Summary
The diurnal cortisol profile has been implicated in multiple physical and mental health conditions in children and adolescents; however, current knowledge regarding the stability of the diurnal cortisol profile is largely based on adults. Developmental changes throughout childhood and adolescence warrant examination of the stability of the diurnal cortisol profile during this stage in the lifecourse. The aim of the present study was to conduct a comprehensive evaluation of the diurnal cortisol profile in children and adolescents. Participants (N = 233; M = 12.40, SD = 1.83; 44.2% girls) in the Healthy Heart Project collected saliva samples, completed demographic questionnaires, and recorded bed and waking time. Intra-class correlations were calculated to evaluate the stability of aggregate and single sample measures of the diurnal cortisol profile. Total cortisol concentration (AUCTG, AUCAG) and maximum sample were the most stable cortisol measures (ICCavg = 0.54). Dynamic measures (AUCI, slope; ICCavg = 0.22) and other single sample measures (awake, lunch, dinner, bedtime, morning random, day random; ICCavg = 0.28) were less stable. Of the developmentally relevant covariates tested, sleep duration, adrenarche, and time of awakening were most associated with cortisol values. Altogether, the diurnal cortisol profile yielded moderate to high stability in children and adolescents. These findings can inform methodological decisions regarding cortisol sampling protocols for children and adolescents.
Keywords: Cortisol, Stability, Children, Adolescents, HPA axis, Methodology
Cortisol, the end product of the hypothalamic pituitary adrenal (HPA) axis, is released in a circadian fashion and in response to stress. The circadian rhythm of cortisol, or the diurnal cortisol profile, has been implicated in multiple physical and mental health conditions in children and adolescents, including asthma (Wolf et al., 2008; Dreger et al., 2010), bipolar disorder (Ellenbogen et al., 2006), major depression (Van den Bergh and Van Calster, 2009; Adam et al., 2010), and sleep disturbances (El-Sheikh et al., 2008), among others. However, the measurement of cortisol varies widely, and few studies have considered the stability of the diurnal cortisol profile in children and adolescents.
The diurnal cortisol profile is characterized by cortisol levels that peak roughly 30 min after awakening (morning acrophase) and gradually decline throughout the day, reaching nadir at bedtime (Fries et al., 2009). The diurnal cortisol profile is described using aggregate measures such as: the awakening response, diurnal slope, and the total concentration of cortisol over the day. The awakening response refers to the rise in cortisol by 50–75% (approximately 4–15 nmol/L) during the first hour post-awakening (Clow et al., 2004). Two awakening response measures are commonly derived: total amount of cortisol released during the awakening response (AUCAG; area under the awakening response relative to ground or zero; see Table 1) and dynamic increase in the amount of cortisol secreted following awakening (AUCI; area under the curve relative to increase or awakening cortisol value; Pruessner et al., 2003). Diurnal slope is characterized as the decline in cortisol over the day and is calculated as the slope of the line from the awakening or maximum value to the last measured point. Normal slopes are declining and characterized by negative values; blunted slopes are flattened with values closer to zero. Diurnal slope can be determined by standard linear regression or by rise over run (i.e., difference between first and last cortisol values divided by the time between sampling; Adam and Kumari, 2009); the comparability of these two formulas is unknown. Total cortisol concentration (AUCTG) represents the overall secretory activity of the HPA axis throughout the day. AUCTG is estimated as the area under the diurnal profile of all measured time points (excluding the awakening response), relative to ground. Finally, single sample measures including morning (any single sample during the morning; Lupien et al., 2001), awake, and bedtime (Backhaus et al., 2004; Cohen et al., 2006), are also commonly reported in the adult and child literature. Additionally, some researchers collect cortisol at unstandardized time points (e.g., inconsistent collection times due to participant scheduling; El-Sheikh et al., 2008).
Table 1.
Mathematical formulae for cortisol measures.
| Formulas | Symbol | Equation | |
|---|---|---|---|
| Total cortisol level during awakening responsea | AUCAG |
|
|
| Dynamic increase of awakening responsea | AUCI |
|
|
| Total cortisol level during the daya | AUCTG |
|
|
| Diurnal slope anchored to awake using regression | slopeawake regression |
|
|
| Diurnal slope anchored to awake using rise over run | slopeawake to last |
|
|
| Diurnal slope anchored to maximum using regression | slopemax regression |
|
|
| Diurnal slope anchored to maximum using rise over run | slopemax to last |
|
Note: i denotes cortisol samples within 1 h post-awakening; s denotes cortisol sample; s0 denotes awake cortisol sample; t denotes time sample taken in hours; k denotes all samples taken (excluding cortisol samples within 1 h post-awakening); ; Savg denotes average cortisol of Sk, where the first sample is S0; l denotes the last sample taken; smax denotes maximum cortisol sample within 1 h post-awakening; ; Savg.m denotes average cortisol of Sk, where the first sample is smax.
Formulas based on Pruessner et al. (2003).
