Abstract
The mammalian retina consists of multiple cell layers including photoreceptor cells, which are light sensing neurons that play essential functions in the visual process. Previously, we identified mutations in SPATA7, encoding spermatogenesis associated protein 7, in families with Leber Congenital Amaurosis (LCA) and juvenile Retinitis Pigmentosa (RP), and showed that Spata7 null mice recapitulate the human disease phenotype of retinal degeneration. SPATA7 is expressed in the connecting cilium of photoreceptor (PR) cells in the mouse retina, as well as in retinal pigment epithelium (RPE) cells, but the functional role of Spata7 in the RPE remains unknown. To investigate whether Spata7 is required in PRs, the RPE, or both, we conditionally knocked out Spata7 in photoreceptors and RPE cells using Crx-Cre and Best1-Cre transgenic mouse lines, respectively. In Spata7 photoreceptor-specific conditional (cKO) mice, both rod and cone photoreceptor dysfunction and degeneration is observed, characterized by progressive thinning of the outer nuclear layer and reduced response to light; however, RPE-specific deletion of Spata7 does not impair retinal function or cell survival. Furthermore, our findings show that both Rhodopsin and RPGRIP1 are mislocalized in the Spata7Flox/−; Crx-Cre cKO mice, suggesting that loss of Spata7 in photoreceptors alone can result in altered trafficking of these proteins in the connecting cilium. Together, our findings suggest that loss of Spata7 in photoreceptors alone is sufficient to cause photoreceptor degeneration, but its function in the RPE is not required for photoreceptor survival; therefore, loss of Spata7 in photoreceptors alters both rod and cone function and survival, consistent with the clinical phenotypes observed in LCA and RP patients with mutations in SPATA7.
Keywords: Retinitis Pigmentosa, Leber Congenital Amaurosis, SPATA7, Photoreceptors, Retinal Degeneration, Conditional Knockout, RPE, Retinal Function
1. INTRODUCTION
The mammalian retina consists of a variety of cell types that have specialized roles in visual processing and function together to allow for normal vision. Photoreceptors are light-sensing neurons in the retina which function by absorbing photons from the visual field that triggers the phototransduction cascade, converting light into electrical impulses. The retinal photoreceptor layer is composed of rods, which are responsible for sensing low light and peripheral vision, and cones, which are responsible for color and high-resolution vision. Loss of rod and/or cone function can result in several forms of retinal dystrophy (RD), and mutations in several genes have been identified to be associated with RD (Bok, 2007; Daiger et al., 2014; Nash et al., 2015; Riaz and Baird, 2016). For instance, mutations in over 22 genes have been associated with Leber Congenital Amaurosis (LCA, MIM 204000), and over 80 disease loci have been identified for Retinitis Pigmentosa (RP, MIM 268000). LCA is a highly heterogeneous form of inherited retinal dystrophy that manifests congenitally and is characterized by severe visual loss in which both rods and cones are affected (Sharif and Sharif, 2017; Weleber et al., 1993). In contrast, RP is a form of retinal degeneration that has a later onset, and involves progressive degeneration of retinal photoreceptors in which rods are primarily affected, and cones often degenerate subsequently (Fahim et al., 1993). Both LCA and RP are the most common inherited forms of RD eye diseases which result in visual impairment in humans.
Previously, we identified disease-causing mutations in SPATA7, encoding spermatogenesis associated protein 7 (MIM 609868), in families with LCA and juvenile RP (Wang et al., 2009). Following this report, additional studies have identified SPATA7 mutations in patients with these diseases, clearly demonstrating a causal link between SPATA7 and human retinal disease (Kannabiran et al., 2012; Mackay et al., 2011; Matsui et al., 2016; Mayer et al., 2015; Perrault et al., 2010; Watson et al., 2014). Recently, we examined the effects of germline disruption of Spata7 and showed that Spata7 knockout (KO) mice exhibit early onset retinal degeneration, and the phenotype can be rescued by gene therapy (Eblimit et al., 2015; Zhong et al., 2015). SPATA7 is localized in the connecting cilium (CC) of photoreceptor cells in the mouse retina, where it interacts with RP GTPase regulator interacting protein 1 (RPGRIP1) and plays a role in trafficking of Rhodopsin across the CC to the outer segment. It is also expressed in the basal body of the primary cilium in retinal pigment epithelium (hTERT-RPE-1) cells (Eblimit et al., 2015). Additionally, Spata7 mRNA is also expressed in the ganglion and inner nuclear layers in the retina, as well as human spermatocytes and the brain (Mackay et al., 2011; Perrault et al., 2010; Wang et al., 2009).Expression of SPATA7 is not restricted to just one cell type in the retina and the germline null mouse model does not allow study of Spata7 function in a cell-type specific manner. Therefore, we used a conditional gene targeting to ablate Spata7 specifically in the RPE and/or photoreceptors as an approach to investigate its cell type-specific function in retinal degeneration and obtain mechanistic insights into its role in retinal function and survival.
In this study, we generated a conditional Spata7 knockout (cKO) mouse allele to determine which cell type requires Spata7 function for photoreceptor survival. To ablate Spata7 in post-mitotic photoreceptors, we utilized the Crx-Cre line, which expresses Cre in developing photoreceptors in the retina (Furukawa et al., 2002; Furukawa et al., 1997). Additionally, to conditionally delete Spata7 in RPE cells, we used the Best1-Cre transgenic mouse line (Iacovelli et al., 2011). Conditional deletion of Spata7 resulted in a strong photoreceptor phenotype in the Spata7Flox/−; Crx-Cre retina, characterized by progressive degeneration of photoreceptor cells with concomitant loss of retinal function. This phenotype is consistent with the Spata7 KO phenotype previously reported, recapitulating the disease phenotype in patients harboring SPATA7 mutations. In contrast, Spata7Flox/−; Best1-Cre mice did not exhibit in any apparent morphological changes in the retina. Together, these findings suggest that ablation of Spata7 in photoreceptors alone is sufficient to cause retinal degeneration, and loss of Spata7 in RPE fails to cause photoreceptor cell death.
