Abstract
Background
The Intratumoral Microvessel Density (IMVD) is commonly used to quantify tumoral vascularization and is usually assessed by pan-endothelial markers, such as CD31. Endoglin (CD105) is a protein predominantly expressed in proliferating endothelium and the IMVD determined by this marker measures specifically the neovascularization. In this study, we investigated the CD105 expression in pediatric rhabdomyosarcoma and assessed the neovascularization by using the angiogenic ratio IMVD-CD105 to IMVD-CD31.
Methods
Paraffin-embedded archival tumor specimens were selected from 65 pediatric patients affected by rhabdomyosarcoma. The expression levels of CD105, CD31 and Vascular Endothelial Growth Factor (VEGF) were investigated in 30 cases (18 embryonal and 12 alveolar) available for this study. The IMVD-CD105 to IMVD-CD31 expression ratio was correlated with clinical and pathologic features of these patients.
Results
We found a specific expression of endoglin (CD105) in endothelial cells of all the rhabdomyosarcoma specimens analyzed. We observed a significant positive correlation between the IMVD individually measured by CD105 and CD31. The CD105/CD31 expression ratio was significantly higher in patients with lower survival and embryonal histology. Indeed, patients with a CD105/CD31 expression ratio < 1.3 had a significantly increased OS (88%, 95%CI, 60%–97%) compared to patients with higher values (40%, 95%CI, 12%–67%). We did not find any statistical correlation among VEGF and EFS, OS and CD105/CD31 expression ratio.
Conclusion
CD105 is expressed on endothelial cells of rhabdomyosarcoma and represent a useful tool to quantify neovascularization in this tumor. If confirmed by further studies, these results will indicate that CD105 is a potential target for combined therapies in rhabdomyosarcoma.
Electronic supplementary material
The online version of this article (10.1186/s12885-017-3947-4) contains supplementary material, which is available to authorized users.
Keywords: Rhabdomyosarcoma, Endoglin (CD105), CD105/CD31 ratio, Prognostic marker
Background
Rhabdomyosarcoma (RMS) is the most common type of soft tissue sarcoma (STS) in children and young adults, accounting for up to 5% of all childhood cancers and for about 40% of pediatric STS [1]. Embryonal (ERMS) and alveolar (ARMS) RMS are the two major histologic subtypes. ARMS is associated with PAX3/7-FOXO1 gene fusions and with a poor prognosis, often being metastatic at diagnosis [2]. Although during the last three decades, multimodal treatment strategies have substantially improved the prognosis of localized RMS, for metastatic disease the prognosis remains dismal [3]. Therefore, new targets and tailored therapies directed against the metastatic process are needed for these patients. The formation of new blood vessels is a requirement for tumor growth and metastatic spread and many regulators of tumor angiogenesis have been identified in different types of cancer [4]. Studies on inhibitors of angiogenesis have shown antitumor activity in pediatric sarcoma models, including RMS, mostly in combination with other drugs [5–7], and several trials showed promising results for selected clinical indications [8–10]. The quantification of tumor vasculature is a useful indicator of angiogenesis, by helping patients stratification prior to anti-angiogenic therapy and monitoring patient response. One often-quantified aspect of tumor vasculature is the Intratumoral Microvessel Density (IMVD). IMVD is commonly used as a surrogate marker to quantify angiogenic activity and is usually assessed by pan-endothelial markers, such as CD34 and CD31 [10–13]. However, these markers are not tumor endothelial-specific, as they are also expressed on pre-existing/mature vasculature and on large vessels [14, 15]. Recent studies have shown that IMVD assessed by detection of Endoglin (CD105) is more specifically associated with tumor neovascularization [16–20] and represents a significant prognostic marker in several tumors [19–24]. CD105 is a transforming growth factor β (TGF-β) transmembrane co-receptor required for angiogenesis [25] and is highly expressed on the surface of actively proliferating microvascular endothelial cells, forming immature, highly permeable tumor neovessels [26]. In line with its supportive role in tumor neoangiogenesis, CD105 is up-regulated by hypoxia [27–29]. The expression of CD105 has been reported on the tumor vasculature of several sarcomas, including Kaposi sarcoma, angiosarcoma, leiomyosarcoma, chondrosarcoma and gastrointestinal stromal tumor and correlated with worse survival for some of these tumors [21, 30–33]. In this study, we aimed to investigate if CD105 was expressed in pediatric RMS and assess the neovascularization by using the angiogenic ratio IMVD-CD105 to IMVD-CD31. For this purpose, we evaluated the immunohistochemical expression of CD105, CD31 and VEGF in a retrospective series of pediatric patients with RMS. In order to define the proliferation fraction of the endothelium we compared the CD105 microvessels count with CD31 immunoexpression obtaining the CD105/CD31 expression ratio. In the cases where the CD105/CD31 expression ratio is higher, the angiogenesis is increased because CD105 marks the neoformed vessels [26] whereas CD31 is also expressed in mature vessels [18]. This ratio has been reported to have a prognostic value and be a potential predictor of response to anti-VEGF therapy [34–36].
Methods
Study population
Tumor tissue specimens from 65 patients with RMS who underwent surgical resection or biopsy of their primary tumor at the Bambino Gesù Children’s Hospital from 2005 to 2016 were retrospectively reviewed. Among these, we selected 30 appropriate paraffin embedded tissue blocks. The criteria for selecting the patients were based on the availability of an adequate tumor specimens obtained before any treatment and detailed clinical information. Patients’ clinical details, information on therapy and follow-up were collected retrospectively from the medical files. The median age at diagnosis was 48.5 months (range 1–199) with a sex ratio of 1. The most frequent primary site was head and neck (6 parameningeal and 2 non parameningeal patients respectively), followed by orbit (5 patients), pelvis (4 patients), genitourinary non-bladder or prostate (3 patients), extremity (2 patients), genitourinary bladder or prostate (1 patient), and other localizations (7 patients). This series include 18 patients with ERMS and 12 with ARMS. The study was approved according to local institutional guidelines.
Patient variables analyzed
Patient- and tumor-related prognostic factors considered were: age at diagnosis (favorable if ≥ 12 or < 120 months and unfavorable if <12 or ≥ 120 months), primary tumor size (≤ 5 cm versus > 5 cm), tumor site favorable (orbit, genitourinary non bladder/prostate, head and neck non parameningeal) and unfavorable (parameningeal, extremities, genitourinary bladder-prostate and “other site”), histology (embryonal versus alveolar) and COG risk stratification [37].
