Abstract
Background: Understanding factors associated with treatment intensity may help ensure higher value healthcare.
Objective: To investigate factors associated with Medicare costs among prospectively identified, seriously ill older adults and examine if baseline prognosis influences the impact of these factors.
Design/Subjects: Prospective observation of Health and Retirement Study cohort with linked Medicare claims.
Measurements: We identified people with incident serious illness (a serious medical condition, for example, metastatic cancer or functional impairment); calculated subjects' one-year mortality risk; and then followed them for one year. We examined relationships between individual and regional characteristics and total Medicare costs, and then stratified analyses by one-year mortality risk: low, moderate, and high.
Results: From 2002 to 2012, 5208 subjects had incident serious illness: mean age 78 years, 60% women, 76% non-Hispanic white, and 39% hospitalized in the past year. During one-year follow-up, 12% died. Total Medicare costs averaged $20,607. In multivariable analyses, indicators of poor health (e.g., cancer, advanced heart and lung disease, multimorbidity, functional impairment, and others) were significantly associated with higher costs (p < 0.05). However, among those with high mortality risk, health-related variables were not significant. Instead, African American race (rate ratio [RR] 1.56) and moderate-to-high spending regions (RR 1.31 and 1.54, respectively) were significantly associated with higher costs. For this high-risk population, residence in high-spending regions was associated with $31,476 greater costs among African Americans, and $11,162 among other racial groups, holding health constant.
Conclusions: Among seriously ill older adults, indicators of poor health are associated with higher costs. Yet, among those with poorest prognoses, nonmedical characteristics—race and regional practice patterns—have greater influence on treatment. This suggests there may be novel opportunities to improve care quality and value by assuring patient-centered, goal-directed care.
Keywords: : disparities, health services research, Medicare
Introduction
By 2040, it is projected that 1 out of every 3 dollars spent in the United States will be spent on healthcare.1 Healthcare reform debates have highlighted concentrated healthcare costs among a small proportion of the population and considered policy proposals to identify this “high-cost” group and reduce their costs.2 While this discussion of the high-cost population in the United States has often focused on the population at the end of life, among adults with the highest healthcare costs, only 11% are in their last year of life.3,4 Yet, most research has used decedent samples to study healthcare costs and treatment intensity for the seriously ill.
Evidence from decedent studies is difficult to interpret and apply to patient care for three reasons. First, retrospective studies of decedents are subject to potential bias because the researcher does not observe those who survived despite high risk of death, although the empirical importance of such biases has been questioned.5–14 Second, it is problematic to use analyses of decedents to develop policy or clinical interventions, given the prognostic uncertainty associated with serious illness in real clinical settings. Finally, findings from studies limited to decedents and interventions targeting those at the end of life can only offer modest improvements in care value due to the comparatively small size of the end-of-life population and the limited time frame for intervention.3,15
Palliative care, specialized medical care focused on providing relief from the symptoms and stress of a serious illness, is appropriate at any stage of illness regardless of prognosis. In select patient groups, palliative care interventions have been shown to improve quality of life, manage symptoms, improve communication, support patients and families, and lower costs.16–21 Not all patients need all aspects of specialist-level palliative care services and many patients who could benefit receive no palliative care services at all. Efficiently targeting resources to those who need and will benefit from them most is a critical step in providing appropriate, value-driven, and patient-centered care to the seriously ill population.
Using a nationally representative, longitudinal cohort, this study sought to investigate factors associated with treatment intensity (i.e., Medicare costs, and hospital and intensive care unit [ICU] admissions) among prospectively identified, seriously ill older adults, and to assess whether baseline prognosis may influence these factors' impact on treatment. Our conceptual framework22 postulates that patient determinants include (1) factors related to medical need, such as medical conditions and functional status, and (2) nonclinical factors, including socioeconomic status, social supports, and other characteristics rarely measured in clinical settings.7,8,10,23–28 As well, we hypothesized that regional factors (i.e., resource supply, local practice patterns), which are sometimes associated with lower quality or treatment inconsistent with patient preferences,29–39 also affect treatment intensity.31–34,38–41 By illuminating the potential influence of nonclinical factors—those things not representing medical need—these results will help identify people at greatest risk for high treatment intensity, which for some may also be associated with lower quality or preference-discordant care. Such findings could reveal opportunities for clinical or educational interventions to promote person-centered goal-directed treatment.
Methods
Data sources
The study sample is drawn from the Health and Retirement Study (HRS). First assembled in 1992, HRS is a National Institute on aging-funded, longitudinal, and nationally representative cohort study of adults older than 50 years.42 Serial “Core” interviews are conducted every two years and response rates have consistently exceeded 86%. A proxy, usually spouse or adult child, may complete the interview if the subject is unable. The HRS data include a rich array of demographic, social, financial, insurance, health, function, and other characteristics. Over 80% of HRS participants have authorized linkage of their HRS data with Medicare claims. Medicare claims and ICD9 diagnosis codes are used to identify subjects' medical conditions, hospital and ICU admissions, and total Medicare spending. By linking subjects' zip code to hospital referral region (HRR), we captured regional healthcare spending data from the Dartmouth Atlas of Healthcare.
Sample and enrollment
Using linked HRS-Medicare data, we prospectively identified a cohort of people with “incident serious illness,” defined as: first observed diagnosis of one or more severe medical conditions or new functional impairment (i.e., receiving assistance with any of six basic activities of daily living [ADL]).43 Severe medical conditions included the following: cancer (metastatic or hematologic), end-stage renal disease (ESRD), dementia, advanced liver disease or cirrhosis, diabetes with severe complications (ischemic heart disease, peripheral vascular disease, renal disease), hip fracture, advanced chronic obstructive pulmonary disease (COPD) or interstitial lung disease only if using home oxygen or hospitalized for the condition, and advanced congestive heart failure only if hospitalized for the condition. Prior work demonstrated that this seriously ill population has high Medicare costs and high rate of hospitalization, yet a wide range of prognoses, as 87% survive to the next year.43
At each HRS Core interview, beginning in 2002 and continuing through 2012, every subject with continuous Medicare Parts A and B fee-for-service coverage over the preceding 12 months was eligible for enrollment (n = 11,577). Subjects were followed with biennial interviews through 2012 or death resulting in up to 35,215 episodes of eligibility. We enrolled each subject once, at the first interview in which they met the serious illness definition, and then followed them for one year from that date.
