Abstract
Polychlorinated biphenyls (PCB) exposure at low chronic levels is a significant public health concern. Animal and epidemiological studies indicate that low PCB body burden may cause neurotoxicity and be a risk factor for neurodegenerative diseases. In the current study, we measured the ability of two non-dioxin like PCBs, 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) and 2,2′3,5′,6-pentachlorobiphenyl (PCB95), to alter dopamine (DA) levels and metabolism using the dopaminergic PC12 cell line. Our hypothesis is that treatment of PC12 cells with non-toxic concentrations of PCB153 or PCB95 for 12 and 24 h will have different effects due to different congener structures. Levels of DA and of 3,4-dihydroxyphenylacetaldehyde (DOPAL), 3, 4-dihyroxylphenylethanol (DOPET), and 3,4-dihyroxylphenylacetic acid (DOPAC) metabolite, gene expression of the dopamine synthesis enzyme tyrosine hydroxylase (TH) and the vesicular monoamine transporter (VMAT2), and gene expression of the anti-oxidant enzymes Cu/Zn and Mn superoxide oxidase (Cu/ZnSOD, MnSOD), glutathione peroxidase (GPx) and catalase were determined. PCB153 decreased intracellular and extracellular levels of DA after 12 h exposure and this was consistent with an increase in DA metabolites. After 24 h, the level of DA in medium increased compared to the control. In contrast, PCB95 exposure increased the intracellular DA level and decreased DA in medium consistent with a down-regulation of VMAT2 expression at 12 h. After 24 h exposure, PCB95 increased DA levels in media. Expression of TH mRNA increased slightly following 12 h but not at 24 h exposure. MnSOD mRNA increased up to 6–7 fold and Cu/ZnSOD increased less than two-fold after treatment with both congeners. Catalase expression was up-regulated following 24 h exposure to PCB153 and PCB95, but GPx expression was down-regulated after 12 h exposure to PCB95 only. These results suggest that PCB153 and PCB95 are neurotoxic and affect DA turnover with structure-dependent differences between these two congeners.
Keywords: PCB153, PCB95, PC12 cells, Dopamine, DOPAL, DOPAC, DOPET, GPx, VMAT
1. INTRODUCTION
Dopamine (DA) cycles through a complex process of synthesis, storage, release, uptake and receptor activation (Davie, 2008). Tyrosine is the starting point in DA synthesis, is abundant in dietary proteins and taken up into the brain by a low-affinity amino acid transport system (Elsworth and Roth, 1997). Once tyrosine has entered the neuron, tyrosine hydroxylase (TH) converts it to L-DOPA. TH, which is usually phosphorylated by protein kinases, is normally the rate-limiting step in DA synthesis (Elsworth and Roth, 1997). L-DOPA is transformed by aromatic amino acid decarboxylase (AADC) to DA. In dopaminergic neurons, DA is transported from the cytoplasm into synaptic vesicles through the vesicular monoamine transporter (VMAT). In response to a presynaptic action potential, a change in membrane protein conformation allows the influx of calcium ions, which stimulates fusion of vesicles with the neuronal membrane at the synaptic cleft (Kelly, 1993). Once DA is released, it activates several postsynaptic receptors that are associated with a variety of cell signaling mechanisms. Increased extracellular DA may also activate the presynaptic auto-receptor resulting in feedback to inhibit DA synthesis via TH. DA uptake is accomplished via the DA transporter (DAT) back into the presynaptic neuron. If vesicular storage is disrupted and DA levels increase in the cytoplasm, DA has the ability to go through two other pathways. In the second pathway for DA inside the cells, known as the enzymatic pathway, DA is metabolized by monoamine oxidase (MAO) to 3,4-dihydroxyphenylacetaldehyde (DOPAL) which can be further metabolized to 3,4-dihyroxyphenylacetic acid (DOPAC) via aldehyde dehydrogenase (ALDH). As a minor pathway, DOPAL can be reduced to 3,4-dihydroxyphenylethanol (DOPET) via cytosolic aldehyde or aldose reductase (AR) (Jones and Miller, 2008). In addition, a two electron oxidation of L-DOPA or DA may result in the formation of the corresponding quinone and superoxide (Hastings, 2009).
Polychlorinated biphenyls (PCBs) are classified as a group of man-made chemicals which were used in many industrial applications, including in sealants and lubricants and in electrical transformers and capacitors. In the 1980s, PCB production was terminated in most western countries, but contamination from diffuse environmental sources and from pigments which are inadvertently contaminated with PCBs during manufacturing, still continuous (Hu and Hornbuckle, 2010; IARC, 2013). PCBs are highly persistent and ubiquitous pollutants (Safe, 1994). Among the 209 individual PCB congeners, the ortho-substituted congeners constitute a large portion of the PCB residues observed in the environment and in human and animal tissue (Zeng et al., 2016). Among these, PCB153 (2,2′,4,4′,5,5′-hexachlorobiphenyl), a di-ortho substituted, non-dioxin-like congener, is reported to be the most abundant congener in human tissues (Rylander et al., 1998). Another congener, the tri-ortho substituted PCB95 (2,2′,3,5′,6 -pentachloro biphenyl) is a potent inhibitor of the ryanodine receptor and was found to be increased in the brains of certain autism patients (Mitchell et al., 2012; Lesiak et al., 2014).
PCB exposure has been associated with behavioral and cognitive disorders in both human and animal studies (Castoldi et al., 2006) and risk of Parkinson’s disease (Corrigan et al., 1998; Steenland et al., 2006; Petersen et al., 2008). The most consistent neurochemical effect of non-coplanar PCBs is the reduction of DA in cultured cells (Bemis and Seegal, 1999) and in adult rat brain (Tilson and Kodavanti, 1997). PCBs were shown to inhibit neurotransmitter transporters, in particular the Na+ dependent plasma membrane DA and the H+- dependent vesicle monoamine transporter 2 (VMAT2) (Mariussen et al., 2001; Seegal et al., 2010), and to affect DA, DOPAC and homovanillic acid levels in the neostriatum of rats (Dervola et al., 2015). Furthermore, oxidative stress due to increased reactive oxygen species (ROS) or decreased antioxidant defenses, may play a role in PCBs neurotoxicity (Park et al., 2010).
