Abstract
Obesity is an epidemic and costly disease affecting 13% of the adult population worldwide. Obesity is associated with adipose tissue hypertrophy and hyperplasia, as well as pathologic endocrine alterations of adipose tissue including local and chronic systemic low-grade inflammation. Moreover, this inflammation is a risk factor for both metabolic syndrome (MetS) and insulin resistance. Basic and clinical studies demonstrate that foods containing bioactive compounds are capable of preventing both obesity and adipose tissue inflammation, improving obesity-associated MetS in human subjects and animal models of obesity. In this review, we discuss the anti-obesity and anti-inflammatory protective effects of some bioactive polyphenols of plant origin and omega-3 polyunsaturated fatty acids, available for the customers worldwide from commonly used foods and/or as components of commercial food supplements. We review how these bioactive compounds modulate cell signaling including through the nuclear factor-κB, adenosine monophosphate-activated protein kinase, mitogen-activated protein kinase, toll-like receptors, and G-protein coupled receptor 120 intracellular signaling pathways and improve the balance of pro- and anti-inflammatory mediators secreted by adipose tissue and subsequently lower systemic inflammation and risk for metabolic diseases.
Keywords: obesity, inflammation, polyphenols, omega-3 fatty acids
INTRODUCTION
Obesity is a disease of a complex etiology (1,2), which has become a major health problem in the United States and worldwide. Multiple factors contributing to obesity pathogenesis include genetic background, environment, and lifestyle. Importantly, obesity is closely linked with chronic low-grade inflammation (3,4), which in turn may trigger other chronic pathophysiologic conditions such as type 2 diabetes, metabolic syndrome (MetS), and heart diseases (1). Currently, 35.0% men, 40.4% women, and 17.0% of children and adolescent aged 2 to 19 years old in the US are obese (5,6). Moreover, over 600 million adults are obese worldwide according to the World Health Organization (7). Due to its epidemic rates, the obesity emerges as a major health care challenge in most regions of the world.
ADIPOSE TISSUE INFLAMMATION
Obesity is a result of adipose tissue expansion by adipocyte hypertrophy and/or hyperplasia especially in preadipocytes, stroma vascular cells, and stem cells (8). Adipose tissue is a loose connective tissue mainly composed of adipocytes. Besides adipocytes (lipid filled cells), adipose tissue contains fibroblast and immune cells linked together by collagen fibers (9). Two morphologically, anatomically and physiologically distinct adipose tissue types are brown adipose tissue (BAT) and white adipose tissue (WAT) (9). WAT is the most abundant type found in most of organisms and is crucial for energy storing (10). Moreover, WAT has also been well characterized as an endocrine organ (11), which controls a wide range of biological functions. Adipose tissue also acts as a thermal regulator and as a protector for important organs (8). In addition to energy storage and heat production, adipose tissue secretes a wide range of cytokines (9,12, 13), collectively names ‘adipokines’ (10,14). These include pro-inflammatory monocyte chemoattractant protein (MCP)-1, interleukin (IL)-8, IL-6, IL-1, tumor necrosis factor (TNF)-α, and anti-inflammatory IL-10 (15).
Adipose tissue also produces hormones such as angiotensin (Ang)-II (16), known for regulating blood pressure and fluid balance. Other adipose-derived proteins control energy homeostasis (leptin), glucose homeostasis (adiponectin and vistafin), immune regulation (resistin), and angiogenesis and cardiac contractility (apelin) (17,18). In healthy lean individuals, the adipocytes are small in size, insulin sensitive, and primarily secrete anti-inflammatory mediators such as adiponectin, IL-10, IL-4, IL-13, IL-1 receptor antagonist (IL-1Ra), apelin, and transforming growth factor beta (TGFβ) (19,20). By contrast, in obesity, adipocytes are large and the adipose tissue of individuals with obesity is infiltrated by a large number of pro-inflammatory M1 macrophages (21), and secretes cytokines such as TNF-α, IL-6, visfatin, leptin, MCP-1, Ang-II, and plasminogen activator inhibitor-1 (20). As most of these compounds are pro-inflammatory, the “obese” adipose tissue is often referred to as inflamed. Low-grade inflammation in adipose tissue, which is a hallmark of obesity pathophysiology, is strongly associated with significant alterations in the profile of secreted adipokines (22,23).
The etiology of obesity-associated insulin resistance (IR) is intertwined with dyslipidemia, partially caused by enhanced secretion of free fatty acids (FFAs) by “obese” adipocytes, as illustrated in clinical studies comparing obese vs. lean women (24–26). Further, excessive production of pro-inflammatory adipokines by “obese” adipose tissue also causes IR both directly by inhibiting insulin signaling pathway or indirectly through activation of pathways of inflammation (22). As an example, TNF-α secreted by adipose tissue macrophages is directly involved in IR through serine phosphorylation of insulin receptor substrate (IRS)-1 (27). TNF-α also alters adipocyte differentiation and lipid metabolism, thus contributing indirectly to IR (22). Furthermore, Weyer et al. (28) and Arita et al. (29) have shown that plasma adiponectin levels are decreased in individuals with obesity, and inversely correlate with the degree of IR and hyperinsulinemia. These studies suggest that the metabolic syndrome and chronic low-grade inflammation are intertwined with the pathogenesis of obesity.
BIOACTIVE COMPOUNDS OF FOOD
The risk of chronic inflammation and metabolic syndrome are increased in obesity due to pathologic alterations of WAT metabolism and pro-inflammatory bias of secreted adipokines, which have systemic effects on the organism health status (30). Various strategies were developed to cure and/or prevent obesity; and most used approaches are based on suppressing appetite, normalizing the lipid metabolism, and increasing the energy expenditure (31). Numerous other therapeutic drugs, such as phentermine, diethylpropion, sibutramine, and loarcaserine, were developed for obesity treatments; however, some of them exert gastrointestinal and other adverse effects, such as oily stools, high blood pressure, variation in pulse rate, headache, dizziness, nausea, depression, and other serious psychological disorders (32,33). Accordingly, dietary interventions using natural bioactive food compounds have emerged as promising therapeutic tools for obesity and metabolic diseases, with limited deleterious side effects.
Composition of the diet may affect metabolic and endocrine functions as well as overall energy balance (8). Bioactive compounds are generally natural “extra nutritional” constituents which can be found in small quantities in plants and lipid-rich foods (34). The foods containing bioactive compounds improve body metabolism and energy balance. For example, in animal foods, fatty fish such as salmon, mackerel, and herring are enriched in omega-3 (ω-3) polyunsaturated fatty acids (PUFAs). Fruits, vegetables, nuts, seeds, herbs, and spices are plant based foods that are enriched with bioactive phytochemicals. Phenolic compounds, flavonoids, plant sterols, and carotenoids of plant origin have some well-documented protective effects against the prevention of chronic diseases such as coronary heart disease (35). The US Department of Health and Human Services and the US Department of Agriculture recommend a daily dose of 9 servings (about 4.5 cups) of fruits and vegetables, including 4 servings (about 2 cups) of fruits, and 5 servings (about 2.5 cups) of vegetables, based on a 2,000 kcal diet according to the guidelines for Americans to improve overall health and prevent chronic diseases (36). In this review, we have selected bioactive compounds that are commonly used in foods and supplements in the US and other countries for improving health against diseases including obesity; these are summarized in Table 1, and their major mechanisms of action are summarized in Fig. 1. Several other reviews have covered other bioactive compounds as well as some of those discussed here (8,37).
