Abstract
Background
Autoimmune thyroid disease (AITD), which is characterized by an increased presence of thyroid autoantibodies (TAbs), such as antibodies against thyroid peroxidase (TPOAbs) and antibodies against thyroglobulin (TgAbs), has been reported to be associated with rheumatoid arthritis (RA) because AITD and RA both involve autoimmunity. However, few data are available on the incidence of TAbs in Chinese RA patients, and studies on the association between TAbs and joint damage as well as synovitis in RA patients remain sparse. Here, we aimed to evaluate the incidence of TAbs in a consecutive Chinese RA cohort and to investigate whether the elevated presence of TAbs is associated with joint damage and synovitis in RA patients.
Methods
A total of 125 hospitalized RA patients were consecutively recruited. Clinical data and available synovial tissues were collected at baseline, and TAbs and thyroid function were detected by chemiluminescent immunoassay. Patients who tested positive for TPOAbs or TgAbs were classified as the TAbs-positive group, and patients who tested positive for neither TPOAbs nor TgAbs were recruited as the TAbs-negative group. Disease activity was assessed using DAS28-ESR (the disease activity score in 28 joints and including the erythrocyte sedimentation rate). X-ray assessment of the hand/wrist was performed according to the Sharp/van der Heijde-modified Sharp score (mTSS), and patients with an mTSS score >10 were defined as having radiographic joint damage (RJD). Serial tissue sections were stained immunohistochemically for CD3, CD15, CD20, CD34, CD38, and CD68, and synovitis were assessed according to Krenn’s synovitis score.
Results
A total of 44 (35%) patients were positive for either TPOAbs or TgAbs. Importantly, there was a significantly greater percentage of patients with RJD in the TAbs-positive group versus the TAbs-negative group (68% vs. 42%, p = 0.005). Compared with the TAbs-negative group, significantly more CD38-positive plasma cells infiltrated the TAbs-positive synovium, and a higher percentage of patients with high-grade synovitis were observed in the TAbs-positive group (5/8, 63% vs. 5/14, 36%). Moreover, RF positivity and disease activity indicators, including TJC28, DAS28-ESR, and CDAI, were significantly higher in the TAbs-positive group (all p < 0.05). Adjusted logistic regression analysis revealed that positive TAbs (OR 2.999, 95% CI [1.301–6.913]; p = 0.010) and disease duration (OR 1.013, 95% CI [1.006–1.019]; p < 0.001) were independently associated with RJD, and an odds ratio of 2.845 (95% CI [1.062–7.622]) was found for RJD in women with positive TAbs (n = 37) compared with those without TAbs (n = 59) (p = 0.038).
Conclusion
Our data showed that joint destruction was amplified in RA patients with an elevated presence of TAbs, which supports the importance and necessity of TAbs and thyroid function screening and monitoring in RA patient management in clinical practice.
Keywords: Rheumatoid arthritis, Thyroid autoantibodies, Radiographic joint damage
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that is characterized by joint destruction and deformity, affecting millions of people worldwide (McInnes & Schett, 2011). However, RA is more than a symmetrical inflammation of the joints, as increasing evidence supports a higher risk of other autoimmune disorders. Autoimmune thyroid disease (AITD) is one of the most common organ-specific autoimmune diseases, which is characterized by endocrine abnormalities and an elevated presence of thyroid antibodies (TAbs), such as antibodies against thyroid peroxidase (TPOAbs) and antibodies against thyroglobulin (TgAbs). Indeed, the coexistence of RA and AITD has been a subject of interest for several decades (Hijmans et al., 1961; Becker, Ferguson & McConahey, 1963). The two diseases appear to be associated based on their similarities in both genetics, such as HLA-DR B1, CTLA4 and PTPN22 (Raychaudhuri, 2010; Tomer & Huber, 2009), and environmental risk factors, such as infection, vaccines and smoking (De Carvalho, Pereira & Shoenfeld, 2009).
The prevalence of AITD in RA patients investigated in different studies varies considerably among countries, ranging from 0.5% in Morocco (Benamour et al., 1992) to 27% in Slovakia (Lazurova et al., 2009). A higher frequency of AITD or TAbs has been observed in RA patients compared with the general population (Shiroky et al., 1993; Chan, Al-Saffar & Bucknall, 2001; Raterman et al., 2008; Del et al., 2003; Pan, Gu & Shan, 2015), and AITD and TAbs have been found to be associated with RA disease activity (Koszarny et al., 2013; Joshi et al., 2017). Moreover, it is not rare to find arthritis or synovitis resembling RA in some patients with AITD (Punzi et al., 1997), and the presence of TAbs has been found in synovial fluid even prior to their detection in the serum (Punzi et al., 1991). However, to our knowledge, few data are available on the incidence of TAbs in Chinese RA patients, and studies on the association between TAbs and joint damage as well as synovitis in RA patients remain sparse. Do RA patients with positive TAbs run a higher risk of joint damage or experience more severe synovitis than patients without positive TAbs? Is the clustering of positive TAbs and some RA characteristics associated with an amplified joint damage risk? To minimize the risk of selection bias, we performed a consecutive cohort study recruiting hospitalized RA patients to better evaluate the incidence of TAbs in Chinese RA patients and to investigate whether positive TAbs are linked with joint damage and synovitis in RA patients.
Materials and Methods
Patients
Between February 2015 and August 2016, hospitalized patients who fulfilled the 1987 revised criteria of the ACR (Arnett et al., 1988) or the 2010 ACR/European League Against Rheumatism (EULAR) criteria for the classification of RA (Aletaha et al., 2010) were consecutively recruited in the Department of Rheumatology at Sun Yat-Sen Memorial Hospital, Guangzhou, China. All patients were aged ≥ 18 years. The exclusion criteria included the following: individuals overlapping with other autoimmune diseases (e.g., systemic lupus erythematosus, scleroderma, polyarteritis nodosa, dermatomyositis); with hypothalamus or pituitary disease; with serious infection, organ failure, or malignancy; with a history of surgical removal of the thyroid gland; or treated with iodine-containing drugs (e.g., amiodarone, lithium, carbamazepine, phenytoin sodium, interferon-alpha). Written informed consent was obtained from all patients. This study was approved by the Medical Ethics Committee of Sun Yat-Sen Memorial Hospital (identifier: SYSEC-2009-06 and SYSEC-KY-KS-011).
