Abstract
Objectives
KV channels are important regulators of vascular tone, but the identity of specific KV channels involved and their regulation in disease remain less well understood. We determined the expression of KV1 channel subunits and their role in cAMP-mediated dilation in coronary resistance arteries from subjects with and without coronary artery disease (CAD).
Methods
Human coronary arteries and arterioles (HCA) from non-CAD and CAD patients were assessed for mRNA and protein expression of KV1 channel subunits with molecular techniques and for vasodilator response with isolated arterial myography.
Results
Assays of mRNA transcripts, membrane protein expression, and vascular cell-specific localization revealed abundant expression of KV1.5 in vascular smooth muscle cells of non-CAD HCAs. Isoproterenol and forskolin, two distinct cAMP-mediated vasodilators, induced potent dilation of non-CAD arterioles, which was inhibited by both the general KV blocker 4-AP and the selective KV1.5 blocker DPO-1. The cAMP-mediated dilation was reduced in CAD and was accompanied by a loss of or reduced contribution of 4-AP-sensitive KV channels.
Conclusions
KV1.5, as a major 4-AP-sensitive KV1 channel expressed in coronary VSMCs, mediates cAMP-mediated dilation in non-CAD arterioles. The cAMP-mediated dilation is reduced in CAD coronary arterioles, which is associated with impaired 4-AP-sensitive KV channel function.
Keywords: potassium channels, voltage-gated potassium channels, vasodilation, coronary arteries, coronary artery disease
1 INTRODUCTION
Potassium (K+) channels in the vascular smooth muscle cell (VSMC) of resistance arteries and arterioles play a central role in the regulation of vascular tone.1–4 Current evidence indicates that VSMCs express at least four distinct classes of K+ channels: voltage-gated (KV), calcium-activated (KCa), inwardly rectifying (Kir) including ATP-sensitive (KATP), and two pore domain (K2P) K+ channels.1,5 Although the relative importance of each class may vary depending on the species and vascular beds, accumulating studies support the role for KV, particularly shaker-family KV (KV1) channels in the regulation of the resting vascular tone and vasodilation of the microvasculature in response to various endothelial, neurohumoral, and metabolic factors.6–9 For instance, H2O2, an endothelium-derived hyperpolarization factor10–12 as well as a metabolic dilator released from beating cardiac myocytes13,14 activates KV channels to induce dilation in the canine, rat, and porcine coronary circulation.7,15,16 Similarly, stimulation of β2-adrenoceptors (e.g., with isoproterenol), an important mechanism of coronary blood flow increase during exercise,17 activates 4-AP sensitive KV channel currents in rabbit portal vein and rat coronary artery.18,19 Alterations in KV channel function have been found in animal models of hypertension, metabolic syndrome, and diabetes.4,6,20–22. Although KV channels have been studied in various animal models, the functional roles of these channels in the human microvasculature are less well understood.23
Recently, our laboratory reported that KV1.5 is the major type of KV1 channels expressed and active in VSMCs of human adipose arterioles.24 We also found that hydrogen peroxide (H2O2) induces potent smooth muscle-dependent vasodilation that is largely mediated by KV1.5 and large-conductance Ca2+-activated K+ (BKCa) channels in human adipose arterioles from subjects without coronary artery disease (non-CAD).24 This H2O2-induced dilation is reduced in adipose arterioles from subjects with coronary artery disease (CAD) and is accompanied by a loss of KV1.5- but not BKCa-dependent dilation.24 The expression of specific KV channel subunit gene products and proteins varies among species, vascular beds, or vessel size.25 In addition, there is evidence that certain vascular beds such as the coronary circulation may adapt to limit the deleterious effects on vascular function during disease.26,27 It remains unknown whether results obtained in adipose arterioles regarding the expression and function of KV1 channels, as well as their potential alterations in disease, are also applicable to other vascular beds of humans. In the present study, we identified major KV1 channel subtypes in human coronary arteries/arterioles (HCA) using molecular and immunohistochemical approaches. We also examined the functional role of KV1 channels in cAMP-mediated vasodilation induced by the β2-adrenergic receptor agonist isoproterenol and the adenylate cyclase activator forskolin in isolated human coronary arterioles from non-CAD and CAD subjects.
2 MATERIALS AND METHODS
2.1 Tissue acquisition
Fresh human atrial tissues were obtained as discarded surgical specimens (n=21) from patients undergoing cardiopulmonary by-pass surgeries. Unused whole hearts (n=22) were acquired from the Wisconsin Organ Donor Network. De-identified patient demographic data were collected using the Generic Clinical Research Database at the Medical College of Wisconsin. All procedures were approved by the Institutional Review Board of the Medical College of Wisconsin/Froedtert Hospital. Patient demographic information is summarized in Table 1.
Table 1.
Patient demographics (n=43)
| Atria (n=21)
|
Donor heart (n=22)
|
|||
|---|---|---|---|---|
| non-CAD (n=7) | CAD (n=14) | non-CAD (n=13) | CAD (n=9) | |
|
|
|
|
|
|
| Gender, Male/Female | 4(57%)/3(43%) | 6(43%)/8(57%) | 5(38%)/8(62%) | 6(67%)/3(33%) |
| Age, yr (mean ± SEM) | 62±5 | 63±3 | 43±5 | 60±3 |
| Body mass index (mean ± SEM) | 32±3 | 27±2† | 27±2 | 31±2 |
| Underlying diseases/Risk factors | ||||
| Coronary artery disease (CAD) | 0 | 14(100%) | 0 | 9(100%) |
| Hypertension | 4(57%) | 9(64%) | 3(23%) | 6(67%) |
| Hyperlipidemia | 3(43%) | 7(50%) | 1(8%) | 2(33%) |
| Diabetes mellitus | 0 | 0 | 0 | 1(11%) |
| Atrial fibrillation | 0 | 4(29%) | 1(8%) | 0 |
| Congestive heart failure | 0 | 3(21%) | 0 | 0 |
| Myocardial infarction | 0 | 3(21%) | 0 | 4(44%) |
| None of the above | 0 | 0 | 8(62%) | 0 |
Total of 1 patient with no information on height and weight.