In the present study, we investigated the effects of PCB153 and PCB95 on PC12 cells. These dopaminergic cells are known to synthesize, store, release and metabolize catecholamine in a manner similar to the mammalian central nervous system and have previously been used for PCB research (Shain et al., 1991; Angus and Contreras, 1996; Park et al., 2010; Langeveld et al., 2012). The aim was to investigate the targets and pathways involved in PCB153 and PCB95 induced neurotoxicity and protection responses. To our knowledge this is the first study that identifies the effects of single congeners on DA metabolites, especially DOPAL and DOPET, and describes alteration of antioxidant enzymes in PC12 cells.
2. MATERIALS AND METHODS
2.1. Materials
The rat pheochromocytoma PC12 cell line (Cat. # CRL-1721 FZ, Lot # 61925403; received June 2015) and RPMI1640 medium (Cat #30-2001) were obtained from American Tissue Culture Collection (ATCC). Collagen IV was from Sigma Aldrich (Cat # 9007-34-5). All other cell culture components such as Fetal Bovine Serum (FBS), Penicillin- streptomycin (PS), Horse Serum (HS), Hank’s Balanced Salt Solution (HBSS) and phosphate buffered saline (PBS) were obtained from Invitrogen (Carlsbad, CA, USA). Carboxy-H2DCFDA was purchased from Thermos Fisher Scientific. PCB153 and PCB95 were provided by Dr. H.J. Lehmler of the Iowa Superfund Research Program at the University of Iowa. PCB 95 was synthesized using the Suzuki coupling of 2,5-dichlorobenzene boronic acid and 2,3,6-trichloroiodobenzene according to a published method (Joshi et al., 2011). The synthesis and characterization of the PCB 153 batch used in this study has been reported previously (Rignall et al., 2013). Both congeners had a purity of > 99% based on relative peak area, as determined using gas chromatography-mass spectrometry (Holland et al., 2017). All PCR reagents and kits were obtained from Qiagen (Valencia, CA, USA). All other chemicals were purchased from Sigma (St. Louis, MO, USA), unless otherwise noted.
2.2. Cell Culture
PC12 cells were maintained in RPMI 1640 with 10% HS, 5% FBS and 1% penicillin-streptomycin in a humidified incubator at 37° C with 5% CO2 in Collagen IV coated 75 cm flask. The medium was changed every other day. For most experiments cells were plated in collagen-coated 6 well plates in 5 ml medium at a density of 1.5 × 106 per well.
2.3. Measurement of Cell Viability
To identify a non-toxic concentration of PCBs in PC12 cells, 100,000 cells in 0.3 ml medium per well were seeded in collagen-coated 24-well plates. The PCB congeners were dissolved in DMSO and cells were treated with multiple concentrations (0.001–100μM) of PCB153 or PCB95 for 24 h in serum-free medium. The final concentration of DMSO in medium was 0.5%. Cell viability was determined using 5μM of resazurine in medium. Cells were treated with resazurine solution for 30 minutes and the fluorescence intensity in each well was measured at 535 nm excitation and 590 nm emission wavelengths with a microplate reader (GeniosPro from TECAN, Seestrasse, Switzerland). The fluorescence intensity correlates with the number of living cells in each well, since living cells are able to metabolize non-fluorescent blue resazurin (oxidized form) to the red, fluorescing resorufin (reduced form). Each concentration data point was measured in triplicate wells and each experiment was preformed three times. Data are shown as percent of solvent control.
2.4. HPLC Analysis of DA and Its Metabolites
An Agilent 1200 Series Capillary HPLC system coupled with a photodiode array detector set to absorbance at 202nm and 280nm was used for quantification of DA, DOPAC, DOPAL and DOPET. The method used followed what is described in (Mexas et al., 2011). Briefly, after seeding the cells in collagen coated 6-well plates they were kept in the same growth condition for 48 h. Media were removed to eliminate interaction of DA and its metabolites with serum proteins. Cells were washed and incubated in HEPES-buffered medium containing 115 mM NaCl2, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.5 mM glucose, 1 mM NaH2PO4, and 15 mM HEPES (pH 7.4) to which DMSO (vehicle control), PCB153 or PCB95 (5 and 10 μM PCBs) was added. Cells were then incubated for 12 or 24 h at 37° C in 5% CO2. For measurement of extracellular DA and its metabolites, HEPES-buffered media were collected. 100 μL aliquots were taken and perchloric acid was added to 5% (v/v), samples were spun at 10,000× g for 10 min. 40 μL of supernatant from each cell sample was injected into an HPLC to measure extracellular DA and DA metabolites. For intracellular measurements, the cells in the plate were washed with 1xPBS, scraped and collected in 200 μL of 1xPBS (pH 7.4). Cell lysate was sonicated at 2 sec intervals for 10 times.100 μL aliquots were taken and protein was precipitated by addition of 5% (v/v) of perchloric acid and samples were spun at 10,000× g for 10 min to pelletize precipitated protein. 40 μL of supernatant from each cell sample were injected into an HPLC to measure intracellular DA and DA metabolites. The separation was completed employing a Phenomenx Luna C18 column and a mobile phase of 6% acetonitrile (v/v) in 0.1% trifluoroacetic acid and HPLC-grade water. The flow rate was 50 μL per minute. Protein concentration was determined using a Thermo Scientific Pierce BCA Protein Assay kit and a Molecular Devices Spectra-Max plate reader at 562 nm absorbance. A standard curve was used to calculate protein concentrations. The level of DA and DA metabolites are expressed as ng/mg protein.
2.5. Quantitative Real Time PCR
Cells exposed to PCBs in 6-well plates as described above were used for analysis of gene transcription. Total RNA was extracted from cells after each treatment regimen using an RNeasy mini kit from Qiagen (Valencia, CA, USA) following the manufacturer’s procedure. RNA was quantified and analyzed for purity with a NanoDrop2000 from Thermo Fisher Scientific (Wilmington, DE, USA). A 260/280 ratio of ~2.0 was accepted as pure. Total RNA was reverse transcribed utilizing a high capacity cDNA reverse transcription kit obtained from Applied Biosystems (Foster City, CA, USA). The cDNA was later used for quantitative real-time RT-PCR (qRT-PCR) employing an Eppendorf RealPlex thermal cycler. The applied PCR program started with 95° C for 10 min followed by 35 cycles at 95° C for 30 s, 55° C for 30 s, and 72° C for 1 min. The primers for the quantification of selected genes are listed in Supplemental Table 1. Beta-actin was chosen to serve as the house-keeping gene for the analyses. An amplification threshold in the linear range of each sample was selected to calculate the cycle threshold (CT) for each sample. The relative mRNA levels were calculated as follows:
2.6. Statistical Analysis
All experiments were performed at least three times in triplicate. Data from three experiments was combined and analyzed together. The data are expressed as mean± SEM of three experiments. Statistical analysis was conducted using Two-way ANOVA along with a Tukey’s multiple comparison test to confirm the difference between groups. Statistical significance was calculated in SAS 9.3 or GraphPad prism 6. A p-value of <0.05 was considered to be statistically significant.
