Abstract
Background
Women with node-positive breast cancer have high risk for recurrence. We evaluate the impact of approximated tumor subtype and response to chemotherapy on long-term outcomes in a node-positive cohort receiving neoadjuvant chemotherapy.
Methods
ACOSOG Z1071 enrolled cT0-4N1-2 breast cancer patients treated with neoadjuvant chemotherapy from 2009-2011. Factors impacting breast cancer-specific survival (BCSS) and overall survival (OS) were analyzed.
Results
Median follow-up of 701 eligible patients was 4.1 years (0.4–6.5). Ninety patients (12.8%) died from breast cancer. Approximated subtype and chemotherapy response were significantly associated with BCSS and OS (p<0.0001). BCSS and OS was highest in patients who achieved pathologic complete response (pCR) (p<0.0001 and p<0.0001, respectively).
5-year BCSS was highest in HER2-positive disease (95.8%; 95%CI: 87.7–98.6), followed by hormone receptor (HR)-positive/HER2-negative (80.4%; 95%CI: 73.2–85.9) and lowest in triple-negative (TNBC) (74.8%; 95%CI: 66.6–81.2; p<0.0001). Similar patterns were seen in OS.
In TNBC (n=174), 5-year BCSS was higher in patients with pCR versus residual disease (89.8%; 95%CI: 78.8–95.3 versus 65.8%; 95% CI: 54.5–74.9; p=0.0013). In HR-positive/HER2-negative (n=318) disease, BCSS was 100% in patients with pCR and 78.3% (95% CI: 70.4–84.3) in those with residual disease (p=0.018). In HER2-positive disease (n=204) there was no difference between pCR and residual disease (96.0%; 95%CI: 83.6–99.1 versus 95.8%; 95%CI: 81.4–99.1; p=0.77).
Conclusion
In node-positive breast cancer patients treated with neoadjuvant chemotherapy, BCSS and OS were associated with approximated subtype and chemotherapy response and were lowest in TNBC patients with residual disease. 5-year BCSS was >95% in HER2-positive disease independent of chemotherapy response.
Keywords: Breast cancer-specific survival, Overall survival, Node-positive breast cancer, Neoadjuvant chemotherapy
Introduction
Nodal staging in breast cancer remains important because it provides prognostic information and stratifies patients into higher and lower risk groups with respect to recurrence and mortality due to breast cancer. In patients with biopsy confirmed node-positive disease at initial presentation, the use of chemotherapy prior to surgical intervention (neoadjuvant chemotherapy) is often considered. Delivery of chemotherapy to patients in the neoadjuvant setting is associated with several advantages; increased rates of breast-conserving surgery, downstaging of disease in the regional nodal basins and the opportunity to assess response to therapy [1–6]. Eradication of disease from the breast and the lymph nodes, pathologic complete response (pCR), has been shown to be associated with improved survival when compared to the presence of residual disease after chemotherapy [7].
Invasive breast cancers are routinely classified into approximated subtypes based on the status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) on immunohistochemistry studies of the primary tumor. Tumors overexpressing HER2 are known to be biologically more aggressive; however, currently available HER2-targeted therapies are highly effective and have been shown to improve outcomes when patients receive HER2-targeted therapy with chemotherapy. In hormone receptor (HR) positive disease (ER positive and/or PR positive), endocrine therapy is an important component of systemic treatment and is usually given in the adjuvant setting. For triple-negative breast cancer (TNBC), defined as ER negative, PR negative, and HER2 negative, chemotherapy is the only current systemic therapy option. Studies evaluating administration of chemotherapy in the neoadjuvant setting have shown that response rates to chemotherapy vary by tumor subtype [8–11] and that pCR is associated with prognosis [7, 12, 13].
In this study, we report the five-year breast cancer-specific survival (BCSS) and overall survival (OS) rates in women with node-positive breast cancer who were treated with neoadjuvant chemotherapy and enrolled on the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial. We also examine the impact of approximated subtype on response to chemotherapy and survival.
Methods
ACOSOG Z1071 was a prospective clinical trial that enrolled 756 patients with clinical cT1-3, N1-2 breast cancer treated with neoadjuvant chemotherapy across 136 institutions from 2009–2011. The primary endpoint of the trial, false negative rate of sentinel lymph node surgery, was previously reported [14]. All patients underwent axillary dissection. Data regarding clinical stage at presentation, ER, PR, and HER2 status, chemotherapy delivered, surgical procedure, pathologic stage, and use of adjuvant treatment, were collected prospectively on the case report forms at the treating institution and reported to the ACOSOG (Alliance) Statistics and Data Center. Breast cancer approximated subtype was defined by hormone receptor (HR) status from combined ER and PR status and HER2 status as follows: HR positive, HER2-negative (ER positive and/or PR positive, and HER2-negative); HER2-positive (HER2 3+ by immunohistochemistry or amplified by FISH or ISH); and triple negative (ER <1%, PR <1% and HER2-negative). Pathologic complete response was defined as no residual invasive disease in the breast or axillary lymph nodes.
The extent of disease in the surgical specimen was evaluated by the pathologist at the local site and residual disease was staged using the yp-stage designation of the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. Additionally, reporting on the residual cancer burden (RCB) was requested on the case report forms and, where available, analysis of the RCB class was performed. Calculation of RCB provides a standardized procedure for pathologic evaluation of post-neoadjuvant specimens. Educational materials with detailed instructions and an online calculator to determine RCB are posted on a publically available website [15, 16]. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Statistical Considerations
Pathologic response rates were summarized with binomial estimates and 95% confidence intervals (CIs). Comparisons of categorical baseline variables were made among groups with a chi-square test and with a t-test or ANOVA as appropriate for continuous variables. BCSS and OS were measured from the time of surgery and estimated with a Kaplan-Meier estimator. BCSS events were defined as deaths due to breast cancer. Patients were censored if they died from another cause or if they were alive at the time of analysis. Overall survival events were defined as death due to any cause and patients who were alive at the time of analysis were censored. Univariable and multivariable Cox models were used to generate hazard ratios with 95% confidence intervals (CIs). The multivariable model was adjusted for known prognostic variables. Statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chair following Alliance policies. The database used for these analyses was locked October 5, 2016. The trial was registered on clinicaltrials.gov with trial identifier NCT00881361.