Table 1.
Summary of some major functional foods, their biological effects and signaling mechanisms
| Food | Functional compound | Biological function | Signaling pathways or mechanisms | Reference |
|---|---|---|---|---|
| Ginger | Ginger extract | Anti-obesity/Anti-inflammatory | ↑ PPAR-δ | 42 |
| Gingerols | Anti-inflammatory | ↓ PGEs, COX1&2, lipoxygenase, leukotrienes, PG synthetase | 43, 45 | |
| Zingerone | Anti-inflammatory | ↓ NF-κB | 48 | |
| Turmeric | Curcumin | Anti-inflammatory | ↓ Phosphorylation of MAPK, Wnt/β-catenin, NF-κB, PAI-1 | 57, 58, 60, 61 |
| ↑ Adiponectin | 61 | |||
| Anti-obesity | ↓ FASN | 59 | ||
| ↑ FA oxidation, AMPK | 57, 51 | |||
| Garlic | Garlic extract | Anti-obesity | ↓ Adipose tissue, TG, Free FA, weight gain, SREBP-1C, PPAR-γ | 68, 69, 71 |
| Anti-inflammatory | ↑ IL-10 | 74 | ||
| ↓ TNF-α, IL-1α, IFN-γ | 74 | |||
| Alliin | Anti-inflammatory | ↓ Phosphorylation of ERK1/2, IL-6, MCP-1 | 73 | |
| Soy beans | Isoflavones (soy proteins) | Anti-obesity | ↓ Regulate adipose tissue body weight, BMI, adiposity, fat pad, SREBP-1C, ACC, FASN | 78, 80, 81, 88, 90, 91, 93 |
| Anti-inflammatory | ↓ TNF-α, MCP-1, IL-6, lipid accumulation | 85, 86, 94 | ||
| ↑ Adiponectin | 88 | |||
| Bilberry | Polyphenols1) | Anti-inflammatory | ↓ Adipocyte differentiation, PPAR, SREBP-1C, NF-κB, CRP, IL-6, IL-15 | 96, 97 |
| Grape | Polyphenol (procyanidin) | Anti-inflammatory | ↓ NF-κB, TNF-α, IL-6 | 98, 99 |
| ↑ Adiponectin | ||||
| Grape, wine | Resveratrol | Anti-obesity | ↓ Lipogenesis | 101 |
| ↑ FA oxidation | ||||
| Strawberry | Anthocyanins2) | Anti-inflammatory | ↓ IL-6 | 102 |
| Blueberries | Anthocyanins2) | Anti-inflammatory | ↓ NF-κB, IL-6, TNF-α, CRP | 103 |
| ↑ Adiponectin | ||||
| Red sweet cherries | Anthocyanins | Anti-inflammatory | ↓ Inflammation | 104, 105 |
| Anti-obesity | ↓ Obesity | 105 | ||
| Tart cherry | Anthocyanins | Anti-inflammatory/Anti-obesity | ↓ NF-κB, adiposity | 106 |
| Omega-3-fatty acids | ALA | Anti-inflammatory | ↓ IL-6, TNF-α, IL1-β, XBP1, sXBP1 | 108, 112 |
| EPA | Anti-inflammatory | ↓ PGE2, COX, MCP-1, IL-6, TNF-α, TLRs, NF-κB | 120, 125, 126 | |
| DHA | ↑ GPR120, PPAR-γ, adiponectin | 14, 121, 122 | ||
| Anti-obesity | ↑ Mitochondrial biogenesis, β-oxidation, AMPK | 117, 118, 123, 107, 106 | ||
| ↓ Adiposity, lipogenesis, visceral fat, body weight | 14, 116 |
PPAR, peroxisome proliferator-activated receptor; PGE, prostaglandin E; COX, cyclooxygenase; PG, prostaglandin; NF-κB, nuclear factor-κB; MAPK, mitogen-activated protein kinase; PAI-1, plasminogen activator inhibitor type-1; FASN, fatty acid synthase; FA, fatty acid; AMPK, adenosine monophosphate-activated protein kinase; TG, triglyceride; SREBP-1C, sterol regulatory element-binding protein 1C; TNF, tumor necrosis factor; IL, interleukin; IFN, interferons; ERK, extracellular-signal-regulated kinase; MCP-1, monocyte chemoattractant protein; BMI, body mass index; ACC, acetyl-coenzyme A carboxylase; CRP, C-reactive protein; ALA, α-linolenic acid; XBP, X-box-binding protein; sXBP, spliced X-box-binding protein; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; GPR120, G-protein coupled receptor 120.
Anthocyanidins, quercetin, epicatechin, and resveratrol.
Pelargonidin sulfate and pelargonidin-3-O-glucoside.
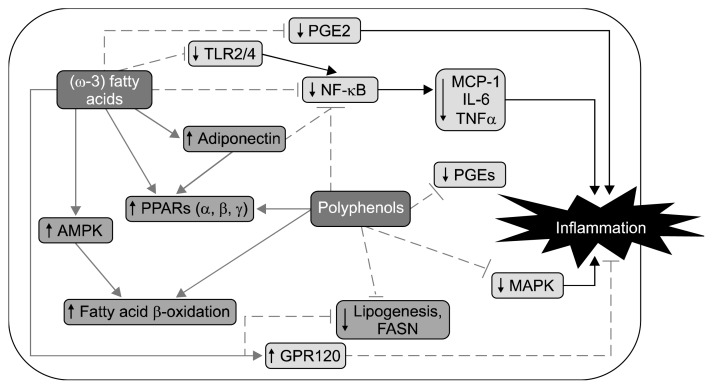
Fig. 1.
Summary of major signaling mechanisms mediating effects of omega-3 (ω-3) fatty acids and some polyphenols. Bioactive compounds such as omega-3 fatty acids and polyphenols discussed in this review modulate cell signaling including various mechanisms including the nuclear factor-κB (NF-κB), adenosine monophosphate-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), toll-like receptors (TLRs), and G-protein coupled receptor 120 (GPR120) intracellular signaling pathways. These effects lead to reduction of inflammation and possibly obesity and associated metabolic diseases. PGE, prostaglandin E; MCP-1, monocyte chemoattractant protein-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; PPAR, peroxisome proliferator-activated receptor; FASN, fatty acid synthase.
GINGER
Ginger (Zingiber officinale), which belongs to Zingiberaceae family, is native to Southern Asia, where it is most commonly used as a spice, especially in the Southeast-Eastern Asian countries (38). Experimental data shows that ginger has multiple health benefits such as digestive stimulant action, antidiabetic effects, lipid lowering and anti-obesity effects, anti-inflammatory effects, protective effects on gastro-intestinal tract, cancer prevention properties, protective effects on liver, kidney, ulcerative colitis, and the absorption of micronutrients (39). Ginger contains both volatile and nonvolatile compounds. Volatile compounds/oil is responsible for the ginger odor (40).
