Abstract
Neurobasal®/B27 is a gold standard culture media used to study primary neurons in vitro. An alternative media (BrainPhys®/SM1) was recently developed which robustly enhances neuronal activity vs. Neurobasal® or DMEM. To the best of our knowledge BrainPhys® has not been explored in the setting of neuronal injury. Here we characterized the utility of BrainPhys® in a model of in vitro mechanical-stretch injury.
METHODS/RESULTS
Primary rat cortical neurons were maintained in classic Neurobasal®, or sequentially maintained in Neurocult® followed by BrainPhys® (hereafter simply referred to as “BrainPhys® maintained neurons”). The levels of axonal markers and proteins involved in neurotransmission were compared on day in vitro 10 (DIV10). BrainPhys® maintained neurons had higher levels of GluN2B, GluR1, Neurofilament light/heavy chain (NF-L & NF-H), and protein phosphatase 2 A (PP2A) vs. neurons in Neurobasal®. Mechanical stretch-injury (50ms/54% biaxial stretch) to BrainPhys® maintained neurons modestly (albeit significantly) increased 24h lactate dehydrogenase (LDH) levels but markedly decreased axonal NF-L levels post-injury vs. uninjured controls or neurons given a milder 38% stretch-injury. Furthermore, two 54% stretch-injuries (in tandem) exacerbated 24h LDH release, increased α-spectrin breakdown products (SBDPs), and decreased Tau levels. Also, BrainPhys® maintained cultures had decreased markers of cell damage 24h after a single 54% stretch-injury vs. neurons in Neurobasal®. Finally, we tested the hypothesis that lentivirus mediated overexpression of the pro-death protein RBM5 exacerbates neuronal and/or axonal injury in primary CNS cultures. RBM5 overexpression vs. empty-vector controls increased 24h LDH release, and SBDP levels, after a single 54% stretch-injury but did not affect NF-L levels or Tau.
CONCLUSION
BrainPhys® is a promising new reagent which facilities the investigation of molecular targets involved in axonal and/or neuronal injury in vitro.
Keywords: Mechanical Stretch-Injury, BrainPhys®, Neurofilaments, TBI, RBM5
1. INTRODUCTION
Cell culture media is widely used for studying neurons in vitro. Different formulations like Neurobasal® or DMEM, plus different types of supplements frequently added to those medium, are commonly employed, and influence experimental variables such as CNS/neurite cell growth and long-term viability (Brewer et al., 1993; Geranmayeh et al., 2015; Harrill et al., 2015; Hogins et al., 2011; Marx, 2013; Ren et al., 2007; Shin et al., 2006; Thomson et al., 2006; Ye and Sontheimer, 1998).
BrainPhys® is a recent advancement in cell culture media for the growth of neurons. It is a new formulation which improves electrophysiological properties in neurons, comparable in efficacy to artificial cerebral spinal fluid (aCSF), and also maintains long-term viability (unlike aCSF which preserves viability for a short time only)(Bardy et al., 2015). For a chemical list and comparison of quantities of neuroactive ingredients in BrainPhys® vs. gold standard Neurobasal®, the reader is directed to the supplemental data accompanying Bardy and colleagues seminal report (Bardy et al., 2015). To the best of our knowledge the utility of BrainPhys® for the study of neuronal death in primary rodent CNS cultures, specifically in the setting of mechanical trauma, has not been evaluated.
Mild/concussive traumatic brain injury (mTBI) is the most common diagnosis of TBI and accounts for ~80–90% of all cases (Blennow et al., 2016; Kraus and Nourjah, 1988). In the majority of patients, neurological symptoms resolve within two weeks post-injury (McClincy et al., 2006). However, ~10–15% of mTBI patients have persistent neurological impairments lasting months after insult, and are subsequently diagnosed with post-concussion syndrome (PCS)(Silverberg and Iverson, 2011). Identifying the biochemical, cell signaling, and neuropathological changes caused by mTBI/PCS may aid in the development of treatments to accelerate recovery.
In adults a single mTBI generally is not believed to cause significant levels of overt neuronal death (Bigler, 2004; Bolton Hall et al., 2016; DeFord et al., 2002; Povlishock et al., 1983). Axonal injury is the major neuropathological feature, and correlates with the manifestation of neurological symptoms (Mac Donald et al., 2011; Wright et al., 2016). However, repetitive mTBI, which exacerbates white matter damage, may cause neuronal death - particularly in vulnerable patients like infants (Goddeyne et al., 2015; Huh et al., 2007). The diagnosis of an uncomplicated mTBI is typically determined by positive findings on neurological assessment but negative findings on cranial CT scan. Clinical development of CSF/plasma axonal-injury biomarkers like neurofilament light chain (NF-L) and total Tau (released from damaged axons) may prove useful in supporting a diagnosis of mTBI (Zetterberg et al., 2013). NF-L in particular has gained considerable interest due to its sensitivity in detecting subtle damage of large-caliber myelinated axons in concussed athletes (Neselius et al., 2012).
In vitro models of mechanical stretch-injury are widely used to study signaling mechanisms activated/altered by trauma in neurons, astrocytes, and in other CNS cells (Ahmed et al., 2000; Ahmed et al., 2002; Ellis et al., 1995; Ellis et al., 2007; Goforth et al., 1999; McKinney et al., 1996; Neary et al., 2003; Tavalin et al., 1995, 1997; Weber et al., 2001; Zhang et al., 1996). The cell injury controller (CIC) is a gas driven commercially available stretch-injury device developed by Ellis et al., (1995), and subsequently used in numerous studies to evaluate the effects of mechanical trauma on CNS cells (Ellis et al., 1995). Here we used the well-known CIC-II injury system to deliver a stretch-injury (50ms/~54% biaxial stretch) to neurons cultured in BrainPhys®. We also performed a comprehensive comparison of cell signaling proteins in BrainPhys® vs. Neurobasal® in uninjured neurons, as well as in stretched neurons, to characterize potential differences in culture systems. We show that BrainPhys® enhances the study of injury-induced changes in axonal neurofilaments, which may be useful for (1) screening new antibodies for the purposes of biomarker development, (2) testing axon-specific rescue therapies, and (3) investigating novel cell signaling mechanisms, such as the pro-death protein RNA binding motif 5 (RBM5) which was further explored here, that may contribute to neuronal/axonal death in the setting of trauma.
2. MATERIALS & METHODS
2.1 Chemicals & Reagents
ThermoFisher Scientific (Pittsburgh, PA, USA): Neurobasal®, B27 supplement, Opti-MEM™, Hanks balanced salt solution (HBSS), magnesium and chloride supplemented PBS, L-Glutamine, Penicillin-Streptomycin, ProLong® Gold Antifade Mountant with DAPI; SIGMA (St. Louis, MO, USA): Bovine Serum Albumin, Normal Goat Serum, Glutamic Acid, Sodium Bicarbonate, HEPES, Bovine DNase I; Corning (Corning, New York, USA): Matrigel Basement Membrane Matrix Phenol Red Free™, Cell Recovery Solution®; STEMCELL Technologies (Cambridge, MA, USA): Neurocult®, BrainPhys®, SM1 supplement. PRIMARY ANTIBODIES: Cell Signaling Technology (Danvers, MA, USA): Mouse Monoclonal Anti-ERK total (Cat# 4696), Rabbit Monoclonal Anti-Phosphorylated ERK (Cat# 4377Rabbit Monoclonal Anti-NMDAR2B (Cat# 4212), Rabbit Monoclonal Anti-CREB total (Cat# 9197), Rabbit Monoclonal Anti-pCREB (Ser133) (Cat# 9198), Mouse Monoclonal Anti-Neurofilament-L (Cat# 2835), Mouse Monoclonal Anti-Neurofilament-H (Cat# 2836), Mouse Monoclonal Anti-Tau 46 (Cat#4019), Mouse Monoclonal Anti-GFAP (Cat# 3670), Rabbit Monoclonal Anti-NeuN (Cat# 12943), Rabbit Monoclonal Anti-GAPDH (Cat# 5174), Rabbit Polyclonal Anti-αTubulin (Cat# 2144), Rabbit Polyclonal Anti-PP2A catalytic subunit (Cat# 2038); ABCAM (Cambridge, MA, USA): Rabbit monoclonal Anti-Glutamate Receptor 1 AMPA subtype (Cat# ab109450), Rabbit Monoclonal Anti-Caspase 9 (Cat# ab184786), Rabbit Polyclonal Anti-Malondialdehyde (Cat# ab6463, Lot# GR110035-6). ATLAS ANTIBODIES (Stockholm, Sweden): Rabbit Polyclonal Anti-RBM5 (Cat#HPA018011, Lot#R07119), Rabbit Polyclonal Anti-RBM10 Cat# HPA034972, Lot#R32333); ENZO Life Sciences (Farmingdale, NY, USA): Mouse Monoclonal Anti-α-Fodrin/αII-Spectrin (Cat# BML-FG6090). TREVIGEN (Gaithersburg, MD, USA): Rabbit Polyclonal Anti-poly (ADP-ribose) (PAR) (Cat# 4336-APC-050, Lot# 22670C11), SECONDARY ANTIBODIES: (ThermoFisher Scientific) Goat Anti-Rabbit IgG (H+L) Cross-Absorbed & HRP Conjugated (Cat# G21234), Goat Anti-Mouse IgG (H+L) Cross-Absorbed & HRP Conjugated (Cat# G21040), Goat Anti-Rabbit IgG (H+L) Cross-Absorbed & Alexa Fluor 594 Conjugated (Cat# A11037), Goat Anti-Mouse IgG (H+L) Cross-Absorbed & Alexa Fluor 488 Conjugated (Cat# A11029).
2.2 Animals
Animal studies were approved by the Institutional Animal Care & Use Committee of the University of Pittsburgh. Euthanasia protocols follow recommendations established by the American Medical Veterinary Association Guidelines for Euthanasia. All steps were taken to prevent animal suffering, and to use the minimum number of subjects needed for statistical significance & analysis. Timed pregnant Sprague Dawley (SD) rats and C57BL/6 mice were purchased from Charles River (Wilmington, MA, USA). Rats and mice were housed in a University of Pittsburgh facility managed by the Division of Laboratory Animal Resources. Rodents had access to ad libitum food/water and maintained on a 12h light/dark cycle. Embryos (~E15–19) were harvested from euthanized dams and cortical brain tissue collected for neuron culture.
