Abstract
Background
The effect of body composition disturbances has been recently in focus. Sarcopenic obesity, a co-occurrence of low muscle mass and high body fat was reportedly predictive of high mortality in patients with cirrhosis. However, the impact of the interacting sarcopenia and overweight on the outcomes after liver transplantation is still unclear.
Methods
We evaluated 200 patients undergoing adult-to-adult living donor liver transplantation at our institution between January 2008 and November 2013 classified according to BMI and psoas muscle index (PMI) on admission to transplant into 4 subgroups; sarcopenic overweight (SO), sarcopenic non-overweight (SN), non-sarcopenic overweight and non-sarcopenic non-overweight (NN). Short-term outcomes and overall post-transplant survival were compared among the four subgroups.
Results
Sarcopenic patients with preoperative low PMI had higher incidence of postoperative bacteremia and major postoperative complications, and poorer overall post-transplant survival than non-sarcopenic patients with normal/high PMI (P<0.001, respectively). Overweight recipients had a significantly higher overall survival (OS) rate than non-overweight patients (P=0.021). SO subgroup (low PMI and BMI ≥25) had statistically indifferent incidence of postoperative bacteremia, major postoperative complications or overall post-transplant survival than other recipients. In contrast, SN subgroup (low PMI and BMI <25) had higher incidence of postoperative bacteremia (P<0.001), major postoperative complications (P<0.001) than the SO subgroup and possessed the poorest OS among the four recipient subgroups (P=0.001).
Conclusions
In living donor liver transplantation, preoperative SO did not confer added significant morbidity or mortality risks than the stand-alone sarcopenia.
Keywords: Living donor liver transplantation (LDLT), overweight, psoas muscle index (PMI), sarcopenia
Introduction
Protein-energy malnutrition is common in patients with end-stage liver disease (ESLD) undergoing liver transplantation (LT), and is associated with increased risk for morbidity and mortality thereafter (1). Sarcopenia, generally defined as progressive and generalized loss of skeletal muscle mass and strength, is frequently encountered in patients with decompensated cirrhosis and was associated with poor post-LT outcomes (2,3). Low skeletal muscle mass was associated with poor overall survival (OS) after living donor LT (LDLT) (4).
At the other extreme of physiognomy, divergent results have been obtained regarding the postoperative outcomes in obese patients versus those of normal body weight (5,6), in particular those undergoing deceased donor liver transplantation (7,8), with some studies even reporting a protective effect of obesity. However, the impact of obesity after LDLT is yet unclear.
Different obesity phenotypes may exist and in particular variation in skeletal muscle mass across obese individuals may confer different health risks. Sarcopenic obesity, a co-occurrence of low muscle mass and high body fat, is an emerging clinical entity that together myosteatosis were associated with higher mortality in patients with cirrhosis (9). However, there is a paucity of evidence on the impact of recipient preoperative sarcopenic overweight (SO) on outcomes post-LT. We hypothesize that SO might confer additional post-transplant morbidity and mortality risks than either of the disorders alone.
The present study was therefore performed to examine the impact of the interacting sarcopenia and overweight recipients’ body habitus on the outcomes after adult-to-adult LDLT.
Methods
Patients
Two hundred thirty-five adult (age ≥18 years) patients underwent adult-to-adult LDLT at Kyoto University Hospital between January 2008 and November 2013. Thirty-five patients who did not undergo preoperative plain computed tomography (CT) imaging at the umbilical level were excluded from the study. Therefore, a total of 200 patients (95 men, 105 women) were enrolled. The study was approved by the Ethics Committee of Kyoto University and conducted in accordance with the Declaration of Helsinki of 1996. Written informed consent was obtained from all patients. The patients’ demographics and clinical characteristics are summarized in Table 1.
Table 1. Patient characteristics.
| Variable | Value |
|---|---|
| Age [years] | 54 [18–69]* |
| Sex (male/female) | 95/105 |
| ABO compatible/incompatible | 140/60 |
| Etiology of liver disease | |
| HCC | 67 |
| Viral hepatitis B or C-related cirrhosis | 38 |
| PBC/PSC | 34 |
| Acute liver failure | 9 |
| Biliary atresia | 19 |
| Metabolic liver diseases | 6 |
| Alcohol | 6 |
| Budd-Chiari syndrome | 4 |
| Others | 17 |
| Child-Pugh classification (A/B/C) | 15/60/125 |
| MELD score | 18 [5–55] |
| Graft (RT/LT/posterior segment/domino) | 102/93/4/1 |
| GRWR | 0.89 (0.54–1.46) |
*, data are given as median [range]. ABO, ABO blood group; GRWR, graft-to-recipient weight ratio; HCC, hepatocellular carcinoma; LDLT, living donor liver transplantation; LT, left lobe graft; MELD, model for end-stage liver disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; RT, right lobe graft.
The selection criteria for the recipients and the surgical techniques for the donor and recipient, and the immunosuppressive regimen have been previously described (10-12). All patients received intravenous antimicrobial prophylaxis with ampicillin (0.5 g) and cefotaxime (0.5 g) twice daily for 3 days starting 30 min before surgery.
Image analysis
We used plain preoperative CT imaging on admission, obtained usually 7 to 14 days before transplantation. The cross-sectional areas of the right and left psoas muscles were measured by manual tracing using preoperative CT imaging at the umbilical level (13,14). The psoas muscle index (PMI) was calculated by normalizing the cross-sectional areas for height (cm2/m2). All preoperative CT imaging was obtained with a multi-detector computed tomography scanner (Aquilion 64, Toshiba Medical Systems, Tochigi, Japan). The technical parameters used for CT were: 120 kV (tube voltage), 0.5 mm × 64 row (detector configuration), tube current modulation, 0.5 sec/rotation (gantry rotation), and 7 mm reconstruction thickness.