Ginger reduces body weight by downregulating the absorption of lipids via inhibiting the fat hydrolysis processes (41). In particular, the calorie burning can also be increased by consuming ginger extract (39). The anti-inflammatory properties of ginger were shown using both in vitro and in vivo models. Misawa et al. (42) studied the anti-obesity properties of ginger extract using C57BL/6 mice. Their study demonstrated that ginger extract can decrease high fat diet (HFD)-induced obesity via activation of the peroxisome proliferator-activated receptor (PPAR)-δ pathway in skeletal muscle and the liver which induces the utilization of fats and the expression of the genes in the skeletal muscle which regulates the oxidation of fatty acid. Furthermore, ginger extract reduced the infiltration of macrophages in adipose tissue of mice fed with ginger extract-enriched high fat diet, compared to HF diet alone (42). Inhibition properties of cyclooxygenase (COX) and lipoxygenase and synthesis of leukotrienes by ginger extract were demonstrated both in vitro and in vivo (43).
Gingerols, shogaols, and paradols are structurally similar compounds of polyphenolic nature found in ginger, which possess major anti-inflammatory properties. Gingerols are the main ginger pungent constituents. They may be converted to shogaols by dehydration (43). Gingerols inhibited prostaglandin (PG) biosynthesizing enzyme (PG synthetase) and leukotrienes biosynthesis enzyme (arachidonate 5-lipoxygenase) in rat basophilic leukemia (RBL-1) cells (44), and inhibited lipopolysaccharide (LPS) induced COX-2 expression in U937 cells (45). Saravanan et al. (46) reported anti-obesity properties of gingerol through the inhibition of dietary fat absorption in the gastrointestinal tract, and its hypophagic and hypolipidaemic activity in male rats in the HFD-induced model of dietary obesity. Ability of gingerols to improve adipocyte differentiation and insulin-dependent glucose uptake was showed in in vitro studies using mouse 3T3-L1 pre-adipocytes (47). Furthermore, the MCP-1 secretion in the same cells, as well as secretion of such mediators of inflammation as TNF-α and nitric oxide (NO) in macrophages, can be significantly inhibited by another ginger phenolic bioactive, zingerone (vanillyacetone) (48). Taken together, these studies demonstrated that ginger components are able to reduce inflammation, lipid storage and absorption and increasing lipid oxidation.
TURMERIC
Turmeric (Curcuma longa) is widely consumed as a spice in India and other Asian countries. Also, it has been used for thousands of years in a medicine of Ayurveda, which means “science of long life”. First records about the turmeric as a versatile medicine are dated 3,000 B.C. (49). Nutritional analysis of turmeric has shown that 100 g turmeric contain 354 kcal; 19 g total fat, including 3 g saturated fat; 65 g of carbohydrates, 38 mg of sodium; about 2.5 g potassium; 21 g fiber, 3 g sugar, 8 g protein, and no cholesterol (49). More than 100 bioactive components have been identified in turmeric (50). The volatile oil is the main component containing turmerone and other coloring agents known as curcuminoids, a group of chemically related bioactive low molecular weight polyphenols (49). Turmeric has well documented anti-inflammatory, anti-obesity, anti-angiogenesis, anti-carcinogenic and antioxidant activities (51,52). Most protective effects of turmeric are attributed to curcuminoids, consisting of 77% curcumin, 17% demethoxycurcumin, and 3% bide-methoxycurcumin (53). Curcumin has been the most studied component of turmeric (54,55).
A randomized control trial conducted by Di Pierro et al. (56) among overweight people with metabolic syndrome showed the ability of curcumin to reduce weight and omental adipose tissue. Both in vitro and in vivo studies have shown the positive influences of curcumin on obesity and its associated metabolic syndrome (8,37). In mice with dietary obesity, supplementing the diet with curcumin decreased adiposity and body weight (51), and increased the oxidation of fatty acid and adenosine monophosphate activated protein kinase (AMPK) activity in adipocytes (57). In vitro, curcumin inhibited the adipocyte differentiation via suppressing the phosphorylation of mitogen-activated protein kinases [mitogen-activated protein kinases (MAPKs); extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinases, and p38] in 3T3-L1 adipocytes (57). MAPK cascades are crucial for the development of adipocytes from 3T3-L1 precursors. In differentiated adipocytes, curcumin further interferes with adipogenesis, through the Wnt/β-catenin signaling pathway (58). Curcumin reduces lipogenesis in fat cells by decreasing the expression of fatty acid synthase (FASN) (59). Moreover, curcumin ameliorated obesity-associated inflammation by preventing the stimulation of nuclear factor κB (NF-κB), a critical pro-inflammatory transcription factor (60,61). Downregulation of the NF-κB reduced expression of TNF-α and MCP-1, thus preventing the infiltration of macrophages into adipose tissue. Curcumin reduced the expression of such potential inflammatory molecules and increased the expression of anti-inflammatory adiponectin (61). Curcumin treatment in C57/BL6 mice increased fatty acid oxidation and AMPK activity in adipocytes (57), and decreased adiposity and body weight (51).
Overall, these studies indicate that curcumin exerts anti-obesity and anti-inflammatory effects in part through adipose tissue, by reducing adiposity, lipid storage, and increasing lipid oxidation.
GARLIC
Garlic (Allium sativum) belongs to the Alliaceae family. Garlic is most popularly used as both a spice and a medicine. It was originated from Central Asia, but it is also currently grown in Mexico, Europe, and North Africa (62). The bioactive components of this plant have been studied for their hypocholesterolemic, hypoglycemic, antioxidant, anticancer, and anti-obesity beneficial biological effects (63). Alliin, ajoene, allicin, diallyl disulfide, diallyl trisulfide, allyl methanethiosulfinate, S-allylcystein, and other numerous sulphur compounds of garlic have been implicated in its anticancer effects (64–66). Allicin (diallyl-dithiosulfinate) is often referred to as the most important garlic bioactive component (62). As the therapeutic efficacy of most of garlic organosulphur bioactives can be compromised during food processing, and cooking protocols are particularly important for garlic containing foods.
Many published studies reported beneficial effects of garlic against obesity, heart diseases, hyperglycemia, and high blood pressure (63,67–70). It was observed that in obese mice, garlic decreases total body adiposity and ameliorates the obesity-associated dyslipidemia via reducing blood triglycerides, FFAs, and total cholesterol (69). Using 3T3-L1 pre-adipocytes, Kim et al. (71) have demonstrated the ability of the garlic compound thiacremonone to interfere with adipocyte differentiation. The anti-adipogenic effects of this sulfur compound are mediated via downregulating the transcription factor PPAR-γ and activating the AMPK. The latter finding was further corroborated by the observation that thiacremonone-induced AMPK activation results in up-regulation of the UCP-2 gene, related to energy expenditure by BAT (71). In a rat model of HFD-induced obesity, administration of 250 mg/kg body weight of aged black garlic with the animal diet reduced weight gain and dyslipidemia (68,72). Ha et al. (68) further showed that garlic extracts modulate lipid and cholesterol metabolism by lowering the liver sterol regulatory element-binding protein 1C (SREBP-1C) mRNA levels.
