Abstract
The impact of epidemic Staphylococcus aureus (S. aureus) on public health is increasing. Because of the abuse of antibiotics, the antibiotic resistance of S. aureus is increasing. Thus, there is an urgent need to develop new immunotherapies and immunoprophylaxes. Previous studies showed that the GapC protein of S. aureus, which is a surface protein with high glyceraldehyde 3-phosphate dehydrogenase activity, transferrin binding activity, and other biological activities, is highly conserved. GapC induces an effective humoral immune response in vivo. However, the B-cell epitopes of S. aureus GapC have not been well identified. Here we used the bioinformatics tools to analyze the sequence of GapC, and we generated protective anti-GapC monoclonal antibodies (mAbs). A protective mAb (1F4) showed strong specificity to GapC and the ability to induce macrophages to phagocytose S. aureus. We screened the motif 272GYTEDEIVSSD282, which was recognized by mAb 1F4, using a phage display system. Then, we used site-directed mutagenesis to identify key amino acids in the motif. Residues G272 D276 E277 I278 and V279 formed the core of the 272GYTEDEIVSSD282 motif. In addition, we showed that this epitope peptide induced a protective humoral immune response against S. aureus infection in immunized mice. Our results will be useful for the further study of epitope-based vaccines against S. aureus infection.
Introduction
Staphylococcus aureus, one of the most important pathogens, causes human soft tissue suppurative infections, pneumonia, endocarditis, osteomyelitis, and septicemia [1]. It is also one of the major pathogens associated with human-related medical devices and artificial joint infections [2, 3]. In animals, it also causes a variety of infections, such as bovine mastitis, chicken suppurative arthritis, and pig abortions, which have been causing huge economic losses to the animal husbandry industry. Over the years, there has been a committment to prevent the S. aureus infections, and antibiotic therapy has always been the main method, but the excessive use and abuse of antibiotics has led to the increasing emergence of resistant strains. Previous studies confirmed that many S. aureus strains are resistance to every clinical antibiotic, such as vancomycin [4, 5], linezolid [6, 7], daptomycin [8] and mupirocin [9]. Therefore, developing new immunotherapies, particularly vaccine control approaches, is being investigated extensively. One strategy is to develop effective monoclonal antibodies (mAbs) and vaccines against S. aureus [10, 11].
Staphylococcus aureus expresses a series of surface proteins that regulate the binding of bacteria to plasma proteins and extracellular matrix components, thereby promoting the colonization and release of toxins at different locations in vivo [12]. Additionally, some of these surface proteins, such as IsdB, SasA and ClfA [13–15], have been tested as candidate targets for vaccines against S. aureus. One of these surface proteins, glyceraldehyde-3-phosphate dehydrogenase C (GapC), possesses GAPDH activity and reversibly catalyzes the conversion of glyceraldehyde-3-phosphate into 1,3-bisphosphoglycerate by phosphorylation. GAPDH is a stimulatory protein that induces the proliferation and differentiation of B cells by inducing intereukin-10 production [16]. Previous studies demonstrated that the S. aureus GapC protein plays a role in the adherence to and internalization into bovine mammary epithelial (MAC-T) cells [17]. Meanwhile, GapC induces an effective humoral immune response and immunoprotection against S. aureus infections, which suggests that GapC might be a good immunodominant antigen [18–20].
As is well known, B-cell immunity provides a natural barrier for a host to block the invasion of pathogens into its cells mainly through B-cell epitope, which are peptide fragments of an antigen that can be recognized and trigger a B-cell immune response [21]. B-cell epitopes are the key for inducing a humoral immune response [22]. B-cell epitopes generally contain 5–7 amino acids, and no more than 20 amino acids. However, the B-cell epitopes on the S. aureus GapC protein have not been well characterized until now. In this study, we generated a mAb, 1F4, that targets GapC. The B-cell epitope recognized by mAb 1F4 was screened, and its core amino acids were analyzed. Animal experiments proved that the GapC epitope peptide recognized by mAb 1F4 induced a protective humoral immune response against S. aureus.
Materials and methods
Mice, ethics statement and production of anti-GapC mouse serum
Specific-pathogen-free BALB/c mice (female, 6–8 weeks) were provided by the Changchun Institute of Biological Products (Changchun, China). The mice were supplied with water and food ad libitum and allowed to acclimatize for a period of 7 days before the corresponding experiment. The humane endpoints were used for all animals involved in the study. Care of laboratory animals and animal experimentation were performed in accordance with animal ethics guidelines and approved by the Animal Ethics Committee of HeiLongJiang BaYi Agricultural University. In the survival study, we used humane endpoints and euthanized mice displaying severe illness prior to the end of our experiments to minimize pain and distress. The criteria for determining when the animals should be euthanized included weight loss, appetite loss, weakness, lack of feeding or drinking, signs of severe organ system dysfunction, ruffled fur, non-responsive to treatment. At the completion of all animal experiments, survivors were euthanized by CO2 exposure with IACUC policy. Death was verified by monitoring cardiac cessation and respiratory arrest. All efforts were made to minimize suffering. The anti-GapC mouse serum was prepared in our lab. BALB/c mice were immunized subcutaneously with 50 μg of GapC emulsified with Freund’s adjuvant. Then, two boosts were immunized with incomplete Freund’s adjuvant at the same dose for 2-week at each interval. Two weeks after the final boost, the BALB/c mice were anesthetized, humanely sacrificed and blood. The anti-GapC mouse serum was isolated from coagulated blood, and the concentration of anti-GapC immunoglobulins in this serum was determined about 100 μg/ml according to our previous study [23].
Bacterial strains, plasmids and cell lines
Staphylococcus aureus strain Newman, a capsular type 5 strain, was obtained from the Eijkman Winkler Laboratory of the University Medical Center, Utrecht, the Netherlands. S. aureus were grown in tryptic soy broth or agar (Difco, Becton-Dickinson, Sparks, MD, USA) at 37°C. The S. aureus gapC gene was cloned into the pET-32a (+) plasmid and expressed in BL21 (DE3) (Novagen, Madison, WI, USA). The mutated versions of the epitope motifs were cloned into pGEX-6p-1 plasmid and expressed in BL21 (DE3). Escherichia coli DH5a (Invitrogen, Carlsbad, CA, USA) was used for cloning purposes. E. coli strains DH5α and BL21 carrying plasmids were grown in Luria-Bertani broth or agar (Difco) at 37°C, in the presence of an antibiotic (100 μg of ampicillin/mL) when necessary. The myeloma cell line SP2/0 was maintained in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum (HyClone, USA) and 100 μg/ml penicillin/streptavidin. All cells were maintained at 37°C in 5% CO2.
