Abstract
Purpose
The purpose was to evaluate the efficacy of the Web-based, Personal Patient Profile-Prostate decision aid versus usual care with regard to decisional conflict in men with localized prostate cancer.
Materials and Methods
A randomized (1:1) controlled, parallel-group, nonblinded trial was conducted in four US regions. Eligible men had clinically localized prostate cancer, an upcoming consult, and spoke/read English or Spanish. Participants answered questionnaires to report decision-making stage, personal characteristics, concerns and preferences plus baseline symptoms and decisional conflict. A randomization algorithm allocated participants to receive tailored education and communication coaching, generic teaching sheets and external websites plus a one-page summary to clinicians (intervention), or the links plus materials provided in clinic (usual care). Conflict outcomes and number of consults were measured at 1-month. Univariate and multivariable models were used to analyze outcomes.
Results
392 men were randomized (198 intervention; 194 usual care) and 305 men returned 1-month outcomes (152; 153). One-month decisional conflict scale (0–100) means were 10.9 (SD=16.7) (intervention) and 9.9 (SD=18.0) (usual care). The multivariable model revealed significantly reduced conflict in the intervention group (−5.00 [−9.40, −0.59]). Other predictors of conflict included income, marital/partner status, decision status, number of consults, clinical site and D’Amico risk classification.
Conclusions
In this multi-center trial, the decision aid significantly reduced decisional conflict. Other variables impacted conflict and modified the decision aid’s effect, notably risk classification, consults and resources. The Personal Patient Profile-Prostate is an effective adjunct for shared decision-making in men with localized prostate cancer.
Keywords: prostatic neoplasms, decision support techniques, medical informatics
Introduction
The American Urological Association 2017 guidelines for clinically localized prostate cancer (LPC)1 begin with counseling patients, incorporating shared decision making, followed by recommendations for appropriate use of active surveillance and active therapies. Men are increasingly engaging in active surveillance of LPC.2,3 For men seeking treatment, there are several options: prostatectomy, external beam radiotherapy, brachytherapy and hormonal therapy. Consulting physicians are challenged to present options with equipoise.4,5 Choosing a course of action for LPC is challenging; both decisional conflict (DC) and uncertainty experienced by men have been documented.6–9 There is evidence that men make a decision by considering personal preferences and factors,6,10,11 and yet, evidence exists that clinicians are making decisions, often without soliciting men’s preferences.12
Our team tested the Personal Patient Profile-Prostate (P3P), 2007–2009, as a preparatory adjunct to the face-to-face consultation.9 Although we found P3P users reported significantly lower conflict related to uncertainty and values clarification over six months post-enrollment, outcomes were influenced by the number of pre-enrollment consults men experienced,13 Internet use, race and income.9 P3P was revised to accommodate low-literacy users and tested with minority men during decision-making.14 We designed a subsequent randomized trial and conducted the study within US health networks in geographically distinct regions.
Materials and Methods
The primary objective of this randomized controlled (1:1), parallel-group, nonblinded trial was to compare decisional conflict between two groups: 1) the P3P decision support intervention plus usual education, and 2) usual education and links to reputable websites. Secondary objectives were to compare decisional regret and satisfaction at 6-months. Here we present the decisional conflict comparison, 1-month post study enrollment.
Sample and Settings
Eligible men had: 1) biopsy-proven prostate cancer, cT1 or cT2 of any risk level (a criterion that the biopsy was conducted enrolling sites was removed one-year after enrollment to increase accrual rates); 2) an upcoming consult at an enrolling site; 3) self-reported ability to read/understand English or Spanish. We excluded men whose records documented any of the following: 1) more than one consult visit; 2) a final care decision; or 3) had begun active surveillance or received any prostate cancer treatment. The study was approved by Dana-Farber/Harvard Cancer Center’s institutional review board and review boards at each site.
Procedures
Research assistants recruited eligible participants by telephone and offered the opportunity to home access or on an iPad in clinic before the consult appointment. Home users agreed to elements of consent online prior to the baseline questionnaire and then signed written consent at the clinic visit; at KP the full consent was completed online. Content was delivered securely utilizing open-source software for computerized patient-reported outcomes (http://cprohealth.org).