3. RESULTS
3.1. PCB95 and PCB153 are cytotoxic in undifferentiated PC12 cells only at high concentrations
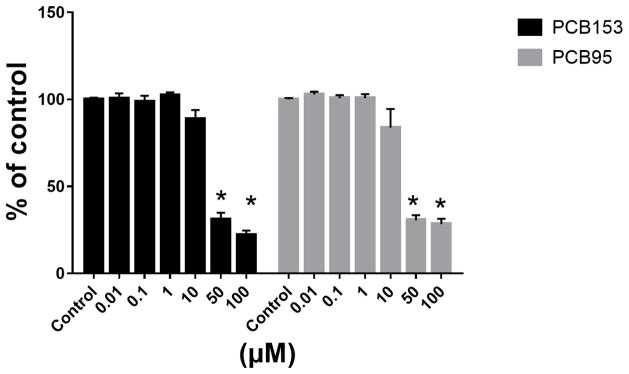
To identify PCB153 and PCB95 concentrations without toxic effects, PC12 cells were exposed to a concentration ranges from 0.001–100 μM. PCBs caused a reduction in viable cells at the two highest concentrations (50 and 100 μM; Figure 1). Therefore, 5 μM and 10 μM of PCBs were employed in the following experiments.
Figure 1.
PCB153 and PCB95 reduce viable cells at concentrations above 10 μM.
PC12 cells were exposed to 0.01–100 μM of PCB153 or PCB95 for 24 h in protein-free medium. Cytotoxicity was measured using the resazurin reduction assay. *p<0.005 compared to the control.
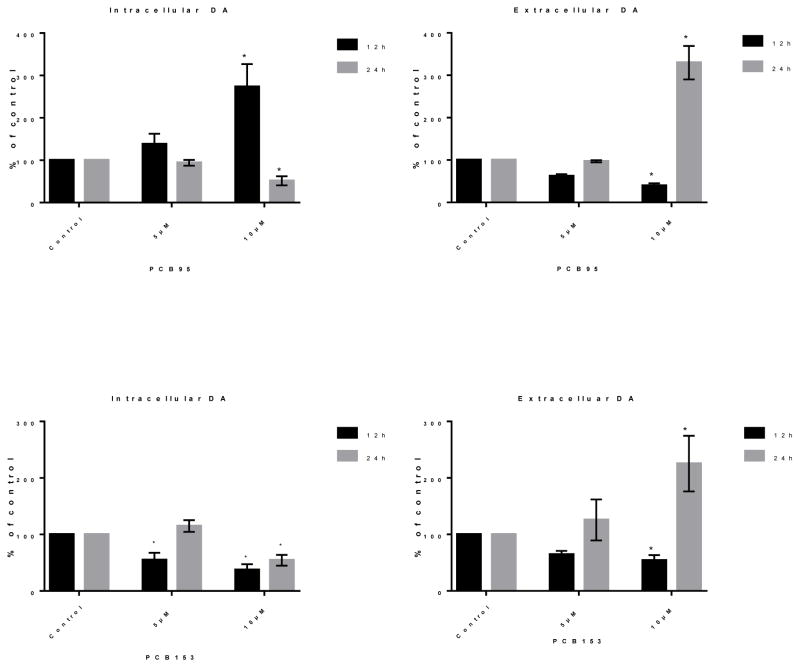
3.2. Non-dioxin like PCBs change intracellular and extracellular DA levels
Effects of PCB153 and PCB95 on levels of DA inside and outside of PC12 cells were investigated. DA levels were different at different time points after exposure of PC12 cells to PCB95 (Figure 2). At 12 h exposure to PCB95, the level of intracellular DA was significantly higher, particularly at 10 μM, while extracellular DA was significantly lower (P<0.05). After 24 h exposure, the DA levels displayed different patterns: intracellular DA levels were significantly diminished at 10 μM but not at 5 μM; the extracellular DA concentration was significantly raised at 10 μM and increased media DA to approximately 200% of the control values.
Figure 2.
Intracellular and extracellular dopamine (DA) levels after 12 and 24 h exposure to PCB95 (top) or PCB153 (bottom). PCB95 increased (12h) then decreased (24h) intracellular DA while the opposite was seen for extracellular DA levels. PCB153 reduced intracellular DA but strongly increased extracellular at the later time point with 10 μM PCB153. Values (mean μM/mg protein± SEM of 3–5 experiments) are depicted as percent of controls. *p<0.05 compared to the control.
Figure 2 also shows the effect of PCB153 on intracellular and extracellular DA levels after 12 and 24 h exposure. As illustrated, the level of intracellular DA was reduced at both time points with 10 μM PCB153. Extracellular DA was significantly reduced after 12 h exposure to 10 μM PCB153, but increased to approximately 150% compared to control after 24 h exposure to 10 μM PCB153.
3.3. PCBs modulate the transcription of DA synthesis and packaging proteins
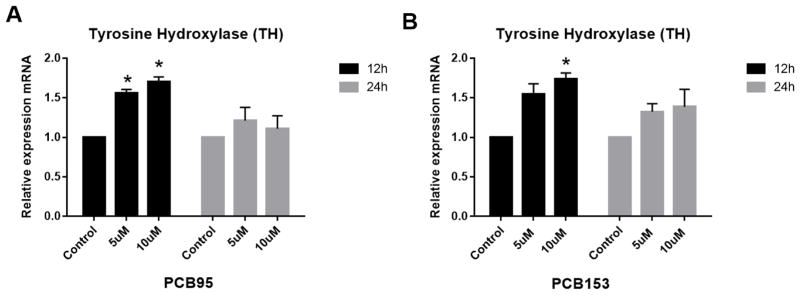
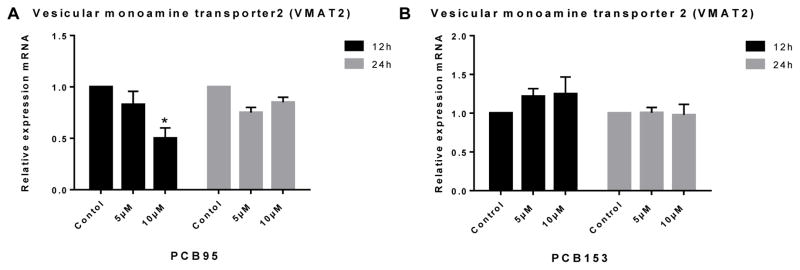
Gene expression analysis in PC12 cells was used to determine whether the alteration in DA levels observed might be due to changes in DA synthesis or DA packaging into vesicles by these congeners. Both PCB153 and PCB95 up-regulated expression of DA’s main synthesis enzyme TH (the rate limiting step for DA synthesis) at 12 h but not at 24 h (Figure 3). VMAT2 was significantly down-regulated at high concentration exposure to PCB95 at 12 h (Figure 4). PCB153 (at both time points of exposure) and PCB95 after 24 h of exposure did not reveal changes in the expression of VMAT2 in PC12 cells (P<0.05).