Results
Of 756 women enrolled on the ACOSOG Z1071 trial, 701 patients met eligibility criteria. Median follow-up was 4.1 years (range 0.4–6.5). Patient and tumor characteristics are summarized in Table 1. As reported previously, [17] pCR rate varied by approximated tumor subtype, being highest in HER2-positive disease and lowest in HR-positive/HER2-negative disease. All 204 patients with HER2-positive disease were treated with a regimen that included chemotherapy and HER2-targeted therapy. Among the 442 patients with HR-positive disease (regardless of HER2 status), 369 (92.5%) received endocrine therapy in the adjuvant setting.
Table 1.
Patient and tumor characteristics overall and by approximated tumor subtype of the eligible patients on ACOSOG Z1071 (Alliance)
| Characteristic | All Patients (n = 701) | Triple-Negative (n = 174) | HER2-Positive (n = 204) | Hormone-Receptor-Positive, HER2-Negative (n = 323) | P Value |
|---|---|---|---|---|---|
|
| |||||
| Patient age, years | 0.22 | ||||
| <50 | 349 (50.2) | 78 (45.1) | 101 (49.8) | 170 (53.3) | |
| ≥50 | 346 (49.8) | 95 (54.9) | 102 (50.3) | 149 (46.7) | |
| Unknown | 6 | 1 | 1 | 4 | |
|
| |||||
| Clinical Tumor category at presentation | 0.28 | ||||
| T0 | 9 (1.3) | 3 (1.7) | 4 (2.0) | 2 (0.6) | |
| Tis | 1 (0.1) | 0 | 1 (0.5) | 0 | |
| T1 | 92 (13.1) | 25 (14.4) | 22 (10.8) | 45 (14.0) | |
| T2 | 384 (54.9) | 103 (59.2) | 104 (51.0) | 177 (55.0) | |
| T3 | 181 (25.9) | 37 (21.3) | 64 (31.4) | 80 (24.8) | |
| T4 | 33 (4.7) | 6 (3.4) | 9 (4.4) | 18 (5.6) | |
| Unknown | 1 | 0 | 0 | 1 | |
|
| |||||
| Clinical Nodal category at presentation | 0.57 | ||||
| N1 | 657 (94.4) | 161 (93.1) | 191 (94.1) | 305 (95.3) | |
| N2 | 39 (5.6) | 12 (6.9) | 12 (5.9) | 15 (4.7) | |
| Unknown | 5 | 1 | 1 | 3 | |
|
| |||||
| Histologic Type | <0.000 | ||||
| IDC | 630 (89.9) | 165 (94.8) | 191 (93.6) | 274 (84.8) | 1 |
| ILC | 39 (5.6) | 4 (2.4) | 5 (2.4) | 30 (9.3) | |
| IMC | 26 (3.7) | 3 (1.7) | 4 (2.0) | 19 (5.9) | |
| Other | 6 (0.9) | 2 (1.2) | 4 (2.0) | 0 | |
|
| |||||
| Pathologic Tumor category at surgery | 235 (33.8) | 82 (47.6) | 103 (51.0) | 50 (15.5) | <0.0001 |
| 251 (36.1) | 51 (29.6) | 63 (31.2) | 137 (42.6) | ||
| T0/Tis | 149 (21.4) | 32 (18.6) | 31 (15.4) | 86 (26.7) | |
| T1 | 58 (8.3) | 6 (3.5) | 5 (2.5) | 47 (14.6) | |
| T2 | 3 (0.4) | 1 (0.6) | 0 | 2 (0.6) | |
| T3 | 5 | 2 | 2 | 1 | |
| T4 | |||||
| Unknown | |||||
|
| |||||
| Pathologic Nodal category at surgery | <0.0001 | ||||
| N0 | 289 (41.3) | 85 (48.8) | 136 (66.7) | 68 (21.1) | |
| N1 | 241 (34.4) | 56 (32.2) | 52 (25.5) | 133 (41.3) | |
| N2 | 130 (18.6) | 27 (15.5) | 14 (6.9) | 89 (27.6) | |
| N3 | 40 (5.7) | 6 (3.4) | 2 (1.0) | 32 (9.9) | |
| Unknown | 1 | 0 | 0 | 1 | |
|
| |||||
| pCR status | <0.0001 | ||||
| pCR both breast and axilla | 195 (27.8) | 66 (42.0) | 92 (45.1) | 37 (11.5) | |
| pCR breast only | 40 (5.7) | 16 (9.2) | 11 (5.4) | 13 (4.0) | |
| pCR axilla only | 92 (13.1) | 19 (10.9) | 42 (20.6) | 31 (9.6) | |
| no pCR | 374 (53.4) | 73 (42.0) | 59 (28.9) | 242 (74.9) | |
|
| |||||
| Residual Cancer Burden class | <0.0001 | ||||
| 0 | 195 (36.6) | 66 (45.8) | 92 (59.0) | 37 (15.9) | |
| I | 33 (6.2) | 9 (6.2) | 12 (7.7) | 12 (5.2) | |
| II | 146 (27.4) | 32 (22.2) | 29 (18.6) | 85 (36.5) | |
| III | 159 (29.8) | 37 (25.7) | 23 (14.7) | 99 (42.5) | |
| Unknown | 168 | 30 | 48 | 90 | |
Abbreviations: pCR = Pathologic complete response.
