Abstract
Background and aims
Methamphetamine use is increasingly prevalent and associated with HIV transmission. Early phase human studies suggested naltrexone reduced amphetamine use among dependent individuals. We tested if extended-release naltrexone (XRNTX) reduces methamphetamine use and associated sexual risk behaviors among high-risk methamphetamine-dependent men who have sex with men (MSM).
Design
Double-blind, placebo-controlled, randomized trial of XRTNX versus placebo over 12 weeks from 2012–2015.
Setting
San Francisco Department of Public Health, California, USA.
Participants
100 community-recruited, sexually-active, actively-using methamphetamine-dependent MSM. Mean age was 43 years, 98% were born male, 55% white, 19% African-American, and 18% Latino.
Interventions
XRNTX 380mg (N=50) or matched placebo (N=50) administered in 3 gluteal injections at 4-week intervals.
Measurements
Regression estimated average level and change in level of positive urines over the period 2 –12 weeks (primary outcomes) and sexual risk behaviors (secondary outcome).
Findings
Ninety percent of visits were completed. By intent-to-treat, participants assigned to XRNTX had similar differences over 2–12 weeks in methamphetamine-positive urines as participants assigned to placebo [IRR 0.95, 95%CI = 0.76 – 1.20; Bayes Factor < 0.3]. Observed urine positivity declined from 78% to 70% in the XRNTX arm and 74% to 64% in the placebo arm. Adherence to injections was 96.7% in the XRNTX arm and 91.3% in the placebo arm. Sexual risk behaviors declined similarly among participants in both arms (all P>0.05). There were no serious adverse events related to study drug, and no differences in frequency of adverse events by treatment arm.
Conclusions
Notwithstanding very high medication adherence for this study, extended-release naltrexone does not appear to reduce methamphetamine use or sexual risk behaviors among methamphetamine-dependent men who have sex with men compared with placebo.
Keywords: methamphetamine, men who have sex with men, HIV, naltrexone, randomized controlled trial
INTRODUCTION
Methamphetamine continues to dominate the synthetic drug market globally, with increasing use in Europe and North America.(1) There were at least 569,000 current methamphetamine users in the United States in 2014,(2) with increasing prevalence in several regions.(3) Methamphetamine use among men who have sex with men (MSM) is prevalent and associated with significant medical and social risks.(4–15) In 2014, 13% of MSM in San Francisco reported current methamphetamine use.(16, 17) MSM accounted for 70% of newly diagnosed HIV infections in 2014(18–20) and multiple studies have demonstrated an independent association between methamphetamine use and risk behaviors among MSM, including high-risk injection,[14] condomless anal intercourse,(21–24) multiple partners,(21, 25) increased duration of sex,(26) having anonymous partners, having sex in a public sex venue, meeting partners in a bathhouse, and exchanging money or drugs for sex.(21–26)
There are no pharmacotherapies approved for treatment of methamphetamine use disorder.(27, 28) Naltrexone, a μ-opioid receptor antagonist approved for alcohol and opioid use disorder treatment, showed promise in reducing amphetamine relapse among dependent, currently abstinent, heterosexuals.(29) Administration of naltrexone also led to reductions in subjective effects of amphetamine in laboratory studies,(30–33) although another study found no effect on reinforcing effects.(34) Methamphetamine, rapidly metabolized into amphetamine, enhances release of mRNA precursors for endogenous opioids that activate μ-opioid receptors and consequently levels of extracellular dopamine.(35–37) Hence, naltrexone was believed to attenuate downstream dopamine release by preventing methamphetamine-inducible endogenous opioids from activating μ-opioid receptors, tempering the reinforcing effects of methamphetamine.(37, 38) Naltrexone has also been associated with a reduction in urge-driven problematic disorders, suggesting potential benefit in HIV risk behaviors. (39–45)
METHODS
To test the hypotheses that naltrexone would reduce methamphetamine use and sexual risk behaviors, we conducted a double-blind, placebo-controlled, randomized trial of monthly injections of extended-release naltrexone (XRNTX, VIVITROL®) among 100 methamphetamine-dependent MSM at the San Francisco Department of Public Health from 2012–2015. The aims were to determine if XRNTX, compared to placebo, reduced (1) methamphetamine use and (2) sexual risk behaviors. Study procedures were approved by the University of California, San Francisco Institutional Review Board (IRB#11-06276).
Recruitment
Potential participants were recruited from clinics, community-based organizations, nightlife venues, websites, snowball referrals, and recruitment flyers. Interested individuals were administered a questionnaire by phone to establish preliminary eligibility and schedule in-person screening. At screening participants gave informed consent and were evaluated for eligibility, including being aged 18 to 65 years; methamphetamine-dependent by Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV-TR (SCID)(46); interested in reducing or stopping methamphetamine use; born male or female and not identifying as female; reporting anal sex with men while under the influence of methamphetamine within prior six months; being methamphetamine metabolite positive by urine; and, for HIV-infected individuals, having a CD4 count >200 cells/mm3, or a CD4 count 100–199 cells/mm3 and HIV viral load <200 copies/mL. Exclusion criteria included any psychiatric condition (e.g. psychosis or suicidality) that in investigators’ judgment precluded safe participation; current use of or urine positive for opioids, or a known medical condition likely to require opioid analgesics; current alcohol dependence by SCID; alanine or aspartate aminotransferase ≥5 times upper limit of normal; estimated glomerular filtration rate <50 mL/min; thrombocytopenia or coagulation disorder; known intolerance or hypersensitivity to naltrexone or other study drug components; acute illnesses requiring prolonged medical care or chronic illnesses likely to progress clinically during participation; pending legal proceedings with high risk for incarceration; participating in another study; and any condition that, in the investigator’s judgment, would interfere with safe participation or adherence to procedures.
