Abstract
Chimeric antigen receptor (CAR) based adoptive T cell therapy is a highly promising treatment for lymphoid malignancies, and CD20 is an ideal target antigen. We previously developed a lentiviral construct encoding a 3rd generation CD20-targeted CAR but identified several features that required additional optimization prior to clinical translation. We describe here several improvements, including replacement of the immunogenic murine antigen-binding moiety with a fully human domain, streamlining the transgene insert to enhance lentiviral titers, modifications to the extracellular IgG spacer that abrogate nonspecific activation resulting from binding to Fc receptors, and evaluation of CD28, 4-1BB, or CD28 and 4-1BB costimulatory domains. We also found that re-stimulation of CAR T cells with an irradiated CD20+ cell line boosted cell growth, increased the fraction CAR-expressing cells, and preserved in vivo function despite leading to a reduced capacity for cytokine secretion in vitro. We also found that cryopreservation of CAR T cells did not impact immunophenotype or in vivo anti-tumor activity compared with fresh cells. These optimization steps resulted in significant improvement in anti-tumor activity in mouse models, resulting in eradication of established systemic lymphoma tumors in 75% of mice with a single infusion of CAR T cells, and prolonged in vivo persistence of modified cells. These results provide the basis for clinical testing of a lentiviral construct encoding a fully human CD20-targeted CAR with CD28 and 4-1BB costimulatory domains and truncated CD19 (tCD19) transduction marker.
Keywords: Chimeric antigen receptors, Adoptive immunotherapy, Non-Hodgkin lymphoma, Gene therapy
Introduction
Non-Hodgkin lymphoma (NHL) is a group of malignancies that occur as a result of uncontrolled expansion of a single lymphocyte clone. Approximately 80% of NHLs are derived from the B-lymphoid lineage (B-NHL) and in the vast majority (> 95%) of cases, the malignant B-NHL cells uniformly express the cell surface marker CD20. CD20 is a non-glycosylated, tetra-spanning, 35 kD phosphoprotein,1–5 which appears to function as a calcium channel involved in the development and differentiation of B cells into plasma cells.6, 7 In normal B-cell differentiation, CD20 is highly expressed during the late pre-B cell through mature B cell stages and is down-regulated in terminally differentiated plasma cells.8 CD20 is also stable on the cell surface with minimal shedding,1, 5, 9 with only trace amounts of soluble antigen,10 and is conserved throughout the natural history of the disease. For these reasons, CD20 is an attractive target for B-NHL treatment, and more than two decades of therapy with CD20-targeted antibodies such as rituximab, obinutuzumab, ofatumumab, ibritumomab tiuxetan, and tositumomab have validated this.11–16 CD20 antibody-based therapy, particularly rituximab, has demonstrated significant anti-tumor activity and improved the overall survival of various lymphoma subtypes in combination with chemotherapy or as maintenance therapy.12–17 As a single agent it is not curative, however, and despite these improved outcomes, more than 20,000 NHL patients continue to die from their disease each year in the United States alone.18 Therefore, alternative therapies are needed for this group of diseases.
One promising approach is adoptive immunotherapy using chimeric antigen receptor (CAR) expressing T cells that specifically target B-cell lineage-restricted tumor-associated antigens.19–29 CAR T cells express a synthetic protein that binds antigen using a single-chain variable fragment (scFv) derived from a monoclonal antibody, which is fused to the CD3ζ T cell receptor signaling domain via spacer and transmembrane domains. Because the antigen recognition function of a CAR derives from an scFv, specificity is independent of major histocompatibility complex haplotype and can target any cell surface antigen to which an antibody can be made. The inclusion of co-stimulatory domains such as CD28 and/or 4-1BB enhance the cytokine secretion, proliferation, and in vivo activity of CAR T cells,30–35 and CARs containing 0,1, or 2 costimulatory domains are termed 1st, 2nd, or 3rd generation CARs, respectively.
We previously reported the results of a pilot trial testing a 3rd generation CAR targeting the CD20 antigen in patients with relapsed B cell lymphomas.27 While the anti-tumor effects appeared to be promising in a small cohort of patients, the CAR expression density was low, potency of the cells was suboptimal due to prolonged ex vivo culture time, and the cell production process was laborious and inefficient. Many of these obstacles were caused by inefficient gene transfer, which our group subsequently addressed by developing a CAR-encoding lentiviral vector. We previously reported the development of this CD20 CAR 3rd generation lentiviral vector, which contained an inducible caspase 9 (iC9) suicide gene and demonstrated promising pre-clinical activity.36 We have identified characteristics of this vector that required additional engineering for optimal function, and we describe here the improvements that led to the development of the construct we have selected for clinical testing.
Materials and Methods
Cell lines
Raji (Burkitt lymphoma), Jurkat (T cell lymphoma), Jeko-1 (mantle cell lymphoma) and K562 (CD20-negative erythroid leukemia) tumor cell lines were purchased from ATCC (Manassas, VA). Granta-519 (mantle cell lymphoma) cell was obtained from DSMZ (Braunschweig, Germany). TM-LCL is an EBV-transformed lymphoblastoid cell line optimized for expansion of T cell cultures.37, 38 Cells were cultured in RPMI 1640 with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine, and incubated in a humidified atmosphere containing 5% CO2 at 37°C. The generation of K562-CD64 cells was previously described.39 Raji-ffLuc and Granta-ffLuc were generated by transduction with a retroviral vector encoding firefly luciferase (ffLuc), Thy1.1, and neomycin resistance gene36, 40 and maintained and selected with 0.8 mg/ml G418 (Promega, Madison, WI). All cell lines were routinely assessed over the course of these experiments to confirm the expected surface marker expression by flow cytometry.
Vector constructs
The lentiviral vectors encoding various CD20-binding scFv CAR constructs were generated by polymerase chain reaction (PCR) and have been previously described.36, 41 All vectors contained a truncated CD19 (tCD19), which is expressed at equimolar amounts with the CAR gene by virtue of separation with a self-cleaving 2A peptide sequence. Flow cytometry experiments confirmed equivalent levels of tCD19 and CAR cell surface expression (data not shown), and tCD19 was thus used as a transduction marker and surrogate for CAR expression. Overlap PCR was used to remove the CAR gene from the iC9_1F5-28-BB-z_tCD19 vector to create the “Empty Vector.” All constructs were confirmed by Sanger sequencing. Lentiviral vectors were pseudotyped with a VSV-G envelope and produced by transient transfection of 293T cells as previously described.41, 42 Supernatants containing CAR vector packaged lentivirus were collected and concentrated 100-fold by centrifugation.
T cell transduction and expansion
Human peripheral blood mononuclear cells (PBMCs) were obtained by apheresis from healthy donors and isolated with Ficoll-Paque (GE health Care Life Sciences, Pittsburgh, PA) density gradient medium by centrifugation. For some experiments, CD14− CD45RA− CD62L+ central memory T cells (TCM) were isolated by immunomagnetic bead selection as previously described.41 In other experiments, CD8+ and CD4+ lymphocytes were positively selected using immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) prior to cryopreservation. T cells were stimulated with anti-CD3/CD28 antibody-coated paramagnetic beads (ThermoFisher [Invitrogen], Waltham, MA) at a 3:1 bead:T cell ratio. T cells were transduced on day 1 after activation by centrifugation at 2100 rpm for 60 minutes at 32°C with concentrated lentiviral vector encoding one of the CD20 CAR constructs supernatant at a multiplicity of infection of 3–13, supplemented with polybrene (8 μg/ml). T cells were expanded in RPMI with 10% FBS, 1% penicillin/streptomycin and 1% L-glutamine, supplemented with recombinant human interleukin 2 (hIL-2) to a final concentration of 50 U/ml. On day 5–6, magnetic beads were removed and T cell expansion was boosted by co-culturing activated T cells with irradiated CD20+ TM-LCLs at a 3:1 ratio of TM-LCL to tCD19+ T cells. The TM-LCL restimulation occurred at day 9–10 in earlier experiments using a 20–21-day total culture time, and day 7 in later experiments using a 15–16-day total culture time before use in mouse and in vitro experiments.