At the time of analysis, 107 patients had died, 90 of breast cancer and 17 from other causes. The five-year BCSS was 83.4% (95% CI: 79.2 to 86.8) and five-year OS was 80.5% (95% CI: 76.1 to 84.2). Univariable and multivariable analyses showing factors impacting BCSS and OS are shown in table 2. On univariable analysis, approximated tumor subtype was associated with BCSS and OS (p<0.0001); patients with HER2-positive disease had the best BCSS and OS followed by those with HR-positive, HER2-negative disease. The worst BCSS and OS were observed in TNBC patients. Response in the breast and the nodes to neoadjuvant chemotherapy was strongly associated with BCSS (p<0.0001) and OS (p<0.0001); increasing pathologic tumor category at surgery and increasing pathologic nodal category at surgery were both strongly associated with reduced BCSS and OS. Clinical tumor category at presentation, use of adjuvant radiation, and type of primary tumor surgery were not statistically significantly associated with BCSS or OS. On multivariable analysis, which included clinical tumor category, approximated tumor subtype, pathologic tumor category, pathologic nodal category, surgical procedure, and use of adjuvant radiation in the model, the statistically significant factors for BCSS and OS were approximated tumor subtype and pathologic nodal category and for OS pathologic tumor category was also significant.
Table 2.
Evaluation of clinical and pathological variables on five-year breast cancer-specific survival and overall survival by univariable and multivariable analysis
| Variable | Breast Cancer-Specific Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
|
| ||||||||
| Clinical Tumor category | ||||||||
| T0–T2 | 1.00 (ref) | 0.093 | 1.00 (ref) | 0.31 | 1.00 (ref) | 0.045 | 1.00 (ref) | 0.34 |
| T3–T4 | 1.44 (0.94–2.22) | 1.28 (0.79–2.09) | 1.49 (1.00–2.21) | 1.24 (0.79–1.96) | ||||
|
| ||||||||
| Approximated Tumor biology | ||||||||
| HR-positive/HER2-negative | 1.00 (ref) | <0.0001 | 1.00 (ref) | 1.00 (ref) | <0.0001 | 1.00 (ref) | <0.0001 | |
| HER2 positive | 0.13 (0.05–0.37) | 0.25 (0.09–0.72) | <0.0001 | 0.28 (0.14–0.55) | 0.56 (0.28–1.14) | |||
| Triple negative | 1.82 (1.19–2.80) | 3.32 (2.07–5.32) | 1.68 (1.12–2.52) | 3.13 (1.99–4.92) | ||||
|
| ||||||||
| Pathologic Tumor category | ||||||||
| T0/Tis | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| T1 | 2.96 (1.45–6.01) | <0.0001 | 2.40 (1.07–5.41) | 0.069 | 2.56 (1.36–4.83) | <0.0001 | 2.16 (1.05–4.47) | 0.044 |
| T2 | 5.26 (2.59–10.67) | 3.61 (1.52–8.57) | 4.53 (2.41–8.52) | 3.30 (1.52–7.16) | ||||
| T3 | 6.36 (2.78–14.51) | 3.03 (1.04–8.82) | 6.46 (3.14–13.33) | 3.20 (1.24–8.30) | ||||
| T4 | — | — | 4.87 (0.63–37.48) | 2.11 (0.23–19.30) | ||||
|
| ||||||||
| Pathologic Nodal category | ||||||||
| N0 | 1.00 (ref) | <0.0001 | 1.00 (ref) | 0.007 | 1.00 (ref) | <0.0001 | 1.00 (ref) | 0.0011 |
| N1 | 2.48 (1.34–4.60) | 1.54 (0.76–3.12) | 2.28 (1.30–3.97) | 1.51 (0.79–2.87) | ||||
| N2 | 4.60 (2.46–8.57) | 2.38 (1.11–5.14) | 4.04 (2.29–7.12) | 2.25 (1.11–4.57) | ||||
| N3 | 7.36 (3.55–15.25) | 4.21 (1.72–10.31) | 7.93 (4.20–14.97) | 4.56 (2.03–10.27) | ||||
|
| ||||||||
| pCR | ||||||||
| Residual disease | 1.00 (ref) | <0.0001 | 1.00 (ref) | <0.0001 | ||||
| pCR | 0.26 (0.12–0.53) | 0.30 (0.16–0.56) | ||||||
|
| ||||||||
| Surgery Performed | ||||||||
| BCS | 1.00 (ref) | 0.26 | 1.00 (ref) | 0.80 | 1.00 (ref) | 0.15 | 1.00 (ref) | 0.80 |
| Mastectomy | 1.29 (0.83–1.99) | 1.06 (0.67–1.70) | 1.34 (0.90–2.00) | 1.06 (0.68–1.63) | ||||
|
| ||||||||
| Radiation | ||||||||
| Yes | 1.00 (ref) | 0.96 | 1.00 (ref) | 0.20 | 1.00 (ref) | 0.94 | 1.00 (ref) | 0.21 |
| No | 0.98 (0.55–1.77) | 1.46 (0.82–2.61) | 1.02 (0.60–1.74) | 1.41 (0.82–2.42) | ||||
|
| ||||||||
| Residual Cancer Burden class | ||||||||
| 0 | 1.00 (ref) | <0.0001 | 1.00 (ref) | <0.0001 | ||||
| I | 2.17 (0.58–8.18) | 1.56 (0.44–5.60) | ||||||
| II | 2.13 (0.89–5.08) | 1.63 (0.75–3.56) | ||||||
| III | 7.75 (3.65–16.44) | 6.85 (3.58–13.10) | ||||||
Abbreviations: pCR = Pathologic complete response; BCS= breast conserving surgery; RCB=Residual cancer burden.
