Abstract
Arbuscular mycorrhizal (AM) fungi are essential elements of soil fertility, plant nutrition and productivity, facilitating soil mineral nutrient uptake. Helianthus annuus is a non-model, widely cultivated species. Here we used an RNA-seq approach for evaluating gene expression variation at early and late stages of mycorrhizal establishment in sunflower roots colonized by the arbuscular fungus Rhizoglomus irregulare. mRNA was isolated from roots of plantlets at 4 and 16 days after inoculation with the fungus. cDNA libraries were built and sequenced with Illumina technology. Differential expression analysis was performed between control and inoculated plants. Overall 726 differentially expressed genes (DEGs) between inoculated and control plants were retrieved. The number of up-regulated DEGs greatly exceeded the number of down-regulated DEGs and this difference increased in later stages of colonization. Several DEGs were specifically involved in known mycorrhizal processes, such as membrane transport, cell wall shaping, and other. We also found previously unidentified mycorrhizal-induced transcripts. The most important DEGs were carefully described in order to hypothesize their roles in AM symbiosis. Our data add a valuable contribution for deciphering biological processes related to beneficial fungi and plant symbiosis, adding an Asteraceae, non-model species for future comparative functional genomics studies.
Introduction
Arbuscular mycorrhizal (AM) symbioses are mutualistic associations between plant roots and soilborne fungi, involving about 80% of land plants. The fungal symbionts are represented by arbuscular mycorrhizal fungi (AMF; phylum Glomeromycota), a key group of beneficial obligately biotrophic microorganisms, which enhance plant growth and nutrition, receiving in exchange plant carbon compounds1. AMF increase the uptake and transfer from the soil to the host plant of mineral nutrients, in particular phosphorus (P), by means of large networks of extraradical hyphae, spreading from mycorrhizal roots to the surrounding environment2,3. Moreover, they provide a number of ecosystem services, improving plant tolerance to biotic and abiotic stresses and reducing the use of chemicals in agriculture4. Beyond improving plant nutrition and health, AMF induce changes in plant physiology, affecting the activity of host cell basic metabolism, i.e. plastid biosynthetic pathways and Krebs cycle5,6 and secondary metabolism, i.e. antioxidant enzymatic systems activity, isoprenoids, polyketides, and polyphenols biosynthesis7,8.
Studies aimed at analysing wide-scale gene reprogramming during the establishment and development of arbuscular mycorrhizal symbioses provided information on the molecular changes occurring in different tissues of a few plant species, such as Medicago truncatula, Lotus japonicus, Pisum sativum, tomato, potato, rice and soybean9–15. Large variations in gene expression were detected, depending on fungal symbiont identity, plant tissue and specific root cell/fungal structure location9,13,16,17. Different species of AM fungal symbionts were able to activate both shared and differential gene expression in their host plants16–20. Most work analysed mycorrhizal root transcriptomes, while only two studies detected up/down regulated genes both in shoot and root tissues, which varied with the host plant tested9,17. The analyses of gene expression in roots, where AM symbiosis is established, revealed changes both in plant and fungal transcriptomes, linked to mycorrhizal establishment and development21–25. Moreover, differential expression of some genes putatively involved in the accommodation of fungal symbionts in root cells during appressorium formation and in arbusculated cells was detected by laser-microdissection13,23,26,27. Several studies focused on root gene expression at different stages of mycorrhizal symbiosis establishment, showing differential activation and modulation of gene expression levels11,13,16,21,28–31.
Most of the data reported so far on transcriptome profiles of mycorrhizal plants were obtained by using microarrays or macroarrays, as well as suppression subtractive hybridization and differential display21,27,32. Such studies provided useful information on mycorrhizal regulated transcripts, although limited to the annotated genes available for the different host plant species and tissues. Recently, the RNA-seq technology was used to detect whole transcripts in mycorrhizal and non-mycorrhizal Solanum lycopersicum fruits and leaves33,34 and Oryza sativa and L. japonicus roots10,14. The use of such a technique showed that important gene functional classes, including post-translational regulation, signalling, transport, biotic and abiotic stresses and hormone metabolism, were differentially regulated in leaves of mycorrhizal tomato plants, compared with non-mycorrhizal ones34. The other work on tomato carried out using RNA-seq showed an up-regulation of genes related to photosynthesis, stress response, transport, amino acid synthesis and carbohydrate metabolism functions, and a down-regulation of genes belonging to cell wall, metabolism and ethylene response pathways gene ontology (GO) classes in fruits produced by mycorrhizal plants, compared with non-mycorrhizal ones33. Handa et al. (2015) found 3,641 differentially expressed genes, mostly up-regulated, during arbuscular mycorrhizal development in L. japonicus roots and belonging to transport and secretion functional classes, whereas Fiorilli et al. (2015) detected 1,035 up-regulated genes in O. sativa mycorrhizal lateral roots compared with non-mycorrhizal ones.
Helianthus annuus belongs to the largest Angiosperm family, the Asteraceae. It is widely cultivated, one of the four most important sources of vegetable oil. Native to North America, it was domesticated by native Americans, before being introduced in Europe and bred to become a globally important crop35. It is generally accepted that even in North America the most widespread cultivars are derived from materials reintroduced from Russia after breeding36, indicating a strong reduction of genetic variability in the cultivars, compared with the wild genotypes, that are adapted to a very large range of environments37. The sunflower genome is large (about 3.3 Gbp) and has recently been sequenced38. It is largely (around 80%) made of repetitive elements39–41 with high genetic polymorphism, especially among wild and domesticated genotypes42. Gene expression in sunflower has been studied in different conditions, for example in response to biotic and abiotic stresses, such as oxidative-stress, temperature, wounding, and pathogen infection43–45. However no data are available on the interaction, in terms of gene induction and/or repression, between sunflower and AMF. Recently, a study on mycorrhizal root colonization in sunflower wild accessions, cultivars, and inbred lines, reported that wild accessions were more susceptible to colonization than cultivars46. Such relationship between susceptibility to AMF and degree of domestication was also reported by other studies47,48 and suggested to be driven by soil characteristics combined with differences in root traits.
Here, for the first time, we used RNA-seq technology in order to assess the expression of differentially regulated genes at early and late stages of mycorrhizal symbiosis establishment in the cultivated sunflower.
Results
AM colonization of sunflower roots
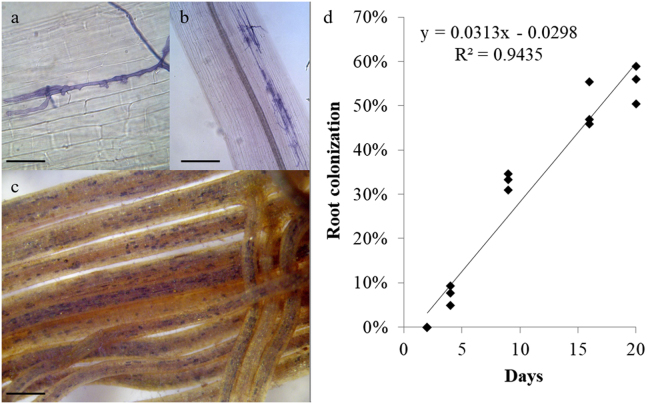
R. irregulare-inoculated roots collected after 2 days of culture did not show any fungal structure, whereas two days later 7.3 ± 1.3% of root length was colonized. At this stage, mycorrhizal colonization was mainly represented by entry points, about 80 ± 21 per root system (0.3 ± 0.06 entry points cm root-1), although only 28% of them developed arbuscules (Fig. 1a,b). Mycorrhizal colonization increased at successive harvests, reaching 49.4 ± 3.0% and 55.1 ± 2.5% after 16 and 20 days of culture, respectively (Fig. 1c,d). After 16 days of culture, entry points raised to 1,230.7 ± 78.4 per root system (4.4 ± 0.4 entry points cm root-1) and 98% of them developed arbuscules.
Figure 1.
Micrographs showing trypan blue (a,b) and succinate dehydrogenase localization and trypan blue staining (c) of Helianthus annuus roots during colonization by the arbuscular mycorrhizal fungus Rhizoglomus irregulare. (a) Appressorium developing a penetration peg on H. annuus roots after 4 days of culture; scale bar = 30 µm; (b) fungal entry points formed by R. irregulare after 4 days of culture; scale bar = 80 µm; (c) H. annuus roots after 16 days of culture, showing extensive mycorrhizal colonization; scale bar = 3 mm; (d) time course of H. annuus mycorrhizal colonization across the different experimental time points. R2 represents the regression coefficient, y indicates the linear equation (n = 3).
cDNA sequencing and aligning on reference predicted transcriptome
Twelve high quality cDNA libraries were prepared using RNA isolated from control and R. irregulare inoculated plants (Table 1).
Tabelle 1.
