Abstract
Background
Aberrant signaling between germ cells and somatic cells can lead to reproductive disease and depends on diffusible signals, including TGFB-family proteins. The TGFB-family protein Gsdf (gonadal soma derived factor) controls sex determination in some fish and is a candidate for mediating germ cell/soma signaling.
Results
Zebrafish expressed gsdf in somatic cells of bipotential gonads and expression continued in ovarian granulosa cells and testicular Sertoli cells. Homozygous gsdf knockout mutants delayed leaving the bipotential gonad state, but then became a male or a female. Mutant females ovulated a few oocytes, then became sterile, accumulating immature follicles. Female mutants stored excess lipid and down-regulated aromatase, gata4, insulin receptor, estrogen receptor, and genes for lipid metabolism, vitellogenin, and steroid biosynthesis. Mutant females contained less estrogen and more androgen than wild types. Mutant males were fertile. Genomic analysis suggests that Gsdf, Bmp15, and Gdf9, originated as paralogs in vertebrate genome duplication events.
Conclusions
In zebrafish, gsdf regulates ovarian follicle maturation and expression of genes for steroid biosynthesis, obesity, diabetes, and female fertility, leading to ovarian and extra-ovarian phenotypes that mimic human polycystic ovarian syndrome (PCOS), suggesting a role for a related TGFB signaling molecule in the etiology of PCOS.
Keywords: Gonad development, GDF9, BMP15, oogenesis, Polycystic ovarian syndrome (PCOS), gonadal soma germ cell interaction, granulosa cells, Sertoli cells, ovarian follicle, insulin signaling, TGFβ
INTRODUCTION
Germ cells and somatic cells interact reciprocally to promote gonad development and gamete maturation (Eppig, 2001). In mouse, gonadal soma factors including Bmp7, Wnt4, and Rspo1 (Jeays-Ward et al., 2003; Ross et al., 2007; Chassot et al., 2008) stimulate proliferation of primordial germ cells (PGCs). Reciprocally, germ cells signal the soma because gonads without germ cells develop aberrantly, leading to, for example, sterile testes in zebrafish (Slanchev et al., 2005; Houwing et al., 2007; Kurokawa et al., 2007; Siegfried and Nusslein-Volhard, 2008; Rodriguez-Mari and Postlethwait, 2011; Tzung et al., 2015).
Signals exchanged between germ cells and somatic cells include members of the transforming growth factor-beta (TGFB) pathway, including ligands (Amh, Bmp2, Bmp8b, Bmp15, and Gdf9) receptors (ACVR1); and downstream effectors (Smad1, Smad4, and Smad5) (Chang and Matzuk, 2001; Aubin et al., 2004; Hanrahan et al., 2004; Clelland et al., 2007; Mendis et al., 2011). Decreased levels of the oocyte-secreted TGFB factors GDF9 and BMP15 occur in polycystic ovarian syndrome (PCOS), a condition affecting 6% of reproductive age women and the most frequently diagnosed problem when couples visit fertility clinics (Diamanti-Kandarakis, 2008; Goodarzi et al., 2011; McAllister et al., 2015). PCOS involves at least two of three signs: hyperandrogenism, infrequent or irregular ovulation, and the accumulation of numerous immature antral follicles (Rotterdam, 2004b; Wei et al., 2014). In addition, people with PCOS often show disrupted insulin profiles, altered gonadotrophin signaling (Diamanti-Kandarakis, 2008), and high levels of AMH (Pigny et al., 2003; Diamanti-Kandarakis, 2008), a granulosa cell-expressed TGFB family member (Durlinger et al., 2002; Pellatt et al., 2010; Seifer and Merhi, 2014).