Approximated tumor subtype
We further evaluated the association of BCSS and OS with approximated tumor subtype. BCSS was best in patients with HER2-positive tumors, with a five-year BCSS of 95.8% (95% CI: 87.7 to 98.6), followed by patients with HR-positive/HER2-negative disease who had five-year BCSS of 80.4% (95% CI: 73.2 to 85.9). Patients with TNBC had a five-year BCSS of 74.8% (95% CI: 66.6 to 81.2). The observed differences in BCSS among the approximated subtypes were statistically significant (p<0.0001).
Similarly, statistically significant differences were observed in OS (p<0.0001), with the highest five-year OS observed in patients with HER2-positive tumors at 93.5% (95% CI: 86.2 to 97.0), followed by HR-positive/HER2-negative disease with five-year OS of 76.6% (95% CI: 69.0 to 82.6). The lowest five-year OS was observed in patients with triple negative disease at 73.1% (95% CI: 64.9 to 79.6).
Dividing the patients with HER2-positive tumors into HER2-positive/HR-positive and HER2-positive/HR-negative, the rate of pCR was significantly higher in HER2-positive/HR-negative disease at 53.6% compared with HER2-positive/HR-positive disease where the pCR rate was 39.2% (p=0.042), however, there was no difference in 5-year OS between HER2-positive/HR-negative at 95.6% (95% CI: 81.7 to 99.0) and HER2-positive/HR-positive at 92.1% (95% CI: 80.7 to 96.9, p=0.56). Similarly, there was no difference in 5-year BCSS between HER2-positive/HR-negative at 95.6% (95% CI: 81.7 to 99.0) and HER2-positive/HR-positive at 95.8% (95% CI: 80.8 to 99.1, p=0.68).
Pathologic response
BCSS and OS were highest in patients with pCR compared to those with residual disease (Figure 1A; p<0.0001 and p<0.0001, respectively). The five-year BCSS was best in patients who experienced a pCR (94.7%; 95% CI: 89.2 to 97.4), followed by patients with residual disease in the breast only (eradication of nodal disease) (93.7%; 95% CI: 85.3 to 97.3) and those with residual disease in the lymph nodes only (eradication of disease in the breast) (92.2%; 95% CI: 70.5 to 98.1). The worst BCSS was observed in patients with residual disease in both the breast and lymph nodes (74.8%; 95% CI: 68.0 to 80.3).
Figure 1.

Breast Cancer-Specific Survival and Overall Survival by: A) Pathologic complete response (pCR) versus any residual disease, and B) Residual Cancer Burden Class (n=532).
In the subset of 532 patients where the RCB class was available, RCB class was associated with BCSS and OS (both p<0.0001, Figure 1B). RCB-III had significantly poorer survival compared with RCB 0/I/II; for OS, the hazard ratio for RCB-III (vs 0/I/II) was 5.22 (3.32, 8.21), p<0.0001 and for BCSS, the hazard ratio for RCB-III (vs 0/I/II) was 4.95 (3.03, 8.07), p<0.0001.
Impact of pathologic response by approximated subtype
As previously reported, pCR rates varied by approximated tumor subtype. Patients with HER2-positive disease had the highest pCR rate (45.1%), followed by TNBC (42.0%). HR-positive/HER2-negative disease had the lowest pCR rate (11.5%), p<0.0001.
Comparing all patients who achieved a pCR, five-year BCSS was high, with rates of 100% for HR-positive/HER2-negative disease and 96.0% (95% CI: 83.6 to 99.1) for HER2-positive, however was lower at 89.8% (95% CI: 78.8 to 95.3) for TNBC (p=0.018). There was no statistically significant difference in OS between the approximated subtypes in patients with a pCR (p=0.086, table 3). In patients withresidual disease after chemotherapy, five-year BCSS was significantly higher in patients with HER2-positive disease at 95.8% (95% CI: 81.4 to 99.1) compared to patients with HR-positive/HER2-negative disease at 78.3% (95% CI: 70.4 to 84.3) and those with TNBC at 65.8% (95% CI: 54.5 to 74.9). These differences were statistically significant, (p<0.0001). Similar patterns of OS by approximated tumor subtype was seen in patients with residual disease (p<0.0001).
Table 3.
Breast Cancer-Specific Survival (BCSS) and Overall survival (OS) by approximated clinical tumor subtype and pathologic response
| pCR | Residual Disease | ||||||
|---|---|---|---|---|---|---|---|
| HR pos/HER2 neg | HER2 positive | Triple negative | HR pos/HER2 neg | HER2 positive | Triple negative | ||
| BCSS | |||||||
| HR | 1.00 | — | — | 1.00 | 0.11 | 2.35 | |
| 95% CI | ref | — | — | ref | 0.03 – 0.44 | 1.50 – 3.68 | |
| 5 yr | 100% | 96.0% | 89.8% | 78.3% | 95.8% | 65.8% | |
| p value | 0.018 | <0.0001 | |||||
| OS | |||||||
| HR | 1.00 | 1.14 | 4.02 | 1.00 | 0.32 | 2.18 | |
| 95% CI | ref | 0.12 – 10.96 | 0.50 – 32.71 | ref | 0.15 – 0.71 | 1.42 – 3.34 | |
| 5 yr | 97.1% | 94.6% | 87.8% | 74.4% | 92.8% | 64.4% | |
| p value | 0.086 | <0.0001 | |||||
Abbreviations: pCR = Pathologic complete response.
