Abstract
Objective
The aim of this study was to investigate the clinicopathological and immunohistochemical (including VEGF, Akt, HSP70, and HSP20 expression) factors that affect the overall and disease-free survival of HCC patients following surgical resection.
Methods
234 patients with HCC following surgical resection were enrolled. Clinicopathological and survival data were analyzed, and immunohistochemical staining was performed on tissue microarray sections using the anti-VEGF, anti-Akt, anti-HSP70, and anti-HSP27 antibodies.
Results
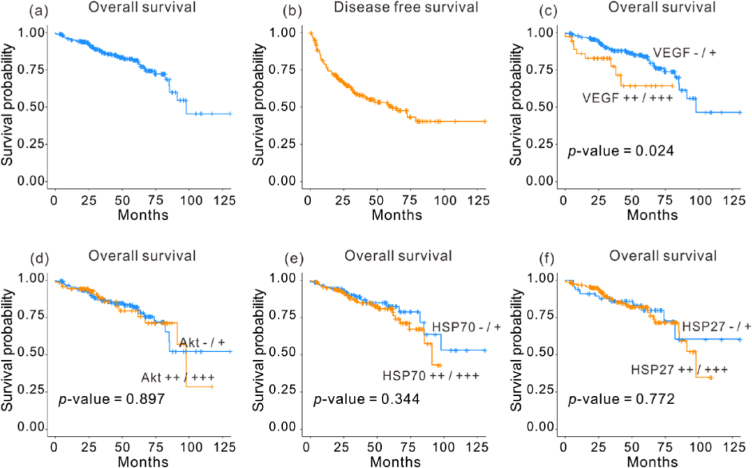
The 3- and 5-year overall survival rates were 86.5 and 81.54%, respectively. Multivariate analysis revealed that VEGF expression (P = 0.017, HR = 2.573) and T stage (P < 0.001, HR = 4.953) were independent prognostic factors for overall survival. Immunohistochemical staining showed that the expression of Akt, HSP70, and HSP27 did not affect the overall survival rate. The 3- and 5-year disease-free survival rates were 58.2 and 49.4%, respectively. Compared to the VEGF(−)/(+) group, the VEGF(++)/(+++) group demonstrated significantly higher proportion of patients with AFP levels > 400 ng/mL, capsule invasion, and microvascular invasion.
Conclusion
VEGF overexpression was associated with capsule invasion, microvascular invasion, and a poor overall survival rate.
Keywords: Hepatocellular carcinoma, hepatic resection, prognostic marker, VEGF
1. Introduction
Surgical resection of hepatocellular carcinoma (HCC) is the treatment of choice for patients with good liver function [1, 2, 3]. Improvements in surgical techniques, instruments, anesthesiology methods, and intensive and general care practices have led to steady improvements in surgical outcomes and prognosis for HCC patients [4]. In these patients, prognosis is not only influenced by pathological characteristics of the tumor, such as its size, stage of differentiation, and vascular invasion, but also by the severity of underlying liver cirrhosis [1, 2, 5, 6, 7, 8].
HCC is a highly vascularized type of tumor. Angiogenesis plays an important role in the development and progression of HCC. Vascular endothelial growth factor (VEGF) is a crucial angiogenic factor and is frequently expressed in HCC [9]. The prognostic impact of VEGF expression has been studied previously [10, 11]. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is commonly activated in human cancers [12]. Increased PI3K/Akt expression has been observed during cell proliferation, adhesion, migration, invasion, and apoptosis [13, 14]. Heat shock proteins (HSP) are involved in the regulation of cell cycle progression and apoptosis during tumorigenesis [15, 16]. HSP70 and HSP27 are potent anti-apoptotic HSPs [17] and their overexpression is associated with poor prognosis in cancer patients [18].
In this study, using immunohistochemical analysis, we investigated the impact of various molecular markers that are known to have crucial roles in tumorigenesis, on the prognosis of HCC patients following surgical resection.
2. Patients and methods
2.1. Patients
Between January 2004 and December 2013, 234 HCC patients who were operated on with curative intention at the Department of Surgery, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea, and whose good quality paraffin-embedded tissue samples were available were included in this study. Patients who had concomitant malignancy or those whose good quality paraffin-embedded tissue samples were not available were excluded from the present study. Clinical, histopathological, and survival data were reviewed and analyzed retrospectively. Levels of alpha-fetoprotein (AFP), Child–Pugh classification, effects of preoperative treatments such as radiofrequency ablation (RFA) or transarterial chemoembolization (TACE), type of operative procedure, and disease recurrence were analyzed. Pathological characteristics, including tumor size and number, satellite nodules, presence of vascular invasion, capsular invasion, and stage of differentiation were examined. All specimens were graded on the basis of the degree of tumor differentiation, using the World Health Organization (WHO) grading system described in the International Classification of Diseases for Oncology (ICD-O) [19]. Patient staging was performed according to the TNM American Joint Committee on Cancer (AJCC) classification system [20]. This study protocol was reviewed and approved by the Institutional Review Board of Korea University Hospital (No. KUGH13082-001).