In the baseline session, the P3P website presented: (1) questionnaires9,15 for which responses were used to create the intervention component: influential personal factors, decision-making stage, decisional control preference, prostate cancer symptoms, age, ethnicity/race; and (2) study measures: DC,16 income, education level, and healthcare utilization variables. After baseline questionnaires were submitted, participants were randomized to the intervention or usual care group in permuted blocks of four, stratified by clinic site, via an algorithm embedded in the software.
The P3P intervention
Participants randomized to P3P were presented the online intervention: education and communication coaching, in the form of text, graphs, and short video clips on medical facts and personally relevant factors to the prostate cancer decision, plus printed teaching sheets as in the prior trial.9,15 Informational topics and tailoring to race, ethnicity and age algorithms in P3P have been previously described.15 Based on usability testing after the first trial,14,17 the software was enhanced with navigation guidance, key terms definitions, larger widgets, and context-sensitive help; content was updated by the investigative. Each clinician of an intervention group patient received a one-page summary of patient-reported information, cueing the provider to symptom issues, concerns and preferences.
Participants in both groups were provided a list of reputable, prostate cancer education websites;18 (Supplemental Table) sites were linked for immediate online viewing and provided on a printed teaching sheet. In both groups, participants received any clinic-specific educational materials and provider consults that were usual preparation for a decision.
Research assistants prompted participants to complete 1-month measures online or by mail (participant preference) at 30+/−7 days from baseline. Participants self-reported any decision and answered the DC Scale (DCS). Medical record review was conducted to verify the decision and document the total number of prostate cancer consults prior to the 1-month follow-up. Research assistants were not blinded to study group assignment; however, all patient-reported outcome measures were self-administered.
Instrument
The DCS has been used in multiple trials aimed at reducing conflict related to health decisions.16,19 In our previous P3P clinical trial we deployed the 16-item, five-subscale version which demonstrated total score reliability coefficients of 0.93–.094.9 Given our adaptation of P3P for low-literacy in this trial, we selected the 10-item, low-literacy version DCS (Table 1). Although subscales can be calculated in this version, here we report the total score. Our rationale for using the total score was previous total score validity, poor construct validity of the support subscale, lack of evidence for a four-factor model in men,19 and subscales based on only two or three items.
Table 1.
Decisional Conflict Scale (DCS) – Low Literacy Version
| Items | Response options and scores |
|---|---|
| 1. Do you know which options are available to you? | Yes (0), Unsure (2), No (4) |
| 2. Do you know the benefits of each option? | Yes (0), Unsure (2), No (4) |
| 3. Do you know the risks and side effects of each option? | Yes (0), Unsure (2), No (4) |
| 4. Are you clear about which benefits matter most to you? | Yes (0), Unsure (2), No (4) |
| 5. Are you clear about which risks and side effects matter most to you? | Yes (0), Unsure (2), No (4) |
| 6. Do you have enough support from others to make a choice? | Yes (0), Unsure (2), No (4) |
| 7. Are you choosing without pressure from others? | Yes (0), Unsure (2), No (4) |
| 8. Do you have enough advice to make a choice? | Yes (0), Unsure (2), No (4) |
| 9. Are you clear about the best choice for you? | Yes (0), Unsure (2), No (4) |
| 10. Do you feel sure about what to choose? | Yes (0), Unsure (2), No (4) |
Statistical Considerations
Originally the study targeted a sample size of 625 and an anticipated 20% attrition rate, for an analytic sample of 500 (250 per study group) at 6 months. A planned interim analysis suggested a larger effect size. Under additional circumstances of fewer sites than anticipated, the revised target sample size was 375, for an analytic sample of 300 participants (150 per study group). The study was designed to have 80% power at a two-sided 0.05 significance level to detect an effect size of 0.325 in DCS scores with a two-sample t-test.
Baseline characteristics and scores were summarized by group and compared using the T-test/Wilcoxon rank-sum test for continuous variables and Fisher’s exact test/Chi-square test for categorical variables. DCS item scores were averaged and multiplied by 25. Scores range from 0 (low conflict) to 100 (high conflict).16 Only DCS questionnaires with complete data were included; no imputation was performed. Cronbach’s alpha coefficients and Pearson’s correlations were calculated. The analytical sample for primary analysis of DCS between study groups included all randomized participants with DCS scores at baseline and 1-month, regardless of how much intervention participants received.
