Abstract
Apoptosis-induced compensatory proliferation (AiP) is a form of compensatory proliferation that is triggered by apoptotic cell death to maintain tissue homeostasis. As such, AiP is essential for many tissue repair processes including regeneration. The apoptotic effectors, termed caspases, not only execute apoptosis, but are also directly involved in the generation of the signals required for AiP. Reactive oxygen species (ROS) play an important role for regenerative processes. Recently, it was shown in Drosophila that apoptotic caspases can mediate the generation of ROS for promoting AiP. This review summarizes and discusses these findings in the context of regenerative processes and cancer.
Keywords: Regeneration, Apoptosis-induced proliferation, Reactive oxygen species, Macrophages, Drosophila
Introduction
Regeneration is a dynamic biological process that is aimed at restoring tissue integrity and maintaining homeostasis following injury. Injury is caused by wounding due to trauma, or pathologies like infection and cancer (1). Regeneration requires a coordinated series of various intracellular, extracellular and intercellular signaling events. Each of these events needs to be tightly regulated to achieve homeostasis and to prevent excessive healing responses. Defects in wound repair can cause non-healing ulcers, or excessive invasive proliferation leading to cancer. Indeed, a classical theory by Harold Dvorak postulates that “tumors are wounds that do not heal” (2). This theory was proposed based on observations that both wounds and tumors share common cellular properties. For example, the composition of the tumor stroma resembled the granulation tissue observed during wound healing. The major difference between wounds and tumors is that in contrast to wound healing, the process is not self-limiting in tumors, and continuous activation of the involved signaling events causes hyperproliferation, invasion, and finally metastasis.
The ability to repair tissue after an injury is a fundamental property of multicellular organisms, though the regenerative responses are quite diverse, and depend on the species, organ and the developmental stage (3). Most species have the capacity to regenerate missing body parts or organs, either completely or with formation of a scar, while a few species like primitive sponges, Hydra, and planarians can regenerate the entire organism from parts of their body (4). Some animals are capable of regenerating their tissue throughout life, while others show a developmental stage-specific restriction of the ability to regenerate. While most mature mammals maintain the ability to regenerate a few select organs such as the liver, they have largely lost the capacity to regenerate lost tissue.
Regeneration follows a succession of events including an immediate wound healing response, followed by formation of a regenerative structure called blastema, leading to proliferation and differentiation, and finally complete restoration of the lost tissue or appendage. Wound healing, the first stage of regeneration, is initiated immediately after an injury, and involves recruitment of immune cells like neutrophils and macrophages, inflammation, and formation of a clot. Actomyosin cables are extended across the wound edge, the extracellular matrix is remodeled, and the wound is closed. Formation of a blastema is a critical step in regeneration, and usually occurs prior to complete wound closure (5). The blastema is the site for regenerative proliferation, and consists of both differentiated cells and stem cells. The cells in the blastema give rise to new cells by multiple different mechanisms including stem cell proliferation, compensatory proliferation, cell cycle re-entry of differentiated cells, dedifferentiation, or transdifferentiation (3). Finally there is remodeling of the newly formed cells to establish epithelial integrity, which restores a fully patterned organ or limb.
Wound healing without blastema formation causes partial restoration of lost tissue, and gives rise to fibrotic scars. Healing by fibrotic scarring rather than by regeneration leads to tissue dysfunction, and can place a huge burden for the health of the animal (4). Therefore, understanding the molecular and cellular basis of regenerative growth has been an area of intense investigation during the past decades. Mammals show restrictive regeneration, and for this reason several different model organisms that show a high degree of regenerative capacity, like hydra, planaria, Drosophila, Xenopus, salamander or zebrafish, are being used with the goal to identify the mechanisms that underlie injury-induced cellular plasticity and regeneration (4). Studying regeneration in various model organisms can provide insight into why mammals have lost the capacity to regenerate, and whether it is possible to restore regeneration in mammals, forming the basis for regenerative medicine.
Apoptosis-induced Compensatory Proliferation (AiP)
Compensatory proliferation is one of the mechanisms by which a regenerative response is initiated post injury. In this mechanism, the uninjured cells increase proliferation to replace the damaged cells, and thus maintain tissue homeostasis (6) (Figure 1A). Studies in several model organisms have revealed that apoptotic cells are one of the driving forces for compensatory proliferation through secretion of mitogenic signals, hence this phenomenon was termed “apoptosis-induced compensatory proliferation” (AiP) (7, 8). Active caspases are the main inducers of AiP. Caspases are conserved cysteine proteases present in cells as inactive zymogens. They are divided into initiator caspases and effector caspases based on the length of their prodomains and their activation process. Initiator caspases like Caspase−2, −8, −9, −10, and Drosophila Dronc have long prodomains, and are activated by incorporation into large apoptotic protein complexes called the apoptosome (Figure 2A,B). Effector caspases like Caspase−3, −6, −7, and Drosophila DrICE and Dcp−1 are characterized by short prodomains, and are activated upon cleavage by upstream initiator caspases (9). After apoptosis induction, caspases are activated in a sequential cascade that culminates in the death of the cell (Figure 2A,B). Recent studies have shown that caspases also function independently of their role in apoptosis, and are involved in inflammation and immunity, cell migration, neurite pruning, cellular remodeling and differentiation, as well as AiP (reviewed in (10, 11)).
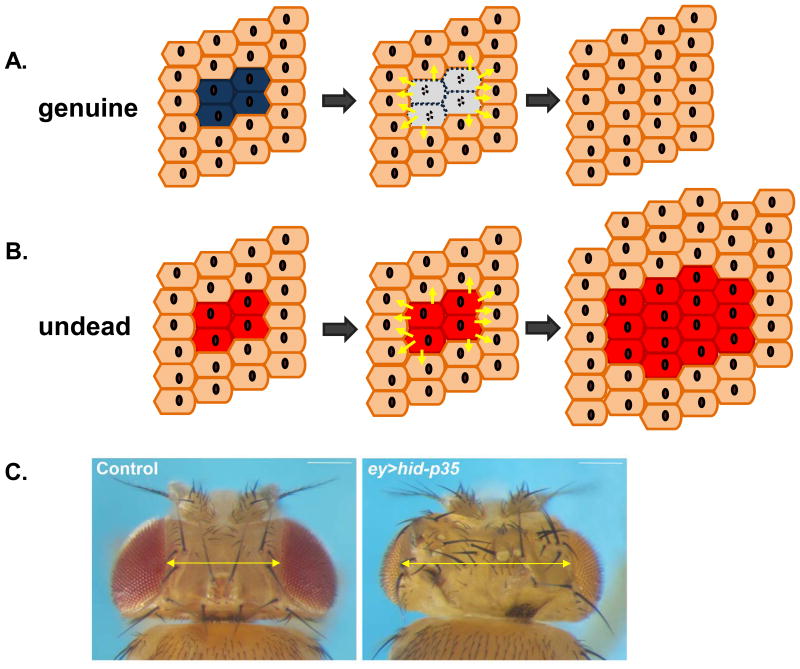
Figure 1. Genuine and Undead Models of Apoptosis-induced Proliferation (AiP).