Anti-inflammatory properties of alliin, a simple L-cysteine derivative in garlic have been extensively studied. Alliin reduced LPS-stimulated inflammation in 3T3-L1 adipocytes by decreasing the phosphorylation of ERK1/2. This effect is associated with decreased gene expression of IL-6 and MCP-1 inflammatory markers (73). Human peripheral blood leukocytes, treated with garlic extracts in vitro, exhibited suppression of TNF-α, IL-1α, interferons (IFN)-γ, and other pro-inflammatory cytokines production along with upregulation of anti-inflammatory IL-10 (74). The above studies demonstrate beneficial effects of garlic components in metabolic diseases by reducing obesity and inflammation and increasing energy expenditure.
SOYBEAN
Soybean (Glycine max), an East Asian legume, is well known for its high quality protein, which contains all nine essential amino acids. The nutritional value of soybean protein may be considered equivalent to meat and animal proteins (75). Soy proteins contain isoflavones, which are phytochemicals, also referred to phytoestrogens, due to their ability to bind estrogen receptors and mediate estrogenic or antiestrogenic effects in mammals (76). Isoflavones are diphenolic compounds capable of reducing oxidative stress (77). Genistein, daidzen, and glycetin are the main isoflavones of soy. Genistein is the major isoflavone in soy and the most reviewed phytoestrogen in soy. All three soy isoflavones regulate adipose tissue without affecting food consumption (78). Furthermore, several studies show favorable role of soy in enhancing health and preventing diseases, such as cholesterol and triglyceride lowering effects (79), reducing adiposity, improving IR as well as protecting against cancer and osteoporosis and ameliorating the symptoms of menopause (80,81). In 1999, the Food and Drug Administration endorsed the claim that 25 g of soy protein per day (approximately 50 mg/d of isoflavones) may lower the risk of heart disease (82). This amount of isoflavones corresponds to the typical daily consumption in Asian countries, however, less than 1 mg of isoflavones is consumed daily in Western countries (83,84).
Many studies reported beneficial effects of soybeans on inflammation in many health conditions (85). Soy proteins inhibit secretion of inflammatory cytokines (TNF-α, MCP-1, and IL-6) and reduce the inflammatory responses in mature murine adipocytes in vitro (86). This finding is in line with inhibiting of LPS-induced inflammation by soy peptides and pancreatic soybean hydrolysates in RAW 264.7 macrophages, reported by Vermont et al. (87). Similarly, Zhang et al. (88) observed that 150 to 450 mg/(kg×d) isoflavone administered with HFD to rats sharply decreased the adiposity and pro-inflammatory adipokine (IL-6, TNF-α, and resistin) levels, and improved blood adiponectin. These alterations in body structure and adipokine profiles correlated with improved insulin sensitivity in animals treated with dietary soy isoflavones (88). A recent randomized clinical trial demonstrated that a nutritional intervention using 30 g soy protein/d as an alternative to animal protein improved body weight, body mass index, and biomarkers associated with cardiovascular risk; including total cholesterol, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, and apolipoprotein B (89). In vivo experiments in rodent obesity models have shown that dietary isoflavones significantly reduced body weight and fat pad weight (90,91). Xiao et al. (92) have shown that soy isoflavones promote lipid clearance in the liver, a major lipogenesis and lipid β-oxidation organ, in weanling Sprague-Dawley rats. It was shown in the same study, that SREBP1 transcription factor expression and its other target genes (acetyl-coenzyme A carboxylase, ATP-citrate lyase, and FASN) were reduced by soy isoflavones (93). Consistent with these findings, soy isoflavones also decrease lipid gathering in differentiated 3T3-L1 cells (94).
Overall, available data demonstrate that soy isoflavones reduce adiposity and inflammation, by reducing lipogenesis, adipogenesis, and improving lipid clearance and may contribute in this manner to metabolic improvements in obesity.
GRAPE, CHERRIES AND BERRIES
Grape, cherries and berries are popular fruits which contain large amounts of polyphenols such as anthocyanins, proanthocyanidins, and resveratrol. Historically, foods, rich in anthocyanins, have been used as a medicine. Anthocyanins (cyanidin-3-glucoside) improve hyperglycemia and insulin sensitivity by inducing the expression of glucose transporter type 4 and decreasing the retinol binding protein 4 (RBP4), which is accompanied by the reduced expression of MCP-1 and TNF-α inflammatory cytokines in white adipose tissue in type 2 diabetic mice (95). Anthocyanidins and bilberry extracts similarly inhibited differentiation of 3T3-L1 adipocytes in vitro and regulated expression of insulin pathway genes, and reduced the IRS-1 phosphorylation. Moreover, both anthocyanidins and bilberry extracts decreased the expression of PPAR-γ (96). In another study, polyphenol-rich bilberry juice reduced expression of NF-κB pathway genes, C-reactive protein (CRP), IL-6, IL-15, and monokine induced by IFN-γ (97). This study also determined effects of polyphenols on LPS-induced NF-κB activation in monocyte cell line and found that quercetin, epicatechin, and resveratrol in bilberry repressed NF-κB activation (97).
A mix of procyanidin (members of proanthocyanidin) flavonoids extracted from grape has shown anti-inflammatory effects on human macrophage-like cell lines and adipocytes (98). This polyphenol rich extract was able to reduce NF-κB pathway and increased the anti-inflammatory adiponectin production (98). Moreover, supplementing the animal diet with grape procyanidins resulted in reduction of TNF-α and IL-6 production in the mesenteric white adipose tissue of male Zucker fa/fa rats prone to spontaneous obesity (99).
Resveratrol, a naturally occurring stress-response polyphenol abundant in grape, grape juice and red wine, reduced the RBP4 and resistin gene expression in 3T3-L1 adipocytes (100). Both these adipokines have been implicated in IR in mice (30,31). Resveratrol also reduced lipogenesis and enhanced fatty acid oxidation in adipocytes (101).
In a clinical study, strawberry anthocyanins, pelargonidin sulfate, and pelargonidin-3-O-glucoside reduced the postprandial inflammation and plasma IL-6, and increased insulin sensitivity in overweight human subjects (102).
Blueberries, another anthocyanin-rich berry, improved the insulin sensitivity in obese insulin-resistant adults (100). In a Zucker rat model of spontaneous obesity, blueberries revealed a wide spectrum of anti-inflammatory activities, including reduced expression of NF-κB, IL-6, and TNF-α in the liver and adipose tissue and lowered concentrations of IL-6, TNF-α, and CRP in plasma while upregulating adiponectin (103).
Consumption of cherries mitigates obesity-associated inflammation and metabolic syndrome. In genetically obese and diabetic db/db mice, a diet supplemented with red sweet cherry powder, ameliorated inflammation and hyperglycemia (104). Anthocyanins, purified from methanol extract of sweet cherries, prevented adipocyte differentiation of 3T3-L1 cells and improved HFD-induced obesity in C57BL/6J (B6) mice. Mice, whose diet was enriched with cherry anthocyanins, improved dyslipidemia and hyperglycemia and reduced plasma levels of chronic inflammatory markers (105). Supplementing the HFD with freeze-dried tart cherries administered to Zucker rats for 90 days, reduced expression of NF-κB and its downstream target genes and decreased total body adiposity, compared to control animals (106).