Clinical assessments
The clinical data of the RA patients were collected at enrollment, as described before (Ma et al., 2015), including the 28-joint tender and swollen joint counts (28TJC and 28SJC, both 0–28), patient and provider global assessments of disease activity (PtGA and PrGA, both 0–10 cm, 10 = worst status), pain visual analog scale (Pain VAS, 0–10 cm, 10 = most pain), Chinese-language version of the Stanford Health Assessment Questionnaire (HAQ, 0–3, 3 = most functional disability; functional limitation was defined as an HAQ score >1), erythrocyte sedimentation rate (ESR, mm/h, normal range: 0–20 mm/h (female), 0–15 mm/h (male)), level of C-reactive protein (CRP, mg/dl, normal range: 0–0.5 mg/dl), level of serum rheumatoid factor (RF, IU/ml, determined by nephelometry (Siemens Healthcare Diagnostics, Munich, Germany), normal range <20 IU/ml), and level of anti-cyclic citrullinated peptide antibody (ACPA, measured by ELISA (Version 2.0, Aesku Diagnostics, Wendelsheim, Germany), normal range <18 U/ml). Disease activity was assessed with DAS28-ESR (the disease activity score in 28 joints based on four variables, including the ESR), DAS28-CRP (the disease activity score in 28 joints based on four variables, including CRP), the simplified disease activity index (SDAI), the clinical disease activity index (CDAI), and the Routine Assessment of Patient Index Data 3 (RAPID3) (Anderson et al., 2012). Disease activity defined by DAS28-ESR was divided into four grades: >5.1 (high disease activity (HDA)), ≥3.2 and <5.1 (moderate disease activity (MDA)), ≥ 2.6 and <3.2 (low disease activity (LDA)), and <2.6 (remission).
Radiographic assessments
Plain radiographs of the bilateral hands and wrists (anteroposterior view) of all included patients were performed to determine the radiographic status at baseline. Joint damage, including the Sharp/van der Heijde-modified total Sharp score (mTSS), joint erosion (JE), and joint space narrowing (JSN), was scored by two experienced observers (MJD from the Department of Rheumatology and YZH from the Department of Radiology) who were blinded to the patients’ clinical data as described previously (Van der Heijde, 2000). Reliability and agreement were assessed based on the intra-class correlation coefficient (ICC): the mean ICC for interobserver agreement was 0.90. Bony erosion was defined as a cortical break by radiography (Van der Heijde et al., 2013). Patients with an mTSS score >10 were defined as having radiographic joint damage (RJD) (Baker et al., 2011).
Chemiluminescent immunoassay for detection of serum TAbs and thyroid function
Blood was obtained from all the included RA patients after overnight fasting, and serum specimens were separated and stored at −80 °C for further analysis. The levels of TPOAbs (normal range: 0–60 IU/ml), TgAbs (normal range: 0–60 IU/ml), free thyroxine (FT4, normal range: 11.5–22.7 pmol/L), free triiodothyronine (FT3, normal range: 3.5–6.5 pmol/L), and thyroid-stimulating hormone (TSH, normal range: 0.55–4.78 pmol/L) were measured using chemiluminescent immunoassay (CLIA, Siemens Healthineers, Massachusetts, USA) according to the manufacturer’s instructions. RA patients with positive TPOAbs or TgAbs were assigned to the TAbs-positive group, and patients with neither positive TPOAbs nor positive TgAbs were assigned to the TAbs-negative group.
Synovial tissues and immunohistochemistry
Synovium was collected by closed Parker-Pearson needle biopsy from one actively inflamed knee joint in RA patient (Schumacher & Kulka, 1972). As described previously (Ma et al., 2014; Zou et al., 2013), serial sealed sections of qualified synovial samples were stained with hematoxylin and eosin (H&E) and also immunohistochemically stained with the commercial mouse monoclonal antibodies (Invitrogen, Carlsbad, CA, USA) according to standard staining protocols: anti-CD3 (clone PS1, T cells), anti-CD15 (clone My1, neutrophils), anti-CD20 (clone L26, B cells), anti-CD34 (clone QB End/10, vascular endothelial cells), anti-CD38 (clone SPC32, plasma cells), and anti-CD68 (clone KP1, macrophages). Irrelevant isotype was used as a negative control. Appropriate positive controls were included to rule out the possibility of an absence of staining due to technical failure.
Synovitis assessments
At least three tissue pieces that contained well-defined synovial lining and sublining areas were included in the analysis for each specimen. Histological changes in H&E-stained sections were graded according to Krenn’s synovitis score (Krenn et al., 2006). The three-component synovitis score included enlargement of the synovial lining layer, increased density of sublining resident cells, and inflammatory infiltration. Each feature was scored from 0–3, and the total synovitis score ranged from 0–9. RA patients were then divided into high-grade (>4) and low-grade (≤4) synovitis groups according to the synovitis score. Synovitis assessments were performed using a Leica DM2500 microscope (Leica Corp., Heidelberg, Germany) by two independent trained investigators (MYQ and LJZ, both from the Department of Rheumatology) who were blinded to the clinical details of the specimens. Differences between the two investigators were resolved by mutual agreement. The densities of cells with positive staining for CD3, CD15, CD20, CD38, and CD68 and the microvessel counts (MVCs, as confirmed based on the presence of CD34-positive endothelial cells and vessel lumen diameter ≤8 red blood cell diameter) were determined by manual counting. Because each high-power field revealed a synovial area of 0.11740 mm2, the densities of cells with positive staining for CD3, CD15, CD20, CD38, CD68, and MVCs were assessed in nine different fields at a magnification of × 400 for a total area of nearly 1 mm2; the densities are given as cells per mm2.
Statistical analysis
IBM SPSS Statistics version 20.0 software (IBM, Armonk, NY, USA) was used for the statistical analyses. For continuous variables, descriptive statistics (median, interquartile range (IQR)) were calculated, and the Mann–Whitney analysis of variance on ranks between two groups was used. For categorical variables, indicators are presented as frequencies and percentages, and the Chi-square test or Fisher’s exact test was used. Univariate and multivariate logistic regression analyses were performed, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to identify risk factors for RJD. Variables were included in the equation when p < 0.05 or removed when p > 0.10 following the step-forward selection rule. Spearman’s rank order correlation test was used to assess the relationship between TAb levels and RA duration. A two-tailed p < 0.05 was considered statistically significant.
Results
Demographic characteristics of RA patients
There were 141 RA patients who fulfilled the inclusion criteria of this study. One patient who was being treated with amiodarone, five patients who were simultaneously diagnosed with malignancies (one hepatocellular carcinoma, one esophageal carcinoma, one breast carcinoma, one nasopharyngeal carcinoma, and one osteosarcoma), and 10 patients who overlapping with other autoimmune diseases (four systemic lupus erythematosus, three scleroderma, two polyarteritis nodosa, one dermatomyositis) were excluded. Finally, a total of 125 RA patients were included in the statistical analysis, of whom 96 (77%) were women. The median disease duration was 60 months, ranging from 12–120 months. Among the included patients, 72% were RF positive, 71% were ACPA positive, and 93% of the patients had bony erosion at baseline. In total, 59 (47%) patients were glucocorticosteroids (GCs) or disease-modifying antirheumatic drugs (DMARDs) therapy naive since six months before enrollment (Table 1).
