Version Changes
Revised. Amendments from Version 2
We want to thank the reviewers for the valuable time spent in the analysis and review of our manuscript. In regards to suggestions, we have added the limitations mentioned by the reviewer in the discussion section.
Abstract
Background: Insulin resistance (IR) evaluation is a fundamental goal in clinical and epidemiological research. However, the most widely used methods are difficult to apply to populations with low incomes. The triglyceride-glucose index (TGI) emerges as an alternative to use in daily clinical practice. Therefore the objective of this study was to determine an optimal cutoff point for the TGI in an adult population from Maracaibo, Venezuela.
Methods: This is a sub-study of Maracaibo City Metabolic Syndrome Prevalence Study, a descriptive, cross-sectional study with random and multi-stage sampling. For this analysis, 2004 individuals of both genders ≥18 years old with basal insulin determination and triglycerides < 500 mg/dl were evaluated.. A reference population was selected according to clinical and metabolic criteria to plot ROC Curves specific for gender and age groups to determine the optimal cutoff point according to sensitivity and specificity.The TGI was calculated according to the equation: ln [Fasting triglyceride (mg / dl) x Fasting glucose (mg / dl)] / 2.
Results: The TGI in the general population was 4.6±0.3 (male: 4.66±0.34 vs. female: 4.56±0.33, p=8.93x10 -10). The optimal cutoff point was 4.49, with a sensitivity of 82.6% and specificity of 82.1% (AUC=0.889, 95% CI: 0.854-0.924). There were no significant differences in the predictive capacity of the index when evaluated according to gender and age groups. Those individuals with TGI≥4.5 had higher HOMA2-IR averages than those with TGI <4.5 (2.48 vs 1.74, respectively, p<0.001).
Conclusions: The TGI is a measure of interest to identify IR in the general population. We propose a single cutoff point of 4.5 to classify individuals with IR. Future studies should evaluate the predictive capacity of this index to determine atypical metabolic phenotypes, type 2 diabetes mellitus and even cardiovascular risk in our population.
Keywords: insulin resistance, triglycerides, glycemia, Metabolic syndrome, cutoff
Introduction
Insulin resistance (IR) is a metabolic condition in which insulin-dependent tissues become less sensitive to insulin action, leading to an imbalance in the metabolism of carbohydrates, lipids and proteins 1. This condition is caused by the influence of different risk factors in the population, such as aging, alcohol consumption, smoking, hypercaloric diets, sedentary lifestyle and obesity 2. Its role in the development of different pathologies, as cardiovascular disease (CVD) 3 and cerebrovascular disease 4, is now recognized, as well as playing an important role in the pathogenesis and clinical outcomes of the metabolic syndrome (MS) 5 and type 2 diabetes mellitus (DM2) 6.
In 1963, Randle and colleagues were among the first to investigate the pathophysiology of IR 7, suggesting the elevation of free fatty acids (FFA) in the splenic circulation as the cornerstone of this disorder. They proposed the glucose fatty acid cycle, called the Randle cycle. Years later, a theory proposed by Shulman 8 surfaced, which continued to support the role of FFA in the pathophysiology of IR. However, these authors suggested that FFA and its products, such as diacylglycerol, acyl-coA and ceramides, activate serin-threonine kinases, which phosphorylate important proteins, inhibit the insulin signaling pathway and subsequently translocated glucose transporter 4 (GLUT4) to the plasma membrane 8.
Although many aspects remain to be clarified in the pathophysiology of IR, its long-term complications have generated great interest in the determination of ideal methods that allow the promotion of an early and accurate diagnosis in risky populations 9. In this sense, the Euglycemic-Hyperinsulinemic Clamp is considered the gold standard for the determination of IR 10, but the high cost and impracticability of this method has promoted the development of new techniques for the estimation of insulin sensitivity. Many mathematical models have been proposed in recent years with the objective of simplifying the measurement of IR 11, highlighting the Homeostasis Model Assessment (HOMA-IR), a validated method to measure IR from serum glucose and fasting serum insulin 12. This index has been studied in our population, which was conducted in 2026 subjects and evaluated the factors related to insulin resistance (defined as HOMA2-IR=2) 13. However, one of the biggest limitations is the lack of accessibility to populations with lower incomes, since all the individuals require insulin blood tests.
Simental-Mendia et al. 14, 15 have proposed and validated a new formula to evaluate IR from the levels of serum triglycerides (TAG) and fasting glucose (FG), which is known as ‘Triglyceride/Glucose Index’ (TGI), this formula is a potential diagnostic tool when other standard methods are not available. Based on the information above, the objective of the study is to determine an optimal cut point of the TGI to determine IR and to evaluate the behavior according to the main sociodemographic characteristics in an adult population from Maracaibo, Zulia, Venezuela.