An evaluation of the impact of pCR on BCSS by approximated tumor subtype yielded a significant difference in patients with TNBC (n=174) between those with a pCR, with a five-year BCSS of 89.8% (95% CI: 78.8 to 95.3) compared to those with residual disease, with five-year BCSS of 65.8% (95% CI: 54.5 to 74.9), p=0.0013 (Figure 2). In the 204 patients with HER2-positive disease, there was no statistically significant difference in BCSS between those patients that achieved a pCR, with 96.0% (95% CI: 83.6 to 99.1) five-year BCSS; compared to those with residual disease, with 95.8% (95% CI: 81.4 to 99.1) five-year BCSS, p=0.77. In the 323 patients with HR-positive/HER2-negative breast cancer, the proportion of patients achieving a pCR was low (11.5%), and there were no BCSS events seen in the patients with a pCR. The five-year BCSS rate of patients with residual disease was 78.3% (95% CI: 70.4 to 84.3). In patients with HR-positive/HER2-negative disease, the five-year OS rates were 97.1% (95% CI 80.9 to 99.6) in patients with a pCR and 74.4% (95% CI 66.2 to 80.9) in patients with residual disease (p=0.033). BCSS and OS by RCB class for each approximated tumor subtype is shown in Figure 3. As RCB class increases, BCSS and OS decrease in HR-positive/HER2-negative and TNBC.
Figure 2.
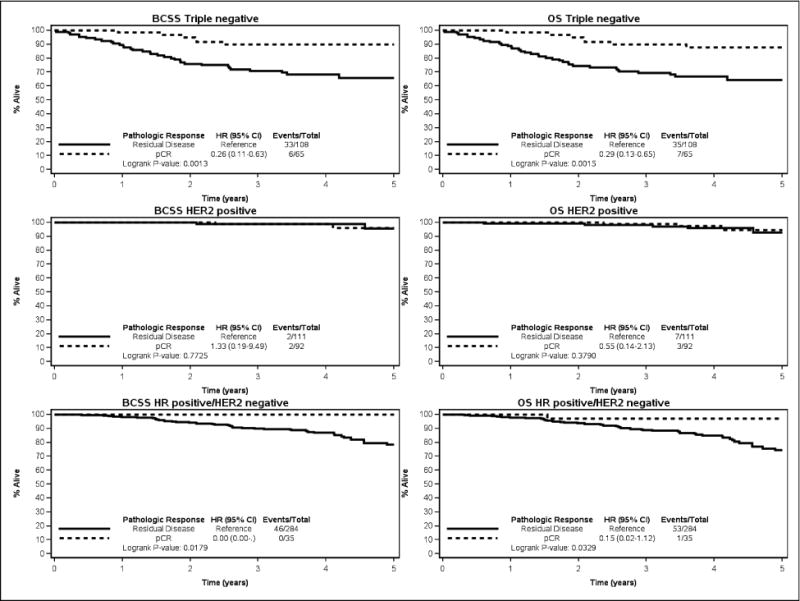
Breast Cancer-Specific Survival and Overall Survival comparing patients with a pathologic complete response (pCR) to those with residual disease in triple-negative breast cancer, HER2-positive breast cancer, and Hormone Receptor (HR)-positive/HER2-negative breast cancer.
Figure 3.

Breast Cancer-Specific Survival and Overall Survival by Residual Cancer Burden class (n=532) in triple-negative breast cancer, HER2-positive breast cancer, and Hormone Receptor (HR)-positive/HER2-negative breast cancer.
Discussion
In this prospective clinical trial of biopsy-proven node-positive breast cancer patients treated with neoadjuvant chemotherapy, we have confirmed that BCSS and OS were associated with approximated tumor subtype and response to chemotherapy. The highest five-year survival rates were seen in the patients with HR-positive/HER2-negative disease who achieved a pCR, although this was a small group of patients and HR-positive tumors are known to have a longer time to relapse. Five-year BCSS was >95% in patients with HER2-positive disease treated with HER2-targeted therapy, regardless of extent of residual disease after neoadjuvant chemotherapy. The poorest survival was seen in TNBC patients with residual disease. Pathologic complete response was a strong indicator of outcome in TNBC and HR-positive/HER2-negative disease.
Assessment of pCR is valuable information for estimating survival as patients with pCR have improved survival compared with those patients with residual disease. This finding has been consistent across studies that have documented that disease response correlates with survival. [7, 13, 18–20] Previous studies, however, have shown that dichotomous distinction of pCR and residual disease is a suboptimal prognostic surrogate. Evaluation of location of residual disease showed that residual disease in the nodes was associated with a worse prognosis compared with residual disease in the breast alone. The greatest difference was seen between those with residual disease in either the breast or nodes only compared to those patients with residual disease in both the breast and nodes. Estimation of extent of disease by Residual Cancer Burden has been proposed as an informative method for assessment of extent of residual disease after neoadjuvant chemotherapy, since RCB class has been shown to correlate with survival. [15, 21–23] As demonstrated in our data, and in keeping with previous reports, the RCB class provides additional discrimination, in particular to identify those patients with the poorest outcome, as identified by RCB class III. RCB-III is a group of patients at significantly higher risk of relapse and identifies a patient group which may benefit from further systemic therapy and an area to focus additional research. The impact of additional systemic therapy in patients with high volume residual disease remains an area under investigation and data from prospective trials are needed to address this question. One prospective randomized study in patients with HER2-negative breast cancer with residual disease after neoadjuvant chemotherapy showed that adjuvant capecitabine resulted in a better recurrence-free survival. [24] The survival curves for RCB-I and II did not demonstrate significant separation in the Z1071 cohort. It may be that longer follow-up is needed in order to demonstrate differences between these RCB classes.