2.2 Vascular Reactivity
Human coronary arterioles (internal diameter, 100 to 200 μm) were dissected from the atrial tissue and mounted in a wire myograph (Danish Myo Technology A/S) for isometric tension recording (Figs. 4–5) as previously described.28 In some experiments (Fig. 6), human coronary arterioles (50 to 150 μm) were cannulated and pressurized (60 mmHg) with two glass micropipettes, and the internal diameter of arterioles was measured with a video system (Boeckeler VIA-100).29 Arterioles were preconstricted with endothelin-1 to approximately 30–50% of the tension developed during exposure to 80 mM high K+ in physiological saline solution (wire myograph) or the baseline internal diameter (cannulated preparation). Relaxation responses to cumulative concentrations of the mixed β2-adrenergic receptor agonist isoproterenol (10−9 to 10−5 M), the adenylate cyclase activator forskolin (10−9 to 10−5 M), or the nitric oxide donor sodium nitroprusside (10−9 to 10−4 M) were determined in the absence and/or presence of 30 minutes preincubation with one of K+ channel modulators, including the general KV1 channel blocker 4-aminopyridine (4-AP; 5 × 10−3 M) and selective KV1.5 channel blocker diphenyl phosphine oxide-1 (DPO-1; 10−6 M). Unless otherwise indicated, experiments were performed on endothelium-intact arterioles and in the presence of the nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (10−4 M) and the cyclooxygenase inhibitor indomethacin (10−5 M). At the end of each experiment, papaverine (10−4 M), an endothelium-independent vasodilator, was added to the vessel bath to determine the maximal dilation for normalization of dilator responses. Vasodilator responses are expressed as a percentage of maximal relaxation relative to endothelin-1 constriction, with 100% representing full relaxation to basal tension or the maximal diameter. The average baseline (cannulated preparation) or normalized (wire myograph) internal diameter of arterioles used in vessel reactivity studies is 168±29 μm (n=31).
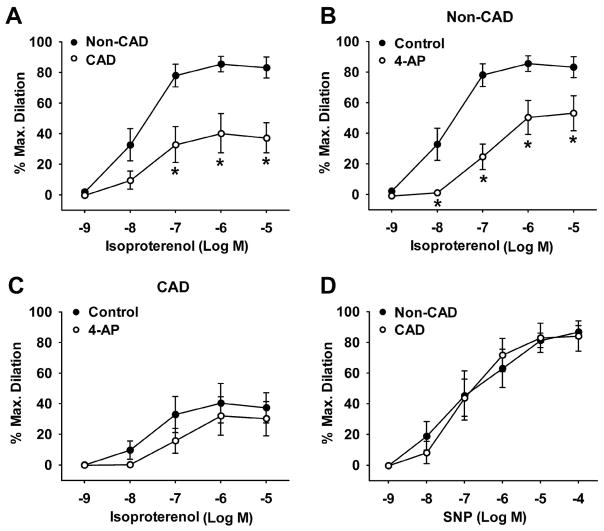
Figure 4. Role of KV channels in isoproterenol-induced dilation of human coronary arterioles from non-CAD and CAD subjects.
The β2-adrenergic receptor agonist isoproterenol induced dose-dependent dilation in non-CAD arterioles, and this dilation was significantly reduced in CAD arterioles (A). The isoproterenol-induced dilation was markedly reduced by the general KV1 channel blocker 4-AP (5 × 10−3 M) in non-CAD (B) but not in CAD vessels (C). Vasodilation to the NO donor sodium nitroprusside (SNP) was similar in non-CAD and CAD arterioles (D). *P<0.05 versus non-CAD or control; n=5–9 vessels/group.
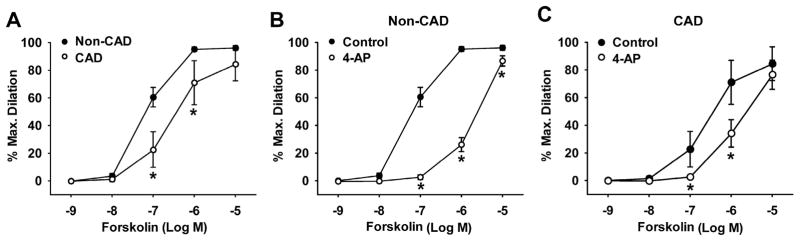
Figure 5. Role of KV channels in forskolin-induced dilation of human coronary arterioles from non-CAD and CAD subjects.
The adenylate cyclase activator forskolin-induced dilation was markedly reduced in CAD arterioles as compared with non-CAD arterioles (A). The dilation was reduced by the general KV1 channel blocker 4-AP (5 × 10−3 M) in non-CAD (B) and to a lesser extent in CAD (C) arterioles. *P<0.05 versus non-CAD or control; n=4–8 vessels/group.
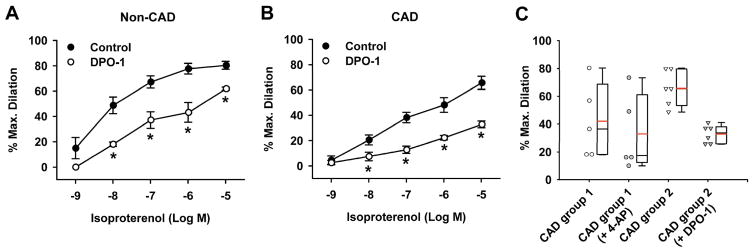
Figure 6. Role of KV1.5 in isoproterenol-induced dilation of human coronary arterioles from non-CAD and CAD subjects.
Isoproterenol-induced dilation was significantly reduced by selective KV1.5 channel blocker DPO-1 (10−6 M) in non-CAD arterioles (A). The dilation was also reduced by DPO-1 in CAD arterioles (B). *P<0.05 versus control; n=3–6 vessels/group. C, Dot density and associated box plots depicting the isoproterenol-induced maximal dilation of individual CAD arterioles used in two vessel groups for determining 4-AP- (Figure 4C) or DPO-1 (Figure 6B)-sensitive component of vasodilation, respectively. Mean dilation is indicated by red lines.