2. MATERIAL AND METHODS
2.1 Generation of a Spata7 Conditional Allele
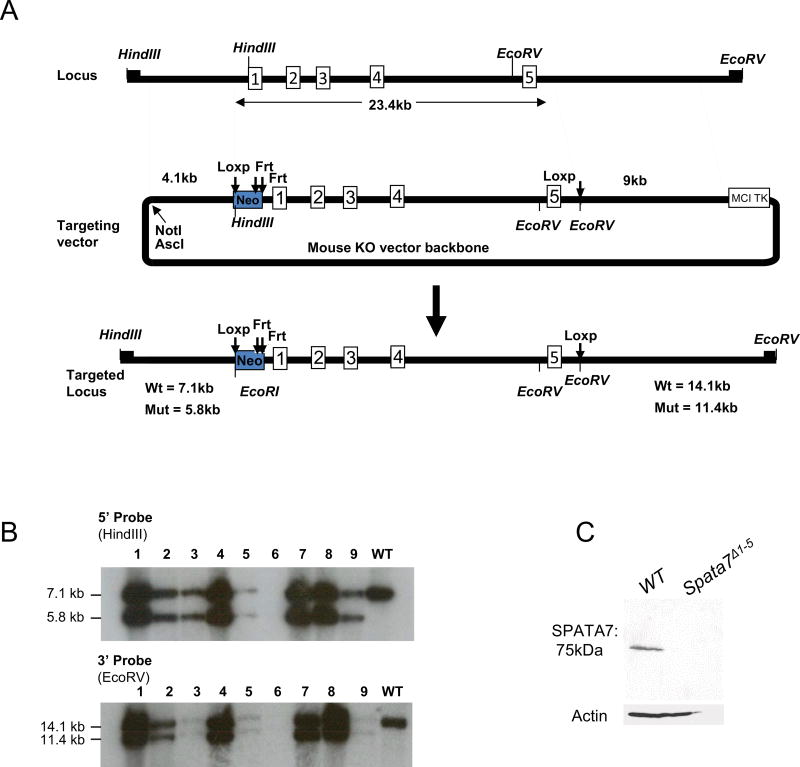
To generate a floxed Spata7 allele (Spata7Flox), AB 2.2 embryonic stem cells (Mouse ES Cell Core Facility at Baylor College of Medicine) derived from the 129 SvEv strain were electroporated with a linearized targeting vector. DNA from embryonic stem cell lines was digested with HindIII or EcoRV and analyzed by Southern blot using 5′ and 3′ probes (flanking the recombination arms and not included in the targeting vector), respectively, and genomic PCR. Three independently targeted cell lines were selected and microinjected into the C57BL6 blastocysts to generate chimeras. One chimera underwent germline transmission. To genotype subsequent F2 and F3 generations of Spata7Flox mice, we used a genomic PCR assay with a pair of primers from the targeted region. These primers are: P1F (5’-CTTGGTCCTGTGAAGGTTCTATG-3’) and P1R (5’- GGATTTACTACGAGCTCAGATTCC-3’), P2F (5’- GGATTAAAGGGGTGTACTCAAGG-3’) and P2R (5’-TTCCACACCAGAATCAGTTCTTT-3’). Mice harboring a floxed Spata7 allele were crossed to Hprt-Cre mice to generate Spata7Δ1–5 knockout mice. To generate cell-type specific loss of Spata7, Spata7Flox/Flox mice were bred with previously generated Spata7null mice to obtain Spata7Flox/− mice. Spata7Flox/− mice were crossed with the Crx-Cre or Best1-Cre mice to obtain the following cKO mutant mice: Spata7Flox/−; Crx-Cre and Spata7Flox/−; Best1-Cre, and the respective littermate controls: Spata7Flox/+; Crx-Cre and Spata7Flox/+; Best1-Cre. Additionally, a combination of Spata7Flox/−, Spata7Flox/+ and Spata7Flox/+; Crx-Cre mouse retinal sections were used as controls in this study. All animals were handled in accordance with the policies on the treatment of laboratory animals by Baylor College of Medicine.
2.2 Measurements of Outer Nuclear Layer (ONL) Thickness
Mouse eyes were dissected, embedded in paraffin, and sectioned along the vertical meridian. The thickness of the outer nuclear layer (ONL) was measured at 20 positions equally spaced along the retina (10 positions each in the superior and inferior hemispheres). For each position, three measurements were taken and the mean value of these three measurements was calculated. Measurements were made using a camera lucida connected to a light microscope, a WACOM graphics tablet (WACOM, Vancouver, WA), and AxioVision LE Rel. 4.1 software (Zeiss, Goettingen, Germany). Before each measurement session, the setup was calibrated using a stage micrometer (Klarmann Rulings, Litchfield, NH). A minimum of three mice were used for each genotype for morphometry analysis. For morphometry statistical analysis, the Student’s T-test was performed (NS= not significant, p>0.05; *p<0.05; **p<0.01; ***p<0.001).
2.3 Electroretinograms
Mice were dark-adapted overnight and anesthetized by a single intraperitoneal injection of 22 mg/kg ketamine, 4.4 mg/kg xylazine, and 0.37 mg/kg acepromazine. Both pupils were dilated with a drop of tropicamide (1.0%) and phenylephrine (2.5%) and then corneas were anesthetized with a drop of proparacaine (1.0%). After 1 min, excess amount of fluid was cleaned and a drop of Goniosoft (2.5%) was placed on each cornea to keep it moistened and provide a good contact between the cornea and the ERG electrode (N1530NNC). All ERG tests were performed under a dim red light and a heating pad was used to keep mice at a constant body temperature of 37.6°C. Six flash intensities (−34, −24, −14, −4, 0 and 10 dB) were used to record scotopic ERG a- and b-waves on a UTAS Visual Diagnostic System and EMWIN software (LKC Technologies, Gaithersburg, MD, USA). Additionally, photopic ERGs were subsequently recorded at various ages. Mice were light-adapted to a 30 cd*s/m2 white background for 2 min after scotopic ERG recordings and then photopic ERGs were recorded with flash intensities of 0, 10, and 25 dB as previously described (Agrawal et al., 2017). A minimum of six mice were used for each genotype for ERG analysis. GraphPad Prism5 software (GraphPad Software, La Jolla, CA, USA) was used to analyze all ERG data, and the Student’s T-test was performed for statistical analysis. (NS= not significant, p>0.05; *p<0.05; **p<0.01; ***p<0.001).
2.4 Western Blot Analysis
Whole retinas from wild-type and mutant mice were homogenized in 100 µl of 1×NETN buffer (20% glycerol, 50mM Tris-HCI pH8.0, 150mM NaCI, 1mM EDTA, 0.5% NP-40) and centrifuged for 20 min at 13,000 rpm at 4°C to harvest supernatant. Proteins were quantified by the BCA protein assay and heated at 95°C in sample application buffer (5% SDS, 15% sucrose, 50mM Na2CO3, 50 mM DTT, 1% 2-mercaptoethanol, bromophenol blue) before being separated on a 10% SDS-PAGE gel (pH 8.8) with a 4% stacking gel (pH 6.8) and then transferred to nitrocellulose membranes at 40 mA overnight at 4°C. Membranes were first blocked with 5% dry milk in Tris-buffered saline Tween 20 (TBST) (0.05M Tris–HCl, pH 8.0, 0.15M NaCl, 0.05% Tween 20) at room temperature for 1 hr and then incubated overnight at 4°C with primary antibodies in 5% dry milk in TBST: 1/1000 rabbit anti-Spata7 (Proteintech Group, 12020-1-AP), and 1/5000 mouse anti-β-Actin (Sigma). Membranes were further incubated with 1/5000 antirabbit IgG horseradish peroxidase (HRP) conjugate (Santa Cruz Biotechnology) in 5% dry milk in TBST for 1 hr at room temperature. Bands were detected with the enhanced chemiluminescence (ECL) plus system (GE Healthcare).
2.5 Immunohistochemistry
Eyes were enucleated from knockout and wild-type mice. Modified Davidson’s Fixative was used to fix eyes overnight for paraffin embedding. Seven micrometer eye sections were cut (Microtome, Leica). Slides were deparaffinized and antigen retrieval was performed by boiling sections in 0.01 M Tris, EDTA buffer (pH 9.0) for 30 min(10 mM sodium Citrate buffer with pH 6 was used for Cre recombinase antigen retrieval), followed by cooling for 30 min at room temperature. Slides were washed in PBS, incubated for 1 hr at room temperature in hybridization buffer (10% normal goat serum, 0.1% Triton X-100, PBS), then incubated overnight in primary antibody diluted in hybridization buffer. Slides were then washed in PBS, incubated with secondary antibody diluted in hybridization buffer at room temperature for 2 hr, washed in PBS, mounted with anti-fade medium (Prolong; Invitrogen) to reduce bleaching, and coverslipped. Fluorescent images were captured with a Zeiss Apotome.2 microscope (Zeiss Axio Imager M2m. Immunohistochemistry with frozen sections was conducted as previously described (Eblimit et al., 2015) and all immunostaining experiments were performed independently using three biological replicates.
2.6 Isolation of mouse RPE and RT-PCR
Mouse retinal pigment epithelial (RPE) cells were isolated from two mouse eyes according to a published method (Sonoda et al., 2009) with slight modifications. The anterior segment, lens, and vitreous were discarded, the posterior eye cup was dissected into four flaps with a razor blade, and then the retina and sclera were gently removed from the RPE layer with forceps. RPE–choroid sheets were incubated in 2% (wt/vol) dispase solution (Gibco, Billings. MT) for 30 min at 37°C with 5% CO2 and then placed in 1% holding buffer (bovine serum albumin - BSA) (Sigma, St. Lois, MI) in phosphate buffered saline (PBS) (VWR International Inc, West Chester, PA) to stop the dispase reaction. After RPE layers were peeled off from the choroid with fine forceps, RPE sheets were placed in Trizol reagent (Invitrogen), total RNA was isolated, and first-strand cDNA was synthesized using the SuperScript™III First-Strand Synthesis System for RT-PCR (Invitrogen). Primers flanking exons 1 and 5 were used for RT-PCR.