Proliferation and cytokine secretion assays
T cells were labeled with 5 μmol/L carboxyfluorescein succinimidyl ester (CFSE) according to manufacturer directions (ThermoFisher [eBioscience], Waltham, MA) and co-cultured with irradiated Raji-ffLuc target cells at an E:T ratio of 1:1 tCD19+ T cells to tumor cells (2 × 105 cells each). Supernatants were collected 24 hours after co-culture, and levels of IL-2, TNF-α, and IFN-γ were measured by Luminex assay as previously described.27, 41 For proliferation analysis, cells were collected after 96 hours and labeled with anti-CD3 and anti-CD19 antibodies. Then, cells were analyzed by flow cytometry, and the percentage of divided cells was determined based on CFSE dye dilution of live CD8+ gated tCD19+ T cells.
Cytotoxicity assays
To assess cytolytic function of CAR T cells, target cells were labeled overnight with 51Cr (PerkinElmer, Waltham, MA), washed, and dispensed at 2 × 103 cells per well in 96 well round bottom plates. Effector CD8+ tCD19+ T cells were added at various effector to target (E:T) ratios. After 4 hours, supernatants were harvested into 96 well Lumaplates, air-dried overnight, and counts assayed with a TopCount (PerkinElmer, Waltham, MA). Specific lysis was calculated as previously described.27, 41
Flow cytometry
To quantify transgene expression in transduced T cells, cells were routinely isolated and stained with anti-CD3 and anti-CD19 antibodies. In experiments to determine detailed immunophenotypes and Fcγ receptor (FcγR) binding of the CAR, cells were stained with anti-CD64 (FCGR1A), anti-CD69 (L78), anti-CD45RA (HI100), anti-CD25 (BC96), anti-CD45RO (UCHL1), anti-CD62L (DREG-56), anti-CCR7 (GO43H7), anti-CD127 (A019D5), anti-CD43 (CD43-10G7), anti-CD95 (DX2), and/or anti-CD57 (HNK-1). To measure CAR T cell persistence in mouse blood, peripheral blood was collected by retro-orbital bleeding, red blood cells lysed by ACK lysis buffer, FcγR blocked by Gamunex-c (Grifols USA, Los Angeles, CA) intravenous immunoglobulin (IVIG), and cells stained with anti-human CD3, anti-mouse CD45 and anti-human CD19. To directly measure expression of the CAR, cells were stained with biotinylated goat anti-human IgG Fc followed by PE-streptavidin. All antibodies were purchased from ThermoFisher (eBioscience, Waltham, MA), BD Biosciences (San Jose, CA), Jackson ImmunoResearch (West Grove, PA), or Biolegend (San Diego, CA). Samples were acquired with a BD FACSCanto, BD LSRII, or BD LSRFortessa, and data were analyzed using FlowJo Software version v10.0.6 (Treestar, Ashland, OR).
Murine xenograft experiments
Six- to 8-week-old randomized male or female NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD/SCID/γ−/−[NSG]) mice were obtained from The Jackson Laboratory or bred in-house. Mice were injected with 5 × 105 Raji-ffLuc or Granta-ffLuc tumor cells via tail vein. At 2 or 7 days after tumor injection (as noted in figure legends), mice were injected with 5 × 106 tCD19+ CAR-modified or empty vector T cells by tail vein. The 2-day or 7-day intervals between tumor injection and T cell infusion were selected to yield different levels of disease burden for the CAR T cells to respond to. In the earlier experiments we used 2-day intervals, but in later experiments as the CAR construct became more optimized and led to more potent activity, we allowed 7 days for the tumor to engraft to evaluate activity against more advanced tumors. Survival and tumor burden were assessed longitudinally for at least 90 days in these experiments. Mice were euthanized per institutional guidelines for symptoms of progressive tumor growth, including hind-limb paralysis or > 20% weight loss. In this disseminated tumor model, tumor response was measured using bioluminescence imaging as previously described.40, 41
Results
Engineering of IgG1 spacer region to abrogate Fc receptor binding
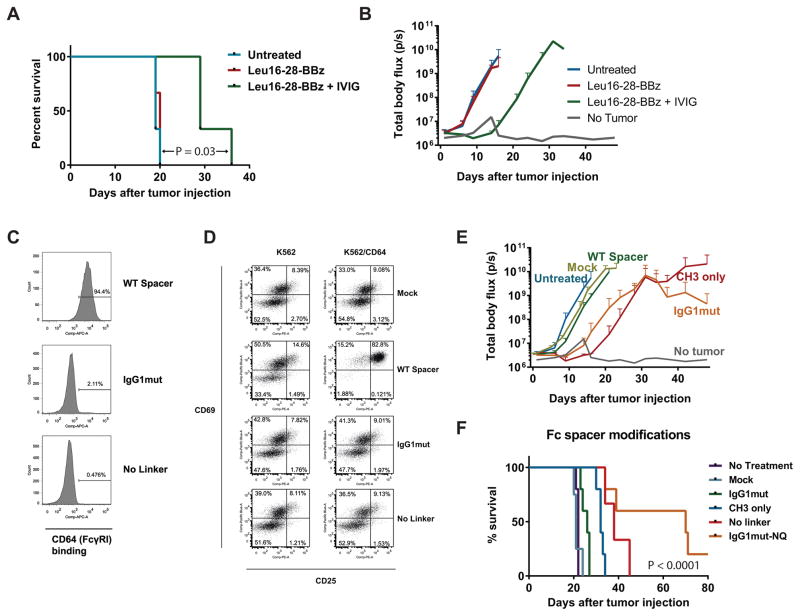
Our group previously developed a lentiviral vector encoding a 3rd generation anti-CD20 CAR (iC9_1F5-28-BB-z_tCD19), which showed potent in vitro function against CD20+ tumor cells, as well as in vivo eradication of disseminated lymphoma xenograft tumors under certain conditions.36 However, this level of anti-tumor activity required 2 infusions of CAR T cells, with treatment of mice only 2 days after tumor inoculation. This contrasted with other CAR studies in which a single infusion of CAR T cells was sufficient to eradicate 5–7 day established tumors.39, 43 Previous studies demonstrated that CARs with a full-length (CH2-CH3) human IgG1 or IgG4 extracellular spacer domain bind FcγRs, triggering antigen-independent CAR signaling that leads to activation-induced cell death (AICD) in vivo.39, 44–46 We speculated that the in vivo function of our CAR, which contained a human IgG1 Fc spacer domain, was suboptimal in part because of FcγR-induced AICD. This hypothesis was supported by experiments showing that pre-treatment of mice with i.v. immune globulin (IVIG) to block FcγRs prior to CAR T cell infusion improved survival compared with control mice receiving CAR T cells alone, which showed no anti-tumor activity (Figure 1A and 1B).
Figure 1. Modified extracellular spacer domain abrogates FcγR binding and improves in vivo CAR T cell function.