TNBC is known to be an aggressive subtype of breast cancer, with poor outcomes compared to other tumor subtypes. [25] This is likely due to the heterogeneity of the disease and the lack of availability of targeted therapy for this subtype. Consistent with other studies, we found that patients with TNBC that achieved a pCR had a significantly better BCSS and OS compared with those with residual disease. [26] In fact, patients with TNBC who experienced a pCR had 5-year BCSS and OS in keeping with the other tumor subtypes. It is reassuring that such patients with “chemosensitive” TNBC, have an excellent outcome, such that additional systemic therapy may not be necessary. This highlights one of the advantages of delivery of chemotherapy in the neoadjuvant setting, as it provides outcome information to the treating physicians and patients early in the course of treatment and can be an opportunity to tailor treatment recommendations based on response. Patients with chemotherapy-resistant TNBC are at the highest risk of recurrence and death due to breast cancer and therefore multiple studies are evaluating the role of additional systemic therapy in these patients. One such study is SWOG 1418 which is randomizing patients with TNBC who have residual disease after neoadjuvant chemotherapy to pembrolizumab versus no additional therapy. [27]
HER2-positive breast cancer is a biologically aggressive subtype of breast cancer and early studies demonstrated poor survival in these patients. However, as understanding of HER2-positive disease advanced and HER2-targeted therapies are now routinely administered, the survival rates have dramatically improved. Studies have shown that the use of HER2-targeted therapy, in addition to chemotherapy, in the neoadjuvant setting resulted in high rates of pathologic complete response [8, 9, 28–33]. During the time that the ACOSOG Z1071 trial was conducted, patients with HER2-positive disease treated in the neoadjuvant setting routinely received chemotherapy and trastuzumab. Currently, treatment for HER2-positive breast cancer in the neoadjuvant setting can include more than one HER2-targeted agent in addition to chemotherapy (trastuzumab and pertuzumab) and results in even higher pCR rates. [10, 12, 31, 34].
The pCR rate observed in patients with HER2-positive disease in Z1071 was 45%. Interestingly, the five-year OS and BCSS were not impacted by the presence of residual disease in this cohort of patients. A prior small study that included 53 patients with HER2-positive disease demonstrated favorable locoregional recurrence rates regardless of response to neoadjuvant chemotherapy. [26] The findings from this study and the current study are in contrast to multiple previous studies which have shown that in patients with HER2-positive disease pCR is associated with improved survival compared to those with residual disease. [7, 11, 13, 33, 35] A meta-analysis of studies by Cortazar et al. which included 1989 patients with HER2-positive disease showed a significant difference in event-free survival between the 586 patients who achieved a pCR and the 1403 patients with residual disease. [7] The finding that there was no difference in survival outcomes in Z1071 in the HER2-positive patients with or without pCR may be due to the relatively small sample size and the relatively short follow-up time. With the addition of pertuzumab, and possibly other HER2-targeted agents in the future, outcomes for patients with HER2-positive disease may continue to improve. Current clinical trials are addressing the role of HER2-targeted therapies without chemotherapy.
Hormone receptor-positive/HER2-negative breast cancer is considered a less aggressive subtype and breast cancer recurrences often occur much later in the course of the disease, with risk of recurrence remaining elevated beyond 10 years. [36] The presence of a target (estrogen receptor) for adjuvant systemic therapy along with the availability of endocrine therapy that is reasonably well tolerated has led to lower rates of breast cancer events in this patient population. Rates of pCR in the patients with HR-positive/HER2-negative disease were low at 11.5%. This is consistent with other studies that have previously shown lower pCR rates in HR-positive disease. [7, 13, 37] Additionally, the number of breast cancer recurrences or deaths in the patients who achieved a pCR was so small that statistical comparison of the impact of pCR on outcome was limited. Since these patients are treated with adjuvant endocrine therapy for 5 years and in some instances 10 years after surgery, they remain at risk for future breast cancer events for over 10 years. [36] One limitation of the Z1071 trial is that luminal A tumors could not be distinguished from luminal B tumors based on the available information.
In HR-positive/HER2-negative disease, alternative strategies for treatment are being explored and utilized in the clinical practice, in particular the use of neoadjuvant endocrine therapy to assess the endocrine responsiveness of the tumor. Patients with an excellent response to neoadjuvant endocrine therapy may be candidates to avoid chemotherapy.[38] Additionally, prognostic indicators such as the 21-gene recurrence score are being used in patients with ER-positive disease, both in node-negative and in some cases in node-positive patients, to evaluate the potential benefit from chemotherapy. In node-positive patients with ER-positive disease, use of the 21-gene recurrence score assay is based mainly on retrospective data. Data from the prospective trial (RxPONDER trial) is awaited. [39] In one retrospective study, use of the 21-gene recurrence score in node-positive disease was shown to decrease the rate of recommendation for adjuvant chemotherapy from 81% to 50%. [40] The West German Plan B prospective trial showed excellent 3-year survival of patients with a recurrence score ≤11 treated without chemotherapy and included patients with node-positive disease. [41]
One of the major limitations of this study is that the patients enrolled in Z1071 were a heterogeneous cohort and were not selected based on tumor subtype. There was no standardized chemotherapy regimen recommended and although RCB reporting was recommended in the protocol it was not completed in a significant proportion (24%) of patients. The follow-up time is relatively short with an average follow-up of four years at the time of this report and additional follow-up is awaited. Additionally, the tumor subtyping is based on approximated subtype using receptor status reported from the local pathology laboratory and not based on central pathology review or molecular analysis of the individual tumors. A large proportion of patients enrolled had HR-positive/HER2-negative disease and practice patterns have shifted toward more use of endocrine therapy in these patients and less chemotherapy. Since HR-positive/HER2-negative tumors have lower pCR rates and longer time to recurrence, the high proportion of these patients in the study limits the interpretation of impact of RCB. We did observe higher rates of RCB-III than previously reported, raising the possibility of over-estimation; however this may be attributable to the fact that all patients enrolled in Z1071 were clinically node-positive at time of diagnosis.