Methods
Sample selection and study design
The Maracaibo City Metabolic Syndrome Prevalence Study (MMSPS) study is a descriptive, cross-sectional, randomized, multi-stage sampling study that was carried out in Maracaibo-Venezuela; the second most populated city in the country with an approximate population of 2,500,000, in order to evaluate the cardiovascular and metabolic risk factors of this locality, during the period May 2007 - December 2009 16. The only inclusion criteria was individuals older than 18 years. The sample (1,986 individuals) was calculated based on the estimates of the population in the city given by the National Institute of Statistics (1,428,043 inhabitants for 2007). A total of 244 individuals (12%) were added through oversampling, in order to increase the accuracy of the estimates obtained from the smaller subgroups of the sample, representing a total of 2230 individuals of both genders. Sampling details have been previously published 16. The study was approved by the Bioethics Committee of the Endocrine and Metabolic Research Center – University of Zulia (approval number: BEC-006-0305). This ethical approval included all future studies that used the data from the MMSPS. All participants signed written consent before being questioned and physically examined by a trained team.
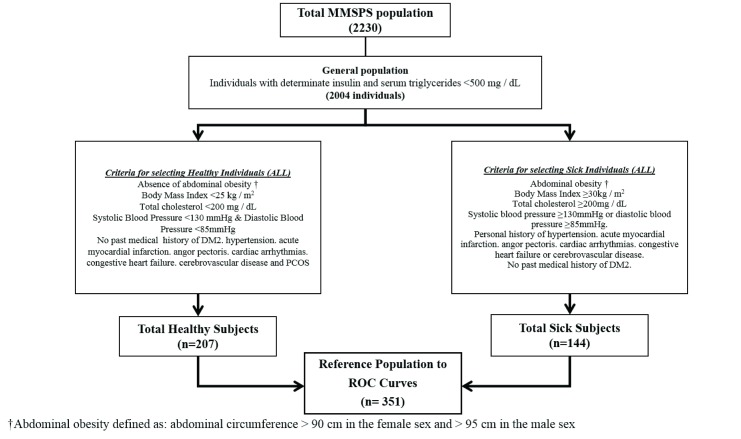
For this analysis, 2004 individuals were selected according to the availability of baseline insulin, in addition to the exclusion of individuals with TAG≥500 mg/dl. Based on this sub-sample, a reference population was selected based on all the following criteria: abdominal obesity, total cholesterol, high blood pressure and personal history of DM2, coronary artery diseases, cardiac arrhythmias, acute cerebrovascular disease, and polycystic ovaries; with the purpose of establishing a subsample of healthy and unhealthy subjects without using definitions or diagnostic criteria that include TAG and glycaemia values to avoid variables correlation a priori ( Figure 1).
Figure 1. Diagram of reference population selection for cut-off points determination of the triglyceride-glucose index in Maracaibo city, Venezuela.
ROC Curves: Receiver operating characteristic curves.
Individual evaluation
The data was gathered through a complete medical history performed by the trained team: the past medical and family history for cardiovascular and endocrine-metabolic diseases was assessed.
Blood pressure and anthropometric evaluation
The auscultation method was used for the measurement of blood pressure, using stethoscopes and calibrated sphygmomanometers adequately validated. The procedure was performed with the individual at rest (at least 15 minutes) and sitting with both feet on the floor; three measurements were taken, with 15 minutes of separation between one measurement and the other. Systolic blood pressure was determined by auscultation of the first Korotkoff noise, while diastolic blood pressure was determined on auscultation of the fifth Korotkoff noise.
Weight was determined through a dielectric balance (Tanita, TBF-310 GS Body Composition Analyzer, Tokyo, Japan), and height was obtained by using vertical tape calibrated in centimeters and millimeters. Individuals were standing and barefoot, with light clothing throughout the evaluation, maintaining a straight posture and head up. Body Mass Index was calculated using the weight/height formula and classified according to the criteria proposed by the World Health Organisation (low weight, normal weight, overweight, Obesity type I, II and III) 17. The measurement of the abdominal circumference was taken with a plastic metric tape in centimeters at equidistant points between the costal ridge and the iliac crest, according to the protocol proposed by the National Salute Institute of the United States 18. Abdominal obesity was defined according to specific cutoff points for our previously determined population, ≥90 cm in women and ≥95 cm in men 19.
