Abstract
Sex differences in cognitive processing and function have been documented in human and animal studies. Females have been found to perform better than males on non-spatial memory tasks, while males tend to outperform females on spatial memory tasks. The neural mechanisms underlying these sexual dimorphisms are unclear. However, it is known that the cholinergic system is critically involved in memory processes, and there are notable differences between males and females in cholinergic system function and receptor expression. In particular, there are sex differences in the processing of information in the frontal cortex and hippocampus. In this study, we examined the roles of muscarinic and nicotinic acetylcholine receptors in the medial frontal cortex (MfC) and ventral hippocampus (VH) on spatial working memory in male and female rats. Local infusions of scopolamine (SCOP) and mecamylamine (MEC) (10, 20, 50 µg/side) were used to antagonize these receptors in each respective brain region during performance in the 16-arm radial arm maze. Infusions of SCOP into the VH caused a significant increase in memory errors in female rats, but had no significant effect on males, while infusions of MEC into the VH had no effect on either sex. Infusions of both SCOP (50 µg/side) and MEC (20 µg/side) into the MfC caused working memory impairments in both sexes. These results show that muscarinic acetylcholine receptors in the VH are differentially vulnerable to spatial memory impairments in females. Ventral hippocampal muscarinic acetylcholine receptors may play a key role in male-female differences in spatial memory.
Keywords: Sex differences, Cholinergic, Muscarinic, Nicotinic, Scopolamine, Mecamylamine, Hippocampus, Frontal cortex, Rats
1. Introduction
Studies have shown that differences exist between males and females in terms of cognitive processing. These differences are particularly prevalent during spatial versus non-spatial cognition/memory tasks. For instance, female rodents have been shown to outperform males during novel object recognition (NOR) tasks; a task of working memory that, in standard form, has no spatial or aversive components (Bettis and Jacobs, 2013; Sutcliffe et al., 2007). However, when a spatial component was introduced to the NOR task in these studies, male rodents showed marked improvement in memory function compared with females. Indeed, a superior performance of males when compared to females was also reported in the radial-arm and Morris water mazes, both tests classically used to assess spatial memory function (Gresack and Frick, 2003; Jacobs et al., 1990; LaBuda et al., 2002; Roof and Stein, 1999; Saucier et al., 2008). The evidence for human cognitive processing tells a similar story to the rodent literature. Women have been shown to perform better than men on verbal and memory tasks (Seidlitz and Diener, 1998), and to be more sensitive than men to the location of objects in a personal space (Saucier et al., 2007; Voyer et al., 2007). By contrast, men were shown to outperform women during a virtual Morris water maze (MWM) task, a simulation of the common spatial working memory task performed with rodents (Astur et al., 1998; Sandstrom et al., 1998). Although some studies report conflicting results (Bimonte et al., 2000; Healy et al., 1999), a preponderance of the evidence still suggests that women perform better during non-spatial tasks, and men perform better on spatially-dependent tasks.
The neural bases for the differences observed in spatial memory between males and females remain poorly understood. Studies looking at gonadal hormones have suggested that levels of estradiol may alter the performance of spatial-related tasks in females; higher levels typically impairing performance (Galea et al., 1995; Pleil and Williams, 2010). The role of testosterone in these tasks is less clear (for review, see Celec et al., 2015). There is also evidence that differences in hippocampal and prefrontal cortical processing between males and females may play a significant role in the results observed during cognitive tasks. These brain regions have long been known to be involved in the processing of information related to both memory and spatial navigation (Diana et al., 2010; Kennedy and Shapiro, 2004; Maguire et al., 1998). Indeed, communication between these regions appears to be critical for processing working memory (for review, see Jin and Maren, 2015; Preston and Eichenbaum, 2013). However, it has been shown that females may rely more heavily on the frontal cortical regions to perform cognitive tasks while males seem to make greater use of the hippocampus (Gur et al., 2002). More recent studies using advanced imaging techniques in humans have confirmed that structural brain differences (including functional connections) correlate with differing behavioral patterns between males and females (Tunc et al., 2016). The mechanisms underlying these structural and behavioral differences have yet to be sufficiently explored.
The cholinergic neurotransmitter system has long been known to be critically involved in cognitive function. Early work by our lab and others showed that administration of nicotine improves performance in cognitive tasks (Buccafusco and Jackson, 1991; Levin et al., 1992; Levin and Rose, 1990). Moreover, it has repeatedly been shown that blockade of both nicotinic (nAChRs) and muscarinic (mAChRs) acetylcholine receptors with mecamylamine (MEC) and scopolamine (SCOP), respectively, results in significant impairments in working memory tasks (Cozzolino et al., 1994; Levin et al., 1989). It is known that sexual dimorphisms exist regarding cholinergic receptor expression and system function (Avissar et al., 1981; Brown and Brooksbank, 1979; Hortnagl et al., 1993; Koylu et al., 1997). How these differences may contribute to spatial memory tasks is less well characterized. The present study was performed to demonstrate the relative roles of both nAChRs and mAChRs during a spatial memory task in male and female rats. It was hypothesized that blocking these receptors would result in differential impairment in radial-arm maze performance in males and females. Because of previous literature detailing the sex differences in cognitive processing between the frontal cortex and hippocampus, these brain areas were chosen as specific sites for determining the roles of nAChRs and mAChRs in spatial memory in males and females. The results of this study will shed light on how cholinergic function contributes to the observed differences in males and females during the performance of spatial memory tasks.