Immunohistochemistry methods
The tissues were fixed with 10% formalin and embedded in paraffin. Consecutive 2.5 μm-thick serial sections were cut, deparaffinised in xylene, rehydrated and washed using double distilled water. These sections were used for immunohistochemical staining for CD105, VEGF and CD31 and human tonsils were used as positive controls for CD105, CD31 and VEGF. For staining with VEGF and CD31, sections were pretreated with DAKO PT link (PT200) in low pH solution (cod. K8005 DAKO North America, CA) for antigen retrieval. As for CD105, sections were pretreated with Proteinase K (cod. S3020 DAKO North America, CA) for 10 min at room temperature. The immunostaining was done at 4 °C overnight using the following monoclonal mouse anti-human antibodies as primary antibody: anti-CD105 (clone SN6h, 1:10, DAKO North America, CA), anti-VEGF (MS-1467-P, 1:200, Thermo Fisher, Fremont, CA), anti-CD31 (IR610, Ready-to-Use, DAKO North America, CA). As the secondary antibody, we used En Vision Flex/HRP (cod. K8024, Ready-to-Use, DAKO North America, CA). The sections were then reacted in chromogen 3,3’-diaminobenzidine to detect the peroxidase activity, counterstained with hematoxylin and mounted.
Measurement of IMVD
Hematoxylin-Eosin staining has been used by an experienced pathologist (RB) in order to select the area of the tumor, the necrotic areas were excluded. The sections were examined using a double-headed light microscope (Leica DM4 B) by two independent operators (RB and VDP), who were not aware of the clinical status of the patients. IMVD was assessed by immunostaining for either CD31 (IMVD-CD31) or CD105 (IMVD-CD105) according to the procedure described by Weidner et al., [11, 38]. The most vascularized area (hot-spots) was identified at low magnification (40X) and then three fields were counted at high magnification (20X). We considered as a countable single microvessel any endothelial cell or endothelial-cell cluster stained and clearly separated from the adjacent microvessels, tumor cells and other connective-tissue elements. The mean of the vessels in three fields was used as CD105 IMVD or CD31 IMVD. CD105 IMVD and CD31 IMVD have been evaluated in two different serial slides, within the same “hot spot” area. In order to define the proliferation fraction of the endothelium, we calculated the CD105/CD31 ratio dividing the IMVD of CD105 by the IMVD of CD31, as previously described [34–36]. Indeed, since CD31 is a pan-endothelial marker and CD105 is primarily expressed by proliferating endothelial cells, this ratio specifically measures the fraction of proliferating endothelial cells.
Evaluation of VEGF
The VEGF expression was estimated according to the percentage of immunoreactive cells in a total of 1000 cells. The tumors were classified into 4 categories based on VEGF staining: negative (0), weak (1+), moderate (2+) and strong (3+). The percentage of positive cells was defined as sporadic (positive cells ≤ 1% and < 10%), focal (positive cells ≤ 10% and < 50%) or diffused (positive cells ≥ 50%). The immuno-histochemical scores were recorded as score 0 (no immunoreactivity), score 1 (1+ with sporadic or focal distribution), score 2 (1+ with diffused distribution or 2+ or 3+ with sporadic distribution), score 3 (2+ with focal or diffused distribution), score 4 (3+ with focal or diffused distribution) [39].
Statistical analysis
Categorical data was represented as counts and proportions, and continuous data as mean and standard deviation or median and range. We analyzed the overall survival (OS) and event-free survival (EFS) defined as the time from diagnosis until the date of death and the date of disease relapse/progression, respectively. The follow-up period was calculated from the date of diagnosis until the last follow-up visit. Correlation between CD105 and CD31 IMVD was examined using the Spearman’s Rho. The ROC (Receiving operation curve) analysis was used in order to find an appropriate cut-off of CD105/CD31 ratio discriminating between death and survival, and event (disease relapse/progression) and non event in terms of sensibility and specificity.
Univariable analysis of time to event data (OS and EFS) was performed through the Kaplan Meier method, Log-rank test and Cox proportional hazard model. Relationships between the CD105/CD31 ratio and clinico-pathological data were assessed using univariable quantile regression analysis. P values less than 0.05 were considered to be statistically significant. Data was analyzed using the STATA software version 13.1.
Results
Clinico-pathological features of RMS patients
Patients’ characteristics are detailed in Table 1.
Table 1.
Characteristics of the 30 patients with Rhabdomyosarcoma
| Pt | Age (mos) | Gender | Histology | Primary site | Primary size (cm) | Metastasis | Nodes | IRSa Group |
COG Risk Groups | EpSSG Risk Groups |
Status | CD105/CD31 ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | M | ARMS | Extremity | > 5 | No | N0 | III | Intermediate | HR/G | NED | 1.1611 |
| 2 | 65 | F | ERMS | Pelvis | > 5 | No | N1 | III | Intermediate | HR/F | NED | 0.6825 |
| 3 | 43 | M | ERMS | Abdomen | > 5 | No | N0 | III | Intermediate | HR/E | NED | 0.7466 |
| 4 | 82 | F | ARMS | Extremity | > 5 | No | N0 | III | Intermediate | HR/G | NED | 1.0460 |
| 5 | 55 | M | ERMS | GU non BP | ≤ 5 | No | N0 | I | Low | LR | NED | 1.4292 |
| 6 | 41 | M | ERMS | Orbit | > 5 | Bone/BM | N0 | IV | High | META | DOD | 0.5133 |
| 7 | 28 | F | ARMS | HN PM | ≤ 5 | No | N0 | II | Intermediate | HR/G | NED | 1.4225 |