2.3 Primary Neuron Culture
In brief, mixed-gender cortical halves were collected from ~E16–17 embryonic brains under a dissecting light microscope (Leica; Buffalo Grove, IL, USA). Harvested tissue was kept on ice in HBSS supplemented with sodium bicarbonate (10mM), HEPES (15mM), and penicillin-streptomycin (100 Units/mL). Cortical halves were dissociated by mechanical shearing using sterile scissors (~1min) in a 1.5mL tube. Cells were then trypsinized for ~8min in a 37°C water bath, quenched, centrifuged (4°C/200g/5min), and resuspended in trituration solution (10mg/mL DNase dissolved in Neurobasal®/B27). Cells were further dispersed by ~10 passes through a fire-polished glass Pasteur pipette. Cortical progenitor cells were centrifuged, resuspended in plating media (see below), and seeded onto BioFlex Silastic plates (Flexcell International Corp.; Burlington, NC, USA) at a density of 1.5×106/well. In pilot studies, using Neruobasal®/B27 (pH ~7.5–7.6 and supplemented with 25μM Glutamic acid, 0.5mM L-Glutamine, and 100Units/mL penicillin-streptomycin per manufacture instructions), we tested several plate coating-conditions including poly-D-lysine, Laminin, Collagen, and Matrigel on Silastic membranes. Only Matrigel improved adherence to Silastic plates in our hands.
Neurocult®/SM1 and Neurobasal®/B27 were also supplemented with 25μM Glutamic acid and 0.5mM L-Glutamine. The details germane to the preparation of plates are as follows: Frozen Matrigel stocks were thawed overnight at 4°C. ~1h prior to seeding cells, 0.5mg/mL Matrigel was diluted (vortexed ~20s) into ice-cold Opti-MEM media and 1mL/well immediately applied (hand-rocked under a laminar flow hood) to ensure an even coating across the entire Silastic surface. Plates were incubated in a 37°C/5% CO2 incubator for ~1h. Neuronal progenitor cells (resuspended in Neurocult®/SM1 or Neurobasal®/B27) were counted on a Cellometer (Nexcelom Bioscience; Lawrence, MA, USA). BioFlex plates were removed from the incubator just prior to cell seeding. Opti-MEM was quickly aspirated away by vacuum suction using sterile glass pipettes and care taken not to touch the membrane surface. 1.5×106 cells was added to each well in 2mL of plating media. Plates were gently rocked side-to-side a few times to ensure even distribution of cells across the membrane surface. Plates were returned to the 37°C/5% CO2 incubator and left undisturbed for 5d. On day in vitro (DIV) 5 and 8, wells were given ½ media exchange (1mL) with BrainPhys®/SM1 or Neurobasal®/B27; vendor instructions state that the first media swap (after plating in Neurocult®) is on DIV5 with BrainPhys®, to promote healthy synaptic activity in primary neurons. During media swaps, plates were removed from the incubator and set on a flat surface under the laminar flow hood (i.e. not tilted as to prevent partial exposure of monolayer neurons to air). 1mL was carefully aspirated off using a 1mL pipette tip and replaced with BrainPhys® or Neurobasal®; fresh media was gently/slowly added by dispensing downward along the plate wall. For injury studies, 750μL of conditioned media (100% C.M.) was collected on DIV9 from each well and mixed with an equal volume of fresh BrainPhys®/SM1 or Neurobasal®/B27 (i.e. to generate 50% C.M.). The remaining media in each well was aspirated away and replaced with 1mL 50% C.M. This was done to (1) further dilute remaining low levels of Neurocult such that injuries were done primarily under conditions of BrainPhys®, and (2) in order to correct for potential intra-well differences in final media volumes on DIV9 caused by uneven evaporative loss across culture days which otherwise could affect the accuracy of LDH analysis.
2.4 Mechanical Stretch-Injury
BioFlex plates were given a nitrogen-gas induced mechanical stretch using the Cell Injury Controller-II device (Custom Design & Fabrication Inc.; Sandston, VA, USA) and returned to 37°C/5% CO2. The injury level was set to either a 50ms/38% biaxial stretch, or a 50ms/54%, biaxial stretch. The % membrane stretch is directly related to the peak well-pressure (psi) achieved, which is recorded by a pressure transducer connected to the CIC device, and regulated by adjusting the source pressure (i.e. nitrogen burst). A peak well-pressure of 2.7psi induces 38% biaxial-stretch. A peak well-pressure of 4.0psi induces 54% biaxial-stretch. Supplementary Table 1–6 shows peak well-pressures achieved across all experiments. Controls were given a 1mL media adjustment (i.e. 50% C.M.) but were not injured. In lentivirus experiments, the 50% C.M. media was prepared separately for the empty-control vector vs. RBM5-overexpression vector; this was done in order to (1) account for any unknown soluble factors due to genotype which may impact injury outcome (e.g. RBM5 overexpression increases TNF-α expression in cancer cells (Wang et al., 2012)), and (2) account for possible differences in uninjured baseline LDH levels needed to accurately calculate % cytotoxicity.
Methods described by Bardy C. et al., and media preparation protocols provided by STEMCELL Technologies Inc., suggested the absence of penicillin-streptomycin antibiotics in BrainPhys®, which may disturb normal electrophysiology of neurons in vitro (Bahrami and Janahmadi, 2013). Thus we also did not include penicillin-streptomycin in Neurocult®/SM1 or BrainPhys®/SM1 medium. Several precautions were taken to maintain sterility. First, we established the CIC device inside a laminar flow hood. Thus all injuries were done under sterile conditions. Second, we limited examination of cells (for quick visualization) using a light microscope until just after the 24h post-injury time point (prior to sample collection on DIV10). BioFlex plates are designed such that the Silastic membrane is slightly elevated above the base of the plastic 6-well plate, which makes it difficult to observe cells using a standard inverted light microscope. The alternative is to use an upright light microscope (using the 10X objective) and carefully lower the objective inside the well (from above) until cells are in focus. This method of observing cells carries greater risk of culture contamination because the protective plate lid must be removed and because the unsterile lens/objective is precariously close to the culture media surface. For these reasons, cells were not visualized during culturing until just prior to sample collection. Using the above protocol as standard practice for each experiment, we observed highly consistent (healthy/viable/morphologically similar) cultures across all experiments.
2.5 LDH Analysis
LDH analysis was done as we reported (Jackson et al., 2013). In brief, at ~24h post-injury, plates were removed from the incubator and 100μL cell lysis buffer was added to an uninjured control (i.e. one well per plate or per virus genotype). Lysis buffer was aspirated up/down 10 times and plates returned to the 37°C/5% CO2 incubator for 10min. Plates were immediately set on ice. 400μL culture media (per well) was collected for each condition (i.e. uninjured control, lysed-uninjured control, or injured) and transferred into a 1.5mL tube. Samples were stored at −80C until downstream analysis. Thawed samples were centrifuged at 15min/4°C/16,000g. LDH release was measured using the LDH-Cytotoxicity Assay Kit II (Abcam) and analyzed on a GloMax-Multi Microplate reader (PROMEGA; Madison, WI, USA). Individual samples were run in duplicate (10μL/well). Negative control was 50% C.M. media prepared on DIV9. % cytotoxicity was calculated by the formula = (Sample LDH – Negative Control)/(Max LDH – Negative Control) × 100.
2.6 Chemical Stimulation of Synaptic Activity
Primary rat cortical neurons were seeded onto PDL coated polystyrene 6-well plates and maintained in Neurobasal®/B27 or Neurocult/SM1 for 5d. At DIV5 and DIV8 cultures were given ½ media exchange with fresh Neurobasal®/B27 or BrainPhys®/SM1, respectively. On DIV9, conditioned media was aspirated off and replaced with 1mL of prewarmed (37°C) 100% fresh Neurobasal®/B27 or BrainPhys®/SM1 containing either vehicle (water/DMSO) or drugs (1mM 4-AP + 25μM bicuculline). Experimental or vehicle treatments were applied for 15min. Cells were washed with ice cold PBS, immediately homogenized in RIPA buffer (with protease/phosphatase inhibitors), and pERK analyzed by Western blot to assess the extent of synaptic NMDAR activation.
Independent supplementary experiments were done to examine the effect of medium on 4AP/Bicuculine induced ERK activation in aged cultures, in both rat and mouse neurons. DIV19-20 primary mouse or rat cortical neurons were grown in Neurobasal®/B27, on PDL coated polystyrene 6-well plates, or grown in BrainPhys®/SM1 on laminin/poly-L-ornithine coated 6-well plates. On the respective day of experimentation, mixed neuron/glia cultures were treated for 15min with vehicle (water/DMSO) or 1mM 4-AP/25μM. Cells were washed with ice cold PBS, homogenized in RIPA buffer, and pERK levels analyzed by Western blot.
2.7 Western Blot
Western blots were done as reported but with minor modifications (Jackson et al., 2015). Matrigel was removed prior to homogenizing cells. The plates remained on ice during the entire procedure. The remaining media was aspirated off (i.e. immediately after collecting/storing media for LDH analysis). Cells were quickly washed once with 2mL/well of ice cold PBS (supplemented with magnesium and calcium). PBS was removed and replaced with 1mL of ice cold Cell Recovery Solution® and incubated (on ice) for 15mins. Cells were gently scraped off Silastic membranes and transferred to a 1.5mL tube. An additional 400μL Cell Recovery Solution® was added to each well and any remaining cells were carefully scraped off and added to the corresponding 1mL (i.e. for a final 1.4mL recovery solution/well in a 1.5mL tube). Cells were given an additional 45min on ice (i.e. 1h total). Tubes were inverted 2–3 times and subsequently centrifuged for 5min/4°C/200g. Recovery solution was carefully removed and discarded without disturbing cell pellet. 1mL ice cold PBS (+magnesium/+calcium) was added to the pellet. Tubes were inverted 2–3 times and subsequently centrifuged for 5min/4°C/200g. PBS was carefully removed and discarded without disturbing cell pellet. 65μL RIPA buffer (supplemented with EDTA and Protease/Phosphatase Inhibitors) was added to cell pellets and mixed by pipetting up/down ~10 times. Samples were stored at −80°C. In preparation for BCA analysis, samples were thawed on ice, sonicated ~20s, and centrifuged 15min/4°C/16,000g. Protein concentrations were measured by BCA as described previously.
10–20μg/well protein extract was loaded onto TGX-precast gels (BioRad; Hercules, CA, USA). Samples were run at 120V. Electrophoresed proteins were transferred to Hybond P 0.2 PVDF membrane (GE Healthcare Life Sciences; Pittsburgh, PA, US). In studies requiring total membrane stain, PVDFs were incubated in 100% methanol for 30s, stained with reversible Swift Membrane Stain (Fisher Scientific), images collected on a flatbed scanner, membranes de-stained for ~2–3min, and washed 5min in TBS. Membranes were blocked for 1h in Tris-buffered saline (TBS) containing Tween-20 (TBS-T) and 7.5% blotting grade milk. Primary antibodies were diluted in TBS-T/milk and incubated on blots overnight on a rocker at 4°C. Membranes were washed three times with TBS (5min per wash), incubated 2h with secondary antibody, washed an additional three times in TBS, incubated 1.5min in ECL detection reagent (ThermoFisher Scientific), and blots exposed to film in a dark room. Films were captured on a 600dpi flatbed scanner and complied in Photoshop (Adobe; San Jose, CA, USA).