2. Materials and methods
2.1. Subjects
Experimental procedures in this study were conducted in accordance with AAALAC guidelines, and were also approved by the Duke University Animal Care and Use Committee. Young adult male and female rats were purchased from Charles River Laboratories (Raleigh, NC, USA), and used in the study. Animals began behavioral training after one week of acclimation to the laboratory housing environment (postnatal day 60). The animals were housed in a temperature-controlled vivarium at Duke University under standard laboratory conditions and were kept on a 12:12 reverse light/dark cycle. Behavioral testing occurred during the active (dark) phase of the light cycle. The animals were housed in groups of three per cage until brain cannulation surgery. After surgery the rats were housed singly to prevent damage to the surgical crown by cage mates. All animals were given unlimited access to water while in their home cage environment. Unlimited food was also provided until behavioral training commenced. Once training began all animals were kept on a food-restricted diet for the remainder of testing procedures, so that the animals’ weights were maintained at approximately 85% of free-feeding levels. All animals progressively gained weight throughout the study.
2.2. Drugs
Scopolamine HCl was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mecamylamine HCl was obtained via the National Institute on Drug Abuse (NIDA) Drug Supply Program. Both drugs were dissolved in artificial cerebrospinal fluid (aCSF), which served as the vehicle for both compounds. The drug doses and vehicle were administered in a repeated measures counterbalanced design with every rat receiving all of the doses and vehicle administration so that dose-effects were not confounded with order of administration. Scopolamine was given first, then mecamylamine.
2.3. Cannulation surgery
Brain cannulation surgeries were performed in a manner similar to Hall et al. (2015). Briefly, upon successful completion of training criteria for the radial-arm maze (see Section 2.4) each rat received a bilateral local infusion cannula implanted via stereotaxic surgery (David Kopf Instruments, Tujunga, CA, USA). Two specific brain areas were selected for the study: the medial frontal cortex (MfC) and ventral hippocampus (VH). Rats were anesthetized with a combination of ketamine (60 mg/kg i.p.) and dexmedetomidine (0.15 mg/kg i.p.) and placed on the stereotaxic instrument. An incision was made down the midline of the head to expose the skull. Coordinates for both brain regions were taken relative to the bregma of the skull. The MfC coordinates were based on Paxinos and Watson (2007) and were as follows: anteroposterior +2.70 mm, lateral ±0.75 mm, dorsoventral −2.70 mm. Coordinates for the VH were derived from Pellegrino et al. (1979), and were: anteroposterior −3.20 mm, lateral ±5.20 mm, dorsoventral −7.00 mm. Cannulae were secured via screw and wire structure and a mixture of carboxylate cement (Durelon™, 3M, St. Paul, MN, USA) that covered the surgical site. Surgical wounds were treated with the topical anesthetic bupivacaine, and all animals were given ketoprofen (5 mg/kg, s.c.) for postoperative pain. Animals were given a five-day recovery period from surgery before behavioral testing with SCOP and MEC treatments began. Upon completion of behavioral testing, animals were sacrificed and brains were removed and placed in a formalin solution. To ensure proper placement of the infusion cannulae, each brain was then sliced on a cryostat. Only those animals with the correct histological localization of the bilateral cannulae for the respective targeted brain region were considered for statistical analysis.
2.4. Behavioral testing
Radial-arm maze tests were conducted on a black, wooden maze, elevated 31 cm off the floor, and consisting of a central platform (50 cm diameter) with 16 arms (10 cm × 60 cm) extending radially from the central platform. Food cups were located 2 cm from the distal end of each arm. Shapes in the form of cardboard cutouts were placed on the walls of the testing room to serve as visual cues and to permit spatial orientation. Habituation to the maze occurred in two 10 min sessions. During habituation, each rat was placed inside a large, round, opaque cylinder and allowed to consume 12 halves of sugar-coated cereal (Froot Loops®; Kellogg's, Battle Creek MI, USA). Once habituated, the rats began training on the radial-arm maze task. During testing, 12 arms of the 16-arm maze were baited to test for working memory, while the 4 remaining arms were left unbaited and used to test for reference memory. The choice of baited arms was randomized among the animals, but the baited arms for each animal were kept constant for the duration of the study. Repeated entries into a baited arm of the maze were scored as working memory errors, and entries into unbaited arms were scored as reference memory errors. Latency was calculated as the total session time in seconds divided by the total number of arms entered by the animal. Each trial began by placing the rat on the central platform inside the opaque cylinder for 10 s, after which the cylinder was removed and the rat was allowed to freely explore the maze. Testing sessions lasted 10 min or until the rat had entered all 12 baited arms, whichever came first. After every session, the maze apparatus was wiped clean with a damp paper towel. Acceptable training criteria consisted of a rat performing 18 sessions of the radial-arm maze task wherein the animal entered at least 8 of the 12 baited arms of the maze.