| 8 | 13 | M | ARMS | Orbit | ≤ 5 | No | N0 | III | Intermediate | HR/G | NED | 1.7949 |
| 9 | 11 | F | ERMS | HN PM | ≤ 5 | No | N1 | III | Intermediate | HR/F | DOD | 1.3006 |
| 10 | 37 | F | ERMS | GU non BP | ≤ 5 | No | N0 | III | Low | SR/C | NED | 0.8942 |
| 11 | 116 | F | ERMS | Pelvis | > 5 | Lung | Nx | IV | High | META | DOD | 1.0158 |
| 12 | 176 | M | ARMS | Retroperitoneum | > 5 | Lung | N1 | IV | High | META | DOD | 1.5517 |
| 13 | 48 | F | ERMS | GU BP | > 5 | No | N0 | III | Intermediate | HR/E | NED | 1.8627 |
| 14 | 49 | F | ARMS | HN PM | > 5 | No | N1 | III | Intermediate | VERY HR | NED | 1.2461 |
| 15 | 17 | M | ARMS | HN non PM | ≤ 5 | No | N0 | II | Intermediate | HR/G | NED | 1.0354 |
| 16 | 1 | M | ERMS | Pelvis | > 5 | No | N0 | II | Low | HR/E | NED | 0.8868 |
| 17 | 1 | F | ARMS | HN PM | ≤ 5 | Multipleb | N1 | IV | High | META | DOD | 1.5608 |
| 18 | 16 | F | ARMS | Chest wall | ≤ 5 | No | N0 | II | Intermediate | HR/G | NED | 0.8651 |
| 19 | 24 | M | ARMS | Orbit | ≤ 5 | No | N0 | III | Intermediate | HR/G | DOD | 2.0024 |
| 20 | 135 | M | ARMS | Chest wall | > 5 | No | N1 | III | Intermediate | VERY HR | DOD | 1.9190 |
| 21 | 26 | F | ERMS | Biliary tracts | ≤ 5 | No | N0 | III | Low | SR/D | NED | 1.1058 |
| 22 | 97 | M | ERMS | Orbit | > 5 | No | N0 | III | Low | SR/C | NED | 1.0274 |
| 23 | 154 | M | ERMS | Orbit | > 5 | No | N0 | II | Low | SR/C | AWD | 0.9020 |
| 24 | 14 | F | ERMS | Abdomen | > 5 | No | N1 | I | Low | HR/F | DOD | 2.3542 |
| 25 | 23 | F | ERMS | Pelvis | > 5 | Lung/Bone | N1 | IV | High | META | NED | 0.7117 |
| 26 | 199 | F | ERMS | HN PM | > 5 | No | N0 | III | Intermediate | HR/E | AWD | 0.9333 |
| 27 | 176 | M | ARMS | GU non BP | ≤ 5 | Bone | N1 | IV | High | VERY HR | NED | 0.5914 |
| 28 | 106 | F | ERMS | HN PM | > 5 | No | N1 | III | Intermediate | HR/F | AWD | 1.0217 |
| 29 | 146 | M | ERMS | HN non PM | > 5 | No | N1 | III | Low | HR/F | AWD | 0.7733 |
| 30 | 189 | M | ERMS | Extremity | > 5 | No | N0 | III | Intermediate | HR/E | AWD | 0.3281 |
(Pt patient, mos months, M male, F female, ARMS alveolar rhabdomyosarcoma, ERMS embryonal rhabdomyosarcoma, GU non BP genitourinary non-bladder or prostate, HN non PM head and neck non-parameningeal, GU BP genitourinary bladder or prostate, HN PM Head and neck parameningeal, BM bone-marrow, N0 no clinical or pathological node involvement, N1 clinical or pathological nodal involvement, NX No information on lymph node involvement, HR High Risk, META metastatic, LR Low Risk, SR Standard Risk, NED no evidence of disease, DOD died of disease, AWD alive with disease, a: post-surgical stage according to Intergroup Rhabdomyosarcoma Study (IRS) grouping system41; b: subcutaneous, liver, pancreas, lung, bone-marrow and paravertebral lesion at L2-L3 level; COG, Children’s Oncology Group)
Patients were staged according to COG-STS risk stratification [37]. PAX3/PAX7-FKHR fusion gene transcripts were evaluated in 9 cases of 12 ARMS and 9 cases of 18 ERMS. PAX3-FKHR fusion gene was positive in 8 ARMS cases, and PAX7-FKHR in 1 ARMS case. None of PAX3/PAX7-FKHR fusion gene was detected in the ERMS examined. The median follow-up of patients was 5 years (range 0.28–11.12 years). Eight patients died of disease (median time from diagnosis 16.5 months, range 5–64). Patients #6, #17 and #24 presented a short follow-up since they died after 5, 7 and 10 months respectively due to rapid disease progression. The immunostaining was performed on pretreatment tumor biopsy specimens. The expression of CD31 and CD105 was localized in endothelial cells in all the specimens analyzed and not expressed by tumor cells. In the tumor CD105 was specifically associated with immature vessels which showed a stronger positivity compared to the large vessels (Additional file 1: Figure S1). VEGF expression was detected mainly in the cytoplasm of the tumor cells or endothelium (Fig. 1). Nineteen tumors (63.3%) showed a VEGF staining score of 1–2, while 11 (36.7%) showed a score of 3–4.
Fig. 1.
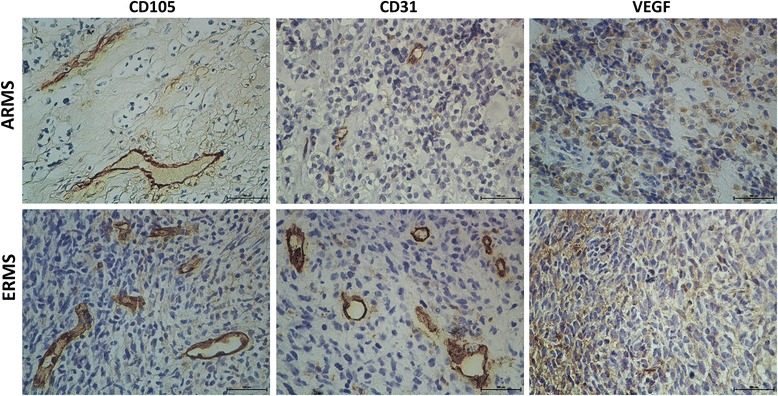
Representative immunostaining for CD105, CD31and VEGF of ARMS and ERMS. Magnification × 200
The ratio of IMVD-CD105 to IMVD-CD31 in RMS primary samples
Analysis of CD105 and CD31 expression demonstrated that the average of CD105-IMVD was not statistically, significantly higher than CD31-IMVD in RMS tissue (P = 0.122 Wilcoxon signed-rank test). We observed a statistically significant positive correlation between the IMVD individually measured by the two markers (Spearman’s rho = 0.86, P = 0.05), (Fig. 2). CD105/CD31 expression ratio in the tumor specimens ranged from 0.32% to 2.35%, with a median value of 1% and a mean of 1.15% (Table 1). The ROC curve analysis was used to determine the optimal cut-off of CD105/CD31 ratio (Fig. 3). EFS showed a cut-off point value of 0.9 with a 90.9% sensitivity and 52.6% specificity (Fig. 3a). OS had a cut-off point value of 1.3 with a 71.4% sensitivity and 78.2% specificity (Fig. 3b). Our analysis demonstrated that ten patients with a CD105/CD31 expression ratio equal or higher than 0.9 (50% of patients in this group) had relapse or disease progression. Only one patient (#6) with a ratio lower than 0.9 (10%) experienced disease progression, but had metastatic disease, which is per se a poor prognostic factor. These results suggest that the CD105/CD31 expression ratio in the primary tumor could be associated with disease aggressiveness.
Fig. 2.
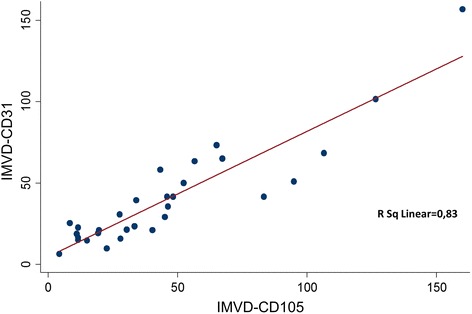
Correlation between CD105-IMVD and CD31-IMVD in rhabdomyosarcoma tumor samples (R2 = 0.83, P < 0.001)
Fig. 3.