(A & B) NSG mice were inoculated i.v. with Raji-ffLuc tumors followed 2 days later by infusion of 5 × 106 CD20-targeted CAR+ T cells (TCM-enriched healthy donor T cells transduced with Leu16-28-BBz and re-stimulated with CD20+ LCL cells), with or without i.p. injection of 30 mg i.v immune globulin (IVIG) per mouse 4 hours prior to T cell infusion. Survival is shown in (A), with the curves compared using a log-rank (Mantel-Cox) test. Tumor burden as measured by bioluminescence imaging (mean ± SD of 3 mice per group) is shown in (B). Results are representative of 2 independent experiments. (C) CD64 (FcγRI) binding was tested using Jurkat cells transduced with a wild-type IgG1 Fc spacer CAR (iC9-SP163-1F5-IgG1-28-BB-z; “WT spacer”), CAR with spacer replacement of IgG1 hinge-linker by IgG2 hinge linker a.a. sequence (iC9-SP163-1F5-IgG1mut-28-BB-z; “IgG1mut”), and a CAR construct with deletion of the IgG1 hinge-linker (“No linker”). Transduced cells were identified by anti-CD19 antibody staining to detect the tCD19 transduction marker, and cells were stained with CD64, 3 days after transduction. The CD19+ population was gated, and frequencies of gated cells are shown in the histograms. (D) TCM T cells were transduced with Mock, WT spacer, IgG1mut, and No Linker constructs, and co-cultured with K562 or K562/CD64 in serum-free medium. To analyze T cell activation, cells were collected after 24 hours of co-culture and stained with CD3, CD19, CD25, and CD69. Frequencies of CD69+ and CD25+cells (gated on CD3+ CD19+ cells) are listed in the quadrants. (E) NSG mice were inoculated i.v. with Raji-ffLuc tumors followed 2 days later by infusion of 5 × 106 CD20-targeted CAR+ T cells transduced with the following 1F5-based 3rd generation lentiviral vectors: WT spacer, the same vector but with CH2 domain deleted (“CH3 only”), or IgG1mut, or mokc-transduced T cells, and tumor burden was measured by bioluminescence over time (n=3 mice/group). (F) Mice were treated as in (E) but also included the No linker CAR and the IgG1mut CAR with N297Q mutation (iC9-SP163-1F5-NQ-28-BB-z; “IgGmut-NQ”). Kaplan-Meier survival curves are shown (n=5 mice/group) and compared using a log-rank test. Tumor burden curves for this experiment are shown in Figure S2 (Supplemental Digital Content).
To further test the impact of Fc- FcγR interactions on CD20 CAR T cell function, we generated several new CAR constructs (Supplemental Digital Content, Figure S1) incorporating various modifications to the extracellular spacer region of the CAR. We first examined whether the CAR Fc spacer can trigger activation of CD20 CAR T cells. Previous studies demonstrated that CAR T cells in the lung upregulated activation markers CD25 and CD69 in the absence of target antigen, so we tested whether different spacer domains also trigger antigen-independent activation after FcγR binding. As shown in Figure 1C, soluble CD64 (FcγRI) binds to CARs with wild type IgG1 spacers, but not to spacers in which the IgG1 hinge linker (first 6 amino acids of the CH2 region) was either replaced with the corresponding amino acids of the IgG2 hinge linker (“IgG1mut”) or removed (“No Linker”). We then assessed the impact of spacer domain-FcγR interactions by co-incubating CAR T cells expressing these constructs with CD64-expressing K562 cells (K562/CD64). As expected, WT spacer CAR cells significantly upregulated CD69 and CD25, indicating activation independent of CD20 binding, but no change in CD25 and CD69 expression compared with basal levels was observed with either the IgG1mut or No Linker CAR T cells.
To examine the anti-tumor activity of these CAR constructs in vivo, we inoculated cohorts of NSG mice with Raji-ffLuc cells, and 2 days later when tumor was disseminated, treated mice with a single infusion of CAR TCM-derived cells with WT or IgG1mut spacers, or a truncated spacer lacking the entire CH2 domain (“CH3-only”). We observed tumor regression and improved survival in the CH3-only and IgG1mut groups, whereas the tumor burden in the group treated with WT spacer CARs was similar to control mice that received mock-transduced T cells or that were left untreated (Figure 1E). Although these spacer modifications appeared to abrogate FcγR binding in vitro, previous studies using CD19 CARs demonstrated that an additional mutation at an N-glycosylation site47 was required to abolish FcγR binding in vivo and fully restore CAR T cell function.39 We therefore incorporated this N297Q mutation into the IgG1mut vector (“IgG1mut-NQ,” also termed “iC9_1F5-NQ-28-BB-z”). This additional mutation resulted in significantly decreased tumor growth and prolonged survival (Figure 1F and Supplemental Digital Content, Figure S2). Together, these results confirm previous studies using CD19, PSCA, and ROR1-targeted CARs demonstrating that modification of the extracellular Fc spacer region of CAR constructs is necessary for robust CAR T cell-mediated anti-tumor activity in NSG xenograft mouse models.39, 44–46, 48
We sought to determine whether the need to include mutations in the spacer could be circumvented by simply removing the CH2 or CH2-CH3 regions. This approach was successful for CD19 and ROR1-targeted CARs, in which truncated extracellular Fc spacer domains (i.e. hinge-only) enhanced cytotoxicity, proliferation, and effector functions compared with a long spacer (Hinge-CH2-CH3).39, 48 We transduced TCM cells with CAR constructs of various lengths and measured cytokine secretion in response to co-culture with Raji cells. As shown in Figure S3 (Supplemental Digital Content), IL-2 and TNF-α production were higher in T cells expressing CARs with long spacers (IgG1mut-NQ and IgG1mut) compared with shorter spacers (CH3 only or Leu16-short). Similarly, cytotoxic activity was superior with longer spacers. These results are consistent with previous findings that a long spacer is required for CD20-targeted CARs,49 as well as with data from our group that intercellular distance is important for optimal CAR function, with a long spacers being needed to target membrane-proximal epitopes such as the CD20 antibody-binding epitopes, in contrast to the short spacers that optimally target epitopes farther from the cell membrane such as CD22 or CD19.50
Maximization of CAR expression
Our previous 3rd generation CAR construct achieved adequate transduction efficiency (10–75%) using a 2nd generation lentiviral vector system (lentiviral backbone vector plus 2 helper plasmids).36 However, after transferring this construct to a 3rd generation self-inactivating lentiviral vector system (backbone plus 3 helper plasmids) suitable for clinical use, we consistently observed suboptimal transduction efficiencies. Achieving high lentiviral titers is crucial from a feasibility standpoint, since impurities from concentrated lentiviral supernatant are toxic to cells, and the volume of supernatant thus becomes limiting for cell viability. Low transduction efficiencies therefore cannot be achieved simply by increasing the volume of supernatant used during transduction. Because the size of the transgene cassette is an important determinant of lentiviral titer, with viral titer being inversely proportional to vector insert size,51 we speculated that the large (4.4 Kb) insert in the iC9_1F5-NQ-28-BB-z_tCD19 tricistronic vector was limiting viral titers and hypothesized that removal of the 1.2 Kb iC9 gene would improve titers.
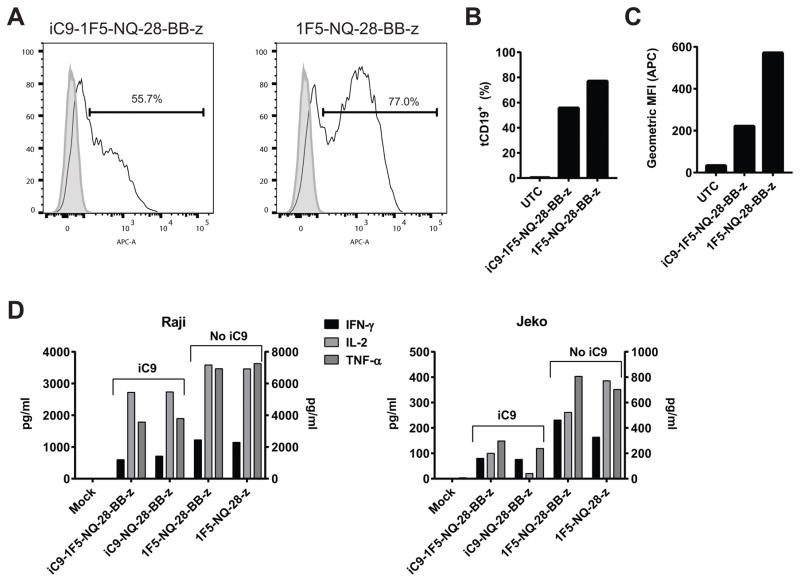
We evaluated differences in transgene expression between TCM cells transduced with iC9_1F5-NQ-28-BB-z_tCD19 and 1F5-NQ-28-BB-z_tCD19 (Figure 2A and B) and found a significant difference in expression (55.7% and 77%, respectively). Additionally, the intensity of expression per cell was also significantly higher in the construct without the iC9 (Figure 2C). Similar results were obtained with 1F5 based 2nd generation CAR vectors containing CD28 or 4-1BB only (data not shown). To determine whether the enhanced transgene expression was associated with improved CAR T cell function, we measured cytokine production of these T cells after stimulation with two different cell lines, Raji and Jeko, and found that for both cell lines, IL-2, TNF-α, and IFN-γ secretion was higher in cells expressing the CAR lacking iC9. Because of the clear improvement in CAR expression and T cell function using the smaller transgene inserts, the constructs we used for further evaluation did not include the iC9 gene.