The DCS at 1-month was compared between groups using analysis of covariance (ANCOVA), linear regression adjusting for baseline DCS. Covariates previously identified as influencing DC (age, education, marital status, working status, income, race/ethnicity, 1-month decisional status, Internet as an information source, clinic) plus new covariates (D’Amico risk classification, number of consults through 1-month) were assessed univariately and then adjusted in multivariable analysis to improve precision of estimating the intervention effect. A backward model selection was conducted and possible two-way interactions with study group were checked. Significant interactions (P ≤ 0.1) were included in the final model. Three clinics with <10 participants each were excluded from the univariate analysis of clinic site and multivariable analysis, resulting in exclusion of 10 participants. Two-sided type III P values were used to assess overall significance in the model, and least-squares means (LS-means) were used to present effects of interactions in the multivariable model. A P-value was deemed statistically significant if ≤ 0.05, and marginally significant values between 0.05 and 0.1. Analyses were performed in SAS 9.4 (SAS Institute, Cary NC).
Results
A total of 392 men (71% consent rate) were enrolled and randomized (198 P3P, 194 UC) from September, 2013 to April, 2016 (Figure 1), when the target sample was reached; 305 (152 P3P, 153 UC) men returned the 1-month DCS questionnaire (retention rate 78%). Table 2 lists the demographic and clinical characteristics plus use of the Internet information prior to the target consult, D’Amico risk classification and clinic sites. There were no baseline group differences. The analytic sample consisted of 276 men with complete DCS data at both baseline and 1-month. Groups remained balanced at 1-month with the exception of age; men ≥ 60 years were significantly more likely to return 1-month measures (p=.05).
Figure 1.
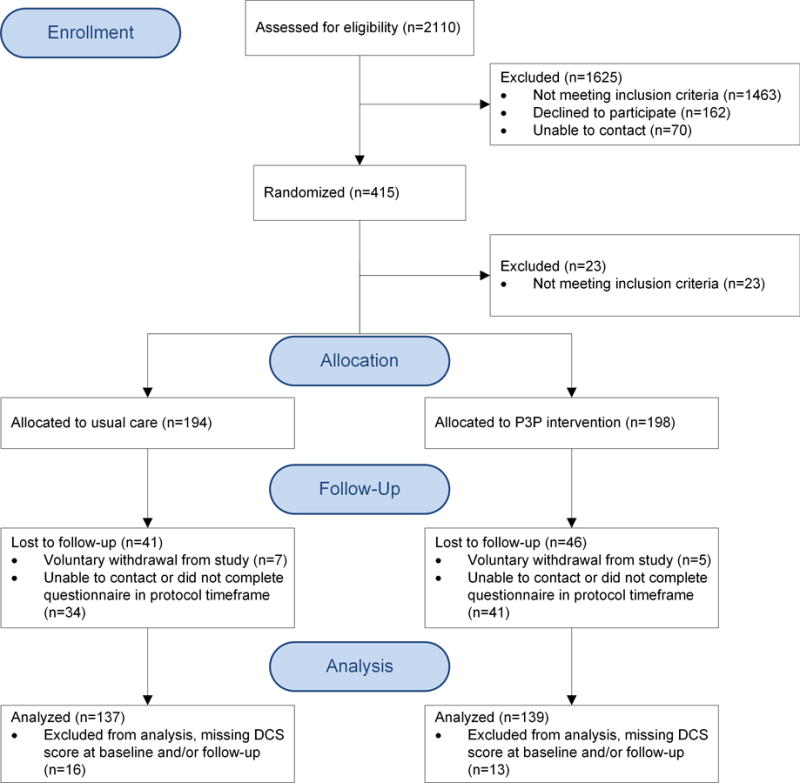
Number of participants screened, enrolled, randomized, and included in analysis.
Table 2.
