Abstract
Background
Short interpregnancy intervals (SIPI) have been associated with increased risks for adverse neonatal outcomes including preterm delivery and infants small for gestational age (SGA). It has been suggested that mechanistically, adverse neonatal outcomes after SIPI arise due to insufficient recovery of depleted maternal folate levels prior to the second pregnancy. However, empirical data are lacking regarding physiological folate levels in pregnant women with SIPI, and relationships between quantified physiological folate levels and outcomes like SGA. Therefore, we sought to test two hypotheses, specifically that compared to controls, women with SIPI would: a) have lower red blood cell folate (RBCF) levels and b) be more likely to have SGA infants (defined as <10th percentile).
Methods
Using data collected in BC, Canada for a larger study on perinatal psychopathology, we documented supplementation use and compared prenatal RBCF levels and proportion of SGA infants between women with SIPI (second child conceived ≤24 months after previous birth, n=26) and matched controls (no previous pregnancies, or >24 months between pregnancies, n=52).
Results
There were no significant differences in either mean RBCF levels (Welch’s t-test, p = 0.7) or proportion of SGA infants (Fisher’s exact test, p = 0.7) between women with SIPI and matched controls.
Conclusion
We report the first data about RBCF levels in the context of SIPI. If confirmed, our finding of no relationship between these variables in this population suggests that continued FA supplementation following an initial pregnancy mitigates folate depletion. We found no relationship between SIPI and SGA.
Keywords: Red Blood Cell Folate, Short Interpregnancy Intervals, Neonatal outcomes, Pre-term delivery, Maternal-Folate Theory, Small for Gestational Age, Folic Acid
Introduction
Low folate levels have been shown to have adverse effects on both pregnant mothers and developing children. Folate plays a critical role in fetal development, and demand for folate increases dramatically during pregnancy due to its involvement in nucleic acid synthesis (Scholl and Johnson 2000). Insufficient folate during pregnancy interferes with cell division, thus hindering fetal development (Lucock 2000). Folic acid (FA) supplementation prior to and during pregnancy decreases the risk of neural tube defects and other congenital anomalies (Anzaku and Musa 2013; Blencowe et al. 2010; van Beynum et al. 2010). This observation drove the implementation of fortification of grains with FA in many other countries, including Canada in 1998 (Blencowe et al. 2010; Centers for Disease Control 1992). However, despite the fortification of food, Health Canada recommends a daily intake of 400 mcg of FA from supplements for all women who have a chance at becoming pregnant.
Shorter intervals (typically 24 months or less) between sequential pregnancies correlate with increased risks for adverse neonatal outcomes such as early neonatal death (Wendt et al. 2012), low birth weight (van Eijsden et al. 2008; Conde-Agudelo et al. 2006; Zhu 2005 ), and preterm delivery (Chen et al. 2015; Scholl et al. 1996; Conde-Agudelo et al. 2006; Ball et al. 2014). It has been suggested that these outcomes may be the result of depletion of maternal nutrient reserves –in particular, folate. Short interpregnancy intervals (SIPI) provide less time between pregnancies to replenish reserves of micronutrients such as folate, thus increasing the risk for negative sequellae in the later child (Smits and Essed 2001; van Eijsden et al. 2008; Conde-Agudelo et al. 2012). However, to our knowledge, no studies to date have empirically investigated physiological maternal folate levels in the context of SIPI. Thus, in this Canadian study, for women with SIPI and matched controls, we aimed to compare a) maternal red blood cell folate levels during pregnancy, and b) frequencies with which infants are born small for gestational age (SGA).
Materials and methods
Overview of procedures
Data used for this study were collected in the context of a larger (n=365), prospective longitudinal study on perinatal psychopathology. The recruitment methods, as well as the procedures for determining red blood cell folate (RBCF) levels have been described (Yaremco et al. 2013). At the enrollment visit during pregnancy (median gestational age = 33 weeks) each participant completed questionnaires regarding demographics, previous pregnancies, medication usage, FA supplementation (including: timing of initiation in relation to conception, as well as current dose and frequency), and had a blood sample collected from which RBCF was measured (see below).
