, Hsu TL, Tu Z, Wu KJ, Lin CH, Lin KI. Galectin-1 and galectin-8 have redundant roles in promoting plasma cell formation. J Immunol. 2011;187(4):1643–1652. doi: 10.4049/jimmunol.1100297. [DOI] [PubMed] [Google Scholar]
- 64.Acosta-Rodriguez EV, Montes CL, Motran CC, Zuniga EI, Liu FT, Rabinovich GA, Gruppi A. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J Immunol. 2004;172(1):493–502. doi: 10.4049/jimmunol.172.1.493. [DOI] [PubMed] [Google Scholar]
- 65.Karimi K, Arck PC. Natural Killer cells: keepers of pregnancy in the turnstile of the environment. Brain Behav Immun. 2010;24(3):339–347. doi: 10.1016/j.bbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 66.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198(8):1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molvarec A, Blois SM, Stenczer B, Toldi G, Tirado-Gonzalez I, Ito M, Shima T, Yoneda S, Vasarhelyi B, Rigo J, Jr, Saito S. Peripheral blood galectin-1-expressing T and natural killer cells in normal pregnancy and preeclampsia. Clin Immunol. 2011;139(1):48–56. doi: 10.1016/j.clim.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 68.Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka N, Habuchi T, Horikawa Y, Hashimoto Y, Yoneyama T, Mori K, Koie T, Nakamura T, Saitoh H, Yamaya K, Funyu T, Fukuda M, Ohyama C. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011;30(15):3173–3185. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki Y, Sutoh M, Hatakeyama S, Mori K, Yamamoto H, Koie T, Saitoh H, Yamaya K, Funyu T, Habuchi T, Arai Y, Fukuda M, Ohyama C, Tsuboi S. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int J Oncol. 2012;40(6):1831–1838. doi: 10.3892/ijo.2012.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jost S, Moreno-Nieves UY, Garcia-Beltran WF, Rands K, Reardon J, Toth I, Piechocka-Trocha A, Altfeld M, Addo MM. Dysregulated Tim-3 expression on natural killer cells is associated with increased Galectin-9 levels in HIV-1 infection. Retrovirology. 2013;10(1):74. doi: 10.1186/1742-4690-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Golden-Mason L, McMahan RH, Strong M, Reisdorph R, Mahaffey S, Palmer BE, Cheng L, Kulesza C, Hirashima M, Niki T, Rosen HR. Galectin-9 functionally impairs natural killer cells in humans and mice. J Virol. 2013;87(9):4835–4845. doi: 10.1128/JVI.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. 2013;190(4):1788–1796. doi: 10.4049/jimmunol.1202814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miko E, Meggyes M, Bogar B, Schmitz N, Barakonyi A, Varnagy A, Farkas B, Tamas P, Bodis J, Szekeres-Bartho J, Illes Z, Szereday L. Involvement of galectin-9/TIM-3 pathway in the systemic inflammatory response in early-onset preeclampsia. PLoS One. 2013;8(8):e71811. doi: 10.1371/journal.pone.0071811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9(1):4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 75.Karlsson A, Christenson K, Matlak M, Bjorstad A, Brown KL, Telemo E, Salomonsson E, Leffler H, Bylund J. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology. 2009;19(1):16–20. doi: 10.1093/glycob/cwn104. [DOI] [PubMed] [Google Scholar]
- 76.Rotshenker S, Reichert F, Gitik M, Haklai R, Elad-Sfadia G, Kloog Y. Galectin-3/MAC-2, Ras and PI3K activate complement receptor-3 and scavenger receptor-AI/II mediated myelin phagocytosis in microglia. Glia. 2008;56(15):1607–1613. doi: 10.1002/glia.20713. [DOI] [PubMed] [Google Scholar]
- 77.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180(4):2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 78.Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, Greaves DR. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol. 2008;28(3):433–440. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 79.Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112(3):389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho MK, Springer TA. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982;128(3):1221–1228. [PubMed] [Google Scholar]
- 81.Vaitaitis GM, Wagner DH., Jr Galectin-9 controls CD40 signaling through a Tim-3 independent mechanism and redirects the cytokine profile of pathogenic T cells in autoimmunity. PLoS One. 2012;7(6):e38708. doi: 10.1371/journal.pone.0038708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 83.Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol. 2007;178(1):436–445. doi: 10.4049/jimmunol.178.1.436. [DOI] [PubMed] [Google Scholar]
- 84.Kojima K, Arikawa T, Saita N, Goto E, Tsumura S, Tanaka R, Masunaga A, Niki T, Oomizu S, Hirashima M, Kohrogi H. Galectin-9 attenuates acute lung injury by expanding CD14-plasmacytoid dendritic cell-like macrophages. Am J Respir Crit Care Med. 2011;184(3):328–339. doi: 10.1164/rccm.201010-1566OC. [DOI] [PubMed] [Google Scholar]