In addition to their roles in mammalian gonad development, TGFB family genes also act in sex determination in some species of fish. In two species of pejerrey, a Y-linked gene encoding Amh (amhy) is the primary sex determinant (Hattori et al., 2012; Yamamoto et al., 2014). In fugu pufferfish, the X-linked sex determinant is a hypoactive allele for Amh receptor type II (Amhr2) (Kamiya et al., 2012; Myosho et al., 2012), mimicking male-to-female sex reversal in amhr2 mutants in Japanese medaka (Matsuda et al., 2002; Nanda et al., 2002; Morinaga et al., 2007). In Luzon ricefish and likely sablefish, the major sex-determining gene is the TGFB family member gsdfY (Myosho et al., 2012; Rondeau et al., 2013). Gsdf (gonadal soma derived factor) was first identified in the somatic gonad of rainbow trout, induced when germ cells invade the genital ridge (Sawatari et al., 2007; Yazawa et al., 2010). Granulosa cells express gsdf in ovaries and Sertoli cells express gsdf in testes (Gautier et al., 2011b). Knockdown experiments show that gsdf stimulates PGC proliferation in trout (Sawatari et al., 2007). Gonads in most teleosts express gsdf (Luckenbach et al., 2008; Shibata et al., 2010; Yazawa et al., 2010; Gautier et al., 2011a; Crespo et al., 2013; Nagasawa et al., 2014) (Robledo et al., 2015; Jiang et al., 2016). In Japanese medaka (Oryzias latipes), gsdf is expressed weakly in gonads of both XX and XY embryos, then increases in males in somatic cells that express dmy/dmrt1by (Shibata et al., 2010). In Japanese medaka, gsdf initiates but does not maintain male fate (Imai et al., 2015; Zhang et al., 2016); in contrast, in tilapia gsdf does not initiate, but maintains dmrt1 expression and testis development (Jiang et al., 2016). In medaka, dmrt1by activates gsdf (Zhang et al., 2016) and suppresses expression of rspo1 and the female pathway, and gsdf activity can rescue sex reversal caused by loss of dmrt1by (Chakraborty et al., 2016). Another Oryzias species eliminates the upstream factor (dmrt1by) and uses gsdf directly as a sex determinant (Myosho et al., 2012).
Gsdf likely plays a special role during the sex change in hermaphroditic fish species. In the sequentially hermaphroditic protogynous three-spot wrasse, gsdf expression turns on as the sex change begins (Horiguchi et al., 2013), while in the protandrous yellowfin sea bream, high gsdf expression appears only in the testis part of the ovotestis (Chen et al., 2015). Zebrafish laboratory strains also pass through a hermaphroditic stage as all juveniles form meiotic oocytes that eventually die in individuals that become males and survive in individuals that become females (Takahashi, 1977; Uchida et al., 2002; Rodriguez-Mari and Postlethwait, 2011; Tzung et al., 2015). These considerations raise the hypothesis that in zebrafish, sex determination might work by manipulating gsdf expression. Here we show that the juvenile bipotential gonad in zebrafish expresses gsdf and confirm that Sertoli cells continue to express gsdf but support cells contacting meiotic and post-meiotic germ cells in transitioning individuals down-regulate gsdf expression (Yazawa et al., 2010; Gautier et al., 2011b). Consistent with Sertoli cell expression of gsdf, immunization of zebrafish against Gsdf protein conjugated to keyhole limpet hemocyanin (KLH) dramatically reduced testis development, although controls were not injected with KLH alone, and all antigens tested had similar effects on the testis (Presslauer et al., 2014). The role that gsdf plays in zebrafish sex determination, however, is as yet unknown. To answer this question, we made gsdf knockout mutations. Results showed that gsdf mutants became either fertile males with enlarged testes or sterile females that accumulated non-vitellogenic follicles. These results rule out the hypothesis that gsdf plays a major role in sex determination in laboratory zebrafish, and show that it provides a somatic signal that regulates follicle maturation and prevents the formation of polycystic ovaries. We showed that gsdf likely arose in the vertebrate genome duplication events along with paralogs bmp15 and gdf9, and because coelacanth possesses gsdf but tetrapods do not (Forconi et al., 2013), the gene was lost in tetrapods; these three paralogs likely share ancestral subfunctions. The ovarian phenotype of zebrafish gsdf mutants mimics PCOS, suggesting a model for PCOS that could be used in screens for therapies.