Patient group assignments
In the present study, sarcopenia was defined as low skeletal muscle mass, denoted by low preoperative PMI. Cutoff points were set as minus 2 standard deviations (−2 SD) below the mean PMI of matched-sex young (<50 years) healthy LT donors at our institution (288 male and 253 female donors): 6.36 for male and 3.92 for female (14). Enrolled patients were assigned to two groups; sarcopenia (n=71) (having a PMI of <6.36 or <3.92 cm2/m2 for male and female recipients, respectively), and non-sarcopenic (n=129); (PMI ≥6.36 or ≥3.92 cm2/m2 for male and female recipients, respectively). Patients were divided according to BMI on admission to two groups, overweight (BMI ≥25 kg/m2) (n=59) and non-overweight (BMI <25 kg/m2) recipients (n=141). A BMI of ≥25 kg/m2 on admission was used as a cutoff point according to the obesity guideline proposed by Japan Society for the Study of Obesity in 2011, which is appropriate for Japanese Asian figures since Asians tend to suffer from metabolic complications of obesity at a lower BMI than others (15). Patients were sub-classified into four subgroups: (I) non-sarcopenic non-overweight (NN) (n=80; 31 males and 49 females) (40%); (II) non-sarcopenic overweight (NO) (n=49; 24 males and 25 females) (24.5%); (III) sarcopenic non-overweight (SN) (n=61; 33 males and 28 females) (30.5%); and (IV) SO (n=10; 7 males and 3 females) (5%) (Figure 1). SO was therefore defined as having a PMI <−2 SD below the mean PMI of matched sex young healthy LDLT donors and a BMI of ≥25 kg/m2 on admission for transplantation.
Figure 1.
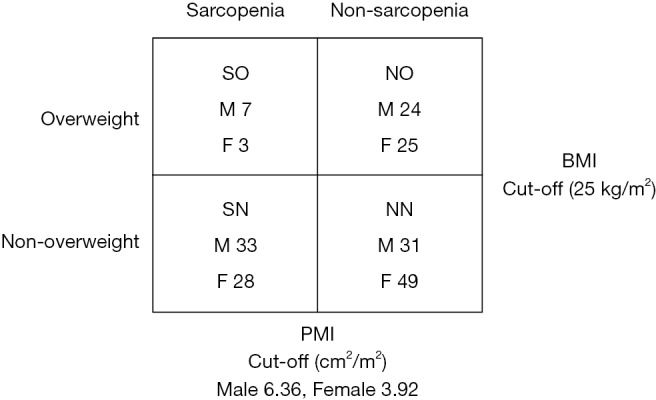
Schematic diagram of subgroup assignments according to BMI and PMI. BMI, body mass index; PMI, psoas muscle index; SO, sarcopenic overweight; NN, non-sarcopenic non-overweight; SN, sarcopenic non-overweight; NO, non-sarcopenic overweight; M, male; F, female.
Postoperative morbidity occurring within 90 days of transplantation was graded according to the validated modified classification of surgical complications by Dindo et al. (16). A Clavien-Dindo score of ≤2 was considered as minor complications, whereas complications with a score of ≥3a were considered major complications. Complications for which surgical or radiologic intervention with antibiotics were needed, such as intra-abdominal abscess, pneumonia, and non-resolving wound infection, were defined as infectious complications.
Infections were defined with the criteria proposed by the Centers for Disease Control and Prevention, which are based on previous reports of liver transplant patients (17). Briefly, post-operative bacteremia was defined as the isolation of bacteria (other than common skin contaminants) from a single blood culture obtained within 90 days of transplantation in the presence of clinical symptoms or signs of infection. Bacteremia caused by common skin contaminants was considered significant only when the organism was isolated from two individual blood cultures, and this finding was accompanied by clinical signs of infection.
Analyzed parameters
The following parameters were analyzed: recipient age, sex, underlying disease, preoperative Child-Pugh score, model for end-stage liver disease (MELD) score, ABO compatibility, donor age, PMI, BMI on admission, prealbumin levels, zinc, branched-chain amino acids (BCAA), cholinesterase, total lymphocyte count, nutritional parameters, and the closely related metabolic parameters including BCAA-to-tyrosine ratio (BTR), tyrosine, and ammonia on admission for transplantation, as well as graft-to-body-weight-ratio, operative time, intra-operative blood loss and erythrocytes infusion units, cold and warm ischemia times, the rate at which major postoperative surgical complications occurred and the incidence of postoperative bacteremia. These parameters were compared first, between the sarcopenic and non-sarcopenic groups; second, between the overweight and non-overweight groups; and third, between the SO subgroup and the other recipients (non-SO). To test our hypothesis that sarcopenia and overweight in combination might be associated with a greater risk than either of the disorders alone, the SO subgroup was also compared to the NO and the SN subgroups.
Post-transplant OS rates were compared among the four recipient subgroups, respectively, with respect to the above-mentioned parameters with a further sex-based sub-stratification of survival analysis to nullify effect modification by gender, if present.