- 85.de la Fuente H, Perez-Gala S, Bonay P, Cruz-Adalia A, Cibrian D, Sanchez-Cuellar S, Dauden E, Fresno M, Garcia-Diez A, Sanchez-Madrid F. Psoriasis in humans is associated with down-regulation of galectins in dendritic cells. J Pathol. 2012;228(2):193–203. doi: 10.1002/path.3996. [DOI] [PubMed] [Google Scholar]
- 86.Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, Matsumura R, Tomioka H, Liu FT, Shirai K. Role of galectin-3 in human pulmonary fibrosis. Allergol Int. 2007;56(1):57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- 87.Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, Cordone S, Delucchi F, Serino M, Federici M, Pricci F, Pugliese G. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54(5):975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Jiang HR, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, Fukada SY, Liu FT, Liew FY, Lukic ML. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol. 2009;182(2):1167–1173. doi: 10.4049/jimmunol.182.2.1167. [DOI] [PubMed] [Google Scholar]
- 89.Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, Leffler H, Lukic ML. Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology. 2012;55(6):1954–1964. doi: 10.1002/hep.25542. [DOI] [PubMed] [Google Scholar]
- 90.Fernandes Bertocchi AP, Campanhole G, Wang PH, Goncalves GM, Damiao MJ, Cenedeze MA, Beraldo FC, de Paula Antunes Teixeira V, Dos Reis MA, Mazzali M, Pacheco-Silva A, Camara NO. A Role for galectin-3 in renal tissue damage triggered by ischemia and reperfusion injury. Transpl Int. 2008;21(10):999–1007. doi: 10.1111/j.1432-2277.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 91.Wu SY, Yu JS, Liu FT, Miaw SC, Wu-Hsieh BA. Galectin-3 negatively regulates dendritic cell production of IL-23/IL-17-axis cytokines in infection by Histoplasma capsulatum. J Immunol. 2013;190(7):3427–3437. doi: 10.4049/jimmunol.1202122. [DOI] [PubMed] [Google Scholar]
- 92.Nobumoto A, Oomizu S, Arikawa T, Katoh S, Nagahara K, Miyake M, Nishi N, Takeshita K, Niki T, Yamauchi A, Hirashima M. Galectin-9 expands unique macrophages exhibiting plasmacytoid dendritic cell-like phenotypes that activate NK cells in tumor-bearing mice. Clin Immunol. 2009;130(3):322–330. doi: 10.1016/j.clim.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 93.Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol. 1999;163(10):5592–5598. [PubMed] [Google Scholar]
- 94.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91(9):3430–3438. [PubMed] [Google Scholar]
- 95.Dias-Baruffi M, Zhu H, Cho M, Karmakar S, McEver RP, Cummings RD. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J Biol Chem. 2003;278(42):41282–41293. doi: 10.1074/jbc.M306624200. [DOI] [PubMed] [Google Scholar]
- 96.Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, −2, and −4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109(1):219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stowell SR, Karmakar S, Arthur CM, Ju T, Rodrigues LC, Riul TB, Dias-Baruffi M, Miner J, McEver RP, Cummings RD. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell. 2009;20(5):1408–1418. doi: 10.1091/mbc.E08-07-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179(3):1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, −2, and −3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283(15):10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stowell SR, Arthur CM, Slanina KA, Horton JR, Smith DF, Cummings RD. Dimeric Galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem. 2008;283(29):20547–20559. doi: 10.1074/jbc.M802495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leonidas DD, Elbert BL, Zhou Z, Leffler H, Ackerman SJ, Acharya KR. Crystal structure of human Charcot-Leyden crystal protein, an eosinophil lysophospholipase, identifies it as a new member of the carbohydrate-binding family of galectins. Structure. 1995;3(12):1379–1393. doi: 10.1016/s0969-2126(01)00275-1. [DOI] [PubMed] [Google Scholar]
- 102.Ackerman SJ, Corrette SE, Rosenberg HF, Bennett JC, Mastrianni DM, Nicholson-Weller A, Weller PF, Chin DT, Tenen DG. Molecular cloning and characterization of human eosinophil Charcot-Leyden crystal protein (lysophospholipase). Similarities to IgE binding proteins and the S-type animal lectin superfamily. J Immunol. 1993;150(2):456–468. [PubMed] [Google Scholar]
- 103.Hirashima M, Ueno M, Kamiya K, Higuchi S, Matsumoto R. Functional heterogeneity of human eosinophil chemotactic lymphokines. Lymphokine Cytokine Res. 1991;10(6):481–486. [PubMed] [Google Scholar]