After meeting criteria for maze training, the rats underwent brain cannulation surgery (see Section 2.3). After recovery from surgery, radial-arm maze sessions began preceded by infusions of either SCOP or MEC into the MfC or VH (depending on the animal/cohort). SCOP and MEC were both infused at a rate of 0.133 µl/min for three minutes, at doses of 10, 20, and 50 µg/side. After the three minute infusion the rats were given an additional 10 min inside their home cages before testing to allow for diffusion of drug. Doses of SCOP and MEC were randomized for each animal in the study, and each animal underwent two rounds of dosing: one round of SCOP followed by a five day washout period followed by one round of MEC. During the study, one day of washout was allowed between drug testing sessions.
2.5. Statistical analysis
The working and reference memory errors and choice latency data were evaluated by analysis of variance with sex as the between subjects factor and drug dose and error type as within subjects factors. As recommended by Snedecor and Cochran (1967) interactions of p < 0.10 were followed up by tests of the simple main effects. A threshold of p < 0.05, two-tailed was used for determining significance for all tests. Only subjects with histologically verified bilateral cannula placements for infusions within the target areas were included in the analysis.
3. Results
3.1. Pre-cannulation training
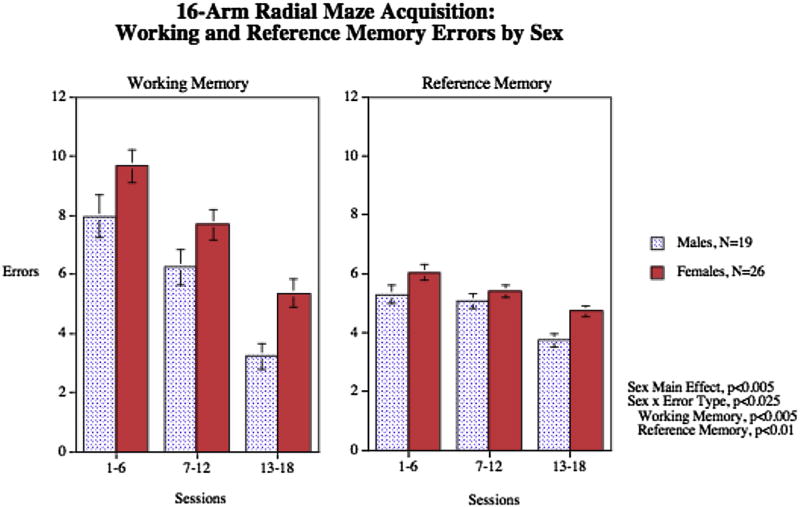
The acquisition data for working and reference memory are shown in Fig. 1. There were significant main effects of sex (F1,43 = 9.49, p < 0.005), error type (F1,43 = 55.76, p < 0.0005) and session block (F2,86 = 49.11, p < 0.0005). There was a significant interaction of sex × error type (F1,43 = 6.02, p < 0.025). Follow-up tests of the sex with the different error types showed that males had significantly (p < 0.05) fewer working and reference (p < 0.01) memory errors than females,.
Fig. 1.
Sex differences in pretreatment acquisition of working and reference memory on the 16-arm radial maze (mean ± sem). Males had significantly fewer working memory errors (p < 0.005) and reference memory errors (p < 0.01) than females, errors±sem.
Working memory errors during the final phase of training (sessions 13–18) averaged 4.5 ± 0.4 and during the aCSF vehicle sessions during drug testing was 5.6 ± 0.6. Reference memory errors during the final phase of training (sessions 13–18) averaged 4.3 ± 0.2 and during the aCSF vehicle sessions during drug testing was 4.0 ± 0.6.