Procedures
Two screening visits assessing for eligibility included physical exam; medical history; mental health and substance use history by SCID; qualitative urine testing for methamphetamine and other substances with MedTox EZ-SCREEN®; blood count, comprehensive metabolic panel, and, for participants born female, pregnancy testing. For otherwise eligible participants testing positive for opioids, a naloxone challenge was administered prior to study drug administration to ensure no risk for opioid withdrawal. Participants who reported being HIV or hepatitis C (HCV)-negative or unknown were given an OraQuick ADVANCE® Rapid HIV-1/2 test or OraQuick® HCV Rapid Antibody Test, respectively, and pooled HIV viral load testing using the Abbott Realtime HIV-1 assay; participants found newly HIV-positive during screening were referred to care and enrollment deferred for three months. HIV-positive participants had CD4 and viral load testing. All participants received HIV risk reduction counseling. Two additional “run-in visits” before enrollment included urine sample collection and assessed participants’ ability to adhere to study schedule. At enrollment, the 1:1 random allocation sequence was generated from a SAS macro program using randomly-selected block sizes (of 2 or 4) to ensure balanced study arms by the study biostatistician. Only the off-site biostatistician and pharmacist knew allocation assignments.
Following enrollment, participants were seen weekly to provide urine for methamphetamine and other substance testing and received 30-minute substance use counseling delivered by staff trained on a manual-driven psychosocial treatment program using cognitive behavioral therapy(47) and motivational interviewing techniques.(48–50) Study drug was administered by study clinicians at enrollment and 4 and 8-week visits. Blood count and comprehensive metabolic panel were done at 4, 8, and 12-week visits, as were physical exams and pregnancy tests, as needed. HIV risk-reduction counseling and testing and HCV testing were repeated for HIV- and HCV-negative participants at 12-week visits. Participants were reimbursed $10 for first screening visit, $25 for second, and $10 for each run-in; $50 for enrollment and weeks 4, 8, and 12, and $15 for other weekly visits. Research staff made reminder calls and/or texts prior to study visit appointments.
Study drug, XR-NTX 380mg and matched placebo, was prepared by an off-site pharmacist in identical-looking vials to maintain double-blind for staff and participants. Clinicians reconstituted and administered study drug from vials numbered to correspond with the treatment allocation sequence. Participants remained on-site for at least 30 minutes after injections to assess for adverse reactions.
If participants opted into genetic testing, a small blood sample was collected at enrollment and stored until enrollment was complete, then tested at the Clinical and Toxicology Research Laboratory of San Francisco General Hospital to determine the presence of the A118G Single-Nucleotide Polymorphism (SNP) thought to be relevant to mediation of XRNTX effects in the opioid receptor μ1 (OPRM1).(51) Genomic DNA was extracted from peripheral blood cells (QIAamp DNA Blood Kit; Qiagen Inc., Valencia, CA). After polymerase chain reaction, A118G genotype was determined with a Taqman 5′ nuclease assay, using allele specific probes (Taqman SNP Genotyping Assay, Catalog #C8950074, Applied Biosystems, Foster City, CA) under the protocol provided with the kit. Three known controls (determined by next-generation sequencing) were used for each genotype. All testing was performed on the Rotor-Gene Q real time PCR system (Qiagen Inc., Valencia, CA).
Measures
Primary outcome was the overall level (2–12 weeks) and change in level of the proportion of methamphetamine-positive urines; all results were confirmed by two staff. Secondary outcomes were sexual HIV risk behaviors, including number of male partners, number of male partners with whom meth was used, episodes of anal sex with serodiscordant partners, episodes of unprotected anal sex with serodiscordant partners, episodes of insertive unprotected anal sex with serodiscordant partners, episodes of receptive unprotected anal sex with serodiscordant partners, study injections administered, and adverse events classified by standard criteria.(52) Audio-computer assisted self-interviews (ACASIs), were completed by participants weekly, addressing recent substance use and cravings. (53, 54) At enrollment and weeks 4, 8 and 12, additional questions assessed substance use treatment, Severity of Dependence (SDS), and sexual risk behaviors.(55–57) We assessed for depressive symptoms with the Center for Epidemiologic Studies Depression scale(58) and screened for psychological distress with the Patient Health Questionnaire-2 (PHQ-2).
Data analysis
Our sample of 100 participants provided 80% power to detect 19–25% change in level of positive urines among participants in the XRNTX group, relative to placebo.(59) Primary outcome data were analyzed by intention-to-treat, without regard to adherence (i.e., all participants were included in efficacy analyses), using a generalized estimating equations (GEE) model with robust standard errors to account for within-participant clustering of binary responses. To obtain direct estimates of risk ratios (RRs), log-link models were used. The form of the model, which omits week 1 (first post-enrollment visit) results, was pre-specified, based on the a priori hypothesis that XRNTX would have gradually increasing efficacy after a short initial delay to achieve steady state levels. The analysis compared trends in urine-positivity from baseline through week 12 modeled as group-specific linear functions of time since randomization. Treatment effect was captured by the divergence of XRNTX and placebo trends at 12 weeks, net of the fitted baseline difference, and was assessed using a test for the time-by-treatment interaction. Use of robust standard errors allowed us to account for within-subject correlation of the responses without making parametric assumptions. Fit of the model was informally assessed by plotting the group-specific fitted trends along with raw percentages. Additionally, we tested the difference in parallel trajectories between 2 and 12 weeks with a model without the interaction term between treatment and time, to estimate the main overall effect of treatment.
Five sensitivity analyses were conducted: 1) including week 1 results; 2) imputing a positive result for all missing urine samples; 3) adjusting for imbalanced baseline characteristics with p<0.10 and baseline correlates of missing or positive urine samples; 4) including participants who completed final visits beyond the maximum allowable visit window; 5) stratifying participants by intensity of methamphetamine use at baseline. All available data were included in each analysis.
In addition, we conducted an as-treated analysis, focusing on the effect of cumulative dose, calculated as the number of study injections received, as a time-dependent covariate. In this analysis, we controlled for the placebo effects of adherence, estimating the as-treated effect by the interaction between arm and cumulative study injections received. We also determined whether treatment effect was modified by μ-opioid receptor (OPRM1) gene polymorphisms in models that stratified by genotype result. Finally, to evaluate for consecutive weeks of continued abstinence, we compared the “number of beyond-threshold weeks of success” (NOBWOS), using the Wilcoxon test.(60)
We used linear, logistic and negative binomial GEE models to assess treatment effects on secondary outcomes, including CES-D scores and sexual risk behaviors. Acceptability of XRNTX was evaluated as percent of study injections completed by arm using the Wilcoxon test. To assess safety, we compared frequency of adverse events of various types by arm using two-tailed Fisher’s exact test. Finally, to evaluate non-significant findings, we calculated Bayes Factor(61) using R (R Foundation for Statistical Computing, Vienna Austria).(62)
RESULTS
Subjects
Target sample was achieved and data collected from September 2012 to September 2015. Figure 1 shows results for screening, study arm assignment and retention; 194 participants provided written informed consent for screening. The most common reasons for ineligibility were: not methamphetamine dependent by SCID (n=15), psychotic disorder (11), exclusionary lab results (n=8), or a medical condition precluding safe participation by clinical judgement (n=8). Two participants were excluded due to current alcohol dependence.