Drawing on existing literature and prognostic indices, we captured all available baseline variables that could feasibly be assessed in a healthcare setting to calculate each subject's risk of one-year mortality.44–48 We constructed a multivariable logit model of actual one-year mortality status based on age, sex, race/ethnicity, U.S. geographic region, marital status, self-reported health, functional status, caregiving needs, body mass index <25, smoking status, history of hospitalization, medical diagnoses, and multimorbidity. Based on the model coefficients, we calculated for each subject a predicted one-year mortality risk. In all analyses, the sample was considered in full and stratified by risk of one-year mortality: low <10%, moderate 10–25%, or high >25%. Details of the estimation are provided in Appendix 1.
Outcome measures
Subjects were followed for up to one year or until death to assess medical treatment intensity. The primary outcome measure of treatment intensity was total Medicare expenditures, adjusted for inflation to 2012 U.S. dollars and for regional pricing differences based on the Centers for Medicare and Medicaid Services (CMS) wage index.49 Secondary outcome measures were more than one hospitalization and any ICU admission for up to one year or death.
Independent variables
We selected patient-level and regional variables that could serve as empirical measures of each construct in the conceptual model of determinants of treatment intensity.22 Patient-level variables reflecting health status included severe medical conditions (described above); comorbidities, identified using Elixhauser ICD9 criteria50 and Medicare claims from the 12 months preceding enrollment; and HRS interview measures: nursing home residence, self-rated health and functional status (i.e., need for any assistance with six basic ADLs). Patient-level nonhealth variables collected from the HRS interview included age, race and ethnicity, sex, marital status, education level, net asset value, non-Medicare insurance coverage (Medicaid, Veterans Administration, Medigap), religiosity, and having relatives live nearby (a proxy for social support). Regional variables included U.S. region (Northeast, South, Midwest, West) and, drawn from the Dartmouth Atlas, mean HRR age-sex-race-adjusted Medicare spending (measured with standardized national prices) for beneficiaries at the end of life,51 categorized as areas with the lowest 25% of spending (reference), middle 50%, and areas with the highest 25% of spending patterns.
Statistical analyses and sensitivity tests
We provide descriptive statistics to report characteristics and one-year outcomes for the full seriously ill cohort, and for subgroups stratified by probability of low, moderate, and high one-year mortality. We then examined bivariate relationships between subjects' social, functional, medical, and regional characteristics and total Medicare costs over one year.
Next, we performed multivariable regression analyses of total costs (generalized linear models with gamma distribution and log link) using the full cohort and stratified by risk of one-year mortality. Gamma coefficients were exponentiated to transform them into rate ratio (RR) estimates. The secondary outcomes (more than one hospitalization and any ICU admissions) were modeled using multivariable logit regression with the full cohort and then stratified by risk of one-year mortality.
In sensitivity analyses of total costs, we examined the effect of each health-related and nonhealth-related variable separately using the full seriously ill cohort and again stratified by probability of low, medium, and high one-year mortality. The models first assessed each health-related variable, adjusted for the nonhealth variables, and then the opposite, each nonhealth variable, adjusted for the health variables.
We used SAS 9.4 (SAS Institute, Cary, North Carolina) and STATA 13 (STATA Corp., College Station, TX) for data management and statistical analyses, respectively. The study was approved by the Mount Sinai School of Medicine Institutional Review Board, the HRS Data Confidentiality Committee, and the CMS Privacy Board.
Results
From 2002 through 2012, 11,577 unique individuals were eligible for enrollment and 5208 were identified with incident serious illness: the first occurrence of a severe medical illness or functional impairment. Mean age was 78 years, 60% were women and 76% non-Hispanic white. Seventy-six percent had at least one serious medical illness, 45% had one or more ADL impairment, and 39% had experienced at least one unplanned hospital admission in the past year. During the one-year follow-up period, 13% of the cohort died (Table 1). Using these baseline data, we calculated risk of one-year mortality (Appendix 1) and examined bivariate associations between one-year total Medicare costs and subjects' personal and regional characteristics (Appendix 2).
Table 1.
Characteristics of Cohort with Incident Serious Illness, Stratified by Probability of Death at One Year
| Full cohort n = 5208 | Low risk of death (<10%) n = 3072 | Moderate risk of death (10–25%) n = 1371 | High risk of death (>25%) n = 765 | |
|---|---|---|---|---|
| Age, mean | 78.1 | 75.5 | 80.5* | 84.4* |
| Female, % | 60.2 | 63.5 | 57.4* | 52.3* |
| Non-Hispanic white, % | 75.6 | 74.1 | 77.5* | 78.2* |
| African American, % | 15.1 | 15.7 | 14.4 | 14.4 |
| Hispanic ethnicity, % | 7.5 | 8.6 | 6.1* | 5.1* |
| Married, % | 46.1 | 52.3 | 39.2* | 33.3* |
| Education, less than high school, % | 35.6 | 33.1 | 38.9* | 39.6* |
| Net asset value, mean, 2012$ | 411,152.60 | 425,145.20 | 398,512.80 | 377,615.60 |
| Medigap insurance, % | 58.7 | 59.2 | 57.3 | 59.1 |
| Medicaid, % | 21.6 | 18.9 | 24.2* | 27.8* |
| Veterans Health Administration, % | 6.4 | 7.1 | 5.8 | 4.6* |
| Nursing home resident, % | 13.3 | 3.2 | 19.0* | 43.5* |
| Self-rated health, poor/fair, % | 59.2 | 44.5 | 74.8* | 90.5* |
| Dementia, % | 22 | 17.9 | 24.2* | 34.2* |
| Metastatic cancer, % | 9 | 3.6 | 12.5* | 24.4* |
| End-stage renal disease, % | 11.9 | 11.1 | 10.5 | 17.3* |
| Advanced heart failure*, % | 6.5 | 2.7 | 9.6* | 16.2* |
| Advanced COPD*, % | 14.9 | 12 | 18.7* | 19.7* |
| Diabetes with end-organ complications, % | 19.8 | 25.1 | 12.1* | 12.5* |
| Advanced liver disease/cirrhosis, % | 5.6 | 7.1 | 3.1* | 4.2* |
| Hip fracture, % | 3 | 2.5 | 3 | 5.1* |
| Three or more comorbidities, % | 84.2 | 79 | 89.9* | 95.2* |
| Any indicator of serious medical illness, % | 75.7 | 74.3 | 74.8 | 83.1* |
| Any ADL impairment, % | 45.2 | 33.6 | 54.4* | 75.4* |
| SMI and ADL impairment, % | 20.9 | 7.9 | 29.2* | 58.6* |
| Any hospital admission in past 12 months, % | 39.3 | 27.8 | 50.1* | 66.3* |
| Resides in region with lowest 25% spending, % | 15.8 | 16.5 | 14.1* | 15.9 |
| Resides in region with middle 50% spending, % | 51.6 | 50.4 | 53.1 | 53.7 |
| Resides in region with top 25% spending, % | 32.6 | 33.1 | 32.8 | 30.3 |
| Outcome measures over one year | ||||
| Total Medicare costs, mean 2012$ | 20,607 | 16,146 | 25,870* | 29,089* |
| Total Medicare costs, median | 7776 | 5615 | 12,498 | 15,716 |
| Inpatient Medicare costs, mean | 8637 | 6925 | 11,104* | 11,087* |
| Hospital nights, mean | 4.7 | 3.5 | 6.2* | 6.8* |
| Any hospital admission, % | 39.1 | 31.8 | 48.7* | 51.2* |
| Multiple hospital admissions, % | 17.8 | 13.8 | 23.3* | 24.3* |
| Multiple Emergency Department visits, % | 24 | 19.9 | 29.3* | 30.8* |
| Any ICU days, % | 17.4 | 14.1 | 22.5* | 21.6* |
| Hospice admission, % | 6.4 | 1.6 | 7.7* | 23.3* |
| Died within one year, % | 12.8 | 4.1 | 16.8* | 40.7* |
| Died in the hospital within one year, %a | 4.3 | 1.7 | 6.6* | 10.8* |
Advanced heart failure = heart failure diagnosis in claims, including primary diagnosis for at least one inpatient admission. Advanced chronic obstructive lung disease = COPD diagnosis in claims, including home oxygen use and/or primary diagnosis for at least one inpatient admission.