Informed consent
Informed consent has been obtained from all individuals included in this study.
2.2. Tissue microarray
Hematoxylin and eosin (H&E) staining for tissue sections obtained from the 234 HCC patients was performed. The stained sections were examined and subjected to immunohistochemical analysis. The corresponding paraffin-embedded tissue blocks were precisely aligned with the pre-marked slides. A high-precision manual tissue arrayer (Beecher Instruments, Silver Spring, MD) was used for sampling 0.6-mm sample cores from the tumor areas of the paraffin-embedded tissue samples in triplicates.
2.3. Immunohistochemical staining
Immunohistochemical staining was performed on the tissue microarray sections. Briefly, paraffin was removed from the samples using xylene. The samples were rehydrated using a graded series of alcohol. Subsequently, they were transferred to citric acid buffer (10 mmol/L) and heated in a microwave oven (700 W, 12 min) for antigen retrieval. Endogenous peroxidases were inhibited using a solution of 3%hydrogen peroxide in methanol. Normal goat serum was used for blocking non-specific binding. Next, the tissue samples were incubated with the respective primary antibody at room temperature for 30 min. Anti-VEGF (VEGF [Ab3], 1:800; Calbiochem, Oncogene Research Products, Cambridge, MA), anti-Akt (V-Akt murine thymoma viral oncogene homolog 1, 1:100; Cell Signaling Technology, Beverly, MA, USA), anti-HSP70 (1:50; Neomarker, Fremont, CA, USA), and anti-HSP27 (1:50; Neomarker) antibodies were used. Samples were washed with PBS-Tween (PBST) and incubated with the biotinylated secondary antibody for 30 min. After washing the samples one more time with PBST, they were incubated with the streptavidin–peroxidase complex for 30 min. Next, 3,3’-diaminobenzidine (DAB; DAKO, Carpinteria, CA, USA), a chromogen, was applied to the samples for 5 min. The slides were counterstained using hematoxylin, rinsed with water, dehydrated, cleared, and cover-slipped. The surrounding non-tumorous liver tissue served as an internal positive control. Negative control was obtained by replacing the primary antibody with non-immunized rabbit serum.
2.4. Immunohistochemical analysis and scoring
For VEGF immunohistochemical staining, majority of tumor cells were stained in the cytoplasm. For Akt, HSP70 and HSP27, any staining either in the cytoplasms or nuclei was regarded as positive.
A histoscore system was used for quantification of the results. Histoscores were determined by multiplying the percentage of positive cells with the intensity of staining. The grades were assigned according to the staining intensity (0, no staining; 1, weak; 2, moderate; and 3, strong staining). Scores from 0 to 5 were assigned according to the percentage of stained cells (0, no stained cells; 1, < 5%; 2, 6–25%; 3, 26–50%; 4, 51–75%; and 5, > 75% stained cells). The final scores were calculated as staining intensities multiplied by the score of the percentage of stained cells in the corresponding area. The final score for each specimen was categorized as follows: 0, negative (−); 1 to 4, weak positive (+); 5 to 9, moderate positive (++); 10 to 15, strong positive (+++).
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20 (SPSS Inc. Chicago, Illinois, USA). Survival time was measured from the date of surgery. Overall survival was defined as the time from surgery until death. Disease-free survival (DFS) was defined as the time from the date of surgery until the date when recurrence was detected. Survival of the patients was calculated using the Kaplan–Meier method. Clinicopathological factors were analyzed using the univariate Kaplan–Meier method. The log-rank test was used for identifying the prognostic factors for overall survival and disease-free survival in HCC patients. Cox proportional hazards model was used for predictors that were considered to be significantly important in the univariate analysis. Groups were compared using Pearson’s chi-square test. A P-value < 0.05 was considered to be statistically significant.
3. Results
3.1. Clinicopathological characteristics
In this study, 196 patients were males and 38 were females. The mean patient age was 56 ± 10.3 years (range, ~29–84 years). The Child–Pugh classification was as follows: 217 patients were categorized as belonging to class A, 16 to class B, and 1 to class C. Forty-five patients underwent preoperative treatments (TACE, 34; RFA, 4; TACE and RFA, 7).
Etiology of cirrhosis was identified as HBV in 182 (87.85%), HCV in 17 (7.3%), alcoholic hepatitis in 11 (4.7%), and cryptogenic in 24 (10.3%) patients. The operative procedures used were as follows: wedge resection, 74; right anterior sectionectomy, 7; right posterior sectionectomy, 13; right hemihepatectomy, 34; left lateral sectionectomy, 18; left hemihepatectomy, 15; bisegmentectomy, 7; central bisectionectomy, 3; trisectionectomy, 2; laparoscopic wedge resection, 29; segmentectomy, 25; laparoscopic left hemihepatectomy, 1; and laparoscopic left lateral sectionectomy, 6 patients.
