Abstract
Background/Aims
A grading system for the endoscopic features of eosinophilic esophagitis (EoE) has recently been validated. The EoE Endoscopic Reference Score (EREFS) incorporates both inflammatory and remodeling features of EoE. High resolution impedance planimetry using the functional luminal imaging probe (FLIP) is a technique for quantification of esophageal remodeling. The aim of this study was to evaluate the association between endoscopic severity with EREFS and esophageal distensibility as measured with the FLIP.
Methods
Upper gastrointestinal endoscopy with biopsies and FLIP were performed in 72 adults with EoE. Endoscopic features of edema, rings, exudates, furrows, and stricture were evaluated using the EREFS system. Esophageal distensibility metrics obtained by FLIP including the distensibility slope and distensibility plateau were compared with EREFS parameters. Bivariate associations between EREFS parameters and histologic eosinophil density were assessed.
Results
Higher ring scores were associated with lower distensibility plateau (rs = −0.46; P < 0.0001). An association was found between severity of exudates and eosinophil density (rs = 0.27; P = 0.02), as well as between furrows and eosinophil density (rs = 0.49; P < 0.0001). Severity of exudates and furrows, and degree of eosinophilia were not associated with the distensibility parameters.
Conclusions
Endoscopic assessment of ring severity can serve as a marker for esophageal remodeling and may be useful for food impaction risk stratification in EoE. Eosinophil count was not significantly associated with esophageal distensibility, consistent with previous reports of dissociation between inflammatory activity and fibrostenosis in EoE. Endoscopic inflammatory features show a mild correlation with histopathology but should not replace histologic indices of inflammation.
In brief
It is difficult to predict the risk of food impaction in eosinophilic esophagitis (EoE). Chen et al. found a correlation between functional measurements of esophageal compliance made with an endoluminal imaging probe (EndoFLIP) and a previously validated score for EoE. In particular, the severity of rings correlated with decreased compliance and this may therefore serve as a predictor for food impaction in these patients.
Introduction
Eosinophilic esophagitis (EoE) is a clinicopathologic disease characterized by esophageal dysfunction and eosinophil-predominant inflammation in the esophagus [1]. In recent years, EoE has become increasingly recognized as a leading cause of dysphagia and food impaction in the adult population [2]. The clinical manifestations of EoE in adults are attributed to esophageal luminal narrowing, which results from the remodeling effects of chronic inflammation. Evidence for esophageal remodeling in EoE includes histologic demonstration of epithelial expansion, subepithelial fibrosis, and angiogenesis, as well as macroscopic demonstration of esophageal strictures and increased thickness of the esophageal mucosa, submucosa, and muscularis propria [3–5].
The high degree of effectiveness of esophageal dilation in providing long-lasting relief of dysphagia in adults with EoE, despite the persistence of inflammation, highlights the central importance of tissue remodeling in symptom generation [6]. However, as opposed to inflammation, which can be evaluated by histopathology, there are currently no validated biomarkers to measure the degree of tissue remodeling and fibrosis in EoE. Standard endoscopic biopsies assess the remodeling changes in the epithelial space but are adequate to evaluate the presence of subepithelial fibrosis in only a minority of cases [7].
One potential means for assessing disease activity that provides information on esophageal remodeling is endoscopic imaging. Characteristic esophageal abnormalities evident on endoscopy in EoE include edema (loss of vascular markings), rings (trachealization), exudates (white plaques), longitudinal furrows (vertical lines), and strictures. However, assessment of the endoscopic findings of EoE can be quite subjective. A recent meta-analysis by Kim et al., which included 100 articles on the endoscopic findings of EoE, found significant heterogeneity among publications in endoscopic descriptions [8]. While the overall level of sensitivity of endoscopy for EoE was modest, prospective studies have reported higher sensitivities, exceeding 90% [9–11].
The lack of a unified nomenclature for describing the endoscopic features of EoE presents a major limitation in endoscopic assessment of disease severity. Recently, we proposed a classification system for endoscopic evaluation of EoE (EREFS) in which the endoscopic features of EoE (edema, rings, exudates, furrows, and strictures) were systematically graded (Table 1) [12]. Intra- and interobserver agreement using EREFS has been independently validated by US and European investigators [12,13]. Endoscopic features measured by EREFS are a major determinant of overall disease severity judged by gastroenterologists [14] and are associated with symptom severity using a recently validated patient-reported outcome instrument for EoE [15].
Table 1.