Figure 2. Absence of iC9 gene improves transduction efficiency and function of CD20 CAR T cells.
Healthy donor PBMC enriched for TCM were activated with anti-CD3/CD28 beads and transduced with a lentiviral vector containing either the iC9-1F5-NQ-28-BB-z construct or the same construct lacking the iC9 gene. Transgene expression was assessed by staining cells with anti-CD19 antibody to detect the tCD19 marker and analyzed by flow cytometry. (A) Histograms representing tCD19 expression of transduced cells (solid line) or untransduced control cells (filled histogram) are shown. The percent transduced cells is quantified in (B) and the geometric mean fluorescence intensity (MFI) of tCD19 expression is shown in (C). These results are representative of 3 independent experiments. (D) To measure cytokine secretion, TCM cells transduced with 1F5-NQ-28-BB-z or 1F5-NQ-28-z, with or without iC9 included, were co-incubated at a 1:1 ratio with irradiated Raji cells (left panel) or Jeko cells (right panel); supernatants were harvested 24 hours later and the indicated cytokines were measured by Luminex assay. IFN-γ and TNF-α are shown on the left y-axes and IL-2 is shown on the right y-axes. These results are representative of 2 independent experiments.
Minimization of immunogenic epitopes
Clinical trials testing adoptive immunotherapy with genetically modified T cells have demonstrated that a potential limitation of this approach is rejection of infused cells by the host immune system.28, 29, 52, 53 Although the transgenic protein contains several potential neoantigenic epitopes at fusion sites between domains, the most immunogenic epitopes are predicted to occur in xenogeneic regions. This was confirmed in epitope mapping studies in patients that developed anti-CAR immune responses in a recent CD19-targeted CAR T cell trial at our center, in which the immune responses mapped to the murine scFv.28, 29 This suggests that replacement of the murine scFv with a humanized or fully human scFv would reduce the immunogenicity of the CAR protein.
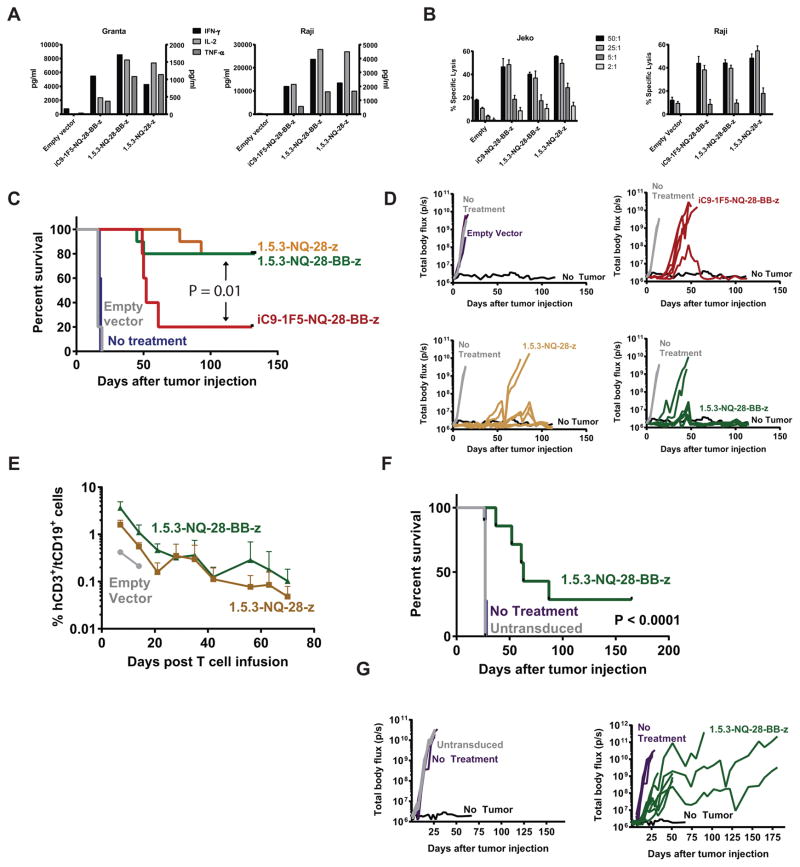
We therefore synthesized a new scFv based on the amino acid sequence of a fully human anti-CD20 antibody54, 55 and replaced the 1F5 murine scFv with it. The parental 1.5.3 antibody exhibits comparable binding affinity to CD20 as rituximab.54 We therefore generated several 1.5.3-containing CAR constructs (Supplemental Digital Content, Figure S1) and found that the in vitro function of T cells expressing these CARs compared favorably with 1F5 CARs, demonstrating potent cytokine secretion and cytotoxicity (Figures 3A and 3B and data not shown).
Figure 3. Fully human CD20 CAR exhibits potent in vitro and in vivo anti-tumor activity.
Healthy donor central memory T cells (TCM) were stimulated with anti-CD3/CD28 beads and transduced the next day with iC9-1F5-NQ-28-BB-z, 1.5.3-NQ-28-BB-z, or 1.5.3-NQ-28-z lentiviral vectors, or a vector encoding only iC9 and tCD19 (“empty vector”), and expanded in vitro. (A) CAR-expressing cells were co-cultured in a 1:1 ratio with irradiated Granta-519 cells (left) or Raji-ffLuc cells (right), and supernatants were harvested 24 hours later and analyzed for the indicated cytokines using a Luminex assay. IL-2 and TNF-α (left axis) and IFN-γ (right axis) levels are shown. (B) Cytotoxicity was measured by using these cells as effectors in a standard 4-hour 51Cr-release assay against either Jeko (left) or Raji-ffLuc (right) cell lines. (C and D) NSG mice were injected i.v. with 5 × 105 Raji-ffLuc cells followed 2 days later by 5 × 106 cells transduced with the vectors described in (A). A Kaplan-Meier curve for overall survival is shown in (C) and tumor burden over time of individual mice as measured by bioluminescence is shown in (D). The survival curves for iC9_1F5-NQ-28-BB-z, 1.5.3-NQ-28-BB-z, and 1.5.3-NQ-28-z were compared using a log-rank (Mantel-Cox) test. (E) Retroorbital blood samples of mice treated with T cells transduced with 1.5.3-NQ-28-z, 1.5.3-NQ-28-BB-z, or an empty vector in the experiment described in C and D were analyzed by flow cytometry for circulating infused CAR T cells. The percentage of cells in the mCD45− hCD3+ hCD19+ gate over time are shown (n = 2 mice per group). (F) NSG mice were injected i.v. with 5 × 105 Granta-ffLuc cells followed 2 days later by either 5 × 106 tCD19+ cells transduced with the 1.5.3-NQ-28-BB-z or an equal number of untransduced cells. A Kaplan-Meier curve for overall survival is shown in (F), with curves compared using a log-rank test, and tumor burden over time of individual mice as measured by bioluminescence is shown in (G).
Costimulatory domain configuration
Several previous studies have shown that the in vitro and in vivo function of CARs containing one (2nd generation) or two (3rd generation) costimulatory domains is superior to that of 1st generation CARs.30–35, 56, 57 However, given the many different variables between CAR constructs that can influence function, including binding avidity, antigen density on target cells, and intercellular distance resulting from CAR synapse formation, all of which could impact the strength of CAR signaling, it cannot be taken for granted that the costimulatory domain that is optimal for one type of CAR will be optimal for another. We previously demonstrated that a 3rd generation CAR containing CD28 and 4-1BB domains outperformed the corresponding 1st generation CAR as well as a 2nd generation CAR containing a CD28 domain only.35 However, the method of CAR gene transfer in these studies was electroporation of T cells with linearized naked DNA plasmids, an inefficient process that resulted in low CAR expression and required multiple rounds of re-stimulation and antibiotic selection of transfected cells, and it is unclear how applicable the findings are to CAR T cells efficiently transduced with lentiviral vectors.