Preparation of recombinant GapC
The full-length gapC gene was amplified by polymerase chain reaction (PCR). The primers used in this study are listed in Table 1. The overlapping PCR conditions were as follows: an initial denaturation step at 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 1 min, followed by a 10-min incubation at 72°C and a final incubation at 4°C for 1 h. The PCR products were cloned into the pET-32a (+) vector and transformed into E. coli strain BL21 (DE3). Recombinant GapC with a six-histidine tag was expressed as described previously [24] and purified with a histidine—affinity chromatography purification system (CTB, China) following the manufacturer’s recommendations.
Table 1. Primers used in this study.
| Primer | Sequence (5’–3’) | Description |
|---|---|---|
| G1 | CGCGGATCCATGGCAGTAAAAGTAGCAA | The forward primer of GapC, plus Bam HI site |
| G2 | CCCAAGCTTTTTAGAAAGTTCAGCTAAG | The reverse primer of GapC, plus Hind III site |
Note: BamH I and Hind III restriction enzyme sites were introduced at the 5’ end of the primers as indicated italics.
Antibody generation and purification
Murine mAbs targeting GapC were generated by the standard hybridoma method described in earlier studies [25, 26]. mAbs were generated in female, 12-week-old BALB/c mice. After three immunizations, the antibody titer of the mice serum was tested. Mice with a titer of 1:10,000 or greater were selected for the cell fusion experiment. Splenocytes were fused with SP2/0 myeloma cells and selected with hypoxanthine-aminopterin-thymidine (HAT) medium [27]. The culture supernatant from the hybridomas was screened by an enzyme-linked immunosorbent assay (ELISA). The mAb-producing hybridomas were cloned by limited dilution of the cells. After four rounds of screening, strongly positive hybridoma cell lines capable of stably secreting the antibodies were screened. The hybridomas (2×106 cells/mouse) were injected intraperitoneally into mice, and after the mice exhibited a swollen abdomen, the ascites fluid was extracted with a syringe. mAb 1F4 was purified using a HiTrapTM Protein G HP column (GE, USA) according to the manufacturer’s recommendations.
Establishment of an ELISA
The optimal working concentrations of the coated antigen and serum were determined by titrations. The purified GapC protein was diluted 1:100, 1:200, 1:400, 1:800, 1:1,600, 1:3,200, 1:6,400, and 1:12,800 with 0.05 M carbonate buffer (pH 9.6), and 100 μl (per well) of each dilution was coated onto microplates (96-well) at 4°C. The liquid in the wells was discarded and then the wells were washed three or four times with phosphate-buffered saline containing 0.5% Tween-20 (PBST). The plates were blocked with 5% nonfat milk for 1 h at 37°C. The serum was diluted 1:100, 1:200, 1:400, 1:1,600, 1:3,200, and 1:6,400 with PBS and added to the wells. After washing, secondary horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Origene, USA) was added and incubated at 37°C for 1 h. After washing, 3,3’,5,5’-tetramethylbenzidine dihydrochloride (Sigma, USA) was added as the substrate. The reaction was terminated with 2 M H2SO4, and the optical density at 450 nm (OD450) was determined using a microplate reader.
Western blot analysis
Recombinant GapC was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% SDS-PAGE). The gel was electrotransferred onto a nitrocellulose membrane (NEB, USA) by western blotting at 115 mA for 90 min at room temperature. The membranes were blocked in blocking buffer for 90 min. After washing three times with PBST (0.5% Tween-20), the membranes were incubated with primary antibodies for 1 h at room temperature. The membrances were washed three times with PBST and incubated with HRP-conjugated goat anti-mouse IgG (Origene) at a 1:5,000 dilution for 1 h. The reaction was detected by a western HRP substrate.
Characterization of mAbs
Hybridoma supernatants and ascites titers were determined by an indirect ELISA. 96-well plates were coated overnight with 1 μg/ml of purified recombinant GapC. After washing three times and blocking with 5% skim milk dissolved in PBS for 1 h at 37°C, 100 μl of the hybridoma cells’ supernatant or ascites fluid were added to the wells and incubated at 37°C for 1 h after washing. Then, the wells were washed three times with PBST, and then incubated with HRP-conjugated goat anti-mouse IgG at 37°C for 1 h. Estimation of enzyme activity was performed using the 3,3’,5,5’-tetramethylbenzidine dihydrochloride (TMB) substrate. The reaction was terminated with 2 M H2SO4, and the OD450 of each well was read using a microplate reader at 450 nm.
The subclasses and specificity of mAb 1F4 were detected by the same method. The mouse mAb Isotyping Kit (GE, UK) was used to detect subclasses, including IgM, IgG1, IgG2a, IgG2, IgG3, IgA, κ chains, and λ chains. The specificity of mAb 1F4 was tested, and the primary antibodies included those recognizing the S. aureus proteins, include IsdB [28], Trap [29], FnbpA [30], and Mntc proteins [31] and the S. dysgalactiae GapC protein [32].
The effect of mAb 1F4 on the GapC GAPDH activity of S. aureus was determined. The GAPDH activity of recombinant GapC was determined by a GAPDH activity assay kit (OMIN, China). The purified GapC was incubated with glyceraldehyde 3-phosphate and NAD+ in a final volume of 1 mL of assay buffer (triethanolamine, Na2HPO4, and EDTA). mAb 1F4 was incubated with the mixture at 37°C for 5 min, the decrease of NADH reflected the level of GAPDH activity by a spectrophotometer at OD340 after incubation.
Screening a random phage-displayed 12 peptide library
According to previous studies, a random PhD-12 phage display peptide library (NEB, Beverly, MA, USA) was screened with the mAbs [33]. 96-well plates were coated with 50 μg/ml of the mAbs (100 μl/well) for 12 h at 4°C. After washing six times with Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBST), 300 μl of blocking buffer was added and incubated at 4°C for 1 h. The diluted phage (1×1011) were incubated at room temperature with the bound mAb for 1 h and shaken slightly. After washing 10 times with TBST, 100 μl of the same mAbs at a concentration of 200 μg/ml were added and incubated with shaking at room temperature for 1h for competitive elution of the bound phages. The phage titer was determined by assaying 1 μl of the phage after elution. The remaining eluate was amplified by infecting 20 ml of E. coli strain ER2738. The culture was shaken vigorously at 37°C for 4.5 h. Finally, the supernatant was collected with polyethylene glycol/NaCl. The phage yield was calculated as: recovery = (eluted phage/input phage) × 100%. After three rounds of biopanning (the concentration of the Tween-20 in the TBST was increased in each round), positive phage clones were screened, and their reactivity with mAb 1F4 was verified by a sandwich ELISA. The single-stranded phage DNAs of the positive phage clones were extracted and sequenced with the primer 5'–TGAGCGGATAACAATTTCAC–3' and analyzed using DNASTAR software.