(A) During genuine AiP, before they die, apoptotic cells (blue) emit signals (yellow arrows) to neighboring surviving cells (brown) to promote compensatory proliferation and maintain tissue homeostasis.
(B) Undead cells (red) emit proliferative signals (yellow arrows) to neighboring normal cells (brown). Because undead cells do not die, they continue to signal, triggering overgrowth. Undead cells themselves also contribute to the overgrowth.
(C) Examples of a normal (control) adult fly head (left) and an undead adult fly head (right). The head capsule is overgrown and displays patterning errors such as additional bristles and ocelli. This is indicated by the yellow double arrow. Genotypes: ey-Gal4 UAS-p35 (control) and ey-Gal4 UAS-hid UAS-p35 (ey>hid-p35; undead).
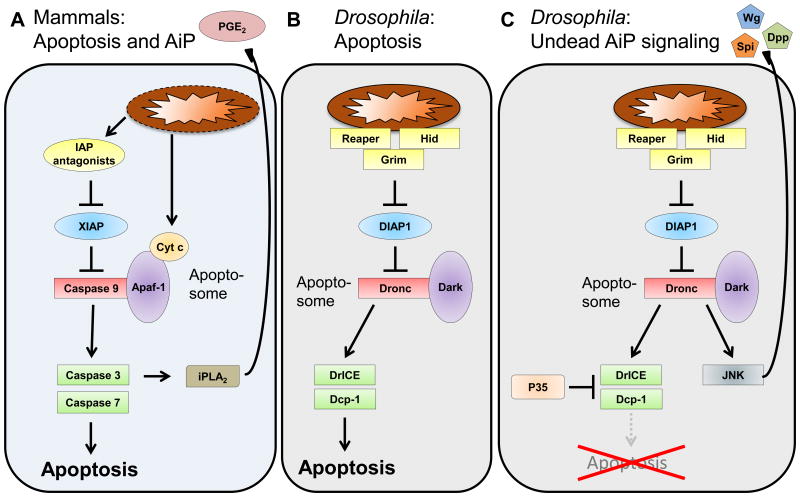
Figure 2. Apoptosis Pathways in Mammals and Drosophila and the undead AiP Model in Drosophila.
(A) The mammalian apoptosis pathway downstream of mitochondria. Mitochondria release IAP-antagonists and Cytochrome c (Cyt c) which trigger caspase activation and apoptosis. The apoptosome is composed of Apaf−1, Cyt c and Caspase−9. Activated Caspase−3 also activates calcium-independent Phospholipase A2 (iPLA2) which generates Prostaglandin E2 (PGE2) for AiP.
(B) The Drosophila apoptosis pathway. The IAP antagonists Reaper, Hid and Grim are localized at mitochondria and trigger apoptosis from there. The Drosophila apoptosome is composed of Dronc and Dark. Cyt c is not involved in Drosophila apoptosis. Homologous proteins to the mammalian apoptosis pathway have the same color as in (A).
(C) The undead AiP pathway in Drosophila. Expression of P35 inhibits the effector caspases DrICE and Dcp−1, thus blocking apoptosis. Dronc is not affected by P35 and can activate Jun N-terminal kinase (JNK) signaling which releases the mitogens Wingless (Wg), Decapentaplegic (Dpp) and Spitz (Spi) for AiP.
AiP initiated by caspases is seen in a variety of model organisms like Hydra, planarians, newts, Drosophila, Xenopus and mice (12). In Hydra, activation of effector caspases in apoptotic cells after mid-gastric amputation triggers release of the mitogen Wnt3, which stimulates proliferation and head regeneration (13). Similarly, in Xenopus tadpoles, tail amputation induces caspase activity at the site of injury, which is essential for regeneration (14). In mice, in an AiP-dependent process termed “Phoenix Rising”, caspases are important for epithelial wound healing, liver regeneration after partial hepatectomy, and tumor repopulation following chemotherapy or radiation. During Phoenix Rising, Caspase−3-induced activation of calcium-independent Phospholipase A2 (iPLA2) and prostaglandin E2 (PGE2) is required for compensatory proliferation (15, 16).
Pioneering work performed in Drosophila over the past decade have helped to identify the mechanisms and signaling events involved in AiP (reviewed in (12, 17, 18)). These genetic models of AiP take advantage of the high regenerative capacity of larval imaginal discs, developing epithelial primordia that give rise to adult structures such as eyes and wings. In these studies apoptotic wounds are induced in eye or wing imaginal discs, either genetically by over-expressing IAP antagonists (like hid or reaper) (Figure 2B,C) (19), or by irradiation, to study the role of active caspases for AiP. However, due to the transient nature of the apoptotic process, studying the non-apoptotic signaling events initiated by caspases is difficult. To overcome this limitation, the “undead” models of AiP were developed (20). In these models, apoptotic signaling is induced by expressing IAP antagonists, but cells are kept alive by co-expressing the baculovirus protein P35 that specifically inhibits the effector caspases DrICE and Dcp−1, but not the initiator caspase Dronc (Figure 2C) (21). Rendering cells in an undead state allows uncoupling of the apoptotic and non-apoptotic functions of Dronc, which can now persistently signal to induce compensatory proliferation leading to hyperplastic overgrowth (Figure 1B) (20, 22–25). For example, if hid and p35 are simultaneously expressed in the developing eye imaginal disc, hyperplastic tissue overgrowth is induced, and adult flies are characterized by enlarged head cuticles with several patterning defects (Figure 1C). The overgrowth phenotype of undead fly heads has been used for genetic screens aimed at identifying genes and mechanisms involved in AiP (26, 27).
Mechanistically, it was soon realized that in undead cells, Dronc promotes the activation of Jun-N-terminal kinase (JNK) signaling as the major inducer of AiP (25, 28) (Figure 2B,C). However, it has been unknown for a long time how active Dronc promotes JNK signaling. Recently it was reported that active Dronc promotes the generation of extracellular reactive oxygen species (eROS), which activate JNK signaling in the undead eye disc tissue (26). If this is the only mechanism by which Dronc activates JNK or if any other mechanisms exist remains to be seen. JNK functions in a positive feedback loop in AiP as it transcriptionally activates hid and reaper, thus amplifying the AiP process (26, 29, 30). Downstream of JNK signaling, undead cells produce and secrete several mitogens - including Wingless (Wg), a WNT-family member, Decapentaplegic (Dpp), a TGF-β family member, and Spitz (Spi), an EGF homolog (Figure 2C) (25, 28, 31). These mitogens then signal to the neighboring cells to initiate proliferation.