- 104.Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, Stevens RL, Hirashima M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273(27):16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 105.Asakura H, Kashio Y, Nakamura K, Seki M, Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T, Saita N, Toyama Y, Takashima H, Nakamura T, Ohkawa M, Hirashima M. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol. 2002;169(10):5912–5918. doi: 10.4049/jimmunol.169.10.5912. [DOI] [PubMed] [Google Scholar]
- 106.Saita N, Goto E, Yamamoto T, Cho I, Tsumori K, Kohrogi H, Maruo K, Ono T, Takeya M, Kashio Y, Nakamura K, Hirashima M. Association of galectin-9 with eosinophil apoptosis. Int Arch Allergy Immunol. 2002;128(1):42–50. doi: 10.1159/000058002. 58002. [DOI] [PubMed] [Google Scholar]
- 107.Imaizumi T, Kumagai M, Sasaki N, Kurotaki H, Mori F, Seki M, Nishi N, Fujimoto K, Tanji K, Shibata T, Tamo W, Matsumiya T, Yoshida H, Cui XF, Takanashi S, Hanada K, Okumura K, Yagihashi S, Wakabayashi K, Nakamura T, Hirashima M, Satoh K. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol. 2002;72(3):486–491. [PubMed] [Google Scholar]
- 108.Laing JG, Robertson MW, Gritzmacher CA, Wang JL, Liu FT. Biochemical and immunological comparisons of carbohydrate-binding protein 35 and an IgE-binding protein. J Biol Chem. 1989;264(4):1097–1110. [PubMed] [Google Scholar]
- 109.Gritzmacher CA, Robertson MW, Liu FT. IgE-binding protein. Subcellular location and gene expression in many murine tissues and cells. J Immunol. 1988;141(8):2801–2806. [PubMed] [Google Scholar]
- 110.Robertson MW, Albrandt K, Keller D, Liu FT. Human IgE-binding protein: a soluble lectin exhibiting a highly conserved inter-species sequence and differential recognition of IgE glycoforms. Biochemistry. 1990;29(35):8093–8100. doi: 10.1021/bi00487a015. [DOI] [PubMed] [Google Scholar]
- 111.Frigeri LG, Zuberi RI, Liu FT. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry. 1993;32(30):7644–7649. doi: 10.1021/bi00081a007. [DOI] [PubMed] [Google Scholar]
- 112.Chen HY, Sharma BB, Yu L, Zuberi R, Weng IC, Kawakami Y, Kawakami T, Hsu DK, Liu FT. Role of galectin-3 in mast cell functions: galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J Immunol. 2006;177(8):4991–4997. doi: 10.4049/jimmunol.177.8.4991. [DOI] [PubMed] [Google Scholar]
- 113.Suzuki Y, Inoue T, Yoshimaru T, Ra C. Galectin-3 but not galectin-1 induces mast cell death by oxidative stress and mitochondrial permeability transition. Biochim Biophys Acta. 2008;1783(5):924–934. doi: 10.1016/j.bbamcr.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 114.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000;30(5):1331–1339. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 115.Kolev M, Le Friec G, Kemper C. The role of complement in CD4(+) T cell homeostasis and effector functions. Semin Immunol. 2013;25(1):12–19. doi: 10.1016/j.smim.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 116.Romaniuk MA, Croci DO, Lapponi MJ, Tribulatti MV, Negrotto S, Poirier F, Campetella O, Rabinovich GA, Schattner M. Binding of galectin-1 to alphaIIb-beta(3) integrin triggers “outside-in” signals, stimulates platelet activation, and controls primary hemostasis. FASEB J. 2012;26(7):2788–2798. doi: 10.1096/fj.11-197541. [DOI] [PubMed] [Google Scholar]
- 117.Pacienza N, Pozner RG, Bianco GA, D’Atri LP, Croci DO, Negrotto S, Malaver E, Gomez RM, Rabinovich GA, Schattner M. The immunoregulatory glycan-binding protein galectin-1 triggers human platelet activation. FASEB J. 2008;22(4):1113–1123. doi: 10.1096/fj.07-9524com. [DOI] [PubMed] [Google Scholar]
- 118.Romaniuk MA, Tribulatti MV, Cattaneo V, Lapponi MJ, Molinas FC, Campetella O, Schattner M. Human platelets express and are activated by galectin-8. Biochem J. 2010;432(3):535–547. doi: 10.1042/BJ20100538. [DOI] [PubMed] [Google Scholar]
- 119.Zappelli C, van der Zwaan C, Thijssen-Timmer DC, Mertens K, Meijer AB. Novel role for galectin-8 protein as mediator of coagulation factor V endocytosis by megakaryocytes. J Biol Chem. 2012;287(11):8327–8335. doi: 10.1074/jbc.M111.305151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saint-Lu N, Oortwijn BD, Pegon JN, Odouard S, Christophe OD, de Groot PG, Denis CV, Lenting PJ. Identification of galectin-1 and galectin-3 as novel partners for von Willebrand factor. Arterioscler Thromb Vasc Biol. 2012;32(4):894–901. doi: 10.1161/ATVBAHA.111.240309. [DOI] [PubMed] [Google Scholar]