Table 1. Comparison of demographic and clinical features between RA patients with and without positive TAbs.
| Parameter | Total (n = 125) | TAbs-positive group (n = 44) | TAbs-negative group (n = 81) | pa |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Women, n (%) | 96(77) | 37(84) | 59(73) | 0.155 |
| Age (years) | 52(46–61) | 51(42–58) | 54(48–62) | 0.071 |
| Disease duration (months) | 60(12–120) | 72(24–120) | 50(12–120) | 0.235 |
| Short (<6 months), n (%) | 13(10) | 3(7) | 10(12) | 0.334 |
| Intermediate (6–24 months), n (%) | 20(16) | 7(16) | 13(16) | 0.984 |
| Long (>24 months), n (%) | 92(74) | 34(77) | 58(72) | 0.492 |
| Age of onset (years) | 46(39–53) | 42(35–49) | 47(41–55) | 0.025 |
| Smoking, n (%) | 19(15) | 7(16) | 12(15) | 0.871 |
| Disease characteristics | ||||
| TJC28 | 6(2–12) | 9(3–13) | 5(1–11) | 0.040 |
| SJC28 | 4(1–10) | 6(2–11) | 3(1–8) | 0.074 |
| Pain VAS | 4(2–6) | 4(3–6) | 4(2–6) | 0.217 |
| PtGA | 5(3–7) | 5(3–7) | 4(3–7) | 0.288 |
| PrGA | 5(3–6) | 6(3–7) | 4(2–6) | 0.081 |
| HAQ | 0.75(0.19–1.25) | 0.88(0.28–1.25) | 0.63(0.13–1.06) | 0.096 |
| Functional limitation, n (%) | 38(30) | 18(41) | 20(25) | 0.060 |
| CRP (mg/L) | 26.5(7.8–50.9) | 24.3(9.8–42.3) | 30.0(5.1–56.4) | 0.576 |
| ESR (mm/h) | 60(37–88) | 66(42–90) | 58(35–85) | 0.399 |
| RF positivity, n (%) | 90(72) | 38(86) | 52(64) | 0.008 |
| RF titer ≥ 3 ULN, n (%) | 76(61) | 34(77) | 42(52) | 0.005 |
| ACPA positivity, n (%) | 89(71) | 32(73) | 57(70) | 0.781 |
| ACPA titer ≥ 3 ULN, n (%) | 71(57) | 26(59) | 45(56) | 0.703 |
| DAS28-CRP | 4.83(3.55–5.68) | 5.10(3.89–5.75) | 4.37(3.49–5.60) | 0.076 |
| DAS28-ESR | 5.45(4.26–6.48) | 6.00(4.97–6.57) | 4.93(4.18–6.47) | 0.036 |
| SDAI | 21(10–33) | 25(16–37.8) | 18(9–31) | 0.084 |
| CDAI | 24.4(13.8–36.8) | 27.1(16.3–37.8) | 21.3(13.1–34.4) | 0.049 |
| RAPID3 | 4.03(1.97–5.39) | 4.39(2.93–5.63) | 3.75(1.78–5.09) | 0.155 |
| Radiographic status | ||||
| Bony erosion, n (%) | 116(93) | 40(91) | 76(94) | 0.547 |
| JNS subscore | 3(0–16) | 8(0–26) | 2(0–11) | 0.088 |
| JE subscore | 9(3–23) | 13(3–34) | 8(3–19) | 0.075 |
| mTSS | 11(4–37) | 19(5–62) | 9(4–31) | 0.076 |
| RJD, n (%) | 64(51) | 30(68) | 34(42) | 0.005 |
| Previous medications, n (%) | ||||
| Naiveb | 59(47) | 18(41) | 41(51) | 0.299 |
| GCs | 52(42) | 20(46) | 32(40) | 0.519 |
| MTX | 35(28) | 13(30) | 22(27) | 0.777 |
| LEF | 24(19) | 7(16) | 17(21) | 0.491 |
| SASP | 7(6) | 2(5) | 5(6) | 0.705 |
| HCQ | 10(8) | 4(9) | 6(7) | 0.740 |
| CysA | 2(2) | 0(0) | 2(3) | NA |
| Biologics | 5(4) | 2(5) | 3(4) | 0.819 |
Notes.
Comparison between the TAbs-positive group and the TAbs-negative group. Data are described as the median (interquartile range) unless stated otherwise.
Without glucocorticosteroids or disease-modifying antirheumatic drugs therapy within the previous six months.
- GCs
- glucorticosteroids
- MTX
- methotrexate
- LEF
- leflunomide
- SASP
- sulfasalazine
- HCQ
- hydroxychloroquine
- CysA
- cyclosporin A
- NA
- not applicable
Thyroid abnormalities in RA patients
The profile of thyroid abnormalities is demonstrated in Table 2. Among the patients, 35 (28%) were positive for TPOAbs, and 27 (22%) were positive for TgAbs, with 44 (35%) patients having either positive TPOAbs or positive TgAbs and 17 (14%) being positive for both. TPOAbs positivity in women was 31%, and TgAbs positivity was 26%. Additionally, significantly higher prevalences of TPOAbs and TgAbs were found in patients with seropositive RF versus those with seronegative RF (36% vs. 9% and 27% vs. 9%, respectively; both p < 0.05). Five (4%) patients were diagnosed with hypothyroidism (two (2%) had clinical hypothyroidism, and three (2%) had subclinical hypothyroidism), and five (4%) patients had hyperthyroidism (three (2%) showed clinical manifestations, and two (2%) showed subclinical hyperthyroidism). All patients with thyroid disorders were in the TAbs-positive group except for 2 patients (1 subclinical hyperthyroidism and 1 subclinical hypothyroidism) in the negative group. Accordingly, FT3, FT4, and TSH abnormalities were all significantly more frequent in the TAbs-positive group than in the TAbs-negative group (21% vs. 6%, 14% vs. 3%, and 18% vs. 3%, respectively; all p < 0.05; Table 2).