2.8 High-Titer Lentivirus
BSL-2 work was approved by the Institutional Biosafety Committee of the University of Pittsburgh. Lentivirus expression plasmids were purchased from Origene (Rockville, MD, USA). We used the pLenti-C-Myc-DDK plasmid (Empty Control Vector, Cat# PS100064) and pLenti-C-Myc-DDK-RBM5_ORF plasmid (Cat# RR213857L1) to overexpress rat RBM5. Packaging plasmids were also purchased from Origene (Cat# TR3002P5). In brief, expression plasmids were amplified using a Maxiprep kit (QIAGEN; Germantown, MD, USA) to obtain high-quality plasmid DNA and concentrations were analyzed on a Genova Nano (JENWAY; Staffordshire, UK). HEK293Ta cells (Genecopoeia; Rockville, MD, USA) were propagated in T225 Flasks. At ~75–80% confluency, cells were transfected with expression plasmids and packing plasmids for ~12h, using MegaTran transfection reagent diluted in Opti-MEM/10%FBS/Penicillin-Streptomycin. Virus-containing media was collected ~36h and ~48h after transfection. Media was passed through a 0.45μM Nalgene filter, concentrated via a Centricon Plus-70 filter unit, and ultracentrifuged for 1.5h/4°C/24,000rpm on a swinging-bucket (SW28) ultra rotor (Beckman-Coulter; Indianapolis, IN, USA). The viral pellet was resuspended in ~200μL sterile Opti-MEM. Virus titer was calculated by measuring viral-associated p24 levels using the QuickTiter™ Lentivirus Titer Kit (Cell Biolabs Inc; San Diego, CA, USA). Neurons were transduced at a multiplicity of infection (MOI) 30 or 60 at the time of seeding (DIV0).
2.9 Immunofluorescence
DIV6 or 10 neurons were placed on ice. The cell culture media was discarded and cells washed with ice cold PBS (+magnesium/+calcium). Cells were immediately fixed with 4% paraformaldehyde and incubated at room temperature under a chemical fume hood for ~30min. Cells were washed once with PBS. In DIV10 stretch-injury experiments, for each 6-well plate, three wells (one condition per well – uninjured, one stretch, two stretch) were incubated with 0.1% Triton-X 100 diluted in PBS for 15min to permeabilize cells, and the other three wells (also encompassing all three conditions) were incubated for an equal time in plain PBS (without 0.1% Triton-X 100); this was done to test if permeabilization negatively or positively affected antibody detection of axonal cytoskeleton proteins NFL and Tau on Silastic surfaces after stretch-injury. Wells were washed three times with PBS, blocked 1h with 20% normal goat serum (NGS)/1% BSA in PBS, and incubated at 4°C overnight with primary antibodies in 3% NGS/PBS. Cells were washed three additional times in PBS and incubated 1.5h at room temperature with secondary antibodies (1:500) in 3% NGS/PBS. Cells were washed three additional times in PBS. PBS was removed one well at a time (in a dimly lit room), and the entire circumference of the Silastic membrane quickly cut out with a scalpel and allowed to drop onto a clean Styrofoam base set below. A small ~1cm×1cm square piece of Silastic membrane was carefully excised from the center of the disc. Using tweezers, square Silastic pieces were placed onto a microscope slide and one to two drops of ProLong® Gold Antifade with DAPI Mounting reagent applied to the top (cell facing) surface. This process was repeated until each slide contained one square piece of Silastic membrane corresponding to an uninjured control, one-stretch, and two-stretch (taken from the same plate). A coverslip was gently set on top of the Silastic squares and slides sealed with clear fingernail polish. Images were captured on a Nikon ECLIPSE Ts2R fluorescent microscope (Melville, NY, USA). Fig. 4–6 show antibody staining for cells given a standard membrane permeabilization step (i.e. non-permeabilized cells had the same staining patterns but weaker overall signal). Cells were also permeabilized for DIV6 studies. Representative images show morphologies which are characteristic across the circumference of wells.
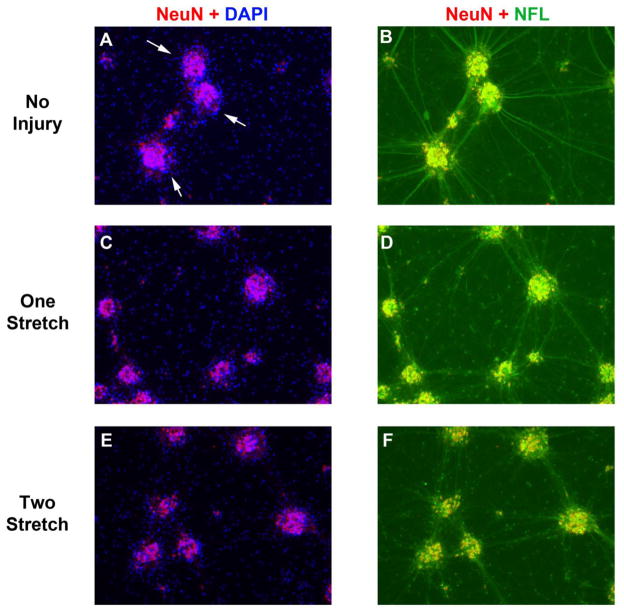
Fig. 4. NF-L Staining After a Single or Multiple Stretch-Injuries.
DIV9 cortical neurons were given a single or multiple 54% biaxial stretch-injuries. Immunofluorescence was done to detect the neuronal marker NeuN (Red) and axonal marker NF-L (Green) 24h later. (a, c, and e) Overlay of nuclear staining NeuN+ neuronal clusters with nuclear DAPI (Blue) in uninjured controls, 1X stretch-injured neurons, and 2X stretch-injured neurons. (b, d, and f) Co-localization of NeuN+ neuronal clusters with NFL+ somata/axons in uninjured controls, 1X stretch-injured neurons, and 2X stretch-injured neurons. Images show 10X magnification.
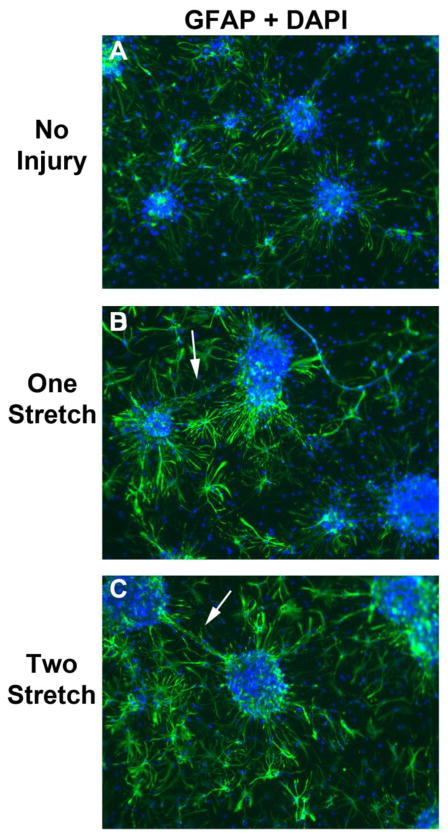
Fig. 6. GFAP Staining After a Single or Multiple Stretch-Injuries.
DIV9 cortical neurons were given a single or multiple 54% biaxial stretche-injuries. Immunofluorescence was done to detect the astrocyte marker GFAP (Green) 24h later, and overlayed with nuclear DAPI staining (Blue). (a, b, and c) Differences in GFAP staining/morphology comparing uninjured controls, 1X stretch-injured neurons, and 2X stretch-injured neurons. Images show 10X magnification.
2.10 Statistics
Western blot densitometry was analyzed using UN-SCAN-IT software (Silk Scientific; Orem, UT, USA). Statistical analysis of LDH results and Western blot densitometry was done in GraphPad Prism. Data were analyzed using a 1-Way-ANOVA followed by Newman-Keuls post-hoc (for multiple group comparisons) and by unpaired T-test (for two group comparison). Data were significant at p<.05. Graphs show mean + SEM.
3. RESULTS
3.1 Comparison of Major CNS Proteins in Primary Rat Neurons Maintained in BrainPhys® vs. Neurobasal Media
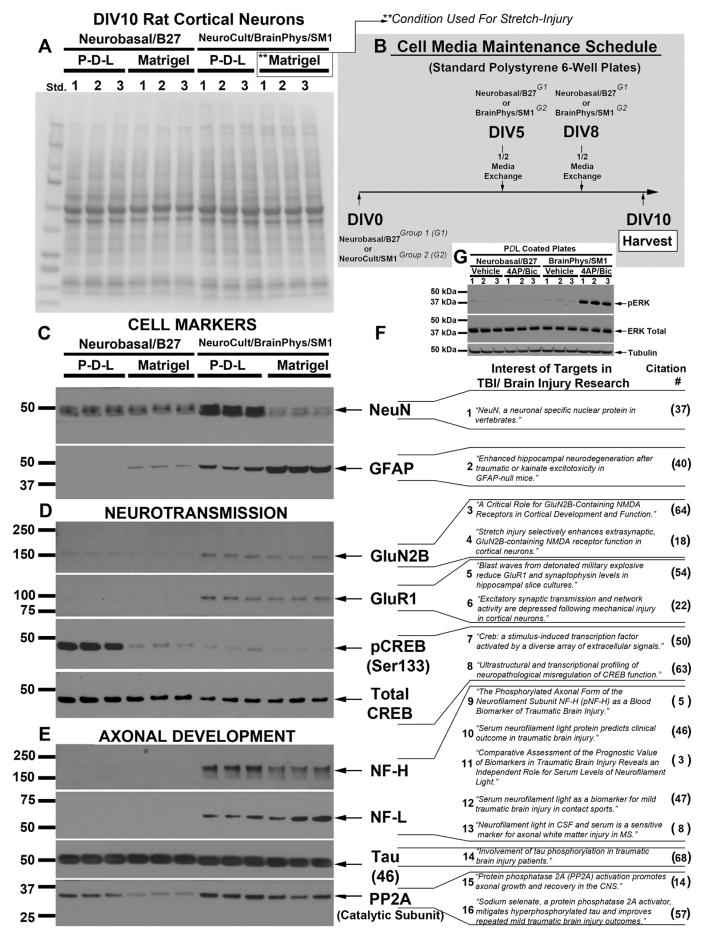
Primary rat cortical neurons, collected on the same isolation day, were grown on standard 6-well polystyrene plates coated with either Poly-D-Lysine (PDL) or Matrigel. All groups received identical media maintenance schedules across culture days (Fig. 1b), and were harvested on DIV10 for Western blot analysis (Fig. 1a; a representative total membrane stain shows equal protein loading/membrane-transfer across groups). Homogenates were analyzed with antibodies to investigate: cell-type specific markers (Fig. 1c), factors involved in neurotransmission (Fig. 1d), and axonal development (Fig. 1e). Fig. 1f shows examples in the literature of each target and its possible role in the setting of TBI and/or neurological injury (NeuN (Mullen et al., 1992), GFAP (Otani et al., 2006), GluN2B (Ferrario et al., 2013; Wang et al., 2011), GluR1(Goforth et al., 2011; Smith et al., 2016), CREB (Shaywitz and Greenberg, 1999; Valor et al., 2010), NF-H (Anderson et al., 2008), NF-L (Al Nimer et al., 2015; Bergman et al., 2016; Shahim et al., 2016; Shahim et al., 2017), Tau (Yang et al., 2017), and PP2A (Cheng et al., 2015; Tan et al., 2016)).