Laboratory analysis
Blood samples were collected between 8:00 A.M. and 10:00 A.M. after an overnight fast (~10 hours). Determination of glucose, total cholesterol, triglycerides, and HDL-C was done with an automated analyzer (Human Gesellschaft fur Biochemica und Diagnostica mbH, Germany). The intra-assay coefficient of variation for total cholesterol, TAG, glucose and insulin was 3%, 5%, 3%, and <10%, respectively. Insulin was determined using an ultrasensitive ELISA double-sandwich method (DRG Instruments GmbH, Germany, Inc.), with a Detection Limit <1 mU/L. The HOMA2IR index was calculated using the software administered by the Oxford Diabetes Center, Endocrinology and Metabolism (available at http://www.dtu.ox.ac.uk/homacalculator/index.php). A cutoff value of ≥2 was used to determine IR 20.
Calculation of TGI
The calculation of the TGI was done using the equation: ln [Fasting TAG (mg/dl) x FG (mg/dl)]/2; thus being expressed on a logarithmic scale 14, 21.
Statistical analysis
Qualitative variables were expressed in absolute and relative frequencies. While the quantitative variables were expressed as arithmetic mean ± SD, with the previous analysis of normality through means of a Geary test. Significant differences between groups were assessed using the Student’s t-test, while an ANOVA was used for comparisons between three or more groups. Data were analyzed through the SPSS v.21 for Windows (IBM Chicago, IL), considering statistically significant results when p <0.05.
ROC curves were plotted in the reference population ( Figure 1) to analyze the predictive capacity and to determine an optimal cutoff point for the TGI. ROC curves were gender-specific using R version 3.4.1. Several indices were calculated to evaluate the optimum cutoff point in the curve. The area under the curve (AUC) is used to establish the ability of the test to obtain an appropriate cutoff where an AUC of 1.00 is considered a perfect diagnostic test 22. Comparisons between AUC were performed using Delong’s test 23. The Youden Index (J) was calculated using the formula [J = sensitivity + specificity-1 = S-(1-Es)] 23, obtaining the value of true positives (sensitivity) and false positives (1-specificity) when J >1. The minimum cutoff point was calculated using the nearest point to 0.1 in the ROC curves formula: square root of [(1-sensitivity) 2 (1-specificity) 2] 24. In addition, probability radius positive [sensitivity/1-specificity] and negative [1-sensitivity/specificity] were calculated to aid in the selection of the cutoff point together with the Youden index. Likelihood values >1 indicate association with the disease, while those <1 indicate association with the absence of the disease 25.
Results
General characteristics of the sample
A total of 2004 individuals were studied, 53.4% (n = 1050) were female, the mean age of the population was 39.6±15.3. The general characteristics of the population are shown in Table 1. The mean TGI in the general population was 4.6±0.3, with higher values in males (males: 4.66±0.34 vs. females: 4.56±0.33, p=8.93×10 -10). The epidemiological behavior of the TGI according to age and ethnicity is shown in Table 2, which shows an increase of the index as age increases. In regards to ethnicity, no statistically significant differences were found between means (p=0.326).
Table 1. General characteristics of the sample studied, Maracaibo city, Venezuela.
| Female (n= 1050) | Male (n=954) | Total (n=2004) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Age (years) | ||||||
| <30 | 308 | 29.3 | 363 | 38.1 | 671 | 33.5 |
| 30–49 | 420 | 40.0 | 350 | 36.7 | 770 | 38.4 |
| ≥50 | 322 | 30.7 | 241 | 25.3 | 563 | 28.1 |
| Ethnicity | ||||||
| Mixed | 794 | 75.6 | 740 | 77.6 | 1534 | 76.5 |
| White Hispanic | 171 | 16.3 | 145 | 15.2 | 316 | 15.8 |
| Afro-Venezuelan | 27 | 2.6 | 32 | 3.4 | 59 | 2.9 |
| American Indian | 47 | 4.5 | 36 | 3.8 | 83 | 4.1 |
| Other * | 11 | 1.0 | 1 | 0.1 | 12 | 0.6 |
| Triglycerides (mg/dl) | ||||||
| <150 | 815 | 77.6 | 644 | 67.5 | 1459 | 72.8 |
| ≥150 | 235 | 22.4 | 310 | 32.5 | 545 | 27.2 |
| Glycaemic status ¶ | ||||||
| Euglycemic | 774 | 73.9 | 662 | 69.4 | 1436 | 71.7 |
| Impaired fasting glucose | 186 | 17.7 | 212 | 22.2 | 398 | 19.9 |
| Type 2 diabetes mellitus | 88 | 8.4 | 80 | 8.4 | 168 | 8.4 |
*Asian and Arabic descent.