Participants
From the participants in the larger study, we selected all those with SIPI, defined as conception of index pregnancy (calculated from date of the last menstrual period) ≤24 months after the date of a previous delivery (Nilsen et al. 2014). Each participant was then matched with two controls, each also chosen from participants in the larger study. We used a pseudo-randomization technique that aims to control for variables that are known to be correlated with the outcomes, but that are not of direct interest to the study (Stuart 2010). Therefore, each SIPI participant was matched to two controls by the following criteria (listed in terms of priority from most critical to least critical): timing of initiation of FA supplementation in relation to the pregnancy, dosage of FA supplement use, and maternal age. Some SIPI participants had been taking folate supplements for >2 years prior to conception of the index pregnancy, which made precise control matching for this variable impossible. Therefore, given that red blood cells (in which we were measuring folate) turn over approximately every 4 months (Hébert 1999), we matched these participants with controls who had been taking FA supplements for ≥4 months prior to conception.
Measurement of RBCF
Procedures for Quantaphose II radioimmunoassay analysis of RBCF used in this study have been described previously (Yaremco et al. 2013).
Assessment of Infant Birthweights
Infant birth weight was confirmed from the hospital discharge record following delivery. In accordance with the guidelines for categorizing infants as SGA, the infants in this study were categorized as SGA when they fell below the 10th percentile for gestational age and sex (Perinatal Services BC 2016).
Analysis
All analysis was carried out in R version 3.3.2 (R Core Team 2016).
We used Welch’s t-test and Fishers exact test respectively, to test our two main hypotheses. Specifically, we hypothesized that compared to controls, women with SIPI would: a) have lower RBCF levels and b) be more likely to have SGA infants. Subsequently, we also further subdivided our SIPI category (<12 months, 12–24 months).
As a secondary, exploratory analyses, we used linear regression to describe the relationships between 1) RBCF levels and: gestational age (days) at delivery infant birthweight (grams), and, interpregnancy interval (months), and 2) interpregnancy interval (months) and: gestational age (days) at delivery, or infant birthweight (grams).
Results
Forty of the 365 participants in the larger study had interpregnancy intervals that met the criteria for SIPI. Of these, 14 had incomplete data and were excluded, leaving 26 participants in the study (median age 33 years; range: 21–40 years). We matched these women (according to the process described above) to 52 controls (median age: 32 years, range: 15–46 years). Demographic data are provided for all participants in Table 1.
Table 1.
Demographics for short interpregnancy interval participants and controls
| Characteristic | SIPI (n=26) n(%) |
Controls (n=52) n(%) |
Total (n=78) n(%) |
|---|---|---|---|
| Ethnicity | |||
| European | 21 (81) | 42 (81) | 63 (81) |
| Asian | 1 (4) | 1 (2) | 2 (3) |
| Mixed | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 4 (15) | 8 (15) | 12 (15) |
| Marital status | |||
| Single | 0 (0) | 3 (6) | 3 (4) |
| Married/living with/partnered | 26 (100) | 49 (94) | 75 (96) |
| Highest education level | |||
| Completed high school | 25 (96) | 32 (61) | 57 (73) |
| Some post-secondary | 24 (92) | 11 (21) | 35 (45) |
| Income | |||
| <$20, 000 | 4 (15) | 0 (0) | 4 (5) |
| $20, 000 – $40, 000 | 4 (15) | 7 (13) | 11 (14) |
| $41, 000 – $60, 000 | 5 (20) | 4 (8) | 9 (12) |
| $61, 000 – $80, 000 | 4 (15) | 13 (25) | 17 (22) |
| $81, 000 – $100, 000 | 6 (23) | 6 (12) | 12 (15) |
| >$100, 000 | 3 (12) | 11 (21) | 14 (18) |
| Psychiatric history | |||
| Major depressive disorder | 18 (69) | 43 (83) | 61 (78) |
| Bipolar disorder | 8 (31) | 8 (15) | 16 (21) |
| Taking psychotropic medication2 | 2 (8) | 4 (8) | 6 (12) |
| Number of Children Prior to Index Pregnancy | |||
| 0 | 0 (0) | 36 (70) | 36 (46) |
| 1 | 25 (96) | 13 (25) | 38 (49) |
| 2 | 0 (0) | 2 (4) | 2 (3) |
| 3 | 1 (4) | 1 (2) | 2 (3) |
Psychotropic medication that participants were taking, that could interfere with folate metabolism, include citalopram, epival, lorazepam, paroxetine, sertraline, topamax (Lambie and Johnson 1985; Linnebank et al. 2011)
All participants were taking supplemental FA (mean 1150mcg/day, range: 800–4800mcg/day), data about duration of use, RBCF levels, and gestational age at delivery are shown in Table 2. One infant was born pre-term (36 weeks), to a woman in the control group.