3.2. Cannulations
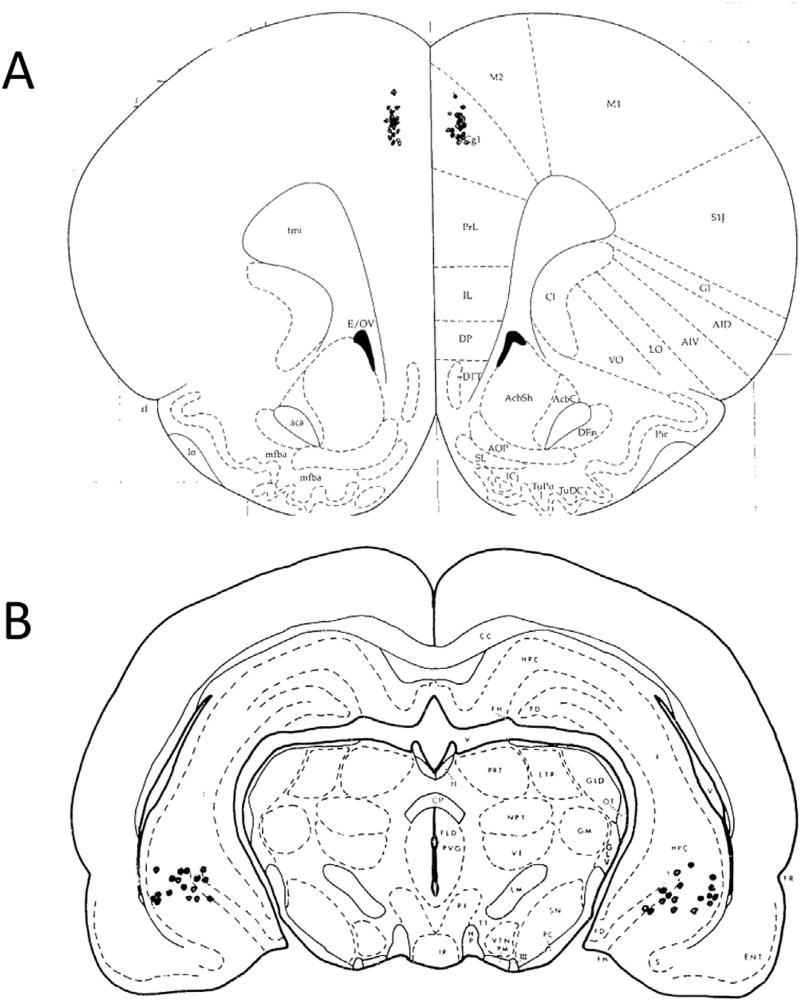
Fig. 2 shows the placements of the bilateral local infusion cannulae for the ventral hippocampal and medial frontal cortical infusion cannulae. Only the subjects with infusion sites within the target area on both sides were included in analysis. For the study of ventral hippocampal infusion effects there were 9 males and 16 females. For the study of medial frontal cortical infusion effects there were 10 males and 10 females.
Fig. 2.
Placements of bilateral cannula in each animal used for data analysis in the study. Animals with incorrect cannula placement were excluded from analysis. A) Medial Frontal Cortex (MfC); B) Ventral Hippocampus (VH).
3.3. Local infusions of scopolamine into the ventral hippocampus
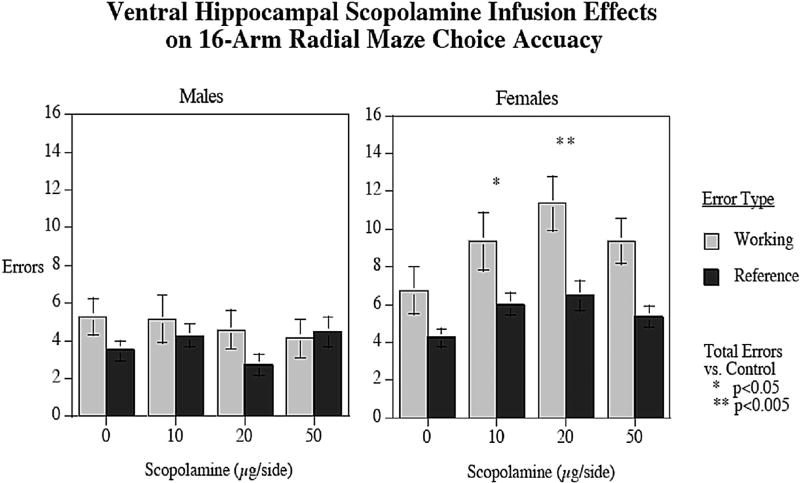
There was a significant overall effect of sex on total working and reference memory errors in the radial arm maze task, with females (14.64 ± 1.18) committing significantly (F1,23 = 13.81, p < 0.005) more total errors than males (8.44 ± 0.69). There was also a significant main effect of working/reference memory errors (F1,23 = 18.26, p < 0.0005) with more working memory errors (7.57 ± 0.74) being committed in the task than reference memory errors (4.84 ± 0.59). Furthermore, there was an interaction between working/reference memory errors × sex (F1,23 = 5.59, p < 0.05), with females committing significantly more errors than males with both error types, but with a greater effect with working (male = 4.75 ± 0.43, female = 9.16 ± 0.92, p < 0.005) than reference memory errors (male = 3.69 ± 0.48, female = 5.48 ± 0.37, p < 0.01). There was also a SCOP dose × sex interaction (F3,69 = 2.31, p < 0.09) that prompted tests of the simple main effects of SCOP effects in each sex. In male rats, there was no significant effect of SCOP into the VH on total working and reference memory errors (Fig. 3). In contrast, with female rats infusions of SCOP into the VH caused significantly more total working and reference errors (F1, 15 = 3.62; p < 0.025. In females, VH local infusion SCOP doses of 10 (p < 0.05) and 20 (p < 0.005) µg/side caused significant increases in total working and reference memory errors compared to performance after infusions of the aCSF vehicle (Fig. 3). There was no significant effect of SCOP treatment on latency in the task for either males or females.