Figure 3.
Tyrosine hydroxylase (TH) expression in PC12 cells treated with PCB95 or PCB153. PC12 cells were treated with PCBs for 12 and 24 h. Both PCBs upregulated TH expression after 12 h but not at 24 h exposures. *p<0.05 compared to the control; data are from 2 to 4 experiments.
Figure 4.
Vesicular monoamine transporter 2 (VMAT2) expression in PC12 cells treated with (A) PCB95 or (B) PCB153. PCB95 significantly downregulated expression of VMAT2 after 12 h but not 24 h exposure. PCB153 did not have any effect on VMAT2 expression. *p<0.05 compared to the control; data are from 2 to 4 independent experiments.
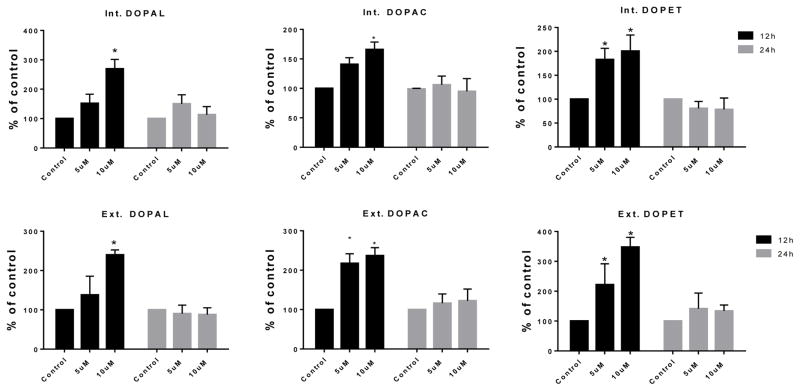
3.4. PCB153 but not PCB95 treatment increased DA catabolism
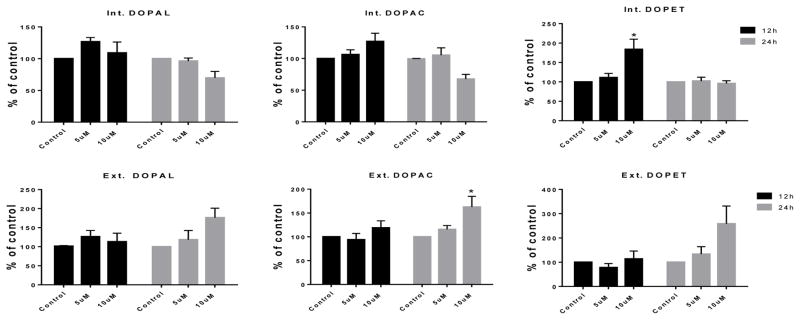
Although levels of DA metabolites were mostly unaffected by PCB95 at either time point (Figure 5), levels of DOPAL, DOPET and DOPAC were significantly increased after 12 h exposure to PCB153 (Figure 6). No significant differences were found for these metabolites after 24 h exposure.
Figure 5.
DA turnover following 12 and 24 h exposure to vehicle only, or 5 or 10μM of PCB95.
Samples underwent HPLC for determination of DA metabolites DOPAL, DOPAC and DOPET. Each value represents the μM/mg protein ±SEM of 3 experiments and data are normalized to their controls. *p<0.05 compared to the control.
Figure 6.
DA turnover following 12 and 24 h exposure to vehicle alone, or 5 or 10μM of PCB153. Samples (from cell extract = int, medium = ext) underwent HPLC analysis for determination of DA the metabolites DOPAL, DOPAC and DOPET. Each value represents the μM/mg protein ±SEM of 3 to 5 experiments. *p<0.05 compared to the control.
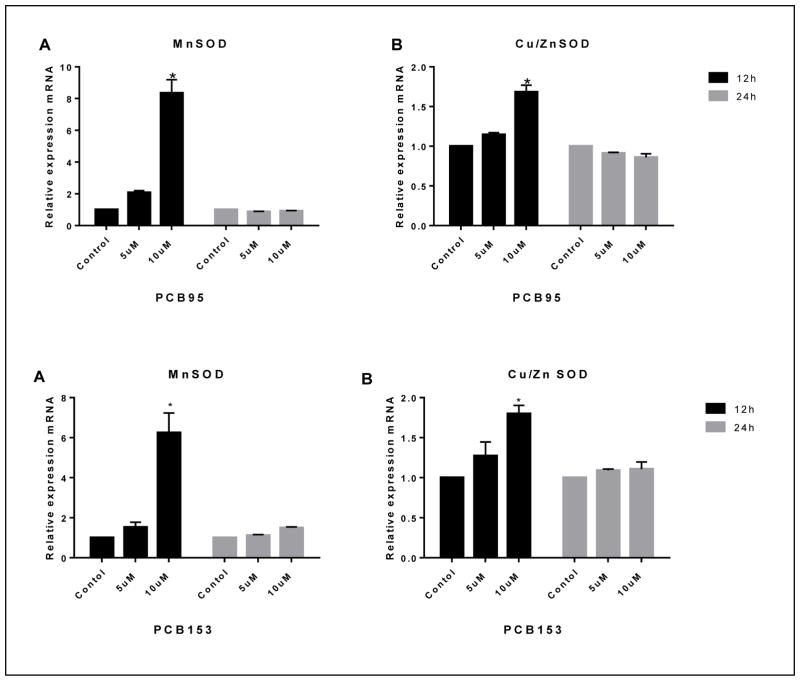
3.5. In PC12 cells antioxidant enzymes expression is altered by PCBs
Figure 7 shows the MnSOD and Cu/Zn SOD mRNA levels after 12 and 24 h exposure to PCB95 and PCB153. Both compounds significantly elevated microsomal MnSOD mRNA levels up to 7 fold at 10 μM after 12 h only. Exposure to either compound exhibited an increase of cytoplasmic Cu/ZnSOD expression and the induction was statistically significant at 10 μM and 12h exposure.