Summary statistics for the Illumina sequencing and mapping against Helianthus annuus reference transcriptome HanXRQ.
| Library nr. (and treatment) | Number of raw reads | Number of reads after trimming | Number of mapped reads on HanXRQ transcriptome | % of mapped reads on HanXRQ transcriptome |
|---|---|---|---|---|
| 1 (C4) | 48,052,338 | 45,262,022 | 42,298,895 | 93.45 |
| 2 (C4) | 22,150,504 | 22,017,463 | 20,335,682 | 92.36 |
| 3 (C4) | 18,724,363 | 18,464,157 | 17,356,730 | 94.00 |
| 4 (C16) | 20,021,732 | 19,825,159 | 18,765,939 | 94.66 |
| 5 (C16) | 55,479,243 | 54,193,826 | 50,403,351 | 93.01 |
| 6 (C16) | 60,628,585 | 59,400,530 | 54,577,580 | 91.88 |
| 7 (M4) | 52,342,074 | 50,361,161 | 46,184,688 | 91.71 |
| 8 (M4) | 18,198,073 | 17,911,868 | 16,717,830 | 93.33 |
| 9 (M4) | 19,798,867 | 18,640,381 | 17,195,413 | 92.25 |
| 10 (M16) | 40,633,983 | 40,186,929 | 32,182,748 | 80.08 |
| 11 (M16) | 21,494,687 | 21,349,112 | 17,098,190 | 80.09 |
| 12 (M16) | 63,492,221 | 62,865,831 | 54,835,563 | 87.23 |
C4 = uninoculated roots after 4 days of culture, C16 = uninoculated roots after 16 days of culture, M4 = mycorrhizal roots after 4 days of culture with Rhizoglomus irregulare, M16 = mycorrhizal roots after 16 days of culture with Rhizoglomus irregulare.
Illumina sequencing generated 441,016,670 sequence reads, each 100 nt in length, encompassing about 29 GigaByte of sequence data. The total number of reads per library ranged from 18.72 to 63.49 million (Table 1). After removal of ambiguous and low-quality reads, 430,478,439 reads, 85 nt in length were retained (Table 1).
Reads from the 12 libraries were aligned to the reference transcriptome of Helianthus annuus 38. The percentage of mapping reads of each sample to the reference predicted transcriptome ranged from 80.08% to 94.66% (Table 1).
Analysis of differentially expressed genes
We studied the expression of 52,243 predicted transcripts included in the Helianthus annuus reference transcriptome38. Analysis was limited to genes with RPKM > 1 in at least one of the three replicates of each treatment. By this method, we selected 33,332 expressed genes.
GO terms analysis performed on the gene expressed in mycorrhizal roots showed 3,650 and 2,613 genes belonging to “biosynthetic process” and “metabolic process” within the macro-functional class Biological Process; “hydrolase activity” (2,795 transcripts) and “nucleotide binding” (2,793 transcripts) were the most abundant terms in Molecular Function class; top GO terms among Cellular Component ontologies were “membrane” (5,866 genes), and “plastid” (1,580 genes) (Fig. 2).
Figure 2.
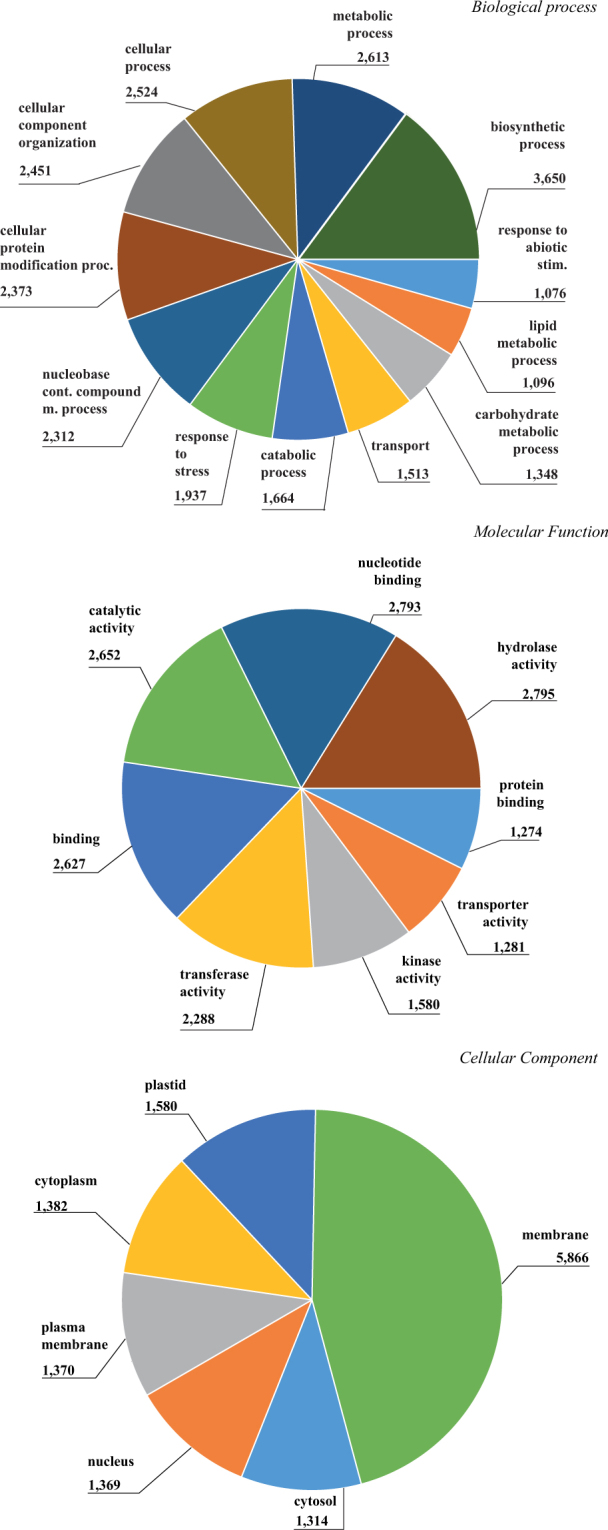
GO slim pie distribution of expressed transcriptome of Helianthus annuus roots, inoculated with Rhizoglomus irregulare. Cut off was set on 1,000 transcripts per GO term.
Overall, 726 differentially expressed genes (DEGs) were detected in sunflower roots inoculated with the AM fungus R. irregulare, compared with uninoculated controls. This relatively low number of DEGs is comparable to those found in other plants inoculated with R. irregulare 10,14.
Differentially expressed genes at the early mycorrhizal stage
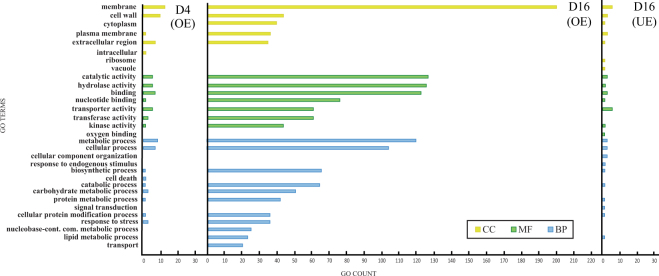
Comparing H. annuus control with mycorrhizal roots 4 days after inoculation (D4), we detected only 19 significantly over-expressed genes. No significant under-expressed transcripts were found in D4 versus control plants. Within biological processes, the most abundant GO-terms of D4 differentially expressed genes were “metabolic process” and “cellular process”; among molecular functions, the most frequent terms were “binding”, “hydrolase activity” and “transporter activity”; cellular component class showed “membrane” and “cell wall” as major terms (Fig. 3).
Figure 3.
GO slim distribution of differentially over and under expressed genes of Helianthus annuus roots during early and late stage of symbiosis (4 days and 16 days after inoculation with Rhizoglomus irregulare). CC = Cellular Component, MF = Molecular Function, BP = Biological Processes.
Amongst differentially expressed genes of D4 plants we identified several transcripts encoding stress response proteins such as germin-like proteins and chitinase, and genes encoding polypeptides with protease activity (Subtilisin-like and carboxypeptidases). Other DEGs included those encoding a nrt1 protein of the ptr family, two UDP-glycosyltransferases, a lysM domain receptor-like kinase 3, an inorganic phosphate transporter, and a glutathione s-transferase (Supplementary Table 1).
Differentially expressed genes at the late mycorrhizal stage
At the second harvesting time point, 16 days after sunflower root inoculation with the AMF R. irregulare (D16), the comparison between control and mycorrhizal roots showed 694 over- and 13 under-expressed genes, respectively (Supplementary Fig. 1).
The greatly increased number of DEGs in D16, compared with D4, may be ascribed to the increasing level of AM root colonization. Furthermore, the number of over-expressed DEGs in D16 plants was considerably higher than that of under-expressed DEGs (Supplementary Fig. 1).
GO terms analysis on D16 DEGs showed that “catalytic activity” and “hydrolase activity” ontologies were the most frequent in the Molecular Function class; the most represented Biological Process terms were “metabolic process” and “cellular processes”; “membrane” and “cell wall” were the two top terms in Cellular Component class (Fig. 3).
Some under-expressed genes were found in mycorrhizal D16 roots, although fewer than the over-expressed transcripts of the same root sample. Regarding Biological Processes, the most represented term was “metabolic process” (2 genes); within Cellular Components the top term was “membrane”; “transporter activity” was the most frequent term in the Molecular Function class (Fig. 3).