Although studies using undead models have provided a lot of insight into the mechanisms of AiP, the fact that cells are kept alive under constant apoptotic stress might alter their signaling properties, thus compromising the relevance for understanding physiological AiP. Consequently, p35-independent models of AiP have been developed, known as the “genuine” or regenerative AiP models. In the genuine models, tissue ablation is induced by a temporal pulse of apoptosis in a spatially-restricted manner. The affected imaginal discs are then allowed to recover and regenerate the lost tissue through AiP (Figure 1A) (32). Studies using the genuine AiP models have mostly corroborated the findings obtained in the undead models (32, 33), although as often seen with the use of different models, there is controversy about the involvement of individual components such as Wg and Dpp in AiP (31). Nevertheless, the studies using genuine models have confirmed the involvement of JNK signaling for inducing AiP (32, 34). Along with JNK signaling, p38 and JAK/STAT signaling pathways are also required for genuine AiP.
Furthermore, as described above for the undead model, ROS are also generated in the genuine models, and are required for activation of p38 and JNK signaling (33).
ROS and ROS signaling
Reactive oxygen species (ROS) are formed upon partial reduction of oxygen, and include superoxide anions (O2−), hydroxyl radical (OH) and hydrogen peroxide (H2O2) (35). They are highly unstable with a relatively short half-life. O2− is generally considered to be the primary ROS and is abundantly generated by different endogenous and exogenous factors. O2− is rapidly dismutated to H2O2, and in the presence of Fe2+ or Cu2+ ions, H2O2 can further be converted to .OH via a process known as Fenton reaction (Figure 3).
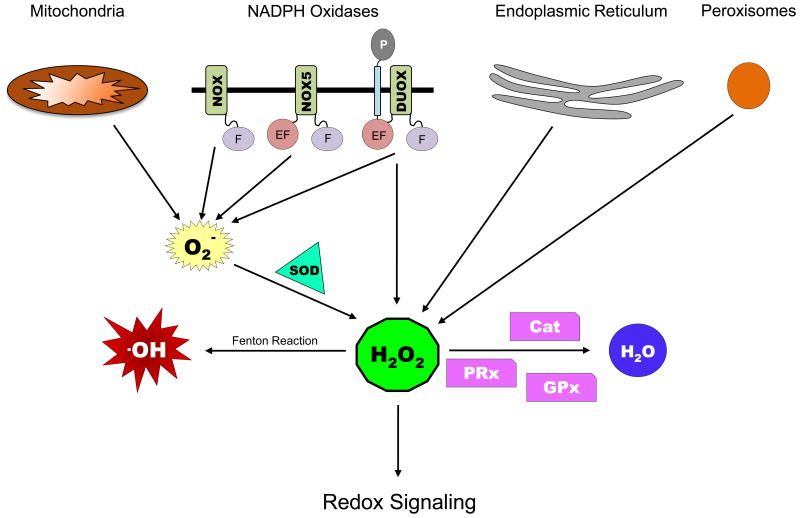
Figure 3. Sources of ROS and Enzymes involved in Redox Signaling.
Abbreviations used: F – Flavin domain; EF – EF hand domain; P – peroxidase homology domain (PHD); SOD – superoxide dismutase; Cat – catalase; GPxs – glutathione peroxidases; PRxs -peroxiredoxins. See text for details.
Mitochondria are the primary source of intracellular O2− (36), followed by other organelles such as endoplasmic reticulum (ER), peroxisomes, and the phagosomes in specialized phagocytic cells that display localized generation of ROS (Figure 3) (37). In mitochondria, the electron transport chain (ETC) complexes transfer electrons from NADH and succinate to synthesize ATP during aerobic respiration. Leaks in the ETC, especially in complex I and III cause a one electron reduction of molecular O2 to form O2− in the mitochondrial matrix (36). In the ER, H2O2 is produced by post-translational oxidative modifications during protein folding (37, 38), while in the peroxisomes, ROS are generated as byproducts of catalytic functions of enzymes involved in various metabolic pathways like α- and β-oxidation of very long chain fatty acids, amino acid catabolism, and others (39, 40).
Another major source of ROS are the membrane-associated NADPH oxidases NOX and dual oxidase (DUOX) (Figure 3). They show widespread localization to different cell and organelle membranes, and account for the generation of extracellular ROS (eROS), in particular via DUOX. They catalyze the reduction of O2 to O2− by using NADPH as an electron donor (41). These enzymes are evolutionarily conserved in eukaryotes. There are total of 7 NOX family members - NOX1−5 and DUOX1−2 in mammals, while in Drosophila there is one homolog for NOX (dNOX) and one for DUOX (dDUOX), and in C. elegans there is no NOX homolog, but 2 DUOX enzymes present (42). NOX proteins are characterized by the presence of a carboxyterminal intracellular Flavin domain that contains binding sites for co-enzymes NADPH and FAD (Figure 3), an amino-terminal hydrophobic domain that forms 6 transmembranal α-helices with four highly conserved heme-binding histidine residues in the transmembrane domain that act as carrier to transport electrons across the membrane, and an additional amino-terminal intracellular Ca2+-binding EF hand domain observed in NOX5. DUOX proteins have a similar structure as NOX5 with the addition of a seventh transmembrane domain and an extracellular peroxidase-homology domain (PHD) (Figure 3). Thus, DUOX proteins can generate O2− through the catalytic core and potentially further process it through its own peroxidase domain (41).
There is a delicate balance between ROS generation and scavenging by the protective antioxidant defenses in the cell. Antioxidant systems present in the cells include enzymes like superoxide dismutase (SOD), catalases, glutathione peroxidases (GPxs) and peroxiredoxins (PRxs) (Figure 3) (38). SODs are responsible for catalyzing the rapid dismutation of highly reactive O2− anions to H2O2: SOD1 converts cytosolic O2−, mitochondrial matrix-associated SOD2 converts O2− generated by mitochondrial ETC, while SOD3 is secreted extracellularly and is responsible for converting extracellular O2− produced by the NADPH oxidases (43). The antioxidant enzymes like catalases, GPxs and PRxs convert H2O2 to H2O and O2. The GPx family proteins reduce H2O2 by oxidizing glutathione, which is then reduced back by glutathione reductase using NADPH as an electron donor, thus normalizing the levels of reduced glutathione in the cells (44). PRx family enzymes contain a redox-sensitive cysteine residue in their active site, which is inactivated by H2O2-mediated oxidation. Thioredoxin acts as an electron donor to reduce and activate PRx, thus completing the catalytic cycle (45).