Statistical analysis
Data are presented as mean ± SD for continuous variables. Continuous variables were analyzed using the Mann-Whitney U test. Categorical variables were compared using the χ2 test or Fisher’s exact test where appropriate. Correlations between two variables were analyzed using Spearman’s rank correlation coefficient. Cumulative OS rates were calculated using Kaplan-Meier methods, and differences between curves were evaluated using the log-rank test or the Mantel-Cox test. A P value<0.05 was considered significant. All statistical data were generated using JMP 11 (SAS Institute, Cary, NC, USA) and Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
There was a positive correlation between PMI and BMI in enrolled cohort (r=0.4657, P<0.0001) (Figure 2). There were no significant differences in patient characteristics or surgical variables between the sarcopenic and non-sarcopenic recipient groups (Table 2), the overweight and non-overweight recipient groups, the SO subgroup and the non-SO recipients, or between the SO and each of the NO and the SN recipient subgroups (Table 3).
Figure 2.
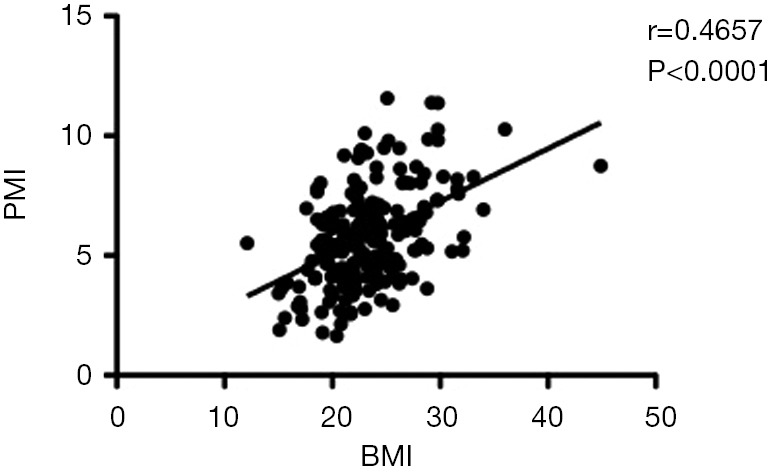
Scatterplot and correlation between BMI and PMI in enrolled patients. BMI, body mass index; PMI, psoas muscle index.
Table 2. Demographics and transplant outcomes of patients classified according to PMI.
| Variable | Low PMI (n=71) | Normal/high PMI (n=129) | P |
|---|---|---|---|
| Preoperative patient characteristics | |||
| Donor age (years) | 41.4±11.9 | 42.0±10.5 | 0.336 |
| Recipient age at transplantation (years) | 50.3±10.4 | 45.9±10.6 | 0.052 |
| Gender (male/female) | 40/31 | 55/74 | 0.063 |
| PMI on admission (cm2/m2) | 3.6±1.5 | 6.9±1.9 | <0.001 |
| BMI on admission (kg/m2) | 24.4±3.3 | 22.7±2.9 | 0.250 |
| Underlying disease | 0.766a | ||
| HCC | 23 | 42 | |
| Viral hepatitis B/C-related cirrhosis | 10 | 28 | |
| PBC/PSC | 11 | 23 | |
| Acute liver failure | 4 | 5 | |
| Biliary atresia after Kasai operation | 9 | 10 | |
| Metabolic liver diseases | 2 | 4 | |
| Alcoholic cirrhosis | 3 | 3 | |
| Budd-Chiari syndrome | 2 | 2 | |
| Others | 7 | 10 | |
| ABO compatibility | 0.584 | ||
| Identical/compatible | 48 | 92 | |
| Incompatible | 23 | 37 | |
| Preoperative Child-Pugh classification | 0.423 | ||
| A, B/C | 24/47 | 51/78 | |
| Preoperative MELD score | 21.3±8.3 | 21.6±10.5 | 0.275 |
| Nutritional status at transplant | |||
| Zinc (µg/dL) | 41.3±10.6 | 42.8±11.1 | 0.544 |
| Prealbumin (mg/dL) | 6.1±2.1 | 7.1±2.3 | 0.543 |
| BCAA (µmol/L) | 499.6±47.8 | 389.6±58.1 | 0.001 |
| Tyrosine (µmol/L) | 139.3±12.3 | 136.5±10.6 | 0.623 |
| BTR | 4.4±0.5 | 2.9±0.5 | 0.003 |
| TLC (/µL) | 836.2±211 | 841.4±211 | 0.734 |
| Ammonia (µg/dL) | 97.5±13.4 | 96.1±18.6 | 0.745 |
| Surgical variables | |||
| Graft type | 0.238 | ||
| Left lobe | 37 | 56 | |
| Right lobe including posterior segment graft | 34 | 73* | |
| GRWR (%) | 0.7±0.1 | 0.8±0.2 | 0.532 |
| Surgical duration (min) | 948±129 | 977±171 | 0.658 |
| Blood loss (mL) | 9,488±3,265 | 9,789±3,280 | 0.915 |
| Intraoperative erythrocyte transfusion (U) | 22.9±11.6 | 20.8±12.7 | 0.756 |
| Cold ischemia time (min) | 68.3±16.2 | 75.3±11.1 | 0.451 |
| Warm ischemia time (min) | 38.3±11.1 | 47.9±16.3 | 0.767 |
| Major postoperative complications, n (%) | 67 (94%) | 95 (74%) | <0.001 |
| Neurological complications | 5 (7%) | 2 (2%) | 0.454 |
| Respiratory complications | 40 (56%) | 28 (22%) | <0.001 |
| Cardiovascular events | 4 (6%) | 1 (1%) | 0.654 |
| Infectious complications | 41 (58%) | 11 (9%) | <0.001 |
| Surgical complications | |||
| Biliary complications | 26 (37%) | 15 (12%) | 0.361 |
| Bile leakage | 18 (25%) | 16 (12%) | 0.543 |
| Biliary stricture | 26 (37%) | 18 (14%) | 0.839 |
| Vascular complications | 8 (11%) | 6 (5%) | 0.073 |
| HAT | 6 (8%) | 4 (3%) | 0.261 |
| PV thrombosis | 3 (4%) | 2 (2%) | 0.987 |
| PV stenosis | 1 (1%) | 1 (1%) | 0.899 |
| HV insufficiency | 2 (3%) | 1 (1%) | 0.187 |
| Incidence of postoperative bacteremia | 54 (76%) | 40 (31%) | <0.001 |
*, including one domino graft; a, P values were compared using Pearson’s χ2 test. ABO, ABO blood group; BCAA, branched-chain amino acids; BTR, BCAA-to-tyrosine ratio; GRWR, graft to recipient weight ratio; HAT, hepatic artery thrombosis; HCC, hepatocellular carcinoma; HV, hepatic vein; MELD, model for end-stage liver disease; PBC, primary biliary cirrhosis; PMI, psoas muscle index; PSC, primary sclerosing cholangitis; PV, portal vein; TLC, total lymphocyte count.