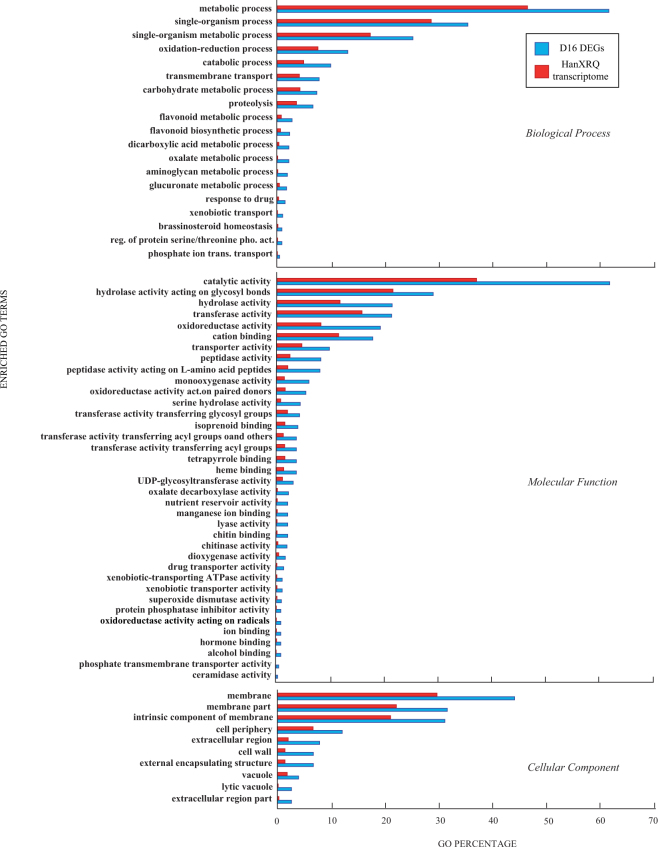
Gene ontology enrichment analysis showed that only the group of over-expressed genes in D16 was significantly enriched (P < 0.05). Using REVIGO, “hydrolases”, “oxidoreductase” and “catalytic activity” categories were enriched in the late stage of colonization: “catalytic activity” were the most enriched among Molecular Functions, and “metabolic process” among Biological Processes (Fig. 4). The most enriched term in the Cellular Component class was “membrane” (Fig. 4).
Figure 4.
Enriched GO term distribution for differentially over-expressed genes of Helianthus annuus roots 16 days after inoculation with Rhizoglomus irregulare. Only summarized enriched GO terms were shown. Abscissa shows percentage of GO terms per D16 DEGs (blue bar) compared to GO terms of whole reference predicted transcriptome (red bar).
Among those over-expressed genes in D16 roots with high fold changes (logFC > 11) we retrieved genes encoding putative transcription factors (an ap2-like ethylene-responsive transcription factor, a DELLA protein, a PHR1), transporter proteins (clathrin assembly protein, an ammonium transporter 3, an ascorbate-specific transmembrane electron transporter, two GDSL esterase lipases) oxidation-reduction activity proteins (two cytochrome p450, several vacuolar-processing enzymes, a putative chitinase 2, several beta-amyrin 28-oxidases, a reticuline oxidase, two SKU5s) and other proteins involved in signal trasduction, cell functioning and metabolism (a vinorine synthase, proteins containing ankyrin repeats, an abscisic acid 8 -hydroxylase 3) (Supplementary Table 1).
In addition, among other over-expressed transcripts at D16 with logFC < 11 were those encoding for proteins involved in stress-defense responses (heat shock proteins, several hydroxyproline-rich glycoprotein, peroxidases, and several lectins) and putative proteins of root remodeling and growth (an acidic endochitinase, two auxin response factors and alpha-tubulins, CDPK, and several ABC transporters) (Supplementary Table 1).
Amongst under-expressed genes of H. annuus mycorrhizal roots at D16 we detected many transcripts encoding for SWEET-like bidirectional sugar transporters, one transcript belonging to a pathogen-related protein and a peroxidase gene. (Supplementary Table 1).
Noteworthy nineteen DEGs were found to be shared among mycorrhizal roots of H. annuus after 4 and 16 days of culture with R. irregulare. All shared transcripts showed an increasing log fold change between the first and the second time point (Table 2).
Tabelle 2.
Differentially expressed genes in H. annuus roots after 4 (D4) and 16 days of culture (D16) with the arbuscular mycorrhizal fungus R. irregulare (log FC: log2 fold change).
| Functional class | Genes identifier | D4 logFC | D16 logFC | Transcript description |
|---|---|---|---|---|
| Catalytic activity (response to stress) | HanXRQChr09g0257681 | 4.32 | 7.57 | germin 2 type 1 |
| HanXRQChr10g0295541 | 3.74 | 6.41 | germin 2 type 1 | |
| HanXRQChr10g0295581 | 4.25 | 7.16 | germin 2 type 1 | |
| HanXRQChr03g0087411 | 7.07 | 11.76 | Mavicyanin | |
| HanXRQChr03g0087431 | 8.46 | 13.28 | Mavicyanin | |
| HanXRQChr05g0159761 | 5.01 | 10.97 | chitinase type 2 | |
| HanXRQChr12g0354981 | 7.77 | 14.36 | Rhicadhesin receptor | |
| Hydrolase activity (protein metabolic process) | HanXRQChr01g0024241 | 6.57 | 10.61 | Subtilisin-like protease |
| HanXRQChr03g0072271 | 6.06 | 9.32 | serine carboxypeptidase II type 3 | |
| HanXRQChr13g0419211 | 4.04 | 11.20 | subtilisin-like protease | |
| HanXRQChr03g0087471 | 5.32 | 10.87 | Subtilisin-like protease SDD1 | |
| Kinase activity and signal transdcution | HanXRQChr06g0174451 | 4.76 | 7.98 | lysM domain receptor kinase type 3 |
| Transferase activity | HanXRQChr11g0350641 | 5.63 | 12.45 | UDP-glycosyltransferase |
| HanXRQChr15g0488781 | 5.10 | 10.79 | UDP-glycosyltransferase | |
| Transporter activity | HanXRQChr15g0472261 | 7.31 | 11.40 | NRT1 PTR |
| HanXRQChr04g0127841 | 4.88 | 11.18 | ammonium transporter 3 member type 1 | |
| HanXRQChr10g0280991 | 4.32 | 11.05 | inorganic phosphate transporter 1 type 4 isoform × 2 | |
| HanXRQChr15g0490031 | 5.42 | 10.10 | Nodulin-26-like | |
| No class | HanXRQChr03g0074111 | 3.99 | 11.30 | Predicted protein |
qRT-PCR validation analysis
In order to validate RNA-seq data, the expression of five genes selected among those differentially expressed between M4 and M16 samples were analysed by qRT-PCR experiments. As shown in Supplementary Figure 2, all genes were differentially expressed, confirming the accuracy of transcriptome profiling obtained with RNA-seq.
Discussion
A large number of terrestrial plant species establish symbioses with AMF, leading to morphological and functional changes in roots. Next generation sequencing techniques can be conveniently used for analysis of transcriptome profiling during plant/fungus interactions. In this work, RNA-Seq experiments in roots of sunflower after R. irregulare inoculation gave a comprehensive view of changes in gene expression, confirming the activation of peculiar AMF regulated genes in sunflower and identifying new genes so far not associated with the establishment of AMF symbioses. Here, we detected a total of 19 and 694 genes over-expressed in mycorrhizal roots at early and late stage of colonization, respectively. Number of DEG is lower to the one retrieved during AM symbiosis in Lotus japonicus and Oryza sativa 10,14. Differences may be related to slightly lower percentage of hyphae colonization in sunflower roots compared to these studies, to different experimental conditions used and to species specific responses.
Among new genes which were not described so far as involved in AMF symbiosis, we identified, for example, those encoding several BAHD-acyltransferases, an oxoglutarate dependent dioxygenase, and a stellacyanin-like protein (Supplementary Table 1).
Overall GO analysis of DEGs showed a number of ontologies: “biosynthetic process”, “hydrolase activity” and “membrane” were the most frequent functional classes. In particular, the GO term “membrane” reflects the major cell membrane rearrangement associated with fungal invasion, “biosynthethic process” suggests host transcriptomic, metabolic and transport activity alteration during symbiosis, and “hydrolase activity” the wide protein recycling associated to cell wall modification (Figs 3 and 4).
Global gene expression data show that D16 mycorrhizal roots were far more active in transcription than D4 root samples. Furthermore, we noticed an unbalanced number of over-expressed genes in comparison with under-expressed genes, as described for other mycorrhizal plant species, for example Lotus japonicus 14,49. Indeed, very few transcripts were significantly under-expressed in sunflower AM roots.
One of the major obstacles in studying AMF symbiosis is the difficulty in synchronizing the initial developmental events leading to the establishment of the symbiosis26. At the early stage time-point (D4), low AMF colonization, mainly represented by entry points with a few developed arbuscules, occurred in inoculated sunflower roots. Consequently, it can be suggested that DEGs in D4 roots could represent genes regulated in the early phases of fungal colonization. During the late phase of symbiosis establishment (D16), when half the length of host roots were colonized by R. irregulare, a higher number of DEGs were found compared to D4, reflecting the advanced stage of root colonization. DEGs were differentially expressed at both time-points, although they were modulated between D4 and D16. This finding is in accordance with the asynchronous nature of the mycorrhizal colonization process, as D16 samples showed fungal structures representative of both early and late stages of the symbiosis, as new entry points may be produced by extraradical hyphae, giving rise to secondary colonization26.
Differentially expressed genes of H. annuus roots in symbiosis with R. irregulare, shared between different time points, encompass genes of symbiosis development, maintenance and functioning and belong to a large number of GO terms. Many of these GO terms and specific genes will be discussed hereafter in relation to their modulation, expression and function.
Differentially over-expressed genes in D4 roots mainly belong to the functional classes “response to stress”, “cell wall” and “transporter activity”, which are largely represented during early interactions between plants and AMF11,28. Interestingly, all D4 DEGs were differentially expressed also in D16 plants, with increasing expression levels at the latter time point (Fig. 3). Indeed, among DEGs involved in plant defense, 3 and 15 transcripts of Germin-like protein were detected in D4 and D16 plants (Table 2) respectively, suggesting a modulation of this multi-gene family as AMF colonization proceeds. Similarly, the number of induced protease-encoding genes increased from 4 to 27 (including genes encoding subtilisins and carboxypeptidases) between the two time points, confirming the involvement of this family in maintaining cell functions and recycling cell wall proteins during mycorrhizal symbioses14,50.