Historically, ROS were thought to be deleterious to the cell causing oxidative stress by indiscriminately damaging proteins, lipids, and nucleic acids. This holds true for the highly reactive O2− and OH radicals, as they can cause irreversible oxidative damage due to their strong oxidizing potential and lipid insolubility, thereby contributing to cellular dysfunction and various pathologies. However, it is becoming more appreciated now that ROS can also mediate important signaling functions, referred to as redox-signaling (46). For example, H2O2 is a perfect candidate to function as second messenger or as signaling molecule due to its relatively long half-life, high stability, and its ability to diffuse across membranes. Indeed oxidation of critical cysteine residues in redox-sensitive proteins is the most studied mechanism by which H2O2 functions as signaling molecule (47). The cellular targets of H2O2 that undergo this reversible cysteine oxidation encompass a vast range of biological processes. Examples include the phosphatases PTEN and PTP1B, kinases like MAPK and redox-reactive transcription factors like YAP1 in yeast and FOXO4 in mammals (37). H2O2 oxidizes the thiol side-chain of cysteine to form reactive sulfenic acid (-SOH) that can form intra- and inter-molecular disulphide (-S-S-) bonds or cyclic sulfenamide (-S-N-) structures, or can undergo hyperoxidation to form sulfinic (-SO2H) or sulfonic (-SO3H) acids (48). These reversible modifications may lead to changes in protein structure, function or activation state.
Along with cysteine thiols, H2O2 can oxidize several other amino acids like methionine, lysine, arginine, proline, histidine and tyrosine (49). Multiple mechanisms have been proposed for understanding how target proteins are selected for oxidation by H2O2. One mechanism proposes colocalization of ROS sources and targets, so that redox signaling events are triggered close to the source of ROS generation (37). For example, NOX proteins are often seen colocalized with putative targets like phosphatases or kinases at the plasma membrane, thereby influencing receptor tyrosine kinase signaling (50). Another mechanism termed as “redox relay” proposes that H2O2 oxidizes the scavenging enzymes like PRx or GPx, which subsequently transfer the oxidation to target proteins (51). This kind of relay process is seen for H2O2-mediated oxidation of apoptosis signaling kinase 1 (ASK1) and downstream phosphorylation of its MAPK substrate p38, which is dependent on formation of a ASK1-PRx1 disulphide intermediate (52). Yet another mechanism termed as “floodgate model” proposes that transient inactivation of scavenging enzymes by hyperoxidation or posttranslational modifications causes accumulation of H2O2 allowing oxidation of target proteins (53). Thus, by localized alterations in the redox buffering capacity, the cell can control ROS flux for selective signaling events (54).
Furthermore, depending on the location of a Cys residue within a protein, not all Cys residues are equally susceptible to H2O2-mediated oxidation (55, 56), further increasing specificity. Another form of physiological ROS regulation involves transport of H2O2 across cell membranes via aquaporins, which are integral membrane proteins involved in transport of water and small-molecule metabolites (57). Aquaporins enhance the membrane permeability of H2O2, and are useful for transporting the extracellular H2O2 produced by NADPH oxidases across the plasma membrane to mediate intracellular signaling cascades (58).
ROS in AiP and Regeneration
Tissue wounding and inflammation are associated with production of ROS, and recent studies show that generation of ROS, especially during the initial stages of wounding and regeneration are essential for an efficient wound healing response. ROS, in particular H2O2 function as an early damage signal to initiate and control different aspects of regenerative responses. In our recent work, we found that extracellular ROS (eROS) are produced by actively proliferating epithelial cells in response to activation of Dronc by pro-apoptotic signals. These eROS are required for the compensatory proliferation in both undead and genuine regenerative models of AiP in Drosophila eye and wing imaginal discs. Dronc triggers generation of eROS via activation of dDUOX specifically, and dNOX to a lesser extent (Figure 4A). Loss of eROS by silencing dDUOX or by expressing extracellular catalases caused an impaired proliferative response (26, 59). In the undead AiP model, eROS are necessary for recruitment and activation of hemocytes, Drosophila macrophage-like immune cells, to the eye imaginal discs. Hemocytes in turn secrete the TNF ortholog Eiger, which activates JNK signaling in the undead epithelial cells, and thus contributes to AiP (Figure 4A) (26). The presence of hemocytes on the overgrown undead epithelial tissue is reminiscent of tumor-associated macrophages (TAMs) seen in many solid tumors in human cancers. TAMs increase the inflammation linked with cancer, and function in tumor progression, immune suppression, angiogenesis and metastasis. High TAM-infiltration of tumors is associated with poor patient prognosis (60). Peripheral blood monocytes are recruited to the tumor, and get “alternatively activated” (M2 phenotype) in response to the tumor microenvironment to differentiate into TAMs. Several tumor-derived factors like chemokines, cytokines and microparticles, extracellular matrix components and immune cells in the tumor microenvironment help polarizing TAMs to adopt a M2-like phenotype (61). Along with these factors, it would be interesting to know if ROS from tumor cells contribute to activating TAMs, as is seen in the case of undead AiP model in Drosophila.
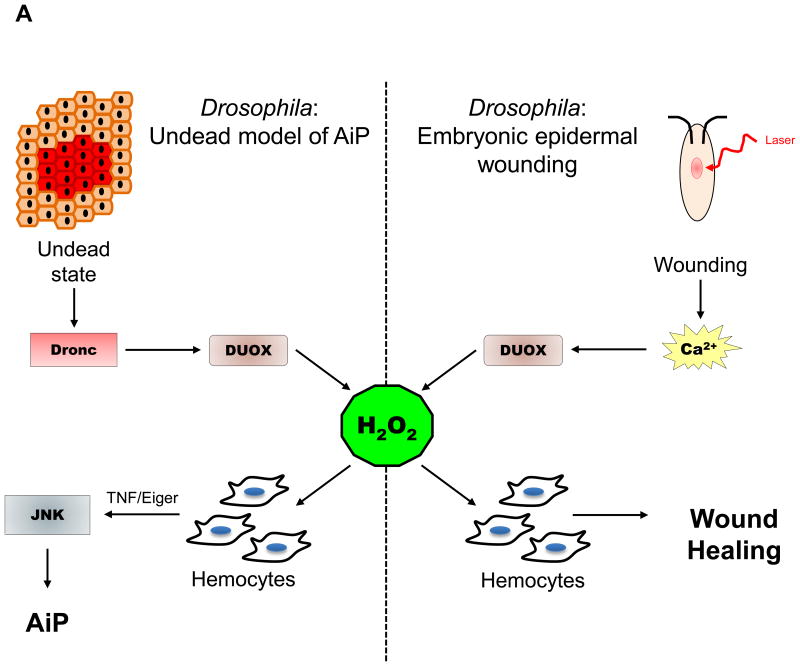
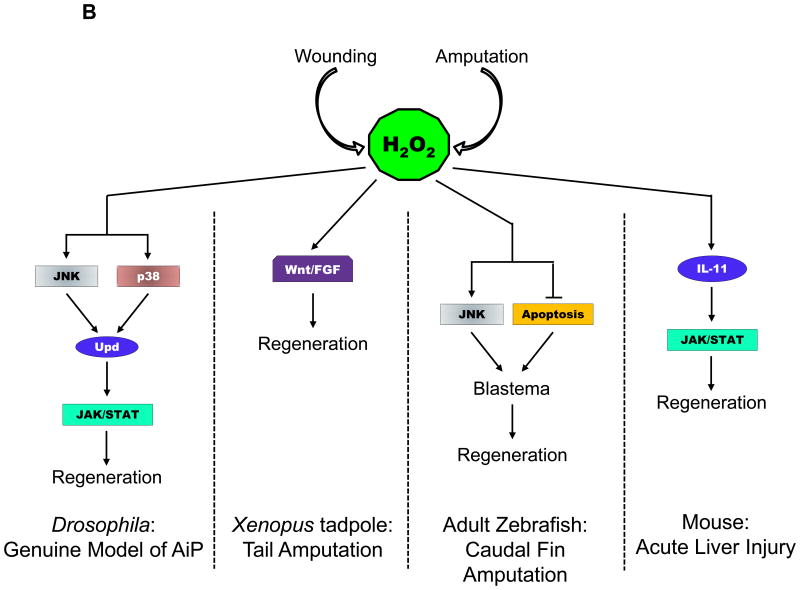
Figure 4. ROS Signaling in AiP and Regeneration in various model organisms.