Table 2. Thyroid abnormalities in RA patients with and without positive TAbs.
| Parameter | Total (n = 125) | TAbs-positive group (n = 44) | TAbs-negative group (n = 81) | pa |
|---|---|---|---|---|
| Thyroid function | ||||
| FT3 (pmol/L) | 4.23(3.70–4.91) | 4.22(3.67–4.61) | 4.31(3.71–4.98) | 0.254 |
| FT3 abnormality, n (%) | 14(12) | 9(18) | 5(6) | 0.019 |
| FT3 elevated | 2(2) | 2(2) | 0(0) | NA |
| FT3 reduced | 12(10) | 7(16) | 5(6) | 0.076 |
| FT4 (pmol/L) | 15.96(14.70–18.21) | 15.25(14.35–18.02) | 16.18(14.92–18.39) | 0.199 |
| FT4 abnormality, n (%) | 8(14) | 6(14) | 2(3) | 0.022 |
| FT4 elevated | 4(7) | 3(7) | 1(1) | 0.125 |
| FT4 reduced | 4(7) | 3(7) | 1(1) | 0.125 |
| TSH (pmol/L) | 1.09(0.55–2.12) | 1.22(0.54–2.36) | 1.01(0.55–1.99) | 0.380 |
| TSH abnormality, n (%) | 10(8) | 8(18) | 2(3) | 0.004 |
| TSH elevated | 5(4) | 4(9) | 1(1) | 0.052 |
| TSH reduced | 5(4) | 4(9) | 1(1) | 0.052 |
| Thyroid disorders, n (%) | ||||
| Hyperthyroidism | 5(4) | 4(9) | 1(1) | 0.052 |
| Clinical hyperthyroidism | 3(2) | 3(7) | 0(0) | NA |
| Subclinical hyperthyroidism | 2(2) | 1(2) | 1(1) | 0.582 |
| Hypothyroidism | 5(4) | 4(9) | 1(1) | 0.052 |
| Clinical hypothyroidism | 2(2) | 2(5) | 0(0) | NA |
| Subclinical hypothyroidism | 3(2) | 2(5) | 1(1) | 0.283 |
Notes.
Comparison between the TAbs-positive group and the TAbs-negative group. Data are described as the median (interquartile range) unless stated otherwise.
Comparison of RA characteristics between patients with and without positive TAbs
There were 44 (35%) patients in the TAbs-positive group. A significantly greater percentage of patients with RJD was observed in the TAbs-positive group versus the TAbs-negative group (68% vs. 42%, p = 0.005; Table 1). Compared with the TAbs-negative group, patients with positive TAbs had RA onset at a significantly younger age (42(35–49) years vs. 47(41–55) years, p = 0.025). RF positivity and disease activity indicators, including TJC28, DAS28-ESR, and CDAI, were significantly higher in the TAbs-positive group (all p < 0.05), with borderline significant differences in SJC28, PrGA, DAS28-CRP, and SDAI as well as in the percentage of functional limitation. Similarly, a significantly higher percentage of patients with HDA was seen in the TAbs-positive group versus the TAbs-negative group (68% vs. 49%, p = 0.043). However, there was no significant difference in other RA clinical features between the two groups, including gender, disease duration, smoking status, and previous medications used since 6 months before enrollment (all p > 0.05; Table 1).
Comparison of synovitis between patients with and without positive TAbs
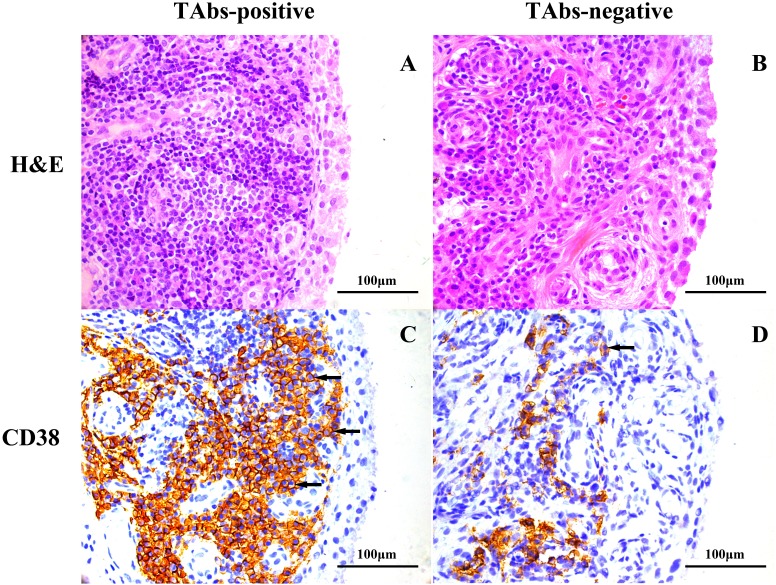
A total of 22 patients had qualified synovial tissues, of whom eight were patients with positive TAbs. Notably, RF positivity and ACPA positivity were both 100% in the eight patients, and were 71% and 79% respectively in the 14 patients with negative TAbs. Significantly more pronounced infiltration of CD38-positive plasma cells was observed in the TAbs-positive synovium (1,354(847–2,096) cells/mm2) than in the TAbs-negative control (274(109–1,252) cells/mm2) (p = 0.048; Table 3). The percentage of patients with high-grade synovitis was also higher in the TAbs-positive group than in the TAbs-negative group (63% vs. 36%), but the difference was not significant (p = 0.221). Representative images of H&E and immunohistochemical staining for CD38 in the synovium of RA patients with and without positive TAbs are shown in Fig. 1. No significant difference between the two groups was observed in the densities of other infiltrated inflammatory cells, including CD3-positive T cells, CD15-positive neutrophils, CD20-positive B cells, CD68-positive macrophages, or MVCs (all p > 0.05; Table 3).
Table 3. Synovial histological features in RA patients with and without positive TAbs.
| Parameter | TAbs-positive group (n = 8) | TAbs-negative group (n = 14) | pa |
|---|---|---|---|
| MVC, /mm2 | 185(87–223) | 135(89–168) | 0.339 |
| CD3+ T cells, /mm2 | 763(242–1,404) | 615(359–1,268) | 1.000 |
| CD15+ neutrophils, /mm2 | 410(103–614) | 340(120–637) | 0.785 |
| CD20+ B cells, /mm2 | 785(144–1,058) | 385(208–2,054) | 0.785 |
| CD38+ plasma cells, /mm2 | 1,354(847–2,096) | 274(109–1,252) | 0.048 |
| CD68+ macrophages, /mm2 | 1,540(1,020–1,818) | 1,003(511–1,681) | 0.195 |
| Krenn’s synovitis score | 5.0(2.5–6.8) | 4.0(2.8–5.0) | 0.267 |
| Low-grade synovitis, n(%) | 3(37) | 9(64) | 0.221 |
| High-grade synovitis, n(%) | 5(63) | 5(36) | – |
| Hyperplasia of lining layer | 2.0(1.3–2.0) | 1.0(1.0–2.0) | 0.105 |
| Inflammatory infiltration | 1.0(1.0–2.0) | 1.0(0.8–1.6) | 0.486 |
| Synovial stroma activation | 2.0(1.0–2.0) | 1.3(1.0–2.0) | 0.439 |
Notes.
Comparison between the TAbs-positive group and the TAbs-negative group. Data are described as the median (interquartile range) unless stated otherwise.
Figure 1. Representative images of H&E and immunohistochemical staining for CD38 in the synovium of RA patients based on TAb status.