The mean tumor size was 3.8 ± 3 cm (range, 0.7–18 cm). As for the number of tumors, 200 (85.5%) patients had a single tumor, 27 (11.5%) had two tumors, and 7 (3%) had more than three tumors. Pathological analysis revealed that the tumors were well differentiated in 19 (8.1%), moderately differentiated in 117 (50%), poorly differentiated in 89 (38%), and undifferentiated in 9 (3.8%) patients. According to the AJCC staging system [20], the following tumor stages were observed: T1 in 133 (56.8%), T2 in 70 (29.9%), T3 in 20 (8.5%), and T4 in 11 (4.7%) patients. The final AJCC stages were stage I observed in 132 (56.4%), stage II in 71 (30.3%), stage III in 30 (12.8%), and stage IV in 1 (0.4%) patients.
Results of immunohistochemical analysis for each molecular marker have been shown in Table 1. Fig. 1 shows immunohistochemical staining patterns for all molecular markers. The patterns of VEGF expression (Fig. 2) were as follows: negative (–) in 99 (42.3%), weak positive (+) in 98 (41.9%), moderate positive (++) in 29 (12.4%), and strong positive (+++) in 8 (3.4%) patients.
Table 1.
The results of immunohistochemical stain for VEGF, Akt, HSP70 and HSP27 in HCC patients
| VEGF | Akt | HSP70 | HSP27 | |
|---|---|---|---|---|
| Negative (-) 0 | 99 (42.3%) | 103(44.0%) | 45(19.2%) | 23(9.8%) |
| Weak (+) 1-4 | 98 (41.9%) | 58(24.8%) | 47(20.1%) | 44(18.8%) |
| Positive(++) 5-9 | 29 (12.4%) | 46(19.7%) | 90(38.5%) | 109(46.6%) |
| Strong (+++) 10-15 | 8(3.4%) | 27(11.5%) | 52(22.2%) | 58(24.8%) |
Figure 1.
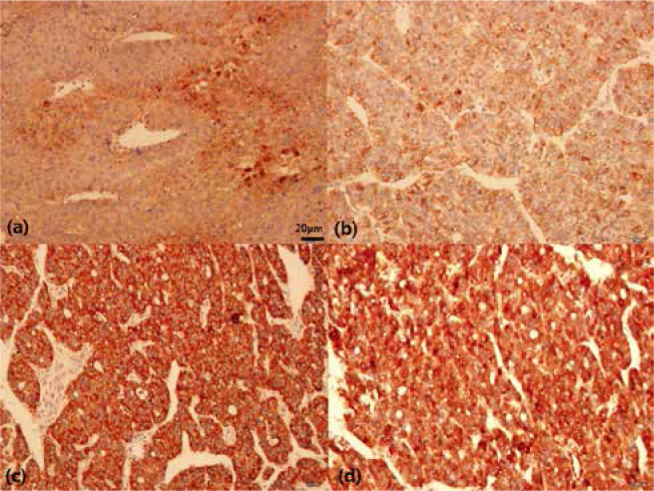
Results of immunohistochemical analysis in HCC. Expression of (a) VEGF in the cytoplasm, (b) Akt, (c) HSP70 and (d) HSP27 in the cytoplasm and nucleus of cancer cells (×200)
Figure 2.
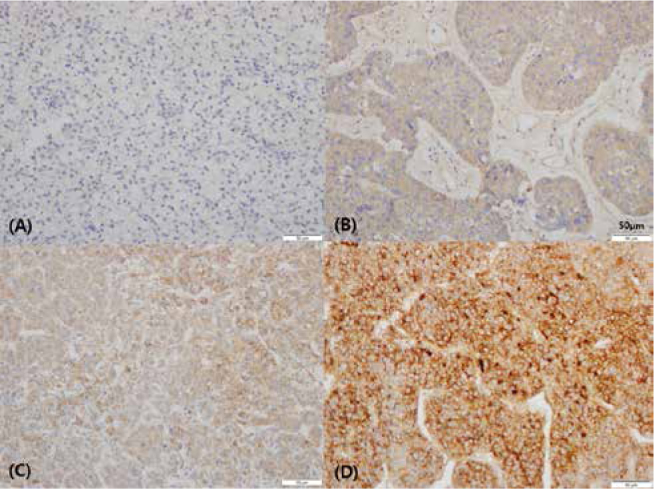
Immunohistochemical analysis for VEGF in HCC. (a) VEGF(-), (b) VEGF(+) (weak positive), (c) VEGF(++) (moderate positive), and (d) VEGF(+++) (strong positive) (x200)
3.2. Clinicopathological and immunohistochemical factors affecting overall survival rates in HCC patients
The 3- and 5-year overall survival rates for the 234 patients who underwent liver resection were 86.5 and 81.54%, respectively (Fig. 3a). Univariate analysis revealed that sex, age, AFP levels, tumor size, tumor number, capsule invasion, microvascular invasion, and tumor grade did not affect overall survival in HCC patients. Child–Pugh class (A vs. B or C; P = 0.048), T stage (T1 or T2 vs. T3 or T4; P < 0.001), and TNM stage (P < 0.001) were the important prognostic factors for overall survival. Univariate analysis showed the expression levels of Akt, HSP70, and HSP27 protein were not correlated with overall survival of patients with HCC (Fig. 3d-f). Expression analysis showed that patients with higher VEGF expression levels [VEGF(++) or VEGF(+++), 5YSR = 64.3%] demonstrated considerably less overall survival rates than those with lower VEGF expression levels [VEGF(–) or VEGF(+), 5YSR = 82.6%, P = 0.024) (Fig. 3c, Table 2).