A statistically significant difference (p < = 0.05) between the higher and low probability of death groups.
Percent died in the hospital among samples, including survivors, excluding 18 who died within one year of core interview but unknown death location.
COPD, chronic obstructive pulmonary disease; ADL, activities of daily living; SMI, serious medical illness.
Subjects were categorized as having low, moderate, or high probability of death. Compared to those with low probability of death, subjects with higher risk were older, less likely to be married, more likely to report poor or fair health, and more likely to have functional impairments, among other differences (all p-values <0.05, Table 1). Those with higher probability of death experienced higher Medicare costs across all categories of spending and had higher rates of admission to the emergency department, hospital, and ICU over the one-year follow-up period. The observed one-year mortality rate in each group was 4% (low), 17% (moderate), and 41% (high).
In multivariable analyses of total Medicare costs using the full sample of seriously ill older adults, specific indicators of poor health (e.g., cancer, ESRD, advanced heart failure and COPD, three or more comorbidities, functional impairment) were significantly associated with higher costs (all p-values <0.05, Table 2). Residing in a high-spending region was also independently associated with higher costs. In models limited to those with low and moderate risk of death in one year, a similar pattern of relationships persisted. Factors indicating poorer health and function (with the exception of dementia) were associated with higher costs.
Table 2.
Factors Associated with Total Medicare Spending over 12 Months, among Cohort with Incident Serious Illness, Stratified by Probability of Death
| Full cohort n = 5208 | Low risk of death (<10%) n = 3072 | Moderate risk of death (10–25%) n = 1371 | High risk of death (>25%) n = 765 | |
|---|---|---|---|---|
| Rate ratios, multivariate analyses | ||||
| Demographics, social, and insurance | ||||
| Age | 1.00 | 0.99 | 0.98* | 0.98* |
| Female | 0.87* | 0.88 | 0.98 | 0.92 |
| African American race | 1.08 | 0.98 | 1.13 | 1.56* |
| Hispanic ethnicity | 0.97 | 0.93 | 1.05 | 1.39 |
| High school degree | 0.96 | 0.98 | 0.97 | 0.98 |
| Net asset value, lowest quartile | 1.02 | 0.99 | 1.00 | 1.04 |
| Religion, very important | 1.01 | 0.97 | 1.07 | 1.16 |
| Relatives nearby | 0.94 | 0.92 | 0.95 | 0.99 |
| Married | 0.91 | 0.94 | 0.85 | 1.15 |
| Nursing home resident | 1.11 | 1.18 | 1.04 | 0.89 |
| Medicaid | 1.17* | 1.21 | 1.02 | 1.38* |
| Veterans Health Administration | 1.08 | 1.05 | 1.17 | 0.99 |
| Medigap insurance | 1.07 | 1.02 | 1.11 | 1.27* |
| Health characteristics | ||||
| Dementia | 0.91 | 0.97 | 0.89 | 0.88 |
| Metastatic cancer | 1.60* | 1.33 | 1.30 | 1.07 |
| End-stage renal disease | 1.73* | 1.89* | 1.58* | 1.52* |
| Advanced heart failure* | 1.44* | 1.62* | 1.32* | 1.10 |
| Advanced COPD* | 1.40* | 1.45* | 1.49* | 1.11 |
| Diabetes with end-organ complications | 1.23* | 1.42* | 1.23 | 1.07 |
| Advanced liver disease/cirrhosis | 0.99 | 1.02 | 0.98 | 1.16 |
| Hip fracture | 1.30* | 1.42 | 1.40 | 0.89 |
| Self-rated health: poor/fair | 1.38* | 1.31* | 1.17 | 0.99 |
| ADL impairment | 1.33* | 1.39* | 1.32* | 1.06 |
| Informal caregiving, 60+ hours/month | 1.26* | 1.43* | 0.97 | 1.08 |
| Three or more comorbidities | 1.49* | 1.42* | 1.47* | 1.12 |
| Region | ||||
| Midwest | 1.02 | 1.10 | 0.85 | 0.93 |
| West | 0.84* | 0.90 | 0.72* | 0.72 |
| South | 1.05 | 1.10 | 1.06 | 0.85 |
| Resides in region with middle 50% end-of-life spending | 1.13* | 1.05 | 1.25* | 1.31* |
| Resides in region with top 25% EOL spending | 1.17* | 1.06 | 1.31* | 1.54* |
Incident serious illness defined as first diagnosis of a serious medical condition (e.g., metastatic cancer) or loss of independence in one or more ADLs.43 Rate ratios = exponentiated coefficients from independent multivariable generalized linear models regressions with gamma family and log link on full sample and each stratum of death probability. Advanced heart failure = CHF diagnosis in claims, including primary diagnosis for ≥1 inpatient admission. Advanced chronic obstructive lung disease = COPD diagnosis in claims, including home oxygen use and/or primary diagnosis for at least one inpatient admission.
p ≤ 0.05.