2.7 Spectral-Domain Optical Coherence Tomography (SD-OCT)
The Ultrahigh resolution Spectral Domain Ophthalmic Imaging System (SD-OCT) (Envisu R2200 SDOIS, Leica Microsystems, Morrisville, NC) was used to perform live-imaging of mouse retinas. Prior to imaging, intraperitoneal injection of a ketamine/xylazine anesthesia cocktail (ketamine, 100 mg/kg; xylazine, 10 mg/kg) was followed by dilation of eyes with one drop of 1% cyclopentolate hydrochloride ophthalmic solution (Baush & Lomb, Rochester, NY) and one drop of 2.5% phenylephrinehydrochloride ophthalmic solution (Falcon Pharmaceuticals, Fort Worth, TX). Prior to dilation, eyes were lubricated using Systane Ultra lubricant drops (Alcon, Fort Worth, TX) and GenTeal Severe lubricant gel (Alcon, Fort Worth, TX) was used post-dilation. Mice were placed in a cylindrical cassette on the rodent alignment stage in front of the optical scanning mouse retina bore. Pupils were adjusted as needed and each eye was imaged along the entire axial length. A minimum of three mice were used for each genotype for OCT analysis, and a representative image was presented in Supplementary Figure 1. Image acquisition, assessment, and processing were performed using the InVivoVue 2.2 Image Acquisition Software (Leica Microsystems).
2.8 Dilutions and sources of antibodies used for immunohistochemistry
Rabbit anti-SPATA7 polyclonal antibody against the C-terminal 15 amino acids (1:2000, custom antibody made by Bethyl Laboratories), mouse anti-Rho (B6-30N, 1:200, a generous gift from W. Clay Smith), rabbit anti-RPGRIP1 (1:500, a gift from Tian Li Sen), rabbit anti-Cre antibody (1:200, Novagen) were used. Secondary antibodies were conjugated with Alexa fluor 488, 568 (1:500, Molecular Probes), and DAPI staining reagent was diluted 1:1000.
3. RESULTS
3.1 Generation of Spata7 photoreceptor-specific conditional knockout mice
Previously, we generated Spata7 germline null mice by deleting Exons 1–10 (Eblimit et al., 2015). To generate Spata7 conditional knockout mice (cKO), we designed a Spata7 conditional allele with loxP sites flanking exons 1 through 5 (Figure 1A). Mice carrying the Spata7 conditional allele were confirmed by genomic Southern blot analysis and PCR (Fig. 1B and data not shown). Spata7Flox/Flox mice were crossed to Hprt-Cre mice to ubiquitously delete Spata7 and generate Spata7Δ1–5 knockout mice. We tested whether SPATA7 is expressed in Spata7Δ1–5 null mice by performing western blotting on retinal extracts from wild-type and Spata7Δ1–5 null adult mice, and no SPATA7 was detected in the null retina (Fig. 1C). These results confirm that the conditional targeting of Spata7 exons1–5 results in complete loss of protein. Spata7Δ1–5 null mice are viable and fertile, and exhibit no gross abnormalities upon examination. Spata7Δ1–5 null mice present retinal phenotypes (data not shown) which are phenotypically indistinguishable from Spata7 null mice (Eblimit et al., 2015). To achieve a maximal level of deletion efficiency, we used Spata7 mutant mice carrying compound heterozygous alleles (Spata7Flox/−). SPATA7 variants in human LCA3 is inherited in an autosomal recessive pattern, and Spata7+/− mice show no detectable retinal degeneration phenotype, suggesting that having one copy of Spata7 is sufficient for normal function of the protein (Eblimit et al., 2015).Therefore, conditional Cre-mediated loss of Spata7 function using the Spata7Flox/− alleles will result in null or nearly null phenotypes at the cellular level. We generated Spata7Flox/− mice by crossing Spata7 null mice with Spata7Flox/Flox mice. For photoreceptor-specific deletion of Spata7, we utilized the Crx-Cre transgenic mouse line, which expresses Cre in developing photoreceptors in the retina (Furukawa et al., 2002; Furukawa et al., 1997), to obtain Spata7Flox/−; Crx-Cre mice.
Figure 1. Generation of Spata7 conditional knockout mice.
(A) Spata7 targeting strategy. Numbered rectangles represent Spata7 exons. (B) Genomic Southern blot of Spata7 targeted allele. Fragments of 5.8 kb and 11.4 kb are detected in a heterozygous mouse using 5’ (HindIII) and 3’ (EcoRV) probes, respectively. (C) Western blot of SPATA7 protein probed using anti-SPATA7 antibody using retinas isolated from wild-type and Spata7Δ1–5 knockout mice. Anti-β-actin antibody was used as a loading control.
3.2 Loss of Spata7 in photoreceptors causes progressive retinal degeneration
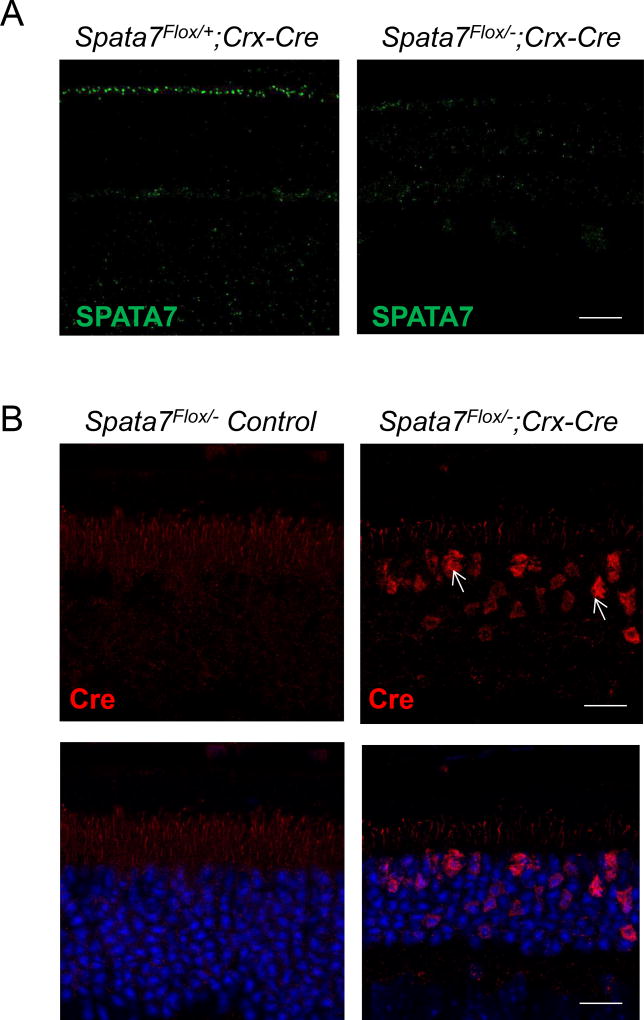
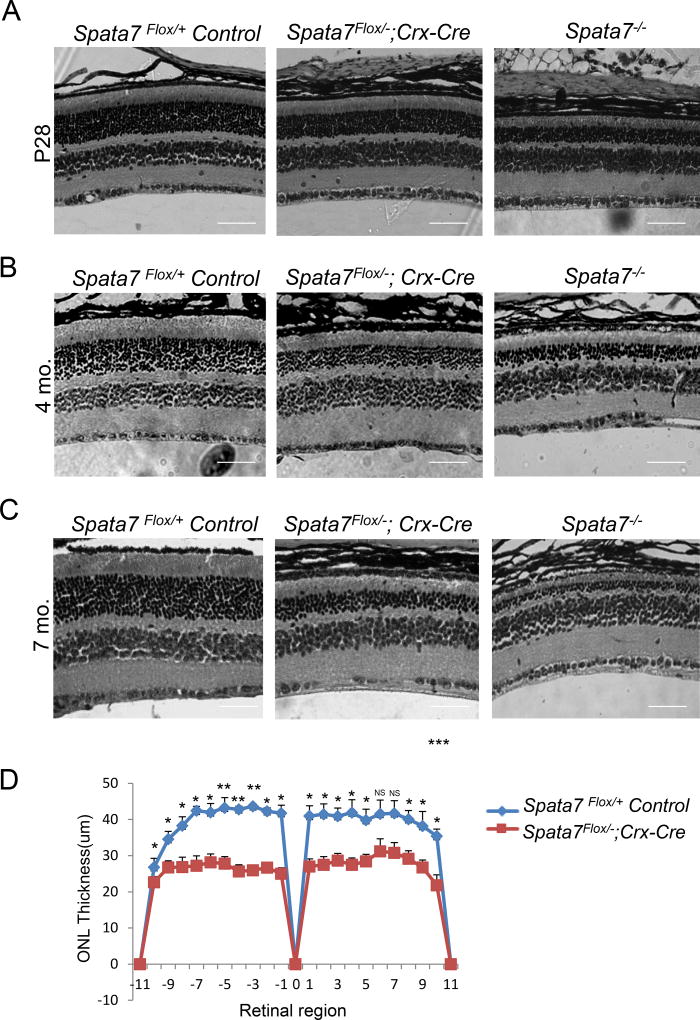