Fig. 1. Protein Changes in DIV10 Cortical Neurons Induced by Maintaining Cultures in Different Medium & Plating Conditions.
(a) Representative total membrane stain showing equal protein loading and membrane transfer across groups. (b) Diagram illustrating time course of culture maintainance in different media treatment groups. Western blots show differences in levels of (c) cell-type specific markers NeuN and GFAP, (d) proteins invovled in neurotransmission including GluN2B, GluR1, and CREB, (e) proteins involved in axonal development including NF-H, NF-L, Tau, and PP2A. (f) Examples in the literature of each target and their importance in the setting of TBI research. (g) Western blot shows stimulation of synaptic activity-induced ERK activiation with 4AP/Bicuculline in DIV9 neurons maintained in Neurobasal®/B27 or BrainPhys®/SM1.
To confirm in primary rat cortical neurons that BrainPhys®/SM1 enhances synaptic activity vs. Neurobasal®/B27, and by the DIV9 time point, plates were treated 15min with a potassium channel blocker (4AP) and GABAA inhibitor (Bicuculline), or vehicle control, and pERK measured by Western blot (Fig. 1G). Stimulation of synaptic activity with 4AP/Bicuculline robustly increased pERK in BrainPhys®/SM1 maintained cultures but not in Neurobasal®/B27 (Fig. 1G). The same results were observed in aged primary mouse cortical neurons and in aged rat neurons (Supplementary Fig. 2).
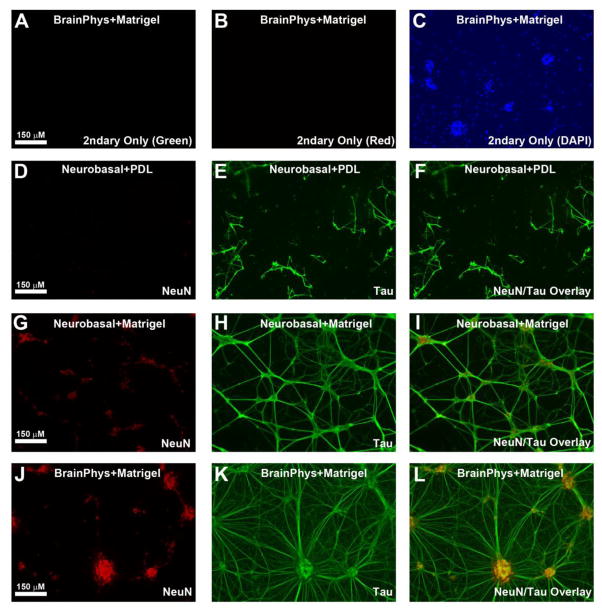
The deformable properties of Silastic membranes are ideal to study the effect of mechanical forces on cells but some cells (like neurons) do not strongly adhere to the Silastic substrate (Ahmed et al., 2000; Cavalcanti-Adam et al., 2002; Slemmer et al., 2002). In pilot studies we tested if various coating conditions (e.g. standard PDL, Laminin, Collagen, or Matrigel) enhance neuronal attachment. Fig. 2 shows the best (i.e. Matrigel) and worst (i.e. PDL) coating conditions in our hands for increasing attachment of DIV6 neurons to the Silastic membrane. Neurobasal®/B27 maintained neurons grown on PDL treated Silastic membrane weakly adhered to the substrate, and usually began to detach (in large sheets) after the first ½ media swap (Fig. 2d–f). Neurobasal®/B27 maintained neurons grown on Matrigel treated Silastic membrane have improved attachment and preferentially exhibited a sparse lattice-like morphology of neuron clusters connected by long axons (Fig. 2g–i). That phenomenon is reported to occur for neurons grown on Matrigel matrix compared to other plating substrates and may partially be mediated by laminin (Sun et al., 2012) Neurocult®/BrainPhys®/SM1 maintained neurons grown on Matrigel treated Silastic membrane strongly adhere to the substrate and developed extensive bundles of long and short axons (Fig. 2j–l).
Fig. 2. The Effect of Medium & Plating Conditions on DIV6 Cortical Neuron Adherance and Morphology on Deformable Silastic Membranes.
DIV6 neurons were grown on Silastic membranes and analyzed by immunofluroresence to detect NeuN (Red) and Tau (Green). (a–b) Secondary only controls show absence of Red/Green signal at equivalent exposures used in subsequent panels. (c) DAPI+ staining (Blue) confirm presence of cells in secondary only controls. (d–f) Detection of NeuN (Red) and Tau (Green) in Neurobasal®/B27 maintained neurons grown on PDL coated Silastic membranes. Cells easily detached under these condtions. (g–i) Detection of NeuN (Red) and Tau (Green) in Neurobasal®/B27 maintained neurons grown on Matrigel coated Silastic membranes. Cells more readily attach under these conditions, and tend to show a lattice-like pattern. (j–l) Detection of NeuN (Red) and Tau (Green) in Neurocult®/BrainPhys®/SM1 maintained neurons grown on Matrigel coated Silastic membranes. Cells strongly attach under these conditions and axons (large-caliber and small) are widespread. Images show 10X magnification.
3.2 Effect of a 38% vs. 54% Biaxial Stretch-Injury on BrainPhys® Maintained Cultures
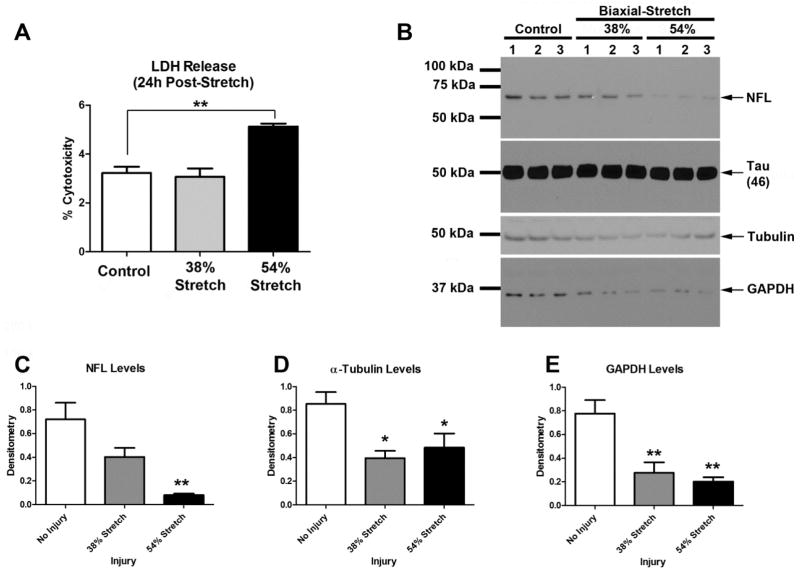
DIV9 cortical neurons were given a single stretch-injury. LDH levels were analyzed 24h later. LDH levels were equivalent in uninjured controls vs. cultures subjected to a 38% stretch-injury (Fig. 3a). In contrast, 24h LDH levels were significantly higher in cultures given a 54% stretch-injury (~1.9% above uninjured controls); notably, this represents only a minor increase in LDH given that % cytotoxicity can reach a maximum of 100 (i.e. on the y-axis) nevertheless it confirms that 54% stretch-injury is sufficient to induce cell damage under our culture conditions.
Fig. 3. Protein Changes Induced by Mechanical Stretch-injury to Neurons Cultured on BrainPhys®/SM1+Matrigel Silastic Membranes.
Day in vitro (DIV) 9 cortical neurons were given a 38% or 54% biaxial stretch, and cell culture media plus protein extracts were harvested 24h later (n=3/group). (a) LDH levels were significantly higher in cultures given a 54% stretch-injury versus uninjured controls, or those given a 38% stretch-injury. (b) Western blots show the effect of mechanical stretch on NF-L, Tau, α-Tubulin, and GAPDH levels at 24h post-injury. (c–e) Densitometric analysis of NF-L, α-Tubulin, and GAPDH, respectively (n=3/group). Data were anlayzed by 1-WAY-ANOVA followed by Newman-Keuls post-hoc. Data were significant at p<.05. Graphs show mean +SEM. (*) = p<.05 versus uninjured control; (**) = p>.001 versus uninjured control.
Next we measured changes in proteins associated with axonal injury. Specifically we focused on NF-L and Tau due to major clinical interest in these targets as injury biomarkers. A single 54% stretch-injury robustly decreased NF-L protein levels whereas the abundant protein Tau was unaffected (Fig. 3b and 3c). We also observed significant decreases in the levels of α-Tubulin (Fig. 3d) and GAPDH (Fig. 3e) at both the 38% and 54% stretch-injury levels. Findings on α-Tubulin are not surprising. Tubulin (α and β) are major components of the axonal cytoskeleton (Kevenaar and Hoogenraad, 2015). Also, in vivo studies show that mTBI mediated axonal injury in mice leads to reduced Tubulin levels in damaged nerves (Tzekov et al., 2016).
Further characterization efforts in BrainPhys® maintained cultures focused on the 54% stretch-injury level because it induced greater molecular changes compared to uninjured controls (specifically with regard to NF-L). Histological changes were examined in cultures given a single stretch-injury versus those given two injuries (in tandem and administered 1h apart). Similar to findings previously observed in DIV6 cultures (Fig. 2), DIV10 neurons were clustered into groups and surrounded by dense axons (Fig. 4a; White arrows show NeuN/DAPI overlay). Large-caliber axonal projections connected neuronal clusters and had strong NF-L+ staining in uninjured controls (Fig. 4b). A single stretch-injury did not affect NeuN+ neuronal clusters (which remained firmly attached to the Silatic membrane) but caused a severe loss of NF-L staining (Fig. 4c and 4d). Two stretch injuries appeared to augment loss of NF-L+ axons (Fig. 4e and 4f).