RESULTS
The gonadal soma expresses gsdf
The hypothesis that gsdf acts in zebrafish sex determination predicts that it should be expressed at or before sex determination, which becomes evident by oocyte death between 19 and 27 days post fertilization (dpf) (Siegfried and Nusslein-Volhard, 2008; Orban et al., 2009). In situ hybridization experiments on tissue sections detected gsdf transcripts in gonads but in no other tissues of zebrafish embryos, larvae, juveniles, or adults. In gonads, gsdf expression had already begun by 8dpf in gonadal somatic cells, which we identified by staining adjacent sections for vasa expression (Yoon et al., 1997) (Fig. 1A, B). In tissue sections prepared under identical conditions, staining of gsdf expression was darker by 12dpf (Fig. 1C, D), continued at 25 and 35dpf (Fig. 1E–H), and appeared in Sertoli cells in testes and in granulosa cells in ovaries (Fig. 1I–K). Double in situ hybridization experiments showed that gsdf is expressed in somatic cells adjacent to germ cells (Fig. 1K), similar to amh-expressing Sertoli cells (Fig. 1L). Adult ovaries expressed gsdf in granulosa cells of pre-vitellogenic follicles (stage-I and stage-III, Fig. 1J), which contrasts with medaka, where expression is weak or not detected in genetic females (Shibata et al., 2010). We conclude that gsdf expression begins before overt sex becomes evident and continues throughout gonad differentiation; thus, gsdf is expressed at a time and place consistent with a role in sex determination in zebrafish.
Figure. 1.
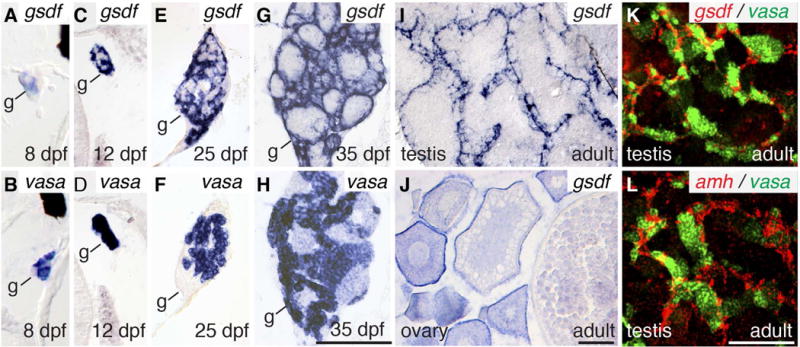
Expression of gsdf. Expression of gsdf in somatic cells (A, C, E, G) and vasa in germ cells (B, D, F, H) of 8dpf (A, B), 12dpf (C, D), 25dpf (E, F), and 35dpf (G, H) animals. Expression of gsdf in 8mpf adult testis (I) and ovary (J). Two color in situ hybridization on adult testis (K) for gsdf (red) and vasa (green) and (L) amh (red) and vasa (green). Black scale bar in H for A-H; black scale bar in J for I, J; white scale bar in L for K, L. All scale bars: 100μm.
Gsdf knockouts
To learn the roles of gsdf, we used TALENs to induce deletion alleles targeting exon-1, which precedes the conserved pro-peptide cleavage site in exon-3 (Massague, 1998; Bottner et al., 2000; Gordon and Blobe, 2008) (Fig. 2A, B). The TALEN target sequence contains a BslI restriction enzyme recognition site that provides evidence for deletion of the target sequence (Fig. 2B). We isolated stable out-of-frame deletion lines (allele designations b1279 (8-nucleotide deletion), b1280 (1-nucleotide deletion), and b1281 (14-nucleotide deletion), (Fig. 2C). To confirm the transcription of the mutated gsdf gene, we made cDNA from the 8 bp deletion, designed primers in exon-1 and exon-2, and sequenced the PCR fragment, predicted to be 275 bp in the wild-type cDNA, 267 bp in the mutant cDNA, and about 1700 bp in the wild-type genomic DNA. Results confirmed the transcription of the -8 mutant gene and verified the sequence of the mutant (Fig. 2C, D). The mutant alleles predict truncated polypeptides of 19 to 39 amino acids including out-of-frame sequences rather than the normal 172 residue Gsdf polypeptide (Fig. 2E). Because these deletion mutants should disrupt the predicted pro-region (Walton et al., 2010) and delete the TGFB domain, they should be gsdf null activity alleles. Although we were not able to develop antibodies to zebrafish Gsdf, qPCR provides evidence for nonsense mediated transcript decay (see below). Because phenotypes of all mutant alleles were similar, results presented below focus on the 8-nucleotide deletion allele.