Saliva sampling protocols to capture the diurnal cortisol profile vary widely (Adam and Kumari, 2009), ranging from two samples on 1 day to six samples over several days. The MacArthur Network (2000) guidelines recommend 3–4 days of sampling to determine total amount of cortisol released (AUCAG, AUCTG), and at least 6 days to capture diurnal slope. Yet the feasibility of these recommendations is often limited by financial resources and participant burden. Thus, several studies have examined the stability of the diurnal cortisol profile in adults to guide sampling protocol decisions. A single day of sampling yields morning cortisol and awakening response values that have low stability (Coste et al., 1994; Hellhammer et al., 2007). Among the awakening response measures, AUCAG is more stable and evidences moderate stability over 2 days of sampling (ICC = 0.69), while AUCI necessitates 5 days of sampling to reach comparable reliability (ICC = 0.65; Hellhammer et al., 2007). Diurnal slope also has moderate stability over 2 days (r = .45–.66; Kraemer et al., 2006). Interestingly, anchoring the diurnal slope to the awake sample (+0 min) rather than the +30 min post-awakening sample, has been found to be more stable in older adults (rs = .63 and .45, respectively; Kraemer et al., 2006). There are no findings regarding the stability of AUCTG or other single sample measures.
The stability of the diurnal cortisol profile in children and adolescents has been minimally investigated. Awakening response is moderately stable across 2 days (AUCAG r = .40–.55; AUCI r = .53; Pruessner et al., 1997; ter Wolbeek et al., 2007; Oskis et al., 2009). However, the robustness of these findings remains unestablished given the methodological limitations of these studies such as including girls only (ter Wolbeek et al., 2007; Oskis et al., 2009) and small samples sizes (N < 50; Pruessner et al., 1997). There is no data regarding the stability of AUCTG or diurnal slope. Single sample measures are moderately stable across 3 consecutive days: awake (α = .49), +30 min post-awakening (α = .77), afternoon (α = .58), and bedtime (α = .75; O’Connor et al., 2005). Similar findings have been reported across 2 consecutive days (Brosnan et al., 2009; Shirtcliff et al., 2005). Thus, while these results suggest some cortisol measures are moderately stable, a more rigorous examination of the stability of the diurnal cortisol profile in children and adolescents is necessary.
Examining the stability of the diurnal cortisol profile in children and adolescents is important because developmental changes in the timing and amount of cortisol released alter the shape of the diurnal profile. The diurnal profile of infants (age 1–2 months) is characterized by two daily cortisol peaks (Larson et al., 1998). At age 2–3 months, an awakening response emerges (Kiess et al., 1995; O’Connor et al., 2005; Oskis et al., 2009). Diurnal slope is not considered stable until age 4 years, which is likely attributable to daytime napping (Gunnar and Donzella, 2002). Furthermore, sleep duration decreases across childhood and adolescence (Wolfson and Carskadon, 1998). Total cortisol concentrations increase steadily from childhood to adolescence (Lupien et al., 2001; Walker et al., 2001; Tornhage, 2002; Gunnar et al., 2009). Pubertal maturation has been inconsistently associated with cortisol with some studies finding an association with increased cortisol (Kiess et al., 1995; Netherton et al., 2004; Oskis et al., 2009), steeper diurnal slope, and reduced awakening response (Adam, 2006), and others reporting no relation (Rosmalen et al., 2005; El-Sheikh et al., 2008). Some evidence suggests these inconsistencies are resolved when pubertal phase (gonadarche or adrenarche) is considered. Thus, the stability of the diurnal cortisol profile should be considered in light of these developmental changes.
Several other covariates alter the diurnal cortisol profile in children and adolescents, including sex, waking time, and season of sampling. Sex differences have not been found for the evening cortisol level; however, girls’ awakening response is greater (Rosmalen et al., 2005). Earlier waking time yields greater morning cortisol levels (Kelly et al., 2008). Season of sampling has inconsistent findings; higher morning cortisol values have been observed both in the winter (short photoperiod; Walker et al., 1997), and spring/summer (long photoperiod; Rosmalen et al., 2005; Matchock et al., 2007). Total cortisol concentration is greater in long photoperiods (Matchock et al., 2007). While the current child and adolescent literature has examined the influence of some covariates, it is based on a limited number of studies and does not include developmentally relevant covariates (e.g., sleep duration, sampling when school in-session).
The question remains whether the diurnal cortisol profile is stable in children and adolescents. Developmental changes throughout childhood and adolescence warrant comprehensive examination of the stability of the diurnal cortisol profile during this stage in the lifecourse. Existing gaps of knowledge in the literature include restrictive samples (girls only), small samples sizes (N < 50), lack of stability data for common cortisol measures (diurnal slope, AUCTG) and limited information about potentially important covariates (sleep duration, school in-session). Thus, significant methodological issues challenge the accuracy and generalizability of previous findings. The aim of the present study was to examine the stability of the diurnal cortisol profile in children and adolescents. Specifically, aggregate and single sample measures of cortisol commonly reported in the literature were evaluated. As a secondary aim, developmentally relevant variables including sex, age, adrenarche, gonadarche, season, school in-session, wake time, and sleep duration were considered as covariates.