- 121.Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and −7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277(44):42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- 122.Cao Z, Said N, Wu HK, Kuwabara I, Liu FT, Panjwani N. Galectin-7 as a potential mediator of corneal epithelial cell migration. Arch Ophthalmol. 2003;121(1):82–86. doi: 10.1001/archopht.121.1.82. [DOI] [PubMed] [Google Scholar]
- 123.Gendronneau G, Sidhu SS, Delacour D, Dang T, Calonne C, Houzelstein D, Magnaldo T, Poirier F. Galectin-7 in the control of epidermal homeostasis after injury. Mol Biol Cell. 2008;19(12):5541–5549. doi: 10.1091/mbc.E08-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Watt DJ, Jones GE, Goldring K. The involvement of galectin-1 in skeletal muscle determination, differentiation and regeneration. Glycoconj J. 2004;19(7-9):615–619. doi: 10.1023/B:GLYC.0000014093.23509.92. [DOI] [PubMed] [Google Scholar]
- 125.Georgiadis V, Stewart HJ, Pollard HJ, Tavsanoglu Y, Prasad R, Horwood J, Deltour L, Goldring K, Poirier F, Lawrence-Watt DJ. Lack of galectin-1 results in defects in myoblast fusion and muscle regeneration. Dev Dyn. 2007;236(4):1014–1024. doi: 10.1002/dvdy.21123. [DOI] [PubMed] [Google Scholar]
- 126.Dias-Baruffi M, Stowell SR, Song SC, Arthur CM, Cho M, Rodrigues LC, Montes MA, Rossi MA, James JA, McEver RP, Cummings RD. Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle. Glycobiology. 2010;20(5):507–520. doi: 10.1093/glycob/cwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cerri DG, Rodrigues LC, Stowell SR, Araujo DD, Coelho MC, Oliveira SR, Bizario JC, Cummings RD, Dias-Baruffi M, Costa MC. Degeneration of dystrophic or injured skeletal muscles induces high expression of Galectin-1. Glycobiology. 2008;18(11):842–850. doi: 10.1093/glycob/cwn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmed H, Du SJ, Vasta GR. Knockdown of a galectin-1-like protein in zebrafish (Danio rerio) causes defects in skeletal muscle development. Glycoconj J. 2009;26(3):277–283. doi: 10.1007/s10719-008-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 130.Ishikawa A, Imaizumi T, Yoshida H, Nishi N, Nakamura T, Hirashima M, Satoh K. Double-stranded RNA enhances the expression of galectin-9 in vascular endothelial cells. Immunol Cell Biol. 2004;82(4):410–414. doi: 10.1111/j.0818-9641.2004.01248.x. [DOI] [PubMed] [Google Scholar]
- 131.Cooper D, Norling LV, Perretti M. Novel insights into the inhibitory effects of Galectin-1 on neutrophil recruitment under flow. J Leukoc Biol. 2008;83(6):1459–1466. doi: 10.1189/jlb.1207831. [DOI] [PubMed] [Google Scholar]
- 132.Norling LV, Sampaio AL, Cooper D, Perretti M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. FASEB J. 2008;22(3):682–690. doi: 10.1096/fj.07-9268com. [DOI] [PubMed] [Google Scholar]
- 133.Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J Immunol. 2008;180(4):2466–2473. doi: 10.4049/jimmunol.180.4.2466. [DOI] [PubMed] [Google Scholar]
- 134.Cooper D, Iqbal AJ, Gittens BR, Cervone C, Perretti M. The effect of galectins on leukocyte trafficking in inflammation: sweet or sour? Ann N Y Acad Sci. 2012;1253:181–192. doi: 10.1111/j.1749-6632.2011.06291.x. [DOI] [PubMed] [Google Scholar]
- 135.Thijssen VL, Poirier F, Baum LG, Griffioen AW. Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood. 2007;110(8):2819–2827. doi: 10.1182/blood-2007-03-077792. [DOI] [PubMed] [Google Scholar]
- 136.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A. 2006;103(43):15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15(8):3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.D’Haene N, Sauvage S, Maris C, Adanja I, Le Mercier M, Decaestecker C, Baum L, Salmon I. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLoS One. 2013;8(6):e67029. doi: 10.1371/journal.pone.0067029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freitag N, Tirado-Gonzalez I, Barrientos G, Herse F, Thijssen VL, Weedon-Fekjaer SM, Schulz H, Wallukat G, Klapp BF, Nevers T, Sharma S, Staff AC, Dechend R, Blois SM. Interfering with Gal-1-mediated angiogenesis contributes to the pathogenesis of preeclampsia. Proc Natl Acad Sci U S A. 2013;110(28):11451–11456. doi: 10.1073/pnas.1303707110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Delgado VM, Nugnes LG, Colombo LL, Troncoso MF, Fernandez MM, Malchiodi EL, Frahm I, Croci DO, Compagno D, Rabinovich GA, Wolfenstein-Todel C, Elola MT. Modulation of endothelial cell migration and angiogenesis: a novel function for the “tandem-repeat” lectin galectin-8. FASEB J. 2011;25(1):242–254. doi: 10.1096/fj.09-144907. [DOI] [PubMed] [Google Scholar]
- 142.Jouve N, Despoix N, Espeli M, Gauthier L, Cypowyj S, Fallague K, Schiff C, Dignat-George F, Vely F, Leroyer AS. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J Biol Chem. 2013;288(4):2571–2579. doi: 10.1074/jbc.M112.418848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269(33):20807–20810. [PubMed] [Google Scholar]