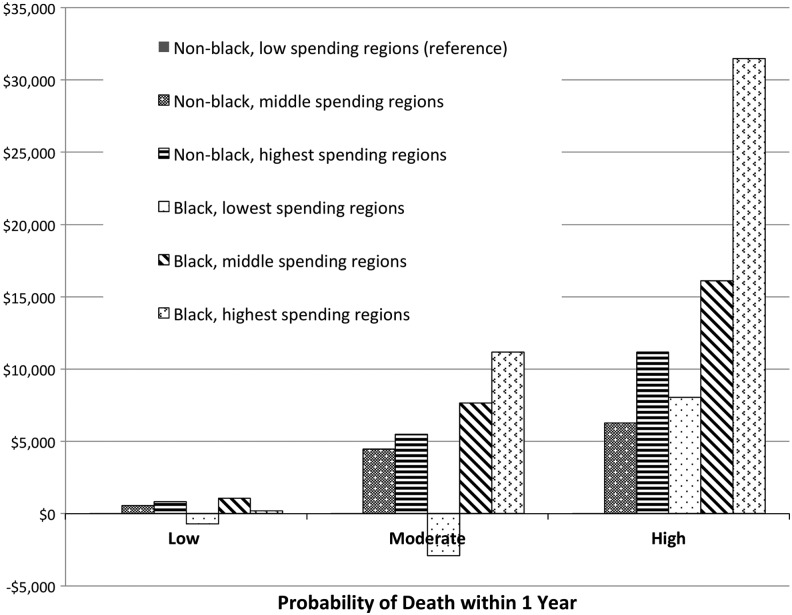
A different pattern emerged in the model limited to those with high risk of death in one year. For this group, health-related variables (excluding ESRD) were not significantly associated with costs. Instead, costs were negatively associated with older age (RR 0.98 per year, p < 0.01), and positively associated with African American race (RR 1.56, p < 0.01) and living in higher spending regions (RR 1.31, p = 0.03 and RR 1.54, p < 0.01 for the middle 50% and highest 25% HRRs, respectively). In the high-risk group, this represents a higher average marginal cost of $16,122 and $31,476 for African Americans residing in middle-spending and high-spending HRRs, respectively, and $11,162 for non-African American subjects in high-spending HRRs (Fig. 1).
FIG. 1.
Average marginal effect of region and race on total Medicare costs, relative to non-black, low spending region, stratified by probability of death.
Analyses of secondary outcomes followed similar patterns. Using the full sample of seriously ill older adults, with multivariable analyses explaining more than one hospitalization within one year, poorer health and function were significantly associated with hospitalizations (Table 3). Residing in the top 75% of spending regions was also associated with multiple hospitalizations (odds ratio [OR] 1.35, p = 0.01 and OR 1.25, p = 0.07 for middle and highest spending HRRs, respectively). In the model limited to those with high risk of death in one year, a different pattern again emerged. As in the overall cost model, health-related variables (excluding ESRD) were no longer significantly associated with multiple hospitalizations, while Medicaid (OR 1.93, p = 0.02), VA coverage (OR 2.59, p = 0.02), and higher spending regions were associated with multiple hospitalizations (OR 1.67, p = 0.07 and OR 1.83, p = 0.05 for middle and highest spending HRRs, respectively). The models of any ICU admission also followed similar patterns, except that insurance status was no longer associated and higher regional spending areas were associated with ICU admissions across the moderate-risk (middle 50% OR 1.57, p = 0.05; highest 25% OR 1.89, p = 0.01) and high-risk groups (middle 50% OR 1.92, p = 0.03; highest 25% OR 1.60, p = 0.15) (Table 4). Sensitivity analyses examining the effect of each health-related and nonhealth-related variable separately revealed the same patterns of relationships as in the fully adjusted models (data not shown).
Table 3.
Factors Associated with Having More Than One Hospital Admission over 12 Months, among Cohort with Incident Serious Illness, Stratified by High and Low Probabilities of Death
| Full cohort n = 5208 | Low risk of death (<10%) n = 3072 | Moderate risk of death (10–25%) n = 1371 | High risk of death (>25%) n = 765 | |
|---|---|---|---|---|
| Odds ratios, multivariate analyses | ||||
| Demographics, social, and insurance | ||||
| Age | 1.00 | 1.01 | 0.99 | 0.98 |
| Female | 0.89 | 0.98 | 0.89 | 0.81 |
| African American race | 1.10 | 0.94 | 1.33 | 1.48 |
| Hispanic ethnicity | 0.63* | 0.52* | 0.71 | 1.51 |
| High school degree | 0.88 | 0.84 | 0.95 | 0.95 |
| Net asset value, lowest quartile | 1.25* | 1.30* | 1.15 | 1.15 |
| Religion, very important | 1.02 | 1.02 | 0.93 | 1.22 |
| Relatives nearby | 0.85* | 0.82 | 0.87 | 0.85 |
| Married | 0.89 | 1.05 | 0.73 | 0.98 |
| Nursing home resident | 0.96 | 0.92 | 1.03 | 0.72 |
| Medicaid | 1.21 | 1.24 | 0.95 | 1.93* |
| Veterans Health Administration | 1.51* | 1.42 | 1.40 | 2.59* |
| Medigap insurance | 1.08 | 1.01 | 1.13 | 1.56 |
| Health characteristics | ||||
| Dementia | 0.84 | 1.01 | 0.78 | 0.73 |
| Metastatic cancer | 1.34* | 0.81 | 1.46 | 0.86 |
| End-stage renal disease | 1.58* | 1.65* | 1.30 | 2.26* |
| Advanced heart failure* | 2.16* | 1.72 | 2.85* | 1.46 |
| Advanced COPD* | 1.54* | 1.62* | 2.06* | 0.86 |
| Diabetes with end-organ complications | 1.28* | 1.57* | 1.34 | 0.99 |
| Advanced liver disease/cirrhosis | 1.17 | 1.12 | 1.34 | 1.74 |
| Hip fracture | 0.99 | 1.11 | 1.20 | 0.68 |
| Self-rated health: poor/fair | 1.50* | 1.45* | 1.32 | 0.89 |
| ADL impairment | 1.21 | 1.19 | 1.59* | 0.96 |
| Informal caregiving, 60+ hours/month | 1.50* | 1.81* | 1.16 | 1.21 |
| Three or more comorbidities | 1.84* | 1.73* | 1.69* | 1.92 |
| Region | ||||
| Midwest | 0.87 | 0.83 | 0.76 | 0.95 |
| West | 0.71* | 0.66 | 0.78 | 0.72 |
| South | 0.85 | 0.83 | 0.93 | 0.70 |
| Resides in region with middle 50% EOL spending | 1.35* | 1.19 | 1.49 | 1.67 |
| Resides in region with top 25% EOL spending | 1.25 | 0.99 | 1.50 | 1.83* |
Incident serious illness defined as first diagnosis of a serious medical condition (e.g., metastatic cancer) or loss of independence in one or more ADLs.43 Odds ratios represent exponentiated coefficients from independent multivariable logistic regressions on full sample and each stratum of death probability. Advanced heart failure = CHF diagnosis in claims, including primary diagnosis for at least one inpatient admission. Advanced chronic obstructive lung disease = COPD diagnosis in claims, including home oxygen use and/or primary diagnosis for at least one inpatient admission.
p ≤ 0.05.