Table 3. Demographics and transplant outcomes of the sarcopenic obese (SO) and sarcopenic non-obese (SN) subgroups.
| Variable | SO (n=10) | SN (n=61) | P |
|---|---|---|---|
| Preoperative patient characteristics | |||
| Donor age (years) | 42.6±10.3 | 40.1±11.2 | 0.321 |
| Recipient age at transplantation (years) | 51.3±10.2 | 46.2±13.2 | 0.732 |
| Gender (male/female) | 7/3 | 33/28 | 0.347 |
| PMI on admission (cm2/m2) | 5.4±1.5 | 5.1±1.4 | 0.439 |
| BMI on admission (kg/m2) | 28.8±2.2 | 19.9±2.3 | <0.001 |
| Underlying disease | 0.853a | ||
| HCC | 2 | 21 | |
| Viral hepatitis B/C-related cirrhosis | 2 | 8 | |
| PBC/PSC | 1 | 10 | |
| Acute liver failure | 1 | 3 | |
| Biliary atresia after Kasai operation | 1 | 8 | |
| Metabolic liver diseases | 1 | 1 | |
| Alcoholic cirrhosis | 1 | 2 | |
| Budd-Chiari syndrome | 0 | 2 | |
| Others | 1 | 6 | |
| ABO compatibility | 0.861 | ||
| Identical/compatible | 7 | 41 | |
| Incompatible | 3 | 20 | |
| Preoperative Child-Pugh classification | 0.784 | ||
| A, B/C | 3/7 | 21/40 | |
| Preoperative MELD score | 19.8±8.6 | 20.2±11.5 | 0.332 |
| Nutritional status at transplant | |||
| Zinc (µg/dL) | 42.6±9.2 | 42.1±11.2 | 0.723 |
| Prealbumin (mg/dL) | 6.7±1.7 | 7.3±1.7 | 0.815 |
| BCAA (µmol/L) | 438.4±42.6 | 530.2±41.1 | 0.001 |
| Tyrosine (µmol/L) | 142.1±11.2 | 135.4±13.6 | 0.324 |
| BTR | 4.4±0.4 | 5.8±0.3 | 0.001 |
| TLC (/µL) | 845.3±254.5 | 843.5±222 | 0.334 |
| Ammonia (µg/dL) | 94.6±11.2 | 95.2±16.4 | 0.945 |
| Surgical variables | |||
| Graft type | 0.719 | ||
| Left lobe | 5 | 32 | |
| Right lobe including posterior segment graft | 5 | 29 | |
| GRWR (%) | 0.8±0.1 | 0.8±0.3 | 0.765 |
| Surgical duration (min) | 949±126 | 975±156 | 0.243 |
| Blood loss (mL) | 9,644±3,134 | 9,635±3,433 | 0.437 |
| Intraoperative erythrocyte transfusion (U) | 22.8±11.5 | 21.2±10.3 | 0.864 |
| Cold ischemia time (min) | 57.3±17.2 | 76.2±11.3 | 0.324 |
| Warm ischemia time (min) | 38.2±12.1 | 40.7±13.2 | 0.931 |
| Major postoperative complications, n (%) | 6 (60%) | 61 (100%) | <0.001 |
| Neurological complications | 1 (1%) | 4 (7%) | 0.913 |
| Respiratory complications | 4 (57%) | 36 (60%) | 0.176 |
| Cardiovascular events | 1 (1%) | 3 (6%) | 0.753 |
| Infectious complications | 5 (50%) | 36 (59%) | 0.291 |
| Surgical complications | |||
| Biliary complications | 4 (40%) | 22 (36%) | 0.443 |
| Bile leakage | 4 (40%) | 14 (23%) | 0.643 |
| Biliary stricture | 3 (30%) | 23 (38%) | 0.192 |
| Vascular complications | 2 (20%) | 6 (10%) | 0.609 |
| HAT | 2 (29%) | 4 (7%) | 0.321 |
| PV thrombosis | 1 (10%) | 2 (3%) | 0.540 |
| PV stenosis | 0 (0%) | 1 (1%) | 0.502 |
| HV insufficiency | 1 (10%) | 1 (1%) | 0.052 |
| Incidence of postoperative bacteremia | 3 (30%) | 51 (84%) | <0.001 |
a, P values were compared using Pearson’s χ2 test. ABO, ABO blood group; BCAA, branched-chain amino acids; BTR, BCAA-to-tyrosine ratio; GRWR, graft to recipient weight ratio; HAT, hepatic artery thrombosis; HCC, hepatocellular carcinoma; HV, hepatic vein; MELD, model for end-stage liver disease; PBC, primary biliary cirrhosis; PMI, psoas muscle index; PSC, primary sclerosing cholangitis; PV, portal vein; SO, sarcopenic obese recipients; SN, sarcopenic non-obese recipients; TLC, total lymphocyte count.