Site-directed mutagenesis assay
To determine the key amino acids of the epitope, we replaced each amino acid residue of the epitopes with alanine. The wild-type and mutated versions of the epitope motifs were synthesized by Comate Bioscience Co.,Ltd and ligated them into the pGEX-6p-1 vector to produce recombinant plasmids. The recombinant plasmids were expressed in BL21 (DE3) to express the fusion protein with a glutathione S-transferase (GST) tag. Using mAb 1F4 as the primary antibody, key amino acids were identified by detecting of the reactivity of various fusion proteins with mAb 1F4 by western blotting.
Confocal laser-scanning microscopic analysis
Staphylococcus aureus (Newman) was grown at 37°C for 4 h, and then 1 ml of the culture was removed and centrifuged at 12,000 × g for 1 min. The supernatant was discarded, and the sediment was washed twice with sterile PBS and then resuspended with 2 ml of PBS. Then, 20 μl of the bacteria was removed, spotted onto the microslides (soaked overnight in 75% ethanol), air-dried. Then, the slides were transferred into the cold acetone for 30 min. After washing the slides for 15 min with PBST, the slides were blocked with 5% skimmed milk at room temperature for 2 h and then washed with PBST for 30 min. After drying, 1:200 diluted anti-GapC serum was added, and mAb 1F4 (1 mg/ml) and SP2/0 (negative control) were used as the primary antibodies and placed at 4°C for 8 h. After washing 30 min with PBST, the slides were incubated with a fluorescein isothiocyanate-conjugated (FITC) secondary goat anti-mouse antibody (Bioss, China) (1:200 dilution) for 5 h at 4°C. Then, the slides were rinsed with water and covered with buffer (glycerol/PBS = 3/1). A LeicaTCS-SP8 confocal laser-scanning microscope was used to examination.
Opsonocytophagic assay
The mice were humanely sacrificed, the peritoneal macrophages were harvested under aseptic conditions, and the macrophagocyte cell concentration was adjusted to 1×105 cells/ml using Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum in a 24-well plates and incubated overnight at 37°C in a 5% CO2 (v/v). DMEM-washed S. aureus Newman strain was adjusted to a concentration of 2×105 colony-forming units (CFU)/ml, resulting in the multiplicity of infection (MOI) of 1–10 [17, 34]. 50 μl of serum was added, and the mixture was incubated at 37°C (5% CO2) for 30 min. Then, the mixture was transferred to the 24-well plates with macrophages at 37°C for 1 h. After incubation, wells were washed 3 times with warm DMEM and extracellular adherent bacteria were killed by adding 0.5 mL of DMEM supplemented with gentamicin (100 μg/mL) and penicillin (5 μg/mL) for 2h [34, 35]. After incubation, the cellular supernatant was discarded, and the cells were washed 3 times with Hank’s balanced salt solution (HyClone). The cells were lysed with cold water at 4°C for 20 min. Viable internalized Newman were counted by plating serial dilutions on tryptic soy agar (in triplicate) of cell lysate and determining the number of CFUs.
Mice immunization and challenge
For the positive immunization assay, female, 6–8-week-old mice were divided into four groups (n = 10). The dosage of each immunogen was 100 μg. The mice were further immunized two more times every 2 weeks. Freund’s adjuvant was used for the first immunization, and incomplete Freund’s adjuvant was used for the second and third immunizations. Two weeks after the third immunization, the mice were challenged intraperitoneally with 5×108 CFU/mouse of S. aureus. Survival was monitored at daily for 15 days after the challenge.
For the passive immunization assay, female, 6–8-week-old mice were passively immunized intravenously with 200 μg of purified mAb 1F4 or SP2/0 cell supernatant (n = 10). Twenty-four hours later, the mice were challenged intraperitoneally with 5×108 CFU/mouse of S. aureus (Newman). Survival was monitored for 7 days after the challenge. All mice were used in accordance with guidelines from the Committee of the Heilongjiang Bayi Agricultural University.
Tertiary structure of the epitope
The PDB (http://www.rcsb.org/pdb/home/home.do) method and the PyMOL tool were used to analyze the tertiary structure of GapC and the epitope. The tertiary structure of Glyceraldehyde-3-phosphate dehydrogenase 1 from S. aureus MRSA252 was used as template [36] and the homology between the template and GapC from S. aureus Newman strain was 100%.
Statistical analysis
All experiments were performed at least twice. Experimental data were analyzed with the Student’s t-test. The main statistical analyses were the opsonophagocytosis of S. aureus and the protection studies. Statistical significance was marked as ‘*’ when p < 0.05, ‘**’ when p < 0.01 and ‘***’ when p < 0.001. p <0.01 was considered to be statistically significant. Values are expressed as means ± standard deviations.
Results
Construction and expression of recombinant GapC
The 1,008-bp gapC gene was obtained by PCR amplification from the S. aureus Newman strain, cloned into the pMD18-T vector, identified by BamHI and HindIII digestion, and sequenced by Beijing Jin Wei Zhi Biological Technology Co., Ltd. The positive clones were compared with the gapC sequence in GenBank, and the results showed that they were 100% identical. To construct a GapC expression vector, the recombinant plasmid pMD18-T-GapC was digested with BamHI and HindIII, and the gapC gene was cloned into the pET-32a (+) plasmid. The recombinant plasmid pET-32a (+)-GapC was transformed into E. coli BL21 (DE3) competent cells. The recombinant plasmids were analyzed by PCR and double digestion with BamHI and HindIII (S1 Fig). The sequencing results demonstrated that the gapC sequence was identical to the known sequence.
Recombinant GapC expression was induced by IPTG and GapC was purified by histidine-affinity chromatography (CTB). SDS-PAGE showed that the molecular weight of recombinant GapC was approximately 55KDa, which is in accordance with the predicted molecular weight of GapC. Moreover, recombinant GapC was further confirmed by western blotting using an anti-His-tag antibody (Fig 1). The results showed that recombinant GapC was expressed correctly.
Fig 1. Expression and purification of recombinant GapC.
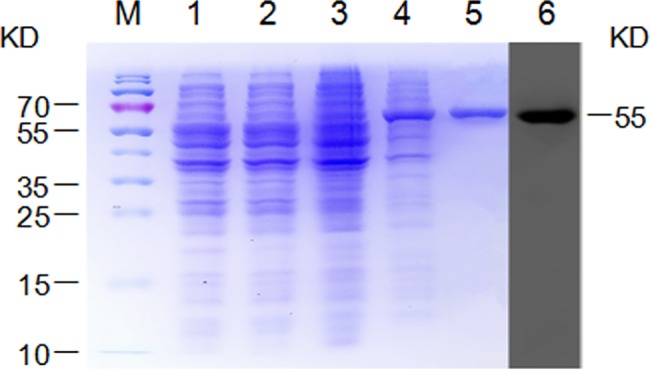
The GapC fusion protein was detected by SDS-PAGE and western blotting using an anti-His antibody. Lane M, protein marker; Lane 1, E. coli BL21 with pET32a (+) prior to isopropyl β-D-1-thiogalactopyranoside (IPTG) induction; Lane 2, E. coli BL21 with pET32a (+) after IPTG induction for 4 h; Lane 3, E. coli BL21 with pET32a (+)/GapC prior to IPTG induction; Lane 4, E. coli BL21 with pET32a (+)/GapC after IPTG induction for 4 h; Lane 5, purified GapC protein; Lane 6, the GapC fusion protein was confirmed by western blotting using anti-His antibody.