Table 4.
Factors Associated with At Least One Intensive Care Unit Admission over 12 Months, among Cohort with Incident Serious Illness, Stratified by High and Low Probabilities of Death
| Full cohort n = 5208 | Low risk of death (<10%) n = 3072 | Moderate risk of death (10–25%) n = 1371 | High risk of death (>25%) n = 765 | |
|---|---|---|---|---|
| Odds ratios, multivariate analyses | ||||
| Demographics, social, and insurance | ||||
| Age | 1.00 | 1.00 | 0.99 | 0.98 |
| Female | 0.74* | 0.80 | 0.78 | 0.67 |
| African American race | 0.94 | 0.80 | 0.91 | 1.66 |
| Hispanic ethnicity | 1.00 | 0.89 | 0.91 | 2.80* |
| High school degree | 1.01 | 0.99 | 1.03 | 1.14 |
| Net asset value, lowest quartile | 1.06 | 1.04 | 1.22 | 0.80 |
| Religion, very important | 1.05 | 1.05 | 1.03 | 1.25 |
| Relatives nearby | 0.88 | 0.86 | 0.92 | 0.83 |
| Married | 0.93 | 1.01 | 0.96 | 0.96 |
| Nursing home resident | 0.87 | 1.32 | 0.91 | 0.52* |
| Medicaid | 1.14 | 1.32 | 0.80 | 1.54 |
| Veterans Health Administration | 1.23 | 1.27 | 1.14 | 1.27 |
| Medigap insurance | 0.94 | 1.01 | 0.85 | 1.04 |
| Health characteristics | ||||
| Dementia | 0.86 | 1.25 | 0.73 | 0.67 |
| Metastatic cancer | 1.09 | 1.80 | 0.73 | 0.48* |
| End-stage renal disease | 1.63* | 1.92* | 1.49 | 1.93* |
| Advanced heart failure* | 1.94* | 1.86* | 2.11* | 1.11 |
| Advanced COPD* | 1.66* | 1.85* | 1.71* | 1.34 |
| Diabetes with end-organ complications | 1.25* | 1.75* | 1.10 | 1.36 |
| Advanced liver disease/cirrhosis | 0.99 | 1.27 | 1.26 | 0.60 |
| Hip fracture | 0.72 | 0.81 | 1.19 | 0.36 |
| Self-rated health: poor/fair | 1.42* | 1.29* | 1.22 | 1.26 |
| ADL impairment | 1.15 | 1.44* | 1.12 | 0.81 |
| Informal caregiving, 60+ hours/month | 1.27* | 1.64* | 0.91 | 0.90 |
| Three or more comorbidities | 1.99* | 1.71* | 2.95* | 1.31 |
| Region | ||||
| Midwest | 1.15 | 1.16 | 0.93 | 1.31 |
| West | 1.00 | 0.95 | 0.90 | 1.14 |
| South | 1.25 | 1.42* | 1.10 | 1.01 |
| Resides in region with middle 50% EOL spending | 1.27* | 1.00 | 1.57* | 1.92* |
| Resides in region with top 25% EOL spending | 1.29* | 0.99 | 1.89* | 1.60 |
Incident serious illness defined as first diagnosis of a serious medical condition (e.g., metastatic cancer) or loss of independence in one or more ADLs.43 Odds ratios represent exponentiated coefficients from independent multivariable logistic regressions on full sample and each stratum of death probability. Advanced heart failure = CHF diagnosis in claims, including primary diagnosis for at least one inpatient admission. Advanced chronic obstructive lung disease = COPD diagnosis in claims, including home oxygen use and/or primary diagnosis for at least one inpatient admission.
p ≤ 0.05.
Discussion
Among a prospectively identified, seriously ill cohort of older adults, factors associated with higher Medicare spending (i.e., advanced illness, functional impairment, multimorbidity, and living in higher spending regions) are similar to those found in previously published mortality follow-back studies.8 In analyses stratified by prognosis, characteristics indicating poorer health are associated with higher treatment intensity in low and moderate mortality risk groups. However, for those with highest risk of death, measures of illness and function were no longer significantly associated with high-intensity treatment. Instead, race and regional spending patterns predict spending and intensive hospital treatment for this group. These data suggest that in the setting of poor prognosis, nonmedical characteristics (i.e., factors likely related to discretionary treatment decisions and not medical need) have greater influences on treatment intensity. Also, more directly relevant to patients' care experiences, we found that among the high-risk group, 30% of African Americans living in high-cost areas were admitted to an ICU in the year following enrollment (compared to 14% of non-blacks in low-spending areas), while 45% had multiple Emergency Department visits and 43% had multiple hospitalizations (compared to 25% and 16%, respectively). Multivariable analyses of these secondary outcomes confirmed the direction of these relationships, but did not reach statistical significance, likely due to inadequate power.