Immunostaining for SPATA7 confirmed its localization to the connecting cilium in the retina of Spata7Flox/+; Crx-Cre control mice, while no signal was detected in the Spata7Flox/−; Crx-Cre adult retina, as expected (Fig. 2A). Additionally, robust Cre-positive staining was observed in the ONL of photoreceptor cells in the Spata7Flox/−; Crx-Cre retina, while it was absent in the Spata7Flox/− control retina (Fig. 2B). These results confirmed that the Spata7 conditional allele was specifically ablated in Cre-expressing photoreceptors. Hematoxylin and eosin (H&E) stained retinal sections from Spata7Flox/−; Crx-Cre mice were examined for morphological defects postnatally. Histological analysis of Spata7 WT, Spata7+/−, Spata7Flox/Flox, Spata7Flox/+, Spata7Flox/−, Spata7Flox/+; Crx-Cre mice showed no morphological defects in the retina at adult stages (6–7 mo. old) (Supp. Fig. 4). In this study, Spata7Flox/−, Spata7Flox/+, and Spata7Flox/+; Crx-Cre mice were used as controls. Histological analysis of the Spata7Flox/−; Crx-Cre retina at postnatal day 28 (P28) revealed a significant retinal degeneration phenotype. Compared to Spata7Flox/−; Crx-Cre retina, the severity of the Spata7 null retina is more pronounced, while the Spata7Flox/+; Crx-Cre mice exhibited no phenotype (Fig. 3A). Retinal morphometry showed a 30% reduction in the thickness of ONL of Spata7Flox/−; Crx-Cre mice at P28 in comparison to the littermate control retinas (Fig. 3D). At 4 months, in Spata7Flox/−; Crx-Cre mice, on average, 8 rows of nuclei remain compared to Spata7Flox/+ control retina which contains 13 rows of nuclei. Similarly, at7 months, an average of 4 rows of nuclei remain in Spata7Flox/−; Crx-Cre mice compared to an average of 13 rows of nuclei present in the Spata7Flox/+ control retina (Fig. 3B, C). This reduction in the overall ONL thickness shows that retinal degeneration is progressive in Spata7Flox/−; Crx-Cre mice. Furthermore, live imaging of the retina of Spata7Flox/−; Crx-Cre mice and control mice at 3, 5, and 7 months using optical coherence tomography (OCT) showed consistent thinning of the ONL (Supp. Fig. 1). Compared to the age-matched control retinas, we observe a reduction in the overall retinal thickness in Spata7Flox/−; Crx-Cre mice.
Figure 2. Loss of SPATA7 expression in the retina of Spata7Flox/−; Crx-Cre mice.
(A) Immunostaining for SPATA7 (green) in retinal sections obtained from Spata7Flox/+; Crx-Cre control mice and Spata7Flox/−; Crx-Cre mice at P28. (B) Immunostaining for Cre-recombinase in Spata7Flox/− control mouse retina and Spata7Flox/−; Crx-Cre adult cKO mouse retina at P28. Nuclei are counter-stained with DAPI (blue). Scale bar = 20 µm. CC: connecting cilium; ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer.
Figure 3. Progressive photoreceptor degeneration in Spata7Flox/−; Crx-Cre conditional knockout mice.
(A) Hematoxylin and eosin (H&E) staining of paraffin-embedded retinal sections obtained from Spata7Flox/+ Control, Spata7Flox/−; Crx-Cre, and Spata7−/− mice at P28 (B) 4 months, and (C) 7 months of age. Progressive thinning of the ONL is observed in Spata7Flox/−; Crx-Cre conditional KO mice and Spata7−/− (germline null) mice starting at P28, while the retina appears normal in Spata7Flox/+ control mice. (D) Retinal morphometry is presented by measuring the thickness of the ONL at 20 equally spaced positions in paraffin embedded sections along the vertical meridian of the retina for three retinas of the same genotype at P28. Three measurements were taken for each position, and a mean value was calculated. Each point represents the mean ±SEM obtained for each group (n ≥3 mouse retinas). Position 0 corresponds to the optic nerve head. Statistical analysis was performed using the Student’s t-test (NS= not significant, p>0.05; *p<0.05; **p<0.01). Scale bar = 40 µm. ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer.
3.3 Defects in response to light in Spata7Flox/−; Crx-Cre mutant mice
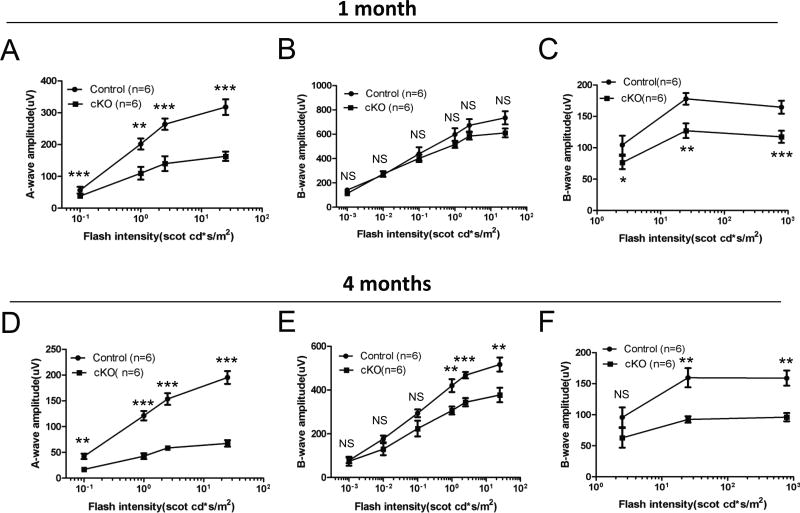
Spata7Flox/+; Crx-Cre mice have normal scotopic (dark-adapted) and photopic (light-adapted) ERG responses, while Spata7Flox/−; Crx-Cre mice at P28 exhibit a significantly reduced response to light, with a 50% reduction in the a-wave amplitude under scotopic conditions (Fig. 4A, B) and a 20–30% reduction under photopic conditions (Fig. 4C), suggesting that both rods and cones are affected. To determine if the functional defects in Spata7 photoreceptor-specific conditional null mice progresses over time, retinal function was reassessed at 4 months of age. Spata7Flox/−; Crx-Cre mice show a further reduction in the scotopic a-wave response, with a 70% reduction in a-wave amplitude and a consequent reduction in scotopic b-waves (Fig. 4D, E). Photopic ERGs, depicting cone function, show a consistent decline in the b-wave amplitude (Fig. 4F). These findings indicate that loss of Spata7 in photoreceptors significantly reduces the response to light in Spata7Flox/−; Crx-Cre mice.