OMEGA-3 POLYUNSATURATED FATTY ACIDS (ω-3 PUFAs)
Dietary fats play a versatile role in whole body homeostasis as a source of energy, components of cell membranes and metabolic precursors for hormones and immune system mediators. Depending on their molecular structure, the dietary fats fall into one of three main categories, namely saturated fatty acids, monounsaturated fatty acids, and PUFAs. According to the location of the first double bond from the methyl (−CH3) end of the molecule, long chain PUFAs are further classified into two major groups: ω-3 and omega-6 (ω-6) fatty acids. ω-3 and ω-6 PUFAs are both essential food constituents as they cannot be synthesized in mammals (107).
Examples for the dietary sources rich in ω-3 fatty acids are fatty marine fish, nuts, and plant oils. However marine fats are rich specifically in very long chain ω-3 fatty acids. The composition of ω-3 PUFAs found in plant sources is mostly enriched with short-chained α-linoleic acid (ALA) which contains 18 carbon atoms (C18:3). Canola (rapeseed), walnut, soybean (up to 10% ALA in total fatty acids) and flaxseed (over 50% ALA in total fatty acids) oils are most widely distributed plant ω-3 PUFA sources. Health benefits of ALA are related with its ability to reduce systemic inflammation. In clinical studies, supplementing the diet with ALA inhibited the IL-6, IL-1β and TNF-α production in peripheral blood mononuclear cells and decreased plasma TNF-α (108). These effects were accompanied by increased proportions of long-chained ω-3 PUFAs: eicosapentaenoic acid (EPA) and docosapentaenoic acid in membranes of neutrophils, monocytes and lymphocytes (108–110). This phenomenon may be explained by the fact that, in mammalian organisms, short chain ALA is converted to some extent to long chain EPA, decosahexaenoic acid (DHA), and other ω-3 PUFAs after a series of elongation, desaturation, and β-oxidation reactions (107,111). Another recent study reported that ALA supplementation decreased expression of genes associated with endoplasmic reticulum stress such as X-box-binding protein (XBP)1 (20%), spliced XBP1 (70%) in subcutaneous adipose tissue in patients with type 2 diabetes (112).
The fatty sea fish species like mackerel, sardines, mullet, salmon, tuna, trout, bluefish, herrings, and anchovies are the major sources of animal fats rich in long chain ω-3 PUFAs (15). Compared to ω-3 PUFA-rich plants, the animal sources of ω-3 PUFAs contain more significant fractions of long-chained EPA (C20:5) and DHA (C22:6). EPA and DHA provide stronger and more rapid health protective effects than ALA due to their high ability to be incorporated into plasma and membrane lipids (113, 114). Moreover, DHA and EPA serve metabolic precursors for biosynthesis of lipid anti-inflammatory mediators such as resolvins, protectins, and maresins (107,115). In most of the countries which have accepted a westernized lifestyle, the diet mainly contains short chain ALA, but lacking in long chain omega-3 PUFAs (111).
Experimental studies have shown that adipose tissue is an anatomical location where the anti-inflammatory effects of ω-3 PUFAs are exerted. In individuals with obesity and laboratory animals, the adipocytes are filled with lipids, are insulin resistant and involved in the pathogenesis of the MetS. Some studies, especially in animals, showed that dietary ω-3 PUFAs are capable of decreasing body adiposity (116,117). Furthermore, these fatty acids increased mitochondria biogenesis and β-oxidation of fatty acid in adipose tissue (117,118). In addition, DHA has ability to inhibit adipocyte differentiation and induce apoptosis in preadipocytes in vitro (119). These mechanisms contribute to anti-obesity effects of long chain ω-3 PUFAs. These fatty acids also reduce inflammation by decreasing the secretion of MCP-1, IL-6, and TNF-α in vitro from LPS-induced human adipose tissue or mature adipocyte cultures (120). Mechanisms mediating actions of long chain ω-3 fatty acids such as EPA and DHA is in part through their lipid mediators protectins and resolvins (120). Further, these fatty acids may act by binding with G protein-coupled receptor 120 (GPR120). Indeed, it was demonstrated that long chain ω-3 fatty acids inhibit the NF-κB pathway in RAW 264.7 mouse macrophages and reduce macrophage-induced adipose tissue inflammation and insulin resistance through GPR120 (121).
The long chain ω-3 PUFAs serve as ligands for PPAR-α and PPAR-γ, the members of the PPAR transcription factors family, which functions in different aspects of regulating energy balance, including lipid metabolism (107, 115,118). EPA and DHA increase secretion of adiponectin in adipose tissue and reduce body weight, in part through PPAR-γ activation in mice fed with a fish oil diet (15, 122). ω-3 PUFAs reduce adiposity and progress of obesity through other key regulatory transcription factors such as PPARs (α, β, and γ) which are involved in adipogenesis and lipid metabolism in mature adipocytes (123). In addition, these fatty acids activate the AMPK pathway, thus inhibiting lipogenesis, and stimulating lipid oxidation, and glucose uptake by adipose tissue (124). Moreover, ω-3 PUFAs inhibit gluconeogenesis in the liver, stimulate glucose transport, and induce mitochondrial biogenesis in skeletal muscle (107). EPA suppresses the liver lipogenesis and steatosis in mice fed with a high fat diet, and prevents visceral fat accumulation and obesity (116). Moreover, ω-3 PUFAs inhibit the inflammatory mediators such as prostaglandins-2 by inhibiting COX activity (125). Obesity-associated inflammation may also be directly modulated by ω-3 PUFAs due to their ability to downregulate the expression of toll-like receptors (TLRs). In mice suffering from LPS-induced systemic inflammation, supplementing the diet with EPA and DHA rich fish oil resulted in decreased levels of IL-1β, IL-6, and TNF-α accompanied by reduced expression of TLR4 gene as well as the genes of NF-κB, MyD88 and other components of TLR4 pathway (126). These data were generated using lean animals fed regular diet. But the protective effects exerted by long chain ω-3 PUFAs against the inflammation, associated with obesity, may be mediated by similar mechanisms, because this kind of inflammation is partially caused by increased intestinal permeability and elevated plasma LPS (127). In sum, the complex spectrum of anti-obesity and anti-inflammatory benefits and diverse mechanisms mediating effects of ω-3 PUFAs point to a promising role for these macronutrients in and for additional research in this area, especially in humans.