2.3 RT-PCR and qPCR
Human coronary arteries and arterioles (100–500 μm) were freshly dissected, cleaned of adherent tissue, and snap-frozen in liquid nitrogen and stored at −80°C until use. Some vessels were denuded of the endothelium to determine the mRNA expression of smooth muscle cells. Total RNA was extracted with RNeasy Fibrous Tissue Mini Kit (Qiagen), and cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen). cDNA (2 ng) was amplified using Platinum PCR Supermix (Invitrogen) and a 45-cycle touch-down protocol with gene-specific primers as previously described.29 Detailed sequence information is also shown in Table 2. Two negative controls, without reverse transcription (RT-) and without template (H2O), were amplified in parallel by using KVα9.3 primers. Human brain total RNA extracted from a normal donor (Agilent Technologies, Santa Clara, CA) was included as a positive control. Human brain tissue is known to express all 11 subunits of KV channels examined in this study.30
Table 2.
Nucleotide sequence of primers used for PCR
| For end-point PCR | ||
|---|---|---|
|
| ||
| Transcript | Forward and Reverse primers | Amplicon size, bp |
| KV α1.1 | 5′-AGT CGC ACT TCT CCA GTA TCC-3′ 5′-TCG GTC AGT AGC TTG CTC TTA T-3′ |
424 |
| KV α1.2 | 5′-TCA AGT TGT CCA GAC ACT CCA A-3′ 5′-TGT GTT AGC CAA GGT ACA GTT G-3′ |
548 |
| KV α1.3 | 5′-CTT CAG GTT TCA GCA GCA TCC-3′ 5′-GCA GGT GGC AGT GGA ATT G-3′ |
395 |
| KV α1.4 | 5′-GAG GCG GAT GAA CCT ACT ACC-3′ 5′-CCC TTT CCC TGA CAC TTC TCC-3′ |
401 |
| KV α1.5 | 5′-CCG TCT ACT TCG CAG AGG CT-3′ 5′-CCA GGC AGA GGG CAT AAA GG-3′ |
465 |
| KV α1.6 | 5′-AGA GGC TGA CGA TGA CGA TTC-3′ 5′-CCG ATG TGG TGT AGG AAG GTA G-3′ |
364 |
| KV α2.1 | 5′-GGC TTG CTC ATC CTC TTC CTT-3′ 5′-TCT CCT GTC TCT TCT GCT CCT T-3′ |
289 |
| KV α9.3 | 5′-GGC TTC TGC TTC TCT TCC TCT-3′ 5′-ACT GGT CCA CAT CAA TGT CCT T-3′ |
297 |
| KV β1.1 | 5′-CAG CAG CCT TAG TCC CTC AG-3′ 5′-GCC GTT CAG CAA CCT CAT CT-3′ |
239 |
| KV β1.2 | 5′-GTG GAG CAG CCG AAC AGA A-3 5′-GCC GTT CAG CAA CCT CAT CT-3′ |
192 |
| KV β1.3 | 5′-ACC TCT CGT GAC TGC CTG T-3′ 5′-GCC GTT CAG CAA CCT CAT CT-3′ |
378 |
| BKCaα | 5′-ATG CGG AAC TCA CCC AAC A-3′ 5′-TCG CCA AAG ATG CAG ACC AC-3′ |
224 |
| β-actin | 5′-GCT CGT CGT CGA CAA CGG CTC-3′ 5′-CAA ACA TGA TCT GGG TCA TCT TCT C-3′ |
353 |
|
| ||
| For real-time PCR (qPCR) | ||
|
| ||
| Transcript | Forward and Reverse | Amplicon size, bp |
|
| ||
| KV α1.5 | 5′-CGTCATCGTCTCCAACTTCAAC-3′ 5′-TTCCGCTGGACTCCTCTGT-3′ |
126 |
| ATP synthase O subunit | 5′-TCTCCTTTAGAAGAAGCCACACT-3′ 5′-GCACAATCATTCCACCCAAGAT-3′ |
124 |
| β-actin | 5′-GCACTCTTCCAGCCTTCCTT-3′ 5′-TGTGTTGGCGTACAGGTCTT-3′ |
114 |
Quantitative PCR was performed using CFX96 C1000 Thermal Cycler (BioRad). The reactions were conducted using 2x SsoAdvanced Universal SYBR Green Supermix (BioRad), KVα1.5-specific primers (Table 2), nuclease-free water and pooled sample cDNA for each non-CAD and CAD group (n=3/group, 2 ng each). Cycle threshold (Ct) values of products were determined using Bio-Rad CFX Manager 3.1 software (BioRad). Relative KVα1.5 gene expression was normalized to average of β-actin and ATP5o genes.31 Comparative Ct method was used to calculate relative expression of KVα1.5 subunit, where CAD was normalized to non-CAD for both intact and denuded pooled samples.
2.4 Immunoblotting
Human coronary arteries and arterioles (200–1000 μm) were freshly dissected, and membrane fraction proteins were isolated by using differential centrifugation method as described previously. 32 Membrane proteins of human brain tissue and total lysates of HEK-293 with or without human KVα1.5 overexpression were included as positive controls as previously described.24 Proteins (20 μg) were separated by 10% SDS-PAGE and membrane were blotted with a primary antibody against a specific KV1 α-subunit (1:2,000 dilution), followed by a horseradish-peroxidase conjugated secondary antibody (1:20,000 dilution). Membranes were developed using the ECL Prime reagent (Amersham). Detailed information on the KV1 antibodies and immunoblotting protocol has been presented in our recent study.24
2.5 Immunohistochemistry
Frozen sections (10 μm) of coronary arteries and arterioles were blocked with 5% normal goat serum and probed with primary antibodies that are specific to each KV1 α-subunit (1:200 dilution) and secondary antibodies (Alexa Fluor 568-conjugated goat anti-rabbit or anti-mouse IgG, 1:400 dilution) as previously described.24 Sections were counterstained with DAPI and images were captured using a confocal fluorescence microscope (model A1-R, Nikon).
2.6 Chemicals
Forskolin and DPO-1 were obtained from Tocris. All other chemicals were purchased from Sigma. Stock solutions were prepared in distilled water, except for the following: forskolin (DMSO); DPO-1 (ethanol); 4-AP (HCl, pH readjusted to 7.4); and indomethacin (10−1 M Na2CO3).