Figure 4. Spata7Flox/−; Crx-Cre mice exhibit reduced amplitudes of scotopic and photopic ERGs.
Quantitative evaluation of scotopic (dark-adapted) a-wave and b-wave amplitudes was performed at (A, B) 1 month and (D, E) 4 months of age. Photopic (light-adapted cone response) amplitudes were also obtained at (C) 1 month and (F) 4 months of age from Spata7Flox/+; Crx-Cre control mice and Spata7Flox/−; Crx-Cre cKO mice. Spata7Flox/+; Crx-Cre mice had normal scotopic and photopic ERG responses, while Spata7Flox/−; Crx-Cre cKO mice exhibited a significantly reduced response to light, with a 50% reduction in the a-wave amplitude under scotopic conditions (A,B) and a 20–30% reduction under photopic conditions (C) at 1 month of age. At 4 months, Spata7Flox/−; Crx-Cre cKO mice showed a further reduction in the scotopic response (D, E) and a consistent reduction in the photopic response (F). Statistical analysis was performed using the Student’s t-test (NS= not significant, p>0.05; *p<0.05; **p<0.01; ***p<0.001). n = 6 mice per genotype.
3.4 Mislocalization of Proteins in photoreceptor-specific Spata7 cKO retina
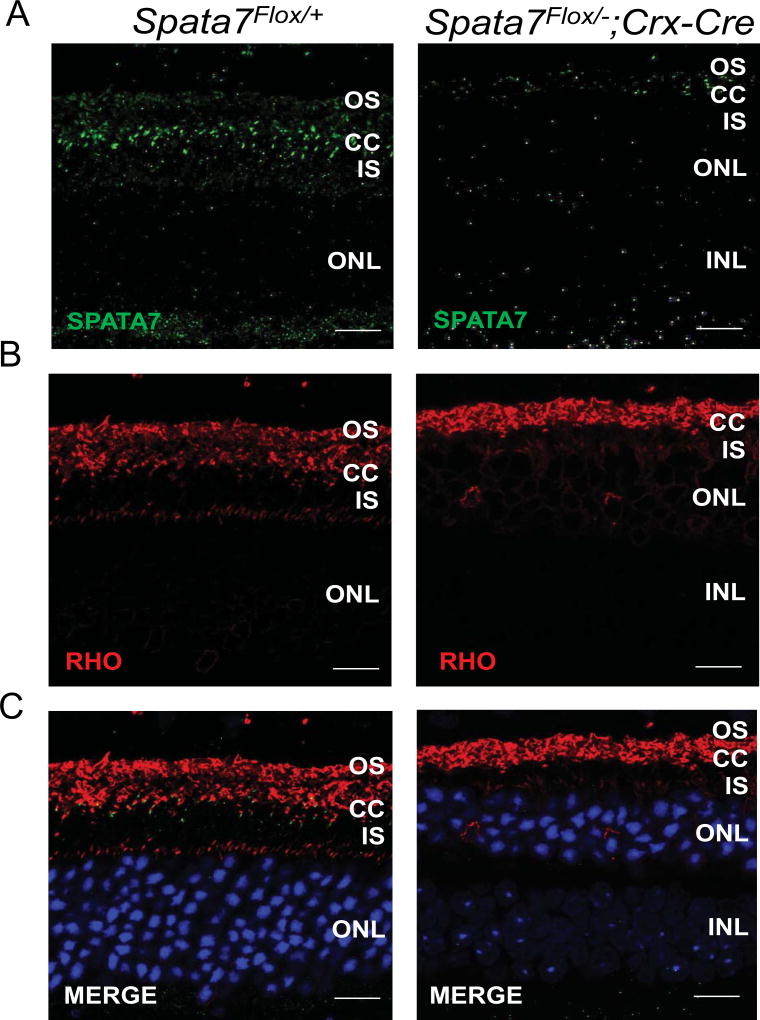
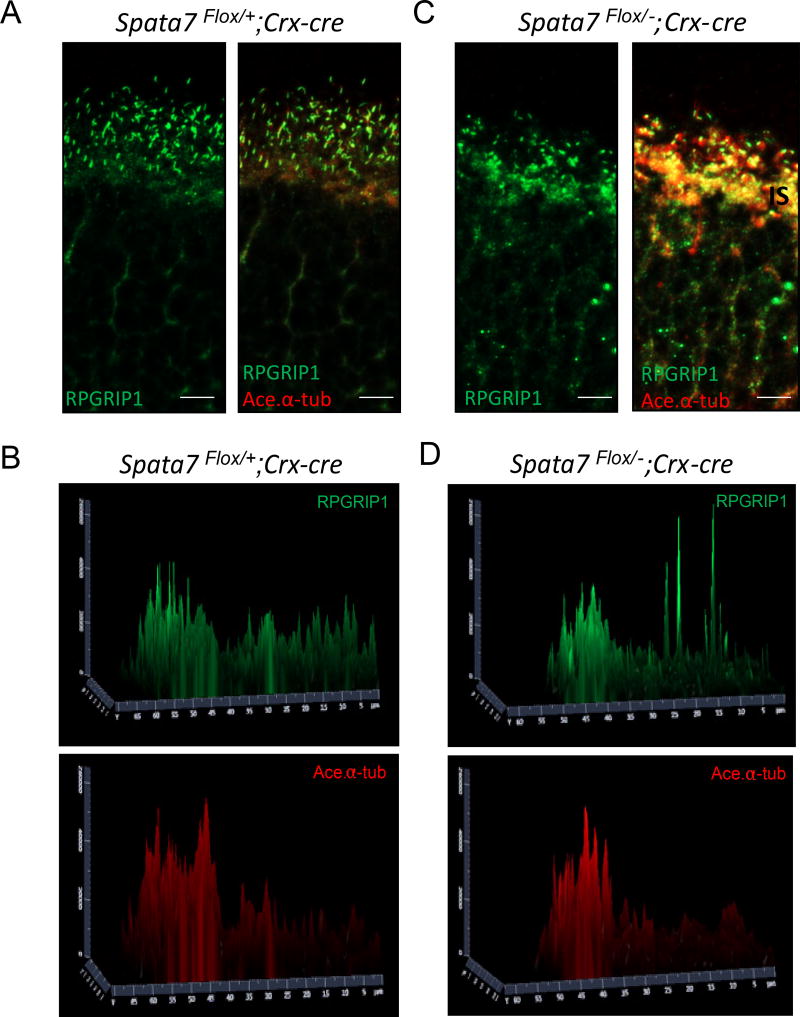
Based on our previous findings, SPATA7 is required for proper localization of Rhodopsin in photoreceptors (Eblimit et al., 2015). Rhodopsin is normally localized to the outer segment and plays an essential function in photoreceptor development and phototransduction. In Spata7 KO mice, Rhodopsin is mislocalized as early as P7, which precedes the photoreceptor degeneration observed at P14 (Eblimit et al., 2015). These findings suggest that photoreceptor apoptosis follows rhodopsin mislocalization and is therefore unlikely to be a secondary consequence resulting from photoreceptor degeneration. To determine whether loss of Spata7 in photoreceptors alone has the same consequence, we performed immunohistochemistry to study Rhodopsin localization in mice at 7 months. Rhodopsin (Rho) is indeed mislocalized in Spata7Flox/−; Crx-Cre mice, while it localizes normally in Spata7Flox/+ control mice (Fig. 5A–C), suggesting that conditional deletion of Spata7 in photoreceptors results in Rho mislocalization, similar to the phenotype observed in Spata7 KO mice. Although we did not test Rho mislocalization at early stages prior to retinal degeneration in Spata7Flox/−; Crx-Cre mice, the consistency of the findings in the adult retina presented here support the previous conclusions that SPATA7 plays a role in Rhodopsin trafficking. Additionally, we also examined localization of RPGRIP1, a previously identified interaction partner of SPATA7 and RPGR (RP GTPase receptor) (Boylan and Wright, 2000; Eblimit et al., 2015). Consistent with the findings observed in Spata7 null mice, mislocalization of RPGRIP1 is evident in the Spata7Flox/−; Crx-Cre retina (Fig. 6). Compared to the control retina, in which RPGRIP1 is localized to the connecting cilium (Fig, 6A, B), RPGRIP1 is mislocalized to the outer nuclear layer (ONL) and photoreceptor synapses in Spata7Flox/−; Crx-Cre retinas, shown at a lower magnification in Supplemental Figure 6, while an exoneme marker, acetylated α-tubulin, remains unchanged (Fig. 6 C, D, Supp. Fig. 6). These results suggest that SPATA7 exerts its role in photoreceptors by functioning as a component of the RPGRIP1-RPGR complex, where it is expressed, and loss of SPATA7 likely destabilizes this complex in the connecting cilium.
