Abstract
Using data from the 1999–2014 National Health and Nutrition Examination Survey (n ~ 46,000), this study documents income disparities in the age patterning of cardiovascular conditions across the lifespan in the U.S. The conditions were assessed from laboratory test results, self-reports of medications used to treat specific conditions, and anthropometric measurements, allowing us to capture whether individuals at given ages had developed the various conditions, regardless of previous diagnosis and treatment. We found evidence of large income disparities in the presence of cardiovascular conditions and risk factors for females, smaller disparities in the same conditions for males, and few disparities that increased with age for either gender. Results were very similar when considering disparities by education instead of income. The findings suggest that the widening socioeconomic gradients in health over the lifespan found in many previous studies—which have generally focused on self-rated health, activity limitations, or diagnosed conditions—reflect, at least to some extent, differences in diagnosis, treatment, and management of health conditions rather than age-related differences in developing them. The findings also suggest that preventive healthcare is not an important source of socioeconomic disparities in cardiovascular health in the U.S., at least for men. The observed patterns of income disparities in cardiovascular conditions over the lifespan are more consistent with theories of early life conditions and the imprinting of health endowments and susceptibilities early in life than with cumulative life exposure or stress hypotheses.
Keywords: Health disparities, Income disparities, Socioeconomic disparities, Lifespan, Cardiovascular health, Biomarkers
Highlights
-
•
Large income disparities in the presence of cardiovascular conditions and risk factors were found for females.
-
•
Smaller disparities in the presence of the same conditions were found for males.
-
•
For most cardiovascular conditions and risk factors, disparities did not increase with age for either gender.
Introduction
Health over the lifespan is a dynamic process that is shaped by when health conditions first appear, how soon conditions are diagnosed, how conditions are managed and treated, and the extent to which symptoms are experienced. Past research, from which we highlight salient examples, has demonstrated widening socioeconomic disparities in morbidity with age in the U.S., during childhood (Case et al., 2002, Fletcher and Wolfe, 2014) and over the lifespan (Case et al., 2002, House et al., 1994, House et al., 2005, Lynch, 2003). A similar pattern has been found for mortality (Crimmins et al., 2011, Woolf and Aron, 2013, Chetty et al., 2016). While the literature to date paints a picture of realized socioeconomic disparities in health at different ages, it provides less information about the underlying processes.
In particular, from most previous studies of the age patterning of health disparities, which focus on self-rated health, activity limitations, or diagnosed conditions, it is impossible to infer the extent to which disparities represent: (1) differential rates of developing health conditions at a given age, (2) differential rates of diagnosis given that conditions present, and (3) differential rates (or types) of treatment given that conditions have been diagnosed. Disparities at all three stages in the process from the development of disease to its treatment are important, and most studies of health over the lifespan implicitly consider combined effects of various stages. For example, studies relying on reports of diagnoses of specific conditions confound the first two stages (developing the condition and having it diagnosed). Understanding where in the process disparities are produced, as well as the extent to which disparities are shaped at each stage, is essential for understanding sources of health disparities and has implications for efforts to narrow them. For instance, large income differences in the age-patterning in whether individuals have developed a given condition would suggest a clear role for prevention, while small income differences at this stage of the process would suggest strategies to encourage screening and diagnosis.
In this study, we systematically document socioeconomic gradients in the first stage—the presence of conditions, whether diagnosed or not—from early adolescence through adulthood in the United States using nationally representative data, and assess the extent to which health disadvantages associated with low socioeconomic status (SES)—characterized by income, and alternatively, education—accumulate, persist, or diminish across the lifespan. We focus on indicators of the leading cause of death in the United States, cardiovascular disease (NCHS, 2015), for which risk factors appear at young ages (Martinson, Teitler & Reichman, 2011) and are measured reliably (NCHS, 2013). We use data from biomarkers and respondent reports of medication use for specific conditions, which together allow us to capture conditions whether or not respondents are aware of them and whether or not they are being treated for them.
Materials and methods
Data
We used data from the continuous National Health and Nutrition Examination Survey (NHANES), which is a comprehensive, nationally-representative health study conducted through the National Center for Health Statistics. The data collection consists of home-administered questionnaires and standardized health examinations (Johnson, Dohrmann, Burt & Mohadjer, 2014), and well-documented public use datasets are available. The cross-sectional study is conducted every year and data are available in 2-year intervals for confidentiality purposes. We pooled all available 2-year sets of data from 1999 through 2014, inclusive (NCHS, 2013). The resulting sample consisted of approximately 46,000 subjects between the ages of 12 and 69 years. Sufficient sample sizes allowed us to stratify analyses by gender and consider narrow age categories. The analytic sample varied across health outcomes because some measures were only available for certain ages.
Measures
We focused on measures of cardiovascular disease and risk factors based on laboratory tests, respondents' reports of taking medications prescribed to treat specific conditions, and anthropometric measurements that were available in NHANES from adolescence to adulthood and have been used to examine SES disparities in an international comparative context (Banks et al., 2006, Martinson, 2012). For each condition, we classified the individual as having the condition if he/she had a positive clinical result as indicated by the NHANES tests for the condition or the individual reported that he/she was currently on medication that is used to treat that condition. For example, we considered both of the following groups as having hypertension: (1) individuals who tested positive for hypertension based on clinical assessments (see below), who may have been aware or unaware that they had the condition, and (2) those who reported being treated for hypertension. Each condition was considered in isolation from all other conditions, except as specifically noted below.
The cardiovascular health conditions and risk factors considered in this study and how they were measured are detailed as follows: (a) Diabetes: glycated hemoglobin ≥ 6.5%, a widely used measure of diabetes that does not rely on a fasting protocol before the blood draw, or current use of medication for diabetes. (b) Low high-density lipoprotein (HDL) cholesterol: HDL < 40 mg/dL, or current use of cholesterol medication. (c) High cholesterol to HDL ratio: ≥ 5, or current use of cholesterol medication. (d) Elevated triglycerides: ≥ 150 mg/dl, or current use of medication for elevated triglycerides. (e) High C-reactive protein (CRP; a marker of inflammation and risk factor for cardiovascular disease, particularly when accompanying other risk factors): ≥ 3 mg/L. (f) Hypertension: mean systolic blood pressure ≥ 140 mm Hg, mean diastolic blood pressure ≥ 90 mm Hg, or current use of medication for hypertension. (g) Obesity: BMI ≥ 30 for adults and International Obesity Task Force cutpoints for those under 18 (Cole, Bellizzi & Flegal, 2000), from heights and weights measured in person.