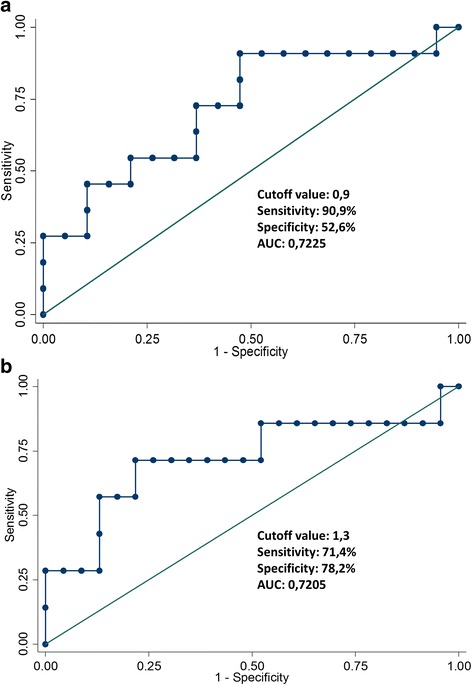
ROC analysis of CD31/CD105 ratio regarding Event-Free survival (a) and Overall survival (b)
Correlation between prognostic factors and outcome in RMS patients
We then investigated the relationship amongst EFS or OS, selected prognostic clinico-pathological parameters (age at diagnosis, tumor size, primary site, histology, COG risk stratification) and the angiogenic CD105/CD31 ratio. As summarized in Table 2, the EFS and OS of patients with high risk RMS, according to COG stratification, resulted dismal, as it was previously reported [37].
Table 2.
Univariable Cox proportional hazards regression for Event Free Survival and Overall Survival
| Variables | EFS | OS | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | IC (95%) | P | Hazard ratio | IC (95%) | P | |
| Age at diagnosis | ||||||
| ≥ 1 < 10 (ref) | – | – | – | – | – | – |
| < 1 ≥ 10 | 2.58 | 0.78;8.53 | 0.121 | 3.02 | 0.74; 12.25 | 0.121 |
| Histology | ||||||
| ARMS (ref) | – | – | – | – | – | – |
| ERMS | 0.63 | 0.19; 2.06 | 0.443 | 0.86 | 0.21; 3.46 | 0.831 |
| Tumor size | ||||||
| ≤ 5 cm (ref) | – | – | – | – | – | – |
| > 5 cm | 1.66 | 0.44;6.28 | 0.453 | 2.17 | 0.43; 10.87 | 0.344 |
| Primary site (location) | ||||||
| Favorable (ref) | – | – | – | – | – | – |
| Unfavorable | 1.11 | 0.32;3.80 | 0.867 | 1.14 | 0.27; 4.80 | 0.851 |
| COG Group | ||||||
| Low (ref) | – | – | – | – | – | – |
| Intermediate | 2.93 | 0.35;24.41 | 0.319 | 1.13 | 011; 10.98 | 0.914 |
| High | 9.76 | 1.04;91.19 | 0.046 | 11.59 | 1.19; 112.25 | 0.034 |
| VEGF score | ||||||
| 1–2 (ref) | – | – | – | – | – | – |
| 3–4 | 0.54 | 0.14;2.06 | 0.373 | 0.46 | 0.93; 2.31 | 0.349 |
| CD105/CD31 Ratio | ||||||
| < 0.9 (ref) | – | – | – | – | – | – |
| ≥ 0.9 | 5.31 | 0.75;46.25 | 0.090 | – | – | – |
| CD105/CD31 Ratio | ||||||
| < 1.3 (ref) | – | – | – | – | – | – |
| ≥ 1.3 | – | – | – | 5.89 | 1.18;29.2 | 0.030 |
(Ref Reference, IC interval confidence, COG Children’s Oncology Group, ERMS embryonal rhabdomyosarcoma, ARMS alveolar rhabdomyosarcoma, VEGF Vascular Endothelial Growth Factor, CD105 Endoglin). In boldface the values statistically significant
Furthermore, in the univariable Cox proportional hazard regression the CD105/CD31 expression ratio resulted to be related with decreased OS (P = 0.03) [38].
Relationship of CD105/CD31 expression ratio with clinico-pathological characteristics and outcome
Based on ROC curves cut-off values, Kaplan-Meier analysis showed that patients with a value of the CD105/CD31 expression ratio < 1.3 had a significantly increased OS (88%, 95%CI = 60%–97%) compared to patients with higher values (40%, 95%CI = 12%–67%; P = 0.013 by the log-rank test), (Fig. 4b). The estimated 5-year EFS was 91% (95%CI = 51%–98%) for patients with a CD105/CD31 ratio lower than 0.9 compared with 45% (95%CI = 22%–65%) for those with a ratio higher or equal to 0.9 (P = 0.054 by the log-rank test) (Fig. 4a). We further evaluated VEGF expression in order to correlate this marker, which is upregulated in RMS [39–43], with the neo-angiogenic ratio. Determinants of CD105/CD31 expression ratio were assessed by univariable quantile regression analysis (Table 3). This ratio was significantly associated with the patients’ survival (P = 0.016) and the embryonal histology (P = 0.019).
Fig. 4.
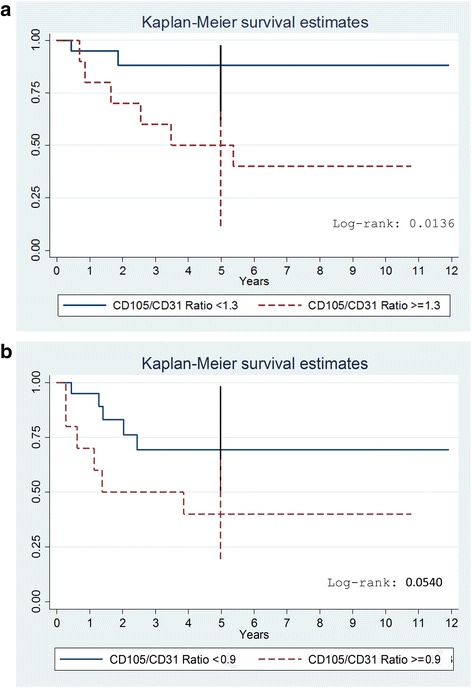
Kaplan-Meier curve for Event-Free survival (a) and Overall survival (b) according to CD105/CD31 ratio groups
Table 3.