1. Method
1.1. Participants
Youth aged 9–18 years were recruited to take part in the Healthy Heart Project, a longitudinal study examining early cardiovascular risk factors, at Concordia University, Montreal, QC. Flyers, postcards, and bookmarks were distributed throughout the community and in primary and secondary schools approved by the Montreal English School Board. Children with serious psychopathology or medication use known to interfere with cardiovascular functioning were excluded. This study was approved by the Concordia University Ethics Review Committee (UH2005-077-4).
1.2. Procedure
Youth and their parents were scheduled for two visits to the laboratory. During the first visit, youth and their parents completed demographic and health questionnaires. Youth were instructed how to use the Salivette sampling device and provided saliva collection kits for home and school. During the second visit, participants returned their saliva samples. Informed consent and youth assent was obtained prior to the study. Participants received monetary compensation for their time.
1.3. Measures
1.3.1. Cortisol
Two sampling protocols were used over the course of the study. For the first protocol (n = 139), saliva samples were collected five times per day for 3 days at home or school. Samples were collected at awakening (awake0), +30 min post-awakening (awake30), +45 min post-awakening (awake45), before lunch, and before dinner. For the second protocol (n = 168), saliva samples were collected six times per day (bedtime sample added) for only 2 days. Participants were instructed to collect saliva samples on at least 1 weekday. The duration of sampling, or the numbers of days that elapsed when collecting samples, ranged from 2 to 64 days.
Saliva samples were collected using the Salivette sampling device (Salimetric, Inc.). Youth were instructed to place the cotton swab under their tongue for at least 30 s. When saturated, it was placed back in the Salivette tube and refrigerated until returned at the second visit. Participants were instructed not to eat or brush their teeth 10 min before taking a sample, consistent with the instructions by Hanrahan et al. (2006). Youth recorded the date and time each sample was taken in a daily log, which was initialed by parents or teachers as a marker of compliance. Compliance was also verified for the awakening (awake0) sample using accelerometry data (supine to sitting; Rotenberg and McGrath, 2011). Participants were highly compliant with saliva sampling (97%). Upon receipt at the laboratory, saliva samples were stored in a sub-zero freezer until they were packaged in dry ice and couriered to the University of Trier, Germany for cortisol assaying. Cortisol levels are robust to environmental conditions associated with the shipping process (Clements and Parker, 1998). Cortisol levels were determined in duplicate using a competitive solid phase time-resolved fluorescence immunoassay with fluorometric end point detection (DELFIA; Dressendörfer et al., 1992). The intra-assay coefficients of variation were less than 11%.
1.3.2. Pubertal stage
Two phases of pubertal development, gonadarche (genital/breast development) and adrenarche (pubic hair growth), were assessed using sex-specific illustrations corresponding to Tanner stages I–V (Growing and Changing Questionnaire; Golding et al., 2001). While visual examination performed by physicians to assess sexual maturation status is the gold standard, it is often not conducted due to concerns about privacy and the sensitivity of the physical examination. Pubertal illustrations have demonstrated good reliability and validity (r = .77–.91; Morris and Udry, 1980; Dorn et al., 1990; Netherton et al., 2004). Pubertal stage was only assessed during the second saliva sampling protocol.
1.3.3. Timing
Season of sampling was based on the timing of the solstice and equinox occurrence (Matchock et al., 2007). Fall/Winter was September 21st to March 20th, and Spring/Summer was March 21st to September 20th. School in-session was defined as September 1st to June 15th; summer break was defined as June 16th to August 31st.
1.3.4. Sleep duration
The quantity of sleep on the night preceding saliva sampling was recorded in the child’s daily logs. Sleep duration was calculated as the time elapsed between bedtime and the time of the awake0 sample. Wake time was set to equal the time of the awake0 sample. Sleep duration was only assessed with the second saliva sampling protocol.
1.4. Sample exclusion criteria
Of the initial 303 participants who were included in the study, participants who did not return any saliva samples (n = 16), took more than 2 weeks to collect saliva samples (n = 7), or did not collect samples on 2 similar days (weekday or weekend; n = 16) were excluded from the sample. Of the remaining participants (n = 264), the majority (97%) collected their saliva samples within 14 days (excluded participants took an average of 24 days to collect samples). Most participants collected their saliva samples on weekdays (n = 233); a subset collected saliva samples only on the weekend (n = 31). Over 50% of the cortisol values differed significantly based on day of data collection (weekday vs. weekend). This is consistent with previous research (Scholtz et al., 2004). Due to the small sample size of participants with weekend only data, stability statistics were limited to weekday data.