Figure 2.
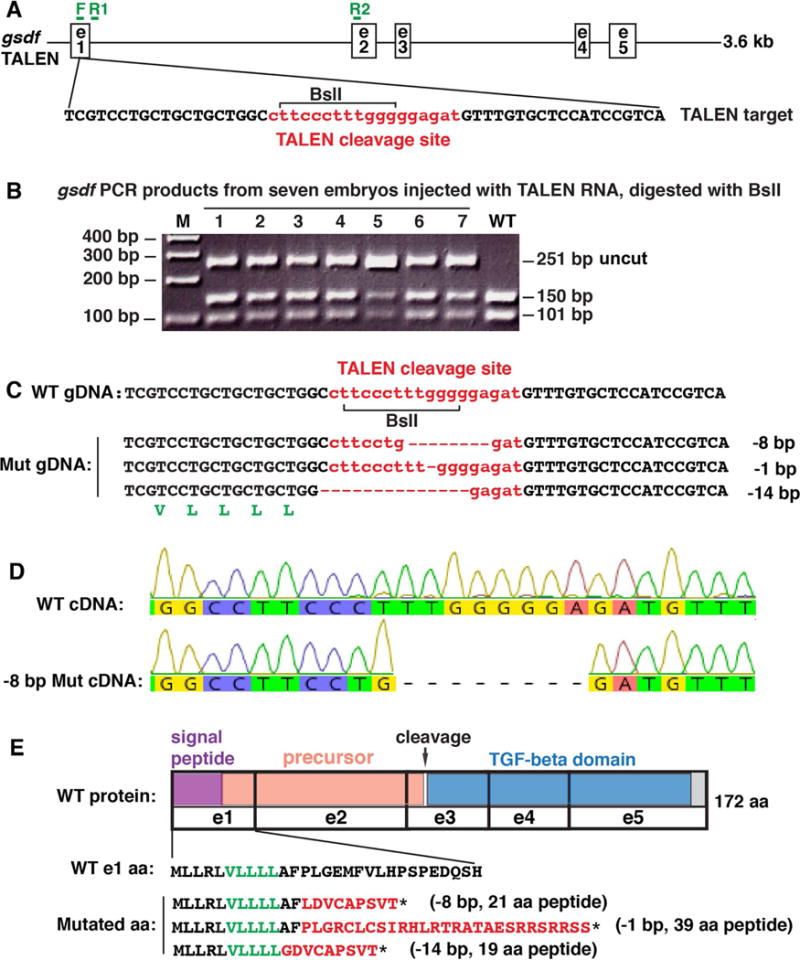
Mutagenesis. A. A 3.6 kb genomic region of gsdf including exons (e) 1–5 with the TALEN target sequence: TALEN cleavage site (small letters in red), TALEN binding sites in black and BslI restriction site (bracket), along with genotyping primer sites (F, R1 and R2 in green). B. PCR analysis of seven G0 injected embryos at 1dpf using genotyping primers F and R1 shows a 251base pair (bp) fragment in wild types (WT) that digests with BslI to produce fragments of 150bp and 101bp. Seven injected embryos (1–7) contained a substantial load of mutations that destroyed the BslI site, leaving the 251 bp fragment undigested, in addition to the WT bands. C. Raising injected embryos and subsequent breeding produced stable lines carrying -8 bp, -1 bp and -14 bp deletions. Talen cleavage site shown in red. A translated portion of gsdf (in green letters) appears below the -14 bp sequence. D. To verify transcription of the -8 bp deletion, we amplified a 275 bp fragment using primer F in exon-1 and R2 in exon-2 shown in Fig. 2A; results verified the 8 bp deletion, which confirmed the genomic sequence in Fig. 2C. E. TALEN-induced mutations are predicted to result in premature stop codons (*). The translated portion of Gsdf (in black and green letters) indicates the wild-type sequence, while red letters indicate the predicted frame-shifted sequences in gsdf mutants. Protein coding domains: purple: signal peptide; salmon: precursor; blue: TGFB-family domain; grey: C-terminus; e: exons; arrow: cleavage site.