A similar induction profile was observed also for genes involved in transporter activity, which increased from 4 to 60 between early and late stage of colonization (Fig. 3, Table 2), confirming the importance of transporter genes modulation during the symbiotic interaction11. Among these DEGs, those encoding an NRT1 PTR protein, involved in NO3- and auxin transport, an inorganic phosphate transporter and the phosphate and water transporter nodulin-26-like protein were confirmed to be expressed at both early and late stages of symbiosis establishment11,51,52.
A LysM-domain receptor kinase encoding gene is the only signal transduction gene identified amongst D4 DEGs. The number of DEGs of this family increased to 7 in D16 roots, including some leucin rich receptors (Table 2, Supplementary Table 1). LysM is required for the earliest cellular and physiological symbiotic responses and its expression was detected in M. truncatula roots 8 days post inoculation28,53.
UDP-glycosyltransferase is one of the stress-responsive gene belonging to the GO term “transferase activity” and was over-expressed even in D4 AM roots, increasing its logFC between D4 and D16 (Table 2). Its activity should combat oxidative stress and pathogen infection. Genes of this family were reported to be differentially expressed in AM plants14,20,54, but they were never previously described in association with the early steps of mycorrhizal establishment.
DEGs observed only at D16, we will discuss them in relation to their functional classes.
The GO term “response to stress” contains several genes encoding transcripts involved in plant stress and defense, and is one of the most represented among DEGs (Figs 3, 4). It has been established that common defense pathways are activated in both pathogenic and beneficial plant-fungal interactions, as a result of fungal elicitors recognised by the plant28,53.
New transcripts implied in fungus-plant relationship are BAHD-acyltransferases, which are here described for the first time in AM symbioses, and a transferase super-family including Vinorine synthases, which play a central role in the endogenous formation of specialized monoterpenoid secondary metabolites, functioning as defensive compounds upon pathogenic infections55,56. Accumulation of terpenoids belonging to phytoalexins was also detected in mycorrhizal roots of Solanum tuberosum and Solanum lycopersicum 33,57.
Another plant defense related gene, encoding Glutathione-S-transferase, is active in M16 sunflower roots. Its activation was already observed in Medicago truncatula 58.
Other over-expressed transcripts encode phytyltransferases; these genes play a role in the isoprenoid metabolic pathway. Usually, isoprenoids are highly expressed as secondary metabolites during AM formation, conferring resistance to stress with activation of plant defense system59–61.
“Catalytic activity” is another wide functional class involved in plant defense, cell wall loosening and oxidative stress defense. DEGs of this functional class encode for germin-like proteins, the monocopper oxidase SKU5 and several peroxidases62,63 (Supplementary Table 1), all known to be induced during AM symbiosis13,29,49,64,65.
Other DEGs encoding defense and stress-responsive proteins were found in mycorrhizal sunflower roots after 16 days of culture. These genes encode pathogen-related proteins, such as chitinases and endoglucanases, and several peroxidases, which were shown to be differentially expressed in other mycorrhizal plant species66–69.
Other defense-related putatively encoded lectins and enzymes involved in isoprenoid and terpenoid metabolism were reported to accumulate specifically in mycorrhizal roots23. In connection with isoprenoid and terpenoid metabolism, an over-expressed gene in D16 mycorrhizal roots encoded a beta-amyrin 28-oxidase that catalyzes the oxidation of beta-amyrin to oleanolic acid70.
Another new transcript involved in symbiosis events is an oxoglutarate dependent dioxygenase encoding gene. This gene, for the first time associated with AM symbiosis, was over-expressed in D16 mycorrhizal roots. Oxoglutarate dependent dioxygenases are involved in alkaloid production, whose content (hyoscyamine and scopolamine) was previously reported to increase in mycorrhizal roots of Datura stramonium 71.
It is interesting to note that some pathogen-related and disease-resistance proteins encoding genes were significantly down-regulated in sunflower AM roots 16 days after inoculation (Supplementary Table 1). Although a partial overlap between symbiotic and pathogenic signaling has been reported72, transcript levels of some of these genes were much lower in plant roots colonized by beneficial fungi than in those infected by root pathogens. This reduced expression was associated with a very restricted localization of transcripts in arbuscule containing cells73. The repression of transcription of some plant defence genes suggests that AMF are able also to trigger mechanisms in plant roots that suppress defence response allowing colonisation to proceed.
Reception of molecular signals and signal transduction are crucial biological events for symbiosis establishment. DEGs with high logFC related to signal formation and transduction encode P450 oxidases (Supplementary Table 1), which are known to be involved in the production of strigolactones and co-expressed both in the plant and in the fungus during AM symbiosis14,74–76.
Signals released by the fungus activate transcription factors in root cells. Among highly over-expressed putative transcription factors, we detected genes encoding a PHR1, several DELLA proteins involved in gibberellin signaling regulation in AM77–80 (Supplementary Table 1), and many ethylene responsive factors (ERFs). PHR1 is a transcription factor belonging to the MYB family that binds to promoters of most genes positively or negatively affected by Pi starvation81,82, hence it may be considered a candidate gene product involved in Pi transport regulation. Ethylene-responsive factors and the ERF Required for Nodulation (ERN) transcription factor are induced in response to AMF, and ERN-like proteins encoding genes showed specific expression in arbusculated cells23.
Some over-expressed transcripts are related to fatty acid metabolism, as for example the GDSL esterase/lipase. The role of these proteins is not fully known, but their remarkable expression during AM symbiosis, mainly in arbusculated cells, has been correlated with the production of signal molecules and membrane components involved in the synthesis of periarbuscular membranes14.
A transcript encoding a calcium-dependent protein kinase 1-like protein was over-expressed in sunflower D16 mycorrhizal roots, compared with controls (Supplementary Table 1). Calcium-dependent protein kinases (CPKs or CDPKs) are important Ca2+ sensors in signaling processes during the establishment of symbioses between host plants and both AMF and rhizobia83,84.
As reported above, previous studies suggested that hormonal balance of mycorrhizal roots is tightly linked to the development of fungal colonization and root structural changes. The expression pattern of genes putatively encoding for auxin and gibberellin biosynthesis showed that transcripts encoding GA oxidases and auxin response factor were over-expressed in D16 mycorrhizal roots (Supplementary Table 1).
Gibberellin content increased in the roots of mycorrhizal plants compared with uninoculated controls in parallel with the accumulation of transcripts involved in their biosynthesis85–87.
The role of auxins in mycorrhizal symbioses is not clear, as biochemical and molecular studies showed variable auxins and auxin-related molecules levels, although data on auxin mutants suggested that auxin perception is required for arbuscule development and mycorrhizal symbiosis establishment, at least by modulating strigolactone levels79,88,89.
A transcript homologous to zeatin O-glucosyltransferase differentially accumulated in D16 mycorrhizal roots (Supplementary Table 1). Such proteins were reported to be involved in cytokinin pathways with the role of catalyzing the formation of o-xylosylzeatin from zeatin29.
Many over-expressed transcripts putatively encoding transporter proteins were found in both D4 and D16 mycorrhizal roots, including an inorganic P transporter, a multidrug resistant ABC transporter, nitrate and ammonium transporters, and aquaporins (Supplementary Table 1). These genes are known to be highly expressed during mycorrhizal interactions and play a pivotal role in symbiotic signaling and nutrition pathways90–93.
ABC transporter genes are required for arbuscule development94 and were over-expressed in D16 mycorrhizal roots samples.
In this work, genes encoding inorganic P, ammonium and nitrate transporters and NIP class of aquaporins were over-expressed in D4 and D16 mycorrhizal roots, and a larger number of highly expressed isoforms was detected at the late stage of the symbiosis, confirming previous data16,29,95,96. The nitrate transporter Nrt1 was shown to play a pivotal role also in auxin transport and consequently in lateral root formation52.
Another newly identified transcript is related to an aquaporin-like encoding gene over-expressed in D16 AM roots that encodes for a stellacyanin-like protein, exhibiting sequence similarity with nodulin-like transporters97 (Supplementary Table 1). A gene encoding for a putative stellacyanin-like protein, CASLP1, was strongly expressed in Capsicum annuum plants after inoculation with different pathogenic bacteria and fungi, possibly due to water losses or to a role of this protein in limiting pathogen spread98. Although at a lower level of over-expression, a probable tonoplast intrinsic protein (TIP) aquaporin was also detected in D16 mycorrhizal roots.
A 9-fold over-expressed sequence recovered from D16 mycorrhizal roots showed homology to a bidirectional SWEET16-like sugar transporter (Supplementary Table 1), characterized as an exporter of sucrose and monosaccharides99, and could play a role in regulating the transfer of plant carbon resources at the plant–fungus interface. Potato SWEETs showed differential expression regulation in response to AMF, although further studies are needed to clarify SWEETs’ role in sugar partitioning during mycorrhizal colonization process100.
Plant cell walls form a dynamic extracellular matrix that controls growth and development and, at the same time, mediates most plant/fungal interactions. During AM symbiosis the cortical layers are reached by fungal hyphae branches that, after contact, lead to unique structures called arbuscules, and this process requires the activation of several genes101. Many transcripts involved in cell wall and membrane shaping, functional to hyphal penetration and arbuscule development, were highly expressed, such as that encoding SKU5 protein, which exhibits sequence similarity with multiple copper ascorbate oxidase and laccase102 (Supplementary Table 1). Other over-regulated transcripts encode carboxypeptidases, subtilisin-like proteases and a type 1 ATPase, all belonging to the GO term Hydrolase and involved in cell remodeling and membrane transport. Here a considerably higher logFC of the expression of some subtilisins was detected in D16 mycorrhizal roots, compared with D4. This result is similar to that reported in roots of L. japonicus inoculated with R. intraradices 50.