1.5. Statistical analyses
Raw cortisol values were square root transformed to address non-normality and skewness. Aggregate measures (AUCAG, AUCI, AUCTG) were calculated for each day of sampling using equations previously reported (cf. Pruessner et al., 2003; Kraemer et al., 2006; see Table 1). Diurnal slope was estimated using two formulas (linear regression, rise over run), each with two different anchoring points (awake0, peak morning sample). To mimic methodological design choices observed in the literature (e.g., morning sample taken when child arrives at school; during classroom visit scheduled at convenient time), two random samples were chosen purposely. Morning random sample was randomly selected from awake0, awake30, or awake45; day random sample was randomly selected from any sample each day. Maximum sample was the highest cortisol value identified each day.
Of the total number of saliva samples (n = 4506), missing data were due to single saliva samples that were either not returned (n = 190, 4.22%) or did not contain enough saliva for assaying (n = 24, 0.53%). Awakening response (AUCAG, AUCI) was defined as missing if the awake0 sample was missing or if the time difference between the collection of the awake and awake45 sample exceeded 1 h. AUCTG and diurnal slope were defined as missing if the awake0 sample, maximum sample and/or at least two other samples were missing. The percentage of missing data for all aggregate measures (AUCAG, AUCI, AUCTG, diurnal slope), ranged from 3% to 25%. Little’s tests showed that the missing aggregate measures were not missing completely at random (χ2 [875, N = 234] = 1242.62, p < .05), but were likely missing at random. This suggests that the missing aggregate measures do not depend on the data itself. Multiple imputation procedures were performed for the aggregate measures. Missing values were imputed 20 times with re-sampling techniques in Amelia II (version 1.2-14). Missing single sample measures were excluded using case-wise deletion (data imputation is not possible).
Classical measurement theory was used to examine the stability of the diurnal cortisol profile. The stability of aggregate and single sample measures was examined using multilevel modeling. Day-to-day sources of cortisol variability (e.g., time of awakening, sleep duration) were nested within individuals. Intra-class correlational analyses were used to evaluate the stability of 14 cortisol measures: AUCAG, AUCI, slopeawake regression, slopemax regression, slopeawake to last, slopemax to last, AUCTG, awakening sample, before lunch sample, before dinner sample, bedtime sample, morning random sample, day random sample, and maximum sample. The intraclass correlation coefficient (ICC) was calculated using the statistical method described by Shrout and Fleiss (1979). The ICC represents the ratio of between-subject variability ( ) to total variability (between-subject variability plus error variability; ); . An ICC ranges from 0 to 1; values greater than 0.8 are considered to be stable. The projected number of days of measurement (m) needed to obtain a stable average cortisol measure (ICCOR = 0.8) was calculated by rearranging the Spearman-Brown formula: m = ICCOR(1 − ICC)/ICC(1 − ICCOR). Classical measurement theory was tested using HLM6 (Scientific Software International) statistical software.
Finally, covariates developmentally relevant to childhood and adolescence (i.e., sex, age, adrenarche, gonadarche, season, school in-session, wake time, sleep duration, sampling duration) were each considered a priori. Covariates were tested using univariate linear regression to examine the extent of their association with the cortisol measures. Analyses were conducted using IBM SPSS Statistics software (Version 20).
2. Results
Participants (N = 233) included children and adolescents aged 9–18 years (M = 12.40 years, SD = 1.82). About half of the sample was female (n = 103; 44.2%) and the majority of participants were of normal body weight (<85th BMI percentile: n = 152; 65%). The majority of parents had a university education (M = 15.88 years, SD = 3.45) and a yearly household income of $73,552 CAN (SD = $51,780). The mean wake time was 7:33 am (SD = 1.4 h) and participants slept on average for 9 h (SD = 1.5 h). The majority of samples were collected within 2 days (70%) while school was in-session (79%). Untransformed cortisol values stratified by age and sex are presented in Table 2.
Table 2.
Un transformed cortisol levels (nmol/L) by age and sex.
| Children (9–12 years), M (SD) | Adolescents (13–18 years), M (SD) | |||
|---|---|---|---|---|
|
|
|
|||
| Boys (n = 71) | Girls (n = 54) | Boys (n = 59) | Girls (n = 49) | |
| Aggregate measures | ||||
| AUCAG | 10.40 (4.63) | 11.88 (7.43) | 11.20 (8.24) | 12.22 (5.81) |
| AUCI | 1.80 (3.54) | 2.18 (7.57) | 1.68 (3.96) | 2.50 (5.38) |
| AUCTG | 61.54 (32.61) | 64.52 (32.81) | 65.97 (44.82) | 72.91 (41.41) |
| Slopeawake | −1.56 (1.83) | −1.18 (3.05) | −.55 (.92) | −.96 (.84) |
| Slopemax | −1.16 (.48) | −1.10 (.42) | −1.14 (.92) | −1.20 (1.03) |
| Single sample measures | ||||
| Awake | 9.97 (6.01) | 10.08 (6.29) | 9.14 (4.84) | 9.56 (4.78) |
| Morning | 11.56 (5.92) | 13.54 (7.06) | 11.17 (5.19) | 12.39 (6.17) |
| Maximum | 16.29 (7.84) | 17.71 (9.13) | 15.12 (6.23) | 18.67 (9.78) |
| Random | 7.53 (6.95) | 9.55 (7.16) | 6.54 (4.74) | 9.20 (6.61) |
| Lunch | 3.92 (3.31) | 4.39 (3.34) | 3.89 (2.27) | 5.00 (3.15) |
| Dinner | 2.27 (4.33) | 3.42 (7.07) | 2.34 (1.66) | 2.40 (1.47) |
| Bedtime | 1.09 (1.13) | 1.89 (3.38) | 1.00 (0.52) | 1.98 (2.40) |
Note: AUCAG, area under curve relative to ground for awakening response; AUCI, area under curve relative to increase; AUCTG, area under curve relative to ground for diurnal profile; Morning, randomly selected sample from three morning samples; Random, randomly selected sample from all samples collected.