- 144.Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269(6):4424–4430. [PubMed] [Google Scholar]
- 145.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem. 1995;270(10):5207–5212. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- 146.Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993;268(16):11750–11757. [PubMed] [Google Scholar]
- 147.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572(2-3):263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 148.Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64(18):6363–6367. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 149.Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan R, Park SH, Chun KH. Galectin-3 increases gastric cancer cell motility by upregulating fascin-1 expression. Gastroenterology. 2010;138(3):1035–1045. doi: 10.1053/j.gastro.2009.09.061. e1031-1032. [DOI] [PubMed] [Google Scholar]
- 150.Shalom-Feuerstein R, Cooks T, Raz A, Kloog Y. Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 2005;65(16):7292–7300. doi: 10.1158/0008-5472.CAN-05-0775. [DOI] [PubMed] [Google Scholar]
- 151.Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem. 2002;277(9):6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- 152.Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. J Biol Chem. 2000;275(46):36311–36315. doi: 10.1074/jbc.M003831200. [DOI] [PubMed] [Google Scholar]
- 153.Oka N, Nakahara S, Takenaka Y, Fukumori T, Hogan V, Kanayama HO, Yanagawa T, Raz A. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005;65(17):7546–7553. doi: 10.1158/0008-5472.CAN-05-1197. [DOI] [PubMed] [Google Scholar]
- 154.Lee YJ, Song YK, Song JJ, Siervo-Sassi RR, Kim HR, Li L, Spitz DR, Lokshin A, Kim JH. Reconstitution of galectin-3 alters glutathione content and potentiates TRAIL-induced cytotoxicity by dephosphorylation of Akt. Exp Cell Res. 2003;288(1):21–34. doi: 10.1016/s0014-4827(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 155.Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, Inohara H, Kanayama HO, Kim HR, Raz A. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004;64(10):3376–3379. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- 156.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93(13):6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bernerd F, Sarasin A, Magnaldo T. Galectin-7 overexpression is associated with the apoptotic process in UVB-induced sunburn keratinocytes. Proc Natl Acad Sci U S A. 1999;96(20):11329–11334. doi: 10.1073/pnas.96.20.11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene. 2001;20(51):7486–7493. doi: 10.1038/sj.onc.1204950. [DOI] [PubMed] [Google Scholar]
- 159.Yang RY, Hsu DK, Yu L, Chen HY, Liu FT. Galectin-12 is required for adipogenic signaling and adipocyte differentiation. J Biol Chem. 2004;279(28):29761–29766. doi: 10.1074/jbc.M401303200. [DOI] [PubMed] [Google Scholar]
- 160.Yang RY, Yu L, Graham JL, Hsu DK, Lloyd KC, Havel PJ, Liu FT. Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. Proc Natl Acad Sci U S A. 2011;108(46):18696–18701. doi: 10.1073/pnas.1109065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, Andre S, Le Bivic A, Gabius HJ, Manninen A, Simons K, Huet G. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169(3):491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R. Requirement for galectin-3 in apical protein sorting. Curr Biol. 2006;16(4):408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 163.Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012;482(7385):414–418. doi: 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vyakarnam A, Dagher SF, Wang JL, Patterson RJ. Evidence for a role for galectin-1 in pre-mRNA splicing. Mol Cell Biol. 1997;17(8):4730–4737. doi: 10.1128/mcb.17.8.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vyakarnam A, Lenneman AJ, Lakkides KM, Patterson RJ, Wang JL. A comparative nuclear localization study of galectin-1 with other splicing components. Exp Cell Res. 1998;242(2):419–428. doi: 10.1006/excr.1998.4111. [DOI] [PubMed] [Google Scholar]
- 166.Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001;29(17):3595–3602. doi: 10.1093/nar/29.17.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang W, Park JW, Wang JL, Patterson RJ. Immunoprecipitation of spliceosomal RNAs by antisera to galectin-1 and galectin-3. Nucleic Acids Res. 2006;34(18):5166–5174. doi: 10.1093/nar/gkl673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paron I, Scaloni A, Pines A, Bachi A, Liu FT, Puppin C, Pandolfi M, Ledda L, Di Loreto C, Damante G, Tell G. Nuclear localization of Galectin-3 in transformed thyroid cells: a role in transcriptional regulation. Biochem Biophys Res Commun. 2003;302(3):545–553. doi: 10.1016/s0006-291x(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 169.Nakahara S, Hogan V, Inohara H, Raz A. Importin-mediated nuclear translocation of galectin-3. J Biol Chem. 2006;281(51):39649–39659. doi: 10.1074/jbc.M608069200. [DOI] [PubMed] [Google Scholar]