Figure 1.
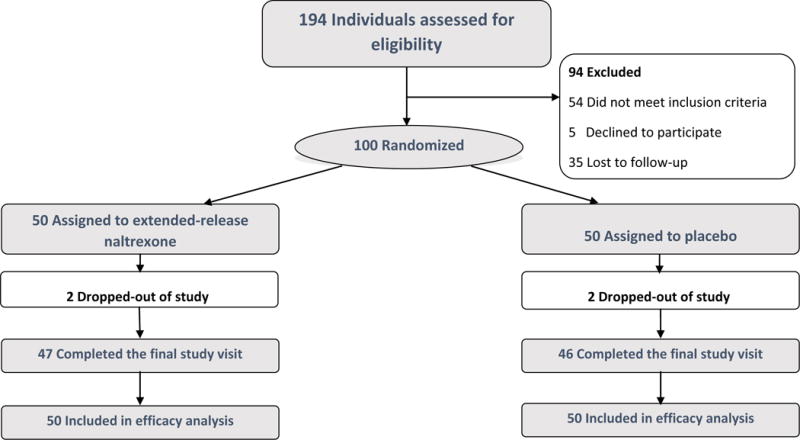
Enrollment and Retention by treatment arm
Participant characteristics were similar in both arms (Table 1), with the exception of: 1) methamphetamine use through rectal administration was reported by a greater proportion of XRNTX participants compared to placebo (52% vs. 16%; P<0.01), and 2) using methamphetamine during sex greater than 50% of the time was more common in the XRNTX arm (80% vs. 46%; P<0.01).
Table 1.
Baseline Characteristics of Trial Participants
| Demographics | No.(%) | |||
|---|---|---|---|---|
| Placebo (n=50) |
Naltrexone (n=50) |
Overall (n=100) |
||
| Age, mean (SD), y | 42.4 (9.0) | 43.9 (8.1) | 43.2 (8.5) | |
| Race/Ethnicity | White | 28 (56.0) | 27 (54.0) | 55 (55.0) |
| African-American | 8 (16.0) | 11 (22.0) | 19 (19.0) | |
| Latino | 9 (18.0) | 9 (18.0) | 18 (18.0) | |
| Asian and Pacific Islander | 3 (6.0) | 1 (2.0) | 4 (4.0) | |
| Other | 2 (4.0) | 2 (4.0) | 4 (4.0) | |
| Current Gender Identity | Male | 47 (94) | 49 (47) | 96 (96) |
| Transgender Female (Male to Female) | 2 (4) | 1 (2) | 3 (3) | |
| Transgender Male (Female to Male) | 1 (2) | 0 (0) | 1 (1) | |
| Education | High school or less | 17 (34.0) | 9 (18.0) | 26 (26.0) |
| Some college | 19 (38.0) | 28 (56.0) | 47 (47.0) | |
| College or above | 14 (28.0) | 12 (24.0) | 26 (26.0) | |
| Income | under $20,000 | 29 (58.0) | 31 (62.0) | 60 (60.0) |
| $20,000–39,999 | 10 (20.0) | 10 (20.0) | 20 (20.0) | |
| $40,000 and above | 11 (22.0) | 8 (16.0) | 19 (19.0) | |
| Employment status | Not employed | 37 (74.0) | 33 (66.0) | 70 (70.0) |
| Part-time | 6 (12.0) | 9 (18.0) | 15 (15.0) | |
| Full-time | 6 (12.0) | 8 (16.0) | 14 (14.0) | |
| Methamphetamine use | ||||
| Frequency of methamphetamine use (past 4 weeks) ‡ | Two days or less per week | 20 (40.0) | 11 (22.0) | 31 (31.0) |
| Three - Seven days per week | 29 (58.0) | 39 (78.0) | 68 (68.0) | |
| Methamphetamine use during sex (past 4 weeks) ‡ | 50% or less of the time | 26 (52.0) | 10 (20.0) | 36 (36.0) |
| Greater than 50% of time | 23 (46.0) | 40 (80.0) | 63 (63.0) | |
| Route of methamphetamine administration | Injection | 25 (50.0) | 26 (52.0) | 51 (51.0) |
| Inserted rectally ‡ | 8 (16.0) | 21 (42.0) | 29 (29.0) | |
| Snorted | 14 (28.0) | 17 (34.0) | 31 (31.0) | |
| Smoked | 40 (80.0) | 42 (84.0) | 82 (82.0) | |
| Ingested orally | 4 (8.0) | 10 (20.0) | 14 (14.0) | |
| Methamphetamine severity of dependence scale (SDS) score, mean (SD) | 5.7 (3.2) | 6.3 (3.4) | 6.0 (3.3) | |
| Methamphetamine visual analog scale (VAS) craving score, mean (SD) | 50.3 (31.0) | 47.8 (30.5) | 49 (30.6) | |
| History of methamphetamine self-help or treatment program | 30 (60.0) | 28 (52.0) | 58 (58.0) | |
| Clinical | ||||
| HIV positive OPRM1 A118G Genotype | 37 (74.0) | 37 (74.0) | 74 (74.0) | |
| Heterozygous (AG) | 12 (24.0) | 13 (26.0) | 25 (50.0) | |
| Homozygous (Wild Type - AA) | 22 (44.0) | 24 (48.0) | 46 (92.0) | |
| Mutant (GG) | 0 0 | 1 (2.0) | 1 (2.0) | |
| Has regular health care provider | 44 (88.0) | 44 (88.0) | 88 (88.0) | |
| Has health insurance | 44 (88.0) | 49 (98.0) | 93 (310.0) | |
| Center for epidemiologic studies depression scale (CES-D) score, mean (SD) | 19.4 (12.1) | 19.4 (13.2) | 19.2 (12.6) | |
characteristics with P<0.10 between treatment arms when compared using the Fisher exact test, and Wilcoxon rank sum tests, as appropriate.