2.7 Statistical Analysis
All data are presented as mean±SEM. Comparisons of concentration-response curves of isolated vessels were performed using 2-way repeated measures analysis of variance (ANOVA), followed by the Student-Newman-Keuls multiple comparison test. P values <0.05 were considered statistically significant.
3. RESULTS
3.1 KV1 subunit mRNA and protein expression in non-CAD human coronary arteries
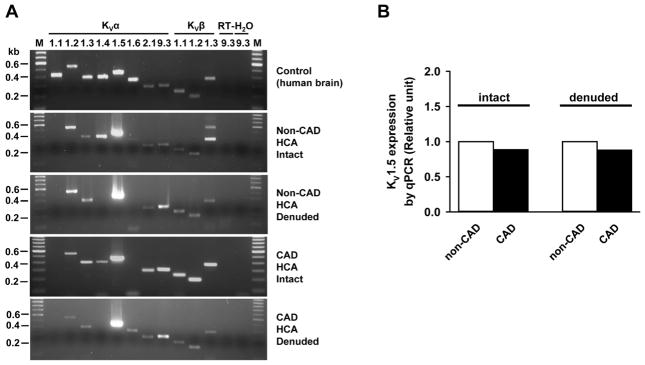
KV1 channels (shaker-related family) have been shown to mediate an important part of KV channel-related function in various vascular beds.25 Using non-CAD human adipose arteries, we recently reported that several KV1 α-subunits, including 1.1, 1.2, 1.4, and 1.5, were consistently found in different arterial samples, with KV1.5 being the most abundantly expressed KV1 α-subunit.24 In this study, we first examined mRNA transcripts of KV1 α and β subunits in HCAs from non-CAD subjects. Selected arteries were denuded of the endothelium with air bubble or 50μm-diameter stainless steel wire to examine the relative mRNA expression of these subunits in smooth muscle cells (n=5/group). As shown in Figure 1A, several KV1 subunits were found to be consistently expressed in both endothelium-intact and -denuded HCAs from non-CAD and CAD subjects, including KVα 1.2, 1.3, 1.5 (most abundant), and KVβ 1.1–1.3. KVα1.1, 1.4 and 1.6 were variably detected in some but not all samples. This mRNA expression profile is generally consistent with that of adipose arterioles, except that variable expression was noticed for KVα1.1 in HCAs but for KVα1.3 in adipose arterioles. As a positive control, the human brain tissue from a normal subject expressed all KV channel subunits examined in this study (Figure 1A). Analysis of relative mRNA expression of KV1.5 normalized to the mean of two housekeeping genes (β-actin and ATP5o) showed a slight decrease (approximately 22%) both in intact and denuded CAD vessels (Figure 1B, pooled sample, n=3/group).
Figure 1. The mRNA expression of KV1 channel subunits in human coronary arteries.
Representative gel images of RT-PCR amplification products from one endothelium-intact and one endothelium-denuded from non-CAD and CAD HCAs are shown in figure 1A. KVα1.2, 1.3, 1.5, 2.1, 9.3 and KVβ1.1–1.3 were consistently found in different arterial samples, whereas KVα1.1 1.4 and 1.6 subunits were variably detected. As a positive control, human brain tissue from a normal subject was found to express all KV channel subunits studied (top). RT-, without reverse transcription; H2O, without template; M, marker. Quantitative PCR revealed a slight reduction of KVα1.5 mRNA in CAD arteries (B).
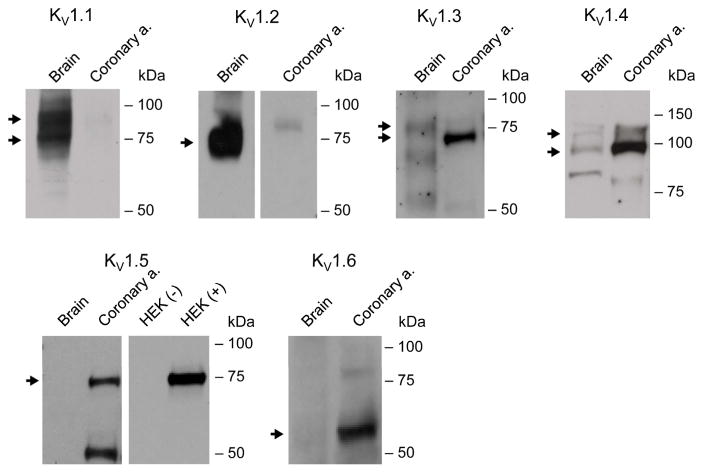
Next, we assessed the protein expression of KV1 channel forming α-subunits using the membrane fraction prepared from non-CAD HCAs. Consistent with mRNA expression from intact vessels, KV1.5 protein, as well as KV1.3 and KV1.4, was detected in all HCAs samples (Figure 2). KV1.6 protein was variably found in non-CAD HCAs, whereas KV1.1 and 1.2 proteins were not detected. As positive controls, the membrane fraction of human brain tissue and total cell lysates of HEK-293 cells overexpressing human KV1.5 were found to express KV1.1–1.2, and KV1.5, respectively.
Figure 2. The protein expression of KV1 channel-forming α-subunits in human coronary arteries.
Western blot analysis of KV1 α-subunits was performed using the membrane fraction of non-CAD HCAs. Consistent with high mRNA expression, KVα1.5 protein was detected in HCAs. KVα1.3, 1.4 and 1.6 were also detected in these samples. Arrow indicates mature forms of KV1 proteins, with the approximate molecular mass values as follows: 75 kDa doublet (1.1), 75 kDa (1.2), 75 kDa doublet (1.3), 110 kDa doublet (1.4), 75 kDa (1.5), and 60 kDa (1.6). Proteins prepared from human brain tissue and HEK-293 with (+) or without (-) human KV1.5 overexpression were included as positive controls.