Monoclonal antibodies to GapC
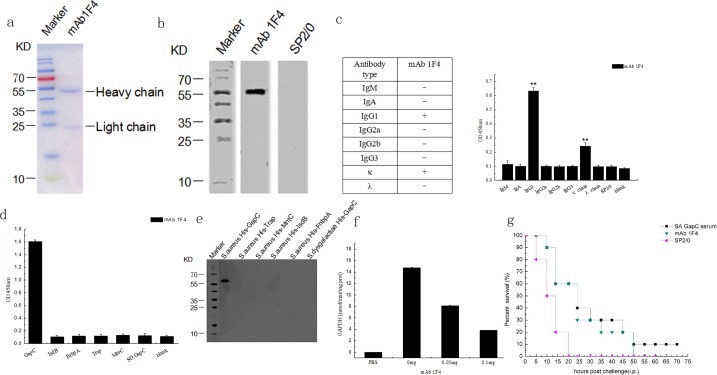
According to the ELISA, the optimal concentration of the purified GapC protein and the positive serum was 1:1,600, as it resulted in the highest P/N value. We generated six hybridoma cell lines. mAbs were screened using ELISA assays according to the optimal dilution concentration. One of the cell lines stably secreted anti-GapC antibody at a high titer, and was referred to as mAb 1F4. Thus, mAb 1F4 was selected for further study. mAb 1F4 was purified from mouse ascites fluid (Fig 2A), and it was characterized by western blotting and an antibodies subclass identification kit. The results showed that mAb 1F4 was belong to the IgG1 subclass and the κ chain (Fig 2B and 2C). Then, we analyzed the specificity of mAb 1F4 by an indirect ELISA, and the results showed that mAb 1F4 recognized only S. aureus GapC, which suggested that the mAb was generated successfully (Fig 2D). Western blotting also showed that mAb 1F4 recognized only the recombinant S. aureus GapC protein, even though the sequence homology between S. aureus and S. dysgalactiae GapC is 63% (Fig 2E). Because GapC is a surface protein that possesses GAPDH activity and reversibly catalyzes the conversion of glyceraldehyde-3-phosphate into 1, 3-bisphosphoglycerate by phosphorylation, the effect of the mAb on GAPDH activity of the recombinant GapC was determined by GAPDH activity assay kit (OMIN, China). The results showed that mAb 1F4 inhibited the GAPDH activity of S. aureus GapC (Fig 2F).
Fig 2. Characterization of mAbs.
(a) The purified 1F4 was analyzed by 12% SDS-PAGE. (b) GapC recognized by the mAb 1F4 was detected by western blotting. (c) The class of the mAb 1F4 was determined to be IgG1 and the κ chain. (d) The specificity of mAb 1F4 toward recombinant FnbpA,Trap, Mntc, IsdB of S. aureus and GapC from S. dysgalactiae was determined by indirect ELISA. (e) The reactivity of mAb 1F4 with the recombinant FnbpA, Trap, Mntc, IsdB proteins of S. aureus and GapC from S. dysgalactiae was determined by western blotting. (f) Effect of 1F4 on GapC GAPDH activity of S. aureus. The OD values were detected at 20 s (A1) and 5 min and 20 s (A2), the decrease of NADH reflected the level of GAPDH activity at OD340. The data represent the means ± SEM (n = 3). (g) Passive immunization with mAb 1F4 protects against S. aureus infection. Mice were injected intraperitoneally with 5×108 CFU of S. aureus (Newman) followed by the intravenous injection of anti-GapC serum, mAb 1F4, or SP2/0 cell supernatant (nagative control) after 24 h (n = 10).
To research the protective effect of mAb 1F4, a passive immunoprotection experiment against S. aureus (Newman) infection was conducted. The results showed that mice in the negative control group died within 20 h after challenge. However, the survival time of mice immunized with mAb 1F4 were 30 h longer than the negative control group (Fig 2G). These results suggest that, although not as effective as GapC anti-serum, mAb 1F4 offered a certain degree of immunoprotection against S. aureus infection.
Phage-displayed epitopes biopanned by the mAb
The random PhD-12 phage display peptide library was screened with mAb 1F4, and the eluted phage titers were determined by sandwich ELISA after three rounds of biological panning. The phages that were non-specifically bound to mAb 1F4 were removed during the panning. The yield of phages with high specific binding to mAb 1F4 increased continuously with three rounds of panning, proving that the specific phages were enriched (S1 Table). We selected 30 clones for mAb 1F4 and analyzed them by sandwich ELISA. Twelve clones showed strong, positive reactions with mAb 1F4 (Fig 3). We sequenced the positive phages and analyzed the results by aligning the GapC amino acid sequences using DNASTAR software. The amino acid sequences of the 12 clones showed the consensus motif GYTEDEIV (S2 Table). The GYTEDEIV motif is located at amino acids 272–279 of the S. aureus GapC and it is likely one of the B-cell epitopes of S. aureus GapC.
Fig 3. Identification of strongly positive phage clones selected by 1F4 using a sandwich ELISA.
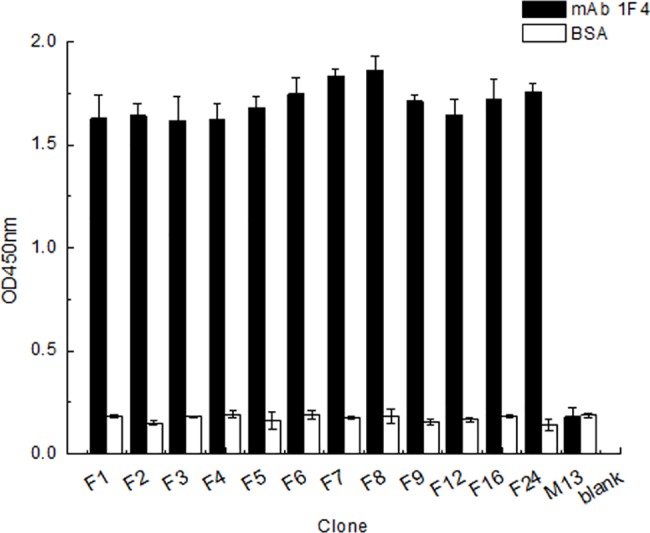
The negative control was wild-type M13 phage and the blank control was E. coli ER2738. BSA-coated wells were used to exclude cross-activity.