Baseline and clinical characteristics by study group
| Study Group | ||||
|---|---|---|---|---|
| UC Control | P3P Intervention | |||
| N | Percent | N | Percent | |
| Age | 59 | 30.4 | 66 | 33.3 |
| < 60 years | ||||
| ≥ 60 years | 135 | 69.6 | 132 | 66.7 |
| Education | 147 | 75.8 | 163 | 82.3 |
| > High school | ||||
| ≤ High school | 44 | 22.7 | 33 | 16.7 |
| Missing/Unknown | 3 | 1.5 | 2 | 1.0 |
| Marital/partner status | 140 | 72.2 | 140 | 70.7 |
| Married/partnered | ||||
| Not married/partnered | 51 | 26.3 | 58 | 29.3 |
| Missing/unknown | 3 | 1.6 | 0 | 0 |
| Race | 57 | 29.4 | 56 | 28.3 |
| Black | ||||
| White Hispanic | 4 | 2.1 | 8 | 4.0 |
| White non-Hispanic | 121 | 62.4 | 119 | 60.1 |
| Others | 12 | 6.2 | 15 | 7.6 |
| Working Status | 90 | 46.4 | 72 | 36.4 |
| Not working | ||||
| Working | 102 | 52.6 | 123 | 62.1 |
| Missing/unknown | 2 | 1.0 | 3 | 1.5 |
| Income | 50 | 25.8 | 47 | 23.7 |
| ≤ 39,999 | ||||
| >39,999 | 125 | 64.4 | 133 | 67.2 |
| Missing/Unknown | 19 | 9.8 | 18 | 9.1 |
| Web as an information source | 43 | 22.2 | 33 | 16.7 |
| No | ||||
| Yes | 135 | 69.6 | 147 | 74.2 |
| Missing/Unknown | 16 | 8.2 | 18 | 9.1 |
| D’Amico risk classification | 30 | 15.5 | 30 | 15.2 |
| High | ||||
| Intermediate | 91 | 46.9 | 103 | 52.0 |
| Low | 71 | 36.6 | 64 | 32.3 |
| Missing/unknown | 2 | 1.0 | 1 | 0.5 |
| Clinic | 16 | 8.2 | 16 | 8.1 |
| ATLVA | ||||
| BIDMC | 52 | 26.8 | 54 | 27.3 |
| BWH | 1 | 0.5 | 0 | 0 |
| DMC | 11 | 5.7 | 10 | 5.1 |
| EUH | 31 | 16.0 | 35 | 17.7 |
| GMH | 10 | 5.2 | 8 | 4.0 |
| LAMC | 19 | 9.8 | 20 | 10.1 |
| LBJ+ | 0 | 0 | 1 | 0.5 |
| NFNY | 2 | 1.0 | 2 | 1.0 |
| SJH | 38 | 19.6 | 40 | 20.2 |
| UMM | 3 | 1.5 | 3 | 1.5 |
| UVA | 11 | 5.7 | 9 | 4.5 |
Site closed after 4 months
ATLVA= Atlanta Veterans Administration, BIDMC=Beth Israel Deaconess Medical Center, BWH=Brigham and Women’s Hospital, DMC= Downey Medical Center, EUH= Emory University Hospital, GMH=Grady Memorial Hospital, LAMC=Los Angeles Medical Center, LBJ= Lyndon B Johnson, NFNY= Niagara Falls, New York, SJH= St. Joseph’s Hospital, UMM=University of Massachusetts Memorial, UVA= University of Virginia
Baseline DCS scores did not differ by group with an overall median of 45 and mean of 43 (SD=25); this level mean score has been associated with feeling unsure about following through with a decision.16 Decisional control preferences and decision-making stages (Table 3) were similar between groups. Our sample majority preferred to share decision-making with the physician and were thinking about the options at the time of enrollment. The DCS had good internal consistency with alpha coefficients of 0.88 and 0.89 at baseline and 1-month, respectively. The Pearson’s correlation between baseline and 1-month DCS scores was 0.27, indicating a significant (P<.0001) but weak correlation.
Table 3.