3.2 Immunofluorescence localization of KV1 α-subunits in human coronary arteries
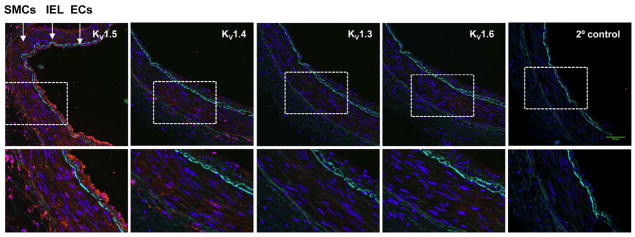
To determine the cell-type specific localization of KV1 α-subunits, we performed immunofluorescence on frozen sections (10 μm) of human coronary arteries and arterioles. KV1 proteins were labeled with Alexa-568 conjugated secondary antibodies which emit red fluorescence upon excitation and images were captured with a confocal fluorescence microscope. Autofluorescence, intrinsic to the internal elastic lamina could be detected in the green fluorescein isothiocyanate channel and was used to visually separate the endothelial cell (EC) from the SMC layer. As shown in Figure 3, KV1.5 was readily detected in both the SMC and EC layers. KV1.4, however, was modestly expressed in SMCs, with little or no EC expression. KV1.3 and KV1.6 proteins were faintly detected in SMCs, with little to no EC expression. Enlarged regions are indicated by the outline (bottom panels). No fluorescent signal was observed in the negative control specimen (2° antibody only), demonstrating specificity of 1° antibodies for their respective KV1 proteins.25
Figure 3. Immunofluorescence localization of KV1 α-subunits in human coronary arteries.
Representative confocal images show KV1 α-subunit proteins (red) in cross tissue sections (10 μm) of an intact non-CAD HCA. Amplified regions indicated by dotted outline are shown in bottom panels. KV1.5 was highly expressed in both smooth muscle cells (SMCs) and endothelial cells (ECs), with a modest expression of KV1.4 in SMCs. KV1.3 and KV1.6 were faintly detected in SMCs and/or ECs. Cell nuclei were stained with DAPI (blue). IEL, internal elastic lamina (IEL, green autofluorescence).
3.3 Role of KV channels in isoproterenol-induced dilation of human coronary arterioles
We next examined the functional role of KV1 channels in mediating physiological vascular responses in HCAs. Previous studies have found that 4-AP-sensitive KV1 channels mediate a major portion of the physiological dilation to the mixed β2-adrenergic receptor agonist isoproterenol in rat small coronary arteries. Furthermore, exposure of these coronary arteries to high glucose (a major risk factor for CAD) impairs the activity and vasodilator function of KV channels in coronary SMCs 19,33–35. It remains unknown whether KV1 channels mediate isoproterenol-induced dilation in HCAs and whether this vasomotor function is altered in diseases such as CAD. As shown in Figure 4A, isoproterenol-induced dilation was markedly reduced in CAD compared with non-CAD arterioles (% dilation at 10−7, 10−6, and 10−5 M isoproterenol, 33±12, 40±13, and 37±10 versus 78±7, 86±5, and 83±7 in non-CAD, respectively, n=5–9; P<0.05). In non-CAD arterioles, the dilation was markedly reduced by 4-AP (5 × 10−3 M), a general KV1 channel blocker (Figure 4B, % dilation at 10−7, 10−6, and 10−5 M isoproterenol, 25±8, 50±11, and 53±11, n=9; P<0.05 versus non-CAD control). In CAD arterioles, however, the dilation was not affected by 4-AP (Figure 4C, n=5). In contrast, dilation to the NO donor sodium nitroprusside (10−9 to 10−4 M) was not significantly altered in CAD arterioles (Figure 4D; n=5/group), suggesting that potential non-specific impairment of vasomotor function in CAD arterioles is unlikely. Together, these results indicate that KV1 channels mediate a significant component of beta-adrenergic dilation in non-CAD HCAs and this KV1-mediated dilation is impaired during CAD.
3.4 Role of KV channels in forskolin-induced dilation of human coronary arterioles
To further confirm the functional role of 4-AP-sensitive KV1 channels, we examined the vasodilator response to forskolin, a pharmacological adenylate cyclase activator that induces non-receptor-mediated vasodilation through a similar cAMP-mediated pathway. Forskolin elicited a potent concentration-dependent dilation of HCAs from non-CAD subjects, whereas the dilation was significantly reduced in CAD subjects (Figure 5A, % dilation at 10−7 and 10−6 M forskolin, 23±13 and 71±16 versus 61±7 95±2 in non-CAD, respectively, n=4–8; P<0.05). 4-AP (5 × 10−3 M) markedly reduced forskolin-induced dilation in non-CAD arterioles (Figure 5B, % dilation at 10−7 and 10−6 M forskolin, 3±2 and 26±5, n=8; P<0.05 versus non-CAD control) but by much less extent in CAD arterioles (Figure 5C, % dilation at 10−7 and 10−6 M forskolin, 3±1 and 34±10, n=4; P<0.05 versus CAD control). These results suggest that the reduced dilation to forskolin in CAD HCAs is partially due to an impairment of 4-AP sensitive KV1 channels.
3.5 Role of KV1.5 channel in isoproterenol-induced dilation of human coronary arterioles
We further evaluated the role of Kv1.5, as a major KV1 α-subunit expressed in HCAs (Figures 1–3), in mediating the vasodilator response to isoproterenol in HCAs. Although our recent study shows that the Kv1.5 channel represents a major isoform of KV1 channels that is functionally impaired in human adipose arterioles from CAD subjects,24 it is important to know whether this impairment extends to the human coronary circulation. Human coronary arterioles were preincubated with the selective KV1.5 blocker DPO-1 (10−6 M). This pretreatment markedly reduced isoproterenol-induced dilation in non-CAD vessels (Figure 6A, % dilation at 10−8, 10−7, 10−6, and 10−5 M isoproterenol, 18±2, 37±7, 43±8, and 43±8 versus 49±7, 67±5, 78±4, and 80±3 in non-CAD control, respectively, n=3; P<0.05). This suggests that KV1.5 serves as an important mediator of β adrenergic receptor-induced dilation in the human coronary microcirculation. Unexpectedly, DPO-1 also significantly reduced the dilation to isoproterenol in CAD vessels (Figure 6B, % dilation at 10−8, 10−7, 10−6, and 10−5 M isoproterenol, 8±3, 13±3, 22±2, and 33±3 versus 21±4, 38±4, 48±6, and 66±5 in CAD control, respectively, n=3; P<0.05). The DPO-1 data seem inconsistent with the apparent loss of 4-AP-sensitive dilation to isoproterenol during CAD (Figure 4C). We examined the contribution of the potential heterogeneous patient groups that have been included in each experimental protocol. As indicated in Figure 6C, baseline vasodilator responses are significantly higher in arterioles included for DPO-1 versus 4-AP protocol.