Fig. 3.
Effects of local infusions of SCOP into the VH on radial-arm maze performance in male and female rats (mean ± sem). Doses of SCOP (10 and 20 µg/side) caused significant increases in working and reference memory errors in females, but not in males, when compared to aCSF vehicle (p < 0.05 and p < 0.005 respectively). There was also a significant sex effect, with females committing significantly more errors overall than males (p < 0.05). errors±sem (n = 9 males and n = 16 females).
3.4. local infusions of mecamylamine into the ventral hippocampus
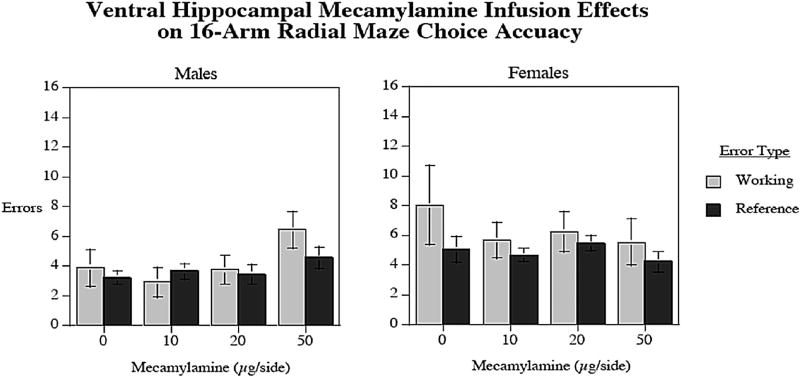
The results of MEC infusions into the VH are presented in Fig. 4. There were no significant differences on working memory errors, reference memory errors, or latency in the task for either males or females.
Fig. 4.
Local infusions of MEC into the VH had no effect on radial-arm maze performance in either males or females. errors±sem (n = 9 males and n = 16 females).
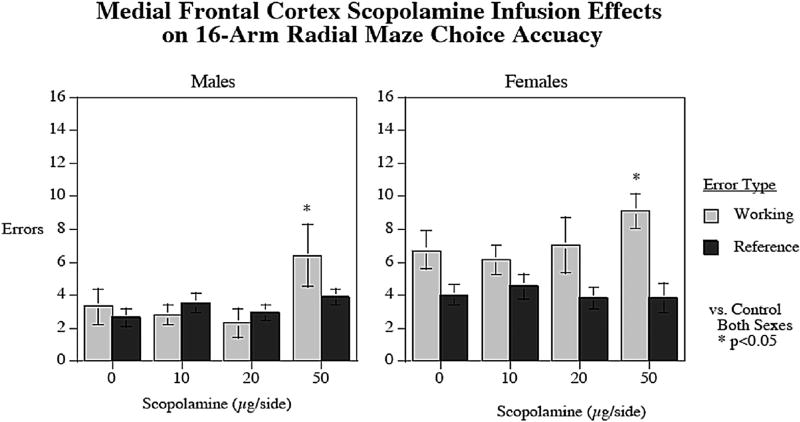
3.5. Local infusions of scopolamine into the medial frontal cortex
There was a significant main effect of sex (F1,18 = 16.15; p < 0.001) on errors, with females (11.25 ± 0.65) committing more total errors than males (6.92 ± 0.86). There was also a significant main effect of working/reference memory type (F1,18 = 11.57; p < 0.005) with more working (5.46 ± 0.59) than reference (3.62 ± 0.31) memory errors. Significant interactions were observed of working/reference memory error type × sex (F1,18 = 6.36; p < 0.025) with a significant (p < 0.001) sex difference with working (male = 3.70 ± 0.67, female = 7.22 ± 0.59) but not reference memory errors (male = 3.22 ± 0.32, female = 4.02 ± 0.52). There was also a significant interaction of SCOP × working/reference memory error type (F3,54 = 3.50; p < 0.025). As shown in Fig. 5, SCOP infusions into the MfC had a significant (p < 0.05) effect increasing working memory errors, with the 50 µg/side dose causing more working memory errors in the maze compared to vehicle treatment regardless of sex (p < 0.05). There was no significant MfC SCOP effect observed on reference memory errors. There was no effect of SCOP infusions into the MfC on task latency.