Key amino acids of the epitope
Every amino acids of the 272GYTEDEIV 279 motif was mutated to alanine to verify the epitope exactly (S3 Table). Plasmid pGEX-6P-1 was used to express the mutated gapC genes which were GST-fusion protein (S2 Fig). Western blotting was performed with mAb 1F4 as the primary antibody. The results showed that the G272A, D276A, E277A, I278A, and V279A mutations completely disrupted the reactivity of the epitope with mAb 1F4 (Fig 4). The results confirmed that the 272GYTEDEIV279 motif was indeed the B-cell epitope of the S. aureus GapC protein, which represents the most basic reactive unit of the epitopes recognized by mAb 1F4.
Fig 4. Precisely defining the epitopes.
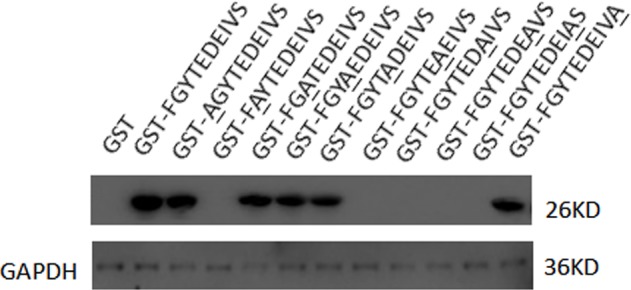
The reactivity of the fusion proteins, in which each amino acid (271FGYTEDEIVS280) was mutated to alanine, with mAb 1F4 was identified by western blotting. The G272A, D276A, E277A, I278A, and V279A mutations completely disrupted the reactivity of the epitope with mAb 1F4.
The epitope recognized by mAb 1F4 is located on the cell surface
To further verify the localization of the 272GYTEDEIV279 epitope on S. aureus Newman cells, we used mAb 1F4 as the primary antibody and a FITC goat anti-mouse antibody as the secondary antibody to perform an indirect immunofluorescence assay. The results showed that the mAb 1F4 group showed obvious green fluorescence and the same bacterial morphology was observed in the brightfield (Fig 5). Moreover, the fluorescence was located on the surface of the bacteria, indicating that the 272GYTEDEIV279 B-cell epitope was exposed on the surface of the bacteria.
Fig 5. Analysis of the 272GYTEDEIV279 epitope by confocal laser-scanning microscopy.
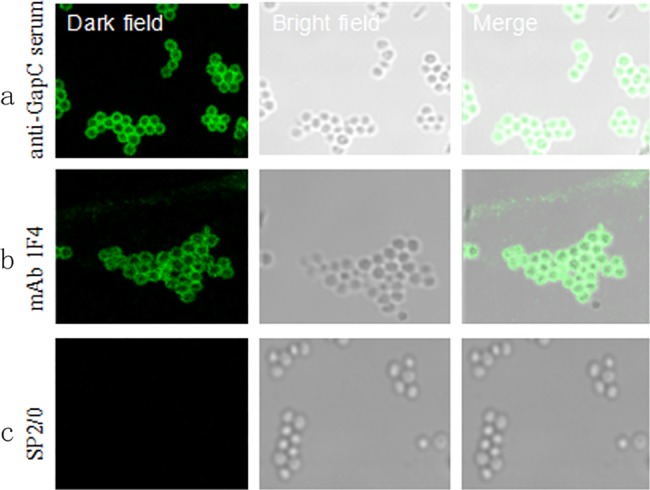
The left panels show dark field fluorescence photos; the middle panels show bright field photos of the bacteria; and the right panels show the two fields combined. (a–c) The S. aureus (Newman) strain was observed. Primary antibodies were S. aureus anti-GapC serum, mAb 1F4, and the SP2/0 supernatant, respectively.
The 272GYTEDEIV279 epitope is highly conserved
To identify whether the selected epitope was conserved in different S. aureus strains and other microbial species, the amino acid sequences of the epitope was compared with those of different strains using the BLAST at the NCBI (Table 2). The results of the homology analysis revealed that the 272GYTEDEIV279 motif is highly conserved in Staphylococcus spp.
Table 2. Alignment of the sequences surrounding the epitope-coding region of the GapC protein from different S. aureus strains.
| Species | Epitope motif |
|---|---|
| Staphylococcus aureus (AQD18932) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus (SGV41094) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus W52298 (EYG72453) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus (SCS75121) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus (SHD85683) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus Chi-8 (EZX77242) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus H85008 (EWH57997) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus DAR5841 (EXN96039) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus subsp. aureusUSA300 (EES93581) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus aureus subsp. aureusUSA300 (ABD21603) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus devriesei (AGB07469) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus epidermidis (AAM28559) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus epidermidis (AIR83029) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus epidermidis (ACH89386) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus argenteus (SGX11541) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus argenteus (SGW47323) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus haemolyticus (ADF49531) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus haemolyticus (KQC20862) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus gallinarum (KIR10623) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus gallinarum (ABC69184) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus xylosus (AAM28566) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus xylosus (AID43350) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus succinus (ACQ91188) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus succinus (ACQ91186) | SNESFGYTEDEIVSSDVVG |
| Staphylococcus petrasii subsp. jettensis (AEM98035) | SNESFGYTEDEIVSSDVVG |
Note: The GenBank accession numbers of the used strains are indicated in parentheses. The homologous amino acid residues of the epitope motif are bold.
anti-epitope serum promotes the phagocytic activity of macrophages
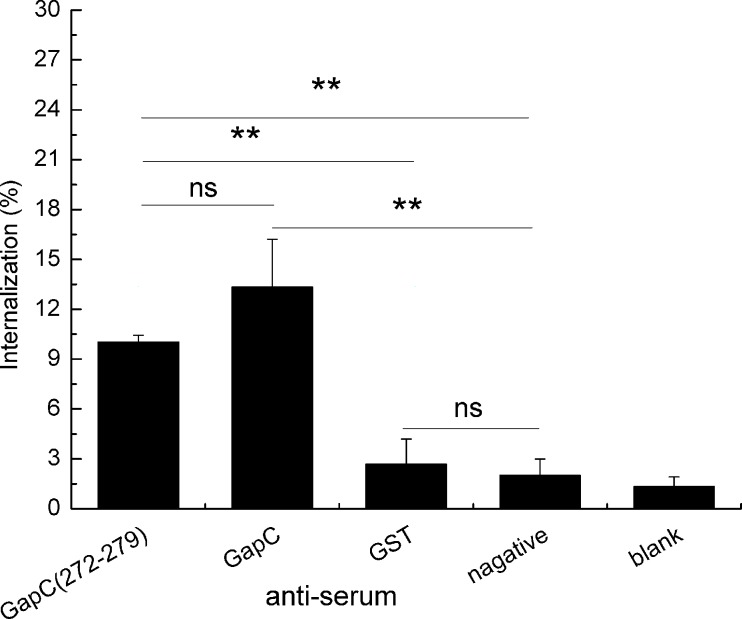
To determine whether the anti-epitope serum had the ability to promote macrophage phagocytosis, we incubated S. aureus with serum. Anti-GapC (272–279) serum was used as the experimental control serum. Anti-GapC serum and negative serum were used as the positive control and negative control serum, respectively. The bacteria were co-cultured with mouse peritoneal macrophages, and the number of phagocytosed bacteria was measured after the cells burst (Fig 6). The results showed that the anti-epitope serum-mediated phagocytosis was significantly higher than that obtained using the GST anti-serum and the negative serum (p < 0.01). The internalization rates of the posotive control and anti-epitope prptide serum group were similar. These results suggest that the 272GYTEDEIV279 B-cell epitope is a functional epitope.