4 DISCUSSION
Bolstered by our recent findings that KV channels contribute less and BKCa channels contribute more to vasodilation of adipose arterioles from patients with CAD,24 the present study extends these observations by defining the expression and vasomotor contribution of KV1 channel subtypes in HCAs. The main new findings are as follows: (1) KV1.5 is a major isoform of KV1 channels expressed in VSMCs of coronary arteries from non-CAD subjects; (2) Isoproterenol and forskolin, two distinct cAMP-mediated vasodilators, induce potent dilation of non-CAD coronary arterioles via activation of 4-AP-sensitive KV (especially KV1.5) channels; and (3) Responses to both cAMP-mediated vasodilators are impaired in CAD coronary arterioles, which is associated with a loss of or reduced contribution of 4-AP-sensitive KV channels. Together, these results provide further support for the important role of VSMC KV1.5 channels in the regulation of vascular tone in the human microcirculation. The impairment of 4-AP-sensitive KV channel function and associated vasodilation is a consistent finding in both the coronary and adipose microvasculature, but a significant 4-AP-sensitive KV (and notably KV1.5) channel component remains in coronary arterioles during CAD.
KV1 channel expression and function in HCAs
The KV channel family consists of 12 subfamilies with over 40 known protein-encoding genes in humans and animals.36 Previous studies have shown the expression and function of specific KV channels are rather heterogeneous among species and vascular beds.25 However, there is a consensus that 4-AP-sensitive shaker-family KV1 are functionally important KV channels in many animal vascular beds and in a few human vasculature studied.25 Using freshly isolated human adipose arteries/arterioles, we recently reported that KV1.5 is the major KV1 channels expressed in VSMCs.24 This is important since KV1.5 mediates metabolic vasodilation in the heart.8 In the present study, immunofluorescence revealed that KV1.5 is also the most abundant KV1 channel-forming α subunit expressed in VSMCs of HCAs. Additional RT-PCR analysis showed that mRNA transcripts of KVα1.2, 1.3, 1.4, 1.5 (most abundant), as well as KVβ1.1–1.3, are present in HCAs from non-CAD subjects. Using immunoblotting analysis, KVα1.3–1.6 but not KV1.1–1.2 were also detected at the protein level. Overall, these observations are consistent with those of our previous study in human adipose arterioles, thus suggesting that the transcriptional and translational regulation of KV1 subunits may be relatively conserved in different vascular beds in humans. Several questions remain to be solved in future studies. As reported in several previous studies,25,37 we found that the number of KV1 α subunits expressed at the mRNA level is larger than that of the functional KV1 channels observed in human arterioles. The role of endothelial KV1 channels such as KV1.5, which was detected in ECs of HCAs, is largely unknown. In addition, the vascular and cell-specific expression of KV1.3, 1.4, and 1.6 channel subunits may require verification with different antibodies.
In this study, we used two different coronary vasodilators to elicit KV-mediated vasodilation. Currently, there are no specific direct KV1 channel openers. We instead used the mixed β2-adrenoceptor agonist isoproterenol, which activates 4-AP-sensitive KV channel currents in rabbit portal vein and rat coronary artery.18,19 Isoproterenol activates the adenylate cyclase-cAMP-protein kinase A pathway that in turn phosphorylates and opens KV channels.18 The adenylate cyclase activator forskolin also induces vasodilation by opening K+ channels, such as KV channels, 38,39 but its action requires no receptor activation. Forskolin increases whole-cell K+ currents in rabbit coronary arteries in a 4-AP-sensitive fashion 38,39 Both isoproterenol and forskolin induced potent dilation in non-CAD coronary arterioles, with a large portion of the dilation inhibited to a similar extent by both the general KV1 blocker 4-AP and the selective KV1.5 blocker DPO-1. Although isoproterenol- and forskolin-induced dilations may involve multiple K+ channel types or other mechanisms, the data with the selective KV1.5 blocker DPO-1 indicate that KV1.5 plays a major functional role in cAMP-mediated coronary dilation. Whether KV1.5 forms a homotetrameric channel complex or associates with other KV subunits remains unclear.
KV1 channel function in coronary arterioles from subjects with CAD
Alterations in KV channel expression and function are key features of several pathological conditions including hypertension, metabolic syndrome, and diabetes.4,6,20–22 In coronary arterioles from patients with CAD, we observed a significant reduction in cAMP-mediated vasodilation that was largely 4-AP-sensitive. The findings that both receptor (isoproterenol) and non-receptor (forskolin) agonist-induced vasodilator responses were impaired in CAD are consistent with the involvement of signaling components downstream of cAMP generation such as KV channels, an important end-effector of vasomotor control. These results, together with our previous findings,24 indicate that 4-AP-sensitive KV channel function is impaired broadly (in both coronary and adipose arterioles) from subjects with CAD. Importantly, this impairment of KV channel function contributes to reduced vasodilation in response to physiological stimuli. In contrast to altered KV-dependent dilation, the NO donor sodium nitroprusside produced dilation that was similar in CAD and non-CAD coronary arteries, indicating specificity for cAMP-mediated dilation in CAD. Interestingly, activation of BKCa channels contribute to NO-induced dilation in several vascular beds,40 and BKCa-mediated dilation of human adipose arterioles was not affected in CAD.24
It is interest that in subjects with CAD, a greater 4-AP-sensitive dilation is observed in coronary compared to adipose arterioles.24 In adipose arterioles, neither 4-AP nor DPO-1 further reduced H2O2-induced vasodilation in CAD. However, in coronary arterioles from CAD subjects, 4-AP-sensitive dilation was still observed. Furthermore, the selective KV1.5 blocker DPO-1 significant inhibited isoproterenol-induced dilation in CAD coronary arterioles. These results suggest there may be vascular bed-specific differences in the extent of KV channel dysfunction in patients with CAD. Previous studies in animal models also suggest that certain vascular beds (e.g., coronary circulation) may adapt in the presence of disease to limit the deleterious effects on vascular function.26,27 The reasons for the persistent 4-AP/DPO-1-sensitive dilator component in coronary but not adipose vessels from subjects with CAD are unclear but could relate to potential heterogeneous patient groups included in different experimental protocols (Figure 6C), biased receptor signaling to isoproterenol, heterogeneous oxidative damage to KV channels in different vascular beds, or compensation by upregulation of other KV α or β subunits in the heart.