Factors associated with CD105/CD31 expression ratio – univariable analysis
| Variables | Number of patients (%) | Univariable Analysis | ||
|---|---|---|---|---|
| Coefficient | IC | P value | ||
| ≥ 1 < 10 (ref) | 21 (70) | – | – | – |
| < 1 ≥ 10 | 9 (30) | − 0.10 | − 0.77;0.56 | 0.757 |
| Histology | ||||
| ARMS (ref) | 12 (40) | – | – | – |
| ERMS | 18 (60) | − 0.49 | − 0.89;-0.08 | 0.019 |
| Tumor size | ||||
| ≤ 5 cm (ref) | 10 (33) | – | – | – |
| > 5 cm | 20 (77) | − 0.28 | − 0.83;0.27 | 0.313 |
| Primary site (location) | ||||
| Favorable (ref) | 12 (40) | – | – | – |
| Unfavorable | 18 (60) | 0.01 | − 0.45;0.47 | 0.962 |
| COG Risk Group | ||||
| Low (ref) | 8 (27) | – | – | – |
| Intermediate | 16 (53) | 0.13 | − 0.53;0.79 | 0.682 |
| High | 6 (20) | − 0.01 | − 0.84;0.81 | 0.977 |
| VEGF score | ||||
| 1–2 (ref) | 19 (63) | – | – | – |
| 3–4 | 11 (37) | 0.41 | − 0.04;0.85 | 0.072 |
| Status | ||||
| Alive (ref) | 22 (73) | – | – | – |
| Dead | 8 (27) | 0.53 | 0.10; 0.95 |
0.016 |
(Ref Reference, IC interval confidence, COG Children’s Oncology Group, ERMS embryonal rhabdomyosarcoma, ARMS alveolar rhabdomyosarcoma, VEGF Vascular Endothelial Growth Factor, CD105 Endoglin). In boldface the values statistically significant
Discussion
Neo-angiogenesis has long been implicated in generating a microenvironment suitable for tumor growth and metastatic spread [44]. Several pro-angiogenic factors have been described and among them VEGF appears to play a central role in the activation of angiogenesis in various cancer [45, 46]. Several efforts have been made to develop therapies focused on the inhibition of the VEGF signaling pathway also in RMS [47, 48]. However these drugs led to transient responses and the complementary/dual inhibition of non-VEGF angiogenic pathways might represent a way to improve anti-angiogenic therapy. A phase I study using an anti-endoglin monoclonal antibody (TRC105) in combination with bevacizumab in adults with advanced cancers showed good tolerance and clinical activity in a VEGF inhibitor-refractory population [49]. A trial testing TRC105 in combination with pazopanib in patients with STS (≥12 years old) is currently ongoing [50]. In this context, methods which enable to quantify tumor angiogenesis are useful surrogate markers of angiogenic activity and response to therapy, and might help stratify patients with RMS for treatment. The IMVD is the most commonly used parameter to quantify intra-tumoral neovascularization and is measured by pan-endothelial markers, such as CD31. CD105 presents a higher specificity for new developing vessels and recent studies have shown that IMVD as determined by this marker has a higher prognostic impact than CD31 in several tumors [21–23]. In particular, IMVD ratio of CD105/CD31 expression, had been used to specifically assess neovascularization showing a prognostic value [34–36]. In the present study, we analyzed for the first time, the CD105 immunoexpression in pediatric RMS and quantified the presence of proliferating endothelial cells by using the CD105/CD31 expression ratio. CD105 was detected in small tumor capillary-like vessels, whereas CD31 presented a more diffused expression in endothelial cells. The significant positive correlation found between the IMVD measured by the two markers is coherent with the association between CD105 expression and other endothelial markers, such as CD31 and CD34, already described in other tumors [51, 52]. We also evaluated whether a correlation between this neoangiogenic ratio and clinic-pathological variables exists. Several prognostic factors, such as the age at diagnosis, primary tumor size, primary site, histology, post-surgical stage and presence or absence of distant metastases are currently used for risk-adapted treatment approaches in clinical trials of RMS patients [53]. Using these parameters, in the univariable survival analysis, we found that the advanced disease, classified according to the COG risk stratification, was a significant predictor of worse OS and EFS. In line with previously reported works, our study confirms that metastatic disease is the main prognostic factor in RMS [3]. The ROC curve for the OS indicated a cutoff point of 1.3, which was used to separate patients with good and poor prognosis. A value of the CD105/CD31 expression ratio < 1.3 was associated with a significantly better patients’ OS. These findings suggest that neovascularization could be an indicator of prognosis in patients with RMS and are supported by the correlation described between the CD105/CD31 expression ratio and aggressive phenotypes in other tumors [35]. Indeed, it has been already reported that IMVD quantified by CD105 correlate with poor survival in patients with breast carcinoma, non-small cell lung cancer and hepatocellular carcinoma [13, 54, 55]. Interestingly, when the histotype was specifically considered, we found that the ERMS correlated significantly with neo-angiogenesis. Kuda et al., previously described that IMVD, assessed by CD31, was higher in ERMS than ARMS [56]. We speculate that this association could be related to the different growth rate displayed by these two RMS histotypes. Indeed, although angiogenesis is a key process activated during cancer invasion and metastasis, highly aggressive histotypes are also able to support their growth through a process known as vasculogenic mimicry (VM) [57]. The generation of non-endothelialized vessel-like channels allows the perfusion of a variety of tumors, enabling them to aggressively proliferate and metastasize [58]. The VM channels are not lined by endothelial cells, but by tumor cells instead, and therefore are not stained by endothelial markers, including CD31 [59]. A higher incidence of VM has been described in tumors presenting necrosis, as well as in ARMS, and has been associated with poor prognosis [60, 61]. The faster growth of ARMS compared to ERMS may explain the different pattern of neovessels in the two variants. No statistically significant differences in CD105/CD31 expression ratio were encountered with respect to age, tumor size, primary tumor location, COG risk groups and VEGF. Despite VEGF overexpression has been reported to be associated with prognosis in RMS patients [42], data regarding the correlation amongst IMVD, VEGF expression and prognosis has shown conflicting results in several tumors including STS and RMS [39, 56, 62–64].
In conclusion, this small proof-of-concept study suggests that CD105 is expressed in endothelial cells of pediatric RMS and that CD105/CD31 expression ratio might be useful to measure the proportion of proliferating endothelial cells in this tumor. Despite the small cohort of patients studied, these data indicate that a high value of CD105/CD31 expression ratio could be related with a “pro-angiogenic” RMS subset of patients with low OS.
Conclusions
If further studies confirm these results in larger cohorts of patients, CD105 may also represent a potential therapeutic target as part of combined therapy in RMS. In particular, an inter-institutional cooperative study would be advisable considering the low frequency of this tumor in the pediatric population. This type of large study could also be a tool to elucidate if the CD105/CD31 expression ratio may be useful for patient’s stratification and/or evaluate response to therapy.
Acknowledgements
We thank Professor Franco Locatelli for critical reading this paper and for his suggestions. We would also like to thank the children’s parents, who gave their informed consent for publication and “Il cuore grande di Flavio” Onlus. Dr. Marta Colletti is a post-doctoral fellow of the Umberto Veronesi Foundation. To Valentina Polcini for proofreading.
Funding
Not applicable
Availability of data and materials
All data generated and analyzed during this study are included in this published article.