Sex ratios and fertility
Sex ratios in zebrafish gsdf mutants were about the same as in wild-type siblings: for example, crossing heterozygotes for the 8-nucleotide deletion allele produced homozygous wild-type offspring with a ratio of 25 males to 33 females (43% males), heterozygotes with a ratio of 54 to 66 (45% males), and homozygous mutants with a ratio of 27 to 33 (45% males) when scored at 4.5 months post fertilization by gonad dissection; other alleles produced similar results. Even old mutants maintained a female phenotype in terms of fin color, body shape, and germ cell development (immature eggs, no sperm). We conclude that in zebrafish, gsdf activity is not the primary genetic sex determinant.
Roles of Gsdf in gonad development
To test for roles of gsdf, we studied the fertility of mutants homozygous for the -1, -8, and -14 alleles. Results showed that, while most homozygous gsdf mutant females at 4.5mpf (months post fertilization) were fertile, by 8mpf, fertility was greatly reduced, and by 18mph, all mutant females tested were sterile (Fig. 3A, B). In males, in contrast, for the -8 allele, for example, 18 of 18 homozygous gsdf mutants were fully fertile and similar results were obtained for other alleles. We conclude that in zebrafish, gsdf activity is essential to maintain female, but not male fertility.
Figure 3.
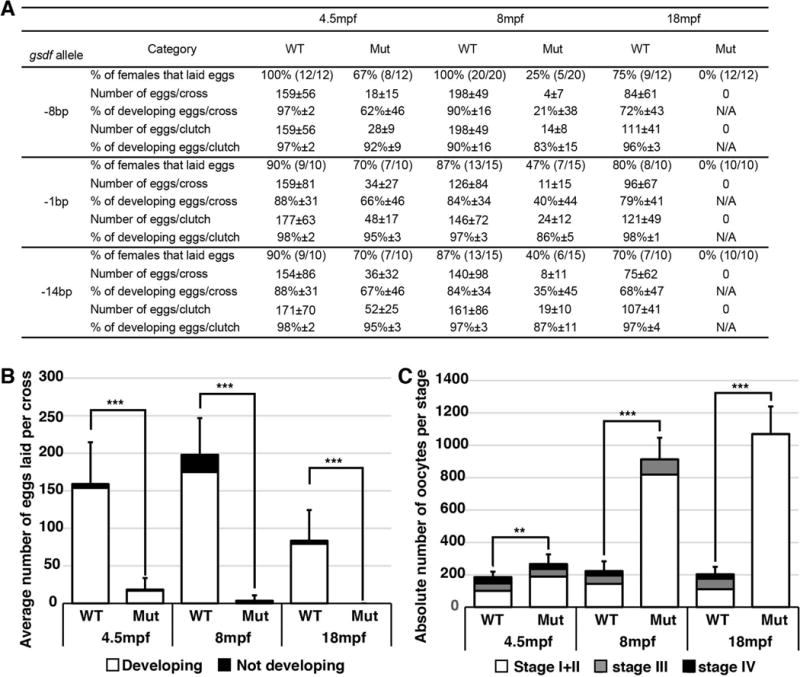
Fertility in gsdf mutant lines. A. Fertility data for the three mutant gsdf alleles compared to wild-type siblings. For each allele, the percentage of females that laid eggs is given and in parentheses appears the number of crosses yielding eggs compared to the number of crosses performed. Other quantities and percentages are given with their respective standard deviation. B. Average number of eggs laid per cross of -8bp gsdf mutant females compared to wild-type siblings at 4.5, 8 and 18 mpf. For each of 12 to 20 crosses for mutant or wild-type genotypes, one wild-type female or one mutant female was paired with three non-sibling wild-type males. Eggs were collected and counted the following day, and their development was followed for at least three days. Eggs developing normally, not developing, or improperly developing were counted. C. Number of oocytes in histological sections of fish homozygous for the -8bp gsdf mutation compared to wild-type siblings at 4.5, 8 and 18 months post fertilization (mpf). Oocytes were categorized in three groups: Stage I + Stage II (white), Stage III (grey) and Stage IV (black) oocytes. The 18 mpf mutant females had only Stage I + Stage II oocytes. Statistical significance: **, 0.01<p<0.001 and ***, p< 0.001.