Study retention and adherence
The mean weekly visit completion rate was 90% (SD= 0.19). Weekly retention was similar among XRNTX and placebo arms (91% [0.17] vs. 89% [0.21]; P=0.42). There were no significant differences in trial completion by arm (XRNTX 94% [n=47], placebo 92% [n=46]; P≥0.99). Four participants dropped out after randomization (2 in each arm). Two additional participants came for their final visit 16 and 34 days past scheduled final visits, outside the maximum allowable window, and were excluded from primary analyses.
Of monthly follow-up visits, 94% (282 out of 300) were completed (XRNTX 95% [143 visits], placebo 93% [139 visits]; P=0.87). Mean number of urine samples collected was 10.5 (XRNTX 10.7, placebo 10.3]; P=0.36); Figure 2 shows the numbers of participants in each group providing urine samples by week. Participants completed 91% of ACASI surveys (329 out of 360; XRNTX 98%, placebo 95%; P=0.201) and 83% of weekly substance use counseling sessions (911 out of 1100; XRNTX 85%, placebo 81%; P=0.06).
Figure 2.
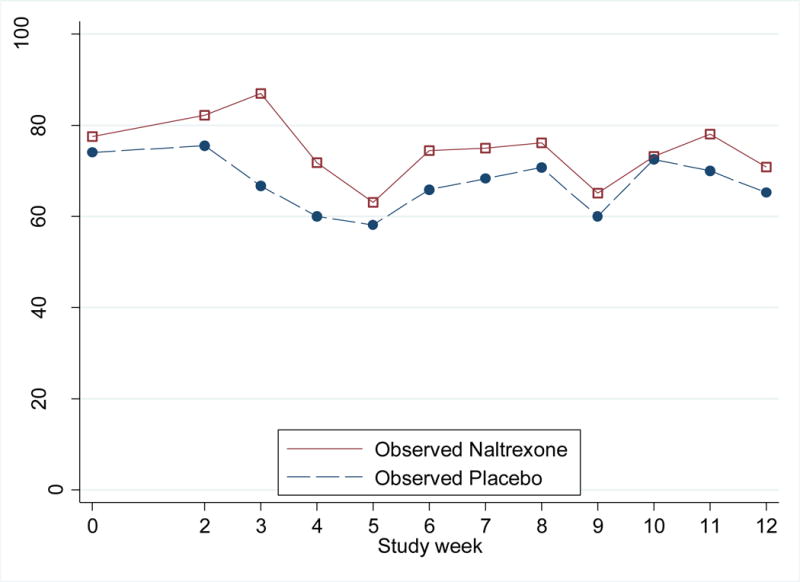
Weekly methamphetamine urine-positivity rates, by treatment arm
When asked to guess treatment assignment at study completion, participants showed no evidence of unblinding: 21 (42%) XRNTX and 16 (32%) placebo participants guessed correctly (P=0.40).
Outcomes
Methamphetamine use
During four pre-enrollment visits, the median number of methamphetamine metabolite-positive urines among enrollees was 3 (IQR: 2–4). At baseline, 75 (75%) participants had positive urines (38 [76%] XRNTX, 37 [74%] placebo; P=0.81). The proportion of positive urines at baseline through month 3 decreased in both arms, from 78% to 70% in XRNTX and 74% to 64% in placebo participants (Table 2 and Figure 2). The intention-to-treat GEE analysis demonstrated a similar risk of testing positive for methamphetamine in XRNTX and placebo arms (IRR 0.95, 95%CI = 0.76 – 1.20). In the model without the interaction term between treatment and time, there was no evidence of a significant main overall treatment effect (IRR 1.10, 95%CI = 0.93 – 1.30).
Table 2.
Weekly methamphetamine urine-positivity rates by treatment arm and overall treatment effect on urine positivity
| Week | IRR (95%CI) | P-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
|
|
||||||||||||||
| Placebo, n(%) | 50 (74) | 45 (76) | 45 (67) | 45 (60) | 43 (58) | 41 (66) | 41 (66) | 41 (68) | 40 (60) | 40 (73) | 40 (70) | 45 (64) | 1.10 (0.93–1.30) | 0.27 |
| XRNTX, n(%) | 49 (78) | 45 (82) | 46 (87) | 46 (72) | 46 (63) | 43 (74) | 44 (75) | 42 (76) | 43 (65) | 41 (73) | 41 (78) | 47 (47) | ||
In checking assumptions of the model for urine positivity, we found no persuasive evidence for departures from linear trend over 12 weeks (P=0.3). The NOBWOS was 0.8 (SD=2.4), with no significant difference in median NOBWOS by arm (XRNTX 0 [IQR=0–0]; placebo 0 [IQR=0–0]; P=0.36). The maximum number of consecutive weeks of continued abstinence was 8 in the XRNTX arm (observed in 1 participant) and 11 in the placebo arm (observed in 2 participants). At trial completion, seventeen participants (17%) achieved abstinence (7 in XRNTX [14%]; 10 in placebo [20%]; P=0.6; see Figure 3).
Figure 3.