Nutritional and metabolic parameters at transplantation
Non-sarcopenic recipients had significantly lower BCAA and BTR preoperative levels than the sarcopenic group, (P=0.001 and P=0.003, respectively) (Table 2). Overweight recipients had significantly lower BCAA and BTR preoperative levels than the non-overweight recipients, (P=0.014 and P=0.011, respectively). Furthermore, significantly lower BCAA and BTR preoperative levels were found in the SO group than in the SN subgroup (P=0.001 and P=0.001, respectively) (Table 3). As for other nutritional and related metabolic parameters, no significant differences were found between the sarcopenic and non-sarcopenic groups, the overweight and non-overweight groups, the SO and non-SO groups, or between the SO and each of the NO or the SN recipients.
Post-transplant outcomes
Of the 200 patients, 162 (81%) developed one or more major postoperative complications. A total of 106 (53%) patients developed one or more infectious complications. Ninety-four patients (47%) developed postoperative bacteremia.
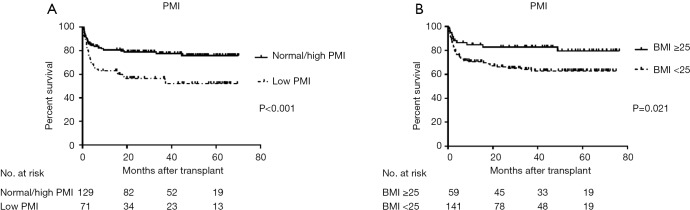
Sarcopenic patients had significantly lower OS (P<0.001) (Figure 3A), higher major postoperative complications rate (P<0.001), especially those infectious in nature (P<0.001), higher incidence of postoperative bacteremia (P<0.001) than non-sarcopenic recipients (Table 2). Surprisingly, overweight recipients had a significantly higher OS (P=0.021) than the non-overweight group (Figure 3B). However, major postoperative complications and incidence of postoperative bacteremia did not significantly differ between both groups (P=0.823 and P=0.057, respectively). There were no significant differences in OS (P=0.054), incidence of major postoperative complications (P=0.543) or postoperative bacteremia (P=0.746) between patients with BMI <18 (n=14) and recipients with BMI ≥18 (n=186).
Figure 3.
Overall post-transplant survival according to preoperative PMI (A) and BMI (B). BMI, body mass index; PMI, psoas muscle index.
The OS (Figure 4), major postoperative complications rate, and incidence of postoperative bacteremia of the SO subgroup did not significantly differ from the other recipients (P=0.748, P=0.082 and P=0.269, respectively). The incidence of postoperative bacteremia and major postoperative complications did not significantly differ between SO and NO subgroups (P=0.601 and P=0.974, respectively). The SO recipients had a significantly lower incidence of postoperative bacteremia and major complications rate than the SN subgroup (P<0.001 and P<0.001, respectively) (Table 3).
Figure 4.
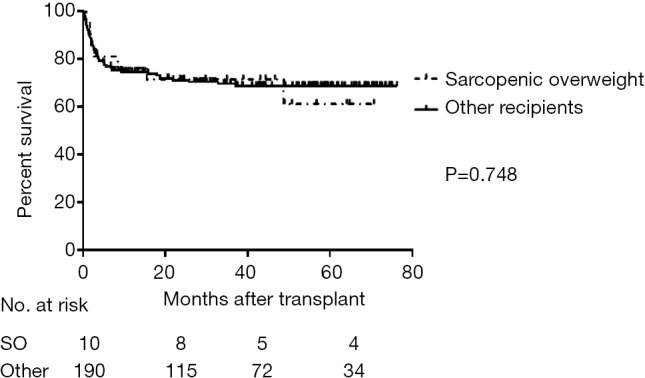
Overall post-transplant survivals of sarcopenic overweight recipients compared to other recipients. SO, sarcopenic overweight subgroup; non-SO, other recipients.
OS among the four subgroups
The median survival time for the entire cohort was 29.7 (range, 0.2–76.3) months. The median follow up period is 56 (range, 0.2–80) months. The 5-year OS for the NO, SO, NN, and SN subgroups were 89.4%, 87.1%, 78.1%, and 55.2%, respectively (overall log-rank: P=0.001) (Figure 5A). Among the four recipient subgroups, the SN subgroup had significantly lower OS compared to the NN subgroup (P=0.004) and to the NO subgroup (P<0.001). Otherwise there were no significant differences in OS among the four recipient subgroups.
Figure 5.