- 170.Nakahara S, Oka N, Wang Y, Hogan V, Inohara H, Raz A. Characterization of the nuclear import pathways of galectin-3. Cancer Res. 2006;66(20):9995–10006. doi: 10.1158/0008-5472.CAN-06-1772. [DOI] [PubMed] [Google Scholar]
- 171.Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, Raz A. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol Cell Biol. 2004;24(10):4395–4406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ueda S, Kuwabara I, Liu FT. Suppression of tumor growth by galectin-7 gene transfer. Cancer Res. 2004;64(16):5672–5676. doi: 10.1158/0008-5472.CAN-04-0985. [DOI] [PubMed] [Google Scholar]
- 173.Hsu DK, Liu FT. Regulation of cellular homeostasis by galectins. Glycoconj J. 2004;19(7-9):507–515. doi: 10.1023/B:GLYC.0000014080.95829.52. [DOI] [PubMed] [Google Scholar]
- 174.Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, Hackeng TM, Kiss R, Kloog Y, Poirier F, Griffioen AW. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70(15):6216–6224. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 175.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI, Croci MC, Shipp MA, Mesri EA, Albini A, Rabinovich GA. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med. 2012;209(11):1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004;19(7-9):543–549. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- 177.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP, Stupirski JC, Mazal D, Osinaga E, Toscano MA, Sundblad V, Rabinovich GA, Salatino M. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73(3):1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- 179.Soldati R, Berger E, Zenclussen AC, Jorch G, Lode HN, Salatino M, Rabinovich GA, Fest S. Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int J Cancer. 2012;131(5):1131–1141. doi: 10.1002/ijc.26498. [DOI] [PubMed] [Google Scholar]
- 180.Ouyang J, Plutschow A, Pogge von Strandmann E, Reiners KS, Ponader S, Rabinovich GA, Neuberg D, Engert A, Shipp MA. Galectin-1 serum levels reflect tumor burden and adverse clinical features in classical Hodgkin lymphoma. Blood. 2013;121(17):3431–3433. doi: 10.1182/blood-2012-12-474569. [DOI] [PubMed] [Google Scholar]
- 181.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76(4):597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 182.Levi G, Teichberg VI. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981;256(11):5735–5740. [PubMed] [Google Scholar]
- 183.Tracey BM, Feizi T, Abbott WM, Carruthers RA, Green BN, Lawson AM. Subunit molecular mass assignment of 14,654 Da to the soluble beta-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J Biol Chem. 1992;267(15):10342–10347. [PubMed] [Google Scholar]
- 184.Hirabayashi J, Kasai K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J Biol Chem. 1991;266(35):23648–23653. [PubMed] [Google Scholar]
- 185.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J Biol Chem. 1995;270(10):5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- 186.Pande AH, Gupta RK, Sumati HK. Oxidation of goat hepatic galectin-1 induces change in secondary structure. Protein Pept Lett. 2003;10(3):265–275. doi: 10.2174/0929866033478960. [DOI] [PubMed] [Google Scholar]
- 187.Clerch LB, Whitney P, Hass M, Brew K, Miller T, Werner R, Massaro D. Sequence of a full-length cDNA for rat lung beta-galactoside-binding protein: primary and secondary structure of the lectin. Biochemistry. 1988;27(2):692–699. doi: 10.1021/bi00402a030. [DOI] [PubMed] [Google Scholar]
- 188.Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, Karmakar S, Mehta P, Dias-Baruffi M, McEver RP, Cummings RD. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem. 2009;284(8):4989–4999. doi: 10.1074/jbc.M808925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Inagaki Y, Sohma Y, Horie H, Nozawa R, Kadoya T. Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur J Biochem. 2000;267(10):2955–2964. doi: 10.1046/j.1432-1033.2000.01311.x. [DOI] [PubMed] [Google Scholar]