CONCLUSION
Obesity is a complex multifactorial disease with the incidence and prevalence rates growing epidemically and globally. Adipose tissue is the anatomic site of manifestation of major pathophysiologic effects of obesity and its associated inflammation. Chronic low-grade inflammation compromises the healthy secretion profile of pro-and anti-inflammatory adipokines and increases the risk of developing insulin resistance, heart and vascular diseases, respiratory disorders, and cancers. Various strategies including lifestyle modifications, dietary supplements and eating patterns have been developed to reduce obesity and inflammation. Numerous clinical and basic, in vivo and in vitro, studies demonstrate the link between the health benefits of the foods containing bioactive compounds and their ability to regulate gene expression in adipose tissue, thus modulating the secretion of adipose cytokines and hormones. The molecular mechanisms of bioactive food compounds are being characterized extensively. Along with its metabolic and inflammation-related complications, the obesity can be ameliorated by including the foods enriched with bioactive compounds into the diet. Bioactive food compounds discussed in this review have a promising potential as an anti-inflammatory components of science-based novel therapeutic diets, which may help patients with obesity. More basic and clinical studies to evaluate the anti-obesity impacts of bioactive food compounds individually or in combination, are warranted. Moreover, educating the people worldwide about nutritional advantages of natural foods, advocating particularly those which are traditional for particular countries and cultures, may be invaluable for preventing the incidence of western diet-induced obesity. This in turn will help halt the obesity epidemic, and ultimately improve the quality of life while reducing the economic burden of obesity.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 2.LeMieux MJ, Kalupahana NS, Scoggin S, Moustaid-Moussa N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J Nutr. 2015;145:411–417. doi: 10.3945/jn.114.202952. [DOI] [PubMed] [Google Scholar]
- 3.Torres-Leal FL, Fonseca-Alaniz MH, Rogero MM, Tirapegui J. The role of inflamed adipose tissue in the insulin resistance. Cell Biochem Funct. 2010;28:623–631. doi: 10.1002/cbf.1706. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. [accessed Jun 2017];Obesity and overweight. 2016 http://www.who.int/mediacentre/factsheets/fs311/en/
- 8.Siriwardhana N, Kalupahana NS, Cekanova M, LeMieux M, Greer B, Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem. 2013;24:613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/S1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 10.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol. 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 11.Barinaga M. “Obese” protein slims mice”. Science. 1995;269:475–476. doi: 10.1126/science.7624769. [DOI] [PubMed] [Google Scholar]
- 12.Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- 13.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2:304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saint-Marc P, Kozak LP, Ailhaud G, Darimont C, Negrel R. Angiotensin II as a trophic factor of white adipose tissue: stimulation of adipose cell formation. Endocrinology. 2001;142:487–492. doi: 10.1210/endo.142.1.7883. [DOI] [PubMed] [Google Scholar]
- 17.Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, Ayav A, Ziegler O, Carpene C, Saulnier-Blache JS, Valet P, Castan-Laurell I. TNFα up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006;20:1528–1530. doi: 10.1096/fj.05-5243fje. [DOI] [PubMed] [Google Scholar]
- 18.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol. 1999;276:E278–E284. doi: 10.1152/ajpendo.1999.276.2.E278. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz JF, Klein S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am J Physiol Endocrinol Metab. 2000;278:E1144–E1152. doi: 10.1152/ajpendo.2000.278.6.E1144. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 27.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor α-induced phosphorylation of insulin receptor substrate-1 (IRS-1): possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 28.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 29.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 30.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. A natural solution for obesity: bioactives for the prevention and treatment of weight gain. A review. Nutr Neurosci. 2015;18:49–65. doi: 10.1179/1476830513Y.0000000099. [DOI] [PubMed] [Google Scholar]
- 32.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Eng J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 33.Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J. 2012;36:13–25. doi: 10.4093/dmj.2012.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitts DD. Bioactive substances in food: identification and potential uses. Can J Physiol Pharmacol. 1994;72:423–434. doi: 10.1139/y94-062. [DOI] [PubMed] [Google Scholar]
- 35.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services and U.S. Department of Agriculture. [accessed Jun 2017];2015–2020 Dietary guidelines for Americans. (8th ed). 2015 https://health.gov/dietaryguidelines/2015/guidelines/
- 37.Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25:1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiengburanatam N, Boonmee A, Sangvanich P, Karnchanatat A. A novel α-glucosidase inhibitor protein from the rhizomes of Zingiber ottensii valeton. Appl Biochem Biotechnol. 2010;162:1938–1951. doi: 10.1007/s12010-010-8971-7. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan K. Ginger rhizomes (Zingiber officinale): a spice with multiple health beneficial potentials. Pharma-Nutrition. 2017;5:18–28. doi: 10.1016/j.phanu.2017.01.001. [DOI] [Google Scholar]
- 40.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 41.Han LK, Gong XJ, Kawano S, Saito M, Kimura Y, Okuda H. Antiobesity actions of Zingiber officinale Roscoe. Yakugaku Zasshi. 2005;125:213–217. doi: 10.1248/yakushi.125.213. [DOI] [PubMed] [Google Scholar]
- 42.Misawa K, Hashizume K, Yamamoto M, Minegishi Y, Hase T, Shimotoyodome A. Ginger extract prevents high-fat diet-induced obesity in mice via activation of the peroxisome proliferator-activated receptor δ pathway. J Nutr Biochem. 2015;26:1058–1067. doi: 10.1016/j.jnutbio.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Grzanna R, Lindmark L, Frondoza CG. Ginger–an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 44.Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull. 1992;40:387–391. doi: 10.1248/cpb.40.387. [DOI] [PubMed] [Google Scholar]
- 45.Lantz RC, Chen GJ, Sarihan M, Sólyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–128. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B. Anti-obesity action of gingerol: effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J Sci Food Agric. 2014;94:2972–2977. doi: 10.1002/jsfa.6642. [DOI] [PubMed] [Google Scholar]
- 47.Sekiya K, Ohtani A, Kusano S. Enhancement of insulin sensitivity in adipocytes by ginger. Biofactors. 2004;22:153–156. doi: 10.1002/biof.5520220130. [DOI] [PubMed] [Google Scholar]
- 48.Woo HM, Kang JH, Kawada T, Yoo H, Sung MK, Yu R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007;80:926–931. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 49.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakayama R, Tamura Y, Yamanaka H, Kikuzaki H, Nakatani N. Two curcuminoid pigments from Curcuma domestica. Phytochemistry. 1993;33:501–502. doi: 10.1016/0031-9422(93)85548-6. [DOI] [Google Scholar]