Baseline decisional control preference and stage
| Study Group | ||||
|---|---|---|---|---|
| UC control | P3P Intervention | |||
| N | Percent | N | Percent | |
| Decisional Control Preference | 62 | 32.0 | 64 | 32.3 |
| Myself | ||||
| Share | 116 | 59.8 | 123 | 62.1 |
| Doctor | 9 | 4.6 | 7 | 3.5 |
| Missing/Unknown | 7 | 3.6 | 4 | 2.0 |
| Stage of Decision | 15 | 7.7 | 13 | 6.6 |
| Haven’t started | ||||
| Haven’t started but want to start | 25 | 12.9 | 34 | 17.2 |
| Currently thinking about options | 97 | 50.0 | 90 | 45.5 |
| Close to choosing | 21 | 10.8 | 25 | 12.6 |
| Made a decision but willing to reconsider | 17 | 8.8 | 22 | 11.1 |
| Made decisions and won’t change my mind | 16 | 8.2 | 12 | 6.1 |
| Missing/Unknown | 3 | 1.5 | 2 | 1.0 |
| Current option (asked only of those who were close or had made a decision) | 5 | 2.6 | 4 | 2.0 |
| Active Surveillance | ||||
| External beam radiation therapy | 6 | 3.1 | 8 | 4.0 |
| Brachytherapy | 6 | 3.1 | 8 | 4.0 |
| Surgery | 30 | 15.5 | 35 | 17.7 |
| Combo-Radio | 1 | 0.5 | 1 | 0.5 |
| Other | 0 | 0 | 3 | 1.5 |
| Missing/unknown (were not close/made) | 146 | 75.3 | 139 | 70.2 |
By 1-month follow-up, all 305 participants except one (intervention group) had attended a physician consultation, 237 (78%) had attended two or more consults, and 269 (88%) participants had made a care decision. For intervention and UC groups, median 1-month DCS scores were 0 and 0, and means 10.9 (SD=16.7) and 9.9 (SD=18.0), respectively. In univariate ANCOVA, there was no significant difference by group in DCS score (Table 4). A higher DCS score was associated with not working, lower income, no decision by 1-month, and lower D’Amico risk. DC varied significantly between sites, with greatest conflict reported by participants enrolled at Emory University Hospital.
Table 4.
Univariate association of selected variables with 1-month decisional conflict score and final multivariable model, ANCOVA approach (N=276)
| Univariate Association | Multivariable model+ | |||
|---|---|---|---|---|
| Estimate (95% CI) | P-value | LS mean (95% CI) | P-value | |
| Study groups: P3P vs UC | −0.96 | 0.63 | −5.00 (−9.40, −0.59) | 0.03 |
| Age: <60 vs. ≥ 60 | 0.37 | |||
| Education: > HS vs. ≤ HS | 0.53 | |||
| Married/partnered vs. others | 0.89 | 5.18 (1.11, 9.26) | 0.01 | |
| Not working vs. working | 3.87 (−0.11, 7.85) | 0.06 | ||
| Household income: ≤ 39,999 vs. >39,999 |
7.36 (2.53, 12.19) | 0.003 | 8.69 (4.43, 12.96) | <.0001 |
| Race: White vs. non-white | 0.15 | |||
| Web as information source: No vs. Yes |
0.48 | |||
| Decided at 1-month: No vs. Yes |
20.65 (16.42, 24.89) | <.0001 | 20.11(16.10, 24.13) | <.0001 |
| D’Amico risk: Low vs. high/intermediate |
4.07 (−0.05, 8.18) | 0.05 | 4.29 (0.80, 7.78) | 0.02 |
| Consults: < 2 vs. ≥ 2 | 8.78 (4.17, 13.38) | 0.0002 | 6.04 (1.83, 10.26) | 0.005 |
| Clinic+ (UVA as reference) | 0.05 | 0.02 | ||
| ATLVA | 1.28 (−11.04, 13.60) | 1.07 (−9.27, 11.41) | ||
| BIDMC | 2.13 (−6.71, 10.98) | 3.52 (−3.60, 10.65) | ||
| DMC | 6.56 (−5.74, 18.87) | 6.39 (−3.56, 16.34) | ||
| EUH | 10.41 (1.21, 19.61) | 11.02 (3.49, 18.56) | ||
| GMH | 7.76 (−5.34, 20.86) | 3.37 (−8.63, 15.36) | ||
| LAMC | 5.07 (−4.87, 15.01) | 4.75 (−3.66, 13.15) | ||
| SJH | 0.85 (−8.34, 10.05) | 3.15 (−4.39, 10.69) | ||
| Study group & marital status† | 0.06 | |||
| Study group & number of consults† | 0.07 | |||
Interaction effects are illustrated in Figure 2
Three clinics with fewer than 10 participants each were excluded
HS=high school, LS=least square; UVA= University of Virginia, ATLVA= Atlanta Veterans Administration, BIDMC=Beth Israel Deaconess Medical Center, DMC= Downey Medical Center, EUH= Emory University Hospital, GMH=Grady Memorial Hospital, LAMC=Los Angeles Medical Center, SJH= St. Joseph’s Hospital
In the multivariable model, the P3P intervention group reported an estimated 5 units (95% CI, 0.59–9.40) lower DCS score (p=0.03) at 1-month than the usual care group. A higher DCS score was associated with lower income, not making a decision by 1-month, low D’Amico risk and clinic site. A marginally significant interaction was detected between study group and marital status (p=0.06), suggesting that single men in the intervention group had lower DC than all other participants (Figure 2a). Another marginal interaction was observed between study group and number of consults (p=0.07), indicating that having < 2 consults in the usual care group resulted in higher DC than all other participants (Figure 2b).