- 190.Kadoya T, Oyanagi K, Kawakami E, Hasegawa M, Inagaki Y, Sohma Y, Horie H. Oxidized galectin-1 advances the functional recovery after peripheral nerve injury. Neurosci Lett. 2005;380(3):284–288. doi: 10.1016/j.neulet.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 191.Jaffe EK. Morpheeins-a new structural paradigm for allosteric regulation. Trends Biochem Sci. 2005;30(9):490–497. doi: 10.1016/j.tibs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 192.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and −9. Biochemistry. 1994;33(47):14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 193.Guevremont M, Martel-Pelletier J, Boileau C, Liu FT, Richard M, Fernandes JC, Pelletier JP, Reboul P. Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann Rheum Dis. 2004;63(6):636–643. doi: 10.1136/ard.2003.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12(5):616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 195.Nishi N, Shoji H, Seki M, Itoh A, Miyanaka H, Yuube K, Hirashima M, Nakamura T. Galectin-8 modulates neutrophil function via interaction with integrin alphaM. Glycobiology. 2003;13(11):755–763. doi: 10.1093/glycob/cwg102. [DOI] [PubMed] [Google Scholar]
- 196.Nishi N, Itoh A, Shoji H, Miyanaka H, Nakamura T. Galectin-8 and galectin-9 are novel substrates for thrombin. Glycobiology. 2006;16(11):15C–20C. doi: 10.1093/glycob/cwl028. [DOI] [PubMed] [Google Scholar]
- 197.Merkle RK, Cummings RD. Asparagine-linked oligosaccharides containing poly-N-acetyllactosamine chains are preferentially bound by immobilized calf heart agglutinin. J Biol Chem. 1988;263(31):16143–16149. [PubMed] [Google Scholar]
- 198.Brewer CF. Thermodynamic binding studies of galectin-1, −3 and −7. Glycoconj J. 2004;19(7-9):459–465. doi: 10.1023/B:GLYC.0000014075.62724.d0. [DOI] [PubMed] [Google Scholar]
- 199.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, Kasai K. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572(2-3):232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 200.Dam TK, Gabius HJ, Andre S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44(37):12564–12571. doi: 10.1021/bi051144z. [DOI] [PubMed] [Google Scholar]
- 201.Sorme P, Kahl-Knutsson B, Huflejt M, Nilsson UJ, Leffler H. Fluorescence polarization as an analytical tool to evaluate galectin-ligand interactions. Anal Biochem. 2004;334(1):36–47. doi: 10.1016/j.ab.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 202.Lepur A, Salomonsson E, Nilsson UJ, Leffler H. Ligand induced galectin-3 protein self-association. J Biol Chem. 2012;287(26):21751–21756. doi: 10.1074/jbc.C112.358002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectins-1, −2 and −3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric poly-saccharides. Glycobiology. 2004;14(2):157–167. doi: 10.1093/glycob/cwh018. [DOI] [PubMed] [Google Scholar]
- 205.Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric Galectin-1 Binds with High Affinity to {alpha}2,3-Sialylated and Non-sialylated Terminal N-Acetyllactosamine Units on Surface-bound Extended Glycans. J Biol Chem. 2005;280(7):5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- 206.Zhuo Y, Chammas R, Bellis SL. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem. 2008;283(32):22177–22185. doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Carlsson S, Oberg CT, Carlsson MC, Sundin A, Nilsson UJ, Smith D, Cummings RD, Almkvist J, Karlsson A, Leffler H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology. 2007;17(6):663–676. doi: 10.1093/glycob/cwm026. [DOI] [PubMed] [Google Scholar]
- 208.Miyanishi N, Nishi N, Abe H, Kashio Y, Shinonaga R, Nakakita S, Sumiyoshi W, Yamauchi A, Nakamura T, Hirashima M, Hirabayashi J. Carbohydrate-recognition domains of galectin-9 are involved in intermolecular interaction with galectin-9 itself and other members of the galectin family. Glycobiology. 2007;17(4):423–432. doi: 10.1093/glycob/cwm001. [DOI] [PubMed] [Google Scholar]
- 209.Poland PA, Rondanino C, Kinlough CL, Heimburg-Molinaro J, Arthur CM, Stowell SR, Smith DF, Hughey RP. Identification and characterization of endogenous galectins expressed in Madin Darby canine kidney cells. J Biol Chem. 2011;286(8):6780–6790. doi: 10.1074/jbc.M110.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105(28):9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Carlsson S, Carlsson MC, Leffler H. Intracellular sorting of galectin-8 based on carbohydrate fine specificity. Glycobiology. 2007;17(9):906–912. doi: 10.1093/glycob/cwm059. [DOI] [PubMed] [Google Scholar]
- 212.Kopitz J, von Reitzenstein C, Burchert M, Cantz M, Gabius HJ. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth-controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J Biol Chem. 1998;273(18):11205–11211. doi: 10.1074/jbc.273.18.11205. [DOI] [PubMed] [Google Scholar]