H&E staining, high-grade synovitis (Krenn’s synovitis score = 6) in a TAbs-positive RA patient (A) and low-grade synovitis (Krenn’s synovitis score = 3.5) in a TAbs-negative RA patient (B); immunohistochemical staining, expression of CD38 in synovium of a TAbs-positive RA patient (C) and a TAbs-negative RA patient (D). Significantly more pronounced amounts of CD 38-positive plasma cells infiltrated the TAbs-positive synovium versus the TAbs-negative control. The black arrows point to CD38-positive cells located at the sublining area of the synovium. (A–D), original magnification, ×400.
Risk factors for RJD in RA patients
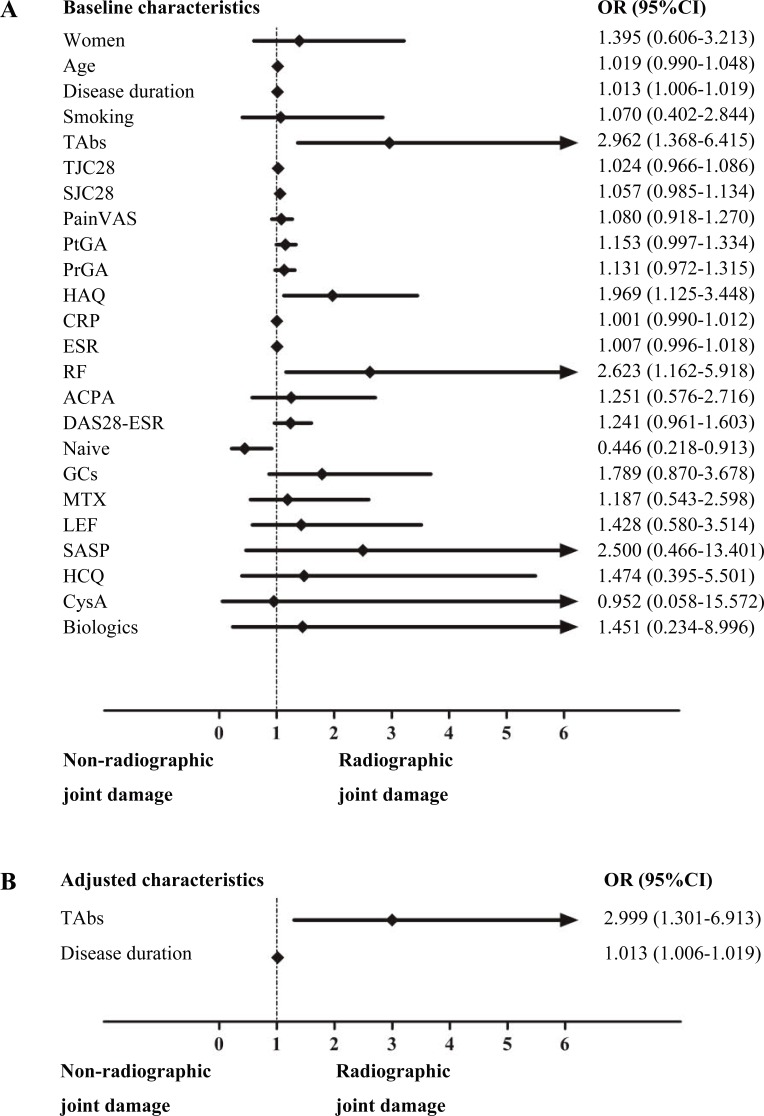
Logistic regression analysis was performed to investigate the risk factors for RJD (Fig. 2). Univariate logistic regression analysis performed on demographic and disease characteristics as well as TAbs status showed that medication-naive status was a protective factor (OR 0.446, 95% CI [0.218–0.913]; p = 0.027), whereas a longer disease duration (OR 1.013, 95% CI [1.006–1.019]; p < 0.001), a higher HAQ score (OR 1.969, 95% CI [1.125–3.448]; p = 0.018), positive RF (OR 2.623, 95% CI [1.162–5.918]; p = 0.020), and positive TAbs (OR 2.962, 95% CI [1.368–6.415]; p = 0.006) were risk factors for RJD. Multivariate logistic regression analysis was performed to control for confounding factors. The result revealed that positive TAbs (OR 2.999, 95% CI [1.301–6.913]; p = 0.010) and disease duration (OR 1.013, 95% CI [1.006–1.019]; p < 0.001) were independently associated with RJD. No significant correlations were found neither between TPOAb levels and disease duration (r = 0.082, p = 0.366) nor TgAb levels and disease duration (r = − 0.057, p = 0.530).
Figure 2. Logistic regression analysis of the risk factors for RJD in RA patients.
Univariate logistic regression analysis of the risk factors for RJD (A); multivariate logistic regression of the risk factors for RJD after adjusting for confounding factors as mentioned above in the univariate logistic regression analysis (B).
Further analysis of positive TAbs yielded an OR of 2.845 (95% CI [1.062–7.622]) for RJD when comparing women in the TAbs-positive group (n = 37) with those in the TAbs-negative group (n = 59) (p = 0.038). Subgroup studies revealed that a high titer of ACPA (defined as an ACPA level ≥ 3 times the upper limit of the normal range (ULN)) was also a risk factor for RJD (OR 2.107, 95% CI [1.025–4.328]; p = 0.043). After adjusting for confounding factors, as mentioned above for the univariate logistic regression analysis, a high titer of ACPA remained significantly associated with RJD (OR 2.467, 95% CI [1.076–5.655]; p = 0.033).
Discussion
This study demonstrated that the prevalence of TAbs is 35% and positive TAbs are independently associated with RJD in RA patients. There was significantly more pronounced infiltration of CD38-positive plasma cells in the TAbs-positive synovium than in the negative control. Additionally, RA patients with positive TAbs had RA onset at a younger age, and higher TJC28 as well as disease activity was also observed in patients with positive TAbs than in those without positive TAbs. The results importantly indicated a significant association between positive TAbs and joint destruction as well as clinical disease activity in RA, possibly due to more infiltration of plasma cells in the TAbs-positive synovium. To the best of our knowledge, this is the first study to obtain evidence that joint destruction might be amplified in RA patients with an elevated presence of TAbs.