We also considered (h) Metabolic Syndrome, an epidemiological construct that is commonly used as a marker for both cardiovascular disease and diabetes, defined as having at least three of the following five conditions (Alberti et al., 2010; Beltrán-Sánchez et al., 2013): (1) abdominal adiposity (waist circumference ≥ 102 cm for males; ≥ 88 for females); (2) hypertension (mean systolic blood pressure ≥ 130 mm Hg, mean diastolic blood pressure ≥ 85 mm Hg, or currently on medication for hypertension); (3) elevated triglycerides ( ≥ 150 mg/dl, or current use of medication for elevated triglycerides); (4) low HDL cholesterol (< 40 mg/dL for males and < 50 mg/dL for females, or current use of cholesterol medication); and (5) elevated fasting blood glucose (≥ 100 mg/dl or current use of medication for diabetes). Metabolic syndrome represents a cluster of risk factors that together increase an individual's likelihood of developing cardiovascular disease by two-fold and of developing diabetes by five-fold (Alberti et al., 2010). We separately considered (i) abdominal adiposity and (j) elevated fasting blood glucose, as defined above; the other components of metabolic syndrome are closely related to conditions b, d, and f. Finally, we considered (k) an indicator of whether a respondent had any of the abovementioned conditions, a through j.
We classified individuals as living in households with income at or below the poverty level (adjusted for family size and cost of living index) or in one of the following income/poverty (poverty ratio) intervals: ≤ 1 (≤ 100% of the poverty level), 1.01–2 (between 100 and 200% of the poverty level), 2.01–3 (between 200 and 300% of the poverty level), 3.01–4 (between 300 and 400% of the poverty level), and > 4 (over 400% of the poverty level). The U.S. dollar equivalent of the poverty threshold (which corresponds to an income to poverty threshold ratio of 1) in 2014 was approximately $19,000 for a family of three, so the lowest income category corresponded to individuals in families of three earning less than $19,000 and the highest corresponded to individuals in families of three earning $76,000 or more per year (DeNavas-Walt & Proctor, 2015). Poverty ratios in the United States reflect income levels and are also used for qualification for most social and health programs and benefits.
We restricted analyses to those aged 12 to 69 and use 5-year age bands in most analyses to ensure sufficient cell sizes. The lower end of the age range was determined by the available data. The upper bound was set to attenuate the effects of selective mortality, which is likely to be most pronounced at older ages. Although at least one study found little evidence that selective mortality altered patterns of educational disparities in health by age (Beckett, 2000), another found evidence that mortality accounts for narrowing health disparities by education at older ages (Dupre, 2007). The smallest age by income cell included 146 subjects – females, ages 55–59 whose family income to poverty ratios were between 3.01 and 4, inclusive. Most cells are much larger.
Analytic methods
We used the svy suite of commands in Stata 14 SE (Stata, 2015) and the NHANES-provided weights to adjust for oversampling and non-response in the cross-sectional data. All analyses were conducted separately for females and males. We focused primarily on patterns of absolute disparities in conditions over the lifespan, because relative disparities mask substantial differences in overall prevalence by age.
We plotted the prevalence of each health condition or risk factor by age and income categories, providing a visual depiction of the extent of income disparities and their patterning by age. We also provided statistical measures of the extent and age patterning of disparities. For each condition and gender, we identified the age category within which the condition emerged (defined as afflicting at least 5% of the overall gender-specific sample) and calculated, from the beginning of that age category to age 69, the age-adjusted difference in the prevalence of that condition between the highest and lowest income categories. In addition, we calculated the differences in prevalence within each age interval. Statistical significance of the differences in prevalence of the condition across all 5 income categories was assessed using chi-square tests. Statistical differences in the magnitude of disparities between females and males were also calculated. We conducted supplementary analyses using level of education attained (less than high school, high school or GED, some college, or college graduate), another measure of SES used in many studies of health disparities, instead of income, for individuals ages 25 to 69.
Results
The weighted sample characteristics and rates of cardiovascular conditions and risk factors by gender are representative of the U.S. population (Table 1). The distribution of household income across the five poverty ratio categories do not reflect income quintile distributions. Rather, about one third of individuals were in the highest income/poverty ratio category (> 4) and the fractions in the lowest category (≤ 1) and in each of the 2.01–3 and 3.01–4 categories were below 20%. Education was slightly skewed towards the lowest category (high school or less) because we included individuals as young as 12 years of age. The prevalence rates of health conditions and markers of disease varied substantially by condition, from 6% for diabetes to 56% with high waist circumference for females, and 7% with diabetes to 44% with high fasting glucose for males. More than two-thirds of both females and males had at least one of any of the conditions considered.
Table 1.
Weighted sample characteristics and rates of cardiovascular and metabolic health conditions by gender, United States, 1999–2014.
| Females, Age 12–69 | Males, Age 12–69 | |
|---|---|---|
| Mean age | 38.8 | 38.2 |
| Income/poverty ratio | ||
| ≤1 | 17.8 | 14.9 |
| 1.01–2 | 20.1 | 19.2 |
| 2.01–3 | 14.8 | 14.9 |
| 3.01–4 | 13.5 | 13.9 |
| > 4 | 33.8 | 37.0 |
| Years of education | ||
| 0–12 | 39.0 | 43.0 |
| 13–15 | 33.3 | 29.4 |
| 16+ | 27.6 | 27.5 |
| Race/ethnicity | ||
| Non-Hispanic White | 65.8 | 66.8 |
| Non-Hispanic Black | 12.9 | 11.4 |
| Hispanic | 14.6 | 15.4 |
| Non-Hispanic Other | 6.7 | 6.4 |
| Cigarette smoking % | 20.6 | 25.3 |
| Drink ≥5x/week | 3.8 | 8.8 |
| No health insurance | 14.5 | 17.3 |
| Percent with: | ||
| Diabetes | 5.9 | 7.1 |
| Low HDL | 18.5 | 35.7 |
| High cholesterol/HDL ratio | 19.8 | 35.7 |
| High triglycerides | 28.0 | 37.1 |
| High C-reactive proteina | 43.2 | 27.6 |
| Hypertension | 21.2 | 22.5 |
| Obesity | 34.3 | 30.6 |
| Metabolic syndromea | 33.1 | 36.4 |
| High waist circumference | 55.8 | 36.5 |
| High fasting glucose | 28.5 | 44.2 |
| Any cardiovascular condition or risk factor | 68.9 | 69.3 |
| N | 23,478 | 22,339 |
only assessed for those aged 20–69.
