Abstract
Objectives
To assess the prevalence and types of arrhythmias in Saudi OSA patients and to identify predictors of arrhythmia in this group of patients.
Methods
This case-control study included all patients who underwent level I attended overnight polysomnography between 2009 and 2012. Electrocardiographic data collected during sleep studies of patients with and without OSA were manually reviewed.
Results
The study comprised 498 patients (394 OSA patients and 104 non-OSA patients (controls). The prevalence of arrhythmia in OSA patients was higher than that in the controls (26.9% vs. 11.5%; p=0.001). Comparing OSA patients and controls showed: premature atrial contraction (10.2%vs.2.9%;p=0.019), premature ventricular contraction (PVC) (19.3%vs.9.6%;p=0.02), non-isolated PVC (bi/tri/qua) 10.8%vs.2.3%;p=0.04) and atrial fibrillation (1.6%vs.0%;p=0.001). Multiple logistic regression analysis revealed that, patients with OSA had twice the odds of having any cardiac arrhythmia (OR 1.91; CI 95% 1.27-3.11; p <0.05).
Conclusions
Patients with OSA had a higher prevalence of arrhythmia compared to controls, and OSA is a predictor of arrhythmia during sleep.
Keywords: Atrial fibrillation; Sleep Apnea, Obstructive, Arrhythmias, Cardiac
INTRODUCTION
Patients with obstructive sleep apnea (OSA) are at risk of developing cardiac arrhythmia1. The mechanisms by which OSA induces arrhythmias have not been fully elucidated; however, many studies have allowed several biologically plausible interactions to be proposed2. Hypoxemia, reoxygenation, hypercapnia, negative intrathoracic pressure, arousal, and sleep deprivation that are associated with OSA can impact cardiac status and electrical stability in relation to sympathetic activation, vagal stimulation, left atrial enlargement, and systemic inflammation2,3. Downstream from the elicitation of these cardiovascular disease mechanisms, OSA is also associated with hypertension, systolic and diastolic ventricular dysfunction, and cardiac and cerebral ischemic events; therefore, OSA can increase the risk of arrhythmias directly and indirectly through its influence on other cardiovascular pathologies2-5.
The association between OSA and cardiac arrhythmias, including ventricular arrhythmias, bradyarrhythmias, and atrial fibrillation (AF), has been reported1. However, the prevalence of nocturnal arrhythmia in patients with OSA in Arabs is unknown. Racial differences in the presentation and complications of patients with OSA have been documented6-9. Moreover, previous studies have shown that Saudi (Arabs) patients with OSA are relatively younger, morbidly obese and have a high prevalence of comorbid conditions such as hypertension, diabetes mellitus, hypothyroidism and bronchial asthma10,11. Therefore, it is possible that the prevalence of nocturnal arrhythmia in patients with OSA in this population is different from that reported Western population given the differences in patient characteristics, risk factor profiles, and management patterns11. Accordingly, we designed this study to explore the association between OSA and cardiac arrhythmia in a large population of Saudi (Arabs) patients referred for a sleep study with a clinical suspicion of OSA.