Figure 5. Spata7Flox/−; Crx-Cre (cKO) mice exhibit Rhodopsin mislocalization.
(A) SPATA7 was detected in retinal paraffin sections of Spata7Flox/− mice, while little to no immunostaining was detectable in the retina of Spata7Flox/−; Crx-Cre mice. (B, C) Localization of rhodopsin (Rho; red) is altered in Spata7Flox/−; Crx-Cre cKO mice, where it is mislocalized to the inner segment and ONL of photoreceptor cells, compared to Rho localization in the OS of Spata7Flox/+ control retinas at 7 month of age. Nuclei are counter-stained with DAPI (blue). Scale bar = 20 µm. OS: outer segment; CC: connecting cilium; IS: inner segment; ONL: outer nuclear layer.
Figure 6. RPGRIP1 mislocalization in Spata7Flox/−; Crx-Cre retina.
(A) Immunostaining for RPGRIP1 was performed in Spata7Flox/+; Crx-Cre frozen retinal sections, where RPGRIP1 (green) co-localization was observed with acetylated-α tubulin (red) marker in the connecting cilium. (C) In Spata7Flox/−; Crx-Cre retinas, mislocalization of RPGRIP1 is evident, where it is detectable in the IS and ONL regions at P40. (B, D) Surface plot quantification of RPGRIP1 and Acetylated- α tubulin intensity in the OS, CC, IS, and ONL compartments. OS: outer segment; CC: connecting cilium; IS: inner segment; ONL: outer nuclear layer. Scale bar = 10 µm.
3.5 Characterization of Spata7 RPE-specific conditional knockout mice
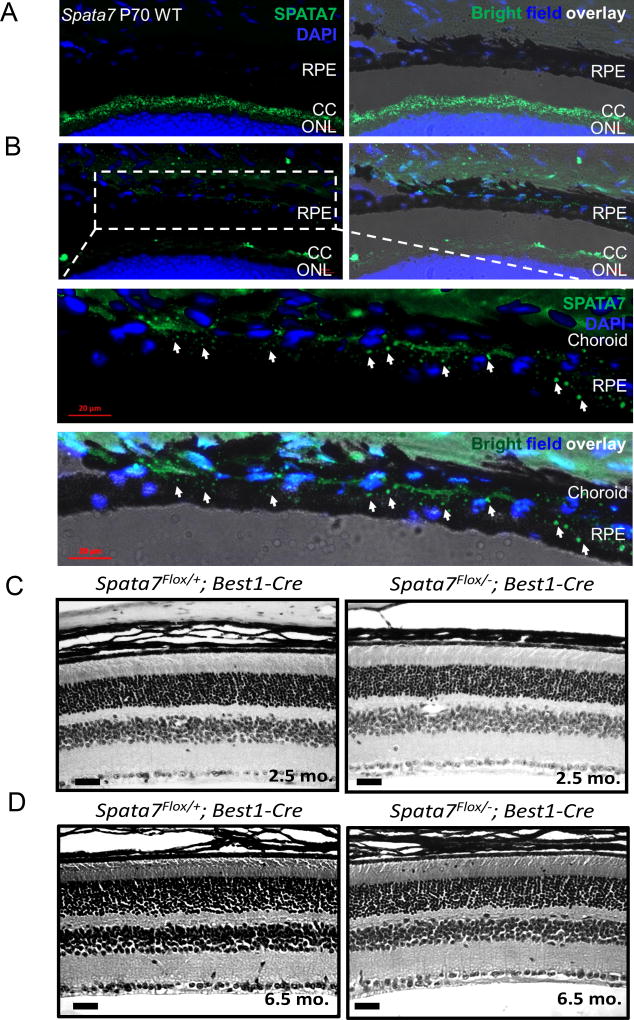
SPATA7 has been previously reported to be expressed in human RPE (hTERT RPE-1) cells (Eblimit et al., 2015). We performed RT-PCR on the RPE isolated from the adult mouse retina, the RPE+ choroid, and whole retina from WT, Spata7+/− and Spata7 null mice respectively (Suppl. Fig. 2). Furthermore, we performed immunostaining on retinal cryosections and detected SPATA7 specifically in the CC (Fig. 7A). We refocused on the RPE region to overcome the difference in the focal plane between RPE and photoreceptor cells, which allowed us to visualize SPATA7 expression in the RPE and choroid regions in the mouse retina (Fig, 7B). Furthermore, we merged SPATA7 with the bright field image to clearly demonstrate that SPATA7 is expressed in the RPE. Together, these results confirm that SPATA7 is expressed in RPE. To determine whether loss of Spata7 specifically in the RPE results in a retinal phenotype, we crossed Spata7Flox/−mice with Best1-Cre transgenic mice, which expresses Cre specifically in RPE cells (Iacovelli et al., 2011). To confirm the expression of Cre in the RPE of Spata7Flox/−; RPE-Cre mice, we performed immunostaining of Cre recombinase in adult retinal sections, and detected strong Cre positive signals in the RPE at low and high magnifications (Supp. Fig. 5 A, B, respectively). We assessed the overall retinal morphology of 2.5-month-old Spata7Flox/−; RPE-Cre retinas by H&E staining and observed no obvious photoreceptor degeneration (Fig. 7C). We also examined retinas of Spata7Flox/− ; RPE-Cre and Spata7Flox/+; RPE-Cre control mice at 6.5 months of age, and consistently observed no significant differences in the overall retinal morphology (Fig. 7D). Since no phenotypes are observed at this late stage, our data suggest that loss of Spata7 in the RPE alone is not sufficient to cause readily detectable retinal degeneration.
Figure 7. Spata7Flox/−; RPE-Cre conditional KO mice lack a retinal degeneration phenotype.
(A) SPATA7 expression (green) in the connecting cilium (left) and overlay of expression with bright field imaging of frozen mouse retinal sections (right). DAPI was used for nuclear counterstaining (blue). (B) Enhanced re-focusing of the SPATA7 signal in the RPE and choroid region at high magnification. Expression of SPATA7 (green) is indicated in the RPE by arrows. Scale bar= 20 µm. (C) Cross sections of 2.5-month-old RPE-specific Spata7 cKO retinas show no obvious photoreceptor degeneration and appear normal compared to Spata7Flox/+;Best1-Cre control retinal sections. (D) At 6.5 months, Spata7Flox/−; RPE-Cre and Spata7Flox/− ; RPE-Cre control mice consistently appear normal with no morphological defects present in retinas obtained from Spata7Flox/− ; RPE-Cre mice. RPE= retinal pigment epithelium, CC= connecting cilium, ONL= outer nuclear layer, OS= outer segment, INL= inner nuclear layer. Scale bar = 20 µm.