Abbreviations
- AIEOP
Italian Association of Pediatric Hematology/Oncology
- ARMS
Alveolar Rhabdomyosarcoma
- CD105
Endoglin
- COG
Children’s Oncology Group
- EFS
Event-Free Survival
- EpSSG
European Pediatric Soft Tissue Sarcoma Study Group
- ERMS
Embryonal Rhabdomyosarcoma
- IMVD
Intratumoral Microvessel Density
- OS
Overall Survival
- RMS
Rhabdomyosarcoma
- STS
Soft Tissue Sarcoma
- TGF-β
Transforming Growth Factor beta
- VEGF
Vascular Endothelial Growth Factor
- VM
Vasculogenic Mimicry
Additional file
Immunostaining of CD105 for ERMS and ARMS. Magnification × 200. (PDF 266 kb)
Authors’ contributions
VDP help in the histological revision, analyzed the results and helped to draft the manuscript, IR collected the data of patients, RB performed histological diagnosis, LR performed the statistical analysis, MP and MCB cut the paraffin blocs and performed immunohistochemistry, AG helped to perform the figures in the manuscript, MC helped to draft the manuscript, RR reviewed the manuscript, DO reviewed the manuscript, AC helped to select the cases, HP reviewed the manuscript, GMM selected the cases and helped to draft the manuscript, ADG designed the study, interpreted the results and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Informed consent to participate at the study was obtained from parent or legal guardian of the patient.
Consent for publication
Written informed consent for publication of their clinical details and clinical images was obtained from the parent or guardian of the patient.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-017-3947-4) contains supplementary material, which is available to authorized users.
Contributor Information
Virginia Di Paolo, Phone: (+39)06 68593516, Email: virginia.dipaolo@opbg.net.
Ida Russo, Phone: (+39)06 68593707, Email: ida.russo@opbg.net.
Renata Boldrini, Phone: (+39)06 68592154, Email: renata.boldrini@opbg.net.
Lucilla Ravà, Phone: (+39)06 68592741, Email: lucilla.rava@opbg.net.
Marco Pezzullo, Phone: (+39) 06 68592660, Email: marco.pezzullo@opbg.net.
Maria Chiara Benedetti, Phone: (+39)06 68592154, Email: mchiara.benedetti@opbg.net.
Angela Galardi, Phone: (+39)06 68593516, Email: angela.galardi@opbg.net.
Marta Colletti, Phone: (+39)06 68593516, Email: marta.colletti@opbg.net.
Rossella Rota, Phone: (+39)06 68592648, Email: rosella.rota@opbg.net.
Domenico Orlando, Phone: (+39)06 68593516, Email: domenico.orlando@opbg.net.
Alessandro Crocoli, Phone: (+39)06 68592012, Email: alessandro.crocoli@opbg.net.
Hector Peinado, Email: hps2002@med.cornell.edu.
Giuseppe Maria Milano, Phone: (+39)06 68592444, Email: giuseppemaria.milano@opbg.net.
Angela Di Giannatale, Phone: (+39)06 68593707, Email: angela.digiannatale@opbg.net.
References
- 1.Gurney JG, Young JL, Jr, Roffers SD, Smith MA, Bunin GR, et al. Soft tissue sarcomas. In: LAG R, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. Bethesda: National Cancer Institute, SEER Program; 1999. pp. 111–123. [Google Scholar]
- 2.Sorensen PH, Lynch JC, Qualman SJ, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children’s oncology group. J Clin Oncol. 2002;20:2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 3.McDowell HP, Foot AB, Ellershaw C, Machin D, Giraud C, Bergeron C. Outcomes in paediatric metastatic rhabdomyosarcoma: results of the International Society of Paediatric Oncology (SIOP) study MMT-98. Eur J Cancer. 2010;46(9):1588–1595. doi: 10.1016/j.ejca.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 4.de Castro JG, Puglisi F, de Azambuja E, El Saghir NS, Awada A. Angiogenesis and cancer: a cross-talk between basic science and clinical trials (the “do ut des” paradigm) Crit Rev Oncol Hematol. 2006;59(1):40–50. doi: 10.1016/j.critrevonc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Maris JM, Courtright J, Houghton PJ, Morton CL, Kolb EA, Lock R, Tajbakhsh M, Reynolds CP, Keir ST, Wu J, Smith MA. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51(1):42–48. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Mokhtari RB, Sheikh R, Wu B, Zhang L, Xu P, Man S, Oliveira ID, Yeger H, Kerbel RS, Baruchel S. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. 2011;17(17):5656–5667. doi: 10.1158/1078-0432.CCR-11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keir ST, Morton CL, Wu J, Kurmasheva RT, Houghton PJ, Smith MA. Initial testing of the multitargeted kinase inhibitor pazopanib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;59(3):586–588. doi: 10.1002/pbc.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navid F, Baker SD, McCarville MB, Stewart CF, Billups CA, Wu J, Davidoff AM, Spunt SL, Furman WL, McGregor LM, Hu S, Panetta JC, Turner D, Fofana D, Reddick WE, Leung W, Santana VM. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin Cancer Res. 2013;19(1):236–246. doi: 10.1158/1078-0432.CCR-12-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glade Bender JL, Lee A, Reid JM, Baruchel S, Roberts T, Voss SD, Wu B, Ahern CH, Ingle AM, Harris P, Weigel BJ, Blaney SM. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol. 2013;31(24):3034–3043. doi: 10.1200/JCO.2012.47.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giatromanolaki A, Koukourakis MI, Theodossiou D, Barbatis K, O'Byrne K, Harris AL, Gatter KC. Comparative evaluation of angiogenesis assessment with anti-factor-VIII and anti-CD31 immunostaining in non-small cell lung cancer. Clin Cancer Res. 1997;3(12 Pt 1):2485–2492. [PubMed] [Google Scholar]
- 11.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 12.Offersen BV, Pfeiffer P, Hamilton-Dutoit S, Overgaard J. Patterns of angiogenesis in nonsmall-cell lung carcinoma. Cancer. 2001;91(8):1500–1509. doi: 10.1002/1097-0142(20010415)91:8<1500::AID-CNCR1158>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka F, Otake Y, Yanagihara K, Kawano Y, Miyahara R, Li M, Yamada T, Hanaoka N, Inui K, Wada H. Evaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res. 2001;7(11):3410–3415. [PubMed] [Google Scholar]
- 14.de la Taille A, Katz AE, Bagiella E, et al. Microvessel density as a predictor of PSA recurrence after radical prostatectomy. A comparison of CD34 and CD31. Am. J Clin Pathol. 2000;113:555–562. doi: 10.1309/02W2-KE50-PKEF-G2G4. [DOI] [PubMed] [Google Scholar]