Figure 7.
MnSOD and Cu/ZnSOD gene expression in PC12 cells treated with PCB95 (top) or PCB153 (bottom). Both, PCB95 and PCB153, highly upregulated MnSOD 6–7 folds following 12 h exposure (A). They also upregulated Cu/ZnSOD but to a much smaller extend (B). *p<0.0 compared to the control; data are from 2–4 experiments.
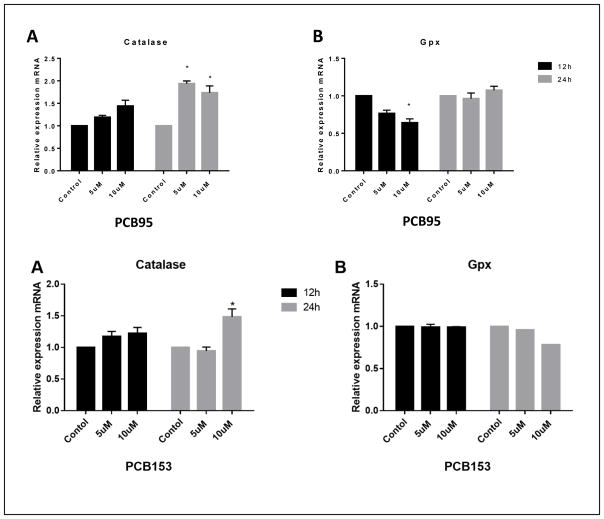
Figure 8 shows the effect of PCB95 and PCB153 on mRNA expression of catalase and glutathione peroxidase (GPx). The impact of the two PCB congeners on these two antioxidant enzymes was different. While levels of catalase mRNA were significantly up-regulated after 24h exposure to either congener, no effect with PCB153 on GPx expression was seen. PCB95 exposure caused reduced levels of GPx mRNA after 12 h exposure but not 24 h (P<0.05
Figure 8.
Catalase and glutathione peroxidase gene expression in PC12 cells after exposure to PCB95 (top) or PCB153 (bottom). Both PCBs upregulated catalase, which was significant after 24h exposure while only PCB95 reduced GPx expression and only after 12h exposure. *p<0.05 compared to the control; data are from 2 to 4 experiments.
4. DISCUSSION
A common finding after PCB exposure is a reduction in intracellular DA levels (Tilson and Kodavanti, 1997; Bemis and Seegal, 1999), which is in agreement with our findings in this study. Several mechanisms have been proposed for this effect. Diminished levels of DA can result from inhibition of TH activity and/or L-aromatic acid decarboxylase (L-AADC) activity (Angus and Contreras, 1996), the two main enzymes that contribute to DA synthesis (see scheme 1). Others have observed that in rats a commercial Aroclor PCB mixture at concentrations similar to those found in human brains decreased the DA re-uptake transporter DAT which can be expected to result in disturbance of the DA system and loss of dopaminergic cells (Caudle et al., 2006). These changes are hallmark features of PD.
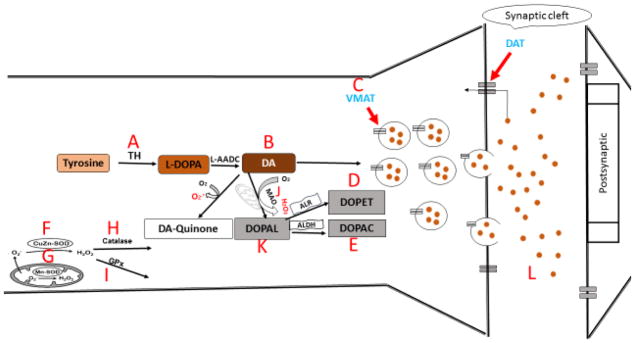
Scheme 1. Schematic summary of the effects of PCB 95 and 153 on dopaminergic (PC12) neuronal cells.
As described in the text, the immediate (12h exposure) main effects were that PCB153 decreased intra- and extracellular DA (B↓, L↓) and increased DOPAL, DOPAC, and DOPET (D↑,E↑,K↑), suggesting enhanced DA catabolism as mechanism for DA reduction. On the other hand, PCB95 increased intracellular DA (B↑) and decreased extracellular DA (L↓) with a reduction in VMAT transcription (C↓), suggesting decreased sequestration and release. Also, both congeners increased SOD transcription (G↑, F↑) and catalase was upregulated at the later time point (H↑), but PCB95 also downregulated GPs (I↓) at the early time point, suggesting transient oxidative stress or oxidative stress-related gene regulation.
(A) tyrosine hydroxylase (TH), (B) intracellular dopamine (DA), (C) vesicular monoamine transporter (VMAT), (D) the dopamine metabolite 3,4-dihydroxyphenyl ethanol (DOPET), (E) the dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), (F) Cu,Zn superoxide dismutase (CuZn-SOD), (G) Mn superoxide dismutase (Mn-SOD), (H) catalase, (I) glutathione peroxidase (GPx), and (J) monoamine oxidase, (K) 3,4-dihydroxyphenylacetaldehyde (DOPAL), (L) extracellular DA.
Another potential source of toxicity may come from DA metabolites. As depicted in scheme 1, DA catabolism results in two reactive products, the aldehyde DOPAL and hydrogen peroxide. DOPAL can be further metabolized to produce the less toxic acid product, DOPAC, or through a minor pathway the ethanol, DOPET (Doorn et al., 2014). In addition to DA homeostasis disruption, increased production of several DA metabolites such as DOPAL is associated with several neurological diseases (O’Brien et al., 2005). Cells exposed to DOPAL showed protein modifications, enzyme inhibition and finally protein aggregation (Marchitti et al., 2007). It has been hypothesized that increased DOPAL is a factor in the loss of DA neurons resulting in Parkinson’s disease (PD) (Goldstein et al., 2013). Whether this pathway is involved in the increased risk of PD in PCB exposed populations in not known. Furthermore, oxidative stress due to the imbalance between the steady-state levels of reactive oxygen species (ROS) and a biological system’s ability to remove the resulting reactive species or to repair the resulting damage by antioxidant defenses, may play a role in PCBs neurotoxicity (Park et al., 2010). DA catabolism can generate ROS in addition to pro- or anti-oxidant DA metabolites and L-DOPA and DA can be oxidized to the corresponding quinone with the formation of ROS (Cadet and Brannock, 1998; Smythies, 2000; Izumi et al., 2005; Meiser et al., 2013). Neuronal cells are already stressed by high ROS levels and reactive species like DA quinone have been implicated in neuronal cell death and neurodegenerative diseases (Izumi et al., 2005; Zhou and Lim, 2009; Belluzzi et al., 2012; Pacelli et al., 2015). ROS and highly reactive quinones may damage vital components of the cell and ROS may also act as secondary messengers through redox signaling and regulation of gene expression (Liou and Storz, 2010). The most effective enzymatic antioxidant defenses within cells are superoxide dismutases (MnSOD and Cu/ZnSOD), catalase (CAT) and glutathione peroxidase (GPx)(Matés et al., 1999), and reduction in the prevalence or activity of either enzyme is another pathway to increased intracellular ROS and a potential mechanism of neurotoxicity.