2.1. Stability and number of days of measurement needed
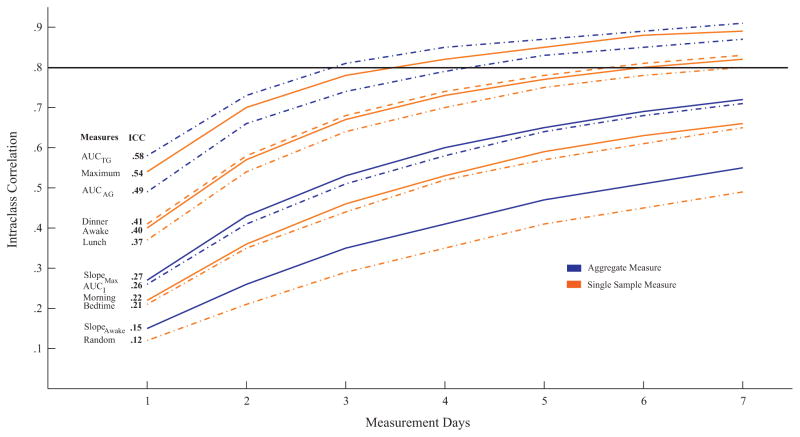
The ICCs and the projected number of days of measurement (m) needed to reach optimal stability (ICC = 0.8) are presented in Fig. 1. The stability of the aggregate measures ranged from 0.16 to 0.58; the stability of the single sample measures ranged from 0.12 to 0.54. Aggregate measures representing the total amount of cortisol released (AUCAG, AUCTG) were more stable than the measures describing the dynamic response of the HPA axis (AUCI, diurnal slope). Diurnal slope was more stable when anchored to the maximum sample as compared to the awake sample (mean ICC = 0.27 vs. 0.15, respectively). There was no difference between linear regression and rise over run formulas (both mean ICC = 0.21); thus, the mean is presented. Among the single sample measures, cortisol was most stable for the maximum sample, followed by the awake and dinner samples.
Figure 1.
The stability of aggregate and single sample cortisol measures over 1 week based on intra-class correlations (ICC). AUCAG, area under the curve relative to ground for the awakening response; AUCI, area under the curve relative to increase; AUCTG, area under the curve relative to ground for the diurnal profile; Morning, randomly selected sample from three morning samples; Random, randomly selected sample from all samples collected. Note: Line at .8 signifies optimal stability, and changes in line style used to ease interpretation of the figure.
The relation between number of days of sampling and stability of cortisol is further depicted in Fig. 1. Maximum cortisol value and aggregate measures of total amount of cortisol (AUCAG, AUCTG) measured over 3–4 days demonstrate optimal stability (ICC = 0.8). Awake, lunch, and dinner samples necessitated measurement for 1 week to attain optimal stability. Finally, over 2 weeks of measurement were necessary for the dynamic cortisol measures (AUCI, slopemax, slopeawake) and other single sample measures (morning random, day random, bedtime).
2.2. Influence of covariates
Among the covariates, sleep duration accounted for the greatest amount of variance in cortisol (M = 5.5%). Longer sleep duration was significantly associated with steeper diurnal slopeawake, lower AUCI, and AUCTG, as well as a trend (p = .07) towards reduced awake and greater bedtime cortisol levels (see Table 3). Accounting for 5.0% of the variance, duration of sampling period was negatively associated with cortisol, such that a longer timelapse between sampling days resulted in significantly lower values for AUCAG and AUCTG, and trended towards significantly lower values for AUCI. Pubertal stage based on adrenarche accounted for 3.7% of the variance in cortisol. Participants who were at later stages of pubic hair development had significantly flatter diurnal slopes (slopeawake and slopemax). Pubertal stage based on gonadarche (0% of variance) was not significantly associated with any cortisol values. Later time of awakening (3.0% of variance) was significantly associated with lower AUCTG. When school was in-session (2.7% of variance), AUCI was higher, dinner was lower, and slopemax was steeper than compared when on summer break. For season of sampling (2.5% of variance), during the spring/summer, diurnal slopemax was flatter. With respect to age (2.5% of variance), older adolescents had greater AUCI values and flatter slopeawake; no other age related differences were found. Females (2.1% of variance) had significantly higher AUCI, maximum, lunch, and bedtime measures than males, and a trend towards greater AUCAG and morning random cortisol measures.