¶Criteria according to the ADA 2016 consensus
Table 2. Epidemiological behavior of the triglyceride-glucose index in the general population according to sociodemographic variables, in Maracaibo city, Venezuela.
| n | TGI (n=2004) | ||
|---|---|---|---|
| Mean±SD | p * | ||
| Gender | 8.93×10 −10 | ||
| Female | 1050 | 4.56±0.33 | |
| Male | 954 | 4.66±0.34 | |
| Age (years) | 6.21×10 −77 | ||
| <30 | 671 | 4.43±0.28 | |
| 30–49 | 770 | 4.65±0.33 | |
| ≥50 | 563 | 4.77±0.31 | |
| Ethnicity | 0.326 | ||
| Mixed | 1534 | 4.60±0.33 | |
| White Hispanic | 316 | 4.62±0.35 | |
| Afro-Venezuelan | 59 | 4.68±0.32 | |
| American Indian | 83 | 4.60±0.31 | |
| Other ¶ | 12 | 4.51±0.28 | |
* Student’s t- test (for more than two groups one-way ANOVA was used).
¶ Asian and Arabic descent.
SD: standard deviation
Post-hoc Tukey: <30 years vs 30–49 years, p=5.09x10 −9; <30 years vs ≥50 years, p=5.09x10 −9; 30–49 years vs ≥50 years, p=5.10x10 −9.
When assessing the reference population (n=351); healthy: n=207 – unhealthy: n=144, a similar behavior of the TGI was found according to age and ethnicity ( Table 3). In addition, there were no differences in TGI mean between the general and reference population.
Table 3. Epidemiological behavior of the triglyceride-glucose index in the reference population according to sociodemographic variables, in Maracaibo city, Venezuela.
| TGI (n=351) | |||
|---|---|---|---|
| n | Mean±SD | p * | |
| Gender | 5.83-10 -6 | ||
| Female | 190 | 4.41±0.29 | |
| Male | 161 | 4.56±0.32 | |
| Age (years) | 8.42×10 -28 | ||
| <30 | 178 | 4.31±0.29 | |
| 30–49 | 118 | 4.61±0.31 | |
| ≥50 | 55 | 4.72±0.23 | |
| Ethnicity | 0.413 | ||
| Mixed | 268 | 4.48±0.32 | |
| White Hispanic | 52 | 4.48±0.34 | |
| Afro-Venezuelan | 8 | 4.59±0.26 | |
| American Indian | 20 | 4.39±0.23 | |
| Other ¶ | 3 | 4.25±0.26 | |
*Student’s t-test (for more than two groups one way-ANOVA was used).
¶ Asian and Arabic descent.
SD: Standard deviation.
Post-hoc Tukey: <30 years vs 30–49 years, p=5.09×10 -9; <30 years vs ≥50 years, p=5.09x10 -9; 30–49 years vs ≥50 years, p=0.03.
Cutoff points for the TGI in the reference population by gender
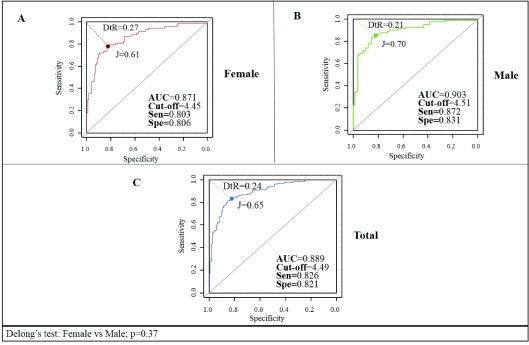
For the determination of cutoff points of the TGI, ROC curves were plotted for the reference population by gender ( Figure 2). An AUC of 0.889 (95% CI: 0.854-0.924) was obtained for the general population with a proposed cutoff of 4.49 (82.6% sensitivity, and 82.1% specificity), while the AUC calculated for males was 0.903 (95% CI: 0.856-0.950) and for females was 0.871 (95% CI: 0.818-0.925); Delong's test: p=0.37. The cutoff points and index calculated according to the ROC curves are shown in Table 4.
Figure 2. Receiver Operating Characteristic curves for the triglyceride-glucose index in the reference population by gender, in Maracaibo city, Venezuela.
DtR, Distance to ROC; J, Youden Index; AUC, are under the curve; sen, sensitivity; spe, specificity.