Fig. 5.
Local infusions of SCOP (50 µg/side) into the MfC caused significant increases in working memory error in both male and female rats in the radial-arm maze compared to aCSF vehicle (p < 0.05). No effect was observed on reference memory errors as a result of SCOP treatment. Females also committed significantly more errors overall in the task (p < 0.001). errors±sem (n = 10 males and n = 10 females).
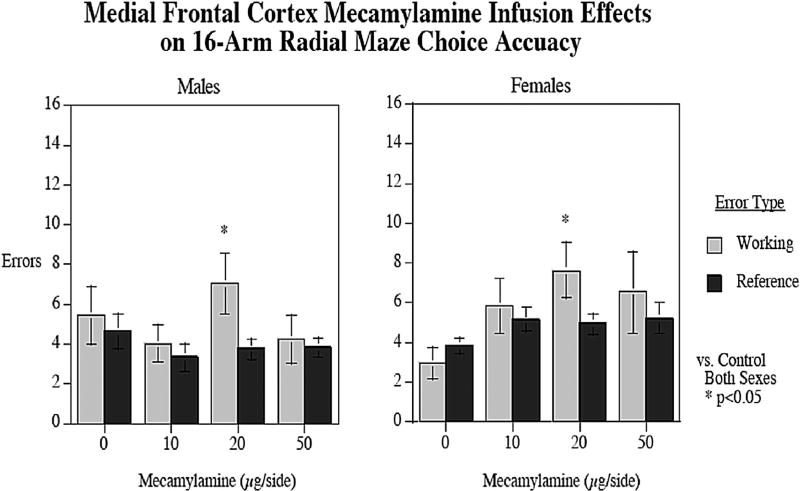
3.6. Local infusions of mecamylamine into the medial frontal cortex
Fig. 6 shows the effect of local infusions of MEC into the MfC on radial-arm maze performance. There was a significant main effect of working/reference memory type (F3,18 = 5.80; p < 0.05) with more working (5.42 ± 0.56) than reference memory errors (4.30 ± 0.27). There was a significant interaction between the dose of MEC and working/reference memory errors (F3,18 = 2.89; p < 0.05). The 20 µg/side MEC dose into the MfC caused a significant (p < 0.025) increase in working memory errors regardless of sex. Since this study used a repeated measures design, in which each animal is given each of the drug treatments, the overlap of standard error bars is not necessarily indicative of the lack of significance of drug treatment. Rather, it is the consistency of the effect between doses that drives the significance determination. Infusions of MEC into the MfC had no significant effect on reference memory errors or response latency.
Fig. 6.
Effects of local infusions of MEC into the MfC on radial-arm maze performance. At a dose of 20 µg/side, infusions of MEC caused significant increases in working memory errors in both males and females, but had no effect on reference memory errors (p < 0.05). errors±sem (n = 10 males and n = 10 females).
4. Discussion
The results of this study reinforce the findings from previous literature showing that male rats outperform female rats during memory tasks that carry a spatial component. Male rats in our study clearly committed fewer working memory errors than females while performing the 16-arm radial-arm maze task as shown in Fig. 1, which displays choice accuracy for the rats prior to drug treatment. We found significant differences in radial-arm maze performance during the SCOP phase of the experiments in the VH, with females having a significant memory impairment due to SCOP treatment whereas the males were not affected by the same dose range of SCOP. There were no statistically significant differential sex effects on response to MEC or in response to the SCOP in the MfC. Sex differences in choice accuracy with vehicle infusions intercurrent with the drug challenges was less robust possibly due to carryover effects of the drug actions in the same animals before the vehicle infusion. Overall, our results from this study confirm what has typically been reported regarding an advantage for male rodents in spatial memory tasks (Jonasson, 2005) and give insight into the cholinergic component and brain region that may underlie the sexually dipmorphic performance.
The most significant finding of this study is that infusions of SCOP into the VH resulted in significantly more working and reference memory errors in females, but not in males, during the 16-arm maze task. We have previously found similar results in the radial-arm maze in female rats using SCOP (Kim and Levin, 1996), and the compound is a commonly used tool to induce working memory impairments in rodent models (Buccafusco, 2009; Klinkenberg and Blokland, 2010). However, this is the first time we have directly compared both male and female performance using this paradigm, and it is very interesting that SCOP infusions did not cause memory impairments in males when infused into the VH. These results suggest that mAChR mechanisms in the VH may play a more significant role in females for spatial memory processing. Moreover, infusions of MEC into the VH had no observed effect on maze performance in either males or females, suggesting that mAChR mechanisms in this brain area may be a key factor in the differences observed between males and females during spatial memory tasks. It should be noted that in our previously published study neither administration of mAChR nor nAChR agonists into the VH resulted in any improvement in female radial-arm maze performance (Kim and Levin, 1996). Perhaps there is some optimal level of VH cholinergic function below which causes memory impairments, and above which has no discernable benefit. Importantly, there were no significant effects on latency observed during the task for either sex as a result SCOP infusions into the VH. Therefore, it is unlikely that the increases in working and reference memory errors we found in this study were the result of off-target effects such as sedation.