In this study, we attempted to gain insight into the effects of PCBs on the above parameters. We used two non-dioxin like congeners that differ in the number of chlorines (5 vs 6) and their position (2 meta chlorines in both plus 3 ortho chlorines in PCB95 vs 2 ortho- and 2 para-chlorines in PCB153) to gain more insight into the mechanisms of PCBs neurotoxicity. These congeners were chosen because of their prevalence (PCB153) and importance as potential neurotoxicant (PCB95) (Mitchell et al., 2012). No dioxin-like PCB (no ortho substitution) were included, since these seem to have no or very limited neurotoxic activity (Shain et al., 1991). Five and 10 μM concentrations were used, since they did not produce cytotoxic effects, to avoid confounding effects due to cell death. These concentrations are high but environmentally relevant, since total PCB serum concentrations in the ppm range were observed in occupational exposure in the 1970s/80s and in PCB poisoning episodes in China and Taiwan, although nowadays the serum levels are usually significantly lower (IARC, 2013). Treatment of PC12 cells with PCB153 or PCB95 produced different outcomes, suggesting different modes of action for these two congeners. Scheme 1 illustrates the numerous potential targets for the PCB congeners in dopaminergic cells and summarizes the major findings.
PCB153 (10 μM) decreased intra- and extracellular DA levels after 12 h with significant increase in extracellular DA after 24 h. These results are similar to what Seegal and colleagues reported for PCB153 with respect to alteration of DA levels using both in vivo and in vitro experiments, including a decrease in intracellular DA level and an increase in extracellular DA (Seegal et al., 1989; Seegal et al., 1990; Bemis and Seegal, 2004). In our PC12 cells TH transcription was increased at 12 h but no change in VMAT expression was seen. These results seem contradictory to the lower DA levels. However, the reduced intra and extracellular DA at 12 h could be explained by elevated DA catabolism as indicated by the elevated levels of DA metabolites DOPAL, DOPAC and DOPET (Figure 6, scheme 1). All 3 measured metabolites were increased in cells and in medium after 12 h exposure to PCB153, suggesting increased biotransformation of DA as a mechanism. Increased DA turnover was also reported with Aroclor 1254 in another cell line, MN9D cells (Lee and Opanashuk, 2004). Bemis and Seegal also reported that PCBs such as PCB153 increased levels of DOPAC in synaptosomes (Bemis and Seegal, 2004). Competitive inhibition of VMAT2 is known to cause elevated DA turnover through the enzymatic pathways and reduced intracellular DA. Comparing the effects of known VMAT inhibitors with those of PCBs, they concluded that this was the most likely cause for the reduced intracellular DA.
We noticed that the levels of DOPAL, DOPAC and DOPET were elevated after 12 h exposure to PCB153 but not at 24 h exposure. In male rat brains at post-natal-day 21 MAO-B activity (scheme 1: J) in the cerebellum was shown to be significantly decreased, up to 45%, after exposure to PCB153 in utero (Roda et al., 2012). Although the methods in the cited study differed considerably from the present study, it may suggest a possible reason for the two phases, i.e. first increased metabolism when intracellular DA is high due to acute competitive inhibition of VMAT, followed by normalization later due to reduced MAO transcription and activity.
PCBs are potent competitive inhibitors of DAT, which is responsible for the re-uptake of extracellular DA (Mariussen et al., 2001). Inhibition of re-uptake can be expected to lead over time to elevated extracellular DA levels as seen after 24 h exposure to PCB153. As a consequence, elevated extracellular DA may activate presynaptic auto-receptors which would down-regulate TH and the synthesis of DA within cells (Wolf and Roth, 1990). This may explain why we saw normalization of TH expression after 24 h exposure to PCB153.
Interestingly, PCB95, a known neurotoxic PCB, produced similar cytotoxicity as did PCB153, however, DA changes inside and outside the cells were markedly different as was PCB95’s effect on TH and VMAT transcription. We noticed that after12 h exposure to PCB95, levels of DA were elevated inside the cells and levels of extracellular DA were reduced which may be due to the observed down-regulation of VMAT (Figure 4) and up-regulation of TH (Figure 3) after 12 h exposure to PCB95. Decreased export and increased synthesis likely explain the effects of PCB95 on intra- and extracellular DA levels after 12 h.
In addition, PCB95 at 10 μM had very different effects on DA metabolite levels compared to PCB153. The latter increased all three metabolites in cells and medium after 12 h exposure with normalized levels at 24 h while PCB95 significantly increased only intracellular DOPET at 12 h and extracellular DOPAC at 24 h. Extracellular DOPAL and DOPET were also elevated after 24 h but not statistically significantly. This lack of strong, early DA catabolism distinguishes PCB95 from PCB153. In this respect, PCB95 is more similar to the PCB mixture Aroclor 1254, which caused a similar fast increase in intracellular DA in PC12 cells followed by a decrease in DA levels without increased DA metabolites (Seegal et al., 1989).
After 24 h exposure to PCB95 the DA distribution was reversed, showing reduced intracellular and elevated extracellular levels. Non-sequestered DA accumulates in cells which could result in inhibition of TH (Kumer and Vrana, 1996). However, based on the results from our study, the effect of PCB95 on DA levels is likely through changes in gene expression of TH and VMAT, which were both normalized at 24h exposure, and not through direct inhibition.