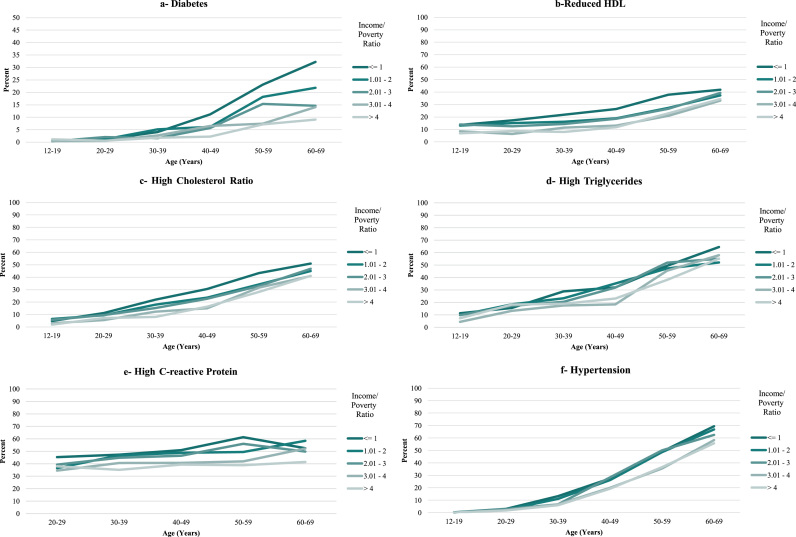
Over the entire age ranges at which conditions were present, income disparities among females tended to be large (Table 2a). The smallest disparity was for high triglycerides, for which there was a 7.4 percentage point difference in prevalence between the highest and lowest income categories, with an insignificant difference across all 5 income categories. For the other conditions, the absolute differences in prevalence between the highest and lowest income categories ranged from 10 to 21.4 percentage points, with an 18.7 percentage point difference for any condition (Fig. 1a through k and Table 2a); all of the differences in prevalence of these conditions were statistically significant across all 5 income categories, with the exception of high triglycerides and high fasting glucose.
Table 2a.
Age patterning of income disparities in cardiovascular and metabolic health conditions, females, United States, 1999–2014.
| Females |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 - 15 | 15 - 19 | 20 - 24 | 25 - 29 | 30 - 34 | 35 - 39 | 40 - 44 | 45 - 49 | 50 - 54 | 55 - 59 | 60 - 64 | 65 - 69 | Total | ||||||||||||||
| Diabetes | x | x | x | x | x | x | x | 12.1 | * | 11.4 | *** | 21.7 | † | 23.3 | 23.3 | * | 17.4 | *** | ||||||||
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | |||||||||||||||||||||
| Low HDL | 6.4 | * | 7.1 | * | 9.9 | *** | 9.1 | * | 16.2 | *** | 11.5 | † | 14.5 | *** | 15.3 | *** | 17.2 | ** | 12.6 | * | 9.2 | 4.4 | 11.0 | *** | ||
| (0.02) | (0.01) | (0.01) | (0.00) | (0.00) | (0.01) | (0.00) | (0.00) | (0.00) | (0.05) | (0.29) | (0.84) | (0.00) | ||||||||||||||
| High cholesterol/HDL ratio | 3.3 | ** | 2.9 | * | 3.7 | * | 6.6 | 12.3 | * | 15.2 | ** | 13.7 | *** | 16.0 | *** | 15.0 | ** | 16.0 | *** | 9.6 | 8.4 | 10.0 | *** | |||
| (0.08) | (0.04) | (0.29) | (0.04) | (0.00) | (0.00) | (0.00) | (0.00) | (0.01) | (0.09) | (0.15) | (0.52) | (0.01) | ||||||||||||||
| High triglycerides | 4.0 | 4.3 | -0.6 | † | -1.9 | 7.1 | 13.2 | 14.4 | *** | 5.8 | 10.0 | 13.4 | *** | 10.5 | 6.3 | 7.4 | * | |||||||||
| (0.19) | (0.37) | (0.39) | (0.91) | (0.14) | (0.13) | (0.00) | (0.14) | (0.47) | (0.13) | (0.53) | (0.33) | (0.27) | ||||||||||||||
| High C-reactive protein | x | x | 10.4 | 8.5 | 9.0 | 15.1 | 6.3 | 18.3 | 19.2 | 27.2 | * | 8.4 | 16.0 | 12.9 | ||||||||||||
| (0.22) | (0.11) | (0.48) | (0.00) | (0.14) | (0.00) | (0.01) | (0.00) | (0.02) | (0.01) | (0.00) | ||||||||||||||||
| Hypertension | x | x | x | x | 6.7 | *** | 8.4 | 8.7 | 9.4 | 7.3 | ** | 20.0 | *** | 11.7 | 16.0 | † | 12.0 | † | ||||||||
| (0.00) | (0.02) | (0.00) | (0.10) | (0.01) | (0.00) | (0.06) | (0.00) | (0.00) | ||||||||||||||||||
| Obesity | 8.4 | 16.1 | *** | 10.8 | ** | 29.5 | *** | 25.0 | *** | 22.1 | ** | 15.2 | ** | 13.5 | *** | 15.3 | ** | 18.3 | *** | 14.5 | * | 16.0 | * | 16.8 | *** | |
| (0.00) | (0.00) | (0.01) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.02) | (0.01) | (0.00) | ||||||||||||||
| Metabolic syndrome | x | x | 8.0 | *** | 6.8 | 11.7 | * | 24.3 | 22.9 | * | 19.4 | 23.5 | * | 36.5 | *** | 11.2 | 24.0 | * | 21.4 | *** | ||||||
| (0.04) | (0.03) | (0.00) | (0.00) | (0.01) | (0.09) | (0.00) | (0.00) | (0.37) | (0.14) | (0.00) | ||||||||||||||||
| High waist circumference | 11.8 | 18.1 | *** | 14.7 | *** | 19.7 | ** | 22.2 | *** | 26.5 | *** | 22.1 | *** | 17.2 | *** | 18.9 | *** | 13.9 | *** | 7.1 | ** | 7.4 | ** | 20.2 | *** | |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.12) | (0.11) | (0.00) | ||||||||||||||
| High fasting glucose | 2.7 | 3.5 | 9.4 | † | 10.0 | 5.9 | 21.4 | 16.6 | 13.9 | 23.9 | *** | 16.9 | 13.0 | 23.2 | *** | 12.2 | * | |||||||||
| (0.33) | (0.51) | (0.08) | (0.01) | (0.07) | (0.00) | (0.02) | (0.12) | (0.00) | (0.44) | (0.31) | (0.00) | (0.45) | ||||||||||||||
| Any cardiovascular condition or risk factor | 15.5 | * | 19.7 | *** | 14.1 | *** | 13.4 | † | 16.4 | * | 25.8 | *** | 19.0 | ** | 15.4 | ** | 12.3 | ** | 10.9 | 5.6 | -0.1 | 18.7 | *** | |||
| (0.00) | (0.00) | (0.00) | (0.01) | (0.00) | (0.00) | (0.00) | (0.00) | (0.01) | (0.00) | (0.06) | (0.48) | (0.00) | ||||||||||||||
Magnitudes of disparities (the top cell in each pair) were calculated as percentage point differences between prevalences of each condition in the highest and lowest income categories. P-values from chi-square tests for statistical significance of differences in prevalence of each condition across all 5 income categories are in parentheses. Each “x” indicates that, for a particular age range, a condition or risk factor did not yet afflict at least 5% of the gender-specific sample.