MATERIALS AND METHODS
Subjects and design
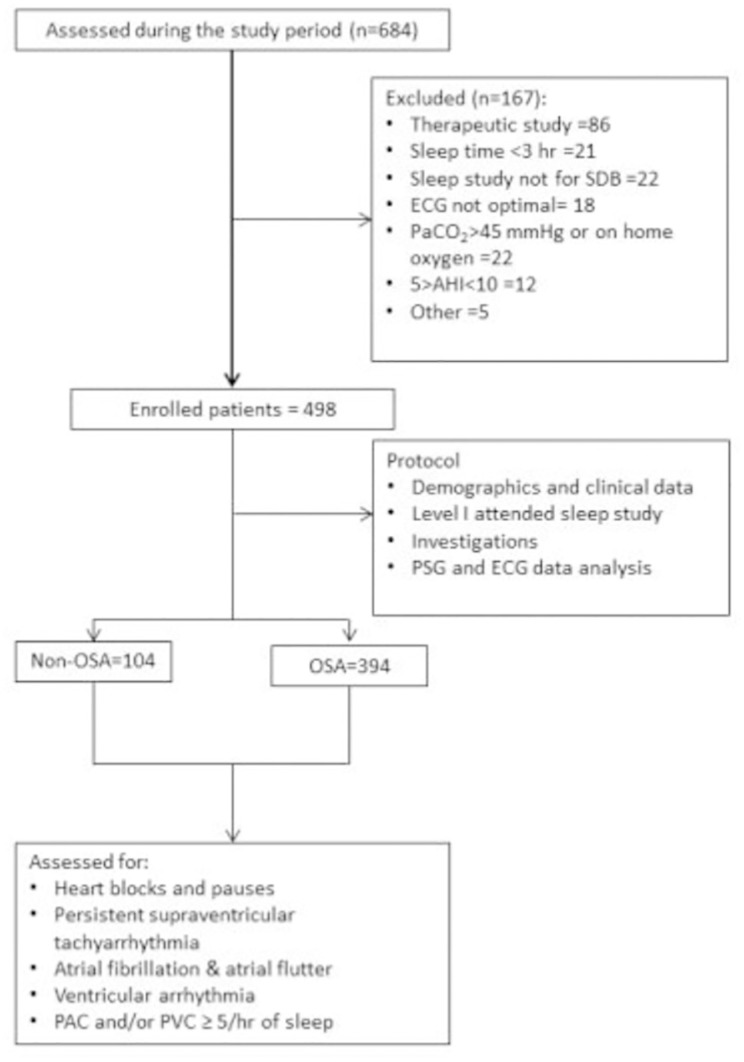
This case-control study comprised all consecutive patients who were referred to the sleep clinic with a clinical suspicion of OSA and underwent type I attended overnight polysomnography (PSG) in the University Sleep Disorders Center (USDC) at King Khalid University Hospital between 2009 and 2012 were reviewed. Using a case-control design, we examined the association between OSA and cardiac arrhythmias in this cohort. Cases (Exposed) were those with OSA defined as apnea hypopnea index (AHI) more than or equal to 10 events/hour. We included all patients’ male and females who are more than or equal to 18 years of age. Patients were excluded if the diagnostic PSG recording was < 3 h duration if they have sleep disorders other than OSA (e.g., narcolepsy or movement disorder), or referral was for therapeutic PSG study only (e.g., continuous positive airway pressure titration). Moreover, patients with daytime hypercapnia (PaCO2>45 mm Hg), with cardiac pacemakers and on home oxygen therapy were excluded. As per the USDC protocol, all patients with sleep-disordered breathing underwent arterial blood gas (ABG) analysis and thyroid function tests. Figure 1 shows the patient flow diagram.
Figure 1.
Patient flow diagram.
The study was approved by the institutional review board in the college of medicine, King Saud University.
Sleep data
All patients underwent a standard level I attended overnight sleep study with neurological, cardiac and respiratory monitoring using Alice® diagnostic equipment (Philips, Respironics Inc., Murrysville, PA, USA). The following parameters were monitored, four leads of electroencephalography (EEG: C1-A4, C2-A3, O1-A4, O2-A3), electrooculography (EOG), chin electromyography (EMG (lead II)), electrocardiography (EKG), oxygen saturation, chest and abdominal wall movements, air flow (thermistor and nasal pressure). As per the American Academy of Sleep Medicine Scoring Manual, a single channel Lead II (right arm to left hip) was used for recording EKG12. Scoring of sleep studies was performed manually according to established scoring criteria12. An apnea is defined as a complete cessation of airflow for =10 seconds. A hypopnea is a =30% decrease in airflow from baseline of more than or equal to 10 seconds associated with a 3% oxygen desaturation or arousal. The desaturation index was defined as the number of desaturation events (more than or equal to 3% decrease in oxygen saturation from the pre-event baseline) per hour of sleep.
The apnea-hypopnea index (AHI) quantifies the number of apneas and hypopneas per hour of sleep. Patients with AHI <5 were used as control (non-exposed).
Demographics and comorbidities data
Data collected included the following; age, sex, body mass index, hypertension, diabetes mellitus, ischemic heart disease, congestive heart failure, dyslipidemia, renal failure, stroke, COPD, bronchial asthma, thyroid disease. Comorbidities were determined from the patient medical file.