DA neurons are particularly vulnerable to oxidative stress given that they have elevated bioenergetics (Pacelli et al., 2015) and levels of ROS increase during inherent auto-oxidation and metabolism of L-DOPA and DA to DA quinones (Kumer and Vrana, 1996; Miller et al., 1996). The main way to avoid ROS mediated cellular damage is to increase the intracellular antioxidant defense capability. Results of recent studies have suggested antioxidants provide neuroprotection towards PCBs (Park et al., 2010). Thus, in this study, we examined the possible changes in gene expression of four main antioxidant enzymes: MnSOD, Cu/ZnSOD, catalase and GPx.
The results of gene expression analyses in this study demonstrate that PCB153 and PCB95 altered mitochondrial MnSOD expression. In PC12 cells, PCB153 and PCB95 at 10 μM up-regulated MnSOD at 12 h. Subsequently, the level of MnSOD mRNA decreased, returning to normal levels at 24h. Cu/ZnSOD mRNA expression was also significantly up-regulated after 12 h exposure with either PCB153 or PCB95 and returned to normal expression after 24 h. The up-regulation in MnSOD and Cu/ZnSOD by both PCB congeners was likely due to an increase of superoxide by PCBs. It has been reported that PCB153 caused an increase of steady state levels of intracellular O2·− and H2O2 in cells in vitro and an adaptive increase in MnSOD and Cu-ZnSOD activity (Zhu et al., 2009).
Unlike the effects on SOD transcription levels, the two PCB congeners differed in their effects on catalase and GPx expression. PCB153 up-regulated catalase expression after 24 h at 10 μM but not 5 μM, but did not have any effect on GPx. PCB95 also increased catalase mRNA level, but at both concentrations and down-regulated GPx after 12 h exposure at 10 μM, which normalized after 24 h exposure. This shows that even within the group of non-dioxin like PCBs there are differences in effects on antioxidant enzymes.
In summary, both PCB congeners tested ultimately caused a decrease in intracellular DA levels similar to what was reported by others with striatal slices, synaptosomes and PC12 cells (Shain et al., 1991; Chishti et al., 1996; Bemis and Seegal, 2004). However, PCB153 produced a transient increase in potentially more reactive DA metabolites, in contrast to PCB95, which caused a transient increase in intracellular DA levels, suggesting different modes of action of these two congeners and different pathways of toxicity. This may have implications for risk assessment. Further studies with a series of PCB congeners to identify structure-activity relationships are needed. Also, at this point, we have measured mRNA levels of VMAT2 and TH and do not know about activity or expression of DAT; however, these data will be determined in the future as altered ratio’s of these proteins (e.g., DAT:VMAT2) could be harmful to dopaminergic cells (Richardson et al., 2006). Our data demonstrate that PCBs could play a role in reducing tissue DA concentration via different mechanisms and thus could possibly lead to changes in behavior and motor function. Ultimately, these changes could progress over time resulting in neurodegenerative disorders. Studies like ours may suggest approaches for development of neuroprotective therapeutics to restrict PCBs long-term adverse health effects.
Supplementary Material
Highlights.
PCB153 decreased intracellular dopamine (DA) and increased (24 h) extracellular DA.
PCB153 increased DA metabolites DOPAC, DOPET, and DOPAL.
PCB95 first increased (12 h) then decreased (24 h) intracellular DA.
PCB95 down-regulated VMAT and GPx.
Both PCB congeners may be neurotoxic, but through different mechanisms.
Acknowledgments
We would like to thank Dr. William D. Klaren for his helpful advice, Susanne Flor for training and help with laboratory techniques, and Drs. Sandhya M. Vyas and Xueshu Li from the Iowa Superfund Research Program Synthesis Core for the synthesis and characterization of the two PCB congeners. Funding was provided by the Iowa Superfund Research Program P42 ES013661; S.H.E. was partially supported by fellowships from the University of Iowa Graduate College and the Iraq-USA training program funded by Iraq.
Abbreviations
- PCB
polychlorinated biphenyl
- PCB153
2,2′,4,4′,5,5′-Hexachlorobiphenyl
- PCB95
2,2′,3,5′,6-pentachlorobiphenyl
- PC12
rat pheochromocytoma cell line
- DOPAL
3,4-Dihydroxyphenylacetaldehyde
- DOPAC
3,4-Dihydroxyphenylacetic acid
- L-DOPA
L-3,4-dihydroxyphenylalanine
- MAO
Monoamine oxidase
- ADHD
Attention deficit hyperactivity disorder
- ROS
Reactive Oxygen Species
- DOPET
3,4-dihydroxyphenylethanol
- VMAT
Vesicle Monoamine Transporter
- AR
Aldehyde/Aldose Reductase
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angus WG, Contreras ML. Effects of polychlorinated biphenyls on dopamine release from PC12 cells. Toxicology letters. 1996;89:191–199. doi: 10.1016/s0378-4274(96)03810-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi E, Bisaglia M, Lazzarini E, Tabares LC, Beltramini M, Bubacco L. Human SOD2 modification by dopamine quinones affects enzymatic activity by promoting its aggregation: possible implications for Parkinson’s disease. PLoS One. 2012;7:e38026. doi: 10.1371/journal.pone.0038026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environmental health perspectives. 1999;107:879–885. doi: 10.1289/ehp.99107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicological sciences: an official journal of the Society of Toxicology. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Blandini F, Randine G, Samuele A, Manzo L, Coccini T. Brain monoaminergic neurotransmission parameters in weanling rats after perinatal exposure to methylmercury and 2, 2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) Brain Res. 2006:1112. doi: 10.1016/j.brainres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, Miller GW. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson’s disease-associated dopamine toxicity. Toxicological sciences: an official journal of the Society of Toxicology. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Fisher JP, Seegal RF. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996;17:653–660. [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Experimental neurology. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Davie CA. A review of Parkinson’s disease. British medical bulletin. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- Dervola KS, Johansen EB, Walaas SI, Fonnum F. Gender-dependent and genotype-sensitive monoaminergic changes induced by polychlorinated biphenyl 153 in the rat brain. Neurotoxicology. 2015;50:38–45. doi: 10.1016/j.neuro.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Doorn JA, Florang VR, Schamp JH, Vanle BC. Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons. Parkinsonism & related disorders. 2014;20(Suppl 1):S73–75. doi: 10.1016/S1353-8020(13)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Roth RH. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson’s disease. Experimental neurology. 1997;144:4–9. doi: 10.1006/exnr.1996.6379. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG. The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson’s disease. J Bioenerg Biomembr. 2009;41:469–472. doi: 10.1007/s10863-009-9257-z. [DOI] [PubMed] [Google Scholar]