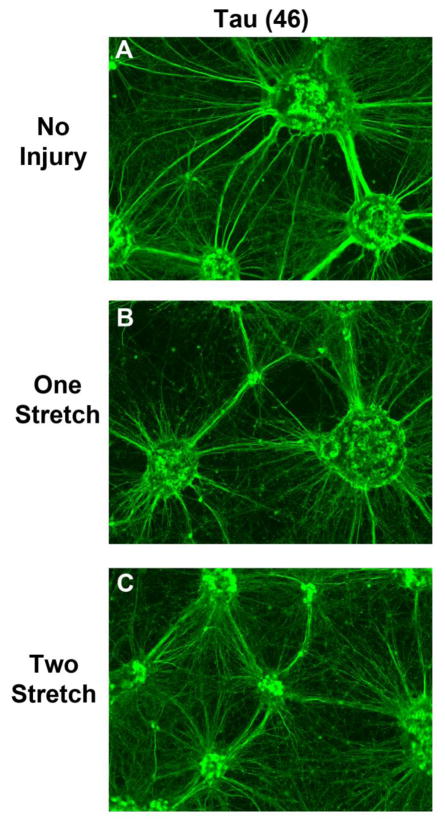
Tau staining (as previously seen) revealed a mass of high complexity short and long axonal processes in DIV10 cultures (Fig. 5a). Tau staining also appeared to show a loss of signal relative to uninjured controls (particularly in long axons) after stretch-injury (Fig. 5b and 5C). We used the Tau 46 monoclonal antibody which is well characterized and its target specificity has been confirmed in Tau KO mice (Petry et al., 2014).
Fig. 5. Tau Staining After a Single or Multiple Stretch-Injuries.
DIV9 cortical neurons were given a single or multiple 54% biaxial stretche-injureis. Immunofluorescence was done to detect the axonal marker Tau (Green) 24h later. (a, b, and c) Differences in Tau staining/morphology comparing uninjured controls, 1X stretch-injured neurons, and 2X stretch-injured neurons. Images show 10X magnification.
Compared to other plating conditions (Fig. 1c), GFAP levels were highest in BrainPhys®+Matrigel. Therefore we also examined the effect of stretch-injury on astrocyte activation under BrainPhys® culture conditions. GFAP staining was relatively homogenous (low-moderate expression) in astrocytes directly adjacent to neuron clusters and in areas further removed from neuron clusters (Fig. 6a). Stretch-injury markedly increased GFAP staining intensity particularly in astrocytes directly adjacent to neuron clusters (Fig. 6b and 6c). We also observed what appeared to be astrocytic foot processes contacting some of the injured large-caliber axons projecting between neuronal clusters (White Arrows).
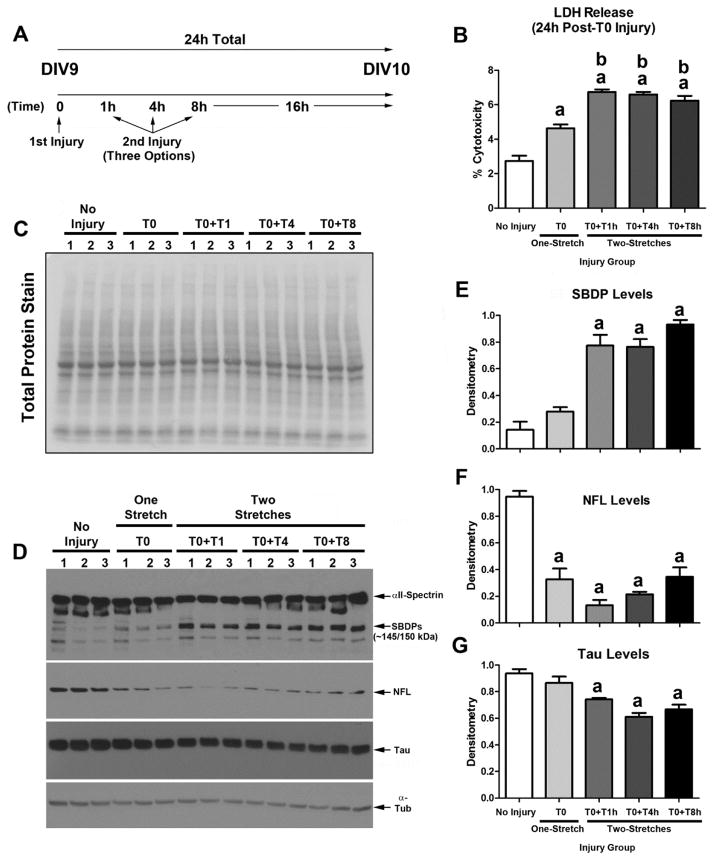
3.3 Multiple Stretches Exacerbate Damage in BrainPhys® Maintained Cultures
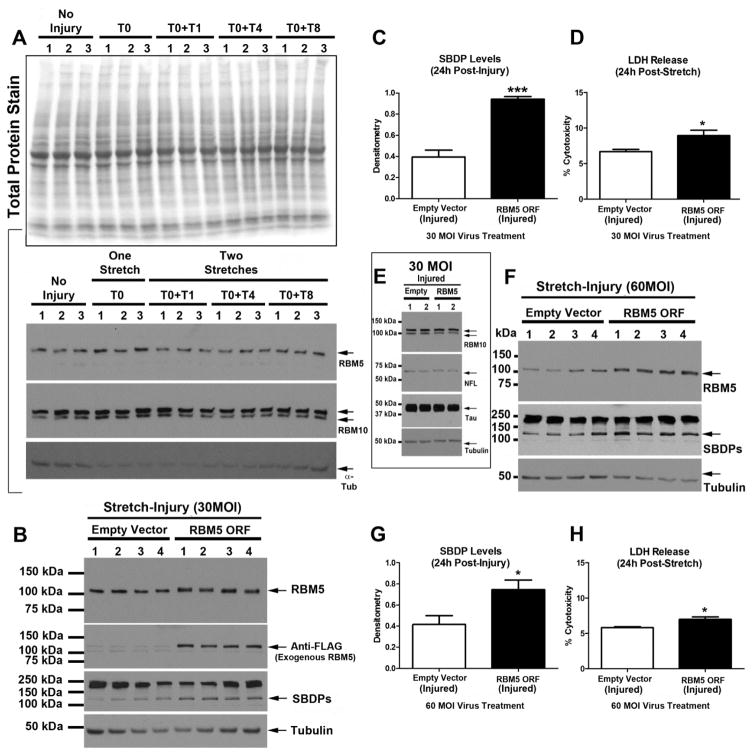
Immunohistological assessment of NF-L suggested that multiple stretch injuries exacerbate axonal injury (Fig. 4). To quantify decreased levels of axonal markers under different injury conditions, we measured protein changes in NF-L and Tau by Western blot in DIV10 cultures. Furthermore, we tested if a longer delay between the first and second stretch-injury (i.e. by 1h vs. 4h vs. 8h) also affected cell death outcomes or markers of axonal injury (Fig. 7a). 24h LDH release in cultures given two injuries was significantly increased relative to uninjured controls or vs. cultures given a single stretch (Fig. 7b). We used a reversible PVDF membrane stain to show equal protein loading across groups (Fig. 7c). Next, as an additional marker of cell damage (i.e. in addition to LDH) we measured αII-Spectrin breakdown products (SBDPs), as well as markers of axonal injury including NF-L and Tau (Fig. 7d). Two stretch-injuries led to a significant increase in 24h 145/150kDa SBDP levels vs. uninjured controls (Fig. 7e). Furthermore, as expected, two stretch-injuries significantly decreased NF-L levels vs. uninjured controls but did not differ from cultures given a single stretch-injury (Fig. 7f). Tau by contrast was significantly decreased (vs. uninjured controls) only in cultures given two stretch-injuries (Fig. 7g).
Fig. 7. Protein Changes Induced by Mutlple Stretch-injuries in BrainPhys®/SM1+Matrigel Maintained Cultures.
DIV9 cortical neurons were given a single or multiple 54% biaxial stretch-injuries, and cell culture media plus protein extracts were harvested 24h later (n=3/group). (a) Diagram showing a time course of experimental injury variables. T0 = 1st injury which is denoted as time zero. T1h = a 2nd injury adminstered at 1h after the first injury. T4h = a 2nd injury adminstered at 4h after the first injury. T8h = a 2nd injury adminstered at 8h after the first injury. Samples were collected 24h from the start of T0 in all groups. (b) LDH levels were significantly higher in cultures given a single 54% stretch-injury versus uninjured controls. LDH levels were further augmented by multiple stretch-injuries. (c) Representative total membrane stain shows equal protein loading/transfer of samples across groups. (d) Westerm blots show the effect of stretch-injury on αII-Spectrin breakdown products (SBDPs), NFL, Tau, and Tubulin. (e–g) Densitometric analysis of SBDPs, NF-L, and Tau, respectively (n=3/group). Data were anlayzed by 1-WAY-ANOVA followed by Newman-Keuls post-hoc. Data were significant at p<.05. (a) = a significant post-hoc difference comparing injury group versus uninjured control. (b) = a significant post-hoc difference comparing the single stretch-injury group (T0 only) versus a multiple stretch-injury group. Graphs show mean +SEM.
3.4 Neurobasal®/B27 Exacerbates Markers of Damage in Stretch-Injured Neurons
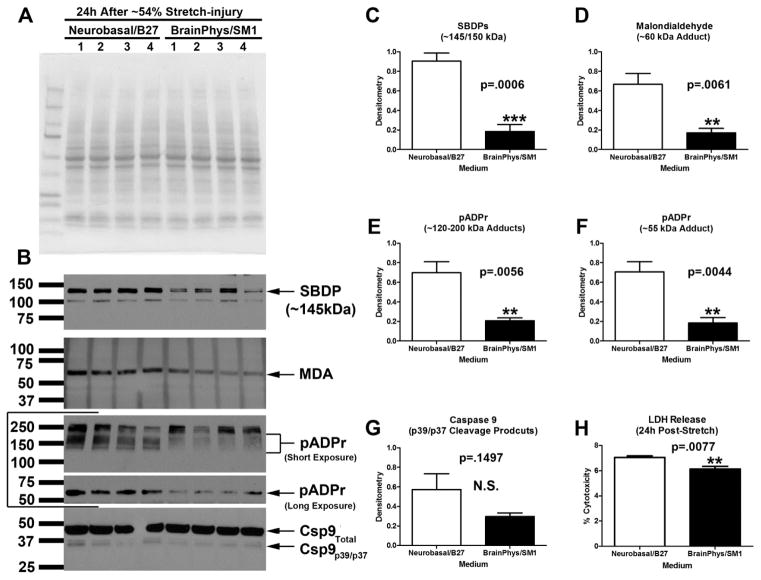
Next we compared the effect of media on 24h post-injury outcomes after a single ~54% stretch. Extracts were collected from Neurobasal®/B27 or BrainPhys®/SM1 maintained cultures and analyzed by Western blot. Protein loading was equal across groups (Fig. 8A). Levels of SBDPs (Fig. 8B and 8C), MDA (Fig. 8B and 8D), and pADPr (Fig. 8B, 8E, and 8F) were significantly increased in injured Neurobasal®/B27 maintained cultures vs. BrainPhys®/SM1. Levels of cleaved (active) caspase-9 were not significantly different between groups (Fig. 8B and 8G). 24h LDH release was significantly increased in injured Neurobasal®/B27 maintained cultures vs. BrainPhys®/SM1.