Fig 6. anti-epitope serum-mediated phagocytosis of S. aureus Newman.
PBS-washed Newman (2×105 CFU) was incubated with 50 μl of the serum for 30 min. The negative control was incubated with the negative serum and the blank control comprised only phagocytes and bacteria. Differences in the internalization rates between anti-epitope peptide serum group and anti-negative serum group was statistically significant. The data represent the means ± SEM (n = 3). Statistical significance was measured using a Student’s t-test (**, p < 0.01).
The 272GYTEDEIV279 epitope peptide has an immunoprotective against S. aureus infection
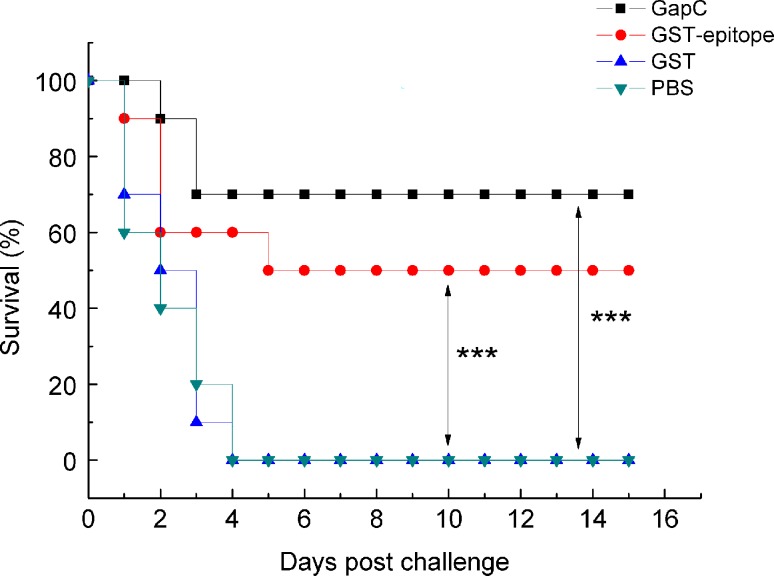
To detect the ability of the epitope to induce an immune response in vivo, we selected 6–8 week-old female BALB/c mice for immunization. An indirect ELISA was used to detect antibody titers of immunized mice 10 days after the third immunization. The results showed that the antibody titer of the GapC protein and the GST-epitope peptide was 1:256,000 and 1:32,000. These results indicated that the B-cell epitope may also induce immune protection. Then, we challenged the mice intraperitoneally with S. aureus. The recombinant GapC group had the lowest mortality rate of 30%, and the immunoprotection of the 272GYTEDEIV279 epitope had the second lowest mortality rate of 50%. In contrast, GST and PBS did not induce protective immune responses, and both this two groups of mice died within a short time after lethal challenge (Fig 7). These results suggest that the 272GYTEDEIV279 B-cell epitope could induce strong immunoprotection against S. aureus infection, which is consistent with previous results.
Fig 7. The 272GYTEDEIV279 epitope peptide confers protection against lethal challenges with S. aureus Newman.
For the prophylaxis study, immunized and control mice (6 weeks old, n = 10) were challenged intraperitoneally with S. aureus (equivalent to 5 × 108 CFU) two weeks after the last immunization. Mice were monitored for 15 days. The survival rates of GapC, GST-epitope were 70% and 50%, respectively. Statistical differences were determined by Student’s t-test. (***, p < 0.001).
Tertiary structure of the epitope
Bioinformatics is an efficient way to analyze proteins. Thus, the PDB (http://www.rcsb.org/pdb/home/home.do) method and the PyMOL tool were used to analyze the tertiary structure of GapC and the epitope (Fig 8). The results showed that the epitope 272GYTEDEIVSSD282 has a coli structure and exposed on the surface of GapC protein.
Fig 8. Structural analysis of the linear B-cell epitope.
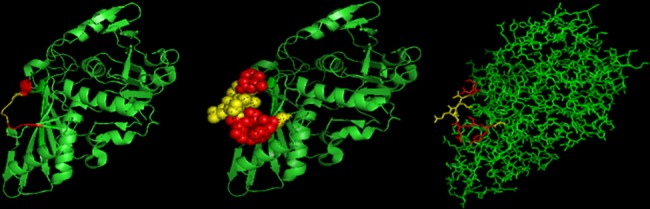
The PDB Protein Data Bank method was used to analyze the three-dimensional model of S. aureus GapC. The residues of the linear B-cell epitope that are recognized by mAb 1F4 are highlighted in red and shown in cartoon, stick, and dot formats, respectively. The core amino acids are shown in yellow.
Discussion
Staphylococcus aureus is rapidly developing resistance to antibiotics, which leads to life-threatening infections, especially in patients whose immune systems have been compromised by other diseases. The prolonged use of antibiotics inhibits antibiotic-sensitive strains and alters the intestinal flora, resulting in S. aureus infections, for instance, S. aureus enteritis. Similarly, S. aureus also causes many medical device-related infections. For animals, S. aureus also causes various animal infections. Because of the prevalence of these infections and the lack of new antibiotics, immunotherapy for S. aureus has begun to be studied extensively. Previous studies have shown that traditional inactivated vaccines and capsular polysaccharide vaccines are not ideal for immunoprotection [37, 38]. One reason is that S. aureus has a variety of capsular types and lacks cross-immune protection between the capsular types. Moreover, the immunogenicity of capsular polysaccharides is weak, which prevents the induction of a strong immune response. In recent years, many studies have focused on subunit vaccines [39, 40]. A variety of antigenic proteins has shown good immunogenicity and can induce organisms to produce an effective immune response. However, because of a plethora of pathogenic factors and the complicated nature of S. aureus pathogenesis, clinical tests of subunit vaccines produced with a single antigen are still unsatisfactory [41]. Multi-antigen subunit vaccines can achieve significantly improved the immune protection, especially in the presence of adjuvants that can induce cellular immune responses, thereby effectively stimulating a host to produce effective cellular and humoral immune responses. However, the large antigenic molecules and limited expression capacity severely constrains the number of subunits that can be incorporated into multi-antigen subunit vaccines. In contrast, epitope vaccines contain only protective epitopes and are relatively safe, and a more effective immune response can be achieved by connecting several epitopes. Therefore, identifying effective B-cell epitopes of surface antigens is critical for the generation of epitope vaccines against S. aureus.