Table 3.
Standardized beta coefficients for cortisol measures and developmentally relevant covariates.
| Demographic | Pubertal | Methodological | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Female | Age | Gonadarche | Adrenarche | Summer | School | Sampling | Wake time | Sleep | |
| Aggregate measures | |||||||||
| AUCAG | .12 | .06 | .05 | .03 | −.08 | −.12 | −.22* | −.06 | −.09 |
| AUCI | .13* | .16* | .12 | .12 | −.08 | .13* | −.13 | −.07 | −.30* |
| AUCTG | .11 | .07 | .03 | .09 | −.08 | −.09 | −.28* | −.18* | −.30* |
| Slopeawake | .10 | .12* | .13 | .18* | .04 | .04 | .06 | −.04 | −.23* |
| Slopemax | .06 | −.03 | .06 | .14* | .16* | −.15* | .08 | −.04 | −.02 |
| Single sample measures | |||||||||
| Awake | .03 | −.07 | .12 | .01 | .04 | .09 | −.05 | .06 | −.16 |
| Morning | .13 | −.01 | .01 | .11 | .02 | −.02 | −.09 | .01 | −.07 |
| Maximum | .13* | .05 | .11 | .15 | −.01 | −.09 | −.11 | −.01 | −.07 |
| Random | .20* | −.02 | .07 | .02 | .07 | −.06 | −.06 | .02 | −.03 |
| Lunch | .15* | .10 | −.09 | .02 | .04 | .05 | −.10 | .08 | .03 |
| Dinner | .09 | .02 | .04 | .12 | .08 | −.16* | −.10 | .28* | .13 |
| Bedtime | .18* | .08 | −.13 | −.09 | .04 | .01 | −.05 | .13 | .17 |
Note: AUCAG, area under curve relative to ground for awakening response; AUCI, area under curve relative to increase; AUCTG, area under curve relative to ground for diurnal profile. Morning, randomly selected sample from three morning samples; Random, randomly selected sample from all samples collected.
p < .05.
3. Discussion
Cortisol is used extensively as a marker of HPA-axis activity in children and adolescents within psychoneuroendocrinology research. Despite its frequent use, the stability of the diurnal cortisol profile has not been established in children and adolescents. In the current investigation, the stability of cortisol and the influence of covariates was tested. Specific cortisol values included aggregate (AUCAG, AUCI, slopeawake regression, slopeawake to last, slopemax regression, slopemax to last, AUCTG) and single sample measures (awake, morning, maximum, random, lunch, dinner, bedtime). Overall, the total concentration of cortisol (AUCTG, AUCAG) and maximum sample were the most stable cortisol values, reaching moderate stability with only 2 days of measurement. To reach comparable stability, dynamic measures that capture the responsivity of the HPA-axis (AUCI, diurnal slope) required three to 7 days of measurement. The stability of diurnal slope was greater when slope was anchored to the maximum sample, rather than awake; this was consistent regardless of the formula used to calculate slope (regression, rise over run). Single sample measures (awake, lunch, dinner) were moderately stable with 3 days of measurement, but the bedtime and the morning random sample required 4 days of measurement. As expected, the day random sample chosen to reflect common practice in the field whereby a convenience sample is taken (e.g., single sample during lab visit), was the least stable and required over 1 week of daily samples to reach moderate stability.
The stability results were largely consistent with those previously reported in a limited number of studies. The observed moderate to high stability of AUCAG over 2 days was consistent with previously reported child (Oskis et al., 2009) and adult findings (Pruessner et al., 1997; Edwards et al., 2001a,b; Hellhammer et al., 2007). In contrast, the low stability of AUCI was inconsistent with Oskis’ finding with child participants of moderate stability (Oskis et al., 2009); however, they were consistent with other adult findings (Edwards et al., 2001a; Hellhammer et al., 2007). Finally, moderate to high stability of single sample measures (awake, dinner) was largely consistent with another study reporting 3 days of sampling with youth (O’Connor et al., 2005).
To examine the influence of covariates on diurnal cortisol profile, several key covariates were purposely selected based on recommendations of the MacArthur Network (2000), their extensive use in the adult literature, and their developmental relevance. Although there is no consistent consideration of covariates in the child literature, sex, age, pubertal stage, time of awakening, and season of sampling have been previously used. In the current study, the influence of other developmentally relevant covariates (sleep duration, school in-session) was also considered. Overall, the covariates accounted for only a small amount of the variance of the cortisol values (less than 10%). Sleep duration, sampling duration, adrenarche, and time of awakening accounted for the greatest variability in the cortisol values. As well, many cortisol values differed depending on whether samples were collected while school was in-session or during the summer break. Notably, each cortisol value had different covariates emerge as relevant; thus, researchers should pay particular attention to carefully select covariates upon choosing their cortisol measure of interest.