Figure 2.
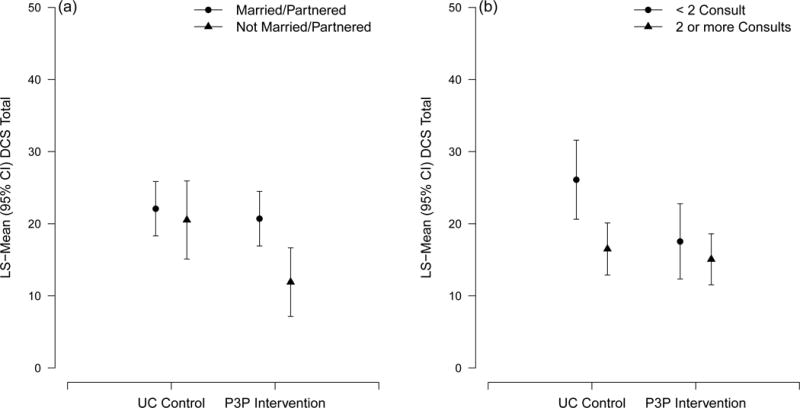
a–b: Four-group Least Square mean plot showing interactions detected in the multivariable model for DCS score: (a) between study group and marital status; (b) between study group and number of consults.
Discussion
In a second multi-center randomized trial, the P3P for men with clinically LPC significantly reduced DC when most men are contemplating an approach to management, over the few weeks post-biopsy. A racially diverse sample was enrolled of typical ages for LPC diagnoses and D’Amico risk classifications were distributed as expected.20 Given that eligibility was limited to men who had one post-biopsy consult, we are confident that we enrolled men at the earliest point during decision-making.
The t-test of DCS scores at 1-month revealed no differences between study groups. Unlike other studies21,22 which measured conflict immediately (same day) after interventions, our trial focused on the conflict experienced during the near-term decision-making period, approximated to one-month from enrollment. As noted in our previous multi-center trial of P3P,9 demographics and clinical situations, were influential during this decision-making period with regard to DC. This multivariable analysis illustrated such an influence and, when adjusted for, the P3P intervention significantly reduced decisional conflict. Other investigative groups have demonstrated positive effects of DA for localized prostate cancer,23,24 but not in a geographically and racially diverse US sample.
Marital status and income were influential predictors of DC. No other publication has described marital status as a predictor of outcome DCS. The interaction between study group and marital/partner status suggests that P3P had a stronger benefit for single men who may have had less help for information gathering. Alternatively, being in a relationship may have created more conflict as the man considered another person, relative to survival and adverse outcomes. No other investigative group has analyzed and reported the influence of income on outcomes. Yet our findings reveal income as an important predictor of DC, both as an independent variable and in interaction analysis with study group. Having an annual household income ≤ $40,000 was associated with higher DC, again indicating that men with fewer resources were at disadvantage.
No decision by 1-month was a strong predictor for the DCS score. Men who remained undecided were likely experiencing more DC. Men who sought consultation from more than one provider between study entry and 1-month reported less DC. Further, the interaction between number of consults and study group reveals the DC jeopardy for men in the control group who had only one consult; the intervention provided men with only one consult a result similar to those with multiple consults in either group. Clearly, exposure to multiple consults with specialists had beneficial effect on DC. Health systems will want to consider methods of providing at least two consultations.