In D16 mycorrhizal roots, transcripts encoding proteins functioning in cell wall and membrane shaping were detected, such as a ripening-related protein, a Clathrin vesicles protein, some vacuolar processing enzymes and Ankyrin-repeat containing proteins, as previously reported52,103,104 (Supplementary Table 1).
EG45 domain is typical of the expansin family105, a superfamily of proteins that play a pivotal role in cell wall loosening, thus increasing root cell wall plasticity. Expression of these proteins is triggered by fungal contact or by a diffusible fungal factor and is a prerequisite to accommodate the fungus in plant root cells27,106. Expansin-encoding genes showed up-regulation in mycorrhizal roots of M. truncatula and C. sativus, and their products were detected both in the interface matrix and in mycorrhizal root cell walls28,107. A gene encoding a protein similar to Prunus armeniaca major allergen, over-expressed in D16 mycorrhizal roots, showed a high similarity to a β-expansin108,109 (Supplementary Table 1).
Many D16 over-expressed genes belong to the GO term “carbohydrate metabolic process” (Fig. 2). Genes included in this GO, such as those encoding endoglucanases, galacturonases, hydroxyproline-rich glycoproteins, cellulose synthase and ascorbate oxidase, are mainly related to root cell wall degradation and remodeling and were expressed in root cells containing fungal structures101,110,111.
Tubulin encoding genes were over-expressed in D16 mycorrhizal roots (Supplementary Table 1), confirming previous findings on tubulin upregulation in S. lycopersicum roots colonized by R. intraradices, compared with uncolonized roots112.
Plant-fungal symbiosis stimulates new adventitious roots contributing to lateral root growth as showed in symbioses of Allium porrum and Prunus cerasifera with F. mosseae and R. intraradices 113,114. Auxin response factors (ARF) are transcription factors involved in adventitious root formation and regulate auxin responsive genes. The auxin response factor 18-like transcript detected here (Supplementary Table 1) was not reported before in mycorrhizal plants, whereas other transcripts encoding putative auxin response factors were induced during colonization of O. sativa and M. truncatula by R. irregulare 18,65. Such an expression pattern might be related, as in other ARFs, to lateral root formation and growth115.
Conclusions
In this work, a comprehensive transcriptome analysis of roots of sunflower in symbiosis with the AM fungal symbiont R. irregulare was carried out adding data from a plant species belonging to Asteraceae, the largest Angiosperm family, which was not investigated yet in this respect. Hence, this is a valuable contribution to deciphering gene expression related to plant symbiosis, for future comparative functional genomics analyses. Results confirmed the importance of AM-modulated transcription of genes involved in plant defence, signal transduction, hormonal balance, nutrient transport and host cell shape in the activation of mechanisms controlling AMF establishment and spread in plant roots.
The repertoire of genes regulated at both early and late stages of the symbiosis allowed us to confirm AMF-regulated genes as well as to identify new genes which were not previously described as involved in AMF symbiosis, for example those encoding a BAHD-acyltransferase, an oxoglutarate dependent dioxygenase, and a stellacyanin-like protein.
Such results can guide application research targeted at exploiting metabolic changes occurring in commercially-valuable crops when colonized by arbuscular mycorrhizal symbionts in field conditions, in pursuit of sustainable and resilient agricultural processes.
Further studies are in progress to elucidate gene expression in the development of AM symbiosis by the comparison of transcriptome profiling in cultivated and wild genotypes of sunflower, showing different susceptibility to AMF.
Materials and Methods
Plant material and treatments
Fourty seeds of H. annuus HA412-HO inbred line (USDA acc. number PI 642777) were germinated on moistened filter paper in Petri plates. The root system of one week old plantlets was individually placed between a 90-mm diameter membrane (cellulose acetate and cellulose nitrate mixture, 0.45 µm pore diameter size, MF-Millipore™) and a 100-cm2 nylon net (41 µm mesh, Millipore™). Spores, mycelium and finely cut colonised roots of R. irregulare (formerly Rhizophagus irregularis) IMA6, obtained from pot-culture soil after wet sieving through a 100-µm-mesh size sieve, were spread onto all the nylon net surface of 20 plantlets. Uninoculated control plantlets received 10 ml of a filtrate, obtained by sieving the same pot-culture soil through a 50 μm pore diameter sieve and through Whatman N. 1 paper, to ensure a common AM fungal-associated microflora. In both inoculated plants and uninoculated controls another 90-mm membrane was placed on the nylon net, and these “sandwich systems” were placed in sterile 150-mm Petri plates containing steam-sterilized quartz grit. After sealing with Parafilm M, the lower half of each plate was wrapped in aluminium foil and plants were maintained in a growth chamber at 24 °C. Plants were supplied weekly with 6 ml half strength Hoagland’s solution. The progression of mycorrhizal colonization was monitored across time-points 48 hours apart on 3 replicate plants, by localizing succinate dehydrogenase activity116 and by staining roots with Trypan blue117. Percentage of AM fungal root colonization was determined by the gridline intersect method118.
RNA extraction
On the basis of root colonization data, RNA-seq analyses were carried out on roots collected from 3 replicate H. annuus mycorrhizal and control plantlets harvested after 4 and 16 days of culture.
Whole roots were ground in liquid nitrogen and total RNA was isolated using the Logemann procedure119,120. Purification from genomic DNA was performed by digestion with DNaseI (Roche). Finally, RNA was purified with phenol/chloroform and precipitated with standard procedures. RNA quality was evaluated by electrophoretic, spectrophotometric and bioanalyzer analysis using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA).
RNA sequencing and mapping procedures
For high-throughput sequencing, mRNA deriving from the experiment was converted to cDNA and 12 cDNA libraries (three replicates of mycorrhizal and control root systems at two harvesting times) were constructed with TruSeq RNA Sample Prep Kit (Illumina), according to the manufacturer’s protocol (Illumina Inc., San Diego, CA, USA). Illumina HiSeq 2000 sequencing yielded single-read sequences with length of 100 bp. The quality of the reads was checked using FastQC (v. 0.11.3) and overall quality was improved by trimming the reads using Trimmomatic121, with following parameters: crop = 95, headcrop = 10, minlen = 85.
High quality RNA-Seq reads were mapped using the CLC genomics workbench (v. 9.5.3).
Traces of ribosomal RNA contamination were removed from all libraries by mapping against sunflower ribosomal sequences downloaded from the NCBI database repository. The parameters used for ribosomal filtering were: length fraction = 0.5 and similarity fraction = 0.8.
cDNA sequences were mapped to the reference transcriptome of Helianthus annus inbred line XRQ38 (https://www.heliagene.org/HanXRQ-SUNRISE/) using stringent parameters (length fraction = 0.8 and similarity fraction = 0.8) but retaining mismatch penalties = 2 and gap penalties = 3 as set by CLC default parameters. Sequence reads are deposited in the Sequence Repository Archive (SRA) under the ID SRP124596.
Differential expression analysis
Gene expression level was calculated as reads per kilobase per million reads mapped (RPKM) as described in Mortazavi et al., (2008). Besides unique reads, reads that occurred up to ten times were also included in the mapping analysis, because this strategy correctly estimates the expression of paralogous genes122. Gene expression was filtered to ensure RPKM > 1 in at least one library.
Raw counts deriving from CLC aligner were analyzed with the R statistical package EdgeR123. A pairwise comparison test was performed between mycorrhizal (M) and control libraries (C) after 4 and 16 days of inoculation with fungus (C4 vs. M4 and C16 vs. M16). The resulting P values were corrected for the FDR124 and significant genes identified with an FDR corrected P value < 0.05. The fold changes (FC) between controls and treatments were considered significant when the expression value of a gene was at least two-fold higher or lower than the control, thus splitting transcripts in two groups: up-regulated and down-regulated.
Transcriptome functional analysis
GO terms for differentially expressed annotated transcripts were retrieved using a Blast2GO file provided by HanXRQ portal (https://www.heliagene.org/HanXRQ-SUNRISE/).
GO enrichment analysis with Fisher’s exact test on differential expressed genes versus whole predicted transcriptome was performed with Blast2GO analysis tools125 using FDR corrected P value < 0.05. Overall enriched GO terms were summarized using REVIGO with allowed similarity of 0.4126.
GO slim (Blast2GO tool) was performed in order to reduce the complexity of GO terms for functional analysis of differentially expressed transcripts.
qRT-PCR analysis
We used qRT-PCR to validate RNA-seq expression profiles of five genes selected from those differentially expressed between M4 and M16 samples. One gene encoding E3 ubiquitin-ligase BRE1-like 1 (genome ID HanXRQChr05g0138451 and one gene encoding mitochondrial ubiquitin ligase activator of NFKB 1 (genome ID HanXRQChr10g0282971) were selected among genes showing stable expression profiles in RNA-seq analyses and used as internal reference genes in qRT-PCR analyses. Complementary DNA from 400 ng total purified RNA was prepared using iScript cDNA synthesis kit (BioRad, Hercules, CA, USA) according to the manufacturer’s protocol.