Arrhythmia data
All EKG records were manually reviewed for specific arrhythmia types by two physicians (AH and NA) blinded to respiratory events and clinical data (one of them is an electrophysiologist (AH)). All types of tachyarrhythmia and bradyarrhythmias were looked for, such as all types of heart blocks and pauses, persistent supraventricular tachyarrhythmia, atrial fibrillation, atrial flutter, and ventricular arrhythmia in particular sustained or non-sustained ventricular tachycardia. Since atrial and ventricular premature extrasystole (PAC and PVC) are very common in normal individuals we capture frequent PAC and/or PVC more than or equal to 5/hour of sleep.
Statistical analysis
Differences in categorical variables between respective comparison groups were analyzed using Chi-square test or Fisher’s exact test. Continuous variables were analyzed using a t-test or Mann-Whitney U test based on the satisfaction of normality assumption. p-values are reported as 2-sided test results, with a 5% level of significance for each test.
Multiple logistic regression analysis was used to identify the relation between OSA and arrhythmia. Variables considered for inclusion were baseline demographic characteristics (age, sex), medical history (diabetes mellitus, hypertension, hyperlipidemia, ischemic heart disease, present or past history of smoking, bronchial asthma, thyroid disease, the severity of OSA (based on AHI), heart failure, and stroke. We used logistic regression analysis to examine the association between OSA and nocturnal cardiac arrhythmias after adjusting for the above variables.
All analyses were performed using Statistical Package for Social Sciences (SPSS), IBM version 22 (SPSS Inc., Chicago, IL, USA) software.
RESULTS
Patient characteristics
A total of 498 patients met the inclusion criteria during the study period. Of these, 394 (79.1%) had OSA and 104 (20.9%) had no-OSA. The characteristics of patients with and without OSA are depicted in Table1. Patients in the total study group were more likely to be male, with the significantly higher proportion of male sex in patients with OSA than patients without OSA. OSA patients were significantly older than patients without OSA. OSA patients had significantly higher rate of diabetes mellitus, hypertension, ischemic heart disease, dyslipidemia, higher BMI, hypothyroidism, and bronchial asthma than patients without OSA. A similar proportion of patients with and without OSA had a history of renal failure, congestive heart failure, and stroke.
Table 1.
Characteristics of patients with and without OSA
| Characteristics | Non OSA (n=104) |
OSA (n=394) |
p-value |
|---|---|---|---|
| Age (yr) | 36.5 ± 13.7 | 48.3 ± 13.9 | <0.001 |
| Apnea Hypopnea Index | 2.4 ± 4 | 45.1 ± 32.4 | <0.001 |
| Sleep Efficiency | 78.1 ± 19.4 | 72.5 ± 18.5 | 0.006 |
| Male | 52(48.6) | 242(61.4) | 0.017 |
| Hypertension | 16(15.4) | 171(43.5) | <0.001 |
| Ischemic heart disease | 1(1) | 35(8.9) | 0.005 |
| Diabetes mellitus | 13(12.5) | 144(36.7) | <0.001 |
| Congestive heart failure | 1(1) | 4(1) | 0.956 |
| Bronchial asthma | 13(12.5) | 111(28.4) | 0.001 |
Arrhythmia rate
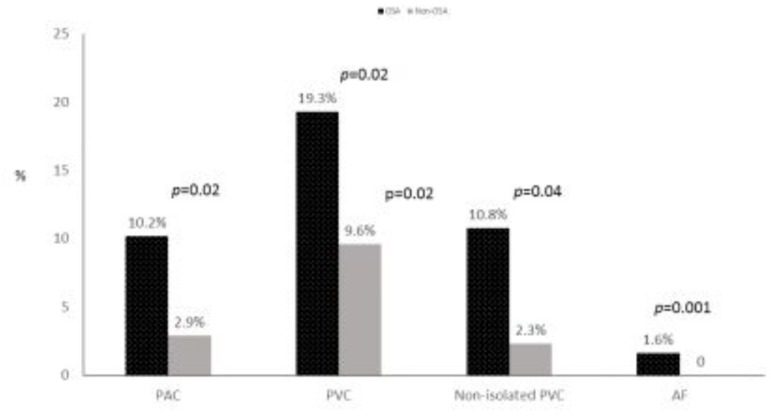
Figure 2 depicts comparisons of arrhythmia between OSA and non-OSA patients. Patients with OSA were significantly more likely than patients without OSA to have arrhythmias (26.9% vs. 11.5%; P=0.001). Proportions of PAC (≥ 5/hr) (10.2% vs. 2.9%; p=0.02), PVC (≥ 5/hr) (19.3% vs. 9.6%; p= 0.02), non-isolated PVC (bi/tri/qua) 10.8% vs. 2.3%; p=0.04) and atrial fibrillation (1.6% vs. 0%; p=0.001) were more significantly higher in patients with OSA than those without OSA. There were no cases of non-sustained or sustained tachycardia, supraventricular tachycardia or conduction delay and heart block. Detected arrhythmias occurred in both NREM and REM sleep in most patients with OSA (87%).