*Difference in disparity is significant between females (presented here) and males (presented in Table 2b). ***<.001, **<.01, *<.05, +<.10
Fig. 1.
Prevalence of Cardiovascular Conditions and Risk Factors, United States, 1999–2014, by Income/Poverty Ratio (Females).
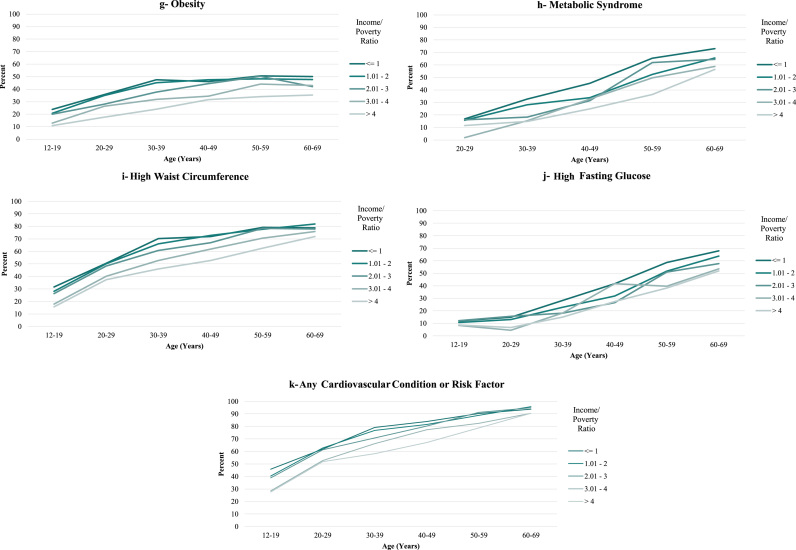
Income disparities were much smaller for males than for females. The largest absolute differences in prevalence between the highest and lowest income categories for males were for diabetes (9.4 percentage points), high CRP (9.6 percentage points), and high fasting glucose (7.3 percentage points, but these were not significantly different across income categories overall). Corresponding disparities in the other conditions ranged from -2.7 to 3.1 percentage points, with a 4 percentage point difference for having any condition (Fig. 2a through k and Table 2b). Furthermore, for obesity and high waist circumference, the poorest males appeared to be thinnest after age 40 (Fig. 2g and i).
Fig. 2.
Prevalence of Cardiovascular Conditions and Risk Factors, United States, 1999-2014, by Income/Poverty Ratio (Males).
For both females and males, there was little evidence that disparities increased with age. A monotonic widening of the income gradient over the lifespan was observed only for diabetes for females and CRP for males and, to a lesser extent, diabetes for males (Figs. 1a, 2a, 2e and Table 2a, Table 2b). Across some conditions (low HDL, high cholesterol ratio, and high triglycerides, for both females and males), disparities appeared largest in middle adulthood (in the 30 s and 40 s). For all other conditions, income disparities—to the extent that they existed—reached their maximum levels very soon after the age at which the conditions emerged at the population level. This pattern was exemplified by the measure of having any condition, for which the absolute difference in prevalence between the highest and lowest income categories for females was 15.5 percentage points in the youngest age bracket (12–15 years), with fluctuations around that level until the late 50 s. For males, the corresponding difference of 8.6 percentage points in the youngest age bracket represented the maximum difference across all age categories.
Table 2b.
Age patterning of income disparities in cardiovascular and metabolic health conditions, males, United States, 1999–2014.