4. DISCUSSION
Photoreceptors are post-mitotic cells that function throughout life in the visual process, in concert with other neurons in the retina. SPATA7, encoding spermatogenesis associated protein 7 (MIM 609868), was previously identified by our lab as a retinal ciliopathy gene in LCA3 and juvenile RP. SPATA7 is expressed in the connecting cilium of photoreceptors, the light sensing neurons in the retina, in RPE cells, as well as in the basal body of the primary cilium in hTERT-RPE-1 cells, which play an essential role in supporting photoreceptor function and survival. SPATA7 is known to function in trafficking rhodopsin, a major G-protein coupled receptor, as well as trafficking and assembly of the ciliary RPGRIP1 protein complex (Eblimit et al., 2015). Recently, we examined the effects of germline disruption of Spata7 and showed that Spata7 null mice exhibit early onset retinal degeneration, recapitulating the human disease phenotype, and that the mouse phenotype can be rescued by gene therapy (Eblimit et al., 2015; Zhong et al., 2015). In this study, we examine the role of SPATA7 independently in two major cell types in the retina, the photoreceptors and the RPE, to address its cell type-specific function in photoreceptor degeneration. Our findings indicate that RPE-specific deletion of Spata7 does not impair retinal function or survival, whereas loss of Spata7 in photoreceptor cells leads to retinal dysfunction and photoreceptor degeneration.
To assess the photoreceptor specific functions of Spata7 in vivo, we used the Crx-Cre transgenic mouse line which allowed us to remove Spata7 early during development since CRE recombinase is selectively expressed in developing photoreceptor cells under the control of the Crx promoter. CRE expression was first detected at embryonic day (E) 12.5 in postmitotic differentiating photoreceptors with prominent expression at E15.5 (Nishida et al., 2003). Spata7Flox/−; Crx-Cre mice exhibited phenotypes in both rods and cones, evident from both morphological and functional analyses of the retina at several postnatal time points. The phenotype observed in Spata7Flox/−; Crx-Cre mice was examined at P28, where more than 30% of photoreceptor cells were already degenerated. However, the onset of the phenotype is likely to be earlier than this time point, similar to the phenotype of Spata7−/− mice, where onset occurs at P14. The post-developmental phenotypes of Spata7Flox/−; Crx-Cre and Spata7−/− mice are consistent with the clinical phenotypes of LCA/juvenile RP patients with mutations in SPATA7, where retinal degeneration occurs at birth or within a few months after birth (Mackay et al., 2011). In humans, the central regions of the retina are well developed at fetal week 20 while the peripheral regions continue to develop, and by 5 months after birth, photoreceptor development is complete (Hendrickson, 2008 #425, Narayanan, 1998 #423). Given the phenotypes of patients with SPATA7 variants and the phenotypes of the Spata7 conditional KO mice, it is likely that SPATA7 is important for photoreceptor maintenance and survival post-developmentally.
In contrast, no retinal defects are observed in RPE-specific Spata7 cKO mice (Spata7Flox/− ; Best1-Cre), even as late as 7 months of age. Comparison of the phenotypes resulting from conditional loss of Spata7 in photoreceptors versus the RPE suggests that while loss of Spata7 in photoreceptors is sufficient to cause photoreceptor cell death, loss of Spata7 in the RPE does not substantially contribute to the phenotype. These findings imply that although SPATA7 expression in the RPE may not be required for photoreceptor survival, it may be involved in different processes with unique functions that remain unknown.
Significantly, loss of Spata7 in photoreceptors alone results in morphological defects observable at P28. Compared to germline Spata7 null mice, the phenotype observed in Spata7Flox/−; Crx-Cre mice is less severe during early stages; however, both the germline and photoreceptor specific-cKO mice have rapid, progressive retinal degeneration during adulthood. The difference between the onset and severity of phenotypes could be explained by the possibility that the Cre recombinase may not be expressed in every photoreceptor cell in the retina or that Cre-mediated recombination does not occur in every cell in which Cre is expressed, resulting in mild mosaicism. Examination of SPATA7 expression by immunostaining at post-natal day 6 (P6) shows a few SPATA7-positive cells (Supp. Fig. 3) in Spata7Flox/−; Crx-Cre mice, indicating that this is a possible reason for the differential degeneration rate. Another possibility, though less likely, is that while loss of SPATA7 in the RPE is not sufficient to cause a phenotype, it may have some minor contribution to the overall retinal degeneration. Furthermore, contribution from other cell types could also be involved since SPATA7 is expressed in the INL and GCL as well.
Our findings show that both Rhodopsin and RPGRIP1 are mislocalized in Spata7Flox/−; Crx-Cre mice, suggesting that loss of Spata7 in photoreceptors can result in altered trafficking of a sub-set of proteins in the connecting cilium. Rhodopsin is a member of the G-protein coupled receptor superfamily that functions in the phototransduction pathway by absorbing photons that activate the G-protein transducin, which leads to a cascade that allows light to be converted into an electrical impulse. Mutations in this gene account for 30–40% of all autosomal dominant RP patients (Parfitt and Cheetham, 2016; Thompson et al., 2014; Zalewska et al., 2014), and over 200 point mutations in Rho have been identified in retinal dystrophy patients (RetNet https://sph.uth.edu/retnet/). Additionally, RPGRIP1, which encodes the RP GTPase interacting protein 1, is associated with autosomal recessive forms of LCA and cone-rod dystrophy (Koenekoop, 2005; Li, 2014; Murga-Zamalloa et al., 2009; Patnaik et al., 2015). RPGRIP1 is also expressed in the connecting cilium and has been suggested to be a structural protein of the ciliary axoneme of the connecting cilium. RPGRIP1, together with its interaction partners RPGR (RP GTPase regulator) and nephrocystin-4 (NPHP4), is involved in ciliary protein trafficking (Boylan and Wright, 2000; Patil et al., 2012; Roepman et al., 2000). In this study, we have shown that RPGRIP1 is mislocalized in Spata7Flox/−; Crx-Cre mice, concordant with the observations in the Spata7 germline null mice. Importantly, this strengthens the hypothesis that SPATA7 function in photoreceptors is likely to be associated with ciliary protein trafficking, where it may work together with RPGRIP1/RPGR as a complex in the connecting cilium. Spata7Flox/−; Crx-Cre mice will be invaluable for studying the role Spata7 plays in protein trafficking through the connecting cilium and gaining molecular insights into the mechanism of Spata7 function in a photoreceptor-specific manner in the retina.
In summary, our data show that loss of Spata7, specifically in photoreceptors, causes loss of photoreceptor function and is required for PR survival. Further, we show that both rod and cone cell function is altered, suggesting that Spata7 is required for both photoreceptor subtypes. Additionally, our results suggest that Spata7 ablation in the RPE does not contribute substantially to photoreceptor degeneration. Together, these findings demonstrate an essential role for Spata7 in photoreceptor cell function and survival.
Supplementary Material
Highlights.
Conditional deletion of Spata7 in the photoreceptors results in photoreceptor dysfunction and degeneration.
RPE-specific deletion of Spata7 does not impair retinal function or cell survival.
Rhodopsin and RPGRIP1 are mislocalized in the Spata7Flox/−; Crx-Cre cKO mice.
Acknowledgments
This work was supported by the National Institutes by grants from the Retina Research Foundation and the National Eye Institute [R01EY020540] to G.M. and R.C., 5T32EY007102-23 to S.A. (PI: Dr Graeme Mardon) and F32 EY024815 to I.A.A. Spata7 knockout mice were generated by the Mouse ES Cell Core at BCM that is partially supported by the BCM IDDRC [Grant Number 5P30HD024064-23] from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Rui Chen, Graeme Mardon, Aiden Eblimit and Smriti Agrawal designed the study and wrote the manuscript. Aiden Eblimit, Smriti Agrawal, Kandace Thomas, Anastassov Ivan Assenov, and Tajiguli Abulikemu performed the experiments and statistical analysis.
CONFLICT OF INTEREST STATEMENT
The authors have declared that no conflicts of interest exist.