Figure 3.
Overall survival curve for the 234 HCC patients following surgical resection, (b) disease-free survival curve, and overall survival curves on the basis of (c) VEGF expression, (d) Akt expression, (e) HSP70 expression, and (f) HSP27 expression.
Table 2.
Clinicopathological and immunohistochemical prognostic factors affecting on overall Survival
| Factor | N | 3YSR | 5YSR | P value | |
|---|---|---|---|---|---|
| Sex | M | 196 | 85.4 | 79.5 | 0.733 |
| F | 38 | 91.8 | 84.8 | ||
| Age | ≤ 60 | 148 | 83.6 | 78.3 | 0.415 |
| > 60 | 86 | 91.7 | 83.4 | ||
| Child-Pugh class | A | 217 | 87.3 | 80.3 | 0.048 |
| B, C | 17 | 76.0 | 65.2 | ||
| AFP | ≤ 400 ng/ml | 186 | 87.6 | 81.5 | 0.740 |
| > 400 ng/ml | 36 | 81.3 | 73.1 | ||
| Tumor size | ≤ 5cm | 187 | 86.8 | 78.9 | 0.585 |
| > 5cm | 47 | 85.5 | 73.3 | ||
| Tumor number | Single | 200 | 84.6 | 79.0 | 0.193 |
| ≥ two | 34 | 97.1 | 86.3 | ||
| T stage | T1, T2 | 203 | 90.8 | 85.6 | <0.001 |
| T3, T4 | 31 | 58.7 | 39.8 | ||
| Capsule invasion | Negative | 173 | 87.9 | 81.9 | 0.263 |
| Positive | 61 | 82.1 | 70.8 | ||
| Microvascular invasion | Negative | 158 | 87.3 | 81.1 | 0.990 |
| Positive | 76 | 84.5 | 78.6 | ||
| Grade | Well or moderate | 136 | 87.1 | 82.2 | 0.678 |
| Poorly or undifferentiated | 98 | 85.7 | 78.1 | ||
| Stage | I | 132 | 87.5 | 84.0 | <0.001 |
| II | 71 | 98.2 | 88.3 | ||
| III | 30 | 57.2 | 37.9 | ||
| IV | 1 | 0 | 0 | ||
| VEGF expression | (-) or (+) | 197 | 88.0 | 82.6 | 0.024 |
| (++) or (+++) | 37 | 77.4 | 64.3 | ||
| Akt | (-) or (+) | 161 | 86.6 | 80.1 | 0.897 |
| (++) or (+++) | 73 | 85.7 | 75.7 | ||
| HSP70 | (-) or (+) | 92 | 88.8 | 82.5 | 0.344 |
| (++) or (+++) | 142 | 84.9 | 78.7 | ||
| HSP27 | (-) or (+) | 67 | 86.0 | 79.9 | 0.772 |
| (++) or (+++) | 167 | 86.4 | 80.1 |
3YSR; 3-year survival rate
5YSR; 5-year survival rate
Multivariate analysis of prognostic factors for overall survival using the Cox proportional hazards model has been shown in Table 3. VEGF expression [P = 0.017, Hazard ratio (HR) = 2.573) and T stage (P < 0.001, HR = 4.953) were identified as independent prognostic factors for overall survival.
Table 3.