Table 2.
Overview of red blood cell folate levels and folic acid supplement use for participants with short interpregnancy intervals and controls
| Characteristics | SIPI (n=26) | Control (n=52) | Total (n=78) | SIPI ≤ 12 (n=13) | SIPI >12 ≤ 24 (n=13) |
|---|---|---|---|---|---|
| Mean RBC Folate (nmol/L) | 1490.58 | 1530.63 | 1517.28 | 1579.27 | 1401.90 |
| Mean Start of FA Supplementation (weeks prior to conception)* | 40.22 | 36.63 | 37.82 | 33.93 | 46.50 |
| Mean gestational age at time of blood draw (weeks) | 31.19 | 32.27 | 31.91 | 30.92 | 31.46 |
| Mean gestational age at delivery (weeks) | 39.58 | 39.77 | 39.71 | 39.77 | 39.38 |
With respect to index pregnancy.
Range: 208 weeks prior to conception to 24 weeks gestation
Relationship between SIPI and RBCF levels
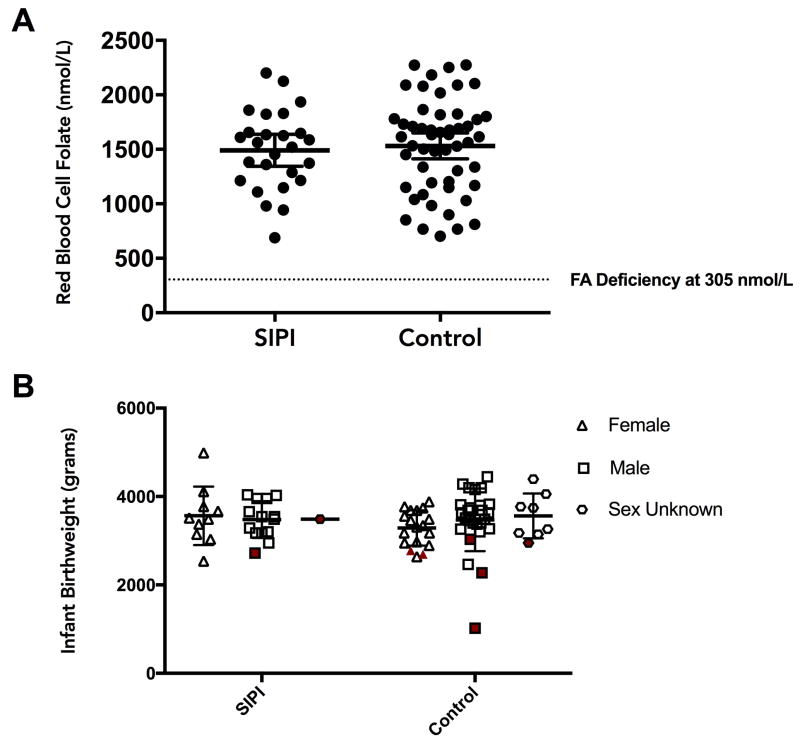
RCBF levels for SIPI and control groups are shown in Figure 1 (Panel A). There was no significant difference in mean RBCF levels between groups (t = −0.4, df = 57.9, p = 0.7), with Cohen’s d = 0.1 (95%CI = −0.37 to 0.58)1.
Figure 1. Comparisons of red blood cell folate levels and infant birthweights between women with short interpregnancy intervals and controls.
Panel A: Comparison of red blood cell folate (RBCF) levels in pregnancy between women with SIPI (n=26) and controls (n=52).
Panel B: Comparison of infant birthweights between women with SIPI (n=26) and controls (n=52). The shaded symbols indicate infants born small for gestational age (SGA). There were 9 infants where the sex was unknown, so birthweights were classified according to the thresholds set in the chart for combined population.