4.1 Study limitations
The present study used blood vessels isolated from surgically removed atrial appendages and unused donor hearts of subjects with a variety of conditions, and thus the non-CAD tissue samples are not truly normal controls. Ethical considerations preclude obtaining tissue from those subjects. To minimize potential confounding effects of the underlying disease, all of the non-CAD tissue samples included in this study are from subjects with no evidence of CAD and no more than 1 risk factor for CAD. Our determination of CAD is based on the patient’s medical record or direct observation of the conduit coronary arteries. We do not pre-select subjects; the majority of CAD patients are undergoing coronary bypass surgery and thus have significant coronary atherosclerosis (e.g., >50% occlusion of the left main coronary or of multiple vessels). We recognize that as a limitation the inherent heterogeneous nature of the CAD population, which is also evident in our analysis of isoproterenol-elicited dilation (Figure 6C). Additional studies to augment sample size would allow the use of statistical approaches to assess the effect of specific risk factors such as sex and age as well as other underlying cardiac conditions on KV channel expression and function.
In the present study, we focused on the role of KV1 channels in cAMP-mediated dilation and did not address the potential contribution of other K+ channels or non-ion channel mechanisms in this dilation. For example, there is evidence that KV7 channels, another distinct family of VSMC KV channels, can be activated by cAMP/protein kinase A and thus contribute to isoproterenol-induced dilation in some vascular beds.41,42 We also did not further explore the mechanisms underlying altered KV1 channel function in CAD coronary arterioles. Consistent with previous findings in human adipose arterioles,24 quantitative PCR suggested a slight reduction mRNA expression of KV1.5 (Figure 1B).
4.2 Clinical Implications
Dysregulation of microvascular function has been implicated in a variety of pathological conditions such as inflammation in visceral fat, obesity-associated insulin resistance, and ischemic heart disease.43–45 This microvascular dysfunction is of particular importance in the coronary circulation, where it serves as an important contributor to myocardial ischemia as well as an independent risk factor for adverse cardiovascular outcomes in patients with CAD.44,45 The heart has near-maximal extraction of oxygen at rest (70–80%) at rest and requires tight, beat-to-beat regulation of blood flow to nearly instantaneously match oxygen supply with its metabolic demand.8,46 Thus, the coronary vascular tone is tightly regulated by endothelial factors (e.g., NO, prostacyclin, and endothelium-derived hyperpolarization factors), neurohumoral factors, metabolic factors, as well as myogenic mechanisms,17,47–54 and this integral regulation allows for the precise control of blood flow in the heart. As key end-effectors of vasomotor control in the coronary circulation, dysfunctional KV channels will negatively impact local blood flow regulation in response to not only endothelial factors such as H2O2 but also to tissue factors such as β-adrenergic transmitters and other metabolic factors.19,20,46 This may induce deficit in regional blood supply and lead to a mismatch in oxygen supply and demand, which over time can impair cardiac function, induce local inflammation, fibrosis, and even heart failure.23
4.3 Perspectives
This study provides evidence that KV1.5, as a major 4-AP-sensitive KV1 channel expressed in human coronary VSMCs, plays an important role in mediating cAMP-mediated dilation of coronary arterioles in the absence of CAD. The cAMP-mediated dilation is reduced in coronary arterioles during CAD, and this impairment is associated with impaired 4-AP-sensitive KV channel function. Future studies are required to elucidate the mechanisms responsible for the regulation of KV1 channels in health and disease and for the potential difference in KV1 channel function and regulation between coronary and other vascular beds of humans.
Acknowledgments
This work was supported by National Institute of Health Grants HL096647 (to D.X.Z). We thank Ryan Nord for organizing tissue sample data.