Figure 2.
Comparisons of arrhythmia between OSA and non-OSA patients.
Arrhythmia and OSA
Table 2 shows the OSA patients stratified by presence or absences of arrhythmia. Of the 394 patents with OSA 104 (26.9%) had an arrhythmia. Patients with OSA and arrhythmia were significantly older than OSA patients without arrhythmia. OSA patients with arrhythmia had significantly higher rate of diabetes mellitus, hypertension, and ischemic heart disease, more than OSA patients without arrhythmia. In addition, arrhythmias were more prevalent in female patients with OSA. A similar proportion of OSA patients with and without arrhythmia had a history of bronchial asthma, hypothyroidism, and dyslipidemia. OSA patients with arrhythmia had significantly lower total sleep time, and sleep efficiency, and desaturation index higher and percent time with SpO2<90% than OSA patients without arrhythmia. There was no difference between the two groups with regard to AHI, central and obstructive apnea index, mean hypopnea index, arousal index and leg movement index.
Table 2.
Comparison between OSA patients with and without cardiac arrhythmia
| Cardiac Arrhythmia | |||
|---|---|---|---|
| Characteristics | No (n=288) | Yes (n=104) | p-value |
| Age (yr) | 45.9 ± 12.5 | 55 ± 15.2 | <0.001 |
| Sex (male) | 185(64.2) | 57(53.8) | 0.06 |
| BMI | 35.9 ± 10.9 | 37.3 ± 8.7 | 0.2 |
| Total sleep time | 332 ± 60.2 | 271 ± 45.9 | 0.04 |
| Sleep Efficiency | 74 ± 18.5 | 68.3 ± 17.8 | 0.006 |
| Apnea Hypopnea Index | 44.1 ± 27.9 | 47.9 ± 26.1 | 0.38 |
| Desaturation index | 31.1 ± 18 | 39.4 ± 29.5 | <0.001 |
| % Time w O2 <90% | 11.2±25.2 | 19.6 ± 32.7 | 0.039 |
| Hypertension | 111(38.7) | 60(56.6) | 0.001 |
| Ischemic heart disease | 18(6.3) | 17(16) | 0.003 |
| Diabetes mellitus | 92(32.1) | 52(49.5) | 0.001 |
| Bronchial Asthma | 84(29.3) | 27(26) | 0.5 |
After adjusting for several independent factors (demographics, cardiovascular risk factors, and comorbidities), patients with OSA had approximately twice the odds of having any cardiac arrhythmia (OR 1.91; CI 95% 1.27-3.11; p <0.05).
DISCUSSION
Our study provides new information on the prevalence of arrhythmia in Arab patients with OSA. Moreover, OSA was an independent predictor of arrhythmias in our patients. In the literature, the reported incidence of arrhythmia in OSA patients ranges from 20% to 50%3. The explanation for this wide range includes the difference in arrhythmia definition, characteristics of the population or the sample studied (e.g., age), study design and sleep-disordered breathing (SDB) severity. The current study showed a prevalence of 26%, which is at the lower end of the range; however, the study group is relatively younger than studied samples in previous studies1,3,13,14. Our study revealed that patients with OSA had a significantly increased prevalence of PACs, PVCs, and AF compared with patients without OSA.
AF is commonly reported in patients with OSA. In some reports, the incidence of AF ranged from 4.7% to 32% while the rate of AF was somewhat lower in our study 1.6%15. Moreover, age is a risk factor for arrhythmia, specifically AF. The mean age in our study was 10 to 15 years younger than what reported in other studies3,13,16,17. Therefore, the lower incidence of AF in the current study may be associated with their markedly younger age in our study group.