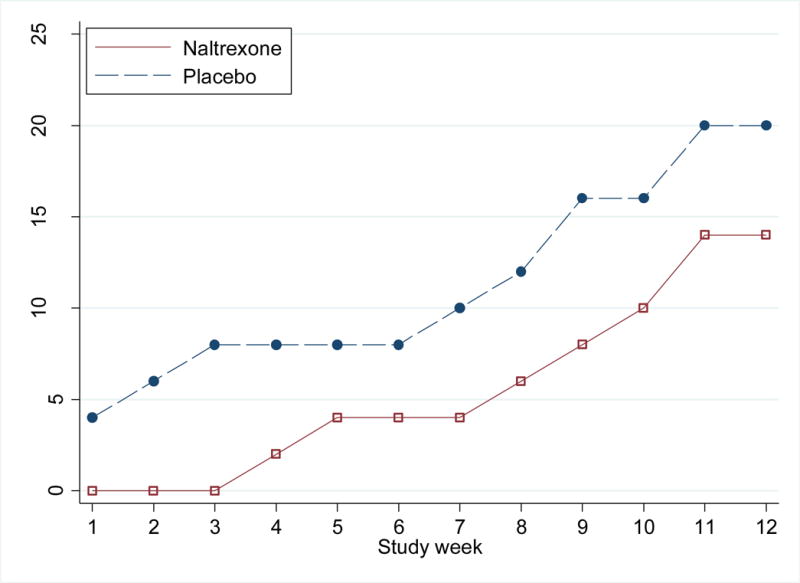
Proportion of Participants who were abstinent from methamphetamine until the end of study, by week and treatment arm
The placebo urine positivity rate (observed 74%; expected 70–90%) and missing urine sample rates (observed 12%; expected 17%) were within parameters assumed in sample size calculations, although within-subject correlation of urines (observed 38%; expected 50–90%) was lower than assumed. Using observed parameters, the updated minimum detectable net percent reduction in urine positivity was at the upper range of a priori estimates. Based on the 19–25% urine positivity rate reductions that the study had 80% power to detect as the theoretical effect of XRNTX on this outcome, we obtained Bayes Factors of 0.13 to 0.29, depending on how the theoretical effect was specified, all below the guideline threshold of 0.3 to establish a null effect.
Sensitivity Analyses
There was no significant effect observed in favor of XRNTX in sensitivity analyses after including week 1 urine results (IRR 1.02, 95% CI 0.81–1.28; P=0.88); imputing of positive results for missing urines (IRR 0.92, 95% CI 0.74–1.1; P=0.44); adjusting for imbalanced baseline methamphetamine use through rectal administration (IRR 0.95, 95% CI 0.75–1.20; P=0.65); controlling for baseline correlates of missing or positive urines (all models P>0.05); or including final visit data from participants who completed visits beyond the pre-specified date allowable (IRR 0.96, 95% CI 0.76–1.20; P=0.70). There was no significant effect of stratifying by baseline intensity of methamphetamine use (effect of treatment over time p-value = 0.41 and 0.77 for light and heavy users, respectively).
Craving, severity of dependence, depressive symptoms
At baseline, mean methamphetamine-craving score by visual analog scale (VAS) was 49 (SD = 31) overall (XRNTX 48 [SD = 30] placebo 50 [SD = 31]). At the 12-week visit, mean score was 29 (SD = 27) in XRNTX and 27 (SD = 26) in placebo arms. Mean methamphetamine-craving scores decreased 19 points overall (95%CI 12–25; P≤0.001), with no significant difference between arms over follow-up (1.5 points, 95%CI −11.4 to 14.3; P=0.82). Mean baseline SDS was 6 (SD = 3) overall (XRNTX 6 [SD = 3], placebo 6 [SD = 3]) and decreased 1 point (95%CI 0.4–1.5; P=0.001), with no significant difference between arms over follow-up (−0.1 point; 95%CI −1.2 to 1.1; P=0.9). Baseline mean CES-D was 19 (SD = 12) overall (XRNTX 19 [SD = 13], placebo 19 [SD = 12]), and decreased 3 points (95%CI 0.5–5; P=0.014), with no significant difference between arms over follow-up (−1.0 point; 95%CI −5 to 3; P=0.7).
Sexual risk behaviors
Baseline frequency of sexual risk behaviors and subsequent decline were similar in XRNTX and placebo arms, demonstrating no effect of XRNTX over placebo (Table 3).
Table 3.
Changes in Sexual Risk Behavior by Treatment Arm
| Mean events
|
Risk Ratio | 95%CI | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4-wk visit | 8-wk visit | 12-wk visit | |||||
| Number of male partners with whom meth was used | Placebo | 4.22 | 3.31 | 2.33 | 2.06 | 0.75 | (0.44 – 1.28) | 0.293 |
|
| ||||||||
| XRNTX | 5.98 | 4.56 | 3.09 | 2.65 | ||||
|
|
||||||||
| Number of male partners | Placebo | 4.61 | 3.75 | 2.71 | 2.51 | 0.76 | (0.49 – 1.18) | 0.219 |
|
| ||||||||
| XRNTX | 6.32 | 4.88 | 3.28 | 3.15 | ||||
|
|
||||||||
| Episodes of anal sex with serodiscordant partners | Placebo | 2.04 | 1.67 | 1.02 | 1.11 | 1.26 | (0.59 – 2.68) | 0.553 |
|
| ||||||||
| XRNTX | 1.6 | 0.92 | 0.59 | 1.13 | ||||
|
|
||||||||
| Episodes of unprotected anal sex with serodiscordant partners | Placebo | 1.69 | 1.31 | 0.69 | 0.87 | 1.41 | (0.57 – 3.48) | 0.459 |
|
| ||||||||
| XRNTX | 1.32 | 0.76 | 0.39 | 0.96 | ||||
|
|
||||||||
| Episodes of insertive unprotected anal sex with serodiscordant partners | Placebo | 1.1 | 1.04 | 0.36 | 0.72 | 0.99 | (0.35 – 2.81) | 0.978 |
|
| ||||||||
| XRNTX | 0.92 | 0.5 | 0.28 | 0.54 | ||||
|
|
||||||||
| Episodes of receptive unprotected anal sex with serodiscordant partners | Placebo | 0.82 | 0.92 | 0.42 | 0.28 | 2.35 | (0.81 – 6.82) | 0.117 |
|
| ||||||||
| XRNTX | 0.64 | 0.4 | 0.35 | 0.5 | ||||
Legend: Sexual behaviors are self-reported activity within past 4 weeks. Serodiscordant partner is defined as HIV-positive person with HIV-negative or unknown HIV-status partner, or HIV-negative person with HIV-positive or unknown HIV-status partner.
Medication adherence
Participants received a mean of 2.8 (SD: 0.5) study injections, with no difference by arm (XRNTX 2.9 [0.3], placebo 2.7 [0.6]; P=0.21). Ninety percent of XRNTX participants received all three study injections, compared to 82% of placebo participants (P=0.2).
As-Treated Analysis
In the as-treated analysis, the effect of treatment on methamphetamine positivity rate for greater number of cumulative study injections received was not significantly different by arm (β coefficient= −0.015, 95%CI −0.08 to 0.05, P=0.66). The effect of receiving an injection in past month on monthly methamphetamine positivity rate was not significantly different between arms (β-coefficient= −0.036, 95%CI −0.27 to 0.20, P=0.76).