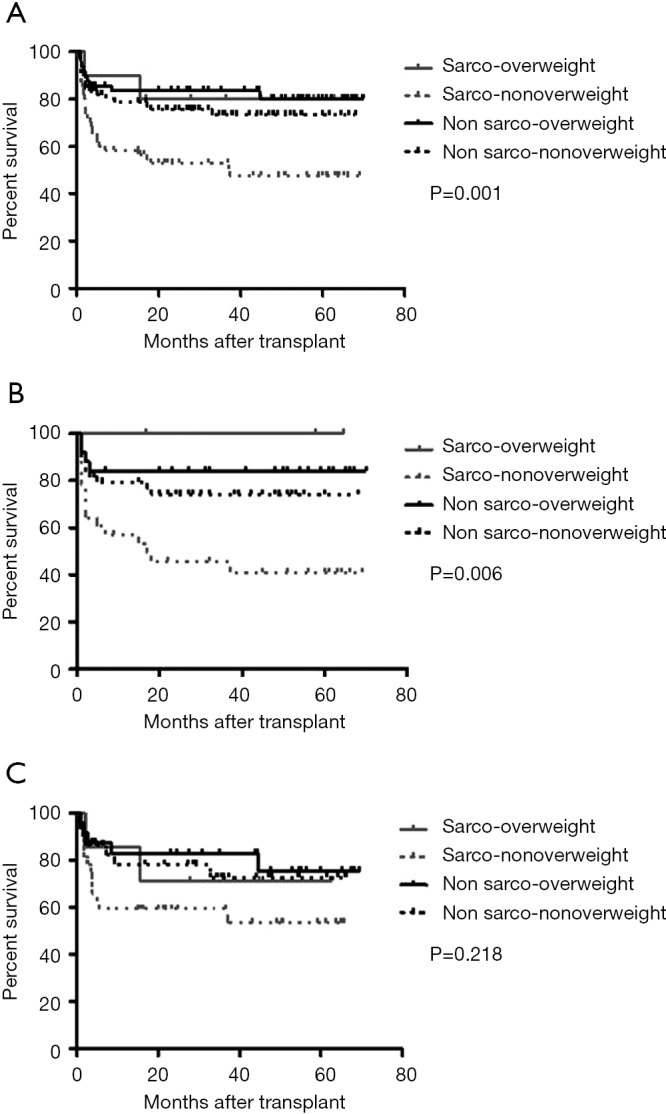
Overall post-transplant survival among the four recipient subgroups (A), female recipient subgroups (B), and male recipient subgroups (C). NO, non-sarcopenic overweight subgroup; NN, non-sarcopenic non-overweight subgroup; SO, sarcopenic overweight subgroup, SN, sarcopenic non-overweight subgroup.
Upon sex-based stratification, female SN recipients retained a significantly lower OS than their female NN and NO counterparts (overall log-rank: P=0.006) (Figure 5B). While, on the other hand, there were no significant differences in OS among the four male recipient subgroups (overall log-rank: P=0.218) (Figure 5C).
Discussion
This retrospective study identified preoperative sarcopenia to be associated with a higher incidence of postoperative bacteremia, major complications, especially those infectious in nature and a poor post-transplant OS. SO did not confer added post-transplant morbidity or mortality risks than the stand-alone sarcopenia. However, on the other hand, SN was associated with higher incidence of major postoperative complications, bacteremia and the poorest OS among recipient entities, especially in females.
The mechanisms by which sarcopenia increases the mortality risk are not fully understood. Muscle mass functions as a source of amino acids for protein synthesis and gluconeogenesis in stress and starvation (18). Skeletal muscle loss leads to contractile insufficiency, metabolic impairment and myokine dysregulation, which contribute to disability, impaired immunity and risk of sepsis-related death in cirrhotic patients (19). In LT, the majority of total post-transplant deaths usually occur in the early post-operative period before discharge. Thus, the occurrence of major postoperative complications is of great concern, not only for the short-term outcomes but also for a poor OS. Sarcopenic patients might have a little reserve to adequately respond to operative stress; thus, they experience more major postoperative complications and poor OS. We speculate that sex-based differences in OS of male and female recipients among the four recipient subgroups might stem out from a difference between feminine and masculine malnutrition patterns. In females, metabolic adaptations shift toward using fat rather than amino acids to spare muscle protein, so that fat stores in females are used to meet their metabolic needs until muscle mass remains the exclusive energy store (20).
Surprisingly, overweight seemed to adversely improve OS after transplant. However, after subgroup or gender sub-classification, it did not retain an independent prognostic value for a better post-transplant OS. Such an apparent survival benefit for the obese was previously described in the elderly and cancer patients as the obesity paradox (5,6). Wong et al. found a better survival in the overweight or mildly obese recipients and even concluded that obesity alone should not be a contraindication for LT (21). Similar to the present study, some recently reported studies failed to show a negative impact of obesity per se on postoperative complications (22) or OS (7) after LT. While, other investigators have reported an increase in postoperative infectious complications (8) with decreased survival (23) in obese recipients. Tanaka et al. attributed inferior post-transplant outcomes in morbidly obese (BMI ≥40 kg/m2) to muscle steatosis and pro-inflammatory cytokines produced by adipose tissue (24). We did not find preoperative overweight to increase major postoperative complications. Such discrepancies between our findings and other studies could be due to the smaller number of enrolled obese patients; BMI ≥30 kg/m2 in ten patients or severely obese BMI ≥40 kg/m2 in only one patient in our study for whom such deleterious effects for morbid obesity on post-transplant outcomes were described. We used a BMI ≥25 kg/m2 as a cutoff point appropriate for the Asian body configuration. The increase in body fat percentage was possibly not so profound to affect major morbidity. Another plausible explanation is that a BMI of 18 to 24.9 kg/m2 could partly reflect malnutrition and muscle wasting with a poor OS of the non-obese. Heuer et al. found higher post-transplant mortality and complications among non-alcoholic steatohepatosis (NASH) patients attributed to higher rates of diabetes mellitus (25). However, there were no NASH cases as an indication for LT included among obese recipients group in the present study.