(A) H2O2 attracts immune cells (hemocytes) to sites of wounding or damage in Drosophila. The left panel displays the signaling events in the undead AiP model. The right panel shows the signaling events after laser-induced tissue damage in Drosophila embryos.
(B) H2O2 signaling after wounding or amputation in several regeneration models indicated at the bottom of each panel.
Abbreviations used: TNF – Tumor Necrosis Factor; JNK – Jun N-terminal Kinase; Upd – Unpaired (Interleukin−6 homolog); Wnt – Wg and Int−1; FGF – Fibroblast Growth Factor; IL−11 – Interleukin 11; JAK/STAT – Janus Kinase/Signal Transducers and Activator of Transcription.
ROS-mediated recruitment of immune cells to wound sites is seen in several different model organisms. The role of H2O2 as a chemoattractant for recruiting leukocytes to the site of wounding was first demonstrated in zebrafish. Tail fin wounding of zebrafish larvae led to activation of DUOX at the site of injury, and generation of a tissue-scale gradient of H2O2 that promotes leukocyte chemotaxis to the wound. Loss of H2O2 gradient impaired leukocyte recruitment to the wound causing an imperfect wound healing response (62). Further evidence supporting the role of H2O2 as a chemoattractant was demonstrated by studies in Drosophila embryos. Laser wounding of the embryonic epidermis triggers a rapid calcium flash at the wound edge that activates dDUOX via its EF hand motifs, leading to generation of H2O2 (Figure 4A). H2O2 is responsible for recruitment of embryonic hemocytes to the wound, thus acting as early damage molecule in wound inflammatory response (63, 64).
However, these studies have not addressed the exact mechanism by which H2O2 promotes immune cell chemotaxis in vivo. It will be interesting to determine if H2O2 acts as a primary chemoattractant, or whether it stimulates production of downstream chemoattractants by the epithelial cells, or whether they modulate the extracellular matrix by interacting with the chemotactic plasma membrane receptors to attract immune cells. All these mechanisms are consistent with the paracrine signaling role of H2O2, and may act redundantly in different contexts. Direct chemotactic activity of H2O2 can be mediated via entering the cytoplasm and modulating intracellular signaling events in the immune cells. This was demonstrated in zebrafish larvae, where wound-derived H2O2 activated the Src family kinase Lyn in neutrophils through direct oxidation of a cysteine residue, thereby mediating leukocyte migration to the wound (65). How redox sensing by Lyn instructs directional migration of neutrophils remains to be determined. This was answered in part by a study in Drosophila embryos, where epidermal wounding generated H2O2 that activated the Lyn homolog Src42A in the hemocytes. Activated Src42A phosphorylated and activated Draper-I (a member of CED−1 family of phagocytic receptors), thereby leading to activation of the kinase Shark, and migration of hemocytes to the wounds. Hence, it was proposed that H2O2 acts as an activator signal rather than a chemoattractant to activate a signaling cascade in the macrophages to prime them to respond to wounds (66).
In addition to attracting immune cells to the site of injury, ROS also participate in proliferative responses during compensatory proliferation and regeneration. In Xenopus tadpoles, tail amputation induces sustained production of ROS over the span of regeneration, and decreasing ROS levels, especially early on after amputation, resulting in impaired tail regeneration. Amputation-induced ROS are also produced via NADPH oxidases, and activate Wnt/β-catenin and FGF signaling, thus initiating regeneration (Figure 4B) (67, 68). The role of caspases in ROS production was not investigated in this model, however, caspase−3 activity was previously shown to be important for proliferation during the first 24 hours after amputation (14). In adult zebrafish, caudal fin amputation causes sustained ROS production, unlike the transient ROS response observed after larval tail fin injury. ROS are immediately detected near the lesion after fin amputation, and are tightly regulated during the first 24 hours of regeneration, reaching a peak at 12 and 16 hours post amputation. This sustained ROS generation is specific for the regenerative response and is not observed during wound healing responses. In this regenerative response, ROS generated via enzymatic activity of NOX induce JNK activation and delayed apoptosis (Figure 4B). Both of these parallel processes are important for blastema formation and compensatory proliferation of epithelial cells (69).
In addition to epithelial regeneration, ROS production induced by amputation of the caudal fin also induces neuro-regeneration. In larvae, increased H2O2 produced by DUOX1 at wound sites is important for peripheral sensory axon regeneration and re-innervation of the skin (70). In adult zebrafish, sensory neurons, especially Schwann cells, induce H2O2 production via Hedgehog signaling post amputation, and this H2O2 stimulates the axonal growth and attracts peripheral axons to the regenerating blastema (71). In a genuine AiP model in Drosophila wing discs, ROS generation triggered in response to a transient pulse of apoptosis induced activation of JNK and p38 signaling in the surviving cells that resulted in expression of JAK/STAT pathway ligand Unpaired (Interleukin−6 (IL−6) homolog), leading to regeneration of lost tissue (Figure 4B) (33).
Similarly, another study showed that following acute liver injury in mice, dying hepatocytes produced ROS that induced production of IL−11 (a member of IL−6 family of cytokines). IL−11 triggers activation of JAK/STAT signaling in healthy hepatocytes, which results in compensatory proliferation (Figure 4B). However, it was not investigated whether involvement of caspases in hepatocytes was necessary for production of ROS or downstream expression of IL−11 in this model of liver injury (72). In Hydra, ROS are produced immediately at the wound edges following bisection, and are important for injury-induced cell death and MAPK activation in the head regenerating tips (73). In planaria, amputation of both the head and tail regions induces ROS production at the wound site, which is necessary for regeneration, patterning, polarization of proliferating cells, and early nervous system differentiation. This study provided the first evidence that ROS are involved in anterior body regeneration, and that production of ROS was independent of the orientation of the wound site (74). In mice, partial hepatectomy induced ROS production is tightly regulated by modulating activities of NADPH oxidases and scavenging enzymes. During the regenerative phase, NOX4 is activated while PRxs and catalases are downregulated to facilitate increased H2O2 production. On the other hand, during the termination phase of regeneration, the level of NOX4 is reduced, and that of PRxs and catalases are induced to decrease H2O2 production. In this model, H2O2 triggers two distinct signaling events in a dose-dependent manner. Elevated H2O2 levels induced cell proliferation by activation of ERK signaling, and in contrast, low H2O2 concentrations promoted cell quiescence by activation of p38 signaling. Thus, activity of specific enzymes and dose-dependent signaling by H2O2 triggers the proliferation-quiescence switch to govern liver regeneration after hepatectomy (75).