| Males |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12–15 | 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | Total | |
| Diabetes | x | x | x | x | x | x | 8.4 | 3.2 | 8.3 | 11.0 | 10.9 | 19.3 | 9.4 |
| (0.00) | (0.09) | (0.05) | (0.01) | (0.01) | (0.00) | (0.00) | |||||||
| Low HDL | 1.8 | 1.3 | -2.6 | 9.4 | 12.5 | 5.5 | 0.0 | 2.0 | 3.0 | -4.3 | 4.9 | 4.6 | 2.7 |
| (0.79) | (0.96) | (0.89) | (0.15) | (0.04) | (0.25) | (0.57) | (0.92) | (0.41) | (0.56) | (0.64) | (0.15) | (0.38) | |
| High cholesterol/HDL ratio | -0.9 | -0.8 | -4.8 | 8.1 | 17.2 | 8.9 | -0.6 | 0.7 | 1.0 | -7.3 | 5.4 | 6.4 | 1.6 |
| (0.40) | (0.91) | (0.40) | (0.22) | (0.00) | (0.20) | (0.41) | (0.84) | (0.21) | (0.17) | (0.72) | (0.00) | (0.00) | |
| High triglycerides | 2.5 | 3.3 | -6.8 | 10.1 | 6.7 | 18.9 | 0.4 | 5.2 | 3.4 | -13.6 | 3.2 | -8.0 | 2.5 |
| (0.76) | (0.47) | (0.03) | (0.02) | (0.26) | (0.01) | (0.63) | (0.47) | (0.42) | (0.25) | (0.74) | (0.76) | (0.01) | |
| High C-reactive protein | x | x | 0.0 | 4.8 | 12.4 | 6.8 | 14.2 | 14.1 | 16.9 | 4.4 | 16.1 | 10.8 | 9.6 |
| (0.62) | (0.45) | (0.12) | (0.22) | (0.01) | (0.07) | (0.03) | (0.26) | (0.00) | (0.63) | (0.00) | |||
| Hypertension | x | x | -5.2 | -4.3 | 2.1 | 4.6 | 5.9 | 6.2 | 0.8 | 12.9 | 3.9 | 4.9 | 3.1 |
| (0.00) | (0.41) | (0.95) | (0.34) | (0.13) | (0.26) | (0.99) | (0.00) | (0.39) | (0.48) | (0.01) | |||
| Obesity | 9.4 | 2.5 | -1.1 | -2.0 | 8.2 | 6.8 | -1.1 | -1.1 | -1.0 | -4.3 | -9.4 | -5.3 | 0.8 |
| (0.00) | (0.57) | (0.53) | 0.77) | (0.02) | (0.03) | (0.12) | (0.09) | (0.66) | (0.47) | (0.11) | (0.33) | (0.00) | |
| Metabolic syndrome | x | x | -2.0 | -1.4 | 0.2 | 10.7 | 9.0 | 12.9 | -6.0 | -9.4 | 7.4 | -5.6 | 2.1 |
| (0.00) | (0.17) | (0.61) | (0.24) | (0.64) | (0.61) | (0.70) | (0.39) | (0.61) | (0.99) | (0.03) | |||
| High waist circumference | 5.5 | 1.1 | -4.7 | 1.2 | 4.7 | 1.0 | -1.3 | -8.8 | -6.1 | -7.5 | -15.7 | -12.5 | -2.7 |
| (0.03) | (0.44) | (0.62) | (0.51) | (0.40) | (0.22) | (0.17) | (0.01) | (0.58) | (0.50) | (0.09) | (0.39) | (0.00) | |
| High fasting glucose | 8.1 | 9.8 | 8.2 | 8.1 | 7.6 | 16.5 | 13.1 | 5.2 | -9.9 | 9.8 | 14.6 | -5.9 | 7.3 |
| (0.39) | (0.05) | (0.15) | (0.40) | (0.17) | (0.06) | (0.43) | (0.61) | (0.30) | (0.55) | (0.45) | (0.89) | (0.12) | |
| Any cardiovascular condition or risk factor | 8.6 | 5.3 | -6.1 | 5.6 | 6.1 | 2.3 | 5.1 | 2.3 | 0.3 | 7.3 | 1.3 | 1.3 | 4.0 |
| (0.01) | (0.33) | (0.28) | (0.49) | (0.17) | (0.08) | (0.30) | (0.42) | (0.82) | (0.09) | (0.48) | (0.05) | (0.00) | |
Magnitudes of disparities (the top cell in each pair) were calculated as percentage point differences between prevalences of each condition in the highest and lowest income categories. P-values from chi-square tests for statistical significance of differences in prevalence of each condition across all 5 income categories are in parentheses. Each “x” indicates that, for a particular age range, a condition or risk factor did not yet afflict at least 5% of the gender-specific sample.
For females, the magnitude and age-patterning of disparities across education levels mirrored those across income categories (Table 3a). For males, however, disparities by education were considerably larger than by income (Table 3b). For example, the age adjusted percentage of high school graduates with any condition was 8.6 points higher than that of college graduates. Even so, the magnitudes of education disparities for males were much smaller than (about half) those of females. Finally, the age patterning in disparities by education was similar to that by income in that there was no evidence of systematic widening of disparities over the lifespan.
Table 3a.
Age patterning of educational disparities in cardiovascular and metabolic health conditions, females, United States, 1999–2014.
| Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 - 29 | 30 - 34 | 35 - 39 | 40 - 44 | 45 - 49 | 50 - 54 | 55 - 59 | 60 - 64 | 65 - 69 | Total | |
| Diabetes | x | x | x | x | 5.7 | 6.1 | 13.0 | 14.3 | 16.9 | 10.3 |
| (0.00) | (0.02) | (0.00) | (0.00) | (0.00) | (0.00) | |||||
| Low HDL | 10.6 | 14.7 | 9.6 | 16.8 | 8.0 | 12.6 | 8.6 | 9.6 | 6.1 | 11.3 |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.02) | (0.00) | (0.11) | (0.05) | (0.28) | (0.00) | |
| High cholesterol/HDL ratio | 6.4 | 15.8 | 13.4 | 14.5 | 12.2 | 14.0 | 13.7 | 13.6 | 7.2 | 12.8 |
| (0.01) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.01) | (0.00) | (0.25) | (0.00) | |
| High triglycerides | 5.3 | 8.1 | 14.2 | 19.7 | 8.8 | 13.1 | 21.5 | 23.8 | 5.8 | 14.3 |
| (0.42) | (0.19) | (0.01) | (0.00) | (0.16) | (0.04) | (0.01) | (0.00) | (0.30) | (0.00) | |
| High C-reactive protein | 6.8 | 3.2 | 22.6 | 18.1 | 11.1 | 17.9 | 15.0 | 17.5 | 15.0 | 14.1 |
| (0.24) | (0.25) | (0.00) | (0.00) | (0.04) | (0.00) | (0.01) | (0.00) | (0.04) | (0.00) | |
| Hypertension | x | 2.6 | 7.7 | 9.6 | 11.4 | 11.1 | 13.3 | 18.1 | 13.3 | 12.0 |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.01) | (0.00) | (0.00) | (0.01) | (0.00) | ||
| Obesity | 19.3 | 20.6 | 20.4 | 17.6 | 13.3 | 9.4 | 16.8 | 16.3 | 7.8 | 16.2 |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.03) | (0.00) | (0.00) | (0.27) | (0.00) | |
| Metabolic syndrome | 6.6 | 13.0 | 15.0 | 29.6 | 16.0 | 19.6 | 29.6 | 25.8 | 21.1 | 20.9 |
| (0.32) | (0.03) | (0.01) | (0.00) | (0.02) | (0.01) | (0.00) | (0.00) | (0.03) | (0.00) | |
| High waist circumference | 17.2 | 15.7 | 24.9 | 21.2 | 15.7 | 20.4 | 18.5 | 10.4 | 16.2 | 18.7 |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.01) | (0.00) | (0.00) | |
| High fasting glucose | 3.1 | 3.2 | 16.6 | 13.1 | 13.7 | 14.2 | 9.5 | 15.1 | 17.7 | 12.0 |
| (0.71) | (0.51) | (0.00) | (0.05) | (0.03) | (0.02) | 0.28) | (0.07) | (0.01) | (0.00) | |
| Any cardiovascular condition or risk factor | 14.5 | 10.0 | 27.1 | 22.4 | 15.0 | 16.3 | 15.0 | 12.6 | 5.2 | 16.3 |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.07) | (0.00) | |
Magnitudes of disparities (the top cell in each pair) were calculated as percentage point differences between prevalences of each condition for those who at most graduated high school and those who had a college education or more. P-values from chi-square tests for statistical significance of differences in prevalence of each condition across all 3 educational categories are in parentheses. Each “x” indicates that, for a particular age range, a condition or risk factor did not yet afflict at least 5% of the gender-specific sample.