Few studies have reported data on the stability of the cortisol diurnal profile in children and adolescents. Sex, age, pubertal stage, time of awakening, and photoperiod have been included as covariates, yet they are inconsistently associated with different cortisol measures in youth. For example, puberty has been found to be a significant covariate for morning random sample (Tornhage, 2002; Oskis et al., 2009) and AUCI (Adam, 2006) while others report it is not a significant covariate for any cortisol measure (Rosmalen et al., 2005). Varying methods in the measurement of puberty may account for these inconsistencies. Some researchers measure puberty based on an individual’s score on either genital/breast development (gonadarche) or pubic hair growth (adrenarche; Knutsson et al., 1997; Matchock et al., 2007); others use an aggregate measure (Adam, 2006; Gunnar et al., 2009). When puberty is separated by gonadarche and adrenarche, its relation to cortisol becomes more apparent. Adrenarche has been associated with AUCTG and morning cortisol (Matchock et al., 2007), although there is limited evidence of an association with gonadarche (Knutsson et al., 1997; Matchock et al., 2007). The current finding that adrenarche, rather than gonadarche, was associated with cortisol is consistent with previous research (Matchock et al., 2007). Inconsistent findings for covariates are also reported in studies of cortisol in adults (cf. Pruessner et al., 1997; Edwards et al., 2001b). These inconsistencies may be attributable to measurement issues of cortisol or the covariates, the cortisol measure considered, or inherent variability in the relation between these covariates and cortisol. However, in the current investigation fewer than 30% of the covariates accounted for more than 1% of the variance in the cortisol measure of interest.
The present investigation is not without limitations. First, two different protocols were used to collect saliva samples and covariate information. Thus, sleep duration and pubertal stage were only available for the second collection protocol (59% of participants). Despite this potential power limitation, sleep duration was a robust covariate and accounted for the greatest amount of variance in cortisol values. Second, participants were permitted to choose their saliva collection days over the course of 1 week; however, they were instructed to sample from at least 1 weekday. Most participants collected all samples during weekdays (88.3%); thus, stability could not be evaluated for weekends due to insufficient data. The stability of the diurnal cortisol profile on weekends is unknown. Third, sampling compliance was based on youth-reported time of saliva collection combined with parent- or teacher-initialed times on the daily log sheet. Accelerometry data indicated youth were able to precisely collect saliva at waking and report accurate time of waking (Rotenberg and McGrath, 2011). Sampling noncompliance typically results in skipping the first sample upon awakening (awake0) or sampling later than awakening which would lessen the cortisol awakening response. Finally, the stability of the diurnal cortisol profile is dependent on both the number of days of measurement and the number of samples per day. For example, in a study with adults, AUCTG based on 3 samples collected on 1 day was comparable to AUCTG based on 5 samples per day collected over 3 days (r = .69; Harville et al., 2007). The current study only projected the optimal number of days of measurement. Future research should consider how many samples on a given day of measurement are needed to derive stable estimates of the diurnal cortisol profile in children and adolescents.
Altogether, the diurnal cortisol profile yielded moderate to high stability in children and adolescents, which has potential implications for future studies. The results of the present study contribute to the existing literature by providing empirical data to guide methodological decisions regarding cortisol sampling protocols for children and adolescents. Researchers are often faced with the challenge of ensuring optimal cortisol measurement balanced against limited financial resources and participant burden. Stable estimates of AUCAG, AUCTG, and maximum cortisol can be derived using five or six samples collected on 1 day. Studies that are restricted to 1 day of measurement should consider using these cortisol measures. Multiple days of sampling are necessary for other cortisol measures and recommended when feasible. The cortisol awakening response (AUCI) requires at least 3 weekdays of sampling to yield a stable estimate. Diurnal slope requires three to 7 weekdays of sampling, depending on whether the calculation is anchored to the maximum or awake0 sample, respectively. Single sample measures may also be used when necessary; however, the stability of the measure depends on the specific sample. At least 2 days of sampling is needed for a stable awake sample, and bedtime sample requires at least 5 days of sampling. Additionally, methodological decisions should be made regarding developmentally important covariates to either consider for the sampling protocol or to control for statistically. For example, researchers may wish to only sample data on weekdays or while school is in-session. When these covariates cannot be factored into the methodological design, researchers should account for them statistically. Examination of sleep duration, duration of sampling, adrenarche, and time of awakening should be tested as covariates. In conclusion, these results suggest the diurnal cortisol profile is stable in children and adolescents.
Acknowledgments
Role of funding source
The funding source had no role in the design, analysis or presentation of this study.