It is well recognized that patients suffering from an autoimmune disease have a strong hereditary susceptibility to other autoimmune diseases (Roldan, Alonso & Barrio, 1999). In the previous study, a total of 15,007 adults subjected to health checkups with TAbs and thyroid function testing were recruited from Peking Union Medical College Hospital (Wen et al., 2015). Compared with this Chinese healthy control cohort, significantly higher incidences of both TPOAbs and TgAbs were observed in RA patients (28% vs. 9% and 22% vs. 10%, respectively; both p < 0.001), with a similar tendency found in women (31% vs. 14% and 26% vs. 16%, respectively; both p < 0.001). A meta-analysis including 1021 RA patients and 1,500 healthy controls showed that TAbs positivity in patients with RA was higher than in healthy controls (TgAbs: OR 3.17, 95% CI [2.24–4.49]; TPOAbs: OR 2.33, 95% CI [1.24–4.39]) (Pan, Gu & Shan, 2015). A large cohort study including 800 RA patients from Colombia also showed that the incidence of TAbs was 37.8% for TPOAbs and 20.8% for TgAbs. Further literature review disclosed geographical variation in TAbs positivity and showed that the prevalence ranges from 5–37% for TPOAbs, from 6-31% for TgAbs, and from 11.4–32% for either (Cardenas et al., 2012). However, several other studies showed no difference in the prevalence of TAbs in RA patients compared with the general population (McCoy et al., 2012; Przygodzka & Filipowicz-Sosnowska, 2009). Given the striking discrepancy between these results, controversy persists over this issue. Although different methods for testing for TAbs in different studies and differences in iodine intake between different areas might explain some of the discrepancy, it is reasonable to propose that the presence of TAbs may play a specific role in RA development and/or progression. In the current study, higher incidences of TPOAbs and TgAbs were found in RA patients compared with the reportedly healthy controls, and a similar result was found in women, which was in accordance with most previous studies. Although no correlation between RF titer and TAbs positivity was observed (Yavasoglu et al., 2009), our results indicated a significantly higher prevalence of both TPOAbs and TgAbs in RA patients with seropositive RF than in those without seropositive RF, which further strengthens the hypothesis of an association between positive TAbs and RA. However, this result deserves further consideration because several cases have been reported showing false-positive results in Tg assays due to interference by RF resulting from its heterophile ability to bind to other Fc-region IgG antibodies (Massart, Corcuff & Bordenave, 2008; Astarita et al., 2015).
The association between thyroid abnormalities and RA disease activity has been extensively studied. Thyroid dysfunction has been reported to be linked with a longer duration and an increased prevalence of morning stiffness in RA patients, and disease activity can be greatly improved by correction of the hypothyroid status (Delamere, Scott & Felix-Davies, 1982). Joshi et al. (2017) showed that RA patients with hypothyroidism had higher DAS28-ESR, TJC28, and Pain VAS scores, and significant correlations were observed between TSH levels and ESR as well as DAS28-ESR. Recently, Koszarny et al. (2013) revealed significant positive correlations between TPOAbs and DAS28 and between TgAbs and inflammatory markers, including ESR and CRP; moreover, a higher mean DAS28 was observed in TPOAbs-positive versus TPOAbs-negative patients as well as in TgAbs-positive versus TgAbs-negative patients. In addition, data from a population-based case-control study of incident RA cases (1,998 adult cases, 2,252 controls) revealed a significant association between thyroxin substitution (reflecting an autoimmune thyroid disease) and the risk of developing either ACPA-positive or ACPA-negative RA (Bengtsson et al., 2014). Nevertheless, debate still exists because several studies showed no correlation between thyroid abnormalities and RA disease activity (Atzeni et al., 2009; Cardenas et al., 2012). In the present study, TJC28 and disease activity scores, including DAS28-ESR and CDAI, were significantly higher in patients with positive TAbs than in patients without positive TAbs. Moreover, RA patients with positive TAbs had an increased risk of earlier RA onset. This phenomenon might occur because some TAbs are induced by chronic inflammation in RA patients. Conversely, it could also be reasonable to speculate that positive TAbs may play a role as a trigger or an accelerator in RA development. The observation of Blake et al. (1979) suggests that thyroid autoantibodies may be produced locally in the joints, and some patients have been reported to develop thyroiditis during follow-up (Punzi et al., 1991). Thus, it is possible that a higher TAbs titer may be produced due to more plasma cells or lymphocytes infiltrating the synovial membrane of RA patients with positive TAbs. Accordingly, in the present study, significantly more pronounced numbers of plasma cells infiltrated the synovial tissues of patients in the TAbs-positive group, and a higher percentage of patients with high-grade synovitis was also observed in the TAbs-positive group, although this difference was not significant. Therefore, the presence of more plasma cells in the TAbs-positive synovial tissue might contribute to joint inflammation or destruction. The small size of the qualified synovial samples might explain the lack of significant differences in other indicators of synovitis. Most importantly, although few studies have reported a correlation between thyroid abnormalities and RA joint destruction (Cardenas et al., 2012), our results revealed that positive TAbs were independently associated with joint destruction in RA patients, which possibly suggested a detrimental role for TAbs in joint damage in RA. The mechanisms by which thyroid abnormalities may be linked with RA have not been fully determined yet; however, explanations are suggested by the shared genetics that determine or influence autoantigen presentation and the regulation of the immune response. Indeed, the shared HLA-DRB1 epitope has been demonstrated to be a risk factor not only for RA development but also for joint damage in RA patients, independent of ACPA status (Rojas-Villarraga et al., 2009; Suzuki et al., 2013). Hence, the results obtained in the current study may be partly attributed to sharing of the HLA-DRB1 epitope in RA patients with concurrent positive TAbs. In conclusion, the associations between an elevated presence of TAbs and amplified joint damage, more pronounced infiltration of plasma cells in the synovium, as well as higher levels of disease activity indicators suggest a probably significant and robust effect exerted by positive TAbs, independent of the definition of RJD.
There are several limitations to this study that merit careful consideration. First, data from cross-sectional studies are not sufficiently convincing to draw a clear causal link between the presence of TAbs and joint damage or disease activity in RA. Second, the relatively small number of RA patients and synovial tissues included in this study may have precluded obtaining a meaningful significant difference or a robust conclusion, and further stratification was difficult to achieve. Third, a general population without RA should have been recruited simultaneously as a control group to improve the data analysis. Further studies are needed in future to explore the mechanism of abnormal TAb levels in RA and to confirm their association with joint destruction in a large and prospective case-control study.
Conclusions
Our data showed that positive TAbs were significantly associated with RJD in RA patients. Joint destruction may be amplified in RA patients with an elevated presence of TAbs, which supports the importance and necessity of TAbs and thyroid function screening and monitoring in RA patient management in clinical practice. RA patients with positive TAbs should thus be paid more attention regarding joint destruction or even joint progression during follow-up.
Supplemental Information
A total of 125 RA patients were included in the statistical analysis.
A total of 22 patients had qualified synovial tissues, of whom 8 were patients with positive TAbs.
Acknowledgments
The authors wish to thank the patients and medical staff for their contribution to this study. We also thank Yan-Fang Ye from Sun Yat-Sen Memorial Hospital for her statistical assistance.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 81471597, 81671612 and 81601427), Guangdong Natural Science Foundation (grant no. 2014A030313074, 2016A030313307 and 2017A030313576), Guangdong Medical Scientific Research Foundation (grant no. A2017109). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yu-Lan Chen and Jian-Zi Lin conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables.