Implications for Cancer
ROS have a long history of being involved in the development and progression of cancer and increased ROS level is considered as a hallmark of many tumors (76, 77). Initially, it was thought that ROS would serve as chemical mutagens that would indiscriminately damage cellular macromolecules such as DNA by oxidative stress, and thus are tumorigenic by promoting genomic instability. In addition, the more recent findings that ROS, most notably H2O2, can also mediate several signaling processes in the cell with very specific oxidation targets has verified this view (recently reviewed in (78)). By regulating signaling events that control the cell proliferation rate, alter the metabolic states of the cell and promote angiogenesis, ROS are increasing the tumorigenic potential of the cancerous cells. Increased ROS production in cancer cells can be due to multiple different factors. Oncogenes such as Ras promote the generation of ROS (79–82). The tumor suppressor p53 is known to establish the redox balance by regulating the expression of antioxidants like GPx1 and SOD2, and loss in its activity can increase the oxidative burden in tumor cells (83–85). Increased metabolism of the tumor cells is another factor that increases the ROS levels in these cells (reviewed in (77)). Many human tumors and cultured tumor cell lines show an upregulation of the mRNA expression and/or protein levels of several different NOX enzymes, which are implicated in increasing the cellular ROS levels. Depending on the subcellular localization of these enzymes and the stage of tumorigenesis, NOX-derived ROS can mediate DNA damage causing genomic instability or signal to activate redox-sensitive pathways, to help in initiation and maintenance of tumorigenesis (86, 87). Along with this, a wide variety of human tumors harbor mutations in mitochondrial DNA-encoding ETC proteins, which are responsible for increasing the mitochondrial-derived ROS production (88).
NOX enzymes can regulate the MAPK/ERK and PI3K/Akt/mTOR signaling pathways through H2O2-mediated oxidation of phosphatases involved in these processes (89–91). PTEN is the primary target of H2O2, and oxidation of its active site cysteine (Cys124) inactivates the phosphatase, resulting in constitutive activation of the PI3K pathway (89). Inactivation of protein phosphatase 2A (PP2A) and PTP1B by H2O2-mediated oxidation causes an increase in Akt activation. This leads to an increased cell proliferation response, anchorage-independent growth and survival (90, 92, 93). Mitochondrial ROS are also responsible to activate the hypoxia-inducible factors (HIFs) in hypoxic tumor cells, thus allowing the tumor cells to adapt to the low oxygen microenvironment and help in its survival. Under hypoxic conditions, increased superoxide generated by mitochondrial ETC stabilizes HIF−1α and HIF−2α subunits (94, 95).
It has also been reported that AiP is involved in the etiology of human cancer ((96–106); recently reviewed in (18)). Chemo- and radiotherapy of human patients often aims to induce the death of the tumor cells. However, apoptotic tumor cells in return may generate signals for AiP and despite initial tumor regression, the tumor cells repopulate and the tumor grows back (97–99, 102). Although a direct role of ROS for this type of tumor AiP has not been demonstrated so far, based on the work in several model organisms, it is possible that this is the case. Future work will need to address this question.
ROS have not only a tumor-promoting role, but they can also have a tumor-suppressive function. For example, genetically engineered mice carrying oncogenic K-Ras and B-Raf mutations significantly increased tumor development and mortality upon dietary supplementation of the anti-oxidants N-acetylcysteine (NAC) or Vitamin E, suggesting that ROS prevented tumor growth in these animals (107). Thus, for the application of therapies which potentially target ROS, we need to have a very detailed context-specific understanding of the role of ROS in these tumors. That will be a challenge for the future.
Conclusions
The initial view that ROS are only produced as a by-product of cellular stress, and cause deleterious oxidative damages in the cells has been replaced after the discovery that cells can temporally and spatially control ROS production, and that ROS-mediated signaling events play an important role in different cellular processes. As discussed here, ROS are critical mediators of AiP for regenerative processes and are also involved in tumorigenesis. Future work is needed to reveal the precise role of ROS and the targets of ROS modification during these processes.
Acknowledgments
We would like to thank the members of the Bergmann lab for fruitful discussions in the course of this work. This work was supported by the National Institute of General Medical Sciences (NIGMS) under award number R35 GM118330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sonnemann KJ, Bement WM. Wound Repair: Toward Understanding and Integration of Single-Cell and Multicellular Wound Responses. Annu Rev Cell Dev Biol. 2011;27:237–63. doi: 10.1146/annurev-cellbio-092910-154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dvorak HF. Tumors: Wounds That Do Not Heal. Similarities between Tumor Stroma Generation and Wound Healing. The New England Journal of Medicine. 1986;315(26):1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka EM, Reddien PW. The Cellular Basis for Animal Regeneration. Dev Cell. 2011;21(1):172–85. doi: 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurtner GC, Werner S, Barrando Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 5.Murawala P, Tanaka EM, Currie JD. Regeneration: The ultimate example of wound healing. Semin Cell Dev Biol. 2012;23(9):954–62. doi: 10.1016/j.semcdb.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Haynie JL, Bryant PJ. The effects of X-rays on the proliferation dynamics of cells in the imaginal wing disc of Drosophila melanogaster. Wilhelm Roux's Archives of Developmental Biology. 1977;183(2):85–100. doi: 10.1007/BF00848779. [DOI] [PubMed] [Google Scholar]
- 7.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18(10):467–73. doi: 10.1016/j.tcb.2008.08.001. Epub 2008/09/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollereau B, Perez-Garijo A, Bergmann A, Miura M, Gerlitz O, Ryoo HD, et al. Compensatory proliferation and apoptosis-induced proliferation: a need for clarification. Cell Death Differ. 2012;20(1):181. doi: 10.1038/cdd.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2014:1–14. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feinstein-Rotkopf Y, Arama E. Can't live without them, can live with them: roles of caspases during vital cellular processes. Apoptosis. 2009;14(8):980–95. doi: 10.1007/s10495-009-0346-6. Epub 2009/04/18. [DOI] [PubMed] [Google Scholar]
- 12.Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol. 2012;4(8):1–17. doi: 10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, et al. Apoptotic Cells Provide an Unexpected Source of Wnt3 Signaling to Drive Hydra Head Regeneration. Dev Cell. 2009;17(2):279–89. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Tseng AS, Adams DS, Qiu D, Koustubhan P, Levin M. Apoptosis is required during early stages of tail regeneration in Xenopus laevis. Dev Biol. 2007;301(1):62–9. doi: 10.1016/j.ydbio.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Science signaling. 2010;3(110):ra13–ra. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Q, Li F, Liu X, Li W, Shi W, Liu Ff, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17(7):860–6. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010;3(145):re8. doi: 10.1126/scisignal.3145re8. Epub 2010/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogarty CE, Bergmann A. Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017 doi: 10.1038/cdd.2017.47. Epub 2017/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu D, Woodfield SE, Lee TV, Fan Y, Antonio C, Bergmann A. Genetic control of programmed cell death (apoptosis) in Drosophila. Fly. 2009;3(1):78–90. doi: 10.4161/fly.3.1.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martín FA, Peréz-Garijo A, Morata G. Apoptosis in Drosophila: compensatory proliferation and undead cells. The International journal of developmental biology. 2009;53(8-10):1341–7. doi: 10.1387/ijdb.072447fm. [DOI] [PubMed] [Google Scholar]