We therefore tested CAR T cells expressing CD28 only, 4-1BB only, or CD28 and 4-1BB domains in the fully human 1.5.3 CAR (Supplemental Digital Content, Figure S1). We found that the titers and levels of CAR expression using the 1.5.3-NQ-BB-z construct were consistently lower than with 1.5.3-NQ-28-z or 1.5.3-NQ-28-BB-z, and function was inferior to the other two CARs in a pilot mouse experiment (data not shown). We therefore focused on testing the 1.5.3-NQ-28-z and 1.5.3-NQ-28-BB-z CARs. Stimulating T cells expressing these constructs with Raji or Granta cells induced similar levels of IL-2 and TNF-α secretion in vitro, though IFN-γ secretion appeared to be higher with the 3rd generation CAR (Figure 3A), and both constructs produced higher cytokine levels than the iC9_1F5-NQ-28-BB-z construct. Cytolytic activity against Raji and Jeko cell lines was similar for all three constructs, with minimal activity against tumor cells by an empty vector (Figure 3B). We also conducted in vivo experiments directly comparing the anti-tumor activity of these CARs in mice bearing disseminated Raji-ffLuc lymphoma xenografts. We found that a single injection of CAR T cells led to tumor eradication in approximately 80% of mice receiving either 1.5.3-NQ-28-z or 1.5.3-NQ-28-BB-z, but only 20% for mice treated with the iC9_1F5-NQ-28-BB-z (Figure 3C and 3D). We also compared CAR T cell expansion and persistence in mice, measuring the proportion of human CD3+/tCD19+ cells in the peripheral blood at 1-week intervals after T cell infusion. Mice treated with 1.5.3-NQ-28-BB-z and 1.5.3-NQ-28-z CAR T cells showed comparable levels of CAR T cell expansion and both had detectable CAR T cells for at least 70 days. Expansion of transferred T cells was higher for both CAR T cell groups compared with mice treated empty vector transduced T cells (Figure 3E).
In summary, these results suggest that the in vivo function of 1.5.3-NQ-28-BB-z and 1.5.3-NQ-28-z CARs is similar. We selected the 3rd generation CAR for further pre-clinical optimization because of the theoretical advantage of including a 4-1BB domain based on data demonstrating enhanced persistence, reduced exhaustion, and metabolic advantages associated 4-1BB signaling,58–60 as well as the potential value to the scientific field of gaining more clinical experience with a 3rd generation CAR given the paucity of clinical data using dual costimulatory domains.
We performed an additional experiment to evaluate the anti-tumor activity of the 1.5.3-NQ-28-BB-z CAR vector against a different cell line. We selected mantle cell lymphoma, as a clinically relevant established tumor model in our laboratory. Granta-ffLuc cells were injected by tail vein, followed 2 days later by 1.5.3-NQ-28-BB-z CAR T cells. As shown in Figure 3F and 3G, 1.5.3-NQ-28-BB-z CAR T cells resulted in a doubling of median survival, although they did not eradicate tumors. Overall, these results demonstrated that T cells expressing fully human 1.5.3-NQ-28-BB-z CARs have potent in vitro activity and anti-tumor efficacy and persistence in vivo.
Optimization of cell culture conditions
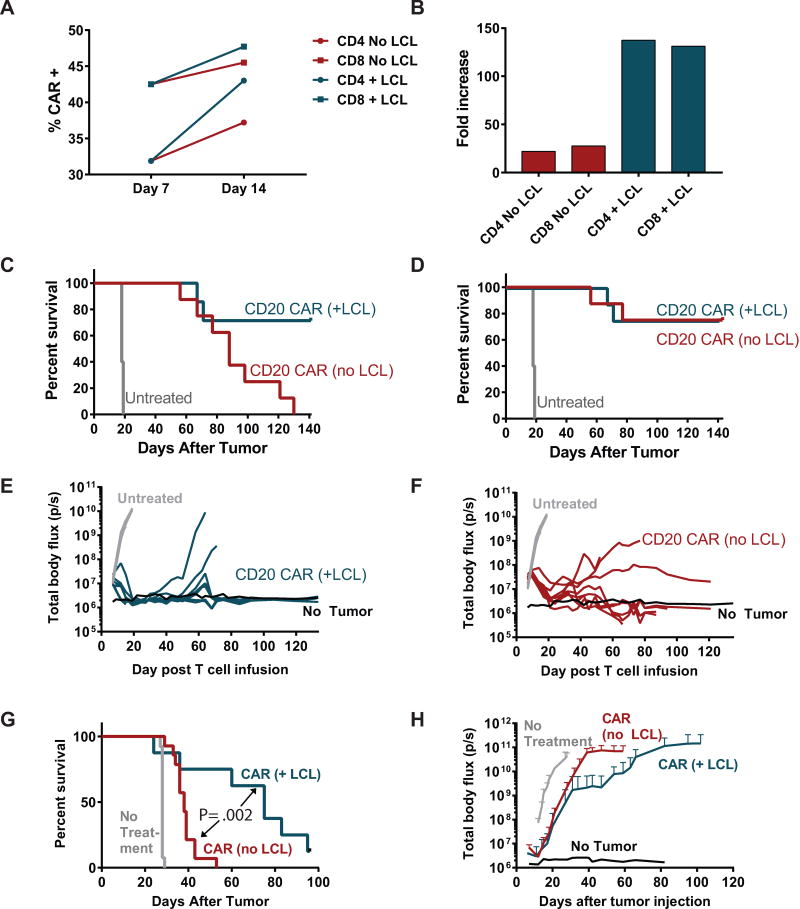
Many strategies have been utilized to obtain sufficient numbers of CAR T cells, but nearly all commonly employed methods involve an initial activation step with paramagnetic beads coated with anti-CD3 and anti-CD28 antibodies, followed by expansion in IL-2 containing medium. In a large CD19 CAR trial currently active at our center,28, 29 there is also a re-stimulation step with γ-irradiated TM-LCL, an EBV-transformed lymphoblastoid cell line expressing high levels of CD19 and CD20, which boosts growth of the T cells and also enriches for CAR+ cells without a sorting step.38 Although the TM-LCL re-stimulation is beneficial in terms of yielding higher numbers of CAR T cells for an infusion product, there is a risk that antigen stimulation could lead to more differentiated or exhausted cells that could have less capacity for in vivo proliferation and anti-tumor activity.
To assess the impact of re-stimulation through the CAR on cell growth, CAR expression, cytokine secretion, and in vivo function, 1.5.3-NQ-28-BB-z CAR T cells were activated with beads, transduced with the CAR vector, and expanded in IL-2 containing medium with or without a re-stimulation step with irradiated CD20+ TM-LCL at day 7. Re-stimulation with TM-LCL led to enrichment of CAR+ cells compared with non-restimulated cells (Figure 4A). Although the change in CAR expression was moderate, total cell yield significantly increased by over 100-fold with re-stimulation in both CD4 and CD8 T cells (Figure 4B). To evaluate the effect of antigen stimulation on CAR T cell function, transduced T cells with or without a TM-LCL re-stimulation were co-cultured with irradiated Granta-519 cells, and cytokine secretion was measured by Luminex assay. We found that secretion of IL-2 and TNF-α was much higher in the non-restimulated cells, a difference that was particularly pronounced in CD8+ cells. IFN-γ was also much more highly secreted by non-restimulated CD8+ cells compared with re-stimulated CD8+ cells, though in CD4+ cells there was a slightly higher level of IFN-γ secretion in re-stimulated cells (Supplemental Digital Content, Figure S4A and S4B). Cytotoxicity also appeared to be slightly higher in non-restimulated CD8+ cells, though the difference was marginal (Supplemental Digital Content, Figure S4C).