Fig. 8. Markers of Damage in Stretch-injured Cultures Maintained in BrainPhys®/SM1 vs. Neurobasal®/B27.
DIV9 cortical neurons were given a single 54% biaxial stretch, and cell culture media plus protein extracts were harvested 24h later (n=4/group). (a) A representative total protein stain shows equal protien loading/membrane transfer across samples. (b) Western blots show levels of SBDP, MDA adducts, pADPr adducts, and activated caspase-9. (c–g) Densitometric analysis of SBDPs, MDA, pADPr (high molecular weight adducts), pADPr (low molecular weight adducts), and caspase-9, respectively (n=4/group). (h) 24h post-injury LDH levels were significnatly higher in cultures given a single 54% stretch-injury and maintained in Neuobasal®/B27 vs. those maintained in BrainPhys®/SM1. Data were anlayzed by unpaired T-test. Data were significant at p<.05. (**) = p<.01. (***) = p<.001. Graphs show mean +SEM.
3.5 Investigation of Novel Therapeutic Targets in BrainPhys® Maintained CNS Cultures
We reported that the nuclear splicing factor RBM5, which is well-known to promote cell death in cancer cells, increases in neurons after a TBI (Jackson et al., 2015). It has not been reported if increased RBM5 levels are directly toxic in primary CNS cells. We first examined if endogenous RBM5 levels, or its paralogue RBM10 (which is also increased after a TBI in vivo (Jackson et al., 2015)), increases in BrainPhys® maintained cultures after a stretch-injury (Fig. 9a). RBM5/RBM10 levels did not increase 24h after a mechanical stretch-injury, which is in contrast to observations after a severe TBI in vivo (Jackson et al., 2015).
Fig. 9. RBM5 Overexpression Increases Vulnerability of Primary CNS Cells to Stretch-Injury.
(a) Total protein stain shows equal protein loading/transfer across groups of non-transduced DIV9 cortical neurons subjected to a single or multiple stretch-injuries. Western blots show levels of RBM5 and RBM10 before and after injury. (b) DIV0 cortical neurons were transduced with 30MOI lentivirus to deliver either an empty-vector (control) or RBM5 open reading frame (ORF, n=4/group). Both virus-control and RBM5-ORF groups were subjeted to a single 54% biaxial stretch on DIV9, and cell culture media plus protein extracts were harvested 24h later (n=4/group). Western blots shows RBM5, anti-FLAG, and SBDPs levels. (c) Densitometric analysis of SBDP levels in injured 30MOI control vs. RBM5-ORF groups (n=4/group). (d) 24h post-injury LDH levels in 30MOI control vs. RBM5-ORF groups (n=4/group). (e) Western blots show RBM10, NFL, and Tau in injured 30MOI control vs. RBM5-ORF groups (n=2/group). (f) DIV0 cortical neurons were transduced with 60MOI lentivirus to deliver either an empty-vector (control) or RBM5-ORF (n=4/group). Both virus-control and RBM5-ORF groups were subjeted to a single 54% biaxial stretch on DIV9, and cell culture media plus protein extracts were harvested 24h later (n=4/group). Western blots shows RBM5 and SBDPs levels. (g) Densitometric analysis of SBDP levels in injured 60MOI control vs. RBM5-ORF groups (n=4/group). (h) 24h post-injury LDH levels in 60MOI control vs. RBM5-ORF groups (n=4/group). Data were anlayzed by unpaired T-test. Data were significant at p<.05. Graphs show mean +SEM. (*) = p<.05; (***) = p>.0001.
Next we tested if forced RBM5 overexpression increased vulnerability of CNS cultures to a subsequent mechanical trauma. Neurons were transduced on DIV0 (day of plating) with 30MOI lentivirus to deliver either an empty-vector control or a vector containing the open reading frame (ORF) of the rat RBM5 gene (RBM5 ORF). Exogenous RBM5 has a DDK tag, detected by anti-FLAG antibodies, and used to distinguish endogenous vs. exogenous RBM5. RBM5 levels were mildly increased at 30MOI compared to empty vector controls (Fig. 9b). An anti-FLAG antibody confirmed the presence of exogenous RBM5. RBM5 overexpressing and control-vector neurons were given a single 54% stretch-injury. RBM5 overexpressing neurons had markedly increased SBDP levels (Fig. 9b and 9c) and modestly, albeit significantly, increased LDH levels (Fig. 9d) 24h post-injury. Markers of axonal damage were not affected by RBM5 overexpression in injured cultures (Fig. 9e). Finally, we tested if higher levels of RBM5 further augmented damage after stretch-injury. Transduction of neurons with 60MOI lentivirus led to a ~2-fold increase in RBM5 levels vs. empty vector controls (Fig. 9f). Both 24h SBDP levels (Fig. 9g) and LDH levels (Fig. 9h) were significantly higher in stretch-injury RBM5 overexpressing neurons. However, 60MOI did not appear to further augment the overall level of injury (e.g. 24h LDH) relative to 30MOI.
4. DISCUSSION
4.1 BrainPhys®+Matrigel Facilitates the Investigation of Neuronal & Axonal-Injury In Vitro
Here we report that BrainPhys® facilitates the investigation of stretch-injured neurons/axons grown on Silastic membranes in vitro. Furthermore, we demonstrate the utility of BrainPhys®+Matrigel cultures for gene modification experiments to explore the function(s) of novel targets in the setting of trauma. Regarding the characterization of molecular changes before and after a stretch-injury in this system, several findings are noteworthy. First, we readily observe robust NF-L staining in “large-caliber” axons (also described as “thick ropelike fascicles” in the in vitro literature (Kosik and Finch, 1987)) linking clusters of neurons. Not surprisingly, NF-L+ axons are easily damaged by mechanical stretch, reflected by a robust loss of NF-L staining and a marked decrease in NF-L protein levels. Germane to young Neurobasal®/B27 maintained cultures studied here, which by comparison have much lower NF-L levels at DIV10 but still produce axons, axonal development is not compromised by the absence of NF-L. Indeed, NF-L KO mice have no overt phenotype in early development but show a shift from large to small caliber axons, and also have a marked loss of NF-H levels (Zhu et al., 1997), which was also seen in Neurobasal®/B27 cultures. Future studies are needed to determine if robust NF-L expression in BrainPhys®/SM1 is somehow linked to increased synaptic activity, particularly since NFs modulate neurotransmission in vivo (Yuan et al., 2015).
Tau staining in contrast localized to both “large-caliber” and to numerous smaller axons. Tau levels did not decrease after a single 54% stretch-injury but did decrease after multiple stretches. We also observed a reproducible decrease in α-Tubulin levels post-injury in BrainPhys®+Matrigel maintained cultures. Tubulin is a major constituent of the axon cytoskeleton (Kevenaar and Hoogenraad, 2015). Thus loss of α-Tubulin after mechanical stretch agrees with findings on reductions in Tau/NF-L here, and supports the notion that this system may be particularly well suited for investigation of subtle molecular changes associated with axonal injury.
4.2 Protein Differences in BrainPhys® vs. Neurobasal® Maintained Cultures
We were surprised by the magnitude of protein changes observed in BrainPhys® vs. Neurobasal® maintained cultures (Fig. 1). Future studies using large-scale 2D gel and/or RNA-seq analysis are needed to better describe the global proteomic/translatome changes in neurons maintained in different medium and at different culture ages. Culture media, which establishes the extracellular environment in vitro, is a major factor which regulates (to a large degree) variability in global gene expression in cells (Guo et al., 2016). Thus, it is not surprising that different neuronal culture media might differentially effect protein expression in neurons; nonetheless the magnitude of changes was considerable. Furthermore, Neurobasal®/B27 enhanced markers of pathology after stretch-injury vs. BrainPhys®. These included increased 24h LDH release (cell death), calpain activation (SBDPs), PARP activity (pADPr adducts), and toxic lipid peroxidation (MDA adducts). These findings are consistent with work by others indicating that Neurobasal® promotes excitotoxicity (Hogins et al., 2011).
Several study limitations must be acknowledged. First, Neurocult®/SM1 may contribute to the differences observed in BrainPhys® maintained neuron cultures, which was not dissected in detail here. Given that this was an initial exploration of the use of BrainPhys® we felt it logical to follow the culture protocol as described by the vendor (i.e. first 5d in Neurocult®/SM1 and thereafter ½ media swaps with BrainPhys®/SM1 at 3d intervals). It is recommend that neurons be cultured in Neurocult®/SM1 initially because it promotes differentiation of embryonic neural progenitors into primary neurons; however, it is unclear if shorter periods of time in Neurocult®/SM1 would be sufficient to induce differentiation and allow for the switch to BrainPhys® sooner. Furthermore, future studies should build upon the foundation of findings here by examining gene expression differences induced by Neurobasal® vs. Neurocult® vs. BrainPhys® vs. DMEM and ± the many different culturing substrates (plastics and coatings) commonly used.
Second, our work does not indicate that BrainPhys®/SM1 is better than Neurobasal®/B27 for in vitro research. Neurobasal®/B27 is a gold standard media and has certain benefits which BrainPhys® may not. For instance, Neurobasal®/B27 has long been touted for its ability to selectively promote neuron growth and limit glial proliferation. Our observation that DIV10 neurons on PDL plates in Neurobasal®/B27 had abundant levels of NeuN but lack GFAP corroborates prior studies indicating that Neurobasal®/B27 is advantageous for maintaining neuron enriched cultures. In contrast, neurons cultured on PDL plates in BrainPhys®/SM1 had NeuN as well as GFAP. Curiously, pCREB was robustly upregulated in pure neuron cultures (PDL plates in Neurobasal®/B27). We do not understand the underlying cause of that difference. Astrocytes are generally protective to neurons in vitro (e.g. for instance mixed glia/neuron cultures require higher levels of glutamate to induce excitotoxicity vs. pure neuron cultures (Amin and Pearce, 1997). We speculate that pure neuron cultures might compensate for the lack of astrocytes by activating self-protective programs, such as pCREB, which is well-known to induce numerous pro-survival genes such as BDNF and Bcl-2(Sakamoto et al., 2011). Together, our findings suggest that Neurobasal®/B27 vs. BrainPhys®/SM1 offer uniquely different advantages for the study of neurons in vitro.
Finally, the protein differences observed in Fig. 1 should be interpreted in the larger context of the developmental time course (which was not studied here). For instance, our findings show that GluN2B and GluR1 are higher in BrainPhys®/SM1 cultures vs. those maintained in Neurobasal®/B27. However, we would not anticipate that these proteins are absent in Neurobasal®/B27 cultures. Indeed, GluN2B and GluR1 are robustly expressed in older DIV20 mixed glia/neurons cultures maintained in Neurobasal®/B27 (Supplementary Fig. 1). Thus, BrainPhys®/SM1 may simply accelerate the development of neurons in vitro. On the other hand, consistent with electrophysiological findings by Bardy and collegues (Bardy et al., 2015), we found that stimulating synaptic activity, induced by a classic method using treatment with 4-AP/Bicuculline (Tauskela et al., 2008), is enhanced in BrainPhys®/SM1 maintained cultures vs. those in Neurobasal®/B27 (Fig. 1 and Supplementary Fig. 2). Therefore the relationship between levels of synaptic-related proteins (like GluN2B and GluR1) vs. the functionality of those synapses is unclear. However, our findings suggest that, at least in our hands, BrainPhys®/SM1 uniquely enhances synaptic activity.