Multivariate analysis of prognostic factors for overall survival and disease free survival
| Prosgnotic factor | category | Overall survival P value | HR | 95% CI |
|---|---|---|---|---|
| Child-Pugh class | A | |||
| B or C | 0.060 | 2.153 | 0.967-4.795 | |
| T stage | T1, T2 | |||
| T3, T4 | <0.001 | 4.953 | 2.591-9.466 | |
| VEGF | (-), (+) | |||
| (++), (+++) | 0.017 | 2.573 | 1.187-5.581 | |
| Prosgnotic factor | category | Disease free survival P value | HR | 95% CI |
| Child-Pugh class | A | |||
| B or C | <0.001 | 3.033 | 1.707-5.390 | |
| T stage | T1, T2 | |||
| T3, T4 | <0.001 | 3.357 | 1.958-5.756 | |
| Capsule invasion | Negative | |||
| Positive | 0.665 | 1.111 | 0.690-1.788 | |
HR; hazard ratio
CI; confidence interval
3.3. Clinicopathological and immunohistochemical factors affecting disease-free survival rates in HCC patients
Of the 234 patients, 101 (43.2%) patients experienced disease recurrence. The patterns of recurrence were as follows: intrahepatic in 80 (79.2%), extrahepatic in 3 (3%), and both intrahepatic and extrahepatic in 18 (17.8%) patients. The 3- and 5-year disease-free survival rates for the 234 patients who underwent liver resection were 58.2 and 49.4%, respectively (Fig 3b). Univariate analysis for disease-free survival showed that sex, age, preoperative AFP level, tumor size, tumor number, and microvascular invasion did not affect overall survival. Child–Pugh class (P < 0.001), T stage (P < 0.001), capsule invasion (P = 0.011), and TNM stage (P < 0.001) were important prognostic factors affecting disease-free survival (Table 4). The expression levels of VEGF, Akt, HSP70, and HSP27 did not significantly affect disease-free survival. Analysis using the Cox proportional hazards model showed that Child–Pugh class (P < 0.001, HR = 3.033) and T stage (P < 0.001, HR = 3.357) were important prognostic factor for disease-free survival in HCC patients (Table 3).
Table 4.
Clinicopathological and immunohistochemical prognostic factors affecting on disease free survival
| Factor | N | 3YSR | 5YSR | P value | |
|---|---|---|---|---|---|
| Sex | M | 196 | 55.4 | 47.5 | 0.112 |
| F | 38 | 72.0 | 60.0 | ||
| Age | ≤ 60 | 148 | 59.2 | 49.2 | 0.825 |
| > 60 | 86 | 56.7 | 49.9 | ||
| Child | A | 217 | 61.4 | 53.2 | <0.001 |
| B, C | 17 | 18.8 | 0 | ||
| AFP | ≤ 400 ng/ml | 186 | 56.8 | 48.2 | 0.945 |
| > 400 ng/ml | 38 | 60.0 | 56.1 | ||
| Tumor size | ≤ 5cm | 187 | 59.3 | 50.8 | 0.707 |
| > 5cm | 54.5 | 44.4 | |||
| Tumor number | Single | 200 | 55.9 | 48.3 | 0.279 |
| ≤ two | 34 | 72.1 | 55.1 | ||
| T stage | T1, T2 | 203 | 62.6 | 54.9 | <0.001 |
| T3, T4 | 31 | 27.5 | 14.6 | ||
| Capsule invasion | Negative | 173 | 62.2 | 53.9 | 0.011 |
| Positive | 61 | 47.2 | 37.8 | ||
| Microvascular invasion | Negative | 158 | 58.0 | 49.3 | 0.938 |
| Positive | 76 | 58.2 | 48.3 | ||
| Grade | Well or moderate | 136 | 58.8 | 47.5 | 0.866 |
| Poorly or undifferentiated | 98 | 57.4 | 51.2 | ||
| Stage | I | 132 | 60.7 | 53.9 | <0.001 |
| II | 71 | 65.4 | 56.5 | ||
| III | 30 | 29.5 | 15.7 | ||
| IV | 1 | 0 | 0 | ||
| VEGF expression | (-) or (+) | 197 | 58.5 | 49.5 | 0.477 |
| (++) or (+++) | 37 | 57.2 | 50.0 | ||
| Akt | (-) or (+) | 161 | 55.0 | 43.3 | 0.114 |
| (++) or (+++) | 73 | 67.6 | 67.6 | ||
| HSP70 | (-) or (+) | 92 | 61.3 | 43.4 | 0.697 |
| (++) or (+++) | 142 | 56.3 | 53.6 | ||
| HSP27 | (-) or (+) | 67 | 60.4 | 48.7 | 0.736 |
| (++) or (+++) | 167 | 56.8 | 49.5 |
3YSR; 3-year survival rate
5YSR; 5-year survival rate
3.4. Comparison of clinicopathological and immunohistochemical factors in patients categorized on the basis of VEGF expression
Table 5 demonstrates the clinicopathological and immunohistochemical differences in patients categorized on the basis of VEGF expression [VEGF(−)/(+) vs. VEGF(++)/(+++) groups]. Compared to the VEGF(−)/(+) group, the VEGF(++)/(+++) group demonstrated a significantly higher proportion of patients with AFP levels higher than 400 ng/mL (P = 0.040), presence of capsule invasion (P = 0.029), and microvascular invasion (P = 0.022). Other prognostic factors were not significantly different between these two groups.
Table 5.