Significant increases in working memory errors were observed in the radial-arm maze as a result of both SCOP (50 µg/side) and MEC (20 µg/side) infusions into the MfC. However, these effects were seen in both males and females, suggesting that these neural mechanisms are likely shared between the sexes. It is interesting, however, that infusions into the MfC had effects on working memory performance, but not reference memory, and this was true for both SCOP and MEC treatments. The MfC contributes greatly to memory processes, and the MfC-VH circuit is particularly crucial to spatial working memory (Burton et al., 2009; Kyd and Bilkey, 2003). Furthermore, cholinergic activity is critical to MfC function. The MfC is densely innervated with cholinergic neurons, and receives projections from the basal forebrain (Eckenstein et al., 1988). It has been shown that both tonic and phasic cholinergic signaling in the MfC are involved in cognitive function (Parikh et al., 2007). Based on our results and others, it may be that the MfC contains an essential cholinergic circuit shared by both sexes that is important for working memory function.
As previously stated, the functional connections between the MfC and hippocampus are crucial to working memory processes. Studies have shown synchronized activity between these brain regions during the performance of working memory tasks (Benchenane et al., 2010; Jones and Wilson, 2005). It has been suggested that the flow of information runs from hippocampus to prefrontal cortex, as the prefrontal firing lags behind that of the hippocampus (Hyman et al., 2005, 2010). A recent study found that VH to prefrontal projections were responsible for encoding task relevant information regarding spatial cues that were important for performing a spatial working memory task (Spellman et al., 2015). Taken with the evidence that males seem to rely more heavily on hippocampal activity during cognitive tasks while females may rely more heavily on PfC mechanisms (Gur et al., 2002), this may explain, in part, the “male advantage” consistently observed in spatial working memory tasks. In light of the results from our current study, these differences may also include a significant mAChR component to the neural processing of spatial cognitive information. Another possibility may be that the differential response by males and females to hippocampal infusions of SCOP could be due to differences in emotional response rather than cognitive response. However, we did not see any of drug infusion effects on response latency in the radial-arm maze indicative of alterations in freezing behavior, indicative of emotional effects of SCOP infusions into the hippocampus.
In summary, the results of our study suggest that mAChR mechanisms in the VH may play a significant role in the sex differences observed during the performance of spatial working memory tasks. Our results also demonstrate that both mAChR and nAChR mechanisms in the MfC are critically important to working memory processes in both males and females. Our findings are consistent with previously published studies demonstrating a “male advantage” in the performance of working memory tasks containing a significant spatial component. Future studies are needed to more fully explore the extent of the differences observed in our study, including which mAChRs may be playing the largest role.
Acknowledgments
This research was supported by the National Institutes of Health (ES022831) and by the U.S. Environmental Protection Agency (83543701). EPA support does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. Dr. Abreu-Villaça was supported by a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) [grant number BEX 2037/15-7].