- Holland EB, Feng W, Zheng J, Dong Y, Li X, Lehmler HJ, Pessah IN. An Extended Structure-Activity Relationship of Nondioxin-Like PCBs Evaluates and Supports Modeling Predictions and Identifies Picomolar Potency of PCB 202 Towards Ryanodine Receptors. Toxicological sciences: an official journal of the Society of Toxicology. 2017;155:170–181. doi: 10.1093/toxsci/kfw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC. Inadvertent Polychlorinated Biphenyls in Commercial Paint Pigments. Environmental Science & Technology. 2010;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. Polychlorinated biphenyls and polybrominated biphenyls. IARC; Lyon: 2013. [Google Scholar]
- Izumi Y, Sawada H, Sakka N, Yamamoto N, Kume T, Katsuki H, Shimohama S, Akaike A. p-Quinone mediates 6-hydroxydopamine-induced dopaminergic neuronal death and ferrous iron accelerates the conversion of p-quinone into melanin extracellularly. J Neurosci Res. 2005;79:849–860. doi: 10.1002/jnr.20382. [DOI] [PubMed] [Google Scholar]
- Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochemical Pharmacology. 2008;76:569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Joshi SN, Vyas SM, Duffel MW, Parkin S, Lehmler HJ. Synthesis of Sterically Hindered Polychlorinated Biphenyl Derivatives. Synthesis (Stuttg) 2011;7:1045–1054. doi: 10.1055/s-0030-1258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RB. Storage and release of neurotransmitters. Cell. 1993;72(Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Langeveld WT, Meijer M, Westerink RH. Differential effects of 20 non-dioxin-like PCBs on basal and depolarization-evoked intracellular calcium levels in PC12 cells. Toxicological sciences: an official journal of the Society of Toxicology. 2012;126:487–496. doi: 10.1093/toxsci/kfr346. [DOI] [PubMed] [Google Scholar]
- Lee DW, Opanashuk LA. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, Wayman GA. The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:717–725. doi: 10.1523/JNEUROSCI.2884-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GY, Storz P. Reactive oxygen species in cancer. Free radical research. 2010;44 doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacological reviews. 2007;59:125–150. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Andersson PL, Tysklind M, Fonnum F. Effect of polychlorinated biphenyls on the uptake of dopamine into rat brain synaptic vesicles: a structure-activity study. Toxicology and applied pharmacology. 2001;175:176–183. doi: 10.1006/taap.2001.9231. [DOI] [PubMed] [Google Scholar]
- Matés JM, Pérez-Gómez C, De Castro IN. Antioxidant enzymes and human diseases. Clinical biochemistry. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11:34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexas LM, Florang VR, Doorn JA. Inhibition and covalent modification of tyrosine hydroxylase by 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite. Neurotoxicology. 2011;32:471–477. doi: 10.1016/j.neuro.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Selhub J, Joseph JA. Oxidative damage caused by free radicals produced during catecholamine autoxidation: protective effects of O-methylation and melatonin. Free radical biology & medicine. 1996;21:241–249. doi: 10.1016/0891-5849(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagen. 2012;53:589–598. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS, Trudeau LE. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr Biol. 2015;25:2349–2360. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Park SH, Jang JH, Chen CY, Na HK, Surh YJ. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology. 2010;278:131–139. doi: 10.1016/j.tox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, Jorgensen PJ, Budtz-Jorgensen E, Grandjean P. Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. Neurotoxicology. 2008;29:584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Rignall B, Grote K, Gavrilov A, Weimer M, Kopp-Schneider A, Krause E, Appel KE, Buchmann A, Robertson LW, Lehmler HJ, Kania-Korwel I, Chahoud I, Schwarz M. Biological and tumor-promoting effects of dioxin-like and non-dioxin-like polychlorinated biphenyls in mouse liver after single or combined treatment. Toxicological sciences: an official journal of the Society of Toxicology. 2013;133:29–41. doi: 10.1093/toxsci/kft034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda E, Manzo L, Coccini T. Application of Neurochemical Markers for Assessing Health Effects after Developmental Methylmercury and PCB Coexposure. Journal of toxicology. 2012;2012:216032. doi: 10.1155/2012/216032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander L, Stromberg U, Dyremark E, Ostman C, Nilsson-Ehle P, Hagmar L. Polychlorinated biphenyls in blood plasma among Swedish female fish consumers in relation to low birth weight. American journal of epidemiology. 1998;147:493–502. doi: 10.1093/oxfordjournals.aje.a009476. [DOI] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Critical reviews in toxicology. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Brosch K, Bush B, Ritz M, Shain W. Effects of Aroclor 1254 on dopamine and norepinephrine concentrations in pheochromocytoma (PC-12) cells. Neurotoxicology. 1989;10:757–764. [PubMed] [Google Scholar]
- Seegal RF, Bush B, Shain W. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicology and applied pharmacology. 1990;106:136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Marek KL, Seibyl JP, Jennings DL, Molho ES, Higgins DS, Factor SA, Fitzgerald EF, Hills EA, Korrick SA, Wolff MS, Haase RF, Todd AC, Parsons P, McCaffrey RJ. Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: a beta-CIT imaging study. Neurobiol Dis. 2010;38:219–225. doi: 10.1016/j.nbd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain W, Bush B, Seegal R. Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicology and applied pharmacology. 1991;111:33–42. doi: 10.1016/0041-008x(91)90131-w. [DOI] [PubMed] [Google Scholar]
- Smythies J. Redox aspects of signaling by catecholamines and their metabolites. Antioxid Redox Signal. 2000;2:575–583. doi: 10.1089/15230860050192332. [DOI] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT, 2nd, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR. Neurochemical effects of polychlorinated biphenyls: an overview and identification of research needs. Neurotoxicology. 1997;18:727–743. [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Annals of the New York Academy of Sciences. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Zeng YH, Tang B, Luo XJ, Zheng XB, Peng PA, Mai BX. Organohalogen pollutants in surface particulates from workshop floors of four major e-waste recycling sites in China and implications for emission lists. Sci Total Environ. 2016;569–570:982–989. doi: 10.1016/j.scitotenv.2016.06.053. [DOI] [PubMed] [Google Scholar]
- Zhou ZD, Lim TM. Dopamine (DA) induced irreversible proteasome inhibition via DA derived quinones. Free Radic Res. 2009;43:417–430. doi: 10.1080/10715760902801533. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kalen AL, Li L, Lehmler HJ, Robertson LW, Goswami PC, Spitz DR, Aykin-Burns N. Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free Radic Biol Med. 2009;47:1762–1771. doi: 10.1016/j.freeradbiomed.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.