We suspect seriously ill patients and their healthcare providers in the low and moderate mortality risk groups are most often encountering medical decisions that are relatively straight-forward, guideline-based, and associated with predictable outcomes (e.g., beginning first-line chemotherapy for metastatic breast cancer). In these groups, indicators of greater illness are associated with greater costs. Those in the high-risk group, however, are more likely to face complex medical decisions that cannot be based upon clinical guidelines alone, are more often best determined by individual patient values and goals for care,52–54 and are typically associated with less predictable outcomes (e.g., ventilator support for respiratory failure in the setting of advanced heart failure, multimorbidity, and functional impairment). Most clinicians, however, lack training in the core palliative care knowledge and skills (symptom management, advanced communication skills, care coordination) that are needed to provide optimal management of these complex patients and hospital systems typically do not provide the resources to overcome these deficiencies. Thus, our findings suggest that nonindividualized care plans, that is, the “glide path,” and local practice patterns may influence care more than patient preference. These findings also support the need for further intervention and research. For example, a hospital or healthcare system could implement a program of triggered palliative care consultations, or require clinicians serving high-risk patients to complete advanced communication skills training.19,55–57 Quality metrics could be developed to assess the concordance of treatment with patient-identified goals of care and providers could be held accountable for high standards through public reporting or payment reform. Any intervention implemented will require further research to evaluate its effect on promoting person-centered, goal-directed care for all seriously ill persons.58–61
This study also revealed the association of other nonhealth factors and treatment intensity within the high-risk group. Consistent with prior research, the African American race was strongly associated with higher costs; further investigation is needed to determine whether this finding reflects patients' preferences or racial disparities at the provider level.8,10,26,62–67 While we hypothesized that higher levels of wealth and education would be associated with lower treatment intensity we did not find significant independent relationships. Medicaid enrollment, an alternative proxy measure for socioeconomic status, however, was significantly associated with higher costs in the high-risk group.
There are limitations to the study. Survey and claims data have limitations in assessing severity of disease. Despite the richness of HRS data, we could not include laboratory or physiologic measures (e.g., ejection fraction, creatinine) in the mortality risk model, nor could we include physician's prognostic estimates. By applying prospective enrollment criteria only at the biennial HRS survey dates, we may not have included those with rapidly progressive illness. As the study data did not include individual patient treatment preferences, we were unable to assess whether treatments provided were consistent with patient goals. Similarly, data were not available to assess issues of culture, trust, or implicit racial biases, which also may influence discretionary medical decision making in this population. Finally, it may appear surprising that diagnoses and multimorbidity were no longer independently predictive of treatment intensity in the high-risk patient group. However, the predictive prognostic model that sorted individuals into low-, moderate-, and high-risk categories depended on these same factors, so that the incremental effect of one or more of these variables in the highest risk category is likely minimal.
Conclusions
Our findings suggest new opportunities and incentives to improve care quality and value for the high-risk geriatric population. Patients at greatest risk for receipt of nonindividualized (i.e., determined by local practice patterns), high-intensity treatments can be prospectively identified. Furthermore, our study highlights the need to expand training in core palliative care knowledge and skills (symptom management, communication skills, care coordination) for all clinicians caring for this population to enhance their ability to elicit patient goals and recommend concordant treatments.49 Finally, automatic identification of this patient population should be considered to facilitate referral to specialist palliative care services, which are specifically designed to care for these patients and have shown promising improvements in patient and family outcomes.16,21,49,55
Acknowledgments
A.S.K. receives support from the National Institute on Aging (NIA) (K23-AG040774), the American Federation for Aging Research, and the National Palliative Care Research Center. J.S.S. receives support from the NIA (P01-AG019783). The Health and Retirement Study is funded by the NIA (U01 AG009740) and the Social Security Administration, and is performed at the Institute for Social Research, University of Michigan.
Appendix Table 1.
Multivariable Regression of One-Year Mortality
| n = 5215 | |
|---|---|
| Age | 1.04* |
| Female | 0.56* |
| African American race | 1.00 |
| Hispanic ethnicity | 0.76 |
| Married | 0.89 |
| Nursing home resident | 1.56* |
| Dementia | 1.02 |
| Metastatic cancer | 5.44* |
| End-stage renal disease | 1.44* |
| Advanced heart failure | 1.96* |
| Advanced COPD | 1.64* |
| Diabetes with end-organ complications | 0.96 |
| Advanced liver disease/cirrhosis | 1.532 |
| Hip fracture | 0.77 |
| Three or more comorbidities | 1.47* |
| Body mass index, <25 | 1.92* |
| Current smoker | 1.32 |
| Self-rated health: poor/fair | 1.88* |
| Difficulty bathing | 0.95 |
| Difficulty managing money | 1.34* |
| Difficulty walking several blocks | 1.85* |
| Difficulty pushing/pulling large objects | 1.16 |
| Any urgent or emergent hospital admission 12 m pre-core | 1.31* |
| Index of ADL impairments | 1.18* |
| Informal caregiving, 60+ hours/month | 1.27* |
| Midwest | 1.28 |
| South | 0.97 |
| West | 1.28 |
Advanced heart failure = heart failure diagnosis in claims, including primary diagnosis for at least one inpatient admission Advanced chronic obstructive lung disease (COPD) = COPD diagnosis in claims, including home oxygen use and/or primary diagnosis for at least one inpatient admission.
p ≤ 0.05.
ADLs, activities of daily living; COPD, chronic obstructive pulmonary disease.
Appendix Table 2.
Unadjusted Bivariate Relationships between Subjects' Social, Functional, Medical, and Regional Characteristics and Total Medicare Costs over One Year, Stratified by Probability of Death at One Year
| Full cohort n = 5208 | Low probability of death (<10%) n = 3072 | Middle probability of death (10–25%) n = 1371 | High probability of death (>25%) n = 765 | |
|---|---|---|---|---|
| Rate ratiosa | ||||
| Demographics, social, and insurance | ||||
| Age | 0.99* | 0.98* | 0.97* | 0.97* |
| Female | 0.88* | 0.93 | 0.99 | 0.80* |
| African American race | 1.31* | 1.12 | 1.39* | 1.78* |
| Hispanic ethnicity | 1.20* | 1.31* | 1.13 | 1.37 |
| High school degree | 0.85* | 0.85* | 0.95 | 0.85 |
| Net asset value, lowest quartile | 1.21* | 1.18* | 1.11 | 1.15 |
| Religion, very important | 0.99 | 0.99 | 1.05 | 1.16 |
| Relatives nearby | 0.97 | 0.92 | 0.99 | 0.97 |
| Married | 0.94 | 0.98 | 0.94 | 1.32* |
| Nursing home resident | 1.14* | 0.87 | 0.96 | 0.73* |
| Medicaid | 1.34* | 1.36* | 1.18 | 1.27* |
| Veterans health administration | 0.96 | 1.01 | 1.01 | 0.91 |
| Medigap insurance | 0.89* | 0.85* | 0.90 | 0.99 |
| Health characteristics | ||||
| Dementia | 0.84* | 0.71* | 0.84* | 0.78* |
| Metastatic cancer | 1.29* | 0.73 | 1.00 | 1.25* |
| End-stage renal disease | 1.79* | 1.71* | 1.73* | 1.80* |
| Advanced heart failure* | 1.53* | 1.31 | 1.29* | 1.23 |
| Advanced COPD* | 1.32* | 1.22* | 1.24* | 1.25 |
| Diabetes with end-organ complications | 1.29* | 1.39* | 1.58* | 1.44* |
| Advanced liver disease/cirrhosis | 0.84 | 0.74* | 1.13 | 1.35 |
| Hip fracture | 1.11 | 0.94 | 1.44 | 0.78 |
| Self-rated health: poor/fair | 1.68* | 1.52* | 1.32* | 1.35 |
| ADL impairment, stable | 1.15* | 1.15 | 1.08 | 0.91 |
| ADL impairment, decline | 1.17* | 1.11 | 1.01 | 0.92 |
| Informal caregiving, 60+ hours/month | 1.37* | 1.43* | 1.04 | 1.18 |
| Three or more comorbidities | 1.83* | 1.57* | 1.75* | 1.75* |
| Region | ||||
| Midwest | 0.98 | 0.98 | 0.90 | 0.92 |
| South | 1.10 | 1.14 | 1.17 | 0.92 |
| West | 0.83* | 0.89 | 0.74* | 0.73 |
| Resides in region with middle 50% end-of-life spending | 1.18* | 1.03 | 1.34* | 1.27 |
| Resides in region with top 25% end-of-life spending | 1.29* | 1.08 | 1.44* | 1.66* |
Rate ratios represent exponentiated coefficients from bivariate generalized linear models with log link and gamma family independently run for each variable in the full sample and within each stratum of death probability. Advanced heart failure = CHF diagnosis in claims, including primary diagnosis for at least one inpatient admission. Advanced chronic obstructive lung disease = COPD diagnosis in claims, including home oxygen use and/or primary diagnosis for at least one inpatient admission.