Comparison of clinicopathological and immunohistochemical results according to VEGF expression
| Factor | VEGF (-) / (+) | VEGF (++) / (+++) | P value | |
|---|---|---|---|---|
| Sex | M | 167 | 29 | 0.333 |
| F | 30 | 8 | ||
| Age | ≤ 60 | 124 | 24 | 0.824 |
| < 60 | 73 | 13 | ||
| Child-Pugh class | A | 180 | 37 | 0.064 |
| B, C | 17 | 0 | ||
| AFP | ≤ 400 ng/ml | 160 | 26 | 0.040 |
| < 400 ng/ml | 26 | 10 | ||
| Size | ≤ 5cm | 154 | 33 | 0.125 |
| < 5cm | 16 | 4 | ||
| Number | Single | 170 | 30 | 0.409 |
| ≥two | 27 | 7 | ||
| T | T1, T2 | 173 | 30 | 0.267 |
| T3, T4 | 24 | 7 | ||
| Capsule invasion | Negative | 151 | 22 | 0.029 |
| Positive | 46 | 15 | ||
| Microvascular nvasion | Negative | 139 | 19 | 0.022 |
| Positive | 58 | 18 | ||
| Grade | Well or moderate | 118 | 18 | 0.203 |
| Poorly or undifferentiated | 79 | 19 | ||
| Stage | I | 117 | 15 | 0.177 |
| II | 56 | 15 | ||
| III | 23 | 7 | ||
| IV | 1 | 0 | ||
| Akt | (-) or (+) | 135 | 26 | 0.834 |
| (++) or (+++) | 62 | 11 | ||
| HSP70 | (-) or (+) | 78 | 14 | 0.841 |
| (++) or (+++) | 119 | 23 | ||
| HSP27 | (-) or (+) | 55 | 12 | 0.577 |
| (++) or (+++) | 142 | 25 | ||
| Recurrence | No | 112 | 21 | 0.991 |
| Yes | 85 | 16 |
4. Discussion
Survival outcome of patients with HCC has considerably improved [4]; however, the rate of disease recurrence following surgical resection is high [11, 21] and the effects of postoperative adjuvant therapy are not satisfactory [22]. In the present study, using clinicopathological and immunohistochemical analysis, we demonstrated that Child–Pugh class, T stage, and VEGF expression were important prognostic factors affecting overall survival following surgical resection in HCC patients.
Long-term prognosis of patients with HCC following surgical resection has remained unsatisfactory due to high recurrence rates of this disease [6, 23]. Previous studies have identified several factors that affected prognosis of patients with HCC following surgical resection. Important prognostic factors associated with increased disease recurrence were serum AFP levels [7], type of tumor [8, 23], tumor size [7, 24], stage of differentiation [23, 24], microvascular invasion [6, 8, 23, 24, 25], satellitosis [7, 21], and resection margin [6, 23].
Microvascular invasion is one of the most significant prognostic factors affecting overall survival or recurrence [6, 8, 23, 25]. It is defined as a microscopic tumor invasion detected in portal or hepatic vein of the surrounding liver tissue, which is contiguous to the tumor [8]. In our study, microvascular invasion was not observed to be an important prognostic factor for survival of HCC patients, although the high VEGF expression group demonstrated significantly higher microvascular invasion than the other groups. Similarly, in a previous study, immunohistochemical analysis showed that VEGF expression in HCCs with microscopic vascular invasions was significantly higher than that in HCCs without microscopic vascular invasions [26]. It is difficult to predict the presence of microvascular invasions preoperatively. Previously, a study aiming to define preoperative predictors of microvascular invasion in multinodular HCC showed that serum AFP levels > 400 mg/L, total tumor diameter > 8 cm, and the presence of > 3 tumors were predictors of microvascular invasion [25].
Angiogenesis involves a cascade of sequential steps that ultimately leads to the neovascularization of tumors. The most potent angiogenic factor involved in tumor progression is VEGF [27]. HCC is a highly vascularized type of tumor. The prognostic impact of VEGF in HCC has been previously reported [9, 10, 26, 27, 28, 29]. In a meta-analysis [29] that aimed at understanding the prognostic impact of serum levels and tissue expression of VEGF, it was observed that high VEGF tissue levels predicted poor overall (HR = 2.15, 95% CI = 1.26–3.68) and disease-free (HR = 1.69, 95% CI = 1.23–2.33) survival. And high serum VEGF levels predicted poor overall (HR = 2.35, 95% CI = 1.80–3.07) and disease-free (HR = 2.36, 95% CI = 1.76–3.16) survival. Tissue and serum VEGF levels appeared to be important prognostic factors for estimating overall survival in HCC patients, and may also be useful for defining prognosis in these patients [29]. In one study, patients belonging to the high VEGF expression group had significantly higher capsular infiltration, vascular invasion, and intrahepatic metastasis rates, thereby suggesting that these tumors were clinically aggressive [30].