Treatment effect by OPRM1 A118G Single-Nucleotide Polymorphisms
Seventy-two participants agreed to be genotyped, of whom, 26 had heterozygous and 46 homozygous (wild type) genotype (see table 1 for the distribution of genotypes by arm). There was no significant effect stratifying by OPRM1 A118G genotype; effect of treatment over time had a p-value of 0.64 and 0.97 for participants with heterozygous and homozygous genotype, respectively.
Safety
There were no differences in frequency of adverse events between arms (P≥0.99). There were three serious adverse events during this study, none of which were deemed related to study drug. Two placebo arm participants were hospitalized, one for left facial drooping and hearing loss in the context of syphilis, and the other for gamma-hydroxybutyric acid overdose. One XRNTX arm participant, who had not received study medication, was hospitalized for self-injurious behavior.
The most common adverse events reported in both arms were: injection site pain (24 [48%] XRNTX, 30 [60%] placebo; P=0.32); hyperglycemia (15 [30%] XRNTX, 12 [24%] placebo; P=0.65); nausea (14 [28%] XRNTX, 10 [20%] placebo, P=0.48); increased ALT (10 [20%] XRNTX, 7 [35%] placebo, P=0.22); and upper respiratory infection (9 [18%] XRNTX, 4 [8%] placebo; P=0.23).
Side-effects reported exclusively among XRNTX participants included hypocalcemia (2 participants [4%]; P=0.50); hypokalemia (2 [4%]; P=0.50), injury NOS (2 [4%]; P=0.50), decreased eGFR (1 [2%]; P≥0.99), dehydration (1 [2%]; P≥0.99), genitourinary tract disorder (1 [2%]; P≥0.99), hypoalbuminemia (1 [2%]; P≥0.99), increased alkaline phosphatase (1 [2%]; P≥0.99), increased creatinine (1 [2%]; P≥0.99), injection site pruritus (1 [2%]; P≥0.99), joint pain (1 [2%]; P≥0.99), muscle cramps (1 [2%]; P≥0.99), peripheral edema (1 [2%]; P≥0.99), suicidal and self-injurious behavior (1 [2%]; P≥0.99).
No XRNTX participants declined study drug due to adverse events; three placebo participants declined study injections (0% vs. 6%; P=0.24) due to injection site pain or nausea from prior study injections.
DISCUSSION
We found no effect of XRNTX on methamphetamine use, methamphetamine craving or sexual risk behaviors among dependent, actively-using MSM when compared to placebo. This result is supported by Bayes Factor analysis which observed values below the guideline threshold to establish a null effect, suggesting that this medication alone is unlike to provide a clinically meaningful benefit in this population. There were no serious adverse events related to study drug and no difference in the frequency of adverse events by study arm.
These findings are aligned with those of many other negative trials of psychotherapeutics for methamphetamine use disorder.(63) Notwithstanding favorable pre-clinical data for many medications, most fail phase II trials. There are few exceptions to this trend, with mixed data for medications such as methylphenidate(64) and bupropion,(65) and some early promise for mirtazapine, which is active in multiple neurotransmitter systems.(66) Similar challenges have affected development of pharmacological treatments for cocaine use disorder, suggesting that treating stimulant use may require targeting multiple neurotransmitter systems simultaneously with a combination of agents.
Our study resulted in remarkably high medication adherence for a study of methamphetamine use. Medication adherence among methamphetamine-dependent individuals is poor in most trials (67–69), yet higher in this study even than in other trials utilizing XRNTX.(70–72) Our approach to administering the injection may have differed somewhat from other clinicians: first, aiming to reduce the common problem of clogged syringes, after drawing up study drug we left the syringe barrel slightly inverted to avoid precipitation toward the needle, and we attached the needle only immediately before injection. Second, we administered study drug into the ventrogluteal site, whereas many clinicians may utilize the prior standard dorsogluteal site which has more pain receptors, nearby nerve bundles, and thicker fat. Infrequent needle clogging and less painful injections may have contributed to adherence.
Our study has limitations. First, our sample was MSM, most of whom had positive HIV serostatus, and results may not be generalizable to other populations. Furthermore, our participants were actively-using methamphetamine, in contrast to an earlier trial of relapse prevention among amphetamine-dependent persons.(29) Second, we studied XRNTX in isolation, whereas there may be benefit when the medication is combined with other agents.(73)
In summary, notwithstanding excellent medication adherence, we found no evidence that XRNTX alone supports reduced methamphetamine use among methamphetamine-dependent, actively-using MSM when compared to placebo. The remarkable level of adherence, however, should promote further research into long-acting medications to treat methamphetamine and other substance use disorders.
Acknowledgments
The study was funded by the National Institute on Drug Abuse (R01DA031678). Study drug was donated by Alkermes, Inc. The authors acknowledge Kara Lynch PhD for conducting the genetic testing. PC has led a study receiving donated ledipasvir-sofosbuvir from Gilead Pharmaceuticals.
Funding Source: National Institute of Health R01DA031678; Study medication donated by Alkermes, Inc.