Abbreviations
- 4-AP
4-aminopyridine
- BKCa channel
large-conductance Ca2+-activated K+ channel
- CAD
coronary artery disease
- DPO-1
diphenyl phosphine oxide-1
- EC
endothelial cell
- HEK-293
human embryonic kidney 293
- HCA
human coronary artery/arteriole
- H2O2
hydrogen peroxide
- KCa channel
Ca2+-activated K+ channel
- KV channel
voltage-gated K+ channel
- KV1 channel
shaker-related voltage-gated K+ channel
- RT-PCR
reverse transcription-polymerase chain reaction
- VSMC
vascular smooth muscle cell
References
- 1.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 2.Jackson WF. Potassium channels and regulation of the microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- 3.Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand. 1998;164:549–557. doi: 10.1046/j.1365-201X.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 4.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 5.Feliciangeli S, Chatelain FC, Bichet D, Lesage F. The family of k2p channels: Salient structural and functional properties. J Physiol. 2015;593:2587–2603. doi: 10.1113/jphysiol.2014.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent k(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD. Contribution of voltage-dependent k+ and ca2+ channels to coronary pressure-flow autoregulation. Basic Res Cardiol. 2012;107:264. doi: 10.1007/s00395-012-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite role of kv1.5 channels in coronary metabolic dilation. Circ Res. 2015;117:612–621. doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwill AG, Noblet JN, Sassoon D, Fu L, Kassab GS, Schepers L, Herring BP, Rottgen TS, Tune JD, Dick GM. Critical contribution of kv1 channels to the regulation of coronary blood flow. Basic Res Cardiol. 2016;111:56. doi: 10.1007/s00395-016-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, Ogasawara Y, Kajiya F. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation. 2003;107:1040–1045. doi: 10.1161/01.cir.0000050145.25589.65. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2o2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: A synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 13.Yada T, Shimokawa H, Hiramatsu O, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Important role of endogenous hydrogen peroxide in pacing-induced metabolic coronary vasodilation in dogs in vivo. J Am Coll Cardiol. 2007;50:1272–1278. doi: 10.1016/j.jacc.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: A feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 15.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2o2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive k+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 16.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive kv currents through s-glutathionylation. Pflugers Arch. 2015;467:285–297. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 18.Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and pka regulate delayed rectifier k+ channels of vascular smooth muscle cells. Am J Physiol. 1998;275:H448–459. doi: 10.1152/ajpheart.1998.275.2.H448. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs camp-mediated dilation by reducing kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 20.Gutterman DD, Miura H, Liu Y. Redox modulation of vascular tone: Focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol. 2005;25:671–678. doi: 10.1161/01.ATV.0000158497.09626.3b. [DOI] [PubMed] [Google Scholar]
- 21.Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. Pathophysiology of voltage-gated k+ channels in vascular smooth muscle cells: Modulation by protein kinases. Prog Biophys Mol Biol. 2010;103:95–101. doi: 10.1016/j.pbiomolbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Joseph BK, Thakali KM, Moore CL, Rhee SW. Ion channel remodeling in vascular smooth muscle during hypertension: Implications for novel therapeutic approaches. Pharmacol Res. 2013;70:126–138. doi: 10.1016/j.phrs.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: Regulation of flow and beyond. Circ Res. 2016;118:157–172. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishijima Y, Cao S, Chabowski DS, Korishettar A, Ge A, Zheng X, Sparapani R, Gutterman DD, Zhang DX. Contribution of kv1.5 channel to hydrogen peroxide-induced human arteriolar dilation and its modulation by coronary artery disease. Circ Res. 2017;120:658–669. doi: 10.1161/CIRCRESAHA.116.309491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42:167–195. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- 26.Bagi Z. Mechanisms of coronary microvascular adaptation to obesity. Am J Physiol Regul Integr Comp Physiol. 2009;297:R556–567. doi: 10.1152/ajpregu.90817.2008. [DOI] [PubMed] [Google Scholar]
- 27.Belin de Chantemele EJ, Stepp DW. Influence of obesity and metabolic dysfunction on the endothelial control in the coronary circulation. J Mol Cell Cardiol. 2012;52:840–847. doi: 10.1016/j.yjmcc.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2o2-induced dilation in human coronary arterioles: Role of protein kinase g dimerization and large-conductance ca2+-activated k+ channel activation. Circ Res. 2012;110:471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. Trpv4-mediated endothelial ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, Greenwood IA. Expression and function of the K* channel KCNQ genes in human arteries. Br J Pharmacol. 2011;162:42–53. doi: 10.1111/j.1476-5381.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang DX, Fryer RM, Hsu AK, Zou AP, Gross GJ, Campbell WB, Li PL. Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res Cardiol. 2001;96:267–274. doi: 10.1007/s003950170057. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated k(+) channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y. Nitration and functional loss of voltage-gated k+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 2004;53:2436–2442. doi: 10.2337/diabetes.53.9.2436. [DOI] [PubMed] [Google Scholar]
- 35.Bubolz AH, Li H, Wu Q, Liu Y. Enhanced oxidative stress impairs camp-mediated dilation by reducing kv channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289:H1873–1880. doi: 10.1152/ajpheart.00357.2005. [DOI] [PubMed] [Google Scholar]
- 36.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International union of pharmacology. Liii. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 37.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated k+ channels in rat small cerebral arteries: Molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase a enhances delayed rectifier k+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 39.Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier k+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol. 1996;74:439–447. doi: 10.1139/o96-048. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka Y, Koike K, Toro L. Maxik channel roles in blood vessel relaxations induced by endothelium-derived relaxing factors and their molecular mechanisms. J Smooth Muscle Res. 2004;40:125–153. doi: 10.1540/jsmr.40.125. [DOI] [PubMed] [Google Scholar]
- 41.Mani BK, Robakowski C, Brueggemann LI, Cribbs LL, Tripathi A, Majetschak M, Byron KL. Kv7.5 potassium channel subunits are the primary targets for pka-dependent enhancement of vascular smooth muscle kv7 currents. Mol Pharmacol. 2016;89:323–334. doi: 10.1124/mol.115.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stott JB, Barrese V, Greenwood IA. Kv7 channel activation underpins epac-dependent relaxations of rat arteries. Arterioscler Thromb Vasc Biol. 2016;36:2404–2411. doi: 10.1161/ATVBAHA.116.308517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scalia R. The microcirculation in adipose tissue inflammation. Rev Endocr Metab Disord. 2013;14:69–76. doi: 10.1007/s11154-013-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berwick ZC, Dick GM, Tune JD. Heart of the matter: Coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52:848–856. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: An update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canty JM, Jr, Iyer VS. Hydrogen peroxide and metabolic coronary flow regulation. J Am Coll Cardiol. 2007;50:1279–1281. doi: 10.1016/j.jacc.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circulation. 1990;82:1–7. doi: 10.1161/01.cir.82.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Bassenge E, Heusch G. Endothelial and neuro-humoral control of coronary blood flow in health and disease. Rev Physiol Biochem Pharmacol. 1990;116:77–165. doi: 10.1007/3540528806_4. [DOI] [PubMed] [Google Scholar]
- 50.DeFily DV, Chilian WM. Coronary microcirculation: Autoregulation and metabolic control. Basic Res Cardiol. 1995;90:112–118. doi: 10.1007/BF00789441. [DOI] [PubMed] [Google Scholar]
- 51.Merkus D, Chilian WM, Stepp DW. Functional characteristics of the coronary microcirculation. Herz. 1999;24:496–508. doi: 10.1007/BF03044220. [DOI] [PubMed] [Google Scholar]
- 52.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol (1985) 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Gutterman DD. Vascular control in humans: Focus on the coronary microcirculation. Basic Res Cardiol. 2009;104:211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncker DJ, Koller A, Merkus D, Canty JM., Jr Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis. 2015;57:409–422. doi: 10.1016/j.pcad.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]