The synthesised cDNA was used for quantitative real-time polymerase chain reaction (qRT-PCR). Primers were designed using Primer3 software (Applied Biosystems) and are reported in Supplementary Table 2. qRT-PCR reactions (20 μL) were carried out with 10 ng of cDNA, 250 nM primers and 1X Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. PCR was run in a StepOne realtime PCR System (Applied Biosystems, Foster City, CA, USA) using the recommended thermal cycling conditions (hold 95 °C, 20 s; 40 cycles 95 °C, 3 s; 60 °C, 30 s).
The qRT-PCR results were obtained from three biological replicates and three technical repeats for each treatment. The relative abundance of transcripts was calculated by using the 2−ΔΔCt method127. For statistical analysis a two tailed t-test was used.
Accession codes
Sequence reads of transcriptome sequencing have been deposited in the NCBI sequence read archive under accession code SRP124596.
Electronic supplementary material
Acknowledgements
This research was supported by the University of Pisa, Italy.
Author Contributions
A.V., L.N., C.S., A.T., A.C., M.G. and T.G. planned and designed the research. A.V., R.B., C.S., A.T. and T.G. performed experiments and collected data. A.V., L.N., R.B., C.S., A.T., K.H.P., D.H., A.C., M.G. and T.G. analysed and interpreted data. A.V., L.N., C.S., A.T., M.G., A.C. and T.G. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18445-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith, S. E. & Read, D. J. Mycorrhizal symbiosis. Cambridge, UK: Academic Press (2008).
- 2.Battini F, Grønlund M, Agnolucci M, Giovannetti M, Jakobsen I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Scientific Reports. 2017;7:4686. doi: 10.1038/s41598-017-04959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepe A, Sbrana C, Ferrol N, Giovannetti M. An in vivo whole-plant experimental system for the analysis of gene expression in extraradical mycorrhizal mycelium. Mycorrhiza. 2017;572:1–10. doi: 10.1007/s00572-017-0779-7. [DOI] [PubMed] [Google Scholar]
- 4.Gianinazzi S, et al. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010;20:519–530. doi: 10.1007/s00572-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 5.Lohse S, et al. Organization and metabolism of plastids and mitochondria in arbuscular mycorrhizal roots of Medicago truncatula. Plant Physiology. 2005;139:329–340. doi: 10.1104/pp.105.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araim G, Saleem A, Arnason JT, Charest C. Root colonization by an arbuscular mycorrhizal (AM) fungus increases growth and secondary metabolism of purple coneflower, Echinacea purpurea (L.) Moench. Journal Agricultural Food Chemistry. 2009;57:2255–2258. doi: 10.1021/jf803173x. [DOI] [PubMed] [Google Scholar]
- 7.Fester T, Hause G. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza. 2005;15:373–379. doi: 10.1007/s00572-005-0363-4. [DOI] [PubMed] [Google Scholar]
- 8.Schliemann W, Ammer C, Strack D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry. 2008;69:112–146. doi: 10.1016/j.phytochem.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Fiorilli V, et al. Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytologist. 2009;184:975–987. doi: 10.1111/j.1469-8137.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 10.Fiorilli V, et al. Host and non-host roots in rice: cellular and molecular approaches reveal differential responses to arbuscular mycorrhizal fungi. Frontiers in Plant Science. 2015;6:636. doi: 10.3389/fpls.2015.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallou A, Declerck S, Cranenbrouck S. Transcriptional regulation of defence genes and involvement of the WRKY transcription factor in arbuscular mycorrhizal potato root colonization. Functional & Integrative Genomics. 2012;12:183–198. doi: 10.1007/s10142-011-0241-4. [DOI] [PubMed] [Google Scholar]
- 12.Grunwald U, et al. Identification of mycorrhiza-regulated genes with arbuscule development-related expression profile. Plant Molecular Biology. 2004;55:553–566. doi: 10.1007/s11103-004-1303-y. [DOI] [PubMed] [Google Scholar]
- 13.Hogekamp C, Küster H. A roadmap of cell-type specific gene expression during sequential stages of the arbuscular mycorrhiza symbiosis. BMC Genomics. 2013;14:306. doi: 10.1186/1471-2164-14-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handa Y, et al. RNA-seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis. Plant and Cell Physiology. 2015;8:1490–1511. doi: 10.1093/pcp/pcv071. [DOI] [PubMed] [Google Scholar]
- 15.Schaarschmidt S, Gresshoff PM, Hause B. Analyzing the soybean transcriptome during autoregulation of mycorrhization identifies the transcription factors GmNF-YA1a/b as positive regulators of arbuscular mycorrhization. Genome Biology. 2013;14:R62. doi: 10.1186/gb-2013-14-6-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massoumou M, et al. Medicago truncatula gene responses specific to arbuscular mycorrhiza interactions with different species and genera of Glomeromycota. Mycorrhiza. 2007;17:223–234. doi: 10.1007/s00572-006-0099-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, et al. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. The Plant Journal. 2007;50:529–544. doi: 10.1111/j.1365-313X.2007.03069.x. [DOI] [PubMed] [Google Scholar]
- 18.Hohnjec N, Vieweg MF, Pühler A, Becker A, Küster H. Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiology. 2005;137:1283–1301. doi: 10.1104/pp.104.056572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deguchi Y, et al. Transcriptome profiling of Lotus japonicus roots during arbuscular mycorrhiza development and comparison with that of nodulation. DNA Research. 2007;14:117–133. doi: 10.1093/dnares/dsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Ráez JA, et al. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. Journal of Experimental Botany. 2010;61:2589–2601. doi: 10.1093/jxb/erq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brechenmacher L, et al. Expression profiling of up-regulated plant and fungal genes in early and late stages of Medicago truncatula-Glomus mosseae interactions. Mycorrhiza. 2004;14:253–262. doi: 10.1007/s00572-003-0263-4. [DOI] [PubMed] [Google Scholar]
- 22.Gomez SK, et al. Medicago truncatula and Glomus intraradices gene expression in cortical cells harboring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biology. 2009;9:10. doi: 10.1186/1471-2229-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogekamp C, et al. Laser micro dissection unravels cell-type-specific transcription in arbuscular mycorrhizal roots, including CAAT-Box transcription factor gene expression correlating with fungal contact and spread. Plant Physiology. 2011;157:2023–2043. doi: 10.1104/pp.111.186635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaude N, Schulze WX, Franken P, Krajinski F. Cell type-specific protein and transcription profiles implicate periarbuscular membrane synthesis as an important carbon sink in the mycorrhizal symbiosis. Plant Signaling & Behavior. 2012;7:461–464. doi: 10.4161/psb.19650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu B, Li W, Liu L, Wei Y, Shi S. Transcriptomes of arbuscular mycorrhizal fungi and litchi host interaction after tree girdling. Frontiers in Microbiology. 2016;7:408. doi: 10.3389/fmicb.2016.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weidmann S, et al. Fungal elicitation of signal transduction-related plant genes precedes mycorrhiza establishment and requires the dmi3 gene in Medicago truncatula. Molecular Plant-Microbe Interactions. 2004;17:1385–1393. doi: 10.1094/MPMI.2004.17.12.1385. [DOI] [PubMed] [Google Scholar]
- 27.Siciliano V, et al. Transcriptome analysis of arbuscular mycorrhizal roots during development of the pre-penetration apparatus. Plant Physiology. 2007;144:1455–1466. doi: 10.1104/pp.107.097980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, et al. Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. The Plant Cell. 2003;15:2106–2123. doi: 10.1105/tpc.014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guether M, et al. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiology. 2009;150:73–83. doi: 10.1104/pp.109.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortu G, et al. Plant genes related to gibberellin biosynthesis and signaling are differentially regulated during the early stages of AM fungal interactions. Molecular Plant. 2012;5:951–954. doi: 10.1093/mp/sss027. [DOI] [PubMed] [Google Scholar]
- 31.Song YY, et al. Hijacking common mycorrhizal networks for herbivore-induced defence signal transfer between tomato plants. Scientific Reports. 2014;4:3915. doi: 10.1038/srep03915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonanomi A, Oetiker JH, Boller T, Wiemken A, Vögeli-Lange R. Symbiosis-related genes of Medicago truncatula induced in the early phase of its interaction with Glomus intraradices. Tri-trophic Interactions in the Rhizosphere IOBC WPRS Bulletin. 2001;24:19–24. [Google Scholar]
- 33.Zouari I, et al. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genomics. 2014;15:221. doi: 10.1186/1471-2164-15-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cervantes-Gámez RG, et al. Arbuscular mycorrhizal symbiosis-Induced expression changes in solanum lycopersicum leaves revealed by RNA-seq analysis. Plant Molecular Biology Reporter. 2016;34:89–102. doi: 10.1007/s11105-015-0903-9. [DOI] [Google Scholar]
- 35.Zukovsky, P. M. Cultivated plants and their wild relatives. Farnham Royal, UK: Commonwealth Agriculture Bureau (1950).
- 36.Semelczi-Kovacs A. Acclimatization and dissemination of the sunflower in Europe. Acta Ethnographica of the Academy of Sviences of Hungary. 1975;24:47–88. [Google Scholar]
- 37.Rogers, C., Thompson, T. & Seiler, G. J. Sunflower species of the United States. Bismarck, North Dakota, USA: National Sunflower Association (1982).