We thank the participants and their families of the Healthy Heart Project, the teachers and principals of the Montreal English School Board, and the research assistants and study coordinators of the Pediatric Public Health Psychology Laboratory at Concordia University. Special thanks to Natasha Hunt and Sabrina Giovanniello for their continued dedication. We also thank John Brand for generating the figure, as well as Sonia Lupien, Mark Ellenbogen, and Wayne Brake for their insightful comments on earlier drafts of this manuscript. This work was made possible through funding support from the Canadian Institute of Health Research (CIHR) Operating Grants (MOP89886 and OCO79897), New Investigator Award (J.J. McGrath MSH95353), Canada Graduate Scholarships Master’s Award (S. Rotenberg), and Health Professional Student Research Award (S. Rotenberg).
Footnotes
Conflict of interest statement
All authors declare that they have no conflict of interest.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology. 2004;29:1184–1191. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Brosnan M, Turner-Cobb J, Munro-Naan Z, Jessop D. Absence of a normal cortisol awakening response (CAR) in adolescent males with Asperger Syndrome (AS) Psychoneuroendocrinology. 2009;34:1095–1100. doi: 10.1016/j.psyneuen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman R. Socioeconomic status, race, and diurnal cortisol decline in the coronary artery risk development in young adults (cardia) study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Coste J, Strauch G, Letrait M, Bertagna X. Reliability of hormonal levels for assessing the hypothalamic–pituitary–adrenocortical system in clinical pharmacology. Br J Clin Pharmacol. 1994;38:474–479. doi: 10.1111/j.1365-2125.1994.tb04386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Nottelmann ED, Inoff-Germain G, Chrousos GP. Perceptions of puberty: adolescent, parent, and health care personnel. Dev Psychol. 1990;26:322–329. [Google Scholar]
- Dreger LC, Kozyrskyj AL, HayGlass KT, Becker AB, MacNeil BJ. Lower cortisol levels in children with asthma exposed to recurrent maternal distress from birth. J Allergy Clin Immunol. 2010;125:116–122. doi: 10.1016/j.jaci.2009.09.051. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001a;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001b;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker CD, Couture S, Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology. 2006;31:1164–1180. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s objective and subjective sleep disruptions: links with afternoon cortisol levels. Health Psychol. 2008;27:26–33. doi: 10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Golding J, Pembray M, Jones R. ALSPAC: the Avon longitudinal study of parents and children. I Study methodology. Paediatr Perinatal Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus—pituitary—adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Appl Nurs Res. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Harville E, Savitz DA, Dole N, Herring H, Thorp JM, Light KC. Patterns of salivary cortisol secretion in pregnancy and implications for assessment protocol. Biol Psychol. 2007;74:85–91. doi: 10.1016/j.biopsycho.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Scholtz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state-and-trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Young R, Sweeting H, Fischer JE, West P. Levels and confounders of morning cortisol collected from adolescents in a naturalistic (school) setting. Psychoneuroendocrinology. 2008;33:1257–1268. doi: 10.1016/j.psyneuen.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendörfer RA, Schriever K, Kessler U, Kounig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37:502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Knutsson U, Dahlgren J, Marcus C, Rosber S, Bronnegard M, Stierna P, Albertsson-Wikland K. Circadian cortisol rhythms in healthy boys and girls: relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Kraemer H, Giese-Davis J, Yutsis M, O’Hara R, Neri E, Gallagher-Thompson D, Taylor CB, Spiegel D. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry. 2006;14:325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Dev Psychobiol. 1998;33:327–337. [PubMed] [Google Scholar]
- Lupien S, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- MacArthur Network. Salivary Cortisol Measurement. 2000 Retrieved from http://www.macses.ucsf.edu/research/allostatic/salivar-ycort.php.
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol Int. 2007;24:969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34:307–316. doi: 10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Rosmalen JGM, Oldehinkel AJ, Ormel J, De Winter AF, Buitelaar JK, Verhhulst FC. Determinants of salivary cortisol levels in 10–12 year old children: a population-based study of individual differences. Psychoneuroendocrinology. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ. Sampling precision of the awakening saliva sample using accelerometry in children and adolescents. Psychosom Med. 2011;73:A114. [Google Scholar]
- Scholtz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend—weekday differences in the cortisol awakening response. Psychosom Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- ter Wolbeek M, van Doornen LJP, Coffeng LE, Kavelaars A, Heijnen CJ. Cortisol and severe fatigue: a longitudinal study in adolescent girls. Psychoneuroendocrinology. 2007;32:171–182. doi: 10.1016/j.psyneuen.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Tornhage CJ. Reference values for morning salivary cortisol concentrations in healthy school-aged children. J Pediatr Endocrinol Metab. 2002;15:197–204. doi: 10.1515/jpem.2002.15.2.197. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children’s Depression Inventory. Psychoneuroendocrinology. 2009;34:791–794. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Walker BR, Best R, Noon JP, Watt GCM, Webb DJ. Seasonal variation in glucocorticoid activity in healthy men. J Clin Endocrinol Metab. 1997;82:4015–4019. doi: 10.1210/jcem.82.12.4430. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and a-amylase in children with asthma and healthy children. Biol Psychol. 2008;78:20–28. doi: 10.1016/j.biopsycho.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]