Previous studies have shown that GapC, which is located on the surface of the cytomembrane of S. aureus, is highly conserved and capable of inducing a protective immune response [18, 20]. Here we generated a mAb against S. aureus GapC. The results showed that mAb 1F4 belonged to the IgG1 subclass and the κ chain. Furthermore, mAb 1F4 only recognized the GapC protein of S. aureus, and passive immunization with mAb 1F4 significantly increased the survival rate of mice after a S. aureus challenge, proving that mAb 1F4 could offer protection against S. aureus infection. To further analyze the minimal unit of the GapC protein that bound to mAb 1F4, we identified the linear B-cell epitope 272GYTEDEIVSSD282 by screening a phage-displayed random 12-mer peptide library with mAb 1F4. The conservation of the B-cell epitope was demonstrated by aligning the epitope sequences of more than 100 different Staphylococcus strains through the BLAST tool at the NCBI, 25 of which were picked randomly and listed in Table 2. The results suggest that the 272GYTEDEIVSSD282 motif, which is highly conserved and capable of inducing a protective immune response, is a highly conserved epitope on the GapC protein of Staphylococcus species. The high degree of conservation provides the possibility for this B-cell epitope to become a potential component of a multi-epitope vaccine, especially to treat Staphylococcus epidermidis infections, which have been increasing gradually in recent years. The results of the site-directed mutagenesis analysis showed that mutating the residues G272, D276, E277, I278, and V279 to alanine completely abrogated the reactivity of GapC with mAb 1F4. Therefore, G272, D276, E277, I278, and V279 constitute the core of the 272GYTEDEIVSSD282 motif.
Analysis of the tertiary structure of the epitope showed that the epitope 272GYTEDEIVSSD282 has a coli structure that is likely exposed on the surface of the GapC protein. A confocal laser-scanning microscopic analysis demonstrated that this B-cell epitope was recognized by purified mAb 1F4, as green fluorescence showed that the 272GYTEDEIVSSD282 epitope was exposed on the bacterial surface. At the same time, the B-cell epitope antisera promoted the opsonophagocytic activity of macrophages. Furthermore, positive immunization with the B-cell epitope peptide showed that it could induce effective immunoprotection against a S. aureus infection. All the above data indicate that the B-cell epitope 272GYTEDEIVSSD282 is a highly conserved, efficient, and immunodominant epitope, and it has great application potential in the future.
Conclusions
The data presented here demonstrate that anti-GapC mAb 1F4 might be used in immunotherapeutic treatment of S. aureus infections, and the B-cell epitope 272GYTEDEIVSSD282 might be a promising epitope for a multi-epitope vaccine.
Supporting information
The full-length GapC gene was cloned into pET-32a vector; Lane M, Trans5K DNA Marker (Left) and Trans2K DNA Marker (Right); Lane 1, pET-32a-GapC digested with BamH I and Hind III; Lane 2, positive clone identified by PCR.
(PDF)
(PDF)
(PDF)
Consensus amino acid motifs are shown in bold and underlined.
(PDF)
The mutational sites are shown in lowercase letters. The mutated amino acids are bold and underlined.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Natural Science Foundation of Heilongjiang Province of China (ZD2016004) and Research Innovation Program for College Graduates of Heilongjiang Bayi Agricultural University (YJSCX2017-Y62).
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998; 339(8): 520–32. doi: 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 2.Sheagren JN. Staphylococcus aureus. The persistent pathogen (Second of two parts). N Engl J Med. 1984; 310(22): 1437–1442. doi: 10.1056/NEJM198405313102206 [DOI] [PubMed] [Google Scholar]
- 3.O’Gara JP, Humphreys H. Staphylococcus epidermidis biofilms: importance and implications. J Med Microbiol. 2001; 50(7): 582–587. doi: 10.1099/0022-1317-50-7-582 [DOI] [PubMed] [Google Scholar]
- 4.Howden BP, Davies JK, Johnson PD, Stinear TP, Gravson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin- intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010; 23(1): 99–139. doi: 10.1128/CMR.00042-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pe ´richon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009; 53(11): 4580–4587. doi: 10.1128/AAC.00346-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locke JB, Hilgers M, Shaw KJ. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700). Antimicrob Agents Chemother. 2009; 53(11): 4580–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendes RE, Deshpande LM, Castanheira M, DiPersio J, Saubolle MA, Jones RN. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Ch. 2008; 52(6): 2244–2246. doi: 10.1128/AAC.00231-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006; 355(7): 653–665. doi: 10.1056/NEJMoa053783 [DOI] [PubMed] [Google Scholar]
- 9.Cadilla A, David MZ, Daum RS, Boyle-Vavra S. Association of high-level mupirocin resistance and multidrug-resistant methicillin resistant Staphylococcus aureus at an academic center in the Midwestern United States. J Clin Microbiol. 2011; 49(1): 95–100. doi: 10.1128/JCM.00759-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verkaik NJ, van Wamel WJ, van Belkum A. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy. 2011; 3(9):1063–73. doi: 10.2217/imt.11.84 [DOI] [PubMed] [Google Scholar]
- 11.Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. 2012; 54(8): 1179–86. doi: 10.1093/cid/cis033 [DOI] [PubMed] [Google Scholar]
- 12.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998; 6(12): 484–488. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Cui Y, Zhang J, Zhu Z, Piao F. Cloning and expression of Staphylococcus aureus surface protein Isdb and its immune experiment in mice. 2011; 27(4): 566–71. [PubMed] [Google Scholar]
- 14.Yang Y, Qian M, Yi S, Li B, Yu R, Guo Q, et al. Monoclonal Antibody Targeting Staphylococcus aureus Surface Protein A (SasA) Protect Against Staphylococcus aureus Sepsis and Peritonitis in Mice. PLoS One. 2016; 11(2): e0149460 doi: 10.1371/journal.pone.0149460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delfani S, Mohabati Mobarez A. Protection of mice against Staphylococcus aureus infection by a recombinant protein ClfA-IsdB-Hlg as a vaccine candidate. Med Microbiol Immunol. 2016; 205(1): 47–55. doi: 10.1007/s00430-015-0425-y [DOI] [PubMed] [Google Scholar]
- 16.Cheung AL, Fischetti VA. The role of fibrinogen in staphylococcal adherence to catheters in vitro. JInfect Dis. 1990; 161: 1177–1186. [DOI] [PubMed] [Google Scholar]