In summary, neoadjuvant chemotherapy is effective at achieving pCR in a substantial proportion of patients with HER2-positive and TNBC. For HER2-positive disease treated with chemotherapy and HER2-targeted therapy, 5-year BCSS and OS are excellent, regardless of response to therapy. For patients with chemotherapy-resistant TNBC, with residual disease after neoadjuvant chemotherapy, alternative strategies should and are being explored to improve patient outcome.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180790, U10CA180858 and U10CA180868. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
References
- 1.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of Neoadjuvant Chemotherapy in Stage II-III Triple Negative Breast Cancer on Eligibility for Breast-Conserving Surgery and Breast Conservation Rates: Surgical Results from CALGB 40603 (Alliance) Ann Surg. 2015 Sep;262(3):434–9. doi: 10.1097/SLA.0000000000001417. discussion 438–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant Chemotherapy for Operable Breast Cancer. Br J Surg. 2007 Oct;94(10):1189–200. doi: 10.1002/bjs.5894. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an International Consensus Conference on the Current Status and Future of Neoadjuvant Systemic Therapy in Primary Breast Cancer. Ann Surg Oncol. 2012 May;19(5):1508–16. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 4.Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and Impact of Documented Eradication of Breast Cancer Axillary Lymph Node Metastases Before Surgery in Patients Treated With Neoadjuvant Chemotherapy. Ann Surg. 1999 Jul;230(1):72–8. doi: 10.1097/00000658-199907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bear HD, Anderson S, Brown A, et al. The Effect on Tumor Response of Adding Sequential Preoperative Docetaxel to Preoperative Doxorubicin and Cyclophosphamide: Preliminary Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003 Nov 15;21(22):4165–74. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of Preoperative Versus Postoperative Chemotherapy on the Extent and Number of Surgical Procedures in Patients Treated in Randomized Clinical Trials for Breast Cancer. Ann Surg. 2006 Sep;244(3):464–70. doi: 10.1097/01.sla.0000234897.38950.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortazar P, Zhang L, Untch M, et al. Pathological Complete Response and Long-term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet. 2014 Jul 12;384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 8.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly Higher Pathologic Complete Remission Rate After Neoadjuvant Therapy with Trastuzumab, Paclitaxel, and Epirubicin Chemotherapy: Results of a Randomized Trial in Human Epidermal Growth Factor Receptor 2-Positive Operable Breast Cancer. J Clin Oncol. 2005 Jun 1;23(16):3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant Chemotherapy with Trastuzumab Followed by Adjuvant Trastuzumab Versus Neoadjuvant Chemotherapy Alone, In Patients with HER2-Positive Locally Advanced Breast Cancer (The NOAH Trial): A Randomised Controlled Superiority Trial with a Parallel HER2-Negative Cohort. Lancet. 2010 Jan 30;375(9712):377–84. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 10.Gianni L, Pienkowski T, Im YH, et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012 Jan;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 11.Untch M, Fasching PA, Konecny GE, et al. Pathologic Complete Response After Neoadjuvant Chemotherapy Plus Trastuzumab Predicts Favorable Survival in Human Epidermal Growth Factor Receptor 2-Overexpressing Breast Cancer: Results from the TECHNO Trial of the AGO and GBG Study Groups. J Clin Oncol. 2011 Sep 1;29(25):3351–7. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 12.Gianni L, Pienkowski T, Im YH, et al. 5-year Analysis of Neoadjuvant Pertuzumab and Trastuzumab in Patients With Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer (NeoSphere): A Multicentre, Open-Label, Phase 2 Randomised Trial. Lancet Oncol. 2016 Jun;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J Clin Oncol. 2012 May 20;30(15):1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 14.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy in Patients With Node-Positive Breast Cancer: The ACOSOG Z1071 (Alliance) Clinical Trial. JAMA. 2013 Oct 9;310(14):1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of Residual Breast Cancer Burden to Predict Survival After Neoadjuvant Chemotherapy. J Clin Oncol. 2007 Oct 1;25(28):4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 16.MDACC. Residual Cancer Burden Calculator. http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3. Accessed April 19 2017.
- 17.Boughey JC, McCall LM, Ballman KV, et al. Tumor Biology Correlates With Rates of Breast-Conserving Surgery and Pathologic Complete Response After Neoadjuvant Chemotherapy for Breast Cancer: Findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014 Oct;260(4):608–14. doi: 10.1097/SLA.0000000000000924. discussion 614–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher B, Bryant J, Wolmark N, et al. Effect of Preoperative Chemotherapy on the Outcome of Women With Operable Breast Cancer. J Clin Oncol. 1998 Aug;16(8):2672–85. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 19.Mamounas EP. Overview of National Surgical Adjuvant Breast Project Neoadjuvant Chemotherapy Studies. Semin Oncol. 1998 Apr;25(2 Suppl 3):31–5. [PubMed] [Google Scholar]
- 20.Kuerer HM, Newman LA, Smith TL, et al. Clinical Course of Breast Cancer Patients With Complete Pathologic Primary Tumor and Axillary Lymph Node Response to Doxorubicin-based Neoadjuvant Chemotherapy. J Clin Oncol. 1999 Feb;17(2):460–9. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 21.Bossuyt V, Provenzano E, Symmans WF, et al. Recommendations for Standardized Pathological Characterization of Residual Disease for Neoadjuvant Clinical Trials of Breast Cancer by the BIG-NABCG Collaboration. Ann Oncol. 2015 Jul;26(7):1280–91. doi: 10.1093/annonc/mdv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provenzano E, Bossuyt V, Viale G, et al. Standardization of Pathologic Evaluation and Reporting of Postneoadjuvant Specimens in Clinical Trials of Breast Cancer: Recommendations from an International Working Group. Mod Path. 2015 Sep;28(9):1185–201. doi: 10.1038/modpathol.2015.74. [DOI] [PubMed] [Google Scholar]
- 23.Symmans WF, Wei C, Gould R, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol. 2017 Apr 1;35(10):1049–60. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toi M, Lee S-J, Lee E, et al. A phase III trial of adjuvant capecitabine in breast cancer patients with HER2-negative pathologic residual invasive diseaseafter neoadjuvant chemotherapy (CREATE-X, JBCRG-04). San Antonio Breast Cancer Symposium; 2015. Abstract S1–07. [Google Scholar]
- 25.Liedtke C, Mazouni C, Hess KR, et al. Response to Neoadjuvant Therapy and Long-term Survival in Patients With Triple-Negative Breast Cancer. J Clin Oncol. 2008 Mar 10;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 26.Yang TJ, Morrow M, Modi S, et al. The Effect of Molecular Subtype and Residual Disease on Locoregional Recurrence in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy and Postmastectomy Radiation. Ann Surg Oncol. 2015 Dec;22(Suppl 3):S495–501. doi: 10.1245/s10434-015-4697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SWOG 1418-Pembrolizumab in Treating Patients With Triple-Negative Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT02954874. Accessed May 3, 2017.