Relationship between SIPI and infants born SGA
Eight infants (two from the SIPI group, and six from the control group) were classified as SGA. Birthweights for infants of mothers in SIPI and control groups are shown in Figure 1 Panel B. There was no significant difference between groups in terms of the proportion of SGA infants (Fisher’s exact test p = 0.7), with Cramer’s V = 0.07 (95%CI = 0 to 0.29).
Exploratory analyses
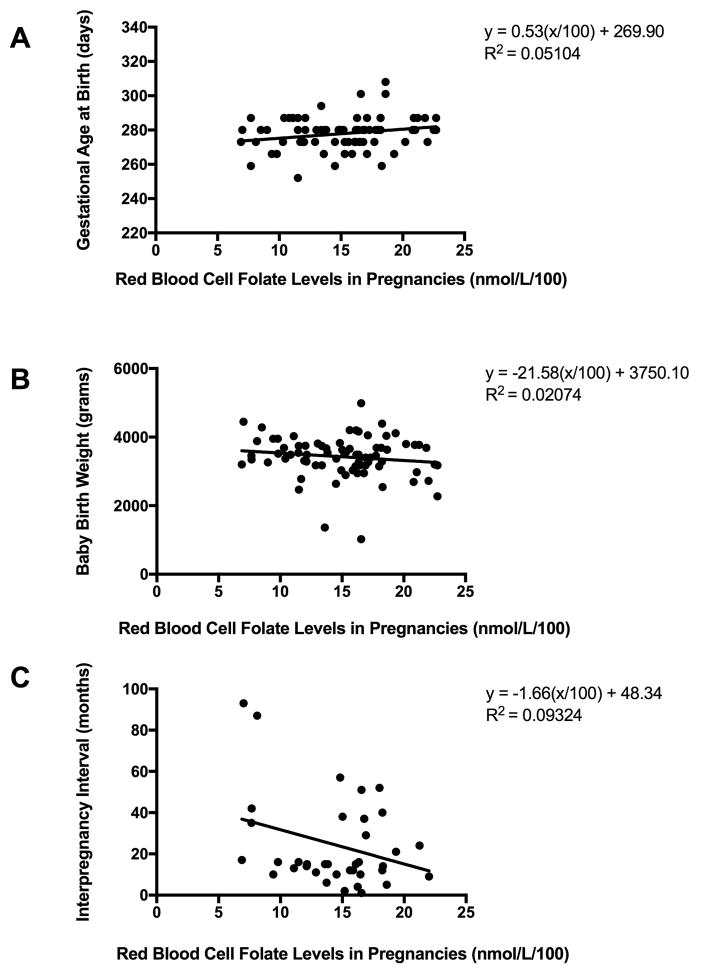
Relationships between RBCF levels and a) gestational age (days) at delivery, b) birthweight (grams), and c) interpregnancy interval (months) across all participants are shown in Figure 2. There was a marginally significant (p=0.047) relationship between RBCF and gestational age at delivery (with a very small effect size). There were no significant relationships between RBCF levels and birthweight (p=0.23), or interpregnancy interval and RBCF levels (p=0.059), small effect sizes were observed. See panels A, B, and C, respectively.
Figure 2. Red blood cell folate levels in pregnancy in relation to other variables.
Panel A: RBCF in relation to gestational age at delivery (days). Data from all women (n=78).
Panel B. RBCF in relation to infant birthweight (grams). Data from all women (n=78).
Panel C. RBCF in relation to interpregnancy intervals. Data from all non-primiparous women (n=39).
There were no significant relationships between interpregnancy interval and: gestational age at delivery (p=0.72) or infant birthweight (p=0.75), see Figure 3, panels A and B, respectively.
Figure 3. Interpregnancy interval in relation to infant birthweight and gestational age at delivery.