Figure 4. Re-stimulation with CD20+ cells in culture improves CAR expression, growth, and preserves in vivo anti-tumor activity.
PBMC from healthy donors were enriched for CD4 or CD8 cells by MACS positive selection, and each subset was separately activated with anti-CD3/CD28 beads and transduced 24 hours later with 1.5.3-NQ-28-BB-z lentiviral supernatant. At day 7, the cells were either re-stimulated with an irradiated CD20+ transformed B cell line (TM-LCL) or continued in culture without re-stimulation. (A) CAR expression of each cell culture at day 7 and day 14, as assessed by anti-human IgG Fc antibody (binds to IgG1 spacer region of CAR). (B) The cell growth of each cell culture up to day 14 is shown, expressed as fold increase from baseline. (C) NSG mice were injected i.v. with 5 × 105 Raji-ffLuc cells, followed 7 days later by infusion of 5 × 106 tCD19+ T cells at a 1:1 ratio of CD4+ tCD19+ : CD8+ tCD19+ cells. Groups received either CAR T cells that had been re-stimulated with TM-LCL (n=7 after excluding one mouse that died at day 12 during anesthesia for imaging), CAR T cells without re-stimulation (n=8), or no treatment (n=5). (C) Overall survival of each group, represented by a Kaplan-Meier curve. (D) Lymphoma-specific survival, including deaths due only to lymphoma progression and excluding deaths from xenogeneic graft-versus-host disease. (E) Tumor burden over time of mice receiving TM-LCL-re-stimulated CAR T cells as measured by bioluminescence imaging. (F) Tumor burden over time of mice receiving non-restimulated CAR T cells as measured by bioluminescence imaging. (G and H) NSG mice were injected i.v. with 5 × 105 Granta-ffLuc cells, followed 7 days later by infusion of 5 × 106 T cells at a 1:1 ratio of CD4+ tCD19+ : CD8+ tCD19+ cells. Groups received either CAR T cells that had been re-stimulated with TM-LCL (n=8), CAR T cells without re-stimulation (n=14), or no treatment (n=13). Overall survival is shown in (G), and tumor burden over time as measured by bioluminescence imaging is shown in (H). Differences between LCL and No LCL survival curves were assessed with a log-rank test.
We also compared the in vivo anti-tumor function of re-stimulated vs non-restimulated CD20 CAR T cells using a model in which NSG mice were treated with T cells 1 week after i.v. injection of Raji-ffLuc tumors. As shown in Figure 4C, treatment with TM-LCL-stimulated CD20 CAR T cells eradicated tumors in approximately 75% of the mice (Figure 4E). Despite the inferior overall survival in the non-restimulated group, no tumor was identified in most of these mice (Figure 4F), which were euthanized primarily as a result of xenogeneic graft-versus-host disease (GVHD). To compare anti-tumor efficacy we evaluated lymphoma-specific survival, which was similar in both groups (Figure 4D). We conducted a second experiment using Granta-ffLuc cells, and in contrast to the Raji model, re-stimulated CAR T cells led to improved survival and better tumor control compared to non-restimulated CAR T cells (Figure 4G and H). Taken together, these results indicated that re-stimulation with CD20+ target cells during cell production significantly boosts the growth of CAR+ T cells without impairing in vivo anti-tumor activity despite the lower capacity for cytokine secretion in vitro in these cells.
Other changes in the cell culture process that we incorporated involved the length of culture and subset composition of the cell product. The initial mouse experiments we conducted (Figure 1) utilized a 20-day CAR T cell expansion process with LCL re-stimulation, but we subsequently found that a shorter 15–16-day process led to improved CAR T cell viability and function (data not shown), and used this for later experiments (Figures 3–5). Additionally, our initial experiments employed magnetically selected TCM cells based on data from our institution and from the National Cancer Institute showing that TCM-derived cells have a superior ability to expand and persist and have enhanced in vivo anti-tumor activity compared with effector or effector memory T cell subsets.61–63 Subsequent experiments at our center showed that a 1:1 ratio of CD8+ TCM to CD4+ naïve or TCM cells was the most effective,64 supporting an earlier publication demonstrating enhanced in vivo anti-tumor activity with a 1:1 ratio of CD4:CD8 CAR T cells.65 This was followed by data from a large CD19-targeted CAR T cell clinical trial at our center, which demonstrated that CD8+ TCM and CD4+ cells at a 1:1 ratio, or in some cases using unselected CD8+ cells instead of CD8+ TCM, yielded potent anti-tumor activity as well as clear dose-response and dose-toxicity relationships.28, 29, 66 Because it is simpler and more cost-effective to perform basic CD4 and CD8 selections rather than the CD14 depletion and CD62L positive selection needed to generate CD8+ TCM, current CAR trials at our center now use a 1:1 CD8:CD4 ratio, without selecting for TCM. As our experiments evolved over time, we adapted the culture conditions in the experiments to mirror the conditions that are used to generate CAR T cell products in the clinical manufacturing facility at our institution, in order to facilitate clinical translation, and thus switched to using a 1:1 CD4:CD8 ratio starting with the mouse experiments in Figure 464, 65.
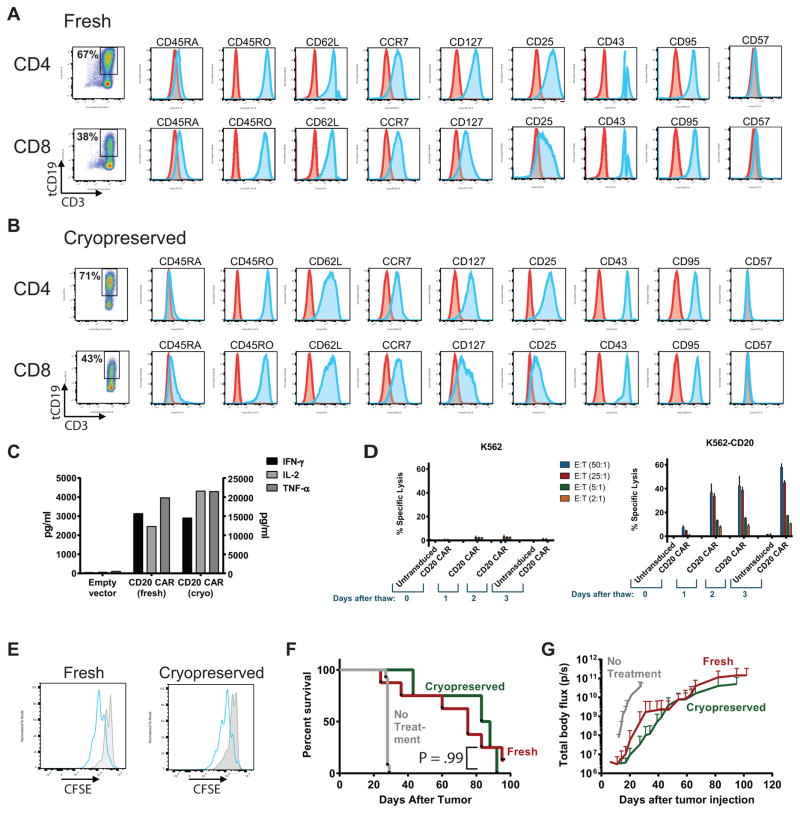
Figure 5. Phenotype and function of CD20 CAR T cells are preserved following cryopreservation.