- 28.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant Therapy With Paclitaxel Followed by 5-Fluorouracil, Epirubicin, and Cyclophosphamide Chemotherapy and Concurrent Trastuzumab in Human Epidermal Growth Factor Receptor 2-Positive Operable Breast Cancer: An Update of the Initial Randomized Study Population and Data of Additional Patients Treated With the Same Regimen. Clin Cancer Res. 2007 Jan 1;13(1):228–33. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 29.Buzdar AU, Suman VJ, Meric-Bernstam F, et al. Fluorouracil, Epirubicin, and Cyclophosphamide (FEC-75) Followed by Paclitaxel Plus Trastuzumab Versus Paclitaxel Plus Trastuzumab Followed by FEC-75 Plus Trastuzumab as Neoadjuvant Treatment for Patients With HER2-Positive Breast Cancer (Z1041): A Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2013 Dec;14(13):1317–25. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and Adjuvant Trastuzumab in Patients With HER2-Positive Locally Advanced Breast Cancer (NOAH): Follow-up of a Randomised Controlled Superiority Trial With a Parallel HER2-Negative Cohort. Lancet Oncol. 2014 May;15(6):640–7. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 31.Singh JC, Mamtani A, Barrio A, et al. Pathologic Complete Response With Neoadjuvant Doxorubicin and Cyclophosphamide Followed by Paclitaxel with Trastuzumab and Pertuzumab in Patients With HER2-Positive Early Stage Breast Cancer: A Single Center Experience. Oncologist. 2017 Feb;22(2):139–43. doi: 10.1634/theoncologist.2016-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib With Trastuzumab for HER2-Positive Early Breast Cancer (NeoALTTO): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet. 2012 Feb 18;379(9816):633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Untch M, Rezai M, Loibl S, et al. Neoadjuvant Treatment With Trastuzumab in HER2-Positive Breast Cancer: Results from the GeparQuattro Study. J Clin Oncol. 2010 Apr 20;28(12):2024–31. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 34.Phase III APHINITY Study: Adjuvant Pertuzumab/Trastuzumab/Chemotherapy Increased Invasive Disease–Free Survival in HER2-Positive Breast Cancer. ASCO Post. 2017 3/2/2017 Edition. [Google Scholar]
- 35.Kim MM, Allen P, Gonzalez-Angulo AM, et al. Pathologic Complete Response to Neoadjuvant Chemotherapy With Trastuzumab Predicts for Improved Survival in Women With HER2-Overexpressing Breast Cancer. Ann Oncol. 2013 Aug;24(8):1999–2004. doi: 10.1093/annonc/mdt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colleoni M, Sun Z, Price KN, et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. J Clin Oncol. 2016 Mar 20;34(9):927–35. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goetz MP, Kalari KR, Suman VJ, et al. Tumor Sequencing and Patient-Derived Xenografts in the Neoadjuvant Treatment of Breast Cancer. J Natl Cancer Inst. 2017 Jul 1;109(7) doi: 10.1093/jnci/djw306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellis MJ, Suman VJ, Hoog J, et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance) J Clin Oncol. 2017 Apr 1;35(10):1061–69. doi: 10.1200/JCO.2016.69.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Angulo AM, Barlow WE, Gralow J, et al. SWOG S1007: A Phase III, Randomized Clinical Trial of Standard Adjuvant Endocrine Therapy With or Without Chemotherapy in Patients With One to Three Positive Nodes, Hormone Receptor (HR)-positive, and HER2-Negative Breast Cancer With Recurrence Score (RS) of 25 or Less. J Clin Oncol. 2011;29(suppl):TPS104. [Google Scholar]
- 40.Peethambaram P, Hoskin T, Day CN, et al. Use of 21-Gene Recurrence Score Assay to Individualize Adjuvant Chemotherapy Recommendations in ER+/HER2-Node Positive Breast Cancer - A National Cancer Database Study. Cancer Research. 2016;77 doi: 10.1038/s41523-017-0044-4. PD 7–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gluz O, Nitz UA, Christgen M, et al. West German Study Group Phase III PlanB Trial: First Prospective Outcome Data for the 21-Gene Recurrence Score Assay and Concordance of Prognostic Markers by Central and Local Pathology Assessment. J Clin Oncol. 2016 Jul 10;34(10):2341–9. doi: 10.1200/JCO.2015.63.5383. [DOI] [PubMed] [Google Scholar]