The PI3K/Akt pathway plays important roles in the regulation of cell growth, survival, and apoptosis inhibition. It also plays an important role in hepatocarcinogenesis. pAkt overexpression was associated with the tumor grade, intrahepatic metastasis, and microvascular invasions [31]. One study showed that inactivation of the Akt/ERK pathway via the upregulation of PTEN resulted in the suppression of proliferation, migration, and invasion of HCC cells, coupled with apoptosis [32]. Another study demonstrated that microRNA-21 decreased PTEN expression levels, which resulted in the activation of Akt and/ or ERK, and enhancement of cell proliferation and epithelial–mesenchymal transition in HCC cells [33]. However, in the present study, immunohistochemical analysis revealed that the expression of Akt was not an important prognostic factor for survival in HCC patients following surgical resection.
In our study, the expression of HSP70 and HSP27 did not affect the overall survival of HCC patients. It has been reported that HSP70 expression was significantly correlated with poor histological grade of differentiation, lack of tumor capsule, and microvascular invasion [34]. HSP70 immunoreactivity was specifically detected in tumor cells and was closely associated with the pathological parameters associated with tumor progression, such as large tumor size, portal vein invasion, and advanced tumor stage [35]. HSP27 expression was previously shown to be associated with the histological grade and survival of HCC patients [36]. Consistent with our results, HSP27 expression did not correlate with clinicopathological factor in overall HCC group in one study, however, the correlation with the prognosis in HBV-associated group was significant [37]. Although very heterogeneous results were obtained in various studies, HSP70 and HSP27 expression levels may play important roles in hepatocarcinogenesis. HSP70 is especially interesting, because it can contribute to tumor progression by promoting tumor cell proliferation in HCC [35]. The anti-apoptotic functions of HSP70 may play an important role in tumor cell proliferation and tumor progression in HCCs, which display high HSP expression [38, 39].
In conclusion, in HCC patients with relatively good liver function, following surgical resection, higher VEGF expression in HCC tissue was a poor prognostic factor in HCC patient. The expression of Akt, HSP70, and HSP27 did not affect overall survival of HCC patients. High VEGF expression was associated with the presence of capsule and microvascular invasion, and consequently with poor overall survival. Molecular targeted therapy is a key advancement for the development of novel HCC drugs. Recently, molecular targeted therapy has been applied in advanced HCCs that could not be surgically treated [40, 41]. Studies for analyzing molecular targets and the underlying pathogenesis mechanisms involved are necessary to predict the outcome(s) of HCC, as well as to understand tumor biology in HCC patients. In the future, the development of novel drugs would be critical for overcoming resistance to chemotherapy in HCC patients.
Acknowledgements
Biospecimens for this study were provided by the Korea University Guro Hospital of Biobank. This research was supported by a faculty research grant from the Korea University (grant number, K0931191).
Footnotes
Conflict of interest statement: Authors state no conflict of interest.
References
- [1].Llovet JM, Bru C, Bruix J.. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in liver disease. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- [2].Bruix J, Sherman M. American Association for the Study of Liver D: Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- [4].Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Annals of surgery. 2011;253(4):745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- [5].Hou YF, Wei YG, Yang JY, Wen TF, Xu MQ, Yan LN, Li B, Chen KF. Microvascular invasion patterns affect survival in hepatocellular carcinoma patients after second hepatectomy. The Journal of surgical research. 2016;200(1):82–90. doi: 10.1016/j.jss.2015.06.069. [DOI] [PubMed] [Google Scholar]
- [6].Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, Taylor BR, Grant DR, Vollmer CM. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. Journal of the American College of Surgeons. 2006;202(2):275–283. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [7].Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147(5):676–685. doi: 10.1016/j.surg.2009.10.043. [DOI] [PubMed] [Google Scholar]
- [8].Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Annals of surgical oncology. 2008;15(5):1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- [9].Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma: a review of literature. Cancer. 2009;115(21):4895–4906. doi: 10.1002/cncr.24537. [DOI] [PubMed] [Google Scholar]
- [10].Tseng PL, Tai MH, Huang CC, Wang CC, Lin JW, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM. et al. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. Journal of surgical oncology. 2008;98(5):349–357. doi: 10.1002/jso.21109. [DOI] [PubMed] [Google Scholar]
- [11].Wang ZL, Liang P, Dong BW, Yu XL, Yu de J. Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective study. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2008;12(2):327–337. doi: 10.1007/s11605-007-0310-0. [DOI] [PubMed] [Google Scholar]
- [12].Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A. Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Molecular cancer research : MCR. 2006;4(7):471–479. doi: 10.1158/1541-7786.MCR-05-0234. [DOI] [PubMed] [Google Scholar]
- [13].Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. Journal of cell science. 2001;114(Pt 13):2375–2382. doi: 10.1242/jcs.114.13.2375. [DOI] [PubMed] [Google Scholar]