- 15.Tomisaki S, Ohno S, Ichiyoshi Y, Kuwano H, Machra Y, Sugimachi K. Microvessel quantification and its possible relation with liver metastasis in colorectal cancer. Cancer. 1996;77:1772–1778. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1722::AID-CNCR46>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Behrem S, Zarkovic K, Eskinja N, Jonjic N. Endoglin is a better marker than CD31 in evaluation f angiogenesis in glioblastoma. Croat Med J. 2005;46(3):417–422. [PubMed] [Google Scholar]
- 17.Yang LY, Lu WQ, Huang GW, Wang W. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer. 2006;6:110. doi: 10.1186/1471-2407-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SL, Gao DL, Zhao ZH, et al. Correlation of matrix metalloproteinase suppressor genes RECK, VEGF, and CD105 with angiogenesis and biological behavior in esophageal squamous cell carcinoma. World J Gastroenterol. 2007;13:6076–6081. doi: 10.3748/wjg.v13.45.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyata Y, Sagara Y, Watanabe S, et al. CD105 is a more appropriate marker for evaluating angiogenesis in urothelial cancer of the upper urinary tract than CD31 or CD34. Virchows Arch. 2013;46:673–679. doi: 10.1007/s00428-013-1463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding S, Li C, Lin S, Yang Y, Liu D, Han Y, Zhang Y, Li L, Zhou L, Kumar S. Comparative evaluation of microvessel density determined by CD34 or CD105 in benign and malignant gastric lesions. Hum Pathol. 2006;37(7):861–866. doi: 10.1016/j.humpath.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Basilio-de-Oliveira RP, Pannain VL. Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell proliferation (Ki67) for gastrointestinal stromal tumors. World J Gastroenterol. 2015;21(22):6924–6930. doi: 10.3748/wjg.v21.i22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavar S, Jelašic D, Seiwerth S, Miloševic M, Hutinec Z, Mišic M. Endoglin (CD 105) as a potential prognostic factor in neuroblastoma. Pediatr Blood Cancer. 2015;62(5):770–775. doi: 10.1002/pbc.25427. [DOI] [PubMed] [Google Scholar]
- 23.Sugita Y, Takase Y, Mori D, Tokunaga O, Nakashima A, Shigemori M. Endoglin (CD 105) is expressed on endothelial cells in the primary central nervous system lymphomas and correlates with survival. J Neuro-Oncol. 2007;82(3):249–256. doi: 10.1007/s11060-006-9281-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhou D, Cheng SQ, Ji HF, Wang JS, Xu HT, Zhang GQ, Pang D. Evaluation of protein pigment epithelium-derived factor (PEDF) and microvessel density (MVD) as prognostic indicators in breast cancer. J Cancer Res Clin Oncol. 2010;136(11):1719–1727. doi: 10.1007/s00432-010-0830-y. [DOI] [PubMed] [Google Scholar]
- 25.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 26.Seon BK, Haba A, Matsuno F, Takahashi N, Tsujie M, She X, Harada N, Uneda S, Tsujie T, Toi H, Tsai H, Haruta Y. Endoglin-targeted cancer therapy. Curr Drug Deliv. 2011;8(1):135–143. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bockhorn M, Tsuzuki Y, Xu L, Frilling A, Broelsch CE, Fukumura D. Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res. 2003;9(11):4221–4226. [PubMed] [Google Scholar]
- 28.Davis TW, O'Neal JM, Pagel MD, Zweifel BS, Mehta PP, Heuvelman DM, Masferrer JL. Synergy between celecoxib and radiotherapy results from inhibition of cyclooxygenase-2-derived prostaglandin E2, a survival factor for tumor and associated vasculature. Cancer Res. 2004;64(1):279–285. doi: 10.1158/0008-5472.CAN-03-1168. [DOI] [PubMed] [Google Scholar]
- 29.Anderberg C, Cunha SI, Zhai Z, Cortez E, Pardali E, Johnson JR, Franco M, Páez-Ribes M, Cordiner R, Fuxe J, Johansson BR, Goumans MJ, Casanovas O, ten Dijke P, Arthur HM, Pietras K. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. J Exp Med. 2013;210(3):563–579. doi: 10.1084/jem.20120662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciernik IF, Krayenbühl Ciernik BH, Cockerell CJ, Minna JD, Gazdar AF, Carbone DP. Expression of transforming growth factor beta and transforming growth factor beta receptors on AIDS-associated Kaposi's sarcoma. Clin Cancer Res. 1995;1(10):1119–1124. [PubMed] [Google Scholar]
- 31.Hara H. Endoglin (CD105) and claudin-5 expression in cutaneous angiosarcoma. Am J Dermatopathol. 2012;34(7):779–782. doi: 10.1097/DAD.0b013e318252fc32. [DOI] [PubMed] [Google Scholar]
- 32.Mitsui H, Shibata K, Mano Y, Suzuki S, Umezu T, Mizuno M, Yamamoto E, Kajiyama H, Kotani T, Senga T, Kikkawa F. The expression and characterization of endoglin in uterine leiomyosarcoma. Clin Exp Metastasis. 2013;30(6):731–740. doi: 10.1007/s10585-013-9574-9. [DOI] [PubMed] [Google Scholar]
- 33.Boeuf S, Bovée JV, Lehner B, van den Akker B, van Ruler M, Cleton-Jansen AM, Richter W. BMP and TGFbeta pathways in human central chondrosarcoma: enhanced endoglin and Smad 1 signaling in high grade tumors. BMC Cancer. 2012;12:488. doi: 10.1186/1471-2407-12-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takase Y, Kai K, Masuda M, Akashi M, Tokunaga O. Endoglin (CD105) expression and angiogenesis status in small cell lung cancer. Pathol Res Pract. 2010;206(11):725–730. doi: 10.1016/j.prp.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Bauman TM, Huang W, Lee MH, Abel EJ. Neovascularity as a prognostic marker in renal cell carcinoma. Hum Pathol. 2016;57:98–105. doi: 10.1016/j.humpath.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Tachezy M, Reichelt U, Melenberg T, Gebauer F, Izbicki JR, Kaifi JT. Angiogenesis index CD105 (endoglin)/CD31 (PECAM-1) as a predictive factor for invasion and proliferation in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Histol Histopathol. 2010;25(10):1239–1246. doi: 10.14670/HH-25.1239. [DOI] [PubMed] [Google Scholar]
- 37.Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the Children’s oncology group (COG) soft-tissue sarcoma committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59(1):5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weidner N. Chapter 14. Measuring intratumoral microvessel density. Methods Enzymol. 2008;444:305–323. doi: 10.1016/S0076-6879(08)02814-0. [DOI] [PubMed] [Google Scholar]