- 21.Meier P, Silke J, Leevers SJ, Evan GI. The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 2000;19(4):598–611. doi: 10.1093/emboj/19.4.598. Epub 2000/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Current biology: CB. 2004;14(14):1262–6. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26(19):7258–68. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Garijo A, Martín FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development (Cambridge, England) 2004;131(22):5591–8. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- 25.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7(4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Fogarty CE, Diwanji N, Lindblad JL, Tare M, Amcheslavsky A, Makhijani K, et al. Extracellular Reactive Oxygen Species Drive Apoptosis-Induced Proliferation via Drosophila Macrophages. Curr Biol. 2016;26(5):575–84. doi: 10.1016/j.cub.2015.12.064. Epub 2016/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Wang S, Hernandez J, Yenigun VB, Hertlein G, Fogarty CE, et al. Genetic models of apoptosis-induced proliferation decipher activation of JNK and identify a requirement of EGFR signaling for tissue regenerative responses in Drosophila. PLoS genetics. 2014;10(1):e1004131. doi: 10.1371/journal.pgen.1004131. Epub 2014/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan Y, Bergmann A. Multiple mechanisms modulate distinct cellular susceptibilities toward apoptosis in the developing Drosophila eye. Dev Cell. 2014;30(1):48–60. doi: 10.1016/j.devcel.2014.05.007. Epub 2014/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shlevkov E, Morata G. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ. 2012;19(3):451–60. doi: 10.1038/cdd.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells BS, Yoshida E, Johnston La. Compensatory Proliferation in Drosophila Imaginal Discs Requires Dronc-Dependent p53 Activity. Curr Biol. 2006;16:1606–15. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez-Garijo A, Shlevkov E, Morata G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development (Cambridge, England) 2009;136(7):1169–77. doi: 10.1242/dev.034017. [DOI] [PubMed] [Google Scholar]
- 32.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative Growth in Drosophila Imaginal Discs Is Regulated by Wingless and Myc. Dev Cell. 2009;16(6):797–809. doi: 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santabárbara-Ruiz P, López-Santillán M, Martínez-Rodríguez I, Binagui-Casas A, Pérez L, Milán M, et al. ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Drosophila Regeneration. PLoS genetics. 2015;11(10):1–26. doi: 10.1371/journal.pgen.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith-Bolton R. Drosophila Imaginal Discs as a Model of Epithelial Wound Repair and Regeneration. Adv Wound Care. 2014;5(6):251–61. doi: 10.1089/wound.2014.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. American Journal of Physiology Lung Cellular and Molecular Physiology. 2000;279(6):L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 36.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7(8):504–11. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonenkov VD, Grunau S, Ohlmeier S, Hiltunen JK. Peroxisomes are oxidative organelles. Antioxid Redox Signal. 2010;13(4):525–37. doi: 10.1089/ars.2009.2996. [DOI] [PubMed] [Google Scholar]
- 40.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2012;1822(9):1363–73. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology. 2004;4(3):181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 42.Ritsick DR, Edens WA, McCoy JW, Lambeth JD. The use of model systems to study biological functions of Nox/Duox enzymes. Biochemical Society Symposium. 2004;71:85–96. doi: 10.1042/bss0710085. [DOI] [PubMed] [Google Scholar]
- 43.Abreu IA, Cabelli DE. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochimica et Biophysica Acta - Proteins and Proteomics. 2010;1804(2):263–74. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochimica et Biophysica Acta - General Subjects. 2013;1830(5):3289–303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287(7):4403–10. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veal EA, Day AM, Morgan BA. Hydrogen Peroxide Sensing and Signaling. Mol Cell. 2007;26(1):1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Miki H, Funato Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J Biochem. 2012;151(3):255–61. doi: 10.1093/jb/mvs006. [DOI] [PubMed] [Google Scholar]
- 49.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–4. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 50.Ushio-fukai M. Localizing NADPH Oxidase – Derived ROS. Science STKE. 2006;349(re8):1–7. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 51.Winterbourn CC. The biological chemistry of hydrogen peroxide. 1. Elsevier Inc; 2013. pp. 3–25. [DOI] [PubMed] [Google Scholar]
- 52.Jarvis RM, Hughes SM, Ledgerwood EC. Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic Biol Med. 2012;53(7):1522–30. doi: 10.1016/j.freeradbiomed.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of Peroxiredoxin I by Phosphorylation Allows Localized H2O2 Accumulation for Cell Signaling. Cell. 2010;140(4):517–28. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Toledano MB, Planson AG, Delaunay-Moisan A. Reining in H2O2 for Safe Signaling. Cell. 2010;140(4):454–6. doi: 10.1016/j.cell.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 56.Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–47. doi: 10.1146/annurev.pharmtox.44.101802.121735. Epub 2004/01/28. [DOI] [PubMed] [Google Scholar]
- 57.Bienert GP, Møller ALB, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282(2):1183–92. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 58.Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A. 2010;107(36):15681–6. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diwanji N, Bergmann A. The beneficial role of extracellular reactive oxygen species in apoptosis-induced compensatory proliferation. Fly. 2017;11(1):46–52. doi: 10.1080/19336934.2016.1222997. Epub 2016/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35(5):585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 61.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459(7249):996–9. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010;20(5):464–70. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 64.Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Current biology: CB. 2013;23(5):424–9. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480(7375):109–12. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans IR, Rodrigues FS, Armitage EL, Wood W. Draper/CED-1 mediates an ancient damage response to control inflammatory blood cell migration in vivo. Curr Biol. 2015;25(12):1606–12. doi: 10.1016/j.cub.2015.04.037. Epub 2015/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol. 2013;15(2):222–8. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galliot B, Crescenzi M, Jacinto A, Tajbakhsh S. Trends in tissue repair and regeneration. Development. 2017;144(3):357–64. doi: 10.1242/dev.144279. [DOI] [PubMed] [Google Scholar]