Endoscopic reference scoring system for the classification and grading of the esophageal features of eosinophilic esophagitis (EREFS).
| Fixed rings | Grade 0 | None |
| Grade 1 | Mild (subtle circumferential ridges) | |
| Grade 2 | Moderate (distinct rings that do not impair passage of a standard diagnostic adult endoscope of outer diameter 8–9.5 mm) | |
| Grade 3 | Severe (distinct rings that do not permit passage of a diagnostic endoscope) | |
| Exudates | Grade 0 | None |
| Grade 1 | Mild (lesions involving <10% of the esophageal surface area) | |
| Grade 2 | Severe (lesions involving >10% of the esophageal surface area) | |
| Furrows | Grade 0 | Absent |
| Grade 1 | Present | |
| Edema | Grade 0 | Absent (distinct vascularity present) |
| Grade 1 | Loss of clarity or absence of vascular markings | |
| Stricture | Grade 0 | Absent |
| Grade 1 | Present |
The endoluminal functional luminal imaging probe (endoFLIP, also known as FLIP) is a novel technology that utilizes high resolution impedance planimetry to evaluate distensibility within a hollow organ. The FLIP device provides data on cross-sectional area (CSA) at multiple sites along the probe while measuring intraluminal pressure during controlled volume distensions of an infinitely compliant bag. The pressure–volume relationships generated allow both the compliance and distensibility of a hollow organ to be determined.
FLIP technology has been used in the esophagus to assess esophagogastric junction (EGJ) distensibility in gastroesophageal reflux disease (GERD) [16] and in treated and untreated achalasia patients [17]. Our previous study demonstrated a substantial reduction in esophageal distensibility in adults with EoE compared with controls that was independent of the degree of esophageal eosinophilia [18]. The quantification of stiffness of the esophageal wall by FLIP provides an objective measure of tissue remodeling at the whole organ level.
The aim of this study was to evaluate the association between endoscopic severity in EoE patients, as graded with the EREFS system, and esophageal distensibility, as measured by FLIP.
Material and methods
Subjects
A total of 72 adults with EoE (51 men, 21 women; age range 18–68) were recruited from the Gastroenterology Clinic at Northwestern Medical Faculty Foundation based on clinical documentation of esophageal symptoms (dysphagia, food impaction, chest pain, or heartburn) and endoscopic biopsies with histopathology that confirmed EoE with ≥15 eosinophils / high power field (eos/hpf) after at least 8 weeks of proton pump inhibitors (PPI). The area of one hpf is 0.2 mm2.
Patients overlapped with the cohort from our previously published study [19]. Patients with prior gastrointestinal surgery or significant cardiopulmonary, neurologic, and psychiatric disorders were excluded from the study. All study participants gave written informed consent and the study protocol was approved by the Northwestern University Institutional Review Board.
Endoscopy
Participants underwent esophagogastroduodenoscopy (EGD) with biopsies under moderate sedation to assess endoscopic EoE findings and the degree of inflammation. A diagnostic adult gastroscope was used (Olympus GIF H180; Olympus Corporation, Tokyo, Japan) unless esophageal luminal narrowing did not allow passage of this scope, in which case a pediatric gastroscope (Olympus GIF-XP160) was used. Still images were taken during the endoscopic procedures, which were performed by one of four esophageal specialists (I.H., J.E.P., P.J.K., N.G.). The endoscopic features were graded using the EREFS system as detailed above. Biopsies were taken from both the distal and proximal esophagus, with four samples being taken from each location.
The FLIP system
A commercially developed imaging probe (EndoFLIP; Crospon Medical Devices, Galway, Ireland) was used to measure esophageal body distensibility based on the concepts and components descried previously [18,20]. Briefly, the device contains a cylindrical bag that can be inflated with a conductive fluid, while simultaneously CSA and intrabag pressure are measured to calculate distensibility.
The FLIP assembly used in this study was 240 cm in length with a 3-mm outer diameter. An infinitely compliant bag mounted on the distal probe was fabricated to assume a cylindrical shape 10 cm in length between tapering ends that were sealed to the assembly. The minimal to maximal range of CSA measureable by the device was 10–491 mm2. The 8-cm segment within the bag that was designed for the impedance planimetry measurement was composed of 17 ring electrodes spaced 5 mm apart. The assembly also contained one solid-state pressure transducer for measuring intrabag pressure. Measurements from the 16 electrode pairs and pressure transducers were sampled at 10 Hz with the data acquisition system and were transmitted to the recording unit, which displayed them in real time as a cylinder of 8 cm in length and varying diameter along its length, reflective of the 16 measured intraluminal diameters.
FLIP protocol
At the time of endoscopy, the FLIP device was placed transorally until the distal end of its bag was straddling the EGJ and the FLIP bag assumed an hourglass shape when distended. The probe was positioned with the most distal recording site 3 cm above the EGJ. CSAs within the esophagus were measured during 2-mL stepwise distensions, beginning with 2 mL and increasing to a maximum of 40 mL (each step lasted for 5–20 seconds). When measurements were interrupted by peristalsis, they were prolonged or repeated to establish stable measurements.