- [15].Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell proliferation. 2000;33(6):341–365. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochemical and biophysical research communications. 2001;286(3):433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- [17].Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. Journal of the National Cancer Institute. 2000;92(19):1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- [18].Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. Journal of the National Cancer Institute. 1993;85(7):570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- [19].Fritz A., Percy C, Jack A, International Classification of Diseases for Oncology (ICD-O) 3rd. Geneva: World Health Organization; 2000. [Google Scholar]
- [20].Edge SB, Byrd DR, Compton CC, AJCC Cancer Staging Manual. 7th. New York: Springer; 2009. [Google Scholar]
- [21].Meniconi RL, Komatsu S, Perdigao F, Boelle PY, Soubrane O, Scatton O. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery. 2015;157(3):454–462. doi: 10.1016/j.surg.2014.10.011. [DOI] [PubMed] [Google Scholar]
- [22].Kim do Y, Ahn SH, Kim SU, Choi SB, Lee KH, Park MS, Park JY, Lee do Y, Han KH, Kim KS. Adjuvant hepatic arterial infusional chemotherapy with 5-fluorouracil and cisplatin after curative resection of hepatocellular carcinoma. Oncology. 2011;81(3-4):184–191. doi: 10.1159/000333827. [DOI] [PubMed] [Google Scholar]
- [23].Kim H, Park MS, Park YN, Kim H, Kim KS, Choi JS, Ahn SH, Han KH, Kim MJ, Kim KW. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei medical journal. 2009;50(6):789–795. doi: 10.3349/ymj.2009.50.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harimoto N, Shirabe K, Ikegami T, Yoshizumi T, Maeda T, Kajiyama K, Yamanaka T, Maehara Y. Postoperative complications are predictive of poor prognosis in hepatocellular carcinoma. The Journal of surgical research. 2015;199(2):470–477. doi: 10.1016/j.jss.2015.06.012. [DOI] [PubMed] [Google Scholar]
- [25].Zhao WC, Fan LF, Yang N, Zhang HB, Chen BD, Yang GS. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2013;39(8):858–864. doi: 10.1016/j.ejso.2013.04.003. [DOI] [PubMed] [Google Scholar]
- [26].Huang GW, Yang LY, Lu WQ. Expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in hepatocellular carcinoma: Impact on neovascularization and survival. World journal of gastroenterology. 2005;11(11):1705–1708. doi: 10.3748/wjg.v11.i11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- [28].Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, Fan ST, Wong J. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Annals of surgery. 2001;233(2):227–235. doi: 10.1097/00000658-200102000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. British journal of cancer. 2009;100(9):1385–1392. doi: 10.1038/sj.bjc.6605017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Deli G, Jin CH, Mu R, Yang S, Liang Y, Chen D, Makuuchi M. Immunohistochemical assessment of angiogenesis in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World journal of gastroenterology. 2005;11(7):960–963. doi: 10.3748/wjg.v11.i7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Grabinski N, Ewald F, Hofmann BT, Staufer K, Schumacher U, Nashan B, Jucker M. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Molecular cancer. 2012;11:85. doi: 10.1186/1476-4598-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y, Gao Q, Su C. Matrine derivative WM130 inhibits hepatocellular carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Cancer letters. 2015;368(1):126–134. doi: 10.1016/j.canlet.2015.07.035. [DOI] [PubMed] [Google Scholar]
- [33].Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L. et al. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer letters. 2013;337(2):226–236. doi: 10.1016/j.canlet.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [34].Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, Cho JW, Yoo BC, Jung GH, Park CK. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World journal of gastroenterology. 2005;11(14):2072–2079. doi: 10.3748/wjg.v11.i14.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Joo M, Chi JG, Lee H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. Journal of Korean medical science. 2005;20(5):829–834. doi: 10.3346/jkms.2005.20.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].King KL, Li AF, Chau GY, Chi CW, Wu CW, Huang CL, Lui WY. Prognostic significance of heat shock protein-27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer. 2000;88(11):2464–2470. doi: 10.1002/1097-0142(20000601)88:11<2464::aid-cncr6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [37].Harimoto N, Shimada M, Aishima S, Kitagawa D, Itoh S, Tsujita E, Maehara S, Taketomi A, Tanaka S, Shirabe K. et al. The role of heat shock protein 27 expression in hepatocellular carcinoma in Japan: special reference to the difference between hepatitis B and C. Liver international : official journal of the International Association for the Study of the Liver. 2004;24(4):316–321. doi: 10.1111/j.1478-3231.2004.0927.x. [DOI] [PubMed] [Google Scholar]
- [38].Kaur J, Ralhan R. Differential expression of 70-kDa heat shock-protein in human oral tumorigenesis. International journal of cancer. 1995;63(6):774–779. doi: 10.1002/ijc.2910630604. [DOI] [PubMed] [Google Scholar]
- [39].Zhao ZG, Shen WL. Heat shock protein 70 antisense oligonucleotide inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. World journal of gastroenterology. 2005;11(1):73–78. doi: 10.3748/wjg.v11.i1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- [41].Nakano M, Tanaka M, Kuromatsu R, Nagamatsu H, Tajiri N, Satani M, Niizeki T, Aino H, Okamura S, Iwamoto H. et al. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study. Cancer medicine. 2015;4(12):1836–1843. doi: 10.1002/cam4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]