4.3 RBM5 Increases the Vulnerability of CNS Cultures to a Subsequent Injury
A key rationale for establishing, and improving-upon, in vitro models which mimic mechanisms of brain injury seen in human patients, is ultimately to develop robust systems for elucidating the contribution of different genes on outcome in the hopes of uncovering new therapeutic targets. RBM5 is a pro-death protein in cancer cells. Its overexpression in malignant cells (1) slows their rate of growth, and (2) increases their vulnerability to die by a subsequent insult (Kobayashi et al., 2011; Loiselle et al., 2016; Rintala-Maki and Sutherland, 2004; Shao et al., 2013; Shao et al., 2012; Su et al., 2016). We previously reported that RBM5 protein levels increase in the mouse brain after a severe TBI (Jackson et al., 2015). Spinal cord trauma also induces neuronal RBM5 expression in rodents (Zhang et al., 2015). However, a pro-death role of RBM5 in primary neurons has yet to be established. Here we confirm that increased RBM5 levels, by lentivirus mediated overexpression, enhances vulnerability to a mechanical insult.
As expected, increasing RBM5 levels led to significantly higher 24h LDH and SBDPs following a single stretch-injury vs. empty-vector controls. These findings show for the first time that RBM5 plays a pro-death role in primary CNS cells. Furthermore, the results demonstrate that BrainPhys®/SM1 is highly amendable to gene manipulation studies, and can theoretically be used to broadly learn more about any molecular target of interest in the context of a mechanical injury. Notably, a key limitation, or advantage depending on the experimental context, addressed in-depth above but which also relates here, is that BrainPhys®/SM1 promotes neuronal and glial survival. Thus more studies are needed to test the pro-death effects of RBM5 overexpression in neuron enriched Neurobasal®/B27 cultures using other types of insults.
In the absence of viral manipulations we did not observe increased RBM5 levels 24h post-injury, which is in contrast to prior findings in a model of severe TBI (Jackson et al., 2015). Thus, the level of injury in our stretch model may need to be increased to trigger endogenous RBM5 upregulation. Moreover, additional pathological mechanisms after an in vivo injury (e.g. hemorrhage, edema, and inflammation) are not replicated in vitro and may also contribute to RBM5 upregulation in vivo. Thus, future studies are warranted to improve upon the stretch-model detailed here by simulating secondary injuries seen after a TBI as well, such as by adding microglia to BrainPhys®/SM1 cultures, which may promote inflammatory-like injury.
Supplementary Material
Highlights.
BrainPhys®/SM1 increases the expression of axonal proteins.
BrainPhys®/SM1 increases the expression of proteins involved in neurotransmission.
Stretch-injury decreases Neurofilament light chain and Tau levels.
Neurobasal®/B27 Exacerbates Stretch-Injury.
Lentivirus mediated overexpression of pro-death RBM5 exacerbates stretch-injury.
Acknowledgments
This work was supported by NIH/NINDS grant R21NS088145 to TCJ and by grant R21NS098057 to TCJ.
Footnotes
Author Contributions: TCJ conceived and designed the experiments. TCJ and SEK performed experiments. TCJ, PMK, EKJ contributed to data analysis and interpretation. TCJ, PMK, EKJ, and SEK contributed to writing the manuscript.
Conflict of Interest: Travis. C. Jackson and Patrick M. Kochanek are Inventors on a patent titled “Small Molecule Inhibitors of RNA Binding Motif (RBM) Proteins for the Treatment of Acute Cellular Injury” (USPTO Patent No# 9,610,266; Assignee: Office of Technology Management of the University of Pittsburgh). The authors declare no other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J Neurochem. 2000;74:1951–1960. [PubMed] [Google Scholar]
- Ahmed SM, Weber JT, Liang S, Willoughby KA, Sitterding HA, Rzigalinski BA, Ellis EF. NMDA receptor activation contributes to a portion of the decreased mitochondrial membrane potential and elevated intracellular free calcium in strain-injured neurons. Journal of neurotrauma. 2002;19:1619–1629. doi: 10.1089/089771502762300274. [DOI] [PubMed] [Google Scholar]
- Al Nimer F, Thelin E, Nystrom H, Dring AM, Svenningsson A, Piehl F, Nelson DW, Bellander BM. Comparative Assessment of the Prognostic Value of Biomarkers in Traumatic Brain Injury Reveals an Independent Role for Serum Levels of Neurofilament Light. Plos One. 2015;10:e0132177. doi: 10.1371/journal.pone.0132177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin N, Pearce B. Glutamate toxicity in neuron-enriched and neuron-astrocyte co-cultures: effect of the glutamate uptake inhibitor L-trans-pyrrolidine-2,4-dicarboxylate. Neurochemistry international. 1997;30:271–276. doi: 10.1016/s0197-0186(96)00092-7. [DOI] [PubMed] [Google Scholar]
- Anderson KJ, Scheff SW, Miller KM, Roberts KN, Gilmer LK, Yang C, Shaw G. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. Journal of neurotrauma. 2008;25:1079–1085. doi: 10.1089/neu.2007.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami F, Janahmadi M. Antibiotic supplements affect electrophysiological properties and excitability of rat hippocampal pyramidal neurons in primary culture. Iranian biomedical journal. 2013;17:101–106. doi: 10.6091/ibj.11242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, Bang AG, Mertens J, Bohnke L, Boyer L, Simon S, Gage FH. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. P Natl Acad Sci USA. 2015;112:E2725–E2734. doi: 10.1073/pnas.1504393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Dring A, Zetterberg H, Blennow K, Norgren N, Gilthorpe J, Bergenheim T, Svenningsson A. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol-Neuroimmunol. 2016:3. doi: 10.1212/NXI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsych Soc. 2004;10:794–806. doi: 10.1017/S1355617704105146. [DOI] [PubMed] [Google Scholar]
- Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic brain injuries. Nature reviews Disease primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- Bolton Hall AN, Joseph B, Brelsfoard JM, Saatman KE. Repeated Closed Head Injury in Mice Results in Sustained Motor and Memory Deficits and Chronic Cellular Changes. Plos One. 2016;11:e0159442. doi: 10.1371/journal.pone.0159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. Journal of neuroscience research. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Cavalcanti-Adam EA, Shapiro IM, Composto RJ, Macarak EJ, Adams CS. RGD peptides immobilized on a mechanically deformable surface promote osteoblast differentiation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002;17:2130–2140. doi: 10.1359/jbmr.2002.17.12.2130. [DOI] [PubMed] [Google Scholar]
- Cheng P, Chen K, Yu W, Gao S, Hu S, Sun X, Huang H. Protein phosphatase 2A (PP2A) activation promotes axonal growth and recovery in the CNS. Journal of the neurological sciences. 2015;359:48–56. doi: 10.1016/j.jns.2015.10.025. [DOI] [PubMed] [Google Scholar]
- DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A, Bullock R, Hamm RJ. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. Journal of neurotrauma. 2002;19:427–438. doi: 10.1089/08977150252932389. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Mckinney JS, Willoughby KA, Liang S, Povlishock JT. A New Model for Rapid Stretch-Induced Injury of Cells in Culture - Characterization of the Model Using Astrocytes. Journal of neurotrauma. 1995;12:325–339. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Willoughby KA, Sparks SA, Chen T. S100B protein is released from rat neonatal neurons, astrocytes, and microglia by in vitro trauma and anti-S100 increases trauma-induced delayed neuronal injury and negates the protective effect of exogenous S100B on neurons. J Neurochem. 2007;101:1463–1470. doi: 10.1111/j.1471-4159.2007.04515.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Ndukwe BO, Ren J, Satin LS, Goforth PB. Stretch injury selectively enhances extrasynaptic, GluN2B-containing NMDA receptor function in cortical neurons. Journal of neurophysiology. 2013;110:131–140. doi: 10.1152/jn.01011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh MH, Baghbanzadeh A, Barin A, Salar-Amoli J, Dehghan MM. Effects of different culture media on optimization of primary neuronal cell culture for in vitro models assay. Iranian Journal of Veterinary Medicine. 2015;9:163–170. [Google Scholar]
- Goddeyne C, Nichols J, Wu C, Anderson T. Repetitive mild traumatic brain injury induces ventriculomegaly and cortical thinning in juvenile rats. Journal of neurophysiology. 2015;113:3268–3280. doi: 10.1152/jn.00970.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Ellis EF, Satin LS. Enhancement of AMPA-mediated current after traumatic injury in cortical neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:7367–7374. doi: 10.1523/JNEUROSCI.19-17-07367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Ren J, Schwartz BS, Satin LS. Excitatory synaptic transmission and network activity are depressed following mechanical injury in cortical neurons. Journal of neurophysiology. 2011;105:2350–2363. doi: 10.1152/jn.00467.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo GJ, Pinello L, Han XP, Lai SJ, Shen L, Lin TW, Zou KY, Yuan GC, Orkin SH. Serum-Based Culture Conditions Provoke Gene Expression Variability in Mouse Embryonic Stem Cells as Revealed by Single-Cell Analysis. Cell Rep. 2016;14:956–965. doi: 10.1016/j.celrep.2015.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill JA, Robinette BL, Freudenrich TM, Mundy WR. Media formulation influences chemical effects on neuronal growth and morphology. In vitro cellular & developmental biology. Animal. 2015;51:612–629. doi: 10.1007/s11626-015-9873-3. [DOI] [PubMed] [Google Scholar]
- Hogins J, Crawford DC, Zorumski CF, Mennerick S. Excitotoxicity Triggered by Neurobasal Culture Medium. Plos One. 2011:6. doi: 10.1371/journal.pone.0025633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. Basic science; repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: a preliminary report. Journal of neurotrauma. 2007;24:15–27. doi: 10.1089/neu.2006.0072. [DOI] [PubMed] [Google Scholar]