The recording unit was set to stop infusing and display an alarm signal if the intrabag pressure exceeded 60 mmHg to prevent unintended dilation of a small-caliber or poorly compliant esophagus.
Data analysis
Distension volume, intrabag pressure, and 16 channels of impedance planimetry data from each subject were exported to MATLAB (The Math Works, Natick, Massachusetts, USA) for further analysis using a customized MATLAB program with four functions: (i) minimization of vascular and respiratory artifacts using a median filter of the intrabag pressure; (ii) isolation of the recording pressure between esophageal contractions using a nonlinear pulse-detection technique; (iii) identification of the distensibility plateau after modeling the esophageal distensibility from the pressure–CSA relationship with a polynomial regression method and extraction of the slope of the best-fit line for the initial phase of distension and curve fitting; and (iv) filtering out the signals related to respiration, vascular pulsation, and esophageal contractions using a wavelet decomposition of data.
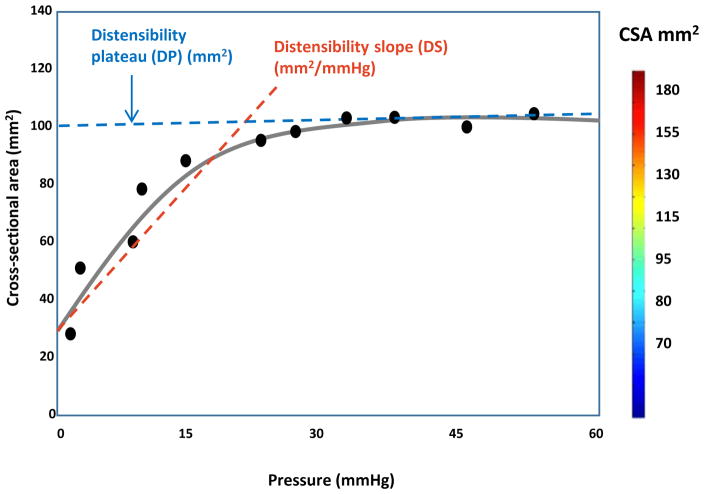
Distensibility was plotted as a function of the narrowest CSA versus intrabag pressure for each subject to derive two distensibility metrics: distensibility slope and distensibility plateau (Fig 1). The luminal CSA increased precipitously with rising intrabag pressure until a certain point, after which little overall change in CSA occurred with further change in pressure. The slope during the initial uptrend of the distensibility curve reflects wall stiffness during serial bag distension and was defined as the distensibility slope (mm2/mmHg). The maximum CSA reached after the plateau was defined as the distensibility plateau (mm2). Our prior study had demonstrated a significant decrease in distensibility slope and distensibility plateau in EoE patients compared with controls [18]. The treating physicians were blinded to the distensibility data, which was assessed independently using a customized MATLAB program.
Fig. 1.
Figure modified from Pandolfino et al. [17]. Distensibility was plotted as a function of the narrowest cross-sectional area versus intrabag pressure. The distensibility metrics – distensibility slope (DS) and distensibility plateau (DP) – were derived from this curve.
The primary endoscopic end points of interest were: (i) endoscopic ring scores based on EREFS classification and scoring system; (ii) severity of exudates and furrows; (iii) severity of eosinophilia on biopsy.
EREFS scores were given during endoscopy by one of the four endoscopists and endoscopic images were separately given EREFS grading by another investigator (J.C.) for confirmation. We used the EREFS scores assigned by the primary endoscopists during the endoscopy. In cases where EREFS scores were incompletely recorded, captured still images were reviewed for EREFS features, except for edema, which is better assessed during endoscopy or with video imaging [12,13]. Mean eosinophil count, determined by the average maximal eosinophil count from distal and proximal esophageal biopsy specimens, and distal eosinophil count were included in the analysis as these were thought to be more relevant than proximal eosinophil count because the distal positioning of the FLIP probe.
Statistical analysis
Descriptive statistics (median and range or interquartile range [IQR] for continuous data and counts with percentages for categorical data) were used to summarize participant demographics, esophageal distensibility, and eosinophil count parameters, along with ring scores (0, 1, 2, 3) and exudates/furrows scores (none, mild, severe). Scatter plots and locally-weighted regression (LOESS) curves were generated to illustrate the association between distensibility plateau and ring score and also to aid in the assessment of assumptions for parametric analyses.