References
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav. Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Avissar S, Egozi Y, Sokolovsky M. Studies on muscarinic receptors in mouse and rat hypothalamus: a comparison of sex and cyclical differences. Neuroendocrinology. 1981;32:295–302. doi: 10.1159/000123175. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bettis TJ, Jacobs LF. Sex differences in memory for landmark arrays in C57BL/J6 mice. Anim. Cogn. 2013;16:873–882. doi: 10.1007/s10071-013-0619-x. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol. Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Brown R, Brooksbank BW. Developmental changes in choline acetyltransferase and glutamate decarboxylase activity in various regions of the brain of the male, female, and neonatally androgenized female rat. Neurochem. Res. 1979;4:127–136. doi: 10.1007/BF00964139. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ. The revival of scopolamine reversal for the assessment of cognition-enhancing drugs. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol. Aging. 1991;12:233–238. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- Burton BG, Hok V, Save E, Poucet B. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav. Brain Res. 2009;199:222–234. doi: 10.1016/j.bbr.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Celec P, Ostatnikova D, Hodosy J. On the effects of testosterone on brain behavioral functions. Front. Neurosci. 2015;9:12. doi: 10.3389/fnins.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino R, Guaraldi D, Giuliani A, Ghirardi O, Ramacci MT, Angelucci L. Effects of concomitant nicotinic and muscarinic blockade on spatial memory disturbance in rats are purely additive: evidence from the Morris water task. Physiol. Behav. 1994;56:111–114. doi: 10.1016/0031-9384(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J. Cogn. Neurosci. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenstein FP, Baughman RW, Quinn J. An anatomical study of cholinergic innervation in rat cerebral cortex. Neuroscience. 1988;25:457–474. doi: 10.1016/0306-4522(88)90251-5. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP, Hampson E. Gonadal hormone levels and spatial learning performance in the Morris water maze in male and female meadow voles, Microtus pennsylvanicus. Horm. Behav. 1995;29:106–125. doi: 10.1006/hbeh.1995.1008. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Male mice exhibit better spatial working and reference memory than females in a water-escape radial arm maze task. Brain Res. 2003;982:98–107. doi: 10.1016/s0006-8993(03)03000-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb. Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Slade S, Allenby C, Kutlu MG, Levin ED. Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology. 2015;99:689–695. doi: 10.1016/j.neuropharm.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SD, Braham SR, Braithwaite VA. Spatial working memory in rats: no differences between the sexes. Proc. Biol. Sci. 1999;266:2303–2308. doi: 10.1098/rspb.1999.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortnagl H, Hansen L, Kindel G, Schneider B, el Tamer A, Hanin I. Sex differences and estrous cycle-variations in the AF64A-induced cholinergic deficit in the rat hippocampus. Brain Res. Bull. 1993;31:129–134. doi: 10.1016/0361-9230(93)90019-8. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front. Integr. Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF, Gaulin SJ, Sherry DF, Hoffman GE. Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Maren S. Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci. Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J. Neurosci. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Levin ED. Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: effects on spatial working memory in rats. Brain Res. 1996;725:231–240. doi: 10.1016/0006-8993(96)00213-2. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci. Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Koylu E, Demirgoren S, London ED, Pogun S. Sex difference in upregulation of nicotinic acetylcholine receptors in rat brain. Life Sci. 1997;61:185–190. doi: 10.1016/s0024-3205(97)00665-6. PL. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cereb. Cortex. 2003;13:444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Mellgren RL, Hale RL. Sex differences in the acquisition of a radial maze task in the CD-1 mouse. Physiol. Behav. 2002;76:213–217. doi: 10.1016/s0031-9384(02)00713-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Persistence of chronic nicotine-induced cognitive facilitation. Behav. Neural Biol. 1992;58:152–158. doi: 10.1016/0163-1047(92)90399-o. [DOI] [PubMed] [Google Scholar]
- Levin ED, McGurk SR, South D, Butcher LL. Effects of combined muscarinic and nicotinic blockade on choice accuracy in the radial-arm maze. Behav. Neural Biol. 1989;51:270–277. doi: 10.1016/s0163-1047(89)90917-5. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rose JE. Anticholinergic sensitivity following chronic nicotine administration as measured by radial-arm maze performance in rats. Behav. Pharmacol. 1990;1:511–520. [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 2007. [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. Plenum Press; New York: 1979. [Google Scholar]
- Pleil KE, Williams CL. The development and stability of estrogen-modulated spatial navigation strategies in female rats. Horm. Behav. 2010;57:360–367. doi: 10.1016/j.yhbeh.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol. Behav. 1999;68:81–86. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman J, Huettel SA. Males and females use different distal cues in a virtual environment navigation task. Brain Res. Cogn. Brain Res. 1998;6:351–360. doi: 10.1016/s0926-6410(98)00002-0. [DOI] [PubMed] [Google Scholar]
- Saucier D, Lisoway A, Green S, Elias L. Female advantage for object location memory in peripersonal but not extrapersonal space. J. Int. Neuropsychol. Soc. 2007;13:683–686. doi: 10.1017/S1355617707070865. [DOI] [PubMed] [Google Scholar]
- Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Sex differences in object location memory and spatial navigation in Long-Evans rats. Anim. Cogn. 2008;11:129–137. doi: 10.1007/s10071-007-0096-1. [DOI] [PubMed] [Google Scholar]
- Seidlitz L, Diener E. Sex differences in the recall of affective experiences. J. Pers. Soc. Psychol. 1998;74:262–271. doi: 10.1037//0022-3514.74.1.262. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; Ames, Iowa: 1967. [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav. Brain Res. 2007;177:117–125. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Tunc B, Solmaz B, Parker D, Satterthwaite TD, Elliott MA, Calkins ME, Ruparel K, Gur RE, Gur RC, Verma R. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos. Trans. R. Soc. Lond B Biol. Sci. 2016;371:20150111. doi: 10.1098/rstb.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Postma A, Brake B, Imperato-McGinley J. Gender differences in object location memory: a meta-analysis. Psychon. Bull. Rev. 2007;14:23–38. doi: 10.3758/bf03194024. [DOI] [PubMed] [Google Scholar]