Interestingly, non-overweight or sarcopenic LDLT candidates had higher BCAA and BTR levels at transplant despite the fact that all enrolled patients received the same perioperative nutritional therapy (4). In patients with cirrhosis, BCAA are mainly metabolized in skeletal muscle where they are utilized in the detoxification of ammonia to glutamine (26). We speculate that the more skeletal muscle mass, the more BCAA consumption, which leads to a decrease in the BTR. On the other hand, the higher the amount of obesity and fat inside the muscle, the more BCAA that are needed for hyperammonemia-induced glutamine synthesis, which leads to a decrease in the BTR.
Sarcopenia is linked to inflammation (27,28). Moreover, sarcopenic obesity is associated with systemic inflammation because adipose tissue synthesizes and secretes various kinds of pro-inflammatory adipokines such as leptin, TNF-α, interleukin (IL)-1 and IL-6 together with decreased adiponectin or IL-15 with effects on the immune system (29). However, in the present study, SO did not confer additional morbidity or mortality risks after LDLT than the stand-alone sarcopenia. Our observations may be partly due to the obesity paradox i.e., the moderately increased fat mass in the enrolled population was not as profound as required to affect major morbidity and it could have served as a metabolic reserve to some extent in such ESLD debilitated patients, especially in female recipients whose malnutrition adaptation is preliminarily fat-consuming. Consistently, SO was not associated with metabolic impairment, poor quality of life or functional limitation in obese women (30), while SN in female recipients was associated with higher postoperative major complications and the poorest OS.
Several limitations of the present study must be acknowledged. First, this was a single-institution retrospective study. However, the study scale was sufficient for follow-up of 200 recipients with diverse indications; yet, the recipients had the same ethnicity, were under homogeneous immunosuppression regimens, and received the same perioperative nutritional therapy; and the surgeons’ skills were uniform. Second, we utilized the psoas muscle as a means to evaluate skeletal mass and skeletal muscle quality at the umbilical level of cross-sectional CT imaging. Other reports have used skeletal muscle mass at the L3 level (31). However, Lim et al. (32) reported that psoas muscle thickness on CT imaging at the level of the umbilicus divided by height might be predictive of mortality, as it might be difficult to precisely identify a given lumbar section because of sacralization of the L5 vertebrae, lumbar wedge fractures, and more pronounced lordosis in patients with refractory ascites. The third limitation of the present study was that there was a possible selection bias because 35 cases (14.9%) were excluded from the study for the reason of having no CT imaging. Several patients had CT imaging performed at outside institutions, and the images were therefore not available. However, these data were excluded at random, and this exclusion criterion was not related to the patients’ general condition or severity of disease. The perioperative factors were not significantly different between the excluded population and the study group (data not shown). Therefore, there was actually little selection bias for patient inclusion in the present study. The forth limitation is that we used a cutoff point of −2 SD of below mean PMI of matched-sex young healthy subjects to define sarcopenia as used before (14). In fact, these cutoff points are most recently adopted in the Japan Society of Hepatology guidelines for sarcopenia in 2016 (33). Several reports have discussed the diagnosis of sarcopenic obesity while indicating no consensus on the definition of sarcopenic obesity using different muscle mass and BMI thresholds. The present study comprised cirrhotic patients as opposed to healthy subjects and of homogenous Asian body constitutions. Therefore, the definitions of sarcopenic obesity used in the previous reports are not necessarily applicable to ESLD cirrhotic patients. The fifth limitation of the present study was that, although calculation of BMI has previously been heavily used to define sarcopenic obesity, measuring the body fat area on CT or calculation of fat-free mass and then fat mass might seem more reliable due to BMI overestimation limitations in ESLD secondary to interstitial edema, pleural effusion, and ascites. However, there has been good agreement between BMI and the % body fat technique for the classification of patients into obese or non-obese categories (34). We did not correct for ascites volume by measuring dry body weight and subtracting ascites fluid volume removed after paracentesis or at LT, or multiplying BMI by serum albumin level. However, similar to our results, studies that corrected BMI for ascites volume did not find mild obesity to significantly decrease post-LT survival (35,36). Moreover, the etiologies and severity of liver diseases according to the Child-Pugh or MELD scores were comparable in the obese and non-overweight groups in the present study. The final limitation of the present study is that we could not collect data on other frailty parameters, including grip strength and levels of exhaustion, as part of sarcopenia definition, along with skeletal muscle mass, due to the study’s retrospective design. We are now conducting a prospective study with larger sample size recording post LT changes in body composition and their possible influence on the outcomes to evaluate the degree of perioperative SO considering grip strength and body composition analysis.
In conclusion, preoperative central sarcopenia and specifically SN in female recipients were associated with higher incidence of postoperative major complications and poor post-transplant OS. Appropriate preoperative interventions, including nutritional therapy and rehabilitation with evaluations of skeletal muscle mass, function, and fat mass, are recommended for good outcomes. Further large-scale prospective studies taking the severity of overweight and sarcopenia into account are warranted to confirm our results.
Acknowledgements
None.