Frequent PVCs were detected in 19.3% in patients with OSA comparable with that reported in previous studies18. Several studies have reported increased complex PVCs and non-sustained ventricular arrhythmia in patients with OSA3. Verrier et al postulated that the decrease vagus nerve activity during REM sleep and unopposed cardiac sympathetic nerve activity in these patients might foster the development of ventricular tachycardia and fibrillation19. On the contrary, Flemons et al reported no difference in the incidence of PVC, ventricular tachycardia, and bradyarrhythmias in patients with and without OSA20. While intermittent hypoxemia and arousals enhance sympathetic nervous activity and may be involved with the tachyarrhymias seen in OSA, the simultaneous hypoxemia and apnea also induce the diving reflex, with cardiac parasympathetic vagal nerve activation.21-23 This may result in severe nocturnal bradyarrhythmias. In a study of 239 OSA patients, bradyarrhythmias occurred only during apnea and hypopnea24. In some studies, these bradyarrhythmias occurred more frequently during REM sleep accompanied by at least a 4% decrease in oxygen saturation24-27. Conversely, in Guilleminault et al. reported, extreme sinus bradycardia in 3 patients during NREM sleep, and sinus arrest was associated with apneas during REM sleep28. These patients had both sinus arrest and extreme sinus bradycardia that were of similar duration28. The lack of a difference in conduction delay between subjects with and without OSA in both the MrOS Sleep Study14 and Sleep Heart Health Study16 was similar to our patients. Perhaps this could be due to the differences in the underlying comorbidities in these two studies compared with data from studies of clinic referral subjects that suggested an association between OSA and bradyarrhythmias and heart failure.
Our study has some limitations that need to be addressed. We evaluated arrhythmia during sleep period only, so we cannot speculate on the arrhythmia in OSA during the wake period. Another limitation is the use of a single EKG channel to classify cardiac arrhythmias. Finally, this is a case-control study; therefore, we cannot establish causality. However, we strived to account for key confounders that may explain this association.
CONCLUSIONS
Patients with OSA had a higher prevalence of arrhythmia compared to controls, and OSA is a predictor of arrhythmia during sleep. Arrhythmia was more prevalent among OSA patients with longer periods of hypoxemia and those with ischemic heart disease. There is a need to modify the practice of polysomnographic interpretation and scoring in order to have a more systematic approach to detect, quantify and report cardiac events. Future studies should examine the impact of co-existing arrhythmia on morbidity and mortality and effect of positive airway pressure therapy on arrhythmias in this group of patients.
REFERENCES
- 1.Patel N, Donahue C, Shenoy A, Patel A, El-Sherif N. Obstructive sleep apnea and arrhythmia: A systemic review. Int J Cardiol. 2017;228:967–970. doi: 10.1016/j.ijcard.2016.11.137. [DOI] [PubMed] [Google Scholar]
- 2.May AM, Van Wagoner DR, Mehra R. OSA and Cardiac Arrhythmogenesis: Mechanistic Insights. Chest. 2017;151(1):225–241. doi: 10.1016/j.chest.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hersi AS. Obstructive sleep apnea and cardiac arrhythmias. Ann Thorac Med. 2010;5(1):10–17. doi: 10.4103/1817-1737.58954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos AR, Guilliam D, Dib SI, Koch S. Race/ethnic differences in obstructive sleep apnea risk in patients with acute ischemic strokes in south Florida. Sleep Breath. 2014;18(1):165–168. doi: 10.1007/s11325-013-0865-9. [DOI] [PubMed] [Google Scholar]
- 7.Scharf SM, Seiden L, DeMore J, Carter-Pokras O. Racial differences in clinical presentation of patients with sleep-disordered breathing. Sleep Breath. 2004;8(4):173–183. doi: 10.1007/s11325-004-0173-5. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Stepnowsky C, Dimsdale J, Marler M, Cohen-Zion M, Johnson S. The effect of race and sleep-disordered breathing on nocturnal BP "dipping": analysis in an older population. Chest. 2002;122(4):1148–1155. doi: 10.1378/chest.122.4.1148. [DOI] [PubMed] [Google Scholar]