- 38.Badouin H, et al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature. 2017;546:148–152. doi: 10.1038/nature22380. [DOI] [PubMed] [Google Scholar]
- 39.Staton SE, et al. The sunflower (Helianthus annuus L.) genome reflects a recent history of biased accumulation of transposable elements. The Plant Journal. 2012;72:142–153. doi: 10.1111/j.1365-313X.2012.05072.x. [DOI] [PubMed] [Google Scholar]
- 40.Natali L, et al. The repetitive component of the sunflower genome as shown by different procedures for assembling next generation sequencing reads. BMC Genomics. 2013;14:686. doi: 10.1186/1471-2164-14-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascagni F, et al. Repetitive DNA and plant domestication: variation in copy number and proximity to genes of LTR-Retrotransposons among wild and cultivated sunflower (Helianthus annuus) genotypes. Genome Biology and Evolution. 2015;7:3368–3382. doi: 10.1093/gbe/evv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vukich M, et al. Genetic variability in sunflower (Helianthus annuus L.) and in the Helianthus genus as assessed by retrotransposon-based molecular markers. Theoretical and Applied Genetics. 2009;119:1027–1038. doi: 10.1007/s00122-009-1106-2. [DOI] [PubMed] [Google Scholar]
- 43.Chaki M, et al. Protein targets of tyrosine nitration in sunflower (Helianthus annuus L.) hypocotyls. Journal of Experimental Botany. 2009;60:4221–4234. doi: 10.1093/jxb/erp263. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Ocaña A, et al. Functional analysis of superoxide dismutases (SODs) in sunflower under biotic and abiotic stress conditions. Identification of two new genes of mitochondrial Mn-SOD. Journal of Plant Physiology. 2011;168:1303–1308. doi: 10.1016/j.jplph.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Ramu SV, et al. Transcriptome analysis of sunflower genotypes with contrasting oxidative stress tolerance reveals individual and combined-biotic and abiotic stress tolerance mechanisms. PLoS ONE. 2016;6:e0157522. doi: 10.1371/journal.pone.0157522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turrini A, et al. Changes in the composition of native root arbuscular mycorrhizal fungal communities during a short-term cover crop-maize succession. Biology and Fertility of Soils. 2016;52:643–653. doi: 10.1007/s00374-016-1106-8. [DOI] [Google Scholar]
- 47.Pérez-Jaramillo JE, Mendes R, Raaijmakers JM. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Molecular Biology. 2016;90:635–644. doi: 10.1007/s11103-015-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leff JW, Lynch RC, Kane NC, Fierer N. Plant domestication and the assembly of bacterial and fungal communities associated with strains of the common sunflower, Helianthus annuus. New Phytologist. 2017;214:412–423. doi: 10.1111/nph.14323. [DOI] [PubMed] [Google Scholar]
- 49.Sugimura Y, Saito K. Transcriptional profiling of arbuscular mycorrhizal roots exposed to high levels of phosphate reveals the repression of cell cycle-related genes and secreted protein genes in Rhizophagus irregularis. Mycorrhiza. 2017;27:139–146. doi: 10.1007/s00572-016-0735-y. [DOI] [PubMed] [Google Scholar]
- 50.Takeda N, Sato S, Asamizu E, Tabata S, Parniske M. Apoplastic plant subtilases support arbuscular mycorrhiza development in Lotus japonicus. The Plant Journal. 2009;58:766–777. doi: 10.1111/j.1365-313X.2009.03824.x. [DOI] [PubMed] [Google Scholar]
- 51.Casieri L, et al. Biotrophic transportome in mutualistic plant–fungal interactions. Mycorrhiza. 2013;23:597–625. doi: 10.1007/s00572-013-0496-9. [DOI] [PubMed] [Google Scholar]
- 52.Gutjahr C, Paszkowski U. Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Frontiers in Plant Sciences. 2013;4:204. doi: 10.3389/fpls.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radutoiu S, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003;425:585–592. doi: 10.1038/nature02039. [DOI] [PubMed] [Google Scholar]
- 54.Mandal S, Upadhyay S, Singh VP, Kapoor R. Enhanced production of steviol glycosides in mycorrhizal plants: a concerted effect of arbuscular mycorrhizal symbiosis on transcription of biosynthetic genes. Plant Physiology and Biochemistry. 2015;89:100–106. doi: 10.1016/j.plaphy.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Ma X, Koepke J, Panjikar S, Fritzsch G, Stöckigt J. Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. The Journal of Biological Chemistry. 2005;280:13576–13583. doi: 10.1074/jbc.M414508200. [DOI] [PubMed] [Google Scholar]
- 56.Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends in Plant Science. 2012;2:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Yao MK, Desilets H, Charles MT, Boulanger R, Tweddell RJ. Effect of mycorrhization on the accumulation of rishitin and solavetivone in potato plantlets challenged with Rhizoctonia solani. Mycorrhiza. 2003;13:333–336. doi: 10.1007/s00572-003-0267-0. [DOI] [PubMed] [Google Scholar]
- 58.Wulf A, et al. Transcriptional changes in response to arbuscular mycorrhiza development in the model plant. Medicago truncatula. Molecular Plant-Microbe Interactions. 2003;4:306–314. doi: 10.1094/MPMI.2003.16.4.306. [DOI] [PubMed] [Google Scholar]
- 59.Zhi-lin Y, Chuan-chao D, Lian-qing C. Regulation and accumulation of secondary metabolites in plant-fungus symbiotic system. African Journal of Biotechnology. 2007;6:1266–1271. [Google Scholar]
- 60.Asensio D, Rapparini F, Peñuelas J. AM fungi root colonization increases the production of essential isoprenoids vs.non essential isoprenoids especially under drought stress conditions or after jasmonic acid application. Phytochemistry. 2012;77:149–161. doi: 10.1016/j.phytochem.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Vranová E, Coman D, Gruissem W. Structure and dynamics of the isoprenoid pathway network. Molecular Plant. 2012;5:318–333. doi: 10.1093/mp/sss015. [DOI] [PubMed] [Google Scholar]
- 62.Sedbrook JC, Carroll KL, Hung KF, Masson PH, Somerville CR. The Arabidopsis SKU5 gene encodes an extracellular glycosyl phosphatidylinositol–anchored glycoprotein involved in directional root growth. The Plant Cell. 2002;14:1635–1648. doi: 10.1105/tpc.002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knecht K, et al. Expression of BvGLP-1 encoding a germin-like protein from sugar beet in Arabidopsis thaliana leads to resistance against phytopathogenic fungi. Molecular Plant-Microbe Interactions. 2010;23:446–457. doi: 10.1094/MPMI-23-4-0446. [DOI] [PubMed] [Google Scholar]
- 64.Doll J, Hause B, Demchenko K, Pawlowski K, Krajinski F. A member of the Germin-Like Protein family is a highly conserved mycorrhiza-specific induced gene. Plant and Cell Physiology. 2003;44:1208–1214. doi: 10.1093/pcp/pcg153. [DOI] [PubMed] [Google Scholar]
- 65.Güimil S, et al. Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proceedings of the National Academy of Sciences USA. 2005;102:8066–8070. doi: 10.1073/pnas.0502999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blilou I, Bueno P, Ocampo JA, Garcia-Garrido JM. Induction of catalase and ascorbate peroxidase activities in tobacco roots inoculated with the arbuscular mycorrhizal Glomus mosseae. Mycological Research. 2000;104:722–725. doi: 10.1017/S095375629900204X. [DOI] [Google Scholar]
- 67.Lambais, M. R., Podila, G. K. & Douds, J. Regulation of plant defense-related genes in arbuscular mycorrhizae. American Phytopathological Society Press, St. Paul, Minneapolis, USA: Current Advances in Mycorrhizae Research, pp. 45–60 (2000).