Ying-Qian Mo, Jin-Jian Liang and Qian-Hua Li performed the experiments, analyzed the data, contributed reagents/materials/analysis tools.
Cheng-Jing Zhou performed the experiments, contributed reagents/materials/analysis tools.
Xiu-Ning Wei, Jian-Da Ma, Ze-Hong Yang and Dong-Hui Zheng performed the experiments.
Lie Dai conceived and designed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the Medical Ethics Committee of Sun Yat-Sen Memorial Hospital (identifier: SYSEC-2009-06 and SYSEC-KY-KS-011).
Data Availability
The following information was supplied regarding data availability:
The raw data have been provided in the Supplemental Files.
References
- Aletaha et al. (2010).Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CR, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- Anderson et al. (2012).Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, Saag KG, O’Dell JR, Kazi S. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care and Research. 2012;64:640–647. doi: 10.1002/acr.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett et al. (1988).Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger T, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthtitis and Rheumatism. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Astarita et al. (2015).Astarita G, Gutierrez S, Kogovsek N, Mormandi E, Otero P, Calabrese C, Alcaraz G, Vazquez A, Abalovich M. False positive in the measurement of thyroglobulin induced by rheumatoid factor. Clinica Chimica Acta. 2015;447:43–46. doi: 10.1016/j.cca.2015.04.039. [DOI] [PubMed] [Google Scholar]
- Atzeni et al. (2009).Atzeni F, Atzeni F, Doria A, Ghirardello A, Turiel M, Batticciotto A, Carrabba M, Sarzi-Puttini P. Anti-thyroid antibodies and thyroid dysfunction in rheumatoid arthritis: prevalence and clinical value. Autoimmunity. 2009;41:111–115. doi: 10.1080/08916930701620100. [DOI] [PubMed] [Google Scholar]
- Baker et al. (2011).Baker JF, George M, Baker DG, Toedter G, Von Feldt JM, Leonard MB. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatology. 2011;50:2100–2107. doi: 10.1093/rheumatology/ker294. [DOI] [PubMed] [Google Scholar]
- Becker, Ferguson & McConahey (1963).Becker KL, Ferguson RH, McConahey WM. The connective-tissue diseases and symptoms associated with Hashimoto’s thyroiditis. New England Journal of Medicine. 1963;268:277–280. doi: 10.1056/NEJM196302072680601. [DOI] [PubMed] [Google Scholar]
- Benamour et al. (1992).Benamour S, Zeroual B, Fares L, El Kabli H, Bettal S. Rheumatoid arthritis in Morocco-a retrospective study of 404 cases. Revue du Rhumatisme et des Maladies Osteo-Articulaires. 1992;59:801–807. [PubMed] [Google Scholar]
- Bengtsson et al. (2014).Bengtsson C, Padyukov L, Kallberg H, Saevarsdottir S. Thyroxin substitution and the risk of developing rheumatoid arthritis; results from the Swedish population-based EIRA study. Annals of the Rheumatic Diseases. 2014;73:1096–1100. doi: 10.1136/annrheumdis-2013-203354. [DOI] [PubMed] [Google Scholar]
- Blake et al. (1979).Blake DR, McGregor AM, Stansfield E, Smith BR. Antithyroid-antibody activity in the snyovial fluid of patients with various arthritides. Lancet. 1979;2:224–226. doi: 10.1016/s0140-6736(79)90238-1. [DOI] [PubMed] [Google Scholar]
- Cardenas et al. (2012).Cardenas RJ, Amaya-Amaya J, Castellanos-de LHJ, Giraldo-Villamil J, Montoya-Ortiz G, Cruz-Tapias P, Rojas-Villarraga A, Mantilla RD, Anaya JM. Autoimmune thyroid disease in rheumatoid arthritis: a global perspective. Arthritis. 2012;2012 doi: 10.1155/2012/864907. Article 864907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Al-Saffar & Bucknall (2001).Chan AT, Al-Saffar Z, Bucknall RC. Thyroid disease in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology. 2001;40:353–354. doi: 10.1093/rheumatology/40.3.353. [DOI] [PubMed] [Google Scholar]
- De Carvalho, Pereira & Shoenfeld (2009).De Carvalho JF, Pereira RM, Shoenfeld Y. The mosaic of autoimmunity: the role of environmental factors. Frontiers in Bioscience. 2009;1:501–509. doi: 10.2741/e46. [DOI] [PubMed] [Google Scholar]
- Del et al. (2003).Del PA, Savastano S, Nuzzo V, Esposito A, Lupoli G. High prevalence of thyroid autoantibodies in newly diagnosed rheumatoid arthritis patients. Clinical and Experimental Rheumatology. 2003;21:137. [PubMed] [Google Scholar]
- Delamere, Scott & Felix-Davies (1982).Delamere JP, Scott DL, Felix-Davies DD. Thyroid dysfunction and rheumatic diseases. Journal of the Royal Society of Medicine. 1982;75:102–106. [PMC free article] [PubMed] [Google Scholar]
- Hijmans et al. (1961).Hijmans W, Doniach D, Rott IM, Holborow EJ. Serological overlap between lupus erythematosus, rheumatoid arthritis, and thyroid auto-immune disease. British Medical Journal. 1961;2:909–914. doi: 10.1136/bmj.2.5257.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi et al. (2017).Joshi P, Agarwal A, Vyas S, Kumar R. Prevalence of hypothyroidism in rheumatoid arthritis and its correlation with disease activity. Tropical Doctor. 2017;47:6–10. doi: 10.1177/0049475515627235. [DOI] [PubMed] [Google Scholar]
- Koszarny et al. (2013).Koszarny A, Majdan M, Suszek D, Wielosz E, Dryglewska M. Relationship between rheumatoid arthritis activity and antithyroid antibodies. Polskie Archiwum Medycyny Wewnetrznej-Polish Archives of Internal Medicine. 2013;123:394–400. doi: 10.20452/pamw.1829. [DOI] [PubMed] [Google Scholar]
- Krenn et al. (2006).Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, Haupl T. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- Lazurova et al. (2009).Lazurova I, Benhatchi K, Rovensky J, Kozakova D, Wagnerova H, Tajtakova M, Shoenfeld Y, Macejova Z. Autoimmune thyroid disease and autoimmune rheumatic disorders: a two-sided analysis. Annals of the New York Academy of Sciences. 2009;1173:211–216. doi: 10.1111/j.1749-6632.2009.04809.x. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2015).Ma J, Wei X, Zheng D, Mo Y, Chen L, Zhang X, Li J, Dai L. Continuously elevated serum matrix metalloproteinase-3 for 3∼6 months predict one-year radiographic progression in rheumatoid arthritis: a prospective cohort study. Arthritis Research & Therapy. 2015;17 doi: 10.1186/s13075-015-0803-2. Article 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2014).Ma JD, Zhou JJ, Zheng DH, Chen LF, Mo YQ, Wei XN, Yang LJ, Dai L. Serum matrix metalloproteinase-3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis. Mediators of Inflammation. 2014;2014 doi: 10.1155/2014/179284. Article 179284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart, Corcuff & Bordenave (2008).Massart C, Corcuff JB, Bordenave L. False-positive results corrected by the use of heterophilic antibody-blocking reagent in thyroglobulin immunoassays. Clinica Chimica Acta. 2008;388:211–213. doi: 10.1016/j.cca.2007.09.024. [DOI] [PubMed] [Google Scholar]