Because of the ordinal nature of ring score, the potentially non-normally distributed outcomes, and relatively sparse counts within specific categories, the nonparametric Spearman’s sample correlation coefficient was employed to examine bivariate relationships between ring/exudates/furrows score and distensibility/eosinophil count parameters. The null hypotheses of no association between ranked values of relevant outcomes were tested at the 5% level of significance. The estimated 95% confidence limits (95%CL) for Spearman’s sample correlation coefficient were also reported. Additionally, we employed the Cochran–Armitage test for trend in order to explore the association between ring score and food impaction.
Because of the exploratory nature of this study and the analyses involved, there were no adjustments for multiple hypothesis tests in order to control type I error rate. As such, marginally significant results should be interpreted with caution.
Results
Demographic data
There were 72 patients included in this study. Table 2 presents a summary of their demographic, treatment, and EGD findings. As is typical for an adult EoE cohort, the patients were predominantly men (71%) with a median age of 37 years. The median age of symptom onset was 30 years (range 5–67). Median diagnostic delay was 3 years (range 0–33), and median disease duration at the time of assessment was 5 years (range 0–49). There were 51 patients (71%) who had a history of atopy (64% with allergic rhinitis, 24% with asthma, and 8% with atopic dermatitis).
Table 2.
Demographics and endoscopic findings of the 72 patients with eosinophilic esophagitis who were entered into this study.
| Median age (range), years | 37 (18–68) | |
| Number of men (%) | 51 (71%) | |
| Median age at symptom onset (range), years | 30 (5–67) | |
| Median diagnostic delay (range), years | 3 (0–33) | |
| Median duration of disease (range), years | 5 (0–49) | |
| History of atopy, n (%) | 51 (71%) | |
| Predominant symptom at the time of EGD and FLIP | None | 1 (1%) |
| Dysphagia | 65 (90%) | |
| Heartburn | 0 (0%) | |
| Chest pain | 5 (7%) | |
| Food impaction | 1 (1%) | |
| Food impaction history, n (%) | 25 (35%) | |
| Treatment at the time of EGD and FLIP | None | 15 (21%) |
| PPI | 45 (63%) | |
| Topical steroids | 3 (4%) | |
| Topical steroids + PPI | 4 (6%) | |
| Diet | 1 (1%) | |
| Diet + PPI | 2 (3%) | |
| Diet + PPI + steroids | 1 (1%) | |
| Research drug | 1 (1%) | |
| EGD findings | Normal | 2 (3%) |
| Rings | ||
| Grade 0 | 5 (7%) | |
| Grade 1 | 43 (60%) | |
| Grade 2 | 18 (25%) | |
| Grade 3 | 6 (8%) | |
| Exudates | ||
| Grade 0 | 33 (46%) | |
| Grade 1 | 34 (47%) | |
| Grade 2 | 5 (7%) | |
| Furrows | ||
| Grade 0 | 13 (18%) | |
| Grade 1 | 56 (78%) | |
| Grade 2 | 3 (4%) | |
| Stricture | 33 (46%) | |
| Narrow caliber | 23 (32%) | |
EGD, esophagogastroduodenoscopy; FLIP, functional luminal imaging probe; PPI, proton pump inhibitor.
At the time of the FLIP study, the predominant symptoms were dysphagia (90%) and chest pain (7%) with 21% of the patients being on no treatment, 63% on PPI alone, 4% on topical steroids alone, 6% on a combination of topical steroid and PPI, 1% on an elimination diet, 3% on an elimination diet and PPI, and 1% on a combination of an elimination diet, PPI, and topical steroid.
The most common endoscopic features were rings in 93% of the patient cohort, followed by furrows in 82% and exudates in 54%. A focal stricture was found in 46%, and a narrow-caliber esophagus was identified in 32%. All of the patients had previously been on a PPI trial for at least 8 weeks to rule out PPI-responsive esophageal eosinophilia.
At the time of the EGD and FLIP, 50 patients (69%) had active eosinophilia with either a distal or proximal eosinophil density of ≥15 per hpf. The median proximal eosinophil count (IQR) was 21.5 (0.0–50.0) eos/hpf, and the median distal eosinophil count (IQR) was 22.5 (1.5–50.0) eos/hpf.
Distensibility according to ring scores
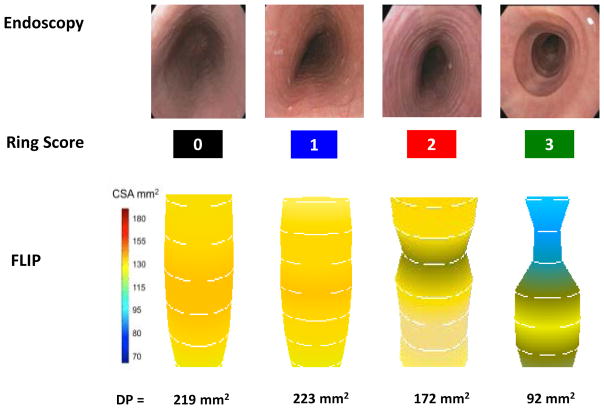
The severity of esophageal rings was scored according to the EREFS grading system: 7% of the patient cohort had a ring grade of 0, 60% had a ring grade of 1, 25% had a ring grade of 2, and 8% had a ring grade of 3. Fig. 2 includes examples of the endoscopic appearances and corresponding FLIP images based on ring severity. High ring grades (grade 2 or 3) are seen as distinct rings endoscopically that show a relationship with luminal narrowing on FLIP imaging. Table 3 presents the median (IQR) of the distensibility parameters according to the ring scores. Severe rings were associated with a lower distensibility slope and distensibility plateau. Spearman’s nonparametric sample correlation coefficient demonstrates a significant association between each of the distensibility parameters and the ring scores. The distensibility plateau had a moderate inverse relationship with ring severity (rs = −0.46; P < 0.0001).
Fig. 2.
Example endoscopic appearance and representative functional luminal imaging probe (FLIP) images during volume distension for each severity of ring score. Luminal narrowing is evident on the FLIP images with moderate to severe rings, and the distensibility plateau (DP) is decreased in severe rings.
Table 3.
Distensibility metrics and distal eosinophil count according to the endoscopic severity of rings, exudates, and furrows.
| Distensibility slope | Distensibility plateau | Distal eosinophil count | |
|---|---|---|---|
| Ring score, median (IQR) | |||
|
| |||
| 0 (n = 5) | 4.58 (2.98–4.60) | 197.0 (160.0–240.0) | 2.0 (1.0–30.0) |
| 1 (n = 43) | 5.65 (3.62–7.61) | 224.5 (184.0–248.0) | 20.0 (1.0–50.0) |
| 2 (n = 18) | 3.51 (2.50–5.20) | 174.5 (124.0–200.0) | 30.0 (15.0–50.0) |
| 3 (n = 6) | 1.48 (0.42–3.30) | 90.0 (60.0–108.0) | 32.5 (0.0–50.0) |
|
| |||
| Spearman’s r (95% CL) | −0.33 (−0.52, −0.11) | −0.46 (−0.63, −0.26) | 0.18 (−0.06, 0.39) |
| P value | P = 0.004 | P < 0.0001 | P = 0.14 |
|
| |||
| Exudates, median (IQR) | |||
|
| |||
| None (n = 33) | 4.60 (3.30–7.61) | 200.0 (160.0–240.0) | 10.0 (1.0–30.0) |
| Mild (n = 34) | 4.60 (2.90–6.00) | 190.0 (171.0–250.0) | 30.0 (5.0–50.0) |
| Severe (n = 5) | 1.51 (0.42–6.18) | 99.0 (80.0–200.0) | 35.0 (30.0–98.0) |
|
| |||
| Spearman’s r (95% CL) | −0.16 (−0.38, 0.08) | −0.15 (−0.37, 0.09) | 0.27 (0.05, 0.48) |
| P value | P = 0.18 | P = 0.22 | P = 0.02 |
|
| |||
| Furrows, median (IQR) | |||
|
| |||
| None (n = 13) | 5.90 (3.30–7.70) | 200.0 (150.0–240.0) | 0.0 (0.0–0.0) |
| Mild (n = 56) | 4.30 (2.53–6.25) | 197.0 (160.0–240.0) | 30.0 (10.0–50.0) |
| Severe (n = 3) | 6.45 (0.39–10.0) | 200.0 (80.0–314.0) | 30.0 (30.0–50.0) |
|
| |||
| Spearman’s r (95% CL) | −0.10 (−0.33, 0.14) | 0.01 (−0.22, 0.24) | 0.49 (0.29, 0.65) |
| P value | P = 0.40 | P =0.92 | P < 0.0001 |
IQR, interquartile range; CL, confidence limits.
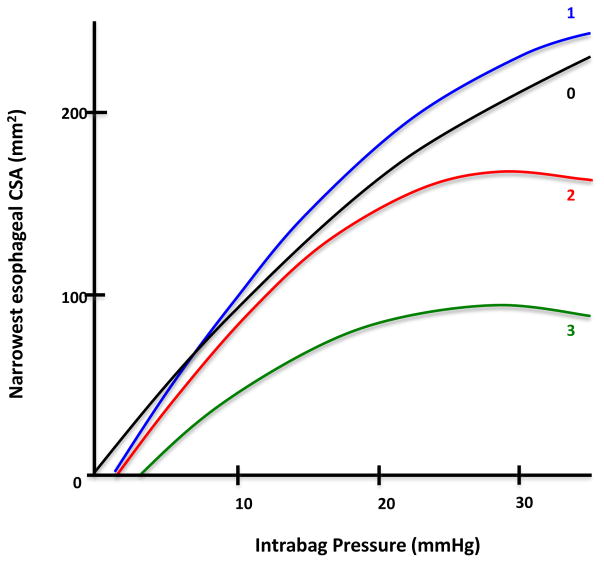
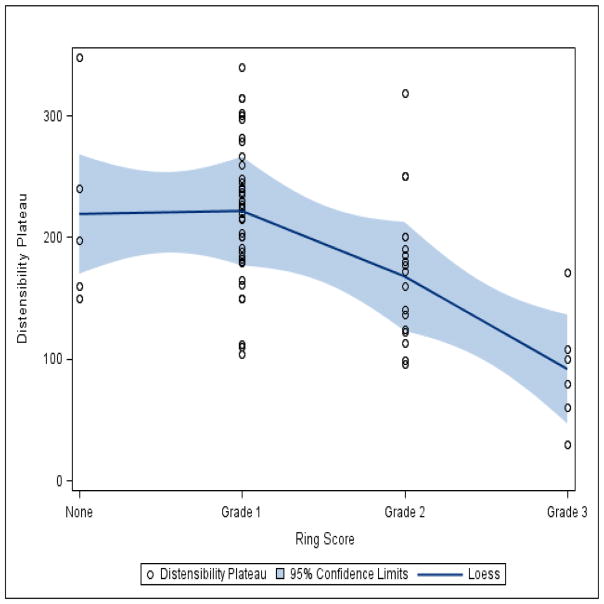
Fig. 3 shows distensibility curves (mean narrowest CSA at various levels of intrabag pressure of patients in each ring group) compiled using summary statistics of pooled patient data for each ring score. The distensibility curves for ring scores of 0 and 1 appear similar to one another. The curves for ring scores of 2 and 3 show decreased slopes and lower distensibility plateaus in comparison, evident by little overall increment in the esophageal CSA despite a change in pressure once the plateau is reached. Fig. 4 presents a LOESS regression curve fitted to the scatterplot of distensibility plateau versus ring scores. This again shows a decrease in the distensibility plateau with increasing ring severity score.
Fig. 3.
Distensibility curves (mean narrowest cross-sectional area [CSA] at various levels of intrabag pressure) compiled using summary statistics of pooled patient data for each ring severity score. The distensibility curves for ring scores of 2 and 3 showed decreased slopes and lower plateaus than those for ring scores of 0 and 1.
Fig. 4.
Locally-weighted regression curve (LOESS) fitted to the scatterplot of distensibility plateau versus ring scores. A decrease in distensibility plateau is evident with increasing ring severity score.
Ring severity and food impaction history
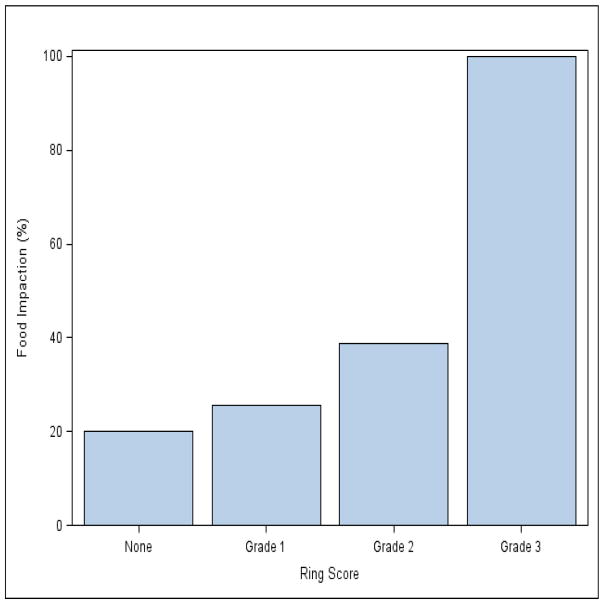
A history of food impaction was reported in 20%, 25%, 39%, and 100% of patients with a ring score of 0, 1, 2, and 3, respectively (Fig. 5). The Cochran–Armitage test for trend result suggested a significant association between ring severity and food impaction history with a P value of 0.002.
Fig. 5.
Histogram showing the percentage of patients with a history of food impaction for each ring score. Ring severity was associated with a significant difference in food impaction history (P = 0.002).
Eosinophil count, inflammatory score, and distensibility
Mean esophageal eosinophil count appeared to be marginally associated with ring severity (rs = 0.25; P = 0.03); however, our results do not suggest evidence of an association between distal esophageal eosinophil count and ring scores (P = 0.14) (Table 3).
Table 3 also includes the median (IQR) of distensibility parameters according to the severity of exudates and furrows. Analyses in general suggest a lack of association between the distensibility parameters and these endoscopic features. However, a modest correlation between mean/distal eosinophil count and severity of exudates/furrows indicates that a higher degree of eosinophil inflammation is associated with worse endoscopic features for furrows and exudates. The results of our analysis did not suggest a significant association between distensibility parameters and degree of esophageal eosinophilia (data not shown).
Discussion
The current clinical paradigm for disease activity assessment in EoE focuses on patient-reported symptom burden and histopathology. Histopathology is a reproducible measure of inflammation but has demonstrated limited direct association with symptom outcomes [22]. Symptom assessment, on the other hand, has intrinsic limitations as patients with EoE often incorporate adaptive behaviors, such as extensive mastication, prolonged meal times, and avoidance of specific food textures, to minimize their dysphagia. In light of this, objective assessment of esophageal remodeling, which is a primary factor responsible for risk of food impaction, becomes a clinically relevant outcome in the management of EoE. Solid food dysphagia is the most common presenting symptom in adult patients with EoE and food impaction necessitating endoscopic removal occurs in 33%–53% of these patients [21].
This study is the first to assess the association between endoscopically detected esophageal features of remodeling and quantitative measurement of esophageal distensibility and compliance. Comparisons between a recently validated endoscopic scoring system, with metrics based on impedance planimetry, revealed significant associations between the severity of esophageal rings and reduction in esophageal distensibility. This lends credence to the utility of endoscopic assessment of ring scores as a determinant of esophageal remodeling in EoE. A recent study involving retrospective chart review of adult patients with EoE found that endoscopy had a poor sensitivity (14.7%; 95% confidence interval [CI] 5.0%–31.1%) and only modest specificity (79.2%; 57.8%–92.9%) for detection of a narrowed esophagus of <21 mm diameter, using barium esophagram as the gold standard for comparison [23]. However, our study supports the usefulness of systematic endoscopic examination as a surrogate, albeit less precise, indicator of remodeling in EoE.
One consequence of esophageal tissue remodeling with decreased esophageal distensibility is increased risk of food impaction. In our earlier study, patients with a history of food impaction tended to have significantly lower distensibility metrics compared with those without a history of food impaction [19]. Among EoE patients with a distensibility plateau <125 mm2, 94% had a history of food impaction compared with 35% with a distensibility plateau of 126–225 mm2. None of the patients with a distensibility plateau >225 mm2 had a history of food impaction. Moreover, patients experiencing food impaction during clinical follow-up had significantly lower distensibility compared with those without food impaction.
Systematic endoscopic examination findings have also been shown to correlate with clinical symptoms. A recent study showed a positive relationship between EREFS scores and a validated adult patient-reported outcome instrument to assess EoE severity [14,15]. In addition, data from our current cohort also showed that endoscopic assessment of ring severity was associated with a significant difference in food impaction history. This supports the clinical relevance of ring severity in terms of the outcome of food impaction.
In this dataset, ring severity was only marginally associated with mean eosinophil count and was not associated with distal eosinophil count, whereas similar analyses suggested a positive association between the severity of furrows/exudates and mean/distal eosinophil count. This indicates dissociation between the degree of tissue remodeling and inflammation. This is consistent with a previous study by Schoepfer et al. [6] that noted relief of dysphagia in over 90% of patients following esophageal dilation in spite of persistent esophageal eosinophilic inflammation.
Our findings are also consistent with prior reports that have linked linear furrows and white exudates found endoscopically with mucosal eosinophilia and eosinophil microabscess formation [24]. The identification of furrows and exudates might be useful in targeting esophageal biopsies. Based on existing studies, however, the absence of these endoscopically identifiable features should not obviate the procurement of esophageal biopsies in patients with suspected EoE. In this study, FLIP quantification of esophageal distensibility was not significantly associated with the degree of mucosal eosinophilia, again indicative of the dissociation between the inflammatory and remodeling process in adults with EoE.
Several limitations of our study exist. Because only adult patients were included, the findings cannot be generalized to pediatric EoE where distinct differences exist in the presence of esophageal remodeling consequences. The differences in presenting symptoms and low prevalence of esophageal strictures in children with EoE indicates that pathophysiologic factors other than distensibility are likely to be important.
The small number of patients included, especially in the highest and lowest ring scores limits the power to detect significant associations, as well as our ability to make inferences regarding those patients that would fall into the extreme ring scores.
In 18 of the 72 patients in our study (25%), the endoscopist did not report the EREFS at the time of endoscopy. In these cases, captured still images of the esophagus were reviewed and EREFS scores were assigned. Because of the difficulty in assessment of edema on still images, we chose to omit this feature from the analyses. A prospective study with video capture and blinded analyses of all five EREFS features is currently underway.
The FLIP balloon had a longitudinal recording segment of 8 cm for impedance planimetry measurement, thereby limiting interrogation of the entire esophageal body. A balloon with increased length is currently being investigated to address this drawback. In addition, treatment at the time of our study was heterogeneous, which precluded the possibility of investigating specific treatment effects with either ring or distensibility scores.
Although the data was prospectively collected, our analysis was retrospective and patients were not followed longitudinally at defined intervals. In addition, patient-reported outcomes were not prospectively obtained using validated instruments to assess the association between symptoms and endoscopic findings.
In summary, our study demonstrated that grading of ring severity using the EREFS endoscopic classification and grading system provides a semiquantitative estimate of tissue remodeling in EoE when compared to quantitative measures of esophageal distensibility by impedance planimetry. The demonstration of furrows and white exudates are macroscopic signs of inflammation associated with microscopic eosinophilic inflammation in EoE. Eosinophil count was not significantly correlated with esophageal distensibility, consistent with previous reports of dissociation between concurrent inflammatory activity and fibrostenosis in EoE. Future research is needed to prospectively assess the utility of the endoscopic characteristics as clinically relevant outcomes in trials of novel therapeutics in EoE.
Acknowledgments
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under grant number R01DK079902.
Footnotes
Competing interests: None.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 2.Kidambi T, Toto E, Ho N, et al. Temporal trends in the relative prevalence of dysphagia etiologies from 1999–2009. World J Gastroenterol. 2012;18:4335–4341. doi: 10.3748/wjg.v18.i32.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Lucendo AJ, Arias A, De Rezende LC, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol. 2011;128:1037–1046. doi: 10.1016/j.jaci.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Mishra A, Wang M, Pemmaraju VR, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–1070. doi: 10.1038/ajg.2009.657. [DOI] [PubMed] [Google Scholar]
- 7.Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:297–316. doi: 10.1016/j.gtc.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HP, Vance RB, Shaheen NJ, et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988–996. e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohil R, Newbury R, Fox L, et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–429. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–1537. 37e1. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Peterson KA, Thomas KL, Hilden K, et al. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci. 2010;55:1313–1319. doi: 10.1007/s10620-009-0859-4. [DOI] [PubMed] [Google Scholar]
- 12.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 13.van Rhijn BD, Warners MJ, Curvers WL, et al. Evaluating the Endoscopic Reference Score for eosinophilic esophagitis: moderate to substantial intra- and interobserver reliability. Endoscopy. 2014;46:1049–1055. doi: 10.1055/s-0034-1377781. [DOI] [PubMed] [Google Scholar]
- 14.Schoepfer AM, Panczak R, Zwahlen M, et al. How do gastroenterologists assess overall activity of eosinophilic esophagitis in adult patients? Am J Gastroenterol. 2015;110:402–414. doi: 10.1038/ajg.2015.32. [DOI] [PubMed] [Google Scholar]
- 15.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255–1266. e21. doi: 10.1053/j.gastro.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwiatek MA, Pandolfino JE, Hirano I, et al. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP) Gastrointest Endosc. 2010;72:272–278. doi: 10.1016/j.gie.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil. 2013;25:496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicodeme F, Hirano I, Chen J, et al. Esophageal Distensibility as a Measure of Disease Severity in Patients with Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101–1107. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon BP, Frokjaer JB, Kunwald P, et al. The functional lumen imaging probe (FLIP) for evaluation of the esophagogastric junction. Am J Physiol Gastrointest Liver Physiol. 2007;292:G377–G384. doi: 10.1152/ajpgi.00311.2006. [DOI] [PubMed] [Google Scholar]
- 21.Desai TK, Stecevic V, Chang CH, et al. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61:795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 22.Hirano I. Therapeutic end points in eosinophilic esophagitis: is elimination of esophageal eosinophils enough? Clin Gastroenterol Hepatol. 2012;10:750–752. doi: 10.1016/j.cgh.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther. 2014;40:1333–1340. doi: 10.1111/apt.12977. [DOI] [PubMed] [Google Scholar]
- 24.Lim JR, Gupta SK, Croffie JM, et al. White specks in the esophageal mucosa: An endoscopic manifestation of non-reflux eosinophilic esophagitis in children. Gastrointest Endosc. 2004;59:835–838. doi: 10.1016/s0016-5107(04)00364-5. [DOI] [PubMed] [Google Scholar]