- Jackson TC, Du L, Janesko-Feldman K, Vagni VA, Dezfulian C, Poloyac SM, Jackson EK, Clark RS, Kochanek PM. The nuclear splicing factor RNA binding motif 5 promotes caspase activation in human neuronal cells, and increases after traumatic brain injury in mice. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:655–666. doi: 10.1038/jcbfm.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TC, Verrier JD, Kochanek PM. Anthraquinone-2-sulfonic acid (AQ2S) is a novel neurotherapeutic agent. Cell death & disease. 2013;4:e451. doi: 10.1038/cddis.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevenaar JT, Hoogenraad CC. The axonal cytoskeleton: from organization to function. Frontiers in molecular neuroscience. 2015;8:44. doi: 10.3389/fnmol.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ishida J, Musashi M, Ota S, Yoshida T, Shimizu Y, Chuma M, Kawakami H, Asaka M, Tanaka J, Imamura M, Kobayashi M, Itoh H, Edamatsu H, Sutherland LC, Brachmann RK. p53 transactivation is involved in the antiproliferative activity of the putative tumor suppressor RBM5. Int J Cancer. 2011;128:304–318. doi: 10.1002/ijc.25345. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Finch EA. Map2 and Tau Segregate into Dendritic and Axonal Domains after the Elaboration of Morphologically Distinct Neurites - an Immunocytochemical Study of Cultured Rat Cerebrum. Journal of Neuroscience. 1987;7:3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JF, Nourjah P. The epidemiology of mild, uncomplicated brain injury. The Journal of trauma. 1988;28:1637–1643. doi: 10.1097/00005373-198812000-00004. [DOI] [PubMed] [Google Scholar]
- Loiselle JJ, Roy JG, Sutherland LC. RBM5 reduces small cell lung cancer growth, increases cisplatin sensitivity and regulates key transformation-associated pathways. Heliyon. 2016;2:e00204. doi: 10.1016/j.heliyon.2016.e00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL. Detection of blast-related traumatic brain injury in U.S. military personnel. The New England journal of medicine. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx V. Cell culture: a better brew. Nature. 2013;496:253–258. doi: 10.1038/496253a. [DOI] [PubMed] [Google Scholar]
- McClincy MP, Lovell MR, Pardini J, Collins MW, Spore MK. Recovery from sports concussion in high school and collegiate athletes. Brain injury. 2006;20:33–39. doi: 10.1080/02699050500309817. [DOI] [PubMed] [Google Scholar]
- McKinney JS, Willoughby KA, Liang S, Ellis EF. Stretch-induced injury of cultured neuronal, glial, and endothelial cells. Effect of polyethylene glycol-conjugated superoxide dismutase. Stroke. 1996;27:934–940. doi: 10.1161/01.str.27.5.934. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. Neun, a Neuronal Specific Nuclear-Protein in Vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neselius S, Brisby H, Theodorsson A, Blennow K, Zetterberg H, Marcusson J. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. Plos One. 2012;7:e33606. doi: 10.1371/journal.pone.0033606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani N, Nawashiro H, Fukui S, Ooigawa H, Ohsumi A, Toyooka T, Shima K, Gomi H, Brenner M. Enhanced hippocampal neurodegeneration after traumatic or kainate excitotoxicity in GFAP-null mice. J Clin Neurosci. 2006;13:934–938. doi: 10.1016/j.jocn.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Petry FR, Pelletier J, Bretteville A, Morin F, Calon F, Hebert SS, Whittington RA, Planel E. Specificity of Anti-Tau Antibodies when Analyzing Mice Models of Alzheimer’s Disease: Problems and Solutions. Plos One. 2014:9. doi: 10.1371/journal.pone.0094251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. Journal of neuropathology and experimental neurology. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Ren P, Guan YQ, Zhang Y. Comparisons among different methods of culturing neural stem cells isolated from human fetal cortex. Fen zi xi bao sheng wu xue bao = Journal of molecular cell biology. 2007;40:79–83. [PubMed] [Google Scholar]
- Rintala-Maki ND, Sutherland LC. LUCA-15/RBM5, a putative tumour suppressor, enhances multiple receptor-initiated death signals. Apoptosis. 2004;9:475–484. doi: 10.1023/B:APPT.0000031455.79352.57. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y, Mattsson N, Andreasen N, Ost M, Zetterberg H, Nellgard B, Blennow K. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Scientific reports. 2016;6:36791. doi: 10.1038/srep36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88:1788–1794. doi: 10.1212/WNL.0000000000003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Yang BX, Zhao LJ, Wang S, Zhang J, Wang K. Tumor suppressor gene RBM5 delivered by attenuated Salmonella inhibits lung adenocarcinoma through diverse apoptotic signaling pathways. World J Surg Oncol. 2013:11. doi: 10.1186/1477-7819-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Zhao L, Wang K, Xu W, Zhang J, Yang B. The tumor suppressor gene RBM5 inhibits lung adenocarcinoma cell growth and induces apoptosis. World J Surg Oncol. 2012;10:160. doi: 10.1186/1477-7819-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annual review of biochemistry. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shin SJ, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, Stice SL. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006;24:125–138. doi: 10.1634/stemcells.2004-0150. [DOI] [PubMed] [Google Scholar]
- Silverberg ND, Iverson GL. Etiology of the post-concussion syndrome: Physiogenesis and Psychogenesis revisited. Neuro Rehabilitation. 2011;29:317–329. doi: 10.3233/NRE-2011-0708. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Matser EJ, De Zeeuw CI, Weber JT. Repeated mild injury causes cumulative damage to hippocampal cells. Brain: a journal of neurology. 2002;125:2699–2709. doi: 10.1093/brain/awf271. [DOI] [PubMed] [Google Scholar]
- Smith M, Piehler T, Benjamin R, Farizatto KL, Pait MC, Almeida MF, Ghukasyan VV, Bahr BA. Blast waves from detonated military explosive reduce GluR1 and synaptophysin levels in hippocampal slice cultures. Experimental neurology. 2016;286:107–115. doi: 10.1016/j.expneurol.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Wang K, Li R, Yin J, Hao Y, Lv X, Li J, Zhao L, Du Y, Li P, Zhang J. Overexpression of RBM5 induces autophagy in human lung adenocarcinoma cells. World J Surg Oncol. 2016;14:57. doi: 10.1186/s12957-016-0815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Huang Z, Liu WW, Yang KX, Sun K, Xing SG, Wang D, Zhang W, Jiang XY. Surface Coating as a Key Parameter in Engineering Neuronal Network Structures In Vitro. Biointerphases. 2012:7. doi: 10.1007/s13758-012-0029-7. [DOI] [PubMed] [Google Scholar]
- Tan XL, Wright DK, Liu S, Hovens C, O’Brien TJ, Shultz SR. Sodium selenate, a protein phosphatase 2A activator, mitigates hyperphosphorylated tau and improves repeated mild traumatic brain injury outcomes. Neuropharmacology. 2016;108:382–393. doi: 10.1016/j.neuropharm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Tauskela JS, Fang H, Hewitt M, Brunette E, Ahuja T, Thivierge JP, Comas T, Mealing GA. Elevated synaptic activity preconditions neurons against an in vitro model of ischemia. The Journal of biological chemistry. 2008;283:34667–34676. doi: 10.1074/jbc.M805624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalin SJ, Ellis EF, Satin LS. Mechanical perturbation of cultured cortical neurons reveals a stretch-induced delayed depolarization. Journal of neurophysiology. 1995;74:2767–2773. doi: 10.1152/jn.1995.74.6.2767. [DOI] [PubMed] [Google Scholar]
- Tavalin SJ, Ellis EF, Satin LS. Inhibition of the electrogenic Na pump underlies delayed depolarization of cortical neurons after mechanical injury or glutamate. Journal of neurophysiology. 1997;77:632–638. doi: 10.1152/jn.1997.77.2.632. [DOI] [PubMed] [Google Scholar]
- Thomson CE, Hunter AM, Griffiths IR, Edgar JM, McCulloch MC. Murine spinal cord explants: a model for evaluating axonal growth and myelination in vitro. Journal of neuroscience research. 2006;84:1703–1715. doi: 10.1002/jnr.21084. [DOI] [PubMed] [Google Scholar]
- Tzekov R, Dawson C, Orlando M, Mouzon B, Reed J, Evans J, Crynen G, Mullan M, Crawford F. Sub-Chronic Neuropathological and Biochemical Changes in Mouse Visual System after Repetitive Mild Traumatic Brain Injury. Plos One. 2016;11:e0153608. doi: 10.1371/journal.pone.0153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valor LM, Jancic D, Lujan R, Barco A. Ultrastructural and transcriptional profiling of neuropathological misregulation of CREB function. Cell death and differentiation. 2010;17:1636–1644. doi: 10.1038/cdd.2010.40. [DOI] [PubMed] [Google Scholar]
- Wang CC, Held RG, Chang SC, Yang LL, Delpire E, Ghosh A, Hall BJ. A Critical Role for GluN2B-Containing NMDA Receptors in Cortical Development and Function. Neuron. 2011;72:789–805. doi: 10.1016/j.neuron.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Wang K, Bacon ML, Tessier JJ, Rintala-Maki ND, Tang V, Sutherland LC. RBM10 Modulates Apoptosis and Influences TNF-alpha Gene Expression. Journal of cell death. 2012;5:1–19. doi: 10.4137/JCD.S9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JT, Rzigalinski BA, Ellis EF. Traumatic injury of cortical neurons causes changes in intracellular calcium stores and capacitative calcium influx. The Journal of biological chemistry. 2001;276:1800–1807. doi: 10.1074/jbc.M009209200. [DOI] [PubMed] [Google Scholar]
- Wright AD, Jarrett M, Vavasour I, Shahinfard E, Kolind S, van Donkelaar P, Taunton J, Li D, Rauscher A. Myelin Water Fraction Is Transiently Reduced after a Single Mild Traumatic Brain Injury - A Prospective Cohort Study in Collegiate Hockey Players. Plos One. 2016:11. doi: 10.1371/journal.pone.0150215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WJ, Chen W, Chen L, Guo YJ, Zeng JS, Li GY, Tong WS. Involvement of tau phosphorylation in traumatic brain injury patients. Acta neurologica Scandinavica. 2017;135:622–627. doi: 10.1111/ane.12644. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Astrocytes protect neurons from neurotoxic injury by serum glutamate. Glia. 1998;22:237–248. doi: 10.1002/(sici)1098-1136(199803)22:3<237::aid-glia3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Yuan A, Sershen H, Veeranna, Basavarajappa BS, Kumar A, Hashim A, Berg M, Lee JH, Sato Y, Rao MV, Mohan PS, Dyakin V, Julien JP, Lee VM, Nixon RA. Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Molecular psychiatry. 2015;20:986–994. doi: 10.1038/mp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9:201–210. doi: 10.1038/nrneurol.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cui Z, Feng G, Bao G, Xu G, Sun Y, Wang L, Chen J, Jin H, Liu J, Yang L, Li W. RBM5 and p53 expression after rat spinal cord injury: implications for neuronal apoptosis. The international journal of biochemistry & cell biology. 2015;60:43–52. doi: 10.1016/j.biocel.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rzigalinski BA, Ellis EF, Satin LS. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274:1921–1923. doi: 10.1126/science.274.5294.1921. [DOI] [PubMed] [Google Scholar]
- Zhu QZ, CouillardDespres S, Julien JP. Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Experimental neurology. 1997;148:299–316. doi: 10.1006/exnr.1997.6654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.