Approximately 30% of patients with sickle cell anemia (SCA) develop chronic kidney disease (CKD) and 14–18% of SCA patients progress to end stage kidney disease [1]. The presence of CKD increases the risk of other systemic complications and reduces the median survival in SCA from 51 years to 29 years [1]. Proteinuria and microalbuminuria have recently been adopted as clinical markers for the detection of the earlier stages of CKD [2]. While the prevalence of proteinuria and albuminuria in SCD is relatively high [3], universal renal concentrating defect may lead to under-detection of urinary protein in clinical settings [4]. To date there are no reliable, specific and sensitive clinical biomarkers for CKD in SCD. We hypothesize combining mass-spectrometry results of unsupervised analyses with the underlying biological process of CKD development in SCA is a feasible approach for discovery and validation of disease-specific diagnostic biomarkers.
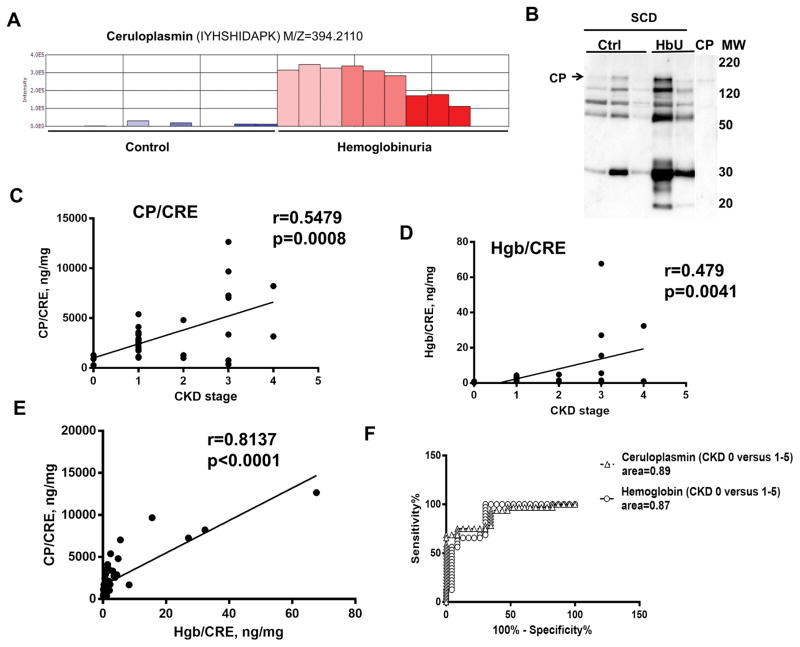
We first applied a mass-spectrometry based approach to analyze urine samples obtained from 20 SCA patients (8 females and 12 males) without albuminuria, defined as urinary albumin-to-creatinine ratio <30 mg/g creatinine. Urinary creatinine, albumin and eGFR were measured as previously described patients [5] (see Supplemental Methods and Supplemental Table 1). Urinary hemoglobin was measured by ELISA and all samples were separated into two groups: hemoglobin/creatinine ratio Hgb/CRE<0.4ng/mg (14 samples, SCA controls) and Hgb/CRE>0.8ng/ml (6 samples). The latter group also contained two samples with hemoglobinuria detected by dipstick as previously reported [5]. In normal controls, Hgb/CRE elvels were less than 0.8ng/ml (11 samples, Supplemental Table 1). Because recent studies demonstrated that hemoglobinuria was associated with CKD progression in SCA patients [5], we conducted mass-spectrometry proteomic analysis on SCA samples with hemoglobinuria and high hemoglobin (6 samples) an compared them to the SCA control samples (see Supplemental Fig. S1 and Supplementary methods). Spectra were analyzed with Proteome Discoverer 1.4 and quantified by SIEVE 2.1 using 3 samples from each group (Supplemental Fig. S2A). Data were normalized by creatinine values. Results from SIEVE 2.1 were exported into the Ingenuity Pathway Analysis. A total of 270 proteins were initially identified (Supplemental Table 2) of which 48 were significantly up- or down-regulated in the samples with hemoglobinuria (p<0.0001, Supplemental Table 3). Out of these 48 dysregulated proteins, we focused on eighteen extracellular proteins (Supplemental Table 4). Ceruloplasmin (CP), a protein involved in iron metabolism, was identified among the 18 extracellular proteins (Supplemental Table 4 and Fig. 1A and Supplemental Fig. S2B). We focused on CP, wherease the rest of the proteins will be validated in future studies. CP was present at much higher levels in the SCA samples with hemoglobinuria compared to SCA controls (Fig. 1A, 31-fold, p=1.8×10−5, Supplemental Table 4).
Figure 1. Ceruloplasmin (CP) in urine correlates with development of CKD in SCD patients.
(A) CP is identified by proteomic analysis of urine. Urine samples with and without hemoglobinuria (N=3 each) were selected from 20 SCD patients without proteinuria and albuminuria. SCD samples without hemoglobinuria were defined as SCD controls. Urine proteins were concentrated and desalted by centrifugation, trypsinized and analyzed by mass spectrometry. Proteins were identified by Proteome Discoverer 1.4 and quantified by SIEVE 2.1. Average intensities shown were derived from integration of ion elution profiles. (B) CP is present in urine samples. Urine samples from SCD without and with hemoglobinuria were separated by SDS PAGE and analyzed by Western blotting with antibodies against CP. Arrow indicates a position of CP. (C–D) Urine ceruloplasmin and hemoglobin correlated with stages of CKD. Correlation analysis was conducted for (C) ceruloplasmin (CP) and (D) hemoglobin (Hgb) to creatinine (CRE) ratios with stages of CKD in 34 SCD patients. Pearson correlation analysis was performed using GraphPad software in 34 SCD patients with different stages of CKD (0–5). (E) Correlation analysis of urinary hemoglobin (Hgb) and CP. CP/CRE and Hgb/CRE ratios are shown. Pearson correlation analysis was performed using GraphPad software. (F) Specificity and sensitivity of urinary ceruloplasminin SCD patients. ROC analysis of urinary CP/CRE and Hgb/CRE is shown for urine samples from SCD patients with stage 0 CKD versus stage 1–5 CKD. The analysis was performed using GraphPad software.