- 9.Pandi-Perumal SR, Abumuamar AM, Spence DW, Chattu VK, Moscovitch A, BaHammam AS. Racial/Ethnic and Social Inequities in Sleep Medicine: The Tip of the Iceberg? J Natl Med Assoc. 2017 doi: 10.1016/j.jnma.2017.04.005. in press. [DOI] [PubMed] [Google Scholar]
- 10.Alharbi M, Almutairi A, Alotaibi D, Alotaibi A, Shaikh S, Bahammam AS. The prevalence of asthma in patients with obstructive sleep apnoea. Prim Care Respir J. 2009;18(4):328–330. doi: 10.4104/pcrj.2009.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alotair H, Bahammam A. Gender differences in Saudi patients with obstructive sleep apnea. Sleep Breath. 2008;12(4):323–329. doi: 10.1007/s11325-008-0184-8. [DOI] [PubMed] [Google Scholar]
- 12.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.1. Darien: American Academy of Sleep Medicine; 2014. www.aasmnet.org [Google Scholar]
- 13.Selim BJ, Koo BB, Qin L, Jeon S, Won C, Redeker NS, et al. The Association between Nocturnal Cardiac Arrhythmias and Sleep-Disordered Breathing: The DREAM Study. J Clin Sleep Med. 2016;12(6):829–837. doi: 10.5664/jcsm.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, et al. Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169(12):1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansukhani MP, Wang S, Somers VK. Sleep, death, and the heart. Am J Physiol Heart Circ Physiol. 2015;309(5):H739–H749. doi: 10.1152/ajpheart.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavergne F, Morin L, Armitstead J, Benjafield A, Richards G1, Woehrle H. Atrial fibrillation and sleep-disordered breathing. J Thorac Dis. 2015;7(12):E575–E584. doi: 10.3978/j.issn.2072-1439.2015.12.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghuram A, Clay R, Kumbam A, Tereshchenko LG, Khan A. A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias. J Clin Sleep Med. 2014;10(10):1155–1160. doi: 10.5664/jcsm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verrier RL, Josephson ME. Impact of sleep on arrhythmogenesis. Circ Arrhythm Electrophysiol. 2009;2(4):450–459. doi: 10.1161/CIRCEP.109.867028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flemons WW, Remmers JE, Gillis AM. Sleep apnea and cardiac arrhythmias. Is there a relationship? Am Rev Respir Dis. 1993;148(3):618–621. doi: 10.1164/ajrccm/148.3.618. [DOI] [PubMed] [Google Scholar]
- 21.Daly MD, Angell-James JE, Elsner R. Role of carotid-body chemoreceptors and their reflex interactions in bradycardia and cardiac arrest. Lancet. 1979;1(8119):764–767. doi: 10.1016/s0140-6736(79)91218-2. [DOI] [PubMed] [Google Scholar]
- 22.Madden BP, Shenoy V, Dalrymple-Hay M, Griffiths T, Millard J, Backhouse L, et al. Absence of bradycardic response to apnea and hypoxia in heart transplant recipients with obstructive sleep apnea. J Heart Lung Transplant. 1997;16(4):394–397. [PubMed] [Google Scholar]
- 23.Somers VK, Dyken ME, Mark AL, Abboud FM. Parasympathetic hyperresponsiveness and bradyarrhythmias during apnoea in hypertension. Clin Auton Res. 1992;2(3):171–176. doi: 10.1007/BF01818958. [DOI] [PubMed] [Google Scholar]
- 24.Becker H, Brandenburg U, Peter JH, Von Wichert P. Reversal of sinus arrest and atrioventricular conduction block in patients with sleep apnea during nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151(1):215–218. doi: 10.1164/ajrccm.151.1.7812557. [DOI] [PubMed] [Google Scholar]
- 25.Koehler U, Schäfer H. Is obstructive sleep apnea (OSA) a risk factor for myocardial infarction and cardiac arrhythmias in patients with coronary heart disease (CHD)? Sleep. 1996;19(4):283–286. [PubMed] [Google Scholar]
- 26.Koehler U, Fus E, Grimm W, Pankow W, Schäfer H, Stammnitz A, et al. Heart block in patients with obstructive sleep apnoea: pathogenetic factors and effects of treatment. Eur Respir J. 1998;11(2):434–439. doi: 10.1183/09031936.98.11020434. [DOI] [PubMed] [Google Scholar]
- 27.Koehler U, Becker HF, Grimm W, Heitmann J, Peter JH, Schäfer H. Relations among hypoxemia, sleep stage, and bradyarrhythmia during obstructive sleep apnea. Pt 1Am Heart J. 2000;139(1):142–148. doi: 10.1016/s0002-8703(00)90321-1. [DOI] [PubMed] [Google Scholar]
- 28.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52(5):490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]