- 69.Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, et al. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep. 2013;3:2084. doi: 10.1038/srep02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rieger S, Sagasti A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol. 2011;9(5):1–12. doi: 10.1371/journal.pbio.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meda F, Gauron C, Rampon C, Teillon J, Volovitch M, Vriz S. Nerves Control Redox Levels in Mature Tissues Through Schwann Cells and Hedgehog Signaling. Antioxidants & redox signaling. 2016;24(6):299–311. doi: 10.1089/ars.2015.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishina T, Komazawa-Sakon S, Yanaka S, Piao X, Zheng DM, Piao JH, et al. Interleukin-11 Links Oxidative Stress and Compensatory Proliferation. Science Signaling. 2012;5(207):ra5–ra. doi: 10.1126/scisignal.2002056. [DOI] [PubMed] [Google Scholar]
- 73.Vriz S, Reiter S, Galliot B. Cell death: a program to regenerate. Curr Top Dev Biol. 2014;108:121–51. doi: 10.1016/B978-0-12-391498-9.00002-4. [DOI] [PubMed] [Google Scholar]
- 74.Pirotte N, Stevens AS, Fraguas S, Plusquin M, Van Roten A, Van Belleghem F, et al. Reactive oxygen species in planarian regeneration: An upstream necessity for correct patterning and brain formation. Oxid Med Cell Longev. 2015:2015. doi: 10.1155/2015/392476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bai H, Zhang W, Qin XJ, Zhang T, Wu H, Liu JZ, et al. Hydrogen peroxide modulates the proliferation/quiescence switch in the liver during embryonic development and posthepatectomy regeneration. Antioxidants & redox signaling. 2015;22(11):921–37. doi: 10.1089/ars.2014.5960. [DOI] [PubMed] [Google Scholar]
- 76.Szatrowski TP, Nathan CF. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991;51(3):794–9. [PubMed] [Google Scholar]
- 77.Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death and Disease. 2016;7(6):e2253–e. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chio IIC, Tuveson DA. ROS in Cancer: The Burning Question. Trends Mol Med. 2017;23(5):411–29. doi: 10.1016/j.molmed.2017.03.004. Epub 2017/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275(5306):1649–52. doi: 10.1126/science.275.5306.1649. Epub 1997/03/14. [DOI] [PubMed] [Google Scholar]
- 80.Ogrunc M, Di Micco R, Liontos M, Bombardelli L, Mione M, Fumagalli M, et al. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21(6):998–1012. doi: 10.1038/cdd.2014.16. Epub 2014/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang JQ, Li S, Domann FE, Buettner GR, Oberley LW. Superoxide generation in v-Ha-ras-transduced human keratinocyte HaCaT cells. Mol Carcinog. 1999;26(3):180–8. Epub 1999/11/13. [PubMed] [Google Scholar]
- 82.Sohn J, Rudolph J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry. 2003;42(34):10060–70. doi: 10.1021/bi0345081. Epub 2003/08/27. [DOI] [PubMed] [Google Scholar]
- 83.Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, et al. p53-Induced Up-Regulation of MnSOD and GPx but not Catalase Increases Oxidative Stress and Apoptosis. Cancer Res. 2004;64(7):2350–6. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 84.Maillet A, Pervaiz S. Redox Regulation of p53, Redox Effectors Regulated by p53: A Subtle Balance. Antioxidants & Redox Signaling. 2012;16(11):1285–94. doi: 10.1089/ars.2011.4434. [DOI] [PubMed] [Google Scholar]
- 85.Kruiswijk F, Labuschagne CF, Vousden KH. P53 in Survival, Death and Metabolic Health: a Lifeguard With a Licence To Kill. Nature Reviews Molecular Cell Biology. 2015;16(7):393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 86.Block K, Gorin Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nature Reviews Cancer. 2012;12(9):627–37. doi: 10.1038/nrc3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roy K, Wu Y, Meitzler Jennifer L, Juhasz A, Liu H, Jiang G, et al. NADPH oxidases and cancer. Clin Sci. 2015;128(12):863–75. doi: 10.1042/CS20140542. [DOI] [PubMed] [Google Scholar]
- 88.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277(23):20336–42. doi: 10.1074/jbc.M111899200. Epub 2002/03/28. [DOI] [PubMed] [Google Scholar]
- 90.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423(6941):769–73. doi: 10.1038/nature01680. Epub 2003/06/13. [DOI] [PubMed] [Google Scholar]
- 91.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? Journal of signal transduction. 2011;2011:792639. doi: 10.1155/2011/792639. Epub 2011/06/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rao RK, Clayton LW. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. 2002;293(1):610–6. doi: 10.1016/S0006-291X(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 93.Ostman A, Frijhoff J, Sandin A, Bohmer FD. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. 2011;150(4):345–56. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- 94.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–8. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 95.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GRS, et al. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177(6):1029–36. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bordonaro M, Drago E, Atamna W, Lazarova DL. Comprehensive suppression of all apoptosis-induced proliferation pathways as a proposed approach to colorectal cancer prevention and therapy. PLoS One. 2014;9(12):e115068. doi: 10.1371/journal.pone.0115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng J, Tian L, Ma J, Gong Y, Zhang Z, Chen Z, et al. Dying tumor cells stimulate proliferation of living tumor cells via caspase-dependent protein kinase Cdelta activation in pancreatic ductal adenocarcinoma. Mol Oncol. 2015;9(1):105–14. doi: 10.1016/j.molonc.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Donato AL, Huang Q, Liu X, Li F, Zimmerman MA, Li CY. Caspase 3 promotes surviving melanoma tumor cell growth after cytotoxic therapy. J Invest Dermatol. 2014;134(6):1686–92. doi: 10.1038/jid.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng X, Tian L, Zhang Z, Yu Y, Cheng J, Gong Y, et al. Caspase 3 in dying tumor cells mediates post-irradiation angiogenesis. Oncotarget. 2015;6(32):32353–67. doi: 10.18632/oncotarget.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng X, Yu Y, He S, Cheng J, Gong Y, Zhang Z, et al. Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett. 2016;385:12–20. doi: 10.1016/j.canlet.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu Q, Peng J, Liu W, He X, Cui L, Chen X, et al. Elevated cleaved caspase-3 is associated with shortened overall survival in several cancer types. Int J Clin Exp Pathol. 2014;7(8):5057–70. [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17(7):860–6. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kurtova AV, Xiao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209–13. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3(110):ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mao P, Smith L, Xie W, Wang M. Dying endothelial cells stimulate proliferation of malignant glioma cells via a caspase 3-mediated pathway. Oncol Lett. 2013;5(5):1615–20. doi: 10.3892/ol.2013.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Z, Wang M, Zhou L, Feng X, Cheng J, Yu Y, et al. Increased HMGB1 and cleaved caspase-3 stimulate the proliferation of tumor cells and are correlated with the poor prognosis in colorectal cancer. J Exp Clin Cancer Res. 2015;34:51. doi: 10.1186/s13046-015-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6(221):221ra15. doi: 10.1126/scitranslmed.3007653. Epub 2014/01/31. [DOI] [PubMed] [Google Scholar]