- 213.Hullin-Matsuda F, Kobayashi T. Monitoring the distribution and dynamics of signaling microdomains in living cells with lipid-specific probes. Cell Mol Life Sci. 2007;64(19-20):2492–2504. doi: 10.1007/s00018-007-7281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kopitz J, Ballikaya S, Andre S, Gabius HJ. Ganglioside GM1/galectin-dependent growth regulation in human neuroblastoma cells: special properties of bivalent galectin-4 and significance of linker length for ligand selection. Neurochem Res. 2012;37(6):1267–1276. doi: 10.1007/s11064-011-0693-x. [DOI] [PubMed] [Google Scholar]
- 215.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230(1):172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 216.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68(2):688–693. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stowell SR, Winkler AM, Maier CL, Arthur CM, Smith NH, Girard-Pierce KR, Cummings RD, Zimring JC, Hendrickson JE. Initiation and regulation of complement during hemolytic transfusion reactions. Clin Dev Immunol. 2012;2012:307093. doi: 10.1155/2012/307093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Debierre-Grockiego F, Niehus S, Coddeville B, Elass E, Poirier F, Weingart R, Schmidt RR, Mazurier J, Guerardel Y, Schwarz RT. Binding of Toxoplasma gondii glycosylphosphatidylinositols to galectin-3 is required for their recognition by macrophages. J Biol Chem. 2010;285(43):32744–32750. doi: 10.1074/jbc.M110.137588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.van den Berg TK, Honing H, Franke N, van Remoortere A, Schiphorst WE, Liu FT, Deelder AM, Cummings RD, Hokke CH, van Die I. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J Immunol. 2004;173(3):1902–1907. doi: 10.4049/jimmunol.173.3.1902. [DOI] [PubMed] [Google Scholar]
- 220.Moody TN, Ochieng J, Villalta F. Novel mechanism that Trypanosoma cruzi uses to adhere to the extracellular matrix mediated by human galectin-3. FEBS Lett. 2000;470(3):305–308. doi: 10.1016/s0014-5793(00)01347-8. [DOI] [PubMed] [Google Scholar]
- 221.Kleshchenko YY, Moody TN, Furtak VA, Ochieng J, Lima MF, Villalta F. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect Immun. 2004;72(11):6717–6721. doi: 10.1128/IAI.72.11.6717-6721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Quattroni P, Li Y, Lucchesi D, Lucas S, Hood DW, Herrmann M, Gabius HJ, Tang CM, Exley RM. Galectin-3 binds Neisseria meningitidis and increases interaction with phagocytic cells. Cell Microbiol. 2012;14(11):1657–1675. doi: 10.1111/j.1462-5822.2012.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Levroney EL, Aguilar HC, Fulcher JA, Kohatsu L, Pace KE, Pang M, Gurney KB, Baum LG, Lee B. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J Immunol. 2005;175(1):413–420. doi: 10.4049/jimmunol.175.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pelletier I, Hashidate T, Urashima T, Nishi N, Nakamura T, Futai M, Arata Y, Kasai K, Hirashima M, Hirabayashi J, Sato S. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J Biol Chem. 2003;278(25):22223–22230. doi: 10.1074/jbc.M302693200. [DOI] [PubMed] [Google Scholar]
- 225.Yang ML, Chen YH, Wang SW, Huang YJ, Leu CH, Yeh NC, Chu CY, Lin CC, Shieh GS, Chen YL, Wang JR, Wang CH, Wu CL, Shiau AL. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. J Virol. 2011;85(19):10010–10020. doi: 10.1128/JVI.00301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology. 2008;371(1):121–129. doi: 10.1016/j.virol.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 227.Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, Sato S, Tremblay MJ. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol. 2005;174(7):4120–4126. doi: 10.4049/jimmunol.174.7.4120. [DOI] [PubMed] [Google Scholar]
- 228.Arthur CM, Cummings RD, Stowell SR. Using glycan microarrays to understand immunity. Curr Opin Chem Biol. 2014;18:55–61. doi: 10.1016/j.cbpa.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stowell SR, Arthur CM, McBride R, Berger O, Razi N, Heimburg-Molinaro J, Rodrigues JP, Noll AJ, von Gunten S, Smith DF, Knirel YA, Paulson JC, Cummings RD. Microbial glycan microarrays define key features of host-microbial interactions. Nat Chem Biol. 2014;10(6):470–476. doi: 10.1038/nchembio.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.St-Pierre C, Manya H, Ouellet M, Clark GF, Endo T, Tremblay MJ, Sato S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J Virol. 2011;85(22):11742–11751. doi: 10.1128/JVI.05351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol. 2008;10(10):2078–2090. doi: 10.1111/j.1462-5822.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite survival. Cell. 2004;119(3):329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]