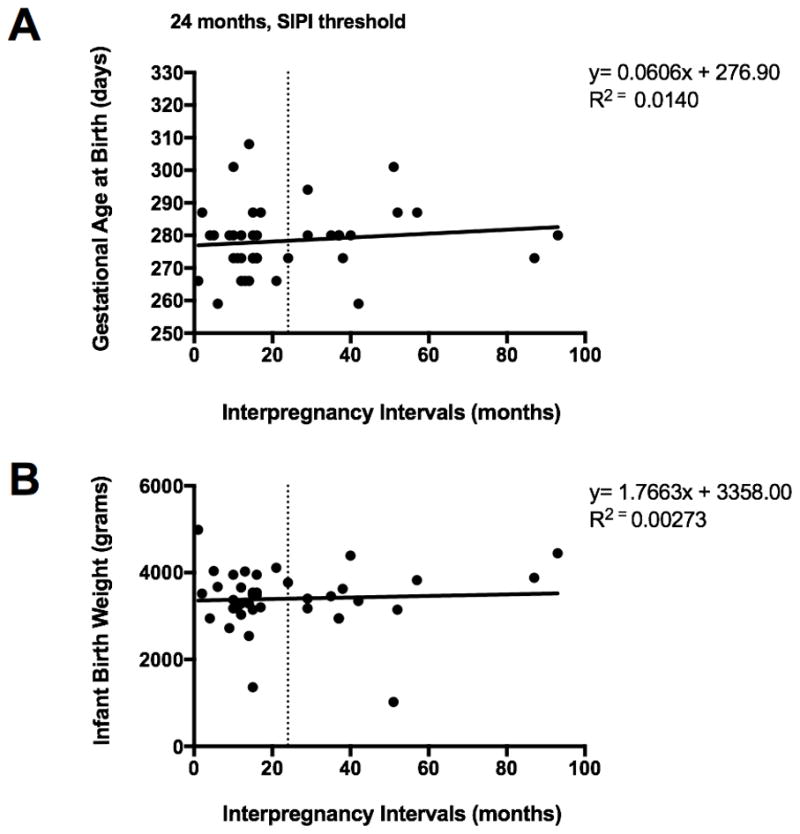
Panel A: Interpregnancy intervals (months) in relation to gestational age at delivery (days). Data from all non-primiparous women (n=39).
Panel B. Interpregnancy intervals (months) in relation to birthweight (grams). Data from all non-primiparous women (n=39).
Discussion
This is the first study to directly examine physiological maternal RBCF levels in women with SIPI, and the first to compare RBCF levels between pregnant women with and without SIPI. We identified no significant difference in RBCF levels between women with SIPI and controls. There are a few possible explanations for these observed results. One possibility is that a difference between the groups exists, but we could not detect it due to the sample size. Alternatively, it is possible that differences exist between these groups in other contexts, but were obscured in this study due to ceiling effects in this specific cohort – that is, the entire group of women studied had folate levels high enough for the effects of any variance to be obscured. With regard to ceiling effects, no women had RBCF levels that would be classified as clinically deficient, defined as <305 nmol/L (Colapinto et al. 2011, see Figure 2), and all were drawn from a population in which FA supplementation was maintained through consumption of fortified grains and/or continued use of FA supplements in the preconception period, with most of the SIPI women reporting to have continued their FA supplementation from their previous pregnancy. Therefore, at least in this population, with adequate FA supplementation, perhaps there is genuinely no significant relationship between SIPI and RBCF. Third, it is also possible that there is genuinely no meaningful relationship between RBCF levels and SIPI. The estimated effect size of SIPI on RBCF levels of 0.1 was in the very small range, but due to sample size the confidence intervals included d values in the moderate range (as high as 0.58). Of note, our exploratory analysis using interpregnancy interval as a continuous variable showed a non-significant trend towards lower RBCF with increased interpregnancy interval (see Figure 1, panel C). Combined, these data undermine confidence in the hypothesis that SIPI produces lower RBCF.
Similarly, there was no significant difference in the proportion of infants born who were classified as SGA between SIPI and control women. There are again a number of potential explanations for these findings. First, it is possible that a difference between the groups exists, but we could not detect it due to the sample size (the number of infants who were classified as SGA was small, n=8). The estimated effect size of the relationship between SIPI and SGA of 0.07 was in the very small range, but again (due to sample size) the confidence intervals include moderate effect sizes. Second, it is possible that the effect of SIPI was related to gestational age at delivery, but not proportion of SGA infants. However, our data do not support a relationship between SIPI and this measure (see Table 2 and Figure 3). Third, it is possible that there is genuinely no meaningful relationship between SIPI and proportion of infants born SGA. In fact, previous findings regarding the relationship between SIPI and fetal growth restriction are mixed.