Table 3b.
Age patterning of educational disparities in cardiovascular and metabolic health conditions, males, United States, 1999–2014.
| Males |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 - 29 | 30 - 34 | 35 - 39 | 40 - 44 | 45 - 49 | 50 - 54 | 55 - 59 | 60 - 64 | 65 - 69 | Total | |
| Diabetes | x | x | x | 3.6 | 1.7 | 6.7 | 7.2 | 10.1 | 11.4 | 6.2 |
| (0.01) | (0.29) | (0.06) | (0.05) | (0.01) | (0.01) | (0.00) | ||||
| Low HDL | 17.9 | 7.5 | 9.2 | 1.8 | 3.8 | 1.7 | -8.0 | 9.1 | 15.5 | 6.1 |
| (0.00) | (0.02) | (0.04) | (0.01) | (0.24) | (0.74) | (0.25) | (0.08) | (0.00) | (0.00) | |
| High cholesterol/HDL ratio | 12.9 | 11.0 | 6.4 | 5.9 | 3.0 | 3.1 | 0.8 | 11.6 | 15.7 | 7.4 |
| (0.00) | (0.00) | (0.16) | (0.01) | (0.28) | (0.38) | (0.98) | (0.01) | (0.00) | (0.00) | |
| High triglycerides | 11.8 | 3.0 | 8.6 | 0.7 | 11.7 | 5.2 | -1.3 | 10.2 | 12.0 | 6.7 |
| (0.09) | (0.27) | (0.26) | (0.03) | (0.05) | (0.64) | (0.78) | (0.11) | (0.14) | (0.03) | |
| High C-reactive protein | 9.5 | 6.2 | 8.2 | 14.1 | 15.1 | 8.6 | 14.7 | 7.4 | 11.8 | 10.9 |
| (0.03) | (0.23) | (0.06) | (0.00) | (0.00) | (0.06) | (0.02) | (0.19) | (0.04) | (0.00) | |
| Hypertension | 0.3 | 3.4 | 6.1 | 7.4 | 11.7 | 3.0 | 10.5 | 13.6 | 8.2 | 7.7 |
| (0.96) | (0.23) | (0.08) | (0.05) | (0.01) | (0.61) | (0.07) | (0.00) | (0.03) | (0.00) | |
| Obesity | 8.6 | 4.0 | 9.8 | 5.1 | 9.5 | 9.6 | 5.2 | 6.2 | 7.9 | 7.3 |
| (0.04) | (0.36) | (0.01) | (0.00) | (0.00) | (0.02) | (0.16) | (0.02) | (0.06) | (0.00) | |
| Metabolic syndrome | 6.4 | 6.0 | 12.8 | 5.2 | 16.4 | 9.7 | 4.8 | 23.2 | 9.6 | 11.2 |
| (0.45) | (0.44) | (0.08) | (0.01) | (0.02) | (0.34) | (0.79) | (0.00) | (0.15) | (0.00) | |
| High waist circumference | 8.8 | 1.2 | 7.0 | 9.0 | 9.9 | 8.5 | 1.3 | 8.0 | 5.4 | 7.0 |
| (0.04) | (0.54) | (0.18) | (0.00) | (0.00) | (0.01) | (0.91) | (0.00) | (0.08) | (0.00) | |
| High fasting glucose | 12.9 | 0.8 | 11.9 | 8.3 | 5.8 | 3.8 | 11.3 | 6.0 | 3.7 | 8.0 |
| (0.04) | (0.94) | (0.04) | (0.09) | (0.57) | (0.76) | (0.16) | (0.46) | (0.81) | (0.02) | |
| Any cardiovascular condition or risk factor | 17.5 | 6.5 | 7.4 | 7.8 | 14.4 | 2.5 | 7.1 | 7.0 | 10.4 | 8.6 |
| (0.00) | (0.08) | (0.06) | (0.00) | (0.00) | (0.01) | (0.01) | (0.00) | (0.00) | (0.00) | |
Magnitudes of disparities (the top cell in each pair) were calculated as percentage point differences between prevalences of each condition for those who at most graduated high school and those who had a college education or more. P-values from chi-square tests for statistical significance of differences in prevalence of each condition across all 3 educational categories are in parentheses. Each “x” indicates that, for a particular age range, a condition or risk factor did not yet afflict at least 5% of the gender-specific sample.
In supplementary analyses (results not shown), we restricted the sample to non-Hispanic whites and found that the patterns were very similar to those for the full sample. Specifically, socioeconomic disparities were much larger for females than males (among males, disparities were apparent only for CRP), and there was no more evidence of widening of disparities over the relevant age range than there was for all race/ethnicities combined. These analyses were conducted as a robustness check on a more homogenous sample—that is, to remove the potentially confounding effects of race/ethnicity. We also conducted sensitivity analyses that controlled for race/ethnicity, and while race/ethnicity was significantly associated with most health conditions, the inclusion of this control did not substantially change the associations between income and health.