- 68.García-Garrido JM, Ocampo JA. Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. Journal of Experimental Botany. 2002;53:1377–1386. doi: 10.1093/jexbot/53.373.1377. [DOI] [PubMed] [Google Scholar]
- 69.Campos-Soriano L, Garcia-Martinez J, San Segundo B. The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Molecular Plant Pathology. 2012;13:579–592. doi: 10.1111/j.1364-3703.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han JY, Kim MJ, Ban YW, Hwang HS, Choi YE. The involvement of b-amyrin 28-oxidase (CYP716A52v2) in oleanane-type ginsenoside biosynthesis in Panax ginseng. Plant and Cell Physiology. 2013;54:2034–2046. doi: 10.1093/pcp/pct141. [DOI] [PubMed] [Google Scholar]
- 71.Zeng Y, et al. Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: current research status and prospectives. Mycorrhiza. 2013;23:253–265. doi: 10.1007/s00572-013-0484-0. [DOI] [PubMed] [Google Scholar]
- 72.Bonfante P, Genre A. Arbuscular mycorrhizal dialogues: do you speak ‘plantish’ or ‘fungish’? Trends in Plant Science. 2015;20:150–154. doi: 10.1016/j.tplants.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Gianinazzi-Pearson V. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. The Plant Cell. 1996;8:1871–1883. doi: 10.1105/tpc.8.10.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koltai H. Implications of non-specific strigolactone signaling in the rhizosphere. Plant Science. 2014;225:9–14. doi: 10.1016/j.plantsci.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nature Chemical Biology. 2014;10:1028–1033. doi: 10.1038/nchembio.1660. [DOI] [PubMed] [Google Scholar]
- 76.Mori N, Nishiuma K, Sugiyama T, Hayashi H, Akiyama K. Carlactone-type strigolactones and their synthetic analogues as inducers of hyphal branching in arbuscular mycorrhizal fungi. Phytochemistry. 2016;130:90–98. doi: 10.1016/j.phytochem.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 77.Harberd NP. Relieving DELLA restraint. Science. 2003;299:1853–1854. doi: 10.1126/science.1083217. [DOI] [PubMed] [Google Scholar]
- 78.Floss DS, Levy JG, Lévesque-Tremblay V, Pumplin N, Harrison MJ. DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences USA. 2013;110:5025–5034. doi: 10.1073/pnas.1308973110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foo E, Ross JJ, Jones WT, Reid JB. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Annals of Botany. 2013;111:769–779. doi: 10.1093/aob/mct041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu N, et al. A DELLA protein complex controls the arbuscular mycorrhizal symbiosis in plants. Cell Research. 2014;24:130–133. doi: 10.1038/cr.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bustos R, et al. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genetics. 2010;6:e1001102. doi: 10.1371/journal.pgen.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Péret B, Clément M, Nussaume L, Desnos T. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science. 2011;16:442–450. doi: 10.1016/j.tplants.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Takeda N, Maekawa T, Hayashi M. Nuclear-localized and deregulated calcium- and calmodulin-dependent protein kinase activates rhizobial and mycorrhizal responses in Lotus japonicus. The Plant Cell. 2012;24:810–822. doi: 10.1105/tpc.111.091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zipfel C, Oldroyd GED. Plant signalling in symbiosis and immunity. Nature. 2017;543:328–336. doi: 10.1038/nature22009. [DOI] [PubMed] [Google Scholar]
- 85.Shaul-Keinan O, et al. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytologist. 2002;154:501–507. doi: 10.1046/j.1469-8137.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- 86.Martín-Rodríguez JÁ, et al. Role of gibberellins during arbuscular mycorrhizal formation in tomato: new insights revealed by endogenous quantification and genetic analysis of their metabolism in mycorrhizal roots. Physiologia Plantarum. 2015;154:66–81. doi: 10.1111/ppl.12274. [DOI] [PubMed] [Google Scholar]
- 87.Martín-Rodríguez JA, et al. Gibberellin–abscisic acid balances during arbuscular mycorrhiza formation in tomato. Frontiers in Plant Science. 2016;7:1273. doi: 10.3389/fpls.2016.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanlon MT, Coenen C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytologist. 2011;189:701–709. doi: 10.1111/j.1469-8137.2010.03567.x. [DOI] [PubMed] [Google Scholar]
- 89.Guillotin B, Couzigou JM, Combier JP. NIN is involved in the regulation of arbuscular mycorrhizal symbiosis. Frontiers in Plant Science. 2016;7:1704. doi: 10.3389/fpls.2016.01704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benedito VA, et al. Genomic inventory and transcriptional analysis of Medicago truncatula transporters. Plant Physiology. 2010;152:1716–1730. doi: 10.1104/pp.109.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonfante P, Genre A. 2010. Mechanisms underlying beneficial plant – fungus interactions in mycorrhizal symbiosis. Nature Communications. 2010;1:48. doi: 10.1038/ncomms1046. [DOI] [PubMed] [Google Scholar]
- 92.Recorbet G, Abdallah C, Renaut J, Wipf D, Dumas-Gaudot E. Protein actors sustaining arbuscular mycorrhizal symbiosis: underground artists break the silence. New Phytologist. 2013;199:26–40. doi: 10.1111/nph.12287. [DOI] [PubMed] [Google Scholar]
- 93.Kapulnik Y, Koltai H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiology. 2014;166:560–569. doi: 10.1104/pp.114.244939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gutjahr C, et al. The half-size ABC transporters STR1 and STR2 are indispensable for mycorrhizal arbuscule formation in rice. The Plant Journal. 2012;69:906–920. doi: 10.1111/j.1365-313X.2011.04842.x. [DOI] [PubMed] [Google Scholar]
- 95.Tan Z, Hu Y, Lin Z. PhPT4 is a mycorrhizal-phosphate transporter suppressed by lysophosphatidylcholine in Petunia roots. Plant Molecular Biology Reporter. 2012;30:1480–1487. doi: 10.1007/s11105-012-0467-x. [DOI] [Google Scholar]
- 96.Chitarra W, et al. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiology. 2016;171:1009–1023. doi: 10.1104/pp.16.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khan JA, Wang Q, Sjölund RD, Schulz A, Thompson GA. An early nodulin-like protein accumulates in the sieve element plasma membrane of Arabidopsis. Plant Physiology. 2007;143:1576–1589. doi: 10.1104/pp.106.092296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kong HY, Jung HW, Lee SC, Choi D, Hwang BK. A gene encoding stellacyanin is induced in Capsicum annuum by pathogens, methyl jasmonate, abscisic acid, wounding, drought and salt stress. Physiologia Plantarum. 2002;115:550–562. doi: 10.1034/j.1399-3054.2002.1150409.x. [DOI] [PubMed] [Google Scholar]
- 99.Chen LQ, et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335:207–210. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- 100.Manck-Götzenberger J, Requena N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Frontiers in Plant Science. 2016;7:487. doi: 10.3389/fpls.2016.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balestrini R, Bonfante P. Cell wall remodeling in mycorrhizal symbiosis: a way towards biotrophism. Frontiers in Plant Science. 2014;5:237. doi: 10.3389/fpls.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balestrini R, et al. Ascorbate oxidase: The unexpected involvement of a ‘wasteful enzyme’ in the symbioses with nitrogen-fixing bacteria and arbuscular mycorrhizal fungi. Plant Physiology and Biochemistry. 2012;59:71–79. doi: 10.1016/j.plaphy.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 103.Genre A, Bonfante P. Cytoskeleton-related proteins in tobacco mycorrhizal cells: γ tubulin and clathrin localization. European Journal of Histochemistry. 1999;43:105–111. [PubMed] [Google Scholar]
- 104.Pumplin N, et al. Medicago truncatula Vapyrin is a novel protein require for arbuscular mycorrhizal symbiosis. The Plant Journal. 2010;61:482–494. doi: 10.1111/j.1365-313X.2009.04072.x. [DOI] [PubMed] [Google Scholar]
- 105.Gookin TE, Hunter DA, Reid MS. Temporal analysis of alpha and beta-expansin expression during floral opening and senescence. Plant Science. 2003;164:769–781. doi: 10.1016/S0168-9452(03)00063-3. [DOI] [Google Scholar]
- 106.Giordano W, Hirsch AM. The expression of MaEXP1, a Melilotus alba expansin gene, is upregulated during the sweet clover–Sinorhizobium meliloti interaction. Molecular Plant-Microbe Interactions. 2004;17:613–622. doi: 10.1094/MPMI.2004.17.6.613. [DOI] [PubMed] [Google Scholar]
- 107.Balestrini R, Cosgrove DJ, Bonfante P. Differential location of alfa-expansin proteins during the accommodation of root cells to an arbuscular mycorrhizal fungus. Planta. 2005;220:889–899. doi: 10.1007/s00425-004-1431-2. [DOI] [PubMed] [Google Scholar]
- 108.Cosgrove DJ, Bedinger P, Durachko DM. Group I allergens of grass pollen as cell wall-loosening agents. Proceedings of the National Academy of Sciences USA. 1997;94:6559–6564. doi: 10.1073/pnas.94.12.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neudecker P, et al. Allergic cross-reactivity made visible solution structure of the major cherry allergen Pru av 1. The Journal of Biological Chemistry. 2001;276:22756–22763. doi: 10.1074/jbc.M101657200. [DOI] [PubMed] [Google Scholar]
- 110.Balestrini R, Josè-Estanyol M, Puigdomènech P, Bonfante P. Hydroxyproline-rich glycoprotein mRNA accumulation in maize root cells colonized by an arbuscular mycorrhizal fungus as revealed by in situ hybridization. Protoplasma. 1997;198:36–42. doi: 10.1007/BF01282129. [DOI] [Google Scholar]
- 111.Siciliano V, Genre A, Balestrini R, deWit PJGM, Bonfante P. Pre-penetration apparatus formation during AM infection is associated with a specific transcriptome response in epidermal cells. Plant Signaling & Behavior. 2007;2:533–535. doi: 10.4161/psb.2.6.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Timonen S, Smith SE. Effect of the arbuscular mycorrhizal fungus Glomus intraradices on expression of cytoskeletal proteins in tomato roots. Canadian Journal of Botany. 2005;83:176–182. doi: 10.1139/b04-160. [DOI] [Google Scholar]
- 113.Berta G, Bonfante-Fasolo P. Anatomy and cytology of hair roots in Calluna vulgaris (L.) Hull. Giornale botanico italiano. 1989;123:9–23. doi: 10.1080/11263508909430242. [DOI] [Google Scholar]
- 114.Berta G, et al. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree physiology. 1995;15:281–293. doi: 10.1093/treephys/15.5.281. [DOI] [PubMed] [Google Scholar]
- 115.Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews Molecular Cell Biology. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 116.Smith SE, Gianinazzi-Pearson V. Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L.: effects of photon irradiance and phosphate nutrition. Australian Journal of Plant Physiology. 1990;17:177–188. doi: 10.1071/PP9900177. [DOI] [Google Scholar]
- 117.Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fung for rapid assessment of infection. Transactions of the British Mycological Society. 1970;55:158IN16–161IN18. doi: 10.1016/S0007-1536(70)80110-3. [DOI] [Google Scholar]
- 118.Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist. 1980;84:489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x. [DOI] [Google Scholar]
- 119.Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Analytical Biochemistry. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- 120.Giordani T, et al. Genome-wide analysis of LTR-retrotransposon expression in leaves of Populus × canadensis water-deprived plants. Tree Genetics and Genomes. 2016;12:75. doi: 10.1007/s11295-016-1036-5. [DOI] [Google Scholar]
- 121.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 123.Robinson MD, McCarthy DJ, Smyth GK. EdgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 125.Conesa A, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 126.Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.