p ≤ 0.05.
ADL, activities of daily living.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Congressional Budget Office. The Long-Term Outlook for Health Care Spending. www.cbo.gov/ftpdocs/87xx/doc8758/MainText3.1.shtml (Last accessed September2, 2013)
- 2.Blumenthal D, Chernof B, Fulmer T, et al. : Caring for high-need, high-cost patients—an urgent priority. N Engl J Med 2016;375:909–911 [DOI] [PubMed] [Google Scholar]
- 3.Aldridge MD, Kelley AS: The myth regarding the high cost of end-of-life care. Am J Public Health 2015;105:2411–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldridge MD, Kelley AS: Epidemiology of serious illness and high utilization of healthcare. In Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Institute of Medicine, The National Academies Press, Washington, DC, 2015 [Google Scholar]
- 5.Bach PB, Schrag D, Begg CB: Resurrecting treatment histories of dead patients: A study design that should be laid to rest. JAMA 2004;292:2765–2770 [DOI] [PubMed] [Google Scholar]
- 6.Kelley AS: Epidemiology of care for patients with serious illness. J Palliat Med 2013;16:730–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley AS, Ettner SL, Morrison RS, et al. : Disability and decline in physical function associated with hospital use at end of life. J Gen Intern Med 2012;27:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley AS, Ettner SL, Morrison RS, et al. : Determinants of medical expenditures in the last 6 months of life. Ann Intern Med 2011;154:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley AS, Ettner SL, Wenger NS, Sarkisian CA: Determinants of death in the hospital among older adults. J Am Geriatr Soc 2011;59:2321–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tschirhart EC, Du Q, Kelley AS: Factors influencing the use of intensive procedures at the end of life. J Am Geriatr Soc 2014;62:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnato AE, Lynn J: Resurrecting treatment histories of dead patients. JAMA 2005;293:1591–1592 [DOI] [PubMed] [Google Scholar]
- 12.Wennberg JE, Fisher ES, Stukel TA, et al. : Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States. BMJ 2004;328:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setoguchi S, Earle CC, Glynn R, et al. : Comparison of prospective and retrospective indicators of the quality of end-of-life cancer care. J Clin Oncol 2008;26:5671–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks GA, Li L, Sharma DB, et al. : Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst 2013;105:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldridge MD, Kelley AS: Epidemiology of serious illness and high utilization of healthcare. In Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Institute of Medicine, The National Academies Press, Washington, DC, 2015 [PubMed] [Google Scholar]
- 16.Bakitas M, Lyons K, Hegel M, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009;302:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brumley R, Enguidanos S, Jamison P, et al. : Increased satisfaction with care and lower costs: Results of a randomized trial of in-home palliative care. J Am Geriatr Soc 2007;55:993–1000 [DOI] [PubMed] [Google Scholar]
- 18.Jordhoy MS, Fayers P, Loge JH, et al. : Quality of life in palliative cancer care: Results from a cluster randomized trial. J Clin Oncol 2001;19:3884–3894 [DOI] [PubMed] [Google Scholar]
- 19.Rabow M, Kvale E, Barbour L, et al. : Moving upstream: A review of the evidence of the impact of outpatient palliative care. J Palliat Med 2013;16:1540–1549 [DOI] [PubMed] [Google Scholar]
- 20.Smith S, Brick A, O'Hara S, Normand C: Evidence on the cost and cost-effectiveness of palliative care: A literature review. Palliat Med 2014;28:130–150 [DOI] [PubMed] [Google Scholar]
- 21.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–742 [DOI] [PubMed] [Google Scholar]
- 22.Kelley AS, Morrison RS, Wenger NS, et al. : Determinants of treatment intensity for patients with serious illness: A new conceptual framework. J Palliat Med 2010;13:807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shugarman LR, Bird CE, Schuster CR, Lynn J: Age and gender differences in Medicare expenditures at the end of life for colorectal cancer decedents. J Womens Health (Larchmt) 2007;16:214–227 [DOI] [PubMed] [Google Scholar]
- 24.Levinsky NG, Yu W, Ash A, et al. : Influence of age on Medicare expenditures and medical care in the last year of life. JAMA 2001;286:1349–1355 [DOI] [PubMed] [Google Scholar]
- 25.Chandra A, Gruber J, McKnight R: Patient cost-sharing and hospitalization offsets in the elderly. Am Econ Rev 2010;100:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Wright AA, Huskamp HA, et al. : Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med 2009;169:480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JX, Rathouz PJ, Chin MH: Comorbidity and the concentration of healthcare expenditures in older patients with heart failure. J Am Geriatr Soc 2003;51:476–482 [DOI] [PubMed] [Google Scholar]
- 28.Chetty R, Stepner M, Abraham S, et al. : The association between income and life expectancy in the United States, 2001–2014. JAMA 2016;315:1750–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasaitis L, Fisher ES, Skinner JS, Chandra A: Hospital quality and intensity of spending: Is there an association? Health Aff (Millwood) 2009;28:w566–w572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skinner J, Chandra A, Goodman D, Fisher ES: The elusive connection between health care spending and quality. Health Aff (Millwood) 2009;28:w119–w123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher ES, Wennberg DE, Stukel TA, et al. : The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Ann Intern Med 2003;138:273–287 [DOI] [PubMed] [Google Scholar]
- 32.Fisher ES, Wennberg DE, Stukel TA, et al. : The implications of regional variations in Medicare spending. Part 2: Health outcomes and satisfaction with care. Ann Intern Med 2003;138:288–298 [DOI] [PubMed] [Google Scholar]