- 39.Miyoshi K, Kohashi K, Fushimi F, Yamamoto H, Kishimoto J, Taguchi T, Iwamoto Y, Oda Y. Close correlation between CXCR4 and VEGF expression and frequent CXCR7 expression in rhabdomyosarcoma. Hum Pathol. 2014;45(9):1900–1909. doi: 10.1016/j.humpath.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Onisto M, Slongo ML, Gregnanin L, Gastaldi T, Carli M, Rosolen A. Expression and activity of vascular endothelial growth factor and metalloproteinases in alveolar and embryonal rhabdomyosarcoma cell lines. Int J Oncol. 2005;27(3):791–798. [PubMed] [Google Scholar]
- 41.Gee MF, Tsuchida R, Eichler-Jonsson C, Das B, Baruchel S, Malkin D. Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene. 2005;24(54):8025–8037. doi: 10.1038/sj.onc.1208939. [DOI] [PubMed] [Google Scholar]
- 42.Barber TD, Barber MC, Tomescu O, Barr FG, Ruben S, Friedman TB. Identification of target genes regulated by PAX3 and PAX3-FKHR in embryogenesis and alveolar rhabdomyosarcoma. Genomics. 2002;79(3):278–284. doi: 10.1006/geno.2002.6703. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Slevin M, Kumar S, Kumar P. The cooperative transforming effects of PAX3-FKHR and IGF-II on mouse myoblasts. Int J Oncol. 2005;27(4):1087–1096. doi: 10.3892/ijo.27.4.1087. [DOI] [PubMed] [Google Scholar]
- 44.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 45.Liu CD, Tilch L, Kwan D, McFadden DW. Vascular endothelial growth factor is increased in ascites from metastatic pancreatic cancer. J Surg Res. 2002;102(1):31–34. doi: 10.1006/jsre.2001.6307. [DOI] [PubMed] [Google Scholar]
- 46.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 47.Chisholm JC., Merks JH., Casanova M., Bisogno G., Orbach D., Gentet JC., Thomassin Defachelles AS, Chastagner PB., Lowis S., Ronghe M., McHugh K., van Rijn R., Hilton M., Bachir J., Fürst-Recktenwald S., Geoerger B., Oberlin O.; BERNIE: Open-label, randomized, phase II study of bevacizumab plus chemotherapy in pediatric metastatic rhabdomyosarcoma (RMS) and non-rhabdomyosarcoma soft tissue sarcoma (NRSTS). J Clin Oncol, 2016; 34 (suppl; abstr 11054).
- 48.Mascarenhas L, Meyers WH, Lyden E, Rodeberg DA. Randomized phase II trial of bevacizumab and temsirolimus in combination with vinorelbine (V) and cyclophosphamide (C) for first relapse/disease progression of rhabdomyosarcoma (RMS): a report from the Children’s Oncology Group (COG). J Clin Oncol. 2014; 32: 5s (suppl; abstract 10003). [DOI] [PMC free article] [PubMed]
- 49.Gordon MS, Robert F, Matei D, Mendelson DS, Goldman JW, Chiorean EG, Strother RM, Seon BK, Figg WD, Peer CJ, Alvarez D, Adams BJ, Theuer CP, Rosen LS. An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2014;20(23):5918–5926. doi: 10.1158/1078-0432.CCR-14-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attia S, Kumar S, Riedel RF., Robinson SI, Conry RM, McKay Boland P, Barve MA, Fritchie K, Seon BK, Alvarez D, Adams BJ, Shazer RL, Theuer CP, Maki RG. A phase 1B/ phase 2A study of TRC105 (Endoglin Antibody) in combination with pazopanib (P) in patients (pts) with advanced soft tissue sarcoma (STS). J Clin Oncol. 2016;34 (suppl; abstr 11016).
- 51.Afshar Moghaddam N, Mahsuni P, Taheri D. Evaluation of Endoglin as an angiogenesis marker in Glioblastoma. Iran J Pathol. 2015;10(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 52.Barresi V, Cerasoli S, Vitarelli E, Tuccari G. Density of microvessels positive for CD105 (endoglin) is related to prognosis in meningiomas. Acta Neuropathol. 2007;114(2):147–156. doi: 10.1007/s00401-007-0251-4. [DOI] [PubMed] [Google Scholar]
- 53.Wexler LH, Meyer WH, Helman LJ. Rhabdomyosarcoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology, vol. 6. Philadelphia: Wolter Kluwer–Lippincott Williams & Wilkins. 2011. pp. 923–53.
- 54.Kumar S, Ghellal A, Li C, Byrne G, Haboubi N, Wang JM, Bundred N. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59:856–861. [PubMed] [Google Scholar]
- 55.Yao Y, Pan Y, Chen J, Sun X, Qiu Y, Ding Y. Endoglin (CD105) expression in angiogenesis of primary hepatocellular carcinomas: analysis using tissue microarrays and comparisons with CD34 and VEGF. Ann Clin Lab Sci. 2007;37:39–48. [PubMed] [Google Scholar]
- 56.Kuda M, Kohashi K, Yamada Y, Maekawa A, Kinoshita Y, Nakatsura T, Iwamoto Y, Taguchi T, Oda Y. FOXM1 expression in rhabdomyosarcoma: a novel prognostic factor and therapeutic target. Tumour Biol. 2016;37(4):5213–5223. doi: 10.1007/s13277-015-4351-9. [DOI] [PubMed] [Google Scholar]
- 57.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156(2):361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao Z, Bao M, Miele L, Sarkar FH, Wang Z, Zhou Q. Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: a systemic review and meta-analysis. Eur J Cancer. 2013;49(18):3914–3923. doi: 10.1016/j.ejca.2013.07.148. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Maniotis AJ, Majumdar D, Pe'er J, Folberg R. Uveal melanoma cell staining for CD34 and assessment of tumor vascularity. Invest Ophthalmol Vis Sci. 2002;43(8):2533–2539. [PubMed] [Google Scholar]
- 60.Chen L, He Y, Sun S, Sun B, Tang X. Vasculogenic mimicry is a major feature and novel predictor of poor prognosis in patients with orbital rhabdomyosarcoma. Oncol Lett. 2015;10(3):1635–1641. doi: 10.3892/ol.2015.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun B, Zhang S, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol. 2004;25(6):1609–1614. [PubMed] [Google Scholar]
- 62.Yudoh K, Kanamori M, Ohmori K, Yasuda T, Aoki M, Kimura T. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br J Cancer. 2001;84(12):1610–1615. doi: 10.1054/bjoc.2001.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chao C, Al-Saleem T, Brooks JJ, Rogatko A, Kraybill WG, Eisenberg B. Vascular endothelial growth factor and soft tissue sarcomas: tumor expression correlates with grade. Ann Surg Oncol. 2001;8(3):260–267. doi: 10.1007/s10434-001-0260-9. [DOI] [PubMed] [Google Scholar]
- 64.West CC, Brown NJ, Mangham DC, Grimer RJ, Reed MW. Microvessel density does not predict outcome in high grade soft tissue sarcoma. Eur J Surg Oncol. 2005;31(10):1198–1205. doi: 10.1016/j.ejso.2005.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this published article.