(A–D) Healthy donor PBMC positively selected for CD4 or CD8 were separately stimulated with anti-CD3/CD28 beads and transduced with lentiviral vector encoding the 1.5.3-NQ-28-BB-z CAR. Cells were re-stimulated with CD20+ TM-LCL cells on day 7, and expanded until day 14, and then either kept in culture (fresh) or formulated in Cryostor medium, placed in a step-down freezer for 3 hours, transferred to liquid nitrogen overnight, and thawed the next day (cryopreserved). Fresh (A) or cryopreserved (B) T cells were evaluated by flow cytometry for the indicated cell surface markers to assess phenotypic markers of differentiation/memory (CD45RA, CD45RO, CD62L, CCR7, CD127), activation (CD25, CD43, CD95), or exhaustion (CD57). Cells were gated on CD3+ tCD19+ cells, and the number of tCD19+ cells, as a surrogate for CAR expression, is shown in the first panel. (C) Fresh or cryopreserved tCD19+ cells were co-cultured at a 1:1 ratio with irradiated Raji-ffLuc cells, and supernatants were harvested 24 hours later and the indicated cytokines measured by Luminex assay. IL-2 values are shown on the right y-axis, and IFN-γ and TNF-α levels are shown on the left y-axis. (D) Fresh or cryopreserved cells were used as effectors in a standard 4-hour 51Cr-release assay using either CD20+ K562 cells (right panel) or parental K562 cells lacking CD20 (left panel) as targets, at the indicated effector:target (E:T) ratios. The data represent the mean (+ SD) of triplicate values. (E) Fresh (left panel) or cryopreserved (right panel) CD8+ CAR+ or untransduced T cells were labeled with CFSE, co-cultured with irradiated Raji-ffLuc cells for 4 days, and then CFSE dilution was evaluated by flow cytometry. CAR+ cells (blue histograms) were gated on CD3+ tCD19+ cells, and untransduced cells (gray filled histograms) were gated on CD3+ cells. (F–G) NSG mice were injected i.v. with 5 × 105 Granta-ffLuc cells, followed 7 days later by infusion of 5 × 106 T cells at a 1:1 ratio of CD4+ tCD19+ : CD8+ tCD19+ cells. Groups received either fresh (red line, n=8) or cryopreserved (green line, n=5) cells (n=5), or no treatment (gray line, n=13). Overall survival is shown in (F), and tumor burden over time as measured by bioluminescence imaging is shown in (G). Differences between fresh and cryopreserved survival curves were assessed with a log-rank test.
Function and immunophenotype of fresh vs. cryopreserved CAR T cells
A question that arises with respect to conducting CAR T cell therapy clinical trials is whether to infuse a fresh cell product or a thawed product that has been cryopreserved. Given the known negative impacts of cryopreservation and thawing on cell viability, there is an intuitive appeal to infusing fresh cells. In addition to avoiding viability losses that require generating a cell number above the target dose, the freezing process could also theoretically impact the function or phenotype of the CAR T cells. However, there are several practical advantages to using cryopreserved cells: 1) logistically the infusion of fresh cells can be problematic since the timing of the infusion is rigid, whereas the use of cryopreserved cells allows more scheduling flexibility around patients’ clinical situations; 2) infusion of a cryopreserved product permits resulting prior to infusion of quality control tests such as sterility cultures that are normally reported after infusion; and 3) ultimately, large scale commercial application of CAR T cells currently requires use of cryopreserved cells from central manufacturing facilities.
We therefore evaluated several phenotypic and functional characteristics of cryopreserved 1.5.3-NQ-28-BB-z CAR T cells compared with fresh cells. First, we used a multi-parameter flow cytometry phenotyping panel to characterize fresh and cryopreserved tCD19+ CD4+ and CD8+ T cells. As shown in Figures 5A and 5B, we did not observe any differences in tCD19+ expression, nor in any activation, memory/differentiation, or exhaustion marker expression between fresh or cryopreserved cells. To evaluate CAR T cell function, we tested whether cytokine production is induced by co-culture with Raji cells. Both fresh and cryopreserved CAR T cells produced similar levels of IFN-γ, IL-2, and TNF-α (Figure 5C). Cytotoxicity was assessed using 51Cr-release assays with K562 and CD20-transduced K562 (K562-CD20) cells as target cells and CAR T cells cultured for various lengths of time after thawing (0, 1, 2, or 3 days) as effectors. We found that cytolytic function immediately after thawing was limited, but improved significantly after a day in culture (Figure 5D). We used a CFSE dilution assay to measure proliferation of tCD19+ CD8+ CAR T cells following stimulation with Raji cells and detected a similar frequency of divided cells in the fresh cells compared with cryopreserved cells (Figure 5E). Finally, we did not observe any significant difference in anti-tumor activity in vivo between fresh and cryopreserved CAR T cells using the Granta-ffLuc NSG mouse model (Figure 5F and 5G). Together, these results demonstrated that cryopreservation of CD20-targeted CAR T cells did not result in any significant changes in transgene expression, immunophenotype, or CAR T cell function.
Discussion
We describe here the pre-clinical optimization steps that led to the generation of the 1.5.3-NQ-28-BB-z_tCD19 lentiviral vector that we have selected for clinical testing. A previous 3rd generation CD20 CAR lentiviral vector developed by our group exhibited potent in vitro function but was ineffective in vivo with a single CAR T cell infusion, contained a potentially immunogenic murine-derived scFv, used an unmodified IgG spacer that left CAR T cells susceptible to AICD caused by FcγR binding, and required transfer into a self-inactivating 3rd generation lentiviral vector backbone suitable for clinical use. We have now addressed these limitations and generated a fully human CD20-targeted CAR T cell vector with potent in vivo anti-tumor function, and have also optimized culture conditions.
One of the key decision points was whether or not to remove the iC9 safety switch. Previous studies have shown that this gene product effects rapid ablation of transduced cells,36, 67 and iC9 would thus be a desirable feature to mitigate against the risk of CAR T cell-mediated toxicities. However, in our hands, inclusion of the iC9 gene reduced the titer of the lentiviral vector to prohibitively low levels. Additionally, as the field gains clinical experience with CAR T cell therapy, management of cytokine release syndrome, the primary acute toxicity of CAR T cell therapy, has become more effective. Most cases of CRS can be successfully treated with tocilizumab and/or dexamethasone without needing to eliminate CAR T cells altogether, and this approach may be particularly effective if applied early in the course of CRS.68 Other anti-inflammatory drugs such as siltuximab, ruxolitinib, or anakinra provide additional means of treating CRS. Another dreaded complication of CAR T cell therapy is cerebral edema, but it is not clear that the presence of a suicide gene in the CAR T cells could avoid this complication, since the as-yet poorly understood steps leading up to the cerebral edema may be irreversibly established by the time the need to activate the suicide gene is perceived. The most practical role for a suicide gene may therefore not be in the management of acute toxicities, but rather to eliminate long-lived CAR T cells causing prolonged B-cell aplasia after tumor eradication has been accomplished. However, this may not require an iC9 gene, as surface transduction markers that are targetable by clinical antibodies have been shown to restore functional B cells after CD19 CAR adoptive transfer.69 The tCD19 marker present in our lentiviral vector construct could also potentially be used as a target by with anti-CD19 therapy such as blinatumomab or antibody-drug conjugates to ablate CAR T cells in the event of prolonged B cell aplasia.
An interesting observation from our studies was that CAR T cells not subjected to a re-stimulation step with a CD20+ cell line during in vitro expansion were associated with a significantly higher rate of xenogeneic GVHD at later timepoints after T cell infusion. We hypothesize that this may be related to the different cytokine secretion profiles observed between re-stimulated and non-restimulated cells, with the latter exhibiting significantly higher levels of cytokine secretion. This finding may have translational relevance to humans, if the lower levels of cytokine secretion by re-stimulated T cells confer a lower risk of cytokine-release syndrome than non-restimulated cells.
The optimal costimulatory domain or combination of co-stimulatory domains for CARs is a matter of much debate and appears to vary to some extent by the particular CAR construct and target antigen being tested. We found that 2nd (CD28-only) and 3rd (CD28 and 4-1BB) generation CARs functioned nearly equivalently in our experiments. Recent studies of CD19 CARs have suggested that 4-1BB costimulation promotes CAR T cells with a central memory phenotype, supports a metabolic profile that leads to enhanced persistence, and mitigates against exhaustion due to repeated CAR signaling,58, 59 whereas CD28 confers a short-lived glycolytic burst and effector memory phenotype.58 Because of these findings, we hypothesized that inclusion of both costimulatory domains may provide both short term potent effector function as well as capacity for long term persistence, and therefore selected the 3rd generation CAR for clinical testing.