Table 4. Cut-off points for triglyceride-glucose index (TGI) selected in the reference population and by gender, in Maracaibo city, Venezuela.
| Gender | TGI ¶ | Sensitivity (%) | Specificity (%) | Youden
index |
ROC
distance |
LR+ | AUC
(95%CI) |
|---|---|---|---|---|---|---|---|
| Female | 4.45 | 80.3 | 80.6 | 0.61 | 0.276 | 4.14 | 0.871
(0.818–0.925) |
| Male | 4.51 | 87.2 | 83.1 | 0.70 | 0.212 | 5.15 | 0.903
(0.856–0.950) |
| Total | 4.49 | 82.6 | 82.1 | 0.65 | 0.249 | 4.61 | 0.889
(0.854–0.924) |
¶Cut-off points selected according to the best combination of indices. Delong’s test= 0.37
Cutoff points for the TGI in the reference population by age
When assessing the predictive capacity of the TGI according to age, a higher AUC was obtained in individuals between the ages of 30 and 50 years old (AUC=0.876; 95% CI: 0.812-0.939); however, when comparing AUCs among age groups, no significant differences were found. The cutoff points obtained according to age are shown in Table 5, with means similar to that proposed for the reference population (<30 years: 4.48 [65.0% sensitivity, and 84.8% specificity]; 30–50 years: 4.51 [84.7% sensitivity, and 78.3% specificity], ≥50 years, 4.51 [86.5% sensitivity, and 66.7% specificity]).
Table 5. Cutoff points for triglyceride-glucose index (TGI) selected in the reference population according to age groups, in Maracaibo city, Venezuela.
| Age
(years) |
TGI ¶ | Sensitivity
(%) |
Specificity
(%) |
Youden
index |
ROC
distance |
LR+ | AUC
(95%CI) |
|---|---|---|---|---|---|---|---|
| <30 | 4.49 | 65.0 | 84.8 | 0.50 | 0.381 | 4.27 | 0.789
(0.684–0.895) |
| 30–50 | 4.51 | 84.7 | 78.3 | 0.63 | 0.265 | 3.89 | 0.876
(0.812–0.939) |
| ≥50 | 4.51 | 86.5 | 66.7 | 0.53 | 0.359 | 2.59 | 0.776
(0.602–0.949) |
¶Cut-off points selected according to the best combination of indices. Delong’s test: <30 vs 30–50 years; p=0.171. <30 vs >50 years; p=0.885. 30–50 vs >50 years; p=0.34.
HOMA2IR according to cutoff points of the TGI
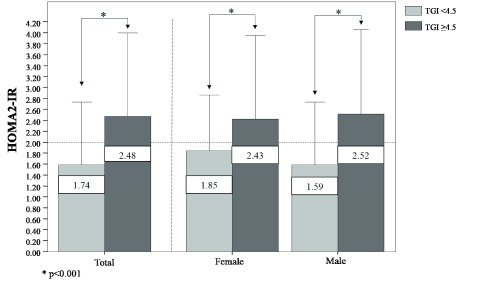
Finally, when assessing HOMA2-IR levels according to the proposed cutoff point of the TGI for the general population ( Figure 3), individuals with TGI≥4.5 exhibited higher levels of HOMA2IR than those who had a TGI <4.5 (2.48 vs 1.74, respectively, p<0.001), with similar behavior by gender.
Figure 3. HOMA2-IR levels according to the specific cutoff point for the triglyceride-glucose index in the general population, in Maracaibo city, Venezuela.
This data is available in both .SAV and .xls forms. BMI: Body Mass Index; BP: Blood Pressure.
Copyright: © 2018 Salazar J et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Discussion
The evaluation of IR is an objective that continues to acquire relevance in clinical and epidemiological research, due to the potential role of this disorder in the pathophysiology of MS 26, the consequent risk of developing DM2 27 and CVD 28. In developing countries with economic difficulties in health systems, such as Venezuela, routine measurements of plasma insulin are not easily accessible, which forces the use of other indices based on the role of glucolipotoxicity as a key element in the development of IR 29. In this way, the TGI has recently been proposed and validated as a useful alternative in clinical settings, and has been well accepted due to the high predictive power observed with respect to other indices 30.
Several studies have shown that the TGI better predicts HOMA-IR levels than variables, such as the TAG/HDL index, visceral adiposity index, leptin, Apo-B/Apo-AI, and lipid parameters 29, 30, representing a very good correspondence with this index 31, which constitutes an important tool with high validity for the clinician when facing limited access to lab work-ups. However, in view of the wide variability of TAG levels according to the ethnic, sociocultural and genetic characteristics of each population, the need arises to evaluate their epidemiological behavior and establish reference values specific to each region.