Ethical Statement: The study was approved by the Ethics Committee of Kyoto University (No. R0061) and conducted in accordance with the Declaration of Helsinki of 1996. Written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Stickel F, Inderbitzin D, Candinas D. Role of nutrition in liver transplantation for end-stage chronic liver disease. Nutr Rev 2008;66:47-54. 10.1111/j.1753-4887.2007.00005.x [DOI] [PubMed] [Google Scholar]
- 2.van Vugt JL, Levolger S, de Bruin RW, et al. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant 2016;16:2277-92. 10.1111/ajt.13732 [DOI] [PubMed] [Google Scholar]
- 3.Masuda T, Shirabe K, Ikegami T, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014;20:401-7. 10.1002/lt.23811 [DOI] [PubMed] [Google Scholar]
- 4.Kaido T, Ogawa K, Fujimoto Y, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 2013;13:1549-56. 10.1111/ajt.12221 [DOI] [PubMed] [Google Scholar]
- 5.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 2013;105:1862-70. 10.1093/jnci/djt310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlberg SE, Schiller JH, Bonomi PB, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013;8:1121-7. 10.1097/JTO.0b013e31829cf942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Protto SE, Quintini C, Reynolds LF, et al. Comparable graft and patient survival in lean and obese liver transplant recipients. Liver Transpl 2013;19:907-15. 10.1002/lt.23680 [DOI] [PubMed] [Google Scholar]
- 8.Hakeem AR, Cockbain AJ, Raza SS, et al. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl 2013;19:551-62. 10.1002/lt.23618 [DOI] [PubMed] [Google Scholar]
- 9.Montano-Loza AJ, Angulo P, Meza-Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126-35. 10.1002/jcsm.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Kiuchi T, Egawa H, et al. Surgery-related morbidity in living donors of right-lobe liver graft: lessons from the first 200 cases. Transplantation 2003;76:158-63. 10.1097/01.TP.0000072372.42396.47 [DOI] [PubMed] [Google Scholar]
- 11.Morioka D, Egawa H, Kasahara M, et al. Outcomes of adult-to-adult living donor liver transplantation: a single institution's experience with 335 consecutive cases. Ann Surg 2007;245:315-25. 10.1097/01.sla.0000236600.24667.a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumitsu K, Hammad A, Kaido T, et al. Validation of Steroid-Free Immunosuppression Regimen after Liver Transplantation. J Clin Gastroenterol Treat 2015;1:008.
- 13.Durand F, Buyse S, Francoz C, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol 2014;60:1151-7. 10.1016/j.jhep.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 14.Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200-5. 10.1016/j.nut.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Misra A, Chowbey P, Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 2009;57:163-70. [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128-40. 10.1016/0196-6553(88)90053-3 [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care 2005;8:61-5. 10.1097/00075197-200501000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr 2014;33:737-48. 10.1016/j.clnu.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Alberino F, Gatta A, Amodio P, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001;17:445-50. 10.1016/S0899-9007(01)00521-4 [DOI] [PubMed] [Google Scholar]
- 21.Wong RJ, Cheung R, Perumpail RB, et al. Diabetes mellitus, and not obesity, is associated with lower survival following liver transplantation. Dig Dis Sci 2015;60:1036-44. 10.1007/s10620-014-3469-8 [DOI] [PubMed] [Google Scholar]
- 22.Werneck M, Afonso RC, Coelho GR, et al. Obese and nonobese recipients had similar need for ventilatory support after liver transplantation. Transplant Proc 2011;43:165-9. 10.1016/j.transproceed.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 23.Hillingsø JG, Wettergren A, Hyoudo M, et al. Obesity increases mortality in liver transplantation--the Danish experience. Transpl Int 2005;18:1231-5. 10.1111/j.1432-2277.2005.00206.x [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Renner EL, Selzner N, et al. The impact of obesity as determined by modified body mass index on long-term outcome after liver transplantation: Canadian single-center experience. Transplant Proc 2013;45:2288-94. 10.1016/j.transproceed.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 25.Heuer M, Kaiser GM, Kahraman A, et al. Liver transplantation in nonalcoholic steatohepatitis is associated with high mortality and post-transplant complications: a single-center experience. Digestion 2012;86:107-13. 10.1159/000339344 [DOI] [PubMed] [Google Scholar]
- 26.Holecek M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition 2013;29:1186-91. 10.1016/j.nut.2013.01.022 [DOI] [PubMed] [Google Scholar]
- 27.Kim EY, Kim YS, Seo JY, et al. The Relationship between Sarcopenia and Systemic Inflammatory Response for Cancer Cachexia in Small Cell Lung Cancer. PLoS One 2016;11:e0161125. 10.1371/journal.pone.0161125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reisinger KW, Derikx JP, van Vugt JL, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr 2016;35:924-7. 10.1016/j.clnu.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 29.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772-83. 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- 30.Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal Study. Obesity (Silver Spring) 2009;17:2082-8. 10.1038/oby.2009.109 [DOI] [PubMed] [Google Scholar]
- 31.Baracos VE, Reiman T, Mourtzakis M, et al. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr 2010;91:1133S-7S. 10.3945/ajcn.2010.28608C [DOI] [PubMed] [Google Scholar]
- 32.Lim S, Kim JH, Yoon JW, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652-4. 10.2337/dc10-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishikawa H, Shiraki M, Hiramatsu A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951-63. [DOI] [PubMed] [Google Scholar]
- 34.Dare AJ, Plank LD, Phillips AR, et al. Additive effect of pretransplant obesity, diabetes, and cardiovascular risk factors on outcomes after liver transplantation. Liver Transpl 2014;20:281-90. 10.1002/lt.23818 [DOI] [PubMed] [Google Scholar]
- 35.Reichman TW, Therapondos G, Serrano MS, et al. "Weighing the risk": Obesity and outcomes following liver transplantation. World J Hepatol 2015;7:1484-93. 10.4254/wjh.v7.i11.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka T, Renner EL, Selzner N, et al. The impact of obesity as determined by modified body mass index on long-term outcome after liver transplantation: Canadian single-center experience. Transplant Proc 2013;45:2288-94. 10.1016/j.transproceed.2012.11.009 [DOI] [PubMed] [Google Scholar]