Discussion
We used data from the nationally-representative National Health and Nutrition Examination Survey to systematically document income disparities in the prevalence of cardiovascular conditions and risk factors across the lifespan in the United States by gender, using relatively narrow age intervals. The conditions were assessed from laboratory test results, reports of taking medications prescribed to treat specific conditions, and anthropometric measurements, allowing us to capture the presence of conditions regardless of whether they had been diagnosed and treated. We intentionally focused on the presence of conditions regardless of diagnosis and treatment in order to ascertain the ages at which income, and alternatively education, disparities in prevalence first appear at the population level.
We found evidence of large socioeconomic disparities in cardiovascular conditions and risk factors for females that were generally constant across the lifespan, smaller disparities in the same conditions for males, and few disparities that increased with age for either gender. These findings are seemingly at odds with prior studies that have generally found a widening of disparities over the lifespan (Case et al., 2002, House et al., 1994, House et al., 2005, Lynch, 2003). However, as indicated earlier, the vast majority of past studies focused on health outcomes, such as self-rated health, activity limitations, and reported conditions, that are influenced by a host of downstream factors including diagnosis, treatment, management, and perceptions of conditions. In order to understand how disparities are produced and to best address them, it is necessary to isolate disparities at different stages of the process from development to treatment of disease. Our study focused on disparities in the first stage—the development of the conditions—by considering measures of the presence of conditions whether they have been diagnosed or not. Only a few previous studies of health disparities (Mensah et al., 2005, Loucks et al., 2007, Walsemann et al., 2016) constructed indicators of cardiovascular disease this way, and only one (Walsemann et al., 2016) considered the age patterning of disparities and that was in the context of racial/ethnic (not SES) disparities for a young adult sample. The study that most closely relates to ours (Zhang & Wang, 2004) focused on obesity and overweight and presented results for the gender-specific age patterning of SES disparities that were consistent with our findings for obesity.
While our findings produce valuable new nationally representative information on health disparities based on well-measured data and careful, systematic analysis, our study is not without limitations. First, we were unable to account for cohort effects. There is some evidence that socioeconomic disparities in obesity, one of the main risk factors for cardiovascular disease, have decreased over recent decades (Ogden and Carroll, 2010, Zhang and Wang, 2004). If disparities in the onset of the other cardiovascular conditions and risk factors we examined have also been decreasing, then our findings would overestimate the degree to which income disparities widen over the lifespan. Since we found little evidence of an age-related widening of disparities, and since the extent of the documented change in disparities over time was very small compared to the very large age effects on the prevalence of cardiovascular conditions and risk factors, it is unlikely that cohort effects would explain the general patterns in disparities we documented across the age range. Second, socioeconomic disadvantage is a much more complex construct than can be captured with simple indicators of income or education. That said, these limited and relatively crude indicators of SES are frequently used in research on health disparities due to their wide availability in national health data. Third, the findings from this study regarding the age patterning of income disparities in cardiovascular conditions cannot be generalized to infectious diseases, cancer, intentional injuries, unintentional injuries, mental illness, or other non-cardiovascular sources of morbidity.
Limitations notwithstanding, the findings from this study lead to several insights about processes underlying health disparities. First, the observation that income disparities are small for most conditions among males suggests that differential access to and use of preventive healthcare is not an important source of income disparities, at least for men and the conditions we studied. Preventive care may or may not be important for females, but our data and findings do not provide clues in this regard as they do for males. The more substantial health disparities that have been observed in prior studies may reflect downstream factors (including diagnosis and treatment) that affect the severity of conditions, the fact that other studies did not focus exclusively on cardiovascular conditions, or possibly the SES measures that were used. SES has traditionally been defined by education, income, and occupation, with each component representing different aspects of SES and often having different relationships to health outcomes (Adler & Newman, 2002). Our educational comparisons (completed college versus no more than high school) contrasted the prevalence of conditions in the upper 27.5% and the lower 43% of the population, whereas our income comparisons (greater than 4 times the poverty threshold versus at or below poverty) were between the upper 37% and the lower 15%. We did find that, for males, disparities by education were somewhat larger than those by income, although as for income disparities, educational disparities were also considerably smaller for males than for females.
Second, the very different levels of socioeconomic disparities by gender are noteworthy and warrant further study. While there is a large literature on health trajectories by gender, there have been fewer studies on gender differences in socioeconomic disparities in health (exceptions include Brown, Richardson, Hargrove & Thomas, 2016; Chang & Lauderdale, 2005; Cummings & Jackson, 2008; Mensah et al., 2005; and Loucks et al., 2007). Even fewer have focused on cardiovascular conditions (exceptions are Mensah et al., 2005 and Loucks et al., 2007), while fewer still have considered socioeconomic disparities in health outcomes by gender and age (exceptions are Brown et al., 2016, which focused on self-rated health, and Zhang & Wang, 2004, which focused on obesity and overweight; Walsemann et al., 2016 is relevant, but focused on racial/ethnic rather than SES disparities). Our findings of larger SES disparities in health for females than males are consistent with this past research and underscore the importance of considering age-related patterns in health separately for males and females and exploring reasons for gender differences. A number of studies have tackled the question of why women's health inequities appear worse than that of men despite women's higher life expectancy (Bird and Rieker, 2008, Courtenay, 2000, Idler, 2003, Yang and Lee, 2009), but there is very little evidence on why income appears to benefit women more than men.
Third, the observation that disparities do not increase with age and generally reach their maximum levels at ages soon after conditions appear, suggests that the biological mechanisms producing disparities in cardiovascular conditions and risk factors (except possibly diabetes and high CRP among males) are not a function of lifetime cumulative exposures to chronic stress (or other social or environmental exposures). While there is considerable evidence that cumulative stress can affect health by inducing neuroendocrine changes via disruptions in the Hypothalamic-Pituitary-Adrenocortical system, the findings from our study suggest that such mechanisms do not produce or account for income disparities in cardiovascular health. Our findings for females are more consistent with epigenetic or immunological theories pointing to the imprinting of lifelong physiological resilience and vulnerabilities in utero and during the first few years of life through environmental effects on the expression of disease-related genes (Meaney, 2001) or the development and regulation of the immune system (Kelly, King & Aminov, 2007).