Urinary CP presence was further validated by ELISA which was normalized using urinary creatinine concentrations. The mean CP/CRE ratios for samples with hemoglobinuria was slightly higher than the mean of CP/CRE ratios for samples without hemoglobinuria (Supplemental Fig. S3, median 1157 ng/mg in samples with hemoglobinuria vs. 755 ng/mg in samples without hemoglobinuria; p=0.192). The presence of CP in urine was further confirmed by Western blot which detected the full size CP (MW=151 kDa) along with several faster migrating bands that might represent proteolytic degradation products of CP (Fig. 1B).
We next analyzed additional proteins involved in iron metabolism including urinary transferrin (TF), and ferritin (Ftn) in the urine of 20 SCA patients described above. TF was detected in the urine samples by mass-spectrometry, but TF levels were similar in the samples with versus without hemoglobinuria (Supplemental Tables 2–4). Urinary Ftn was not detected by mass-spectrometry analysis. Urinary levels of CP, Ftn, TF and Hgb were measured using ELISA assays in 20 samples obtained from SCA patients described above and in an additional 19 samples obtained from healthy subjects without SCA (defined as control for this study). Urinary CP/CRE, TF/CRE and Ftn/CRE ratios were significantly increased in SCA patient samples compared to healthy non-SCD controls (Supplementary Fig. S4A, CP/CRE 914.1±141.4 ng/mg in SCD vs. 129.1±18.81 ng/mg in control, p=4.5×106), (Supplementary Fig. S4B, TF/CRE 341.4±32.66 ng/mg in SC vs. 183.5±30.15 ng/mg in control, p=0.014) and (Supplementary Fig. S4C, TF/CRE 11.4±28.49 ng/mg in SCD vs. 11.18±1.81 ng/mg in control, p=0.0047). We did not find statistically significant difference in urinary Hgb/CRE levels between controls and SCA patients (Supplementary Fig. S4D, 0.867± 0.3968 ng/mg in SCA vs. 1.356± 0.367 ng/mg in control, p=0.4).
Previously, hemoglobinuria was shown to be associated with progression of CKD [5]. To test a relationship between urinary CP levels and CKD stage, we used samples from an additional 34 SCA patients who were at different stages of CKD (0–5) (stage0: 3 patients; stage 1: 19 patients, stage 2: 3 patient, stage 3: 7 patients, stage 4/5: 2 patients). Urinary levels of CP, Ftn, Hgb and TF were measured using ELISA. CP/CRE ratios positively correlated with CKD stage (N= 34, r=0.548, p=0.0008) (Fig. 1C) similar to urinary Hgb/CRE ratios (N= 34, r=0.479, p=0.0041) (Fig. 1D). In contrast, TF/CRE (N= 34, r=0.27, p=0.1063) (Supplementary Fig. S5A) and Ftn/CRE ratios (N= 34, r=0.163, p=0.3580) (Supplementary Fig. S5B) did not correlate with CKD stage. As expected, urine CP/CRE ratios correlated well with urine Hgb/CRE ratios (Fig. 1D, N=54, r=0.814, p<0.0001). ROC analysis showed high sensitivity and specificity for both urinary CP (area under curve 88.99±4.28%; p<0.0001, sensitivity 68.75%, specificity 95.65%) and Hgb (area under curve 86.96±5.20%; p<0.0001, sensitivity 56.25%, specificity 95.65%) in samples with CKD stage 1–5 versus CKD stage 0 (Fig. 1E).
Despite the increased urinary levels of CP, TF and Ftn in the urine of SCA patients, only urinary CP demonstrated overall good correlations with CKD stage and urinary Hgb levels and represent a plausible non-invasive biomarker of CKD risk in SCA patients. Our study suggests that urinary CP may complement urinary free hemoglobin as a non-invasive biomarker for CKD in SCA patients. Our study also highlights the importance of cell-free hemoglobin and iron metabolism in the pathophysiology of CKD in SCA.
Supplementary Material
Acknowledgments
This work was supported by NIH research grants P50HL118006, 1R01HL125005, 5G12MD007597 and K23HL125984. We thank Keona Wynne for technical help.
Footnotes
CONTRIBUTORS
All authors contributed significantly to the study.
References
- 1.Airy M, Eknoyan G. The kidney in sickle hemoglobinopathies. Clinical nephrology. 2017;87(2017):55–68. doi: 10.5414/CN108991. [DOI] [PubMed] [Google Scholar]
- 2.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical practice. 2012;120:c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 3.Drawz P, Ayyappan S, Nouraie M, et al. Kidney Disease among Patients with Sickle Cell Disease, Hemoglobin SS and SC. Clinical journal of the American Society of Nephrology : CJASN. 2016;11:207–215. doi: 10.2215/CJN.03940415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatch FE, Culbertson JW, Diggs LW. Nature of the renal concentrating defect in sickle cell disease. The Journal of clinical investigation. 1967;46:336–345. doi: 10.1172/JCI105535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saraf SL, Zhang X, Kanias T, et al. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. British journal of haematology. 2014;164:729–739. doi: 10.1111/bjh.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.