- 17.Kerro-Dego O, Prysliak T, Perez-Casal J, Potter AA. Role of GapC in the pathogenesis of Staphylococcus aureus. Vet Microbiol. 2012; 156(3–4): 443–447. doi: 10.1016/j.vetmic.2011.11.018 [DOI] [PubMed] [Google Scholar]
- 18.Kerro-Dego O, Prysliak T, Potter AA, Perez-Casal J. DNA-protein immunization against the GapB and GapC proteins of a mastitis isolate of Staphylococcus aureus. Vet Immunol Immunopathol. 2006; 113(1–2): 125–138. doi: 10.1016/j.vetimm.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Fan Z, Ma J, Tong C, Song B, Zhu Z, et al. Cross-protective effect of a novel multi-antigen-chimeric vaccine against Streptococcus and Staphylococcus aureus infection in mice. J Med Microbiol. 2014; 63: 1732–1740. doi: 10.1099/jmm.0.073593-0 [DOI] [PubMed] [Google Scholar]
- 20.Perez-Casal J, Prysliak T, Kerro-Dego O, Potter AA. Immune responses to a Staphylococcus aureus GapC/B chimera and its potential use as a component of a vaccine for S. aureus mastitis. Vet Immunol Immunopathol. 2006; 109(1–2): 85–97. doi: 10.1016/j.vetimm.2005.07.024 [DOI] [PubMed] [Google Scholar]
- 21.Ren J, Song J, Ellis J, Li J. Staged heterogeneity learning to identify conformational B-cell epitopes from antigen sequences. BMC Genomics. 2017; 18(Suppl 2): 113 doi: 10.1186/s12864-017-3493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverstein AM. History of Immunology. Cell immunol. 1982; 67(2): 396–409. [DOI] [PubMed] [Google Scholar]
- 23.Fontaine MC, Perez-Casal J, Song XM, Shelford J, Willson PJ, Potter AA. Immunisation of dairy cattle with recombinant Streptococcus uberis GapC or a chimeric CAMP antigen confers protection against heterologous bacterial challenge. Vaccine. 2002; 20(17–18): 2278–2286. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Zhang H, Fan Z, Zhou X, Yu L, Sun H, et al. Identification of a Concerved Linear B-Cell Epitope of Streptococcus dysgalactiae GapC Protein by Screening Phage-Displayed Random Peptide Library. 2015. June 29;10(6):e0131221 doi: 10.1371/journal.pone.0131221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256(5517): 495–497. [DOI] [PubMed] [Google Scholar]
- 26.Mazzarotto GA, Raboni SM, Stella V, Carstensen S, de Noronha L, Levis S, et al. Production and characterization of monoclonal antibodies against the recombinant nucleoprotein of Araucaria hantavirus. J Virol Methods. 2009; 162(1–2): 96–100. doi: 10.1016/j.jviromet.2009.07.022 [DOI] [PubMed] [Google Scholar]
- 27.Yu Y, Wang H, Zhao L, Zhang C, Jiang Z, Yu L. Fine mapping of a foot-and-mouth disease virus epitope recognized by serotype-independent monoclonal antibody 4B2. J Microbiol. 2011; 49(1): 94–101. doi: 10.1007/s12275-011-0134-1 [DOI] [PubMed] [Google Scholar]
- 28.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006; 188(24): 8421–8429. doi: 10.1128/JB.01335-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korem M, Gov Y, Kiran MD, Balaban N. Transcriptional profiling of target of RNAIII-activating protein, a master regulator of staphylococcal virulence. Infect Immun. 2005; 73(10): 6220–6228. doi: 10.1128/IAI.73.10.6220-6228.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida JF, Brevner NM, Mahi M, Ahmed B, Benbouziane B, Boas PC, et al. Expression of fibronectin binding protein A (FnBPA) from Staphylococcus aureus at the cell surface of Lactococcus lactis improves its immunomodulatory properties when used as protein delivery vector. Vaccine. 2016; 34(10): 1312–1318. doi: 10.1016/j.vaccine.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 31.Salazar N, Castiblanco-Valencia MM, da Silva LB, de Castro I, Monaris D, Masuda HP, et al. Staphylococcus aureus manganese transport protein C (MntC) is an extracellular matrix- and plasminogen-binding protein. PLoS One. 2014; 9(11): e112730 doi: 10.1371/journal.pone.0112730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song B, Yang X, Sun H, Yu L, Ma J, Wu Z, et al. Immunogenicity of amino acids 1–150 of Streptreptococcus GapC displayed on surface of Eschrichia coli. Microb Pathog. 2017; 105: 288–297. doi: 10.1016/j.micpath.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Ning YS, Wang YD, Luo J, Wang W, Dong WQ, et al. Production of mouse monoclonal antibodies against Helicobacter pylori Catalase and mapping the antigenic epitope by phage display library. Vaccine. 2008; 26(9): 1263–1269. doi: 10.1016/j.vaccine.2007.11.055 [DOI] [PubMed] [Google Scholar]
- 34.Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Protein F1 is required for efficient entry of Staphylococcus pyogenes into epithelial cells. J Infect Dis. 1998; 178(1): 147–158. [DOI] [PubMed] [Google Scholar]
- 35.Eyal O, Jadoun J, Bitler A, Skutelski E, Sela S. Role of M3 protein in the adherence and internalization of an invasive Streptococcus pyogenesstrain by epithelial cells. FEMS Immunol Med Microbiol. 2003; 38(3): 205–213. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee S, Dutta D, Saha B, Das AK. Crystal Structure of Glyceraldehyde-3-Phosphate Dehydrogenase 1 from Methicillin-Resistant Staphylococcus aureus MRSA252 Provides Novel Insights into Substrate Binding and Catalytic Mechanism. J Mol Biol. 2010; 401(5): 949–68. doi: 10.1016/j.jmb.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 37.Watson DL, Kennedy JW. Immunisation against experimental staphylococcal mastitis in sheep-effect of challenge with a heterologous strain of Staphylococcus aureus. Aust Vet J. 1981; 57(7): 309–313. [DOI] [PubMed] [Google Scholar]
- 38.Lee JC. An experimental vaccine that targets staphylococcal virulence. Trend Mierobiol, 1998; 6(12): 461–463. [DOI] [PubMed] [Google Scholar]
- 39.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006; 103(45): 16942–16947. doi: 10.1073/pnas.0606863103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman D. Veronate (Inhibitex). Curr Opin Investig Drugs. 2006; 7(2):172–179. [PubMed] [Google Scholar]
- 41.Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infec. 2009; 15(2): 156–164. doi: 10.1111/j.1469-0691.2008.02648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full-length GapC gene was cloned into pET-32a vector; Lane M, Trans5K DNA Marker (Left) and Trans2K DNA Marker (Right); Lane 1, pET-32a-GapC digested with BamH I and Hind III; Lane 2, positive clone identified by PCR.
(PDF)
(PDF)
(PDF)
Consensus amino acid motifs are shown in bold and underlined.
(PDF)
The mutational sites are shown in lowercase letters. The mutated amino acids are bold and underlined.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.