These experiments highlight the importance of testing CAR constructs using animal models, since many of the constructs led to similar proliferation, cytokine secretion, and cytotoxicity in vitro, but had profoundly different anti-tumor efficacy in vivo. Our initial experiments employed an NSG mouse model in which mice were treated with CAR T cells 2 days after lymphoma cell injection. Over the course of the improvements made to the CAR vector and culture conditions we shifted to a more stringent model using 7-day established tumors and were able to eradicate disease in 75% of these mice with a single injection of CAR T cells.
In summary, we have generated a fully human CD20-targeted 3rd generation CAR lentiviral vector, and our data provide rationale for conducting a phase I clinical trial in patients with CD20+ B cell NHL.
Supplementary Material
Acknowledgments
Sources of Funding
B. Till and O. Press have received research funding and royalties from and are inventors of a patent licensed to Mustang Bio, and have also received research funding from Roche/Genentech. S. Riddell and M. Jensen report ownership interest (including patents) in, research funding from, and consulting/advisory board membership for Juno Therapeutics. S. Riddell also is a consultant/advisory board member for Cell Medica. This work was funded by the Giuliani Family Foundation, NIH/NCI K23CA154874 (PI: B.G. Till), Damon Runyon Cancer Research Foundation grant 49-C10 (PI; B.G. Till), NIH/NCI Cancer Center Support Grant P30CA015704 both for Shared Resources (PI: Gary Gilliland) and also as a Recruitment Award (Sub-project PI: B.G. Till), NIDDK DK56465 (PI: S. Heimfeld, core resources), NIH/NCI K08CA151682 (PI: D.J. Green), and a generous gift from the Bezos family.
Bibliography
- 1.Press OW, Howell-Clark J, Anderson S, et al. Retention of B-cell-specific monoclonal antibodies by human lymphoma cells. Blood. 1994;83(5):1390–7. [PubMed] [Google Scholar]
- 2.Bubien JK, Zhou LJ, Bell PD, et al. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121(5):1121–32. doi: 10.1083/jcb.121.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15(9):450–4. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 4.Chang KL, Arber DA, Weiss LM. CD20: A Review. Applied Immunohistochem. 1996;4:1–15. [Google Scholar]
- 5.Press OW, Farr AG, Borroz KI, et al. Endocytosis and degradation of monoclonal antibodies targeting human B- cell malignancies. Cancer research. 1989;49(17):4906–12. [PubMed] [Google Scholar]
- 6.Cragg MS, Walshe CA, Ivanov AO, et al. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–74. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 7.Riley JK, Sliwkowski MX. CD20: a gene in search of a function. Semin Oncol. 2000;27(6 Suppl 12):17–24. [PubMed] [Google Scholar]
- 8.Nadler LM, Korsmeyer SJ, Anderson KC, et al. B cell origin of non-T cell acute lymphoblastic leukemia. A model for discrete stages of neoplastic and normal pre-B cell differentiation. J Clin Invest. 1984;74(2):332–40. doi: 10.1172/JCI111428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–45. [PubMed] [Google Scholar]
- 10.Giles FJ, Vose JM, Do KA, et al. Circulating CD20 and CD52 in patients with non-Hodgkin’s lymphoma or Hodgkin’s disease. Br J Haematol. 2003;123(5):850–7. doi: 10.1046/j.1365-2141.2003.04683.x. [DOI] [PubMed] [Google Scholar]
- 11.Shanehbandi D, Majidi J, Kazemi T, et al. CD20-based Immunotherapy of B-cell Derived Hematologic Malignancies. Curr Cancer Drug Targets. 2017;17(5):423–444. doi: 10.2174/1568009617666170109151128. [DOI] [PubMed] [Google Scholar]
- 12.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 13.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23(9):1984–92. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 14.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–86. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 15.Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–22. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 16.Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081–93. doi: 10.1016/S1470-2045(16)30097-3. [DOI] [PubMed] [Google Scholar]
- 17.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520–31. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 18.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 19.Brentjens RJ, Davila ML, Riviere I, et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–50. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(18 Pt 1):5426–35. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 31.Finney HM, Lawson AD, Bebbington CR, et al. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161(6):2791–7. [PubMed] [Google Scholar]
- 32.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 33.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20(1):70–5. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 34.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18(8):712–25. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 36.Budde LE, Berger C, Lin Y, et al. Combining a CD20 Chimeric Antigen Receptor and an Inducible Caspase 9 Suicide Switch to Improve the Efficacy and Safety of T Cell Adoptive Immunotherapy for Lymphoma. PLoS One. 2013;8(12):e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128(2):189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 38.Terakura S, Yamamoto TN, Gardner RA, et al. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119(1):72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hudecek M, Sommermeyer D, Kosasih PL, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer immunology research. 2015;3(2):125–35. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James SE, Orgun NN, Tedder TF, et al. Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice. Blood. 2009;114(27):5454–63. doi: 10.1182/blood-2009-08-232967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rufener GA, Press OW, Olsen P, et al. Preserved Activity of CD20-Specific Chimeric Antigen Receptor-Expressing T Cells in the Presence of Rituximab. Cancer immunology research. 2016;4(6):509–19. doi: 10.1158/2326-6066.CIR-15-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker PS, Taylor JA, Trobridge GD, et al. Preclinical correction of human Fanconi anemia complementation group A bone marrow cells using a safety-modified lentiviral vector. Gene Ther. 2010;17(10):1244–52. doi: 10.1038/gt.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 44.Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17(10):1206–13. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 45.Jonnalagadda M, Mardiros A, Urak R, et al. Chimeric Antigen Receptors with Mutated IgG4 Fc Spacer Avoid Fc Receptor Binding and Improve T cell Persistence and Anti-Tumor Efficacy. Mol Ther. 2014 doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe N, Bajgain P, Sukumaran S, et al. Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology. 2016;5(12):e1253656. doi: 10.1080/2162402X.2016.1253656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leatherbarrow RJ, Rademacher TW, Dwek RA, et al. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985;22(4):407–15. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- 48.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19(12):3153–64. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zah E, Lin MY, Silva-Benedict A, et al. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer immunology research. 2016;4(6):498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James SE, Greenberg PD, Jensen MC, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol. 2008;180(10):7028–38. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar M, Keller B, Makalou N, et al. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther. 2001;12(15):1893–905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 52.Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117(1):72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 53.Riddell SR, Elliott M, Lewinsohn DA, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2(2):216–23. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 54.Bornstein GG, Queva C, Tabrizi M, et al. Development of a new fully human anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. Invest New Drugs. 2010;28(5):561–74. doi: 10.1007/s10637-009-9291-z. [DOI] [PubMed] [Google Scholar]
- 55.Gazit-Bornstein G, Green L, Yang XD, et al. Antibodies directed to CD20 and uses thereof. Google Patents. 2007 [Google Scholar]
- 56.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 58.Kawalekar OU, O’Connor RS, Fraietta JA, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44(2):380–90. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102(27):9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sommermeyer D, Hudecek M, Kosasih PL, et al. Chimeric antigen receptor-modified T cells derived from defined CD8(+) and CD4(+) subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moeller M, Haynes NM, Kershaw MH, et al. Adoptive transfer of gene-engineered CD4+ helper T cells induces potent primary and secondary tumor rejection. Blood. 2005;106(9):2995–3003. doi: 10.1182/blood-2004-12-4906. [DOI] [PubMed] [Google Scholar]
- 66.Turtle CJ, Hay KA, Hanafi LA, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J Clin Oncol. 2017 doi: 10.1200/JCO.2017.72.8519. JCO2017728519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner R, Leger KJ, Annesley CE, et al. Decreased Rates of Severe CRS Seen with Early Intervention Strategies for CD19 CAR-T Cell Toxicity Management. Blood. 2016:586. ASH Abstracts. [Google Scholar]
- 69.Paszkiewicz PJ, Frassle SP, Srivastava S, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest. 2016;126(11):4262–4272. doi: 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.