The differences in DC between clinic sites are likely related to unknown variables or unexplored relationships between known variables. Emory University Hospital does stand out as a site associated with higher 1-month DC. However, there are no obvious explanations as the site was similar to other academically-affiliated urban sites. In the first P3P trial, we found site differences in baseline DC explained by the distinct socio-demographic characteristics of men at various sites.25 Further analysis of such factors is warranted for these new trial data.
Finally, D’Amico risk classification was among the strong covariates influencing the DCS score. Given the importance of the new AUA guidelines1 and increasing numbers of men with low risk offered active surveillance, our findings illustrate this contemporary dilemma. Men with more options may experience higher conflict. The man with intermediate or high risk disease also may choose between options but typically, all are active treatments.
Limitations include the nonblinded trial design, as P3P delivers an intervention to both patients and clinicians, and significantly fewer men in the younger group (< 60) returned a 1-month outcome measure. Thiel and colleagues26 found a similar age disparity for return of quality of life questionnaires after prostatectomy. Older men may feel more obligated to follow through with a previous agreement. Also, we excluded the lowest accruing sites from the final analytic sample, mainly independent or non-networked practices. Our results are most pertinent to larger health network sites. Finally, we selected the low literacy version of the DCS in order to meet the needs of a diverse sample of men. The fewer items and fewer response options resulted in total score 1-month medians of 0, perhaps limiting the questionnaire’s ability to discriminate between levels of conflict.
Future research is warranted to understand more about reaching men with limited resources. Little support is available for partners of men with LPC; a future development area. Further, P3P could be re-designed to provide optional versions, basic and advanced for men and/or partners with low and high literacy or those who may or may not be Internet-savvy.
Providing clinic access through an Internet-connected device can be accomplished along with headsets. Clinicians are welcome to view a demonstration site (https://p3p.cirg.washington.edu/demo) and refer patients to the active link (https://p3p4me.org/users/login).
Conclusions
In a multi-institutional sample, P3P demonstrated a beneficial effect for men with LPC as they engaged in decision-making for how to manage the cancer. Other variables impacted conflict and modified P3P’s effect, notably risk level and men’s resources. This aid to shared decision-making may be helpful to support men recently diagnosed with LPC.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Taylor Hendel for her skilled manuscript review and preparation. We are grateful to all participants and study site staff.
Funding: National Institutes of Health, National Institute for Nursing Research R01NR009692
Footnotes
CTN: NCT01844999
References
- 1.Sanda M, Chen R, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. American Urological Association Education and Research, Inc; 2017. [Google Scholar]
- 2.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA : the journal of the American Medical Association. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 3.Tsai H-T, Philips G, Taylor KL, et al. Use and Predictors of Expectant Management of Low and Intermediate Risk Localized Prostate Cancer in Elderly Men. Urology Practice. 4:132–9. doi: 10.1016/j.urpr.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer BA, Miller DC, Litwin MS, et al. Variations in quality of care for men with early-stage prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3735–42. doi: 10.1200/JCO.2007.13.2555. [DOI] [PubMed] [Google Scholar]
- 5.Adsul P, Wray R, Boyd D, et al. Perceptions of Urologists About the Conversational Elements Leading to Treatment Decision-Making Among Newly Diagnosed Prostate Cancer Patients. Journal of cancer education: the official journal of the American Association for Cancer Education. 2016 Mar 31; doi: 10.1007/s13187-016-1025-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Berry DL, Ellis WJ, Woods NF, et al. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urologic oncology. 2003;21:93–100. doi: 10.1016/s1078-1439(02)00209-0. [DOI] [PubMed] [Google Scholar]
- 7.Bailey DE, Jr, Wallace M, Mishel MH. Watching, waiting and uncertainty in prostate cancer. J Clin Nurs. 2007;16:734–41. doi: 10.1111/j.1365-2702.2005.01545.x. [DOI] [PubMed] [Google Scholar]