Footnotes
Clinical Trial Registration: clinicaltrials.gov identifier: NCT01449565; registered prior to study initiation
Authors declare no other conflicts of interest.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report 2015. New York: United Nations publication; 2015. (Sales No. E.15.XI.6). [Google Scholar]
- 2.Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Center for Behavioral Health Statistics and Quality; 2015. [Google Scholar]
- 3.Coffin PO. In: National Drug Early Warning System (NDEWS) San Francisco Sentinel Community Site Drug Use Patterns and Trends, 2016. System N. D. E. W., editor. College Park, MD: NDEWS Coordinating Center; 2016. [Google Scholar]
- 4.Shoptaw S, Peck J, Reback CJ, Rotheram-Fuller E. Psychiatric and substance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependent gay and bisexual men seeking outpatient drug abuse treatment. J Psychoactive Drugs. 2003;35(Suppl 1):161–168. doi: 10.1080/02791072.2003.10400511. [DOI] [PubMed] [Google Scholar]
- 5.Tominaga GT, Garcia G, Dzierba A, Wong J. Toll of methamphetamine on the trauma system. Arch Surg. 2004;139:844–847. doi: 10.1001/archsurg.139.8.844. [DOI] [PubMed] [Google Scholar]
- 6.Increasing morbidity and mortality associated with abuse of methamphetamine–United States, 1991–1994. MMWR Morb Mortal Wkly Rep. 1995;44:882–886. [PubMed] [Google Scholar]
- 7.National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2005. Treatment Episode Data Set (TEDS). Highlights - 2003. (DASIS Series: S-27). [Google Scholar]
- 8.Roberts DL, Ball J. Amphetamine and Methamphetamine Emergency Department Visits. 1995–2002 The Drug Abuse Warning Network The DAWN Report. 2004 Jul; [Google Scholar]
- 9.Brecht ML, Greenwell L, Anglin MD. Methamphetamine treatment: trends and predictors of retention and completion in a large state treatment system (1992-2002) J Subst Abuse Treat. 2005;29:295–306. doi: 10.1016/j.jsat.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18:235–242. doi: 10.1097/01.yco.0000165592.52811.84. [DOI] [PubMed] [Google Scholar]
- 12.Curtis EK. Meth mouth: a review of methamphetamine abuse and its oral manifestations. Gen Dent. 2006;54:125–129. quiz 130. [PubMed] [Google Scholar]
- 13.Saini T, Edwards PC, Kimmes NS, Carroll LR, Shaner JW, Dowd FJ. Etiology of xerostomia and dental caries among methamphetamine abusers. Oral Health Prev Dent. 2005;3:189–195. [PubMed] [Google Scholar]
- 14.Williams N, Covington JS., 3rd Methamphetamine and meth mouth: an overview. J Tenn Dent Assoc. 2006;86:32–35. [PubMed] [Google Scholar]
- 15.Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. In: The Economic Cost of Methamphetamine Use in the United States, 2005. Center R. D. P. R., editor. 2009. [Google Scholar]
- 16.Raymond HF. In: SF NHBS Substance Use. Das M, editor. San Francisco: 2010. [Google Scholar]
- 17.Coffin PO. National Drug Early Warning System (NDEWS) San Francisco Sentinel Community Site Drug Use Patterns and Trends. College Park, MD: NDEWS Coordinating Center; 2015. [Google Scholar]
- 18.Purcell D, Johnson C, Lansky A, Prejean J, Stein R, Denning P, et al. Calculating HIV and Syphilis Rates for Risk Groups: Estimating the National Population Size of Men Who Have Sex with Men. National STD Prevention Conference; Atlanta, GA: 2010. [Google Scholar]
- 19.HIV Epidemiology Annual Report, San Francisco Department of Public Health. San Francisco: Department of Public Health; 2014. [Google Scholar]
- 20.HIV Surveillance Report, 2014. Center for Disease Control and Prevention; 2015. [Google Scholar]
- 21.Ober A, Shoptaw S, Wang PC, Gorbach P, Weiss RE. Factors associated with event-level stimulant use during sex in a sample of older, low-income men who have sex with men in Los Angeles. Drug Alcohol Depend. 2009;102:123–129. doi: 10.1016/j.drugalcdep.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawstorne P, Digiusto E, Worth H, Zablotska I. Associations between crystal methamphetamine use and potentially unsafe sexual activity among gay men in Australia. Arch Sex Behav. 2007;36:646–654. doi: 10.1007/s10508-007-9206-z. [DOI] [PubMed] [Google Scholar]
- 23.Koblin BA, Murrill C, Camacho M, Xu G, Liu KL, Raj-Singh S, et al. Amphetamine use and sexual risk among men who have sex with men: results from the National HIV Behavioral Surveillance study–New York City. Subst Use Misuse. 2007;42:1613–1628. doi: 10.1080/10826080701212519. [DOI] [PubMed] [Google Scholar]
- 24.Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor MM, Aynalem G, Smith LV, Montoya J, Kerndt P. Methamphetamine use and sexual risk behaviours among men who have sex with men diagnosed with early syphilis in Los Angeles County. Int J STD AIDS. 2007;18:93–97. doi: 10.1258/095646207779949709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semple SJ, Zians J, Strathdee SA, Patterson TL. Sexual marathons and methamphetamine use among HIV-positive men who have sex with men. Arch Sex Behav. 2009;38:583–590. doi: 10.1007/s10508-007-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karila L, Weinstein A, Aubin H-J, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. British Journal of Clinical Pharmacology. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KM K. The search for medications to treat stimulant dependence. Addiction Science and Clinical Practice. 2008;4:28–35. doi: 10.1151/ascp084228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- 30.Jayaram-Lindstrom N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol. 2004;24:665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- 31.Jayaram-Lindstrom N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008;33:1856–1863. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- 32.Marks KR, Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Separate and Combined Effects of Naltrexone and Extended-Release Alprazolam on the Reinforcing, Subject-Rated, and Cardiovascular Effects of Methamphetamine. Journal of clinical psychopharmacology. 2016;36:213–221. doi: 10.1097/JCP.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks KR, Lile JA, Stoops WW, Rush CR. Separate and combined impact of acute naltrexone and alprazolam on subjective and physiological effects of oral d-amphetamine in stimulant users. Psychopharmacology. 2014;231:2741–2750. doi: 10.1007/s00213-014-3449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoops WW, Pike E, Hays LR, Glaser PE, Rush CR. Naltrexone and bupropion, alone or combined, do not alter the reinforcing effects of intranasal methamphetamine. Pharmacol Biochem Behav. 2015;129:45–50. doi: 10.1016/j.pbb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tien LT, Ho IK, Loh HH, Ma T. Role of mu-opioid receptor in modulation of preproenkephalin mRNA expression and opioid and dopamine receptor binding in methamphetamine-sensitized mice. J Neurosci Res. 2007;85:673–680. doi: 10.1002/jnr.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horner KA, Adams DH, Hanson GR, Keefe KA. Blockade of stimulant-induced preprodynorphin mRNA expression in the striatal matrix by serotonin depletion. Neuroscience. 2005;131:67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Schad CA, Justice JB, Jr, Holtzman SG. Endogenous opioids in dopaminergic cell body regions modulate amphetamine-induced increases in extracellular dopamine levels in the terminal regions. J Pharmacol Exp Ther. 2002;300:932–938. doi: 10.1124/jpet.300.3.932. [DOI] [PubMed] [Google Scholar]