- 33.Mittler JN, Landon BE, Fisher ES, et al. : Market variations in intensity of Medicare service use and beneficiary experiences with care. Health Serv Res 2010;45:647–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wennberg JE, Bronner K, Skinner JS, et al. : Inpatient care intensity and patients' ratings of their hospital experiences. Health Aff (Millwood) 2009;28:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchard RS, Fisher ES, Teno JM, et al. : Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc 1998;46:1242–1250 [DOI] [PubMed] [Google Scholar]
- 36.Teno JM, Fisher ES, Hamel MB, et al. : Medical care inconsistent with patients' treatment goals: Association with 1-year Medicare resource use and survival. J Am Geriatr Soc 2002;50:496–500 [DOI] [PubMed] [Google Scholar]
- 37.Teno JM, Gruneir A, Schwartz Z, et al. : Association between advance directives and quality of end-of-life care: A national study. J Am Geriatr Soc 2007;55:189–194 [DOI] [PubMed] [Google Scholar]
- 38.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR: Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA 2011;306:1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman DC, Fisher ES, Chang C-H, et al. :. Quality of End-of-Life Cancer Care for Medicare Beneficiaries: Regional and Hospital-Specific Analyses. Dartmouth Atlas of Health Care Report, Lebanon, NH, 2010, pp. 1–51 [PubMed] [Google Scholar]
- 40.Fisher ES, Bynum JP, Skinner JS: Slowing the growth of health care costs—lessons from regional variation. N Engl J Med 2009;360:849–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welch HG, Sharp SM, Gottlieb DJ, et al. : Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA 2011;305:1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leff B, Burton L, Mader S, et al. : Satisfaction with hospital at home care. J Am Geriatr Soc 2006;54:1355–1363 [DOI] [PubMed] [Google Scholar]
- 43.Kelley AS, Covinsky KE, Gorges RJ, et al. : Identifying older adults with serious illness: A critical step toward improving the value of health care. Health Serv Res 2017;52:113–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yourman LC, Lee SJ, Schonberg MA, et al. : Prognostic indices for older adults: A systematic review. JAMA 2012;307:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Lindquist K, Segal MR, Covinsky KE: Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 2006;295:801–808 [DOI] [PubMed] [Google Scholar]
- 46.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER: Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med 2009;24:1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan A, Kuo Y-F, Goodwin JS: Predicting life expectancy for community-dwelling older adults from Medicare claims data. Am J Epidemiol 2013;178:974–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harper S, MacLehose RF, Kaufman JS: Trends in the black-white life expectancy gap among US States, 1990–2009. Health Aff 2014;33:1375. [DOI] [PubMed] [Google Scholar]
- 49.Kelley AS, Morrison RS: Palliative care for the seriously ill. N Engl J Med 2015;373:747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elixhauser A, Steiner C, Harris DR, Coffey RM: Comorbidity measures for use with administrative data. Med Care 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 51.Friedman EM, Shih RA, Langa KM, Hurd MD: US prevalence and predictors of informal caregiving for dementia. Health Aff 2015;34:1637–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sudore RL, Fried TR: Redefining the “planning” in advance care planning: Preparing for end-of-life decision making. Ann Intern Med 2010;153:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tulsky JA: Beyond advance directives: Importance of communication skills at the end of life. JAMA 2005;294:359–365 [DOI] [PubMed] [Google Scholar]
- 54.Cooper Z, Koritsanszky LA, Cauley CE, et al. : Recommendations for best communication practices to facilitate goal-concordant care for seriously ill older patients with emergency surgical conditions. Ann Surg 2016;263:1–6 [DOI] [PubMed] [Google Scholar]
- 55.Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Institute of Medicine, The National Academies Press, Washington, DC, 2015 [PubMed] [Google Scholar]
- 56.Back AL, Arnold RM, Baile WF, et al. : Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med 2007;167:453–460 [DOI] [PubMed] [Google Scholar]
- 57.Kelley AS, Back AL, Arnold RM, et al. : Geritalk: Communication skills training for geriatric and palliative medicine fellows. J Am Geriatr Soc 2012;60:332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lynn J, Arkes HR, Stevens M, et al. : Rethinking fundamental assumptions: SUPPORT's implications for future reform. J Am Geriatr Soc 2000;48:S214–S221 [DOI] [PubMed] [Google Scholar]
- 59.Hammes BJ, Briggs LA, Silvester W, et al. :. Implementing a care planning system: How to fix the most pervasive errors in health care. Meeting the Needs of Older Adults with Serious Illness. Springer, New York, 2014, pp. 177–189 [Google Scholar]
- 60.Hanson LC, Schenck AP, Burstin H: Quality and outcome measures. Meeting the Needs of Older Adults with Serious Illness. Springer, New York, 2014, pp. 93. –108 [Google Scholar]
- 61.Tulsky JA: Improving quality of care for serious illness: Findings and recommendations of the institute of medicine report on dying in America. JAMA Intern Med 2015;175:840–841 [DOI] [PubMed] [Google Scholar]
- 62.Barnato AE, Anthony DL, Skinner J, et al. : Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med 2009;24:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnato AE, Berhane Z, Weissfeld LA, et al. : Racial variation in end‐of‐life intensive care use: A race or hospital effect? Health Serv Res 2006;41:2219–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnato AE, Chang C-CH, Saynina O, Garber AM: Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med 2007;22:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson KS: Racial and ethnic disparities in palliative care. J Palliat Med 2013;16:1329–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson KS, Kuchibhatla M, Tanis D, Tulsky JA: Racial differences in hospice revocation to pursue aggressive care. Arch Intern Med 2008;168:218–224 [DOI] [PubMed] [Google Scholar]
- 67.Mack JW, Paulk ME, Viswanath K, Prigerson HG: Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med 2010;170:1533–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]