- 51.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. 2010;2:737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 55.Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57:2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 56.Di Pierro F, Bressan A, Ranaldi D, Rapacioli G, Giacomelli L, Bertuccioli A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur Rev Med Pharmacol Sci. 2015;19:4195–4202. [PubMed] [Google Scholar]
- 57.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 58.Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/β-catenin signaling. Am J Physiol Cell Physiol. 2010;298:C1510–C1516. doi: 10.1152/ajpcell.00369.2009. [DOI] [PubMed] [Google Scholar]
- 59.Zhao J, Sun XB, Ye F, Tian WX. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol Cell Biochem. 2011;351:19–28. doi: 10.1007/s11010-010-0707-z. [DOI] [PubMed] [Google Scholar]
- 60.Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-κB-mediated cytokine expression in adipocytes. Nutr Metab. 2008;5:17. doi: 10.1186/1743-7075-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bradford PG. Curcumin and obesity. Biofactors. 2013;39:78–87. doi: 10.1002/biof.1074. [DOI] [PubMed] [Google Scholar]
- 62.Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J Basic Med Sci. 2013;16:1031–1048. [PMC free article] [PubMed] [Google Scholar]
- 63.Joo H, Kim CT, Kim IH, Kim Y. Anti-obesity effects of hot water extract and high hydrostatic pressure extract of garlic in rats fed a high-fat diet. Food Chem Toxicol. 2013;55:100–105. doi: 10.1016/j.fct.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 64.Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 65.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (garlic) in metabolic syndrome. J Endocrinol Invest. 2015;38:1147–1157. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 66.Omar SH, Al-Wabel NA. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm J. 2010;18:51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arreola R, Quintero-Fabián S, López-Roa RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Grajeda L, Ortuño-Sahagún D. Immunomodulation and anti-inflammatory effects of garlic compounds. Immunol Res. 2015;2015:401630. doi: 10.1155/2015/401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ha AW, Ying T, Kim WK. The effects of black garlic (Allium satvium) extracts on lipid metabolism in rats fed a high fat diet. Nutr Res Pract. 2015;9:30–36. doi: 10.4162/nrp.2015.9.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y, Lee MS, Kim JS, Lee HS. Garlic decreases body weight via decrease of serum lipid and increase of uncoupling proteins mRNA expression. FASEB J. 2007;21:LB59. [Google Scholar]
- 70.Park HS, Choi EJ, Lee JH, Kim GH. Evaluation of Allium vegetables for anti-adipogenic, anti-cancer, and anti-inflammatory activities in vitro. J Life Sci. 2013;5:127–132. doi: 10.1080/09751270.2013.11885219. [DOI] [Google Scholar]
- 71.Kim EJ, Lee DH, Kim HJ, Lee SJ, Ban JO, Cho MC, Jeong HS, Yang Y, Hong JT, Yoon DY. Thiacremonone, a sulfur compound isolated from garlic, attenuates lipid accumulation partially mediated via AMPK activation in 3T3-L1 adipocytes. J Nutr Biochem. 2012;23:1552–1558. doi: 10.1016/j.jnutbio.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Kim I, Kim JY, Hwang YJ, Hwang KA, Om AS, Kim JH, Cho KJ. The beneficial effects of aged black garlic extract on obesity and hyperlipidemia in rats fed a high-fat diet. J Med Plants Res. 2011;5:3159–3168. [Google Scholar]
- 73.Quintero-Fabián S, Ortuño-Sahagún D, Vázquez-Carrera M, López-Roa RI. Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3T3-L1 adipocytes. Mediators Inflamm. 2013;2013:381815. doi: 10.1155/2013/381815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–215. doi: 10.1002/cyto.10133. [DOI] [PubMed] [Google Scholar]
- 75.Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007;4:72–82. doi: 10.7150/ijms.4.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rimbach G, Weinberg PD, de Pascual-Teresa S, Alonso MG, Ewins BA, Turner R, Minihane AM, Botting N, Fairley B, Matsugo S, Uchida Y, Cassidy A. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim Biophys Acta. 2004;1670:229–237. doi: 10.1016/j.bbagen.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Ørgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med. 2008;233:1066–1080. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- 79.Brook JG, Linn S, Aviram M. Dietary soya lecithin decreases plasma triglyceride levels and inhibits collagen-and ADP-induced platelet aggregation. Biochem Med Metab Biol. 1986;35:31–39. doi: 10.1016/0885-4505(86)90055-1. [DOI] [PubMed] [Google Scholar]
- 80.Panneerselvam S, Packirisamy RM, Bobby Z, Sridhar MG. Protective effect of soy isoflavones (from Glycine max) on adipose tissue oxidative stress and inflammatory response in an experimental model of post-menopausal obesity: the molecular mechanisms. Biochem Anal Biochem. 2016;5:266. doi: 10.4172/2161-1009.1000266. [DOI] [Google Scholar]
- 81.Cassidy A, Albertazzi P, Lise Nielsen I, Hall W, Williamson G, Tetens I, Atkins S, Cross H, Manios Y, Wolk A, Steiner C, Branca F. Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Proc Nutr Soc. 2006;65:76–92. doi: 10.1079/PNS2005476. [DOI] [PubMed] [Google Scholar]
- 82.U.S. Food and Drug Administration. [accessed Jun 2017];CFR–code of Federal regulations title. 2017 :21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm.
- 83.Keinan-Boker L, van Der Schouw YT, Grobbee DE, Peeters PH. Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr. 2004;79:282–288. doi: 10.1093/ajcn/79.2.282. [DOI] [PubMed] [Google Scholar]
- 84.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake and body composition in postmenopausal women. Menopause. 2003;10:427–432. doi: 10.1097/01.GME.0000058866.35869.B4. [DOI] [PubMed] [Google Scholar]
- 85.Fanti P, Asmis R, Stephenson TJ, Sawaya BP, Franke AA. Positive effect of dietary soy in ESRD patients with systemic inflammation–correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol Dial Transplant. 2006;21:2239–2246. doi: 10.1093/ndt/gfl169. [DOI] [PubMed] [Google Scholar]
- 86.Kwak SJ, Kim CS, Choi MS, Park T, Sung MK, Yun JW, Yoo H, Mine Y, Yu R. The soy peptide Phe-Leu-Val reduces TNFα-induced inflammatory response and insulin resistance in adipocytes. J Med Food. 2016;19:678–685. doi: 10.1089/jmf.2016.3685. [DOI] [PubMed] [Google Scholar]
- 87.Dia VP, Bringe NA, de Mejia EG. Peptides in pepsin-pancreatin hydrolysates from commercially available soy products that inhibit lipopolysaccharide-induced inflammation in macrophages. Food Chem. 2014;152:423–431. doi: 10.1016/j.foodchem.2013.11.155. [DOI] [PubMed] [Google Scholar]
- 88.Zhang HM, Chen SW, Zhang LS, Feng XF. Effects of soy isoflavone on low-grade inflammation in obese rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:336–339. [PubMed] [Google Scholar]
- 89.Ruscica M, Pavanello C, Gandini S, Gomaraschi M, Vitali C, Macchi C, Morlotti B, Aiello G, Bosisio R, Calabresi L, Arnoldi A, Sirtori CR, Magni P. Effect of soy on metabolic syndrome and cardiovascular risk factors: a randomized controlled trial. Eur J Nutr. 2016;27:1–13. doi: 10.1007/s00394-016-1333-7. [DOI] [PubMed] [Google Scholar]