In regards to our study, the mean TGI was higher in men compared to women. Several studies have reported similar findings 14, 32; however, when plotted ROC curves for the TGI for each gender, significant differences in the AUC values and effect size by gender were not observed. We propose the use of a single cutoff point of 4.5 for the identification of unhealthy individuals in the clinical practice as an easy tool for the clinician.
These findings corresponded to those originally reported by the index’s authors in 2010 15, when evaluating the discriminative ability of this index to determine IR against the euglycemic-hyperinsulinemic clamp in a population of 99 individuals (11 healthy, 34 obese, 22 with prediabetes and 32 with DM2) suggesting a cutoff point of 4.68 with high sensitivity (96.5%) and specificity (85.0%). Based on these findings, several studies have attempted to establish specific cutoff points for their populations, assessing the clinical utility of this index. In 2011, in a Brazilian population, Vasques et al. 33 stipulated that the TGI had a slightly better performance to diagnose IR compared to HOMA2-IR (AUC=0.79 vs AUC=0.77, respectively). Although these investigators did not perform any statistical tests to compare the diagnostic capacity between both indices, the similar behavior allows the use of TGI in clinical settings routinely.
On the other hand, Unger et al. 21 conducted a cross-sectional study in an Argentinean population with the aim of evaluating the discriminative capacity of the TGI for the diagnosis of MS, taking into account that the development of this syndrome is related to IR. They set a cutoff point of 8.8 (sensitivity = 79%, specificity = 86%) to diagnose MS in their population, a value that differs markedly from the values in our region and those originally proposed. These differences have been observed in other studies 34– 36, so considering that the means of TAG do not vary significantly between these and our study, this discrepancy could be attributable to errors in the calculation of the original formula 37.
In regards to age, an increase in the average TGI was observed as age increased, similar to that found by Navarro-González et al. 38 and Cuda et al. 39. This can be associated with the increase in oxidative stress inherent to aging, which would favor the development of IR, as well as the elevation of plasma levels of TAG 40. In this sense, Guerrero-Romero et al. 31 recently evaluated the performance of the TGI to determine IR against the euglycemic-hyperinsulinemic clamp in a young Mexican adult population (mean age: 19.2±1.4), with the goal of establishing a specific cutoff point in this population. However, the cutoff points of 4.55 in men and 4.68 in women were similar to the value proposed previously for the general population, and similar to the one proposed in our study. Also, when we plotted ROC curves for each age group, we did not observe significant differences between AUC neither effect size in Cohen’s d analysis, ruling out the need to establish age-specific cutoff points.
In relation to ethnicity, the small sample in each of the categories precludes the generalization of these results, but it shows that it is necessary to evaluate the behavior of the TGI in the different ethnic groups on a large scale and to determine if there are significant differences between them because there is no data reported on this diagnostic method among various ethnicities.
Base on previous analyzes, the usefulness of the TGI has been extended in several regions of the world, being also used in the screening of other pathological metabolic states in which the IR underlies the fundamental pathophysiological mechanism, such as DM2 41 and atypical metabolic phenotypes 42, 43. In fact, the TGI appears to be a better predictor of incidence of DM2 than TAG 38, weight gain 43 and other IR indices, such as TAG/HDL and HOMA-IR 41. Moreover, Lee et al. performed a retrospective study involving 2900 Korean non-diabetic adults determinate as a cutoff point 8, (AUC=0.751, 95% CI 0.704-0.799) to predict the incidence of DM2 in their population. In addition, only individuals with a TGI >8.8 were associated with a significant risk of DM2 incidence, regardless of the presence of obesity 44.
Additionally, the index can better predict the patient's metabolic status, as it has been shown in discriminating atypical metabolic phenotypes, such as metabolically obese normal weight and healthy obese, as well as their progression throughout time 35. Cutoff points have been determined to discriminate these pathological metabolic status in two populations of Korea 34, 35, which could facilitate the definition of these phenotypes. Future studies in our population should evaluate the utility of this index in the identification of these abnormal pathological statuses, considering an appropriate inference when used in subjects with extremely high triglyceride levels. It is important to mention that within the limitations of this study it is necessary to consider the cross-sectional design and the lack of gold standard during the process of selection of the reference population, thus is difficult to evaluate a direct causal relationship. Likewise, this analysis is limited to a Latin-American population, extrapolating the findings to other ethnicities should be interpreted cautiously; there might be a need for ethnic specific TGI cut-offs, as Korean, Italian and Chinese population have made.