While some have shown significant relationships (e.g. Smits et al. 2013), others have found no association (Basso et al. 1998, Klerman et al. 1998). The area is ripe for a meta-analytical approach.
Our finding of marginally significant association between RBCF and gestational age at delivery is in line with some prior literature suggesting a relationship between folic acid supplementation and preterm delivery. Specifically, one study found that rates of pre-term delivery were lowest in women with pre-conceptional folate supplementation of >1 year (Greenberg et al. 2011). Another study showed that the risk of pre-term delivery was highest amongst women taking <0.25mg folic acid daily (Scholl et al. 1996). Given that the effects seen in this study are small, and that other studies have found conflicting results (Sengpiel et al. 2014) this may warrant further study, particularly in populations where folate status is more variable.
Limitations
As discussed above, our study was conducted in a country where grains are fortified with folate, and involved a relatively small number of predominantly Caucasian women who were taking FA supplements, so is not generalizable beyond this setting, and findings should be considered preliminary.
Conclusion
These preliminary data highlight the importance of follow up studies in a larger cohort of women in pregnancy to better understand relationships between SIPI, folate levels during pregnancy, and adverse neonatal outcomes.
Supplementary Material
Acknowledgments
The study was funded by the Canadian Institute of Health Research (CIHR). JA was supported by the Canada Research Chairs Program, and BC Mental Health and Substance Use Services. The authors thank Roger Dyer and Janette King for the folate analyses, and the Translational Psychiatric Genetics Group (TPGG) for their varied contributions, and the team’s volunteers. All co-authors declare no relevant conflicts of interest.
Footnotes
For descriptive purposes, RBCF data from five women who participated in the main study twice (two sequential pregnancies, separated by a SIPI) are provided in the supplementary figure S1..
References
- Anzaku A, Musa J. Prevalence and associated risk factors for gestational diabetes in Jos, North-central, Nigeria. Arch Gynecol Obstet. 2013;6(5):859–863. doi: 10.1007/s00404-012-2649-z. [DOI] [PubMed] [Google Scholar]
- Ball SJ, Pereira G, Jacoby P, de Kler N, Stanley F. Re-evaluation of link between interpregnancy interval and adverse birth outcomes: Retrospective cohort study matching two intervals per mother. BMJ. 2014;349(jul23 1):g4333. doi: 10.1136/bmj.g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso O, Olsen J, Christensen K. Risk of preterm delivery, low birthweight and growth retardation following spontaneous abortion: A registry-based study in Denmark. Int J Epidemiol. 1998;27(4):642–646. doi: 10.1093/ije/27.4.642. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol. 2010;39(suppl_1):110–121. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Jhangri G, Lacasse M, Kumar M, Chandra S. Relationship between interpregnancy interval and adverse perinatal and neonatal outcomes in Northern Alberta. J Obstet Gynaecol. 2015;37(7):598–605. doi: 10.1016/S1701-2163(15)30197-3. [DOI] [PubMed] [Google Scholar]
- Colapinto C, O’Connor D, Tremblay Mark S. Folate status of the population in the Canadian health measures survey. CMAJ. 2011;183(2):E100–E106. doi: 10.1503/cmaj.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta A. Birth spacing and risk of adverse perinatal outcomes: A meta-analysis. JAMA. 2006;295(15):1809. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermúdez A, Castano F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plan. 2012;43(2):93–114. doi: 10.1111/j.1728-4465.2012.00308.x. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Puho EH, Langmar Z, Acs N, Banhidy F. Possible association of folic acid supplementation during pregnancy with reduction of preterm birth: A population-based study. Eur J Obstet Gynecol Reprod Biol. 2010;148:135–140. doi: 10.1016/j.ejogrb.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Facts | folic acid | NCBDDD | CDC. 2014 Retrieved from https://www.cdc.gov/ncbddd/folicacid/about.html.