References
- Adler N., Newman K. Socioeconomic disparities in health: Pathways and policies. Health Affairs. 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- Alberti K.G., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Banks J., Marmot M., Oldfield Z. Disease and disadvantage in the United States and in England. JAMA. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Beckett M. Converging health inequalities in later life—an artifact of mortality selection? Journal of Health and Social Behavior. 2000;41:106–119. [PubMed] [Google Scholar]
- Beltrán-Sánchez H., Harhay M.O., Harhay M.M., McElligott S. Prevalence and trends of metabolic syndrome in the adult US population, 1999–2010. Journal of the American College of Cardiology. 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird C.E., Rieker P.P. Cambridge University Press; 2008. Gender and health: The effects of constrained choices and social policies. [Google Scholar]
- Brown T.H., Richardson L.J., Hargrove T.W., Thomas C.S. Using multiple-hierarchy stratification and life course approaches to understand health inequalities: The intersecting consequences of race, gender, SES, and age. Journal of Health and Social Behaviour. 2016;57(2):200–222. doi: 10.1177/0022146516645165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A., Lubotsky D., Paxson C. Economic status and health in childhood: The origins of the gradient. American Economic Review. 2002;92(5):1308–1334. doi: 10.1257/000282802762024520. [DOI] [PubMed] [Google Scholar]
- Chang V.W., Lauderdale D.S. Income disparities in body mass index and obesity in the United States, 1971–2002. Archives of Internal Medicine. 2005;165:2122–2128. doi: 10.1001/archinte.165.18.2122. [DOI] [PubMed] [Google Scholar]
- Chetty R., Stepner M., Abraham S., Lin S., Scuderi B., Turner N., Bergeron A., Cutler D. The association between income and life expectancy in the United States, 2001–2014. JAMA. 2016;315(16):1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T.J., Bellizzi M.C., Flegal K.M. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay W.H. Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Social Science Medicine. 2000;50(10):1385–1401. doi: 10.1016/s0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- Crimmins E.M., Preston S.H., Cohen B., editors. Panel on understanding divergent trends in longevity in high-income countries. National Research Council. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- Cummings J.L., Jackson P.B. Race, gender, and SES disparities in self-assessed health, 1974–2004. Research on Aging. 2008;30(2):137–167. [Google Scholar]
- DeNavas-Walt C., Proctor B.D. U.S. Government Printing Office; Washington, DC: 2015. U.S. Census Bureau, Current Population Reports, P60-252, Income and Poverty in the United States: 2014. [Google Scholar]
- Dupre M.E. Educational differences in age-related patterns of disease: Reconsidering the cumulative disadvantage and age-as-leveler hypotheses. Journal of Health and Social Behavior. 2007;48(1):1–15. doi: 10.1177/002214650704800101. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Wolfe B. Increasing our understanding of the health-income gradient in children. Health Economics. 2014;23(4):473–486. doi: 10.1002/hec.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House J.S., Lantz P.M., Herd P. Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans' Changing Lives Study) Journals of Gerontology: Series B. 2005;60B(Special Issue II):S15–S26. doi: 10.1093/geronb/60.special_issue_2.s15. [DOI] [PubMed] [Google Scholar]
- House J.S., Lepkowski J.M., Kinney A.M., Mero R.P., Kessler R.C., Herzog A.R. The social stratification of aging and health. Journal of Health and Social Behavior. 1994;35(3):213–234. [PubMed] [Google Scholar]
- Idler E.L. Discussion: Gender differences in self-rated health, in mortality, and in the relationship between the two. The Gerontologist. 2003;43(3):372–375. [Google Scholar]
- Johnson C.L., Dohrmann S.M., Burt V.L., Mohadjer L.K. National Health and Nutrition Examination Survey: Sample design, 2011–2014. National Center for Health Statistics Vital Health Stat. 2014;2(162) [PubMed] [Google Scholar]
- Kelly D., King T., Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2007;622(1):58–69. doi: 10.1016/j.mrfmmm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Loucks E.B., Rehkopf D.H., Thurston R.C., Kawachi Socioeconomic disparities in metabolic syndrome differ by gender: Evidence from NHANES III. Annals of Epidemiology. 2007;17:19–26. doi: 10.1016/j.annepidem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Lynch S.M. Cohort and life-course patterns in the relationship between education and health: A hierarchical approach. Demography. 2003;40:309–331. doi: 10.1353/dem.2003.0016. [DOI] [PubMed] [Google Scholar]
- Martinson M., Teitler J., Reichman N.E. Health across the life span in the United States and England. American Journal of Epidemiology. 2011;173(8):858–865. doi: 10.1093/aje/kwq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson M.L. Income inequality in health at all ages: A comparison of the United States and England. American Journal of Public Health. 2012;102(11):2049–2056. doi: 10.2105/AJPH.2012.300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24(1):1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mensah G.A., Mokdad A.H., Ford E.S., Greenlund K.J., Croft J.B. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States (2014). : With Special Feature on Adults Aged 55–64. Hyattsville, MD. 2015 [PubMed]
- National Center for Health Statistics. NHANES Analytic Guidelines: September (2013). Version. Hyattsville, MD: National Center for Health Statistics; 2013
- Ogden C.L., Carroll M.D. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2007–2008. National Center for Health Statistics. 2010;6(1):1–6. [Google Scholar]
- StataCorp. Stata: Release 14. Statistical Software (2015). . College Station, TX: StataCorp LP
- Walsemann K., Goosby B.J., Farr D. Life course SES and cardiovascular risk: Heterogeneity across race and by gender. Social Science and Medicine. 2016;152:147–155. doi: 10.1016/j.socscimed.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf S.H., Aron L. National Academies Press; Washington (DC): 2013. U.S. health in international perspective: Shorter lives, poorer health. [PubMed] [Google Scholar]
- Yang Y., Lee L.C. Sex and race disparities in health: Cohort variations in life course patterns. Social Forces. 2009;87(4):2093–2124. [Google Scholar]
- Zhang Q., Wang Y. Socioeconomic inequality of obesity in the United States: Do gender, age, and ethnicity matter? Social Science Medicine. 2004;58(6):1171–1180. doi: 10.1016/s0277-9536(03)00288-0. [DOI] [PubMed] [Google Scholar]