References
- Agrawal SA, Burgoyne T, Eblimit A, Bellingham J, Parfitt DA, Lane A, Nichols R, Asomugha C, Hayes MJ, Munro PM, Xu M, Wang K, Futter CE, Li Y, Chen R, Cheetham ME. REEP6 Deficiency Leads to Retinal Degeneration through Disruption of ER Homeostasis and Protein Trafficking. Hum. Mol. Genet. 2017 doi: 10.1093/hmg/ddx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D. Contributions of genetics to our understanding of inherited monogenic retinal diseases and age-related macular degeneration. Arch. Ophthalmol. 2007;125:160–164. doi: 10.1001/archopht.125.2.160. [DOI] [PubMed] [Google Scholar]
- Boylan JP, Wright AF. Identification of a novel protein interacting with RPGR. Hum. Mol. Genet. 2000;9:2085–2093. doi: 10.1093/hmg/9.14.2085. [DOI] [PubMed] [Google Scholar]
- Daiger SP, Bowne SJ, Sullivan LS. Genes and Mutations Causing Autosomal Dominant Retinitis Pigmentosa. Cold Spring Harb. Perspect. Med. 2014;5 doi: 10.1101/cshperspect.a017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblimit A, Nguyen TM, Chen Y, Esteve-Rudd J, Zhong H, Letteboer S, Van Reeuwijk J, Simons DL, Ding Q, Wu KM, Li Y, Van Beersum S, Moayedi Y, Xu H, Pickard P, Wang K, Gan L, Wu SM, Williams DS, Mardon G, Roepman R, Chen R. Spata7 is a retinal ciliopathy gene critical for correct RPGRIP1 localization and protein trafficking in the retina. Hum. Mol. Genet. 2015;24:1584–1601. doi: 10.1093/hmg/ddu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim AT, Daiger SP, Weleber RG. Nonsyndromic Retinitis Pigmentosa Overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- Furukawa A, Koike C, Lippincott P, Cepko CL, Furukawa T. The mouse Crx 5'-upstream transgene sequence directs cell-specific and developmentally regulated expression in retinal photoreceptor cells. J. Neurosci. 2002;22:1640–1647. doi: 10.1523/JNEUROSCI.22-05-01640.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Iacovelli J, Zhao C, Wolkow N, Veldman P, Gollomp K, Ojha P, Lukinova N, King A, Feiner L, Esumi N, Zack DJ, Pierce EA, Vollrath D, Dunaief JL. Generation of Cre transgenic mice with postnatal RPE-specific ocular expression. Invest. Ophthalmol. Vis. Sci. 2011;52:1378–1383. doi: 10.1167/iovs.10-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannabiran C, Palavalli L, Jalali S. Mutation of SPATA7 in a family with autosomal recessive early-onset retinitis pigmentosa. J. Mol. Genet. Med. 2012;6:301–303. doi: 10.4172/1747-0862.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenekoop RK. RPGRIP1 is mutated in Leber congenital amaurosis: a mini-review. Ophthalmic Genet. 2005;26:175–179. doi: 10.1080/13816810500374441. [DOI] [PubMed] [Google Scholar]
- Li T. Leber congenital amaurosis caused by mutations in RPGRIP1. Cold Spring Harb. Perspect. Med. 2014;5 doi: 10.1101/cshperspect.a017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DS, Ocaka LA, Borman AD, Sergouniotis PI, Henderson RH, Moradi P, Robson AG, Thompson DA, Webster AR, Moore AT. Screening of SPATA7 in patients with Leber congenital amaurosis and severe childhood-onset retinal dystrophy reveals disease-causing mutations. Invest. Ophthalmol. Vis. Sci. 2011;52:3032–3038. doi: 10.1167/iovs.10-7025. [DOI] [PubMed] [Google Scholar]
- Matsui R, McGuigan Iii DB, Gruzensky ML, Aleman TS, Schwartz SB, Sumaroka A, Koenekoop RK, Cideciyan AV, Jacobson SG. SPATA7: Evolving phenotype from cone-rod dystrophy to retinitis pigmentosa. Ophthalmic Genet. 2016;37:333–338. doi: 10.3109/13816810.2015.1130154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AK, Mahajnah M, Zobor D, Bonin M, Sharkia R, Wissinger B. Novel homozygous large deletion including the 5' part of the SPATA7 gene in a consanguineous Israeli Muslim Arab family. Mol. Vis. 2015;21:306–315. [PMC free article] [PubMed] [Google Scholar]
- Murga-Zamalloa CA, Swaroop A, Khanna H. RPGR-containing protein complexes in syndromic and non-syndromic retinal degeneration due to ciliary dysfunction. J Genet. 2009;88:399–407. doi: 10.1007/s12041-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash BM, Wright DC, Grigg JR, Bennetts B, Jamieson RV. Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Transl. Pediatr. 2015;4:139–163. doi: 10.3978/j.issn.2224-4336.2015.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Parfitt DA, Cheetham ME. Targeting the Proteostasis Network in Rhodopsin Retinitis Pigmentosa. Adv. Exp. Med. Biol. 2016;854:479–484. doi: 10.1007/978-3-319-17121-0_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil H, Tserentsoodol N, Saha A, Hao Y, Webb M, Ferreira PA. Selective loss of RPGRIP1-dependent ciliary targeting of NPHP4, RPGR and SDCCAG8 underlies the degeneration of photoreceptor neurons. Cell Death Dis. 2012;3:e355. doi: 10.1038/cddis.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik SR, Raghupathy RK, Zhang X, Mansfield D, Shu X. The Role of RPGR and Its Interacting Proteins in Ciliopathies. J Ophthalmol. 2015;2015:414781. doi: 10.1155/2015/414781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Hanein S, Gerard X, Delphin N, Fares-Taie L, Gerber S, Pelletier V, Merce E, Dollfus H, Puech B, Defoort-Dhellemmes S, Petersen MD, Zafeiriou D, Munnich A, Kaplan J, Roche O, Rozet JM. Spectrum of SPATA7 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum. Mutat. 2010;31:E1241–1250. doi: 10.1002/humu.21203. [DOI] [PubMed] [Google Scholar]
- Riaz M, Baird PN. Genetics in Retinal Diseases. Dev. Ophthalmol. 2016;55:57–62. doi: 10.1159/000431142. [DOI] [PubMed] [Google Scholar]
- Roepman R, Bernoud-Hubac N, Schick DE, Maugeri A, Berger W, Ropers HH, Cremers FP, Ferreira PA. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum. Mol. Genet. 2000;9:2095–2105. doi: 10.1093/hmg/9.14.2095. [DOI] [PubMed] [Google Scholar]
- Sharif W, Sharif Z. Leber's congenital amaurosis and the role of gene therapy in congenital retinal disorders. Int J Ophthalmol. 2017;10:480–484. doi: 10.18240/ijo.2017.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat. Protoc. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MD, Hendy GN, Percy ME, Bichet DG, Cole DE. G protein-coupled receptor mutations and human genetic disease. Methods Mol. Biol. 2014;1175:153–187. doi: 10.1007/978-1-4939-0956-8_8. [DOI] [PubMed] [Google Scholar]
- Wang H, den Hollander AI, Moayedi Y, Abulimiti A, Li Y, Collin RW, Hoyng CB, Lopez I, Abboud EB, Al-Rajhi AA, Bray M, Lewis RA, Lupski JR, Mardon G, Koenekoop RK, Chen R. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am. J. Hum. Genet. 2009;84:380–387. doi: 10.1016/j.ajhg.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CM, El-Asrag M, Parry DA, Morgan JE, Logan CV, Carr IM, Sheridan E, Charlton R, Johnson CA, Taylor G, Toomes C, McKibbin M, Inglehearn CF, Ali M. Mutation screening of retinal dystrophy patients by targeted capture from tagged pooled DNAs and next generation sequencing. PLoS One. 2014;9:e104281. doi: 10.1371/journal.pone.0104281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weleber RG, Francis PJ, Trzupek KM, Beattie C. Leber Congenital Amaurosis. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- Zalewska M, Siara M, Sajewicz W. G protein-coupled receptors: abnormalities in signal transmission, disease states and pharmacotherapy. Acta Pol. Pharm. 2014;71:229–243. [PubMed] [Google Scholar]
- Zhong H, Eblimit A, Moayedi Y, Boye SL, Chiodo VA, Chen Y, Li Y, Nichols RM, Hauswirth WW, Chen R, Mardon G. AAV8(Y733F)-mediated gene therapy in a Spata7 knockout mouse model of Leber congenital amaurosis and retinitis pigmentosa. Gene Ther. 2015;22:619–627. doi: 10.1038/gt.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.