To learn how gsdf acts to maintain female fertility, we examined gonad histology. Histological sections of 22 wild-type fish at the committed but immature gonad stage (35dpf) identified 19 fish with only testis tissue and the remaining three with only ovary tissue (Fig. 4A, B). In contrast, among 22 mutant fish at 35dpf, ten had only testis and six had only ovary, but the remaining six (27%) had either one gonad developing as an ovary and the other as a testis (one fish in Fig. 4C, D and another in Fig. E, F) or individual gonads that contained both ovarian follicles and testis lobules (one individual in Fig. 4G, H). Sections of older fish (8mpf and 18mpf, 10 male and 10 female fish) showed no evidence of hermaphroditism. We conclude that Gsdf accelerates the juvenile-to-adult gonadal transition.
Figure 4.
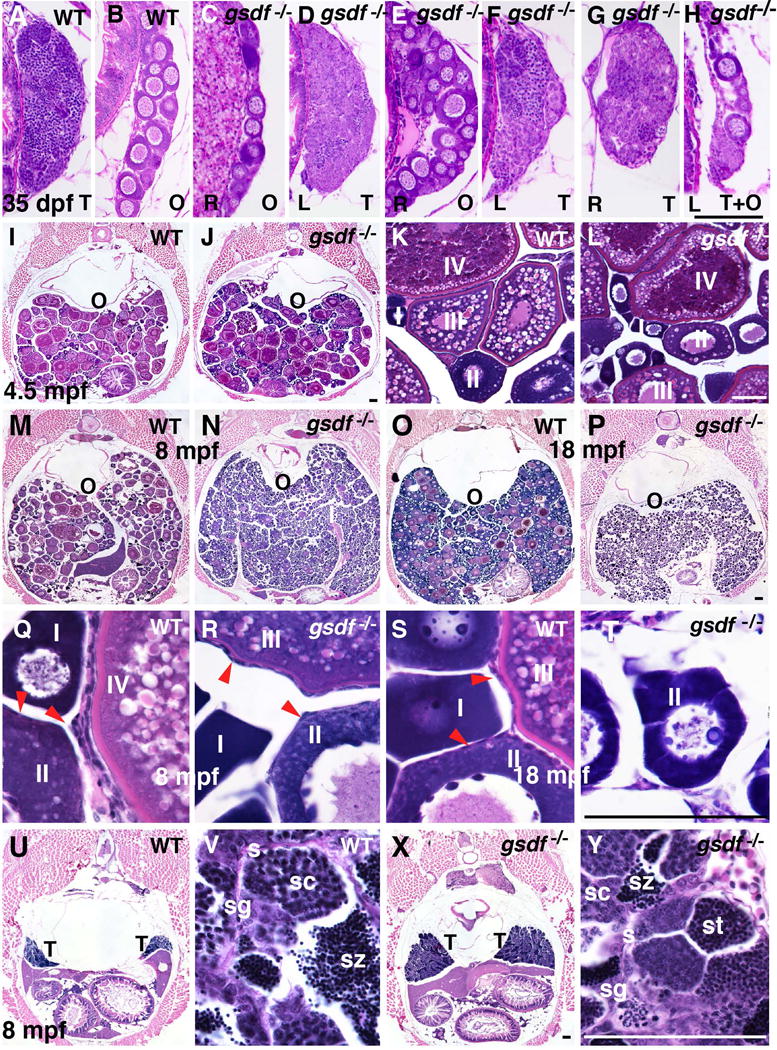
Gonad histology. A-H. Histological sections of 35dpf wild-type fish showed only ovary or testis (A, B), but six of 22 gsdf mutant fish (27%) at 35dpf contained either one ovary and one testis, like the two fish in C-F, or individual gonads containing both ovary and testis like the one fish in G and H. (Abbreviations: O, ovary; T, testis; R, right gonad; L, left gonad). I-T. Adult female ovaries showing low (I, J, M-P), and high (K, L, Q-T) magnification. Cross sections of 4.5mpf of wild types (I, K) and gsdf mutants (J, L) that contained maturing (stage-I, II) and vitellogenic (stage-III and -IV) follicles. 8mpf old wild-type females had gonads filled with maturing (stage-I, -II) and vitellogenic (stage-III and -IV) follicles (M, Q), as well as follicle cells on the surface of oocytes (red arrow heads in panel Q). (N, R) Cross sections of an 8mpf gsