Abstract
Rationale
Several recent studies suggest that Brazil’s Estratégia Saude de Familia (Family Health Strategy-FHS) has contributed to declines in mortality at the national and regional level. Comparatively little is known whether this approach is effective in urban populations with relatively easy access to health services.
Objectives
To use detailed medical data collected as part of São Paulo’s Western Region project to examine whether the FHS program had an impact on child health in São Paulo, Brazil.
Results
No associations were found between FHS and birth weight (OR 1.03, 95% CI 0.93–1.29), gestational length (OR 0.98, 95% CI 0.83–1.15) or stillbirth (OR 1.51, 95% CI 0.75–3.03). FHS eligibility was associated with a 42% reduction in the odds of child mortality (OR 0.58, 95% CI 0.34, 0.91), with largest effect sizes for the early neonatal period (OR 0.18, 95% CI 0.04–0.79).
Conclusions
Community based health delivery platforms may be a highly effective way to reduce neonatal mortality in urban areas of low and middle income countries, even when access to general health services is almost universal.
Keywords: Infant mortality, Estratégia saude de familia, Family health strategy, Progama de saude de familia, Brazil, Community-based programs
Highlights
-
•
Brazil is currently transitioning from a health-center-based to a community-based model
-
•
We find large neonatal mortality reductions in areas with the community based model.
-
•
Community based programs may be effective even in areas with easy healthcare access.
Introduction
Neonatal health continues to be a primary concern for policy makers in low- and middle income countries, with the millennium development goal maternal mortality targets being missed in many countries (Walker, Yenokyan, Friberg, & Bryce, 2013), and close to 3 million neonatal deaths every year (Bhutta et al., 2014; Oestergaard et al., 2011). One increasingly considered strategy to reduce neonatal mortality is community-based home visiting programs, which have been shown to lead to reductions in infant mortality of up to 40% (Baqui et al., 2009; Bhutta et al., 2011; Lassi, Haider, & Bhutta, 2010). Relatively little is known regarding the effectiveness of community-based home-visiting programs outside of South-East Asia, and in particular in settings where access to health services is common and affordable for poor populations as it is generally the case in urban areas of middle income countries. Brazil’s current transition from a center to a home based primary care system offers an ideal setting to directly assess the effectiveness of community-based models.
First proposed in 1991 and created in 1994, Brazil’s Family Health Strategy (FHS) (programa de saúde da família) was initially deployed in small municipalities and became one of the primary health care strategies pursued by the Ministry of Health in 2000 (Sampaio, Mendonça, & Lermen, 2012). The FHS is intensive from a human resource and financial perspective. Under the FHS, areas comprising populations of 3000–4500 people are assigned to and supported by a family health team. Each family health team consists of six community health workers (CHWs), one nurse, two nurse assistants and one general practitioner. Households under the FHS receive a monthly visit by a CHW, who refers members to local health centers whenever needed. During their visits, CHWs are charged with monitoring a range of health conditions including pregnancy, hypertension, diabetes, and communicable diseases such as dengue, tuberculosis and leprosy. For pregnant women, CHWs monitor and encourage pre-natal care attendance, and visit mothers at home within the first few days after their hospital release post-delivery (Aquino, de Oliveira, & Barreto, 2009). This is different from the model traditionally used in Brazil, which primarily relies on patient initiative and offers targeted programs only to special populations based on epidemiological patterns of disease, vulnerability or risk (Morosini & Corbo, 2007).
Both under the FHS and the traditional model, a wide range of services are available at primary health care centers and public hospitals. Each primary health care zone (primary health care unit coverage area) provides basic services for a population of 20,000–40,000 individuals. Under the FHS, each zone is divided into multiple FHS teams, with each community agent responsible for approximately 150 households (Macinko & Harris, 2015). Brazil’s traditional public health care model also offers comprehensive pediatric services, with appointments routinely scheduled one week after birth, and then at 1, 2, 4, 6, 9 and 12 months of age (Ministério da Saúde, 2008). The main difference of the FHS model is that it allows for home-based detection of health problems, as well as home-based support and promotion of access to publicly available services that are often not used due to lack of awareness, lack of time or lack of resources (Bassani et al., 2009, Goldbaum et al., 2005), with large resulting differences in birth outcomes across socioeconomic groups (Macinko et al., 2007, Vettore et al., 2010). Due to large social inequities and the high concentration of specialized health services, scaling up of the FHS model has been slow in São Paulo as well as other (and in particular urban) part of Brazil, resulting in a highly heterogeneous primary care systems within relatively close and highly similar geographic and socioeconomic strata (d׳Avila Viana, Rocha, Elias, Ibanez, & Bousquat, 2008). At the national level, partial FHS coverage has been achieved in over 95% of all municipalities, with an estimated 62% of the total population covered by the program in 2014 (Departamento de Atenção Básica, 2015, Macinko and Harris, 2015).
While several recent studies have shown positive correlations between FHS coverage at the population level and child health outcomes (Brandao et al., 2011, Macinko et al., 2007; Rasella et al., 2010a, Rasella et al., 2010b; Reis, 2014; Rocha & Soares, 2010), the existing literature primarily relies on comparing changes in health outcomes across administrative areas or changes in outcomes within administrative areas over time using panel models. Most of this work builds on the assumption that the rollout and scaling up of the FHS is random, and that population level associations in this setting are representative of the associations between health outcomes and FHS exposure at the individual level, which is not obvious in this setting.
In this paper, we analyze the program in the smaller and more tightly controlled region of São Paulo municipality, where the timing of FHS rollout for each neighborhood was centrally determined based on an initial needs assessment.
Methods
Study setting
The study was conducted in the Butantã-Jaguaré (BJ) region, which is located in the Western Region of São Paulo (see Supplemental Material Figure S1). The area has an estimated population of approximately 380,000, which corresponds to about 3% of the total São Paulo municipality population. São Paulo had an estimated infant mortality rate of 11.49 per 1000 in 2012, substantially below the Brazilian national average of 17 (Ministry of Health of Brazil, 2014). The six administrative districts in the Butantã-Jaguaré region are slightly above the SP average in terms of socioeconomic status as well as child health outcomes, with infant mortality rates varying between 4.4 (Morumbi District) and 10.3 (Villa Sonia) deaths per 1000 live births (Ministry of Health of Brazil, 2014, Prefeitura do Municipio di Sao Paulo, 2014).
Study population
The study population comprised all infants born at the University Hospital between April 1, 2003 (when electronic records were introduced) and November 30, 2012. The University Hospital (HU-USP) is the main public general hospital of the Butantã-Jaguaré region, covering 82% of the births by women covered exclusively by the public national health system (SUS) and about 40% of all births in the region in 2012 (Prefeitura de SP Saúde, 2012).
Hospital birth records
We retrieved detailed electronic records for all deliveries from the hospital’s electronic system, including gestational length, birth weight, delivery mode, APGAR scores and survival status at birth. Births were classified as low birth weight if birth weight was less than 2500 g. Births were classified as pre-term if the estimated gestational length was less than 37 weeks.
Child mortality outcomes
Death records from all children born in Sao Paulo between 2003 and 2012 were obtained from the national vital registration system as well as the Municipality’s mortality information improvement program (PROAIM). According to the district health’ offices internal estimates, these systems capture over 99% of all child deaths (excluding stillbirths) in São Paulo. All records of child deaths among children born in São Paulo Municipality between 2003 and 2012 were extracted, and matched to the births recorded in the hospital’s electronic data system based on children’s birth date and the child’s name. Given the name-based matching, it is possible that some cases were not matched. This could lead to a potential underestimation of overall child mortality levels in our sample, but should not bias our estimation results as long as mismatching occurred systematically which seems rather unlikely. As defined by the PROAIM, we classify neonatal deaths as those that occurred between 0 and 27 days of life; early neonatal death as those occurring between 0 and 6 days and late neonatal as deaths between 7 and 27 days. Deaths occurring between 28 and 364 days of life were classified as post neonatal deaths.
Determining FHS eligibility
To determine whether a birth was covered by the FHS program, mothers’ residential addresses were retrieved from the University Hospital’s electronic system and geocoded. Geocoded addresses were then cross-referenced against period-specific FHS coverage maps provided by the health district office (Secretaria Municipal de Saúde). In the study area, FHS coverage was gradually scaled up in the region between 2001 and 2012 (see Supplemental Information Figures S2 and S3 for details), reaching about 40% the study population by 2012. Births were considered as eligible for FHS if the mother’s home address at the time the child was conceived fell within an area covered by operational FHS teams.
Statistical analysis and empirical strategy
The first section of the empirical analysis provides a description of the FHS rollout over time, as well as a detailed breakdown of the mortality burden in the area. The Butantã-Jaguaré area currently comprises 16 health primary care coverage zones, with active FHS teams covering only selected neighborhoods within 7 selected health zones. Given that public funding for the program was very limited and only gradually increased over time, FHS teams were deployed only to specifically selected areas, with priority given to neighborhoods classified as most vulnerable based on the most recent census data. With limited budget resources, the area with the highest vulnerability index was first chosen for the program, and then more areas gradually added as additional funding became available. While the vulnerability index was primarily based on income and infrastructure, it is possible that more vulnerable areas also had higher initial mortality. To test whether such differences existed prior to the program, we start our analysis by comparing birth and mortality outcomes of FHS and non-FHS areas prior to the actual rollout of the program.
To assess the impact of the FHS model we estimate multivariate logistic models, which explore variations in FHS coverage conditional on area and birth cohort fixed effects. The model estimated can be described as follows:
| (1) |
where is the outcome of interest for child i born in area j and year t, FHS is an indicator for whether the area was covered by a FHS team when the child was conceived, X is a vector of maternal characteristics, and are catchment area (primary health care center) and year fixed effects. In the empirical model, the year fixed effect capture both generic time trends and temporal mortality shocks at the regional level. The area fixed effects capture all time-indifferent variations in local socioeconomic characteristics, health and health care access at the level of the primary health unit. Each observation in our sample corresponds to a birth recorded at Sao Paulo University Hospital between 2003 and 2012. FHS treatment is assigned based on children’s residence and the month of the child’s birth. The assignment of FHS teams is generally done within catchment areas of a given primary health care facility, so that only a certain percentage of mothers from each catchment area benefits from the program. The primary outcome of our analysis is child mortality. However, given the explicit focus of the FHS on prenatal care, we also analyze the following pregnancy outcomes: low birth weight, preterm birth, small for gestational age, stillbirth and Cesarean delivery. Recent reviews suggest that the likelihood of the first four negative outcomes should be reduced through appropriate antenatal care in general (Dowswell et al., 2010) and micronutrient supplementation in particular (Haider & Bhutta, 2015); the same should hold true for cesareans to the extent that they are used to resolve delivery complications.
Given that residuals are likely to display non-zero within-group correlations despite the regional fixed effects (Bertrand, Duflo, & Mullainathan, 2004), all standard errors were clustered at the catchment area (primary health care unit) level.
To test the validity of the estimated results, several robustness checks were implemented: first, general associations between FHS areas (pre-activation of the program) and health outcomes were estimated as a basic placebo test. If it is true that areas selected for FHS were receiving other programs or experiencing different trends, we should detect statistically significant outcome differences prior the program rollout. Second, separate regressions were run on restricted subsamples to minimize the risk of results being driven by specific target areas only. Last, we also show specific cause of death and year specific differences in mortality risk.
All statistical analysis was performed using the Stata© 12 statistical software package.
Ethical clearance was obtained from the University of São Paulo׳s Institutional Review Board.
Results
A total of 27,947 births to mothers residing within the Butantã-Jaguaré area were recorded, the fraction of births from FHS target areas gradually increased over time, with about one third of births covered by the FHS program in 2012.
Table 1 shows average sample characteristics for areas currently not under the FHS programs, as well as FHS areas prior to program rollout. We do not find any statistically significant differences in health outcomes between FHS and non-FHS areas prior to the program launch. On average, stillbirth rates appear slightly lower in FHS areas, while post-neonatal rates appear higher – none of the differences are statistically significant. Some minor differences were found for age structure and the use of Cesarean sections, with mothers from non-FHS areas on average slightly younger, and slightly less likely to use Cesareans.. In general, the prevalence of cesarean sections is rather high, with more than 40% of women over 30 delivering by cesarean section.
Table 1.
Descriptive statistics.
| Never FHS |
FHS target pre intervention |
2-Sample equal means test | ||||
|---|---|---|---|---|---|---|
| Sample: | 18,969 | Sample | 2764 | |||
| N | % | N | % | F-stat | p-Value | |
| Low birth weight | 1376 | (7.26) | 199 | (7.20) | 0.01 | 0.92 |
| Preterm | 1362 | (7.18) | 195 | (7.05) | 0.11 | 0.74 |
| Short for gestational age | 1444 | (7.61) | 204 | (7.38) | 0.51 | 0.49 |
| Stillbirths | 125 | (0.66) | 14 | (0.51) | 1.79 | 0.20 |
| Neonatal deaths | 92 | (0.49) | 14 | (0.51) | 0.03 | 0.87 |
| Post-neonatal deaths | 57 | (0.30) | 15 | (0.55) | 2.50 | 0.13 |
| Cesarian delivery | 6176 | (32.57) | 806 | (29.17) | 12.07 | 0.00 |
| Age under 15 | 122 | (0.64) | 24 | (0.87) | 0.61 | 0.44 |
| Age 15–19 | 3517 | (18.54) | 592 | (21.42) | 11.33 | 0.00 |
| Age 20–34 | 13,108 | (69.10) | 1876 | (67.87) | 3.32 | 0.09 |
| Age 35–39 | 1455 | (7.67) | 180 | (6.51) | 2.73 | 0.12 |
| Age 40 plus | 218 | (1.15) | 34 | (1.23) | 0.15 | 0.70 |
Notes: a) Standard errors are adjusted for clustering at the primary health care unit level. Table covers all hospital births from the Butantã–Jaguaré area over the period 2003–2012 in areas not covered by FHS.
Mortality results
A total of 30,783 deaths among children born in São Paulo between 2003 and 2012 were extracted from the municipality mortality database. 250 mortality records were matched to children born at the University Hospital; 46 of the death records pertained to children born to women outside of the Butantã–Jaguaré region, who were excluded from the analysis due to lacking information on FHS coverage. In terms of mortality, 120 out of 204 cases (58.9%) of all deaths occurred in the first 28 days of life; the most common causes of death were neonatal infections, followed by congenital anomalies, and prematurity.
FHS impact on birth outcomes and child mortality
Table 2 shows the main results from the logistic regression model.. No statistically significant associations were found between FHS coverage and preterm birth, low birth weight or stillbirth; FHS access was associated with an increase in the use of cesarean sections (OR 1.16, 95% CI 1.05–1.29). Maternal age below 20 was associated with an increased risk of low birth weight, preterm birth and stillbirth, as well as a lower likelihood of cesarean section. Increased risk of low birth weight, preterm birth and small for gestational age were also observed for mothers over 40, who were also substantially more likely to deliver via cesarean section (OR 1.91, 95% CI 1.57–2.33) and to experience stillbirths (OR 3.85, 95% CI 1.74–8.53) compared to women aged 20–34.
Table 2.
Multivariate regression results: birth outcomes.
| Low birth weight | Preterm birth | Small for gestational age | Cesarean | Stillbirth | |
|---|---|---|---|---|---|
| Covered by PSF | 1.03 | 0.98 | 0.92 | 1.16⁎⁎⁎ | 1.51 |
| (0.83–1.29) | (0.83–1.15) | (0.74–1.14) | (1.05–1.29) | (0.75–3.03) | |
| Age under 15 | 2.58⁎⁎⁎ | 3.22⁎⁎⁎ | 1.59⁎ | 0.67⁎⁎ | 1.84 |
| (1.72–3.86) | (2.06–5.01) | (0.92–2.73) | (0.48–0.95) | (0.49–6.93) | |
| Age 15–19 | 1.47⁎⁎⁎ | 1.64⁎⁎⁎ | 1.23⁎⁎⁎ | 0.70⁎⁎⁎ | 1.01 |
| (1.30–1.67) | (1.50–1.79) | (1.11–1.37) | (0.65–0.77) | (0.62–1.64) | |
| Age 20–34 | Reference | Reference | Reference | Reference | Reference |
| Group | Group | Group | Group | Group | |
| Age 35–39 | 1.11 | 1.00 | 0.94 | 1.48⁎⁎⁎ | 1.60 |
| (0.91–1.36) | (0.80–1.26) | (0.79–1.10) | (1.32–1.67) | (0.90–2.85) | |
| Age 40+ | 1.80⁎⁎⁎ | 1.44⁎ | 1.48⁎⁎⁎ | 1.91⁎⁎⁎ | 3.85⁎⁎⁎ |
| (1.30–2.50) | (1.00–2.09) | (1.13–1.94) | (1.57–2.33) | (1.74–8.53) | |
| Observations | 27,943 | 27,947 | 27,947 | 27,939 | 27,947 |
Notes: All regressions include year, primary health care facility and FHS area fixed effects. Robust standard errors in parentheses are clustered at the primary health care unit level.
p<0.01.
p<0.05.
p<0.1.
Table 3 shows the results for child mortality, which suggest large protective results for FHS exposure overall, with an estimated odds ratio of 0.56 for child mortality (95% CI 0.34–0.91), and an even smaller (but not statistically significant) odds ratio of 0.39 for neonatal deaths (95% CI 0.13–1.21). Given that virtually all mothers stay in the hospital the first two days after delivery, we show separate results for the first 48 hours and the remaining neonatal period – the estimated results suggest a reduction of 82% in the odds of mortality in this period (OR 0.18, 95% CI 0.04, 0.79). Smaller and non-significant effects were found for the post-neonatal period.
Table 3.
Multivariate regression results: child mortality.
| Child death | Neonatal death | Death in the first 48 h | Neonatal deaths days 3–27 | Post neonatal death | |
|---|---|---|---|---|---|
| Covered by FHS | 0.56⁎⁎ | 0.39 | 2.14 | 0.18⁎⁎ | 0.76 |
| (0.34–0.91) | (0.13–1.21) | (0.29–15.55) | (0.04–0.79) | (0.42–1.38) | |
| Age under 15 | 6.33⁎⁎⁎ | 8.93⁎⁎⁎ | 3.64 | 12.42⁎⁎⁎ | 2.30 |
| (3.67–10.92) | (4.71–16.94) | (0.58–22.73) | (6.78–22.74) | (0.28–19.01) | |
| Age 15–19 | 1.48⁎⁎ | 1.33 | 1.68 | 1.11 | 1.70 |
| (1.03–2.12) | (0.92–1.93) | (0.78–3.66) | (0.59–2.07) | (0.87–3.30) | |
| Age 20–34 | Reference | Reference | Reference | Reference | Reference |
| Group | Group | Group | Group | Group | |
| Age 35–39 | 1.54⁎⁎ | 1.17 | 1.62 | 0.89 | 2.12⁎⁎ |
| (1.06–2.24) | (0.69–1.98) | (0.62–4.19) | (0.37–2.14) | (1.06–4.22) | |
| Age 40+ | 0.99 | 0.81 | 2.05 | NA | 1.28 |
| (0.17–5.95) | (0.14–4.60) | (0.39–10.90) | (0.20–8.21) | ||
| Observations | 27,947 | 26,486 | 21,670 | 26,183 | 26,625 |
Notes: All regressions include year, primary health care facility and FHSarea fixed effects. Robust standard errors in parentheses are clustered at the primary health care unit level.
⁎p<0.1.
p<0.01.
p<0.05.
Remarkably large increases in child mortality risks were also found for young teenage motherhood; compared to the reference group of maternal age 20–34, maternal age <15 was associated with a six fold increase in the odds of child death (OR 6.33, 95% CI 3.67, 10.92), while maternal age 15–19 was associated with a 48% increase in child mortality (OR 1.48, 95% CI 1.03, 2.12).
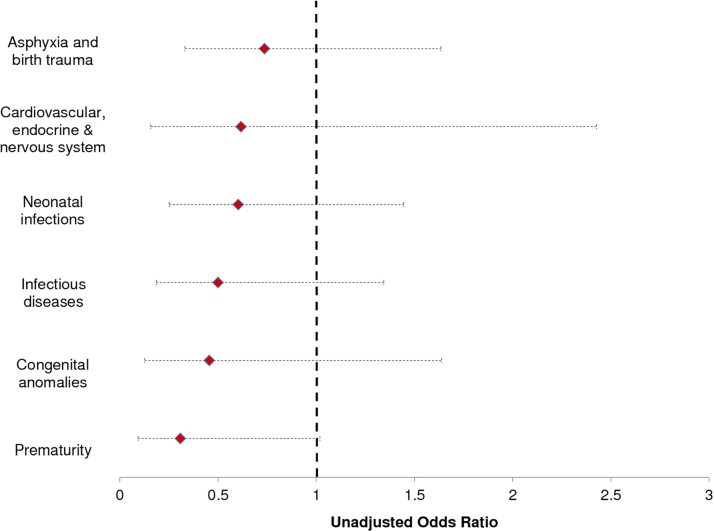
Fig. 1 shows the estimated reductions in the odds of child death by cause of death; access to FHS appears to reduce the risk of mortality from all causes; the small number of deaths in each category however contributes to none of the estimated associations being statistically significant (or statistically different from each other).
Fig. 1.
Mortality Impact by cause of death.
Table 4 shows a series of multivariate robustness checks for the main mortality result. In column (1) of Table 4 we show the results of our placebo test and investigate whether areas selected for FHS had better child mortality outcomes prior to the program starts. Consistent with the patterns shown in Table 1, no pre-intervention differences in health outcomes were found.. Columns (2)–(5) show child mortality estimates for restricted subsamples: column (2) shows the results when areas where the FHS program was initiated prior to the first available data in the sample are excluded; column (3) shows results for a subsample where areas starting the FHS program after 2010 are excluded; column (4) shows results for a subsample excluding the first two years of records, and column (5) shows results excluding the most recent. Across all specifications, the estimated OR on FHS coverage remains constant in the 0.50–0.65 range, with slightly varying degrees of statistical significance.
Table 4.
Sensitivity analysis.
| Treated areas pre FHS(placebo) | Excluding early FHSareas | Excluding late FHSareas | Excluding 2003–2004 births | Excluding 2011–12 births | |
|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | |
| Covered by PSF | 0.99 | 0.54⁎⁎ | 0.57⁎⁎ | 0.65 | 0.55⁎⁎⁎ |
| (0.91–1.08) | (0.33–0.88) | (0.35–0.93) | (0.27–1.52) | (0.36–0.84) | |
| Age under 15 | 5.01⁎⁎⁎ | 6.82⁎⁎⁎ | 6.64⁎⁎⁎ | 6.17⁎⁎⁎ | 5.85⁎⁎⁎ |
| (2.46–10.18) | (3.70–12.58) | (3.86–11.39) | (2.98–12.80) | (3.27–10.46) | |
| Age 15–19 | 1.51⁎⁎ | 1.46⁎⁎ | 1.47⁎⁎ | 1.25 | 1.44⁎ |
| (1.04–2.21) | (1.02–2.09) | (1.01–2.12) | (0.83–1.87) | (0.95–2.19) | |
| Age 20–34 | Reference | Reference | Reference | Reference | Reference |
| Group | Group | Group | Group | Group | |
| Age 35–39 | 1.56⁎⁎⁎ | 1.65⁎⁎⁎ | 1.57⁎⁎ | 1.77⁎⁎⁎ | 1.37 |
| (1.23–1.99) | (1.22–2.24) | (1.08–2.29) | (1.17–2.68) | (0.88–2.14) | |
| Age 40–44 | 1.14 | 1.06 | 1.03 | 1.33 | 1.06 |
| (0.19–6.77) | (0.18–6.16) | (0.17–6.12) | (0.22–7.97) | (0.17–6.69) | |
| Observations | 21,690 | 24,808 | 27,457 | 23,226 | 22,201 |
Notes: Column (1) shows the results from a placebo regression where we regress child mortality on FHS areas prior to program rollout. Columns (2)–(5) repeat the main child mortality specification displayed in column (1) of Table 3 sequentially excluding specific treated areas and birth cohorts. All regressions include year, primary health care facility and FHSarea fixed effects. Robust standard errors in parentheses are clustered at the primary health care unit level. .
p<0.01.
p<0.05.
p<0.1.
In Supplemental Information Figure S4 we show the estimated mortality differential for each birth cohort separately. With the exception of 2005, mortality rates are consistently lower in FHS areas, with an average mortality gap of approximately 4 deaths per 1000 live births; the only year where this estimated difference is statistically significant is 2010.
Discussion
The results presented in this paper suggest that access to FHS teams is associated with rather large reductions in child mortality, with particularly large reductions in the neonatal period. The estimated effect sizes appear large. In a previous FHS evaluation at the municipality level, Aquino et al. (2009) found a 14% reduction in the risk of infant mortality in areas with low initial mortality, which is less than half the reduction we found in our analysis. In theory, one would have expected FHS to improve both prenatal and postnatal care. Empirically, we do not find any differences in any of the birth outcomes, which suggests that the either prenatal care did not improve, or that any improvements in prenatal care did not result in improved birth outcomes. One possible explanation for the low or lacking associations with birth outcomes is the generally high degree of service utilization in the prenatal period across both health care models. The average number of pre-natal consultations in both FHS and non-FHS areas in São Paulo is around seven, which means that improvements in prenatal care – one of the main objectives of the FHS (Aquino et al., 2009)-are relatively hard to achieve.
More generally, it seems important to highlight that the setting studied is likely not representative of many other developing country settings in terms of the structural barriers to health service access. In the densely populated urban areas of Sao Paulo average distances to facilities are relatively small (and transport available), while the vast majority of health services is provided free of charge in public facilities. Given this, the main mechanism through which community-based programs can improve health outcomes is not the provision of standard care, but rather the active outreach to population and early detection of potentially critical health conditions.
In terms of mortality, the main impact of the FHS appears to occur in the neonatal period, i.e. after the mother’s release from the hospital. In many respects, this is the period where the FHS model differs most from the traditional model: according to FHS guidelines, each mother is supposed to receive a home visit by a CHW as well as a nurse within the first few days after hospital release, as well as a follow-up visit to monitor growth within 14 days (Macinko & Harris, 2015). While the data available for this analysis do not allow us to test directly whether these visits explain the observed mortality differences, the observed mortality reductions are consistent in magnitude with the results of a recent trail in Pakistan, which used community health workers for both pre- and postnatal care (Bhutta et al., 2011). Given that the FHS does not seem to have affect antenatal care or birth outcomes, and given that all children in our sample were born at the same hospital, postnatal home visits seem by far the most likely causal mechanism underlying the main results observed.
Compared to the existing literature, the analysis presented has two major strengths: first, by working with individual (child-) level data, we can directly link child health outcomes to FHS treatment, rather than comparing average population level outcomes. All of the existing literature uses ecological designs, comparing average health outcomes at the municipality or district level to average FHS coverage at the same administrative level. These estimates are potentially subject to ecological fallacy, and may not represent true causal impact of the treatment of interest (Macinko et al., 2011; Rasella, Harhay, Pamponet, Aquino, & Barreto, 2014; Rothman, Greenland, & Lash, 2008). Second, by zooming in on one relatively small area in Sao Paulo, we can exclude differences in general health system access or delivery practices, since all children were born at the same hospital. While the selection of specific neighborhoods for the FHS was not random, the rollout of the program was centrally determined by the municipality based on perceived poverty. As we have shown in this paper, the areas selected for the program did not differ from other areas with respect to child mortality prior to the program, which means that confounding through differential pre-trends can be excluded. While it is possible that other health or welfare programs were started in the area over the sample period, none of these programs were – at least to our knowledge-rationed or targeted in ways overlapping with the ESF rollout, which makes time-variant confounding very unlikely as well in our setting. The same is rather unlikely at the municipality level, where it seems possible that municipalities more effective in scaling up ESF may also be more effective in improving other health services or scaling up other government programs over time. Previous studies have tried to overcome this challenge through instrumental variable estimation (Macinko et al., 2010) or dynamic panel modeling (Macinko et al., 2011); we believe that neither approach is necessary in our setting due to the centrally planned rollout of the program.
The analysis presented in this paper also has limitations. First the data available do not allow us to directly assess whether mothers actually used FHS services. The intention-to-treat analysis presented is based on de-jure access to FHS services, without using data on actual use PSF-mother-interactions. The latest estimates from FHS enrollment data within the western region suggest that about 95% of households who are offered FHS services enroll (de Sá, Rebelo, Brentani, Grisi, & Gutierrez, 2012). Enrollees should thus have, but may not have, received the routine visits that are part of the PSF; this means that the true benefits on the actually treated could in theory be larger than the results presented in this study. On the other hand, it is also possible that women not satisfied with FHS services in the area could have chosen another hospital for delivery, which would bias the results in the opposite direction. According to the latest estimates, more than 80% of all women covered under SUS have consistently delivered at the hospital over the study period, so that large selection effects seem unlikely overall (Prefeitura de SP Saúde, 2012).
A second concern related to the empirical approach chosen is that some mortality cases may not have been captured in the vital registration system or may not have been correctly linked to the hospital database. All available data suggests that vital registrations are close to complete at the municipality level; the mortality levels observed at the regional level seem overall highly consistent with the government estimates for the six districts covered. Bias generated by omitted mortality cases seems unlikely overall; even if some mortality cases were missed due to lacking reporting, estimation bias should not occur as long as reporting issues are not related to FHS targeting, which seems reasonable to believe.
A third limitation of the study is that the hospital system does not register socioeconomic status of patients, which may raise concerns that the results presented may reflect differences in socioeconomic status of mothers, or differential socioeconomic trends over time. Given that all empirical models include health and FHS zone fixed effects, the risk of cross-sectional confounding seems small; as the robustness checks presented show, pre-FHS differences in mortality are small and non-significant, and it seems unlikely that FHS neighborhoods would have experienced differential trends in socioeconomic factors over the relatively short project period. Last, the effects observed in this sample may not necessarily extend to the larger Sao Paulo population. The University Hospital primarily attracts the poorer segments of the population covered by SUS, while better off women (with private health insurance) deliver at private hospitals. It seems likely that the FHS may have a smaller impact on better-off populations. It is also rather likely that the University hospital offers substantially higher quality of care compared to other facilities in Brazil (Dowswell et al., 2010), which may attenuate the child health outcome differences generated by the FHS.
Overall, the results presented in this paper suggest a rather strong association between FHS coverage and child survival; further research will be needed to corroborate this finding and to more closely identify the causal mechanisms through which models like the FHS can improve neonatal survival rates.
Conclusion
The results presented in this paper suggest that post-natal home visiting programs such as the ones implemented as part of Brazil’s family health strategy may be a highly effective way to reduce neonatal mortality in urban areas with access to comprehensive health services.
Key messages
Brazil is currently transitioning from a health-center-based to a community-based model
We find that neighborhoods selected for the Family Health Strategy (FHS) program experienced large reductions in mortality in the neonatal period.
The results presented in this paper suggest that the FHS and similarly designed programs may be a highly effective way to reduce neonatal mortality in urban areas with high access to health care.
Ethical clearance
The project was approved by the University Hospital Internal Review Board, record ID CAAE 07569312800000076.
Funding
The project was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP, Grant number: 12/19463-8.
Financial Disclosure: No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
All authors declare that there are no competing interests regarding the present article.
Acknowledgments
The authors would like to thank São Paulo Municipality Health Secretariat, CEINFO for their help and support.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ssmph.2016.01.001.
Contributor Information
Alexandra Brentani, Email: brentani.alexandra@gmail.com.
Sandra Josefina Ferraz Ellero Grisi, Email: sandragrisi@hu.usp.br.
Mauro T. Taniguchi, Email: mttaniguchi@gmail.com.
Ana Paula Scoleze Ferrer, Email: ana.ferrer@hc.fm.usp.br.
Maria Lúcia de Moraes Bourroul, Email: malubourroul@gmail.com.
Günther Fink, Email: gfink@hsph.harvard.edu.
Appendix A. Supplementary material
Supplementary material
References
- Aquino R., de Oliveira N.F., Barreto M.L. Impact of the family health program on infant mortality in Brazilian municipalities. American Journal of Public Health. 2009;99:87–93. doi: 10.2105/AJPH.2007.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqui, A. H., Ahmed, S., Arifeen, S. E., Darmstadt, G. L., Rosecrans, A. M., & Mannan, I., et al. (2009). Effect of timing of first postnatal care home visit on neonatal mortality in Bangladesh: a observational cohort study. [DOI] [PMC free article] [PubMed]
- Bassani D.G., Surkan P.J., Olinto M.T.A. Inadequate use of prenatal services among brazilian women: the role of maternal characteristics. International Perspectives on Sexual and Reproductive Health. 2009;35:15–20. doi: 10.1363/ifpp.35.015.09. [DOI] [PubMed] [Google Scholar]
- Bertrand M., Duflo E., Mullainathan S. How much should we trust differences-in-differences estimates? Quarterly Journal of Economics. 2004;119:249–275. [Google Scholar]
- Bhutta Z.A., Das J.K., Bahl R., Lawn J.E., Salam R.A., & Paul V.K. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? The Lancet. 2014;384:347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Soofi S., Cousens S., Mohammad S., Memon Z.A., & Ali I. Improvement of perinatal and newborn care in rural Pakistan through community-based strategies: a cluster-randomised effectiveness trial. Lancet. 2011;377:403–412. doi: 10.1016/S0140-6736(10)62274-X. [DOI] [PubMed] [Google Scholar]
- Brandao J.R., Gianini R.J., Novaes H.M., Goldbaum M. The family health system: analysis of a health survey in Sao Paulo, Brazil. Journal of Epidemiology and Community Health. 2011;65:483–490. doi: 10.1136/jech.2008.077172. [DOI] [PubMed] [Google Scholar]
- d׳Avila Viana A.L., Rocha J.S., Elias P.E., Ibanez N., Bousquat A. Primary health care and urban dynamics in large cities in Sao Paulo State, Brazil. Cadernos de Saude Publica. 2008;24(Suppl. 1):S79–S90. doi: 10.1590/s0102-311x2008001300013. [DOI] [PubMed] [Google Scholar]
- de Sá J., Rebelo M., Brentani A., Grisi S., Gutierrez M. XIII Congresso brasileiro em informática em saúde; Sao Paulo, Brazil: 2012. GeoHealth web: uma ferramenta para mineração de dados da atenção primária. [Google Scholar]
- Departamento de Atenção Básica. (2015). Histórico de cobertura da saúde da familia. Brasilia, Brazil.
- Dowswell T., Carroli G., Duley L., Gates S., Gulmezoglu A.M., & Khan-Neelofur D. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database of Systematic Reviews. 2010:CD000934. doi: 10.1002/14651858.CD000934.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbaum M., Gianini R.J., Novaes H.M., Cesar C.L. Health services utilization in areas covered by the family health program (Qualis) in Sao Paulo City, Brazil. Revista de Saude Publica. 2005;39:90–99. doi: 10.1590/s0034-89102005000100012. [DOI] [PubMed] [Google Scholar]
- Haider B.A., Bhutta Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews. 2015;11:CD004905. doi: 10.1002/14651858.CD004905.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi Z.S., Haider B.A., Bhutta Z.A. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database of Systematic Reviews. 2010 doi: 10.1002/14651858.CD007754.pub2. [DOI] [PubMed] [Google Scholar]
- Macinko J., de Fátima Marinho de Souza M., Guanais F.C., da Silva Simões C.C. Going to scale with community-based primary care: An analysis of the family health program and infant mortality in Brazil, 1999–2004. Social Science and Medicine. 2007;65:2070–2080. doi: 10.1016/j.socscimed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Macinko J., de Oliveira V.B., Turci M.A., Guanais F.C., Bonolo P.F., Lima-Costa M.F. The influence of primary care and hospital supply on ambulatory care-sensitive hospitalizations among adults in Brazil, 1999-2007. American Journal of Public Health. 2011;101:1963–1970. doi: 10.2105/AJPH.2010.198887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macinko J., Dourado I., Aquino R., Bonolo Pde F., Lima-Costa M.F., & Medina M.G. Major expansion of primary care in Brazil linked to decline in unnecessary hospitalization. Health Affairs. 2010;29:2149–2160. doi: 10.1377/hlthaff.2010.0251. [DOI] [PubMed] [Google Scholar]
- Macinko J., Harris M.J. Brazil׳s family health strategy — delivering community-based primary care in a universal health system. New England Journal of Medicine. 2015;372:2177–2181. doi: 10.1056/NEJMp1501140. [DOI] [PubMed] [Google Scholar]
- Ministério da Saúde . Caderneta de saúde da criança. In: da Saúde Ministério., editor. Secretaria de atenção à saúde. Sao Paulo; Brazil: 2008. [Google Scholar]
- Ministry of Health of Brazil. (2014). Sistema de Informação Sobre Mortalidade (SIM).
- Morosini M.V.G.C., Corbo A.D. Modelos de atenção a saúde da família. EPSJV/Fiocruz; Rio de Janeiro: 2007. [Google Scholar]
- Oestergaard M.Z., Inoue M., Yoshida S., Mahanani W.R., Gore F.M., & Cousens S. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Medicine. 2011;8:e1001080. doi: 10.1371/journal.pmed.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prefeitura de SP Saúde. (2012). Informações em saúde-Mãe Paulistana.
- Prefeitura do Municipio di Sao Paulo . P.d.M.d.S.; Paulo: 2014. Mortalidade Infantil no Municipio die São Paulo. [Google Scholar]
- Rasella D., Aquino R., Barreto M.L. Impact of the Family Health Program on the quality of vital information and reduction of child unattended deaths in Brazil: an ecological longitudinal study. BMC Public Health. 2010;10:380. doi: 10.1186/1471-2458-10-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasella D., Aquino R., Barreto M.L. Reducing childhood mortality from diarrhea and lower respiratory tract infections in Brazil. Pediatrics. 2010;126:e534–e540. doi: 10.1542/peds.2009-3197. [DOI] [PubMed] [Google Scholar]
- Rasella D., Harhay M.O., Pamponet M.L., Aquino R., Barreto M.L. BMJ. 2014. Impact of primary health care on mortality from heart and cerebrovascular diseases in Brazil: a nationwide analysis of longitudinal data; p. 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M. Public primary health care and children’s health in Brazil: evidence from siblings. Journal of Population Economics. 2014;27:421–445. [Google Scholar]
- Rocha R., Soares R.R. Evaluating the impact of community-based health interventions: evidence from Brazil׳s Family Health Program. Health Economics. 2010;19:126–158. doi: 10.1002/hec.1607. [DOI] [PubMed] [Google Scholar]
- Rothman K.J., Greenland S., Lash T.L. Modern epidemiology. 3rd Edition. Lippincott, Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- Sampaio L., Mendonça G., Lermen N.J. Atenção primária à saúde no Brasil. In: Gusso G., JMC L., editors. Tratado de medicina da Família e Comunidade Princípios, formação e prática. Artmed; Sao Paulo, Brazil: 2012. [Google Scholar]
- Vettore M.V., Gama S.G.Nd, Lamarca Gd.A., Schilithz A.O.C., Leal Md.C. Housing conditions as a social determinant of low birthweight and preterm low birthweight. Revista de Saude Publica. 2010;44:1021–1031. doi: 10.1590/s0034-89102010005000045. [DOI] [PubMed] [Google Scholar]
- Walker N., Yenokyan G., Friberg I.K., Bryce J. Patterns in coverage of maternal, newborn, and child health interventions: projections of neonatal and under-5 mortality to 2035. Lancet. 2013;382:1029–1038. doi: 10.1016/S0140-6736(13)61748-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material