Additionally, there are several factors that can influence the TGI values and therefore cutoff points selection; these include lipid-lowering medications, nutritional and physical activity variables which are able to regulate the metabolism of equation components and be potential strategies in the management of IR. However, only the use of lipid-lowering drugs was considered in the selection of the reference population, excluding those subjects who had a regular consumption of statins or fibrates, the role of diet and physical activity will be considered in future studies that determine the effect of environmental components in a multivariate context.
Conclusions
The TGI is an instrument of interest when it comes to identifying IR in the general population. We propose a single cutoff point of 4.5 to identify patients with IR, as we identify the need for standardization of the formula calculation in order to be able to adequately compare the differences observed in various studies. Despite this, due to the easier application in clinical practice, future studies in our population should evaluate the predictive capacity of this index to determine atypical metabolic phenotypes, DM2 and CVD risk.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2018 Salazar J et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
Dataset 1: Data for the study ‘Optimal cutoff for the evaluation of insulin resistance through triglyceride-glucose index: A cross-sectional study in a Venezuelan population’. This data is available in both .SAV and .xls forms. BMI: Body Mass Index; BP: Blood Pressure. doi, 10.5256/f1000research.12170.d171840 45
Funding Statement
This work was supported by the Technological, Humanistic, and Scientific Development Council (Consejo de Desarrollo Científico, Humanístico y Tecnológico; CONDES), University of Zulia (grant nº CC-0437-10-21-09-10).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; referees: 2 approved]
References
- 1. Wilcox G: Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19–39. [PMC free article] [PubMed] [Google Scholar]
- 2. Morigny P, Houssier M, Mouisel E, et al. : Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–66. 10.1016/j.biochi.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 3. Patel TP, Rawal K, Bagchi AK, et al. : Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21(1):11–23. 10.1007/s10741-015-9515-6 [DOI] [PubMed] [Google Scholar]
- 4. Luchsinger JA: Insulin resistance, type 2 diabetes, and AD: cerebrovascular disease or neurodegeneration? Neurology. 2010;75(9):758–759. 10.1212/WNL.0b013e3181eee287 [DOI] [PubMed] [Google Scholar]
- 5. Rojas J, Bermúdez V, Leal E, et al. : Insulinorresistencia E Hiperinsulinemia Como Factores De Riesgo Para Enfermedad Cardiovascular. AVTF. 2008;27(1):30–40. Reference Source [Google Scholar]
- 6. Gastaldelli A: Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;93(suppl 1):S60–S65. 10.1016/S0168-8227(11)70015-8 [DOI] [PubMed] [Google Scholar]
- 7. Randle PJ, Garland PB, Hales CN, et al. : The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789. 10.1016/S0140-6736(63)91500-9 [DOI] [PubMed] [Google Scholar]
- 8. Shulman GI: Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–6. 10.1172/JCI10583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borai A, Livingstone C, Kaddam I, et al. : Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11(1):158. 10.1186/1471-2288-11-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–223. 10.1152/ajpendo.1979.237.3.E214 [DOI] [PubMed] [Google Scholar]
- 11. Singh B, Saxena A: Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1(2):36–47. 10.4239/wjd.v1.i2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthews DR, Hosker JP, Rudenski AS, et al. : Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 13. Bermudez V, Salazar J, Martínez MS, et al. : Prevalence and Associated Factors of Insulin Resistance in Adults from Maracaibo City, Venezuela. Adv Prev Med. 2016;2016: 9405105. 10.1155/2016/9405105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F: The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 15. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. : The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51. 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 16. Bermúdez V, Marcano RP, Cano C, et al. : The Maracaibo city metabolic syndrome prevalence study: design and scope. Am J Ther. 2010;17(3):288–294. 10.1097/MJT.0b013e3181c121bc [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization: Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. Geneva: The Organization;2000; 894:i–xii, 1–253. [PubMed] [Google Scholar]
- 18. Health Statistics: NHANES III reference manuals and reports (CDROM). Hyattsville, MD: Centers for Disease Control and Prevention,1996. Reference Source [Google Scholar]
- 19. Bermúdez V, Rojas J, Salazar J, et al. : Optimal Waist Circumference Cut-Off Point for Multiple Risk Factor Aggregation: Results from the Maracaibo City Metabolic Syndrome Prevalence Study. Epidemiol Res Int. 2014;2014: 718571. 10.1155/2014/718571 [DOI] [Google Scholar]