- 8.Steginga SK, Occhipinti S, Gardiner RA, et al. Prospective study of men’s psychological and decision-related adjustment after treatment for localized prostate cancer. Urology. 2004;63:751–6. doi: 10.1016/j.urology.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Berry DL, Halpenny B, Hong F, et al. The Personal Patient Profile-Prostate decision support for men with localized prostate cancer: a multi-center randomized trial. Urologic oncology. 2013;31:1012–21. doi: 10.1016/j.urolonc.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Showalter TN, Mishra MV, Bridges JF. Factors that influence patient preferences for prostate cancer management options: A systematic review. Patient preference and adherence. 2015;9:899–911. doi: 10.2147/PPA.S83333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saigal CS, Lambrechts SI, Seenu Srinivasan V, et al. The Voice of the Patient Methodology: A Novel Mixed-Methods Approach to Identifying Treatment Goals for Men with Prostate Cancer. The patient. 2016 doi: 10.1007/s40271-016-0203-y. [DOI] [PubMed] [Google Scholar]
- 12.Scherr KA, Fagerlin A, Hofer T, et al. Physician Recommendations Trump Patient Preferences in Prostate Cancer Treatment Decisions. Medical decision making : an international journal of the Society for Medical Decision Making. 2017;37:56–69. doi: 10.1177/0272989X16662841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry DL, Wang Q, Halpenny B, et al. Decision preparation, satisfaction and regret in a multi-center sample of men with newly diagnosed localized prostate cancer. Patient education and counseling. 2012;88:262–7. doi: 10.1016/j.pec.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolpin S, Halpenny B, Sorrentino E, et al. Usability Testing the “Personal Patient Profile-Prostate” in a Sample of African American and Hispanic Men. Comput Inform Nurs. 2016 doi: 10.1097/CIN.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 15.Berry DL, Halpenny B, Wolpin S, et al. Development and evaluation of the personal patient profile-prostate (P3P), a Web-based decision support system for men newly diagnosed with localized prostate cancer. Journal of medical Internet research. 2010;12:e67. doi: 10.2196/jmir.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.User Manual-Decisional Conflict Scale. Ottawa Health Research Institute. 2010 (Accessed 15 Feb, 2017 at http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf.)
- 17.Berry DL, Halpenny B, Bosco JL, et al. Usability evaluation and adaptation of the e-health Personal Patient Profile-Prostate decision aid for Spanish-speaking Latino men. BMC Med Inform Decis Mak. 2015;15:56. doi: 10.1186/s12911-015-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagerlin A, Rovner D, Stableford S, et al. Patient education materials about the treatment of early-stage prostate cancer: a critical review. Annals of internal medicine. 2004;140:721–8. doi: 10.7326/0003-4819-140-9-200405040-00012. [DOI] [PubMed] [Google Scholar]
- 19.Linder SK, Swank PR, Vernon SW, et al. Validity of a low literacy version of the Decisional Conflict Scale. Patient education and counseling. 2011;85:521–4. doi: 10.1016/j.pec.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8146–51. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 21.Chabrera C, Zabalegui A, Bonet M, et al. A Decision Aid to Support Informed Choices for Patients Recently Diagnosed With Prostate Cancer: A Randomized Controlled Trial. Cancer nursing. 2015;38:E42–50. doi: 10.1097/NCC.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 22.Johnson DC, Mueller DE, Deal AM, et al. Integrating Patient Preference into Treatment Decisions for Men with Prostate Cancer at the Point of Care. The Journal of urology. 2016;196:1640–4. doi: 10.1016/j.juro.2016.06.082. [DOI] [PubMed] [Google Scholar]
- 23.Mishel MH, Germino BB, Lin L, et al. Managing uncertainty about treatment decision making in early stage prostate cancer: a randomized clinical trial. Patient education and counseling. 2009;77:349–59. doi: 10.1016/j.pec.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Feldman-Stewart D, Tong C, Siemens R, et al. The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial. Medical decision making : an international journal of the Society for Medical Decision Making. 2012;32:616–26. doi: 10.1177/0272989X11434601. [DOI] [PubMed] [Google Scholar]
- 25.Underhill ML, Hong F, Berry DL. When study site contributes to outcomes in a multi-center randomized trial: a secondary analysis of decisional conflict in men with localized prostate cancer. Health and quality of life outcomes. 2014;12:159. doi: 10.1186/s12955-014-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiel DD, Davidiuk AJ, Broderick GA, et al. Comparison of patient-reported quality of life outcome questionnaire response rates between patients treated surgically for renal cell carcinoma and prostate carcinoma. BMC urology. 2015;15:58. doi: 10.1186/s12894-015-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