- 38.Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- 39.Roth AS, Ostroff RB, Hoffman RE. Naltrexone as a treatment for repetitive self-injurious behaviour:an open-label trial. J Clin Psychiatry. 1996;57:233–237. [PubMed] [Google Scholar]
- 40.Bostwick JM, Bucci JA. Internet sex addiction treated with naltrexone. Mayo Clinic proceedings. 2008;83:226–230. doi: 10.4065/83.2.226. [DOI] [PubMed] [Google Scholar]
- 41.Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2010;22:56–62. [PubMed] [Google Scholar]
- 42.Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2001;13:229–231. doi: 10.1023/a:1014626102110. [DOI] [PubMed] [Google Scholar]
- 43.Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65:982–986. doi: 10.4088/jcp.v65n0715. [DOI] [PubMed] [Google Scholar]
- 44.Raymond NC, Grant JE, Kim SW, Coleman E. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. International clinical psychopharmacology. 2002;17:201–205. doi: 10.1097/00004850-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Fontenelle LF, Oostermeijer S, Harrison BJ, Pantelis C, Yücel M. Obsessive-Compulsive Disorder. Impulse Control Disorders and Drug Addiction, Drugs. 2011;71:827–840. doi: 10.2165/11591790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.First MSRL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-non-Patient Edition (SCID-I/NP, 1/2007 revision) New York: New York State Psychiatric Institute; 2007. [Google Scholar]
- 47.Brief D, Bollinger A, Horton G, LoCastro JS. Relapse Prevention Treatment for Cocaine Addiction: the RPT-C Manual. Bethesda, MD: Medication Development Division, National Institute on Drug Abuse; 1998. [Google Scholar]
- 48.Miller W, Rollnick S. Motivational Interviewing. New York: Guilford Press; 2002. [Google Scholar]
- 49.Miller WR. Motivational interviewing with problem drinkers. Behavioural Psychotherapy. 1983;11:147–172. [Google Scholar]
- 50.DiClemente CC, Prochaska JO, Gilbertini M. Self-efficacy and the stages of self change in smoking. Cognitive Therapy and Research. 1985;9:181–200. [Google Scholar]
- 51.Oslin DW, Berrettini WH, O’Brien CP. Targeting treatments for alcohol dependence: the pharmacogenetics of naltrexone. Addiction biology. 2006;11:397–403. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 52.Health N. I. o., editor. Division of AIDS - National Institute of Allergy and Infectious Diseases. Division of AIDS (DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. Bethesda MD: US Department of Health and Human Services; 2009. [Google Scholar]
- 53.Mezinskis J, D S, Goldsmith J, Cohen M, Somoza E. Craving and withdrawal symptoms for various drugs of abuse. Psychiatric Annals. 1998;28:577–583. [Google Scholar]
- 54.Hartz DT, F-O S, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- 55.Derogatis LR, M N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 56.Gossop M, D S, Griffiths P, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine. Addiction. 1995;90:607–614. doi: 10.1046/j.1360-0443.1995.9056072.x. [DOI] [PubMed] [Google Scholar]
- 57.O’Brien CP, G D, Forman RF, Schweizer E, Pettinati HM. Long-term opioid blockade and hedonic response: preliminary data from two open-label extension studies with extended-release naltrexone. Am J Addict. 2011;20:106–112. doi: 10.1111/j.1521-0391.2010.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng ST, C A, Fung HH. Factorial structure of a short version of the Center for Epidemiologic Studies Depression Scale. International Journal of Geriatric Psychiatry. 2006;21:333–336. doi: 10.1002/gps.1467. [DOI] [PubMed] [Google Scholar]
- 59.Hseih FY, B D, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17:1623–1634. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 60.McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18:414–418. doi: 10.1111/j.1755-5949.2011.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.West R. Using Bayesian analysis for hypothesis testing in addiction science. Addiction. 2016;111:3–4. doi: 10.1111/add.13053. [DOI] [PubMed] [Google Scholar]
- 62.Christie J. Bayes Factors. 2011 [Google Scholar]
- 63.Courtney KE, Ray LA. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. doi: 10.1016/j.drugalcdep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ling W, Chang L, Hillhouse M, Ang A, Striebel J, Jenkins J, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109:1489–1500. doi: 10.1111/add.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heinzerling KG, Swanson AN, Hall TM, Yi Y, Wu Y, Shoptaw SJ. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109:1878–1886. doi: 10.1111/add.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colfax GN, Santos GM, Das M, Santos DM, Matheson T, Gasper J, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68:1168–1175. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coffin PO, Santos G-M, Das M, Santos DM, Huffaker S, Matheson T, et al. Aripiprazole for the treatment of methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:751–761. doi: 10.1111/add.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Das M, Santos D, Matheson T, Santos G-M, Chu P, Vittinghoff E, et al. Feasibility and acceptability of a phase II randomized pharmacologic intervention for methamphetamine dependence in high-risk men who have sex with men. AIDS. 2010;24:991–1000. doi: 10.1097/qad.0b013e328336e98b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colfax GN, S G, Das M, McDermott Santos D, Matheson T, Gasper J, Shoptaw S, Vittinghoff E. Mirtazapine to Reduce Methamphetamine UseA Randomized Controlled Trial. JAMA Psychiatry. 2011;68:1168–1175. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garbutt JC, K H, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 71.Kranzler HR, W DR, Billot L. Naltrexone Depot for Treatment of Alcohol Dependence: A Multicenter, Randomized, Placebo-Controlled Clinical Trial. Alcoholism Clinical and Experimental Research. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- 72.Runarsdottir V, Hansdottir I. Amphetamine Addiction in Iceland and Efficacy of Pharmacotherapy. 2013 [Google Scholar]
- 73.Mooney LJ, Hillhouse MP, Thomas C, Ang A, Sharma G, Terry G, et al. Utilizing a Two-stage Design to Investigate the Safety and Potential Efficacy of Monthly Naltrexone Plus Once-daily Bupropion as a Treatment for Methamphetamine Use Disorder. Journal of addiction medicine. 2016;10:236–243. doi: 10.1097/ADM.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]