- 20. Bermúdez V, Rojas J, Martínez MS, et al. : Epidemiologic Behavior and Estimation of an Optimal Cut-Off Point for Homeostasis Model Assessment-2 Insulin Resistance: A Report from a Venezuelan Population. Int Sch Res Notices. 2014;2014: 616271. 10.1155/2014/616271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Unger G, Benozzi SF, Perruzza F, et al. : Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 2014;61(10):533–40. 10.1016/j.endonu.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 22. Akobeng AK: Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–7. 10.1111/j.1651-2227.2006.00178.x [DOI] [PubMed] [Google Scholar]
- 23. Demler OV, Pencina MJ, D'Agostino RB, Sr: Misuse of DeLong test to compare AUCs for nested models. Stat Med. 2012;31(23):2577–87. 10.1002/sim.5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Böhning D, Böhning W, Holling H: Revisiting Youden's index as a useful measure of the misclassification error in meta-analysis of diagnostic studies. Stat Methods Med Res. 2008;17(6):543–54. 10.1177/0962280207081867 [DOI] [PubMed] [Google Scholar]
- 25. Perkins NJ, Schisterman EF: The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670–5. 10.1093/aje/kwj063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samson SL, Garber AJ: Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1–23. 10.1016/j.ecl.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 27. Lyssenko V, Jonsson A, Almgren P, et al. : Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359(21):2220–2232. 10.1056/NEJMoa0801869 [DOI] [PubMed] [Google Scholar]
- 28. Miller M, Stone NJ, Ballantyne C, et al. : Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. 10.1161/CIR.0b013e3182160726 [DOI] [PubMed] [Google Scholar]
- 29. Er LK, Wu S, Chou HH, et al. : Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS One. 2016;11(3):e0149731. 10.1371/journal.pone.0149731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du T, Yuan G, Zhang M, et al. : Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13(1):146. 10.1186/s12933-014-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, et al. : Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in Young adults. Arch Med Rev. 2016;47(5):382–387. 10.1016/j.arcmed.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 32. Irace C, Carallo C, Scavelli FB, et al. : Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–672. 10.1111/ijcp.12124 [DOI] [PubMed] [Google Scholar]
- 33. Vasques AC, Novaes FS, de Oliveira Mda S, et al. : TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. 10.1016/j.diabres.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 34. Lee SH, Han K, Yang HK, et al. : A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes. 2015;5(4):e149. 10.1038/nutd.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee SH, Yang HK, Ha HS, et al. : Changes in Metabolic Health Status Over Time and Risk of Developing Type 2 Diabetes: A Prospective Cohort Study. Medicine (Baltimore). 2015;94(40):e1705. 10.1097/MD.0000000000001705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abbasi F, Reaven GM: Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60(12):1673–1676. 10.1016/j.metabol.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 37. Hosseini SM: Triglyceride-Glucose (TyG) Index Computation and Cut-Off. Acta Endo (Buc). 2015;11(1):130–131. 10.4183/aeb.2015.130 [DOI] [Google Scholar]
- 38. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, et al. : Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99–105. 10.1016/j.ypmed.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 39. Cuda G, Lentini M, Gallo L, et al. : Fasting triglycerides and glucose index in an unselected consecutive Italian population of outpatients. Riv Ital Med Lab. 2011;7(4):226–227. 10.1007/s13631-011-0032-3 [DOI] [Google Scholar]
- 40. Monickaraj F, Aravind S, Nandhini P, et al. : Accelerated fat cell aging links oxidative stress and insulin resistance in adipocytes. J Biosci. 2013;38(1):113–122. 10.1007/s12038-012-9289-0 [DOI] [PubMed] [Google Scholar]
- 41. Lee SH, Kwon HS, Park YM, et al. : Predicting the Development of Diabetes Using the Product of Triglycerides and Glucose: The Chungju Metabolic Disease Cohort (CMC) Study. PLoS One. 2014;9(2):e90430. 10.1371/journal.pone.0090430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee SH, Han K, Yang HK, et al. : Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008–2010. Clin Endocrinol (Oxf). 2014;82(2):213–220. 10.1111/cen.12502 [DOI] [PubMed] [Google Scholar]
- 43. Navarro-González D, Sánchez-Íñigo L, Fernández-Montero A, et al. : TyG Index Change Is More Determinant for Forecasting Type 2 Diabetes Onset Than Weight Gain. Medicine (Baltimore). 2016;95(19):e3646. 10.1097/MD.0000000000003646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee DY, Lee ES, Kim JH, et al. : Predictive Value of Triglyceride Glucose Index for the Risk of Incident Diabetes: A 4-Year Retrospective Longitudinal Study. PLoS One. 2016;11(9):e0163465. 10.1371/journal.pone.0163465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salazar J, Bermúdez V, Calvo M, et al. : Dataset 1 in: Optimal cutoff for the evaluation of insulin resistance through triglyceride-glucose index: A cross-sectional study in a Venezuelan population. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]