- Greenberg JA, Bell SJ, Guan Y, Yu YH. Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. 2011;4(2):52–29. [PMC free article] [PubMed] [Google Scholar]
- Hébert P, Wells G, Blajchman M, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. NEJM. 1999;340(6):409. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Klerman L, Cliver S, Goldenberg R. The impact of short interpregnancy intervals on pregnancy outcomes in a low-income population. Am J Pub Health. 1998;88(8):1182–1185. doi: 10.2105/AJPH.88.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie D, Johnson R. Drugs and folate metabolism. Drugs. 1985;30(2):145. doi: 10.2165/00003495-198530020-00003. [DOI] [PubMed] [Google Scholar]
- Linnebank M, Moskau S, Semmler A, Widman G, Stoffel-Wagner B, Weller M, et al. Anti-epileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69(2):352. doi: 10.1002/ana.22229. [DOI] [PubMed] [Google Scholar]
- Lucock M, Daskalakis I. New perspectives on folate status: a differential role for the vitamin in cardiovascular disease, birth defects and other conditions. Br J Biomed Sci. 2000;57(3):254–260. [PubMed] [Google Scholar]
- Nilsen R, Mastroiacovo P, Gunnes N, Alsaker ER, Bjørke-Monsen AL, Eussen SJ, et al. Folic acid supplementation and interpregnancy interval. Paed Perinat Epidemiol. 2014;28(3):270–274. doi: 10.1111/ppe.12111. [DOI] [PubMed] [Google Scholar]
- Perinatal Services BC. Birth weight and gestational age chart for British Columbia populations. 2016 Retrieved from http://www.perinatalservicesbc.ca/Documents/Resources/HealthPromotion/BirthCharts/DescriptiveStatisticsandChartsAppendix1.pdf.
- Sengpiel V, Bacelis J, Myhre R, Myking S, Pay AD, Haugen M, et al. Folic acid supplementation, dietary folate intake during pregnancy and risk for spontaneous preterm delivery: A prospective observational cohort study. BMC Preg Childbirth. 2014;14:202. doi: 10.1186/1471-2393-14-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl T, Johnson W. Folic acid: Influence on the outcome of pregnancy. Am J Clin Nutrition. 2000;71(5):1295–1303. doi: 10.1093/ajcn/71.5.1295s. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Dietary and serum folate: Their influence on the outcome of pregnancy. Am J Clin Nutrition. 1996;63(4):520–525. doi: 10.1093/ajcn/63.4.520. [DOI] [PubMed] [Google Scholar]
- Smits L, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. The Lancet. 2001;358(9298):2074–2077. doi: 10.1016/S0140-6736(01)07105-7. [DOI] [PubMed] [Google Scholar]
- Smits J, Elzenga H, Gemke R, Hornstra G, Van Eijsden M. The association between inter-pregnancy interval and birth weight: What is the role of maternal polyunsaturated fatty acid status? BMC Pregnancy Childbirth. 2013;88(1):147–153. doi: 10.1186/1471-2393-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart E. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-ST313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beynum I, Kapusta L, Bakker M, den Heijer M, Blom H, de Walle H. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: A registry-based case-control study in the northern Netherlands. Arch Gynecol Obstet. 2010;287(5):859–863. doi: 10.1093/eurheartj/ehp479. [DOI] [PubMed] [Google Scholar]
- van Eijsden M, Smits L, Van der Wal M, Bonsel G. Association between short interpregnancy intervals and term birth weight: The role of folate depletion. Am J Clin Nutrition. 2008;88(1):147–153. doi: 10.1093/ajcn/88.1.147. [DOI] [PubMed] [Google Scholar]
- Wendt A, Gibbs C, Peters S, Hogue C. Impact of increasing inter-pregnancy interval on maternal and infant health. Paed Perinat Epidemiology. 2012;26(1):239–258. doi: 10.1111/j.1365-3016.2012.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaremco E, Inglis A, Innis SM, Hippman C, Carrion P, Lamers Y, et al. Red blood cell folate levels in pregnant women with a history of mood disorders: A case series. Birth Defects Res A: Clin Mol Teratol. 2013;97(6):416–420. doi: 10.1002/bdra.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BP. Effect of interpregnancy interval on birth outcomes: Findings from three recent US studies. Int J Gynecol Obstet. 2005;89:S25–S33. doi: 10.1016/j.ijgo.2004.08.002. doi:j.ijgo.2004.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.