- 90.Davis J, Higginbotham A, O’Connor T, Moustaid-Moussa N, Tebbe A, Kim YC, Cho KW, Shay N, Adler S, Peterson R, Banz W. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann Nutr Metab. 2007;51:42–52. doi: 10.1159/000100820. [DOI] [PubMed] [Google Scholar]
- 91.Kim HK, Nelson-Dooley C, Della-Fera MA, Yang JY, Zhang W, Duan J, Hartzell DL, Hamrick MW, Baile CA. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J Nutr. 2006;136:409–414. doi: 10.1093/jn/136.2.409. [DOI] [PubMed] [Google Scholar]
- 92.Xiao CW, Wood CM, Weber D, Aziz SA, Mehta R, Griffin P, Cockell KA. Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutr. 2014;9:373. doi: 10.1007/s12263-013-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang C, Pang D, Luo Q, Chen X, Gao Q, Shi L, Liu W, Zou Y, Li L, Chen Z. Soy isoflavones regulate lipid metabolism through an AKT/mTORC1 pathway in diet-induced obesity (DIO) male rats. Molecules. 2016;21:E586. doi: 10.3390/molecules21050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murosaki S, Lee TR, Muroyama K, Shin ES, Cho SY, Yamamoto Y, Lee SJ. A combination of caffeine, arginine, soy isoflavones, and L-carnitine enhances both lipolysis and fatty acid oxidation in 3T3-L1 and HepG2 cells in vitro and in KK mice in vivo. J Nutr. 2007;137:2252–2257. doi: 10.1093/jn/137.10.2252. [DOI] [PubMed] [Google Scholar]
- 95.Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, Ashida H, Horio F, Tsuda T. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74:1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki R, Tanaka M, Takanashi M, Hussain A, Yuan B, Toyoda H, Kuroda M. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr Metab. 2011;8:14. doi: 10.1186/1743-7075-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karlsen A, Paur I, Bøhn SK, Sakhi AK, Borge GI, Serafini M, Erlund I, Laake P, Tonstad S, Blomhoff R. Bilberry juice modulates plasma concentration of NF-κB related inflammatory markers in subjects at increased risk of CVD. Eur J Nutr. 2010;49:345–355. doi: 10.1007/s00394-010-0092-0. [DOI] [PubMed] [Google Scholar]
- 98.Chacón MR, Ceperuelo-Mallafré V, Maymó-Masip E, Mateo-Sanz JM, Arola L, Guitiérrez C, Fernandez-Real JM, Ardèvol A, Simón I, Vendrell J. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine. 2009;47:137–142. doi: 10.1016/j.cyto.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Terra X, Montagut G, Bustos M, Llopiz N, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, Salvadó J, Arola L, Blay M. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Stull AJ. Blueberries’ impact on insulin resistance and glucose intolerance. Antioxidants. 2016;5:44. doi: 10.3390/antiox5040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. J Nutr Biochem. 2011;22:828–834. doi: 10.1016/j.jnutbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 102.Edirisinghe I, Banaszewski K, Cappozzo J, Sandhya K, Ellis CL, Tadapaneni R, Kappagoda CT, Burton-Freeman BM. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br J Nutr. 2011;106:913–922. doi: 10.1017/S0007114511001176. [DOI] [PubMed] [Google Scholar]
- 103.Vendrame S, Daugherty A, Kristo AS, Riso P, Klimis-Zacas D. Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J Nutr Biochem. 2013;24:1508–1512. doi: 10.1016/j.jnutbio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 104.Noratto G, Chew BP, Mertens-Talcott SU. Red sweet cherry ameliorates inflammation in obese diabetic (db/db) mice. FASEB J. 2017;31:S643.21. [Google Scholar]
- 105.Wu T, Tang Q, Yu Z, Gao Z, Hu H, Chen W, Zheng X, Yu T. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int J Food Sci Nutr. 2014;65:351–359. doi: 10.3109/09637486.2013.854749. [DOI] [PubMed] [Google Scholar]
- 106.Seymour EM, Lewis SK, Urcuyo-Llanes DE, Tanone II, Kirakosyan A, Kaufman PB, Bolling SF. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food. 2009;12:935–942. doi: 10.1089/jmf.2008.0270. [DOI] [PubMed] [Google Scholar]
- 107.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci. 2009;116:1–16. doi: 10.1042/CS20070456. [DOI] [PubMed] [Google Scholar]
- 108.Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:385–391. doi: 10.1093/ajcn/85.2.385. [DOI] [PubMed] [Google Scholar]
- 109.Kelley DS, Nelson GJ, Love JE, Branch LB, Taylor PC, Schmidt PC, Mackey BE, Iacono JM. Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids. 1993;28:533–537. doi: 10.1007/BF02536085. [DOI] [PubMed] [Google Scholar]
- 110.Kew S, Banerjee T, Minihane AM, Finnegan YE, Muggli R, Albers R, Williams CM, Calder PC. Lack of effect of foods enriched with plant- or marine-derived n-3 fatty acids on human immune function. Am J Clin Nutr. 2003;77:1287–1295. doi: 10.1093/ajcn/77.5.1287. [DOI] [PubMed] [Google Scholar]
- 111.Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3:1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Holanda Miranda WR, Gomes PM, Beraldo RA, Foss MC, Foss-Freitas MC. Alpha-linolenic acid supplementation effect in endoplasmic reticulum stress and adiponectin in abdominal subcutaneous adipose tissue in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2015;7:A211. doi: 10.1186/1758-5996-7-S1-A211. [DOI] [Google Scholar]
- 113.Simopoulos AP. Omega-3 fatty acids in wild plants, nuts and seeds. Asia Pac J Clin Nutr. 2002;11:S163–S173. doi: 10.1046/j.1440-6047.11.s.6.5.x. [DOI] [Google Scholar]
- 114.Baker EJ, Miles EA, Burdge GC, Yaqoob P, Calder PC. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog Lipid Res. 2016;64:30–56. doi: 10.1016/j.plipres.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 115.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sato A, Kawano H, Notsu T, Ohta M, Nakakuki M, Mizuguchi K, Itoh M, Suganami T, Ogawa Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 2010;59:2495–2504. doi: 10.2337/db09-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulangé A, Vidal H, Slama G, Clément K, Guerre-Millo M, Rizkalla SW. Treatment for 2 mo with n-3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–1679. doi: 10.1093/ajcn/86.5.1670. [DOI] [PubMed] [Google Scholar]
- 118.Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova J, Sponarova J, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce β-oxidation in white fat. Diabetologia. 2005;48:2365–2375. doi: 10.1007/s00125-005-1944-7. [DOI] [PubMed] [Google Scholar]
- 119.Kim HK, Della-Fera M, Lin J, Baile CA. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J Nutr Biochem. 2006;136:2965–2969. doi: 10.1093/jn/136.12.2965. [DOI] [PubMed] [Google Scholar]
- 120.Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F, Césari M, Roche R, Hoareau L. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012;11:175. doi: 10.1186/1476-511X-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-γ-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 123.Madsen L, Petersen RK, Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta. 2005;1740:266–286. doi: 10.1016/j.bbadis.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 124.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 125.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu YH, Li XY, Chen CY, Zhang HM, Kang JX. Omega-3 fatty acid intervention suppresses lipopolysaccharide-induced inflammation and weight loss in mice. Mar Drugs. 2015;13:1026–1036. doi: 10.3390/md13021026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Portune KJ, Benítez-Páez A, Del Pulgar EM, Cerrudo V, Sanz Y. Gut microbiota, diet, and obesity-related disorders–the good, the bad, and the future challenges. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201600252. [DOI] [PubMed] [Google Scholar]