- McCoy et al. (2012).McCoy SS, Crowson CS, Gabriel SE, Matteson EL. Hypothyroidism as a risk factor for development of cardiovascular disease in patients with rheumatoid arthritis. Journal of Rheumatology. 2012;39:954–958. doi: 10.3899/jrheum.111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes & Schett (2011).McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New England Journal of Medicine. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- Pan, Gu & Shan (2015).Pan XF, Gu JQ, Shan ZY. Increased risk of thyroid autoimmunity in rheumatoid arthritis: a systematic review and meta-analysis. Endocrine. 2015;50:79–86. doi: 10.1007/s12020-015-0533-x. [DOI] [PubMed] [Google Scholar]
- Przygodzka & Filipowicz-Sosnowska (2009).Przygodzka M, Filipowicz-Sosnowska A. Prevalence of thyroid diseases and antithyroid antibodies in women with rheumatoid arthritis. Polskie Archiwum Medycyny Wewnetrznej-Polish Archives of Internal Medicine. 2009;119:39–43. [PubMed] [Google Scholar]
- Punzi et al. (1997).Punzi L, Michelotto M, Pianon M, Bertazzolo N, Fagiolo U, Betterle C, Vettor R, Todesco S. Clinical, laboratory and immunogenetic aspects of arthritis associated with chronic lymphocytic thyroiditis. Clinical and Experimental Rheumatology. 1997;15:373–380. [PubMed] [Google Scholar]
- Punzi et al. (1991).Punzi L, Schiavon F, Ramonda R, Cavasin F, Ruffatti A, Todesco S. Anti-thyroid microsomal antibody in synovial fluid as a revealing feature of seronegative autoimmune thyroiditis. Clinical Rheumatology. 1991;10:181–183. doi: 10.1007/BF02207661. [DOI] [PubMed] [Google Scholar]
- Raterman et al. (2008).Raterman HG, Van Halm VP, Voskuyl AE, Simsek S, Dijkmans BA, Nurmohamed MT. Rheumatoid arthritis is associated with a high prevalence of hypothyroidism that amplifies its cardiovascular risk. Annals of the Rheumatic Diseases. 2008;67:229–232. doi: 10.1136/ard.2006.068130. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri (2010).Raychaudhuri S. Recent advances in the genetics of rheumatoid arthritis. Current Opinion in Rheumatology. 2010;22:109–118. doi: 10.1097/BOR.0b013e328336474d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Villarraga et al. (2009).Rojas-Villarraga A, Diaz FJ, Calvo-Páramo E, Salazar JC, Iglesias-Gamarra A, Mantilla RD, Anaya J. Familial disease, the HLA-DRB1 shared epitope and anti-CCP antibodies influence time at appearance of substantial joint damage in rheumatoid arthritis. Journal of Autoimmunity. 2009;32:64–69. doi: 10.1016/j.jaut.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Roldan, Alonso & Barrio (1999).Roldan MB, Alonso M, Barrio R. Thyroid autoimmunity in children and adolescents with Type 1 diabetes mellitus. Diabetes, Nutrition & Metabolism. 1999;12:27–31. [PubMed] [Google Scholar]
- Schumacher & Kulka (1972).Schumacher HJ, Kulka JP. Needle biopsy of the synovial membrane–experience with the Parker-Pearson technic. New England Journal of Medicine. 1972;286:416–419. doi: 10.1056/NEJM197202242860807. [DOI] [PubMed] [Google Scholar]
- Shiroky et al. (1993).Shiroky JB, Cohen M, Ballachey ML, Neville C. Thyroid dysfunction in rheumatoid arthritis: a controlled prospective survey. Annals of the Rheumatic Diseases. 1993;52:454–456. doi: 10.1136/ard.52.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki et al. (2013).Suzuki T, Ikari K, Yano K, Inoue E, Toyama Y, Taniguchi A, Yamanaka H, Momohara S. PADI4 and HLA-DRB1 are genetic risks for radiographic progression in RA patients, independent of ACPA status: results from the IORRA cohort study. PLOS ONE. 2013;8:e61045. doi: 10.1371/journal.pone.0061045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer & Huber (2009).Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. Journal of Autoimmunity. 2009;32:231–239. doi: 10.1016/j.jaut.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heijde (2000).Van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. Journal of Rheumatology. 2000;27:261–263. [PubMed] [Google Scholar]
- Van der Heijde et al. (2013).Van der Heijde D, Van der Helm-van MA, Aletaha D, Bingham CO, Burmester GR, Dougados M, Emery P, Felson D, Knevel R, Kvien TK, Landewe RB, Lukas C, McInnes I, Silman AJ, Smolen JS, Stanislawska-Biernat E, Zink A, Combe B. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Annals of the Rheumatic Diseases. 2013;72:479–481. doi: 10.1136/annrheumdis-2012-202779. [DOI] [PubMed] [Google Scholar]
- Wen et al. (2015).Wen XH, Zhang B, Zhang K, Chai XF, Lian XL, Xu TD. Analysis of the relationship between thyroid antibodies and thyroid disfunction diseases in checkup people. China Medical Herald. 2015;25:69–77. [Google Scholar]
- Yavasoglu et al. (2009).Yavasoglu I, Senturk T, Coskun A, Bolaman Z. Rheumatoid arthritis and anti-thyroid antibodies. Autoimmunity. 2009;42:168–169. doi: 10.1080/08916930802428114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou et al. (2013).Zou C, Zhu L, Li Y, Mo Y, Zheng D, Ma J, Ou-Yang X, Pessler F, Dai L. The association between hepatitis B virus infection and disease activity, synovitis, or joint destruction in rheumatoid arthritis. Clinical Rheumatology. 2013;32:787–795. doi: 10.1007/s10067-013-2170-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A total of 125 RA patients were included in the statistical analysis.
A total of 22 patients had qualified synovial tissues, of whom 8 were patients with positive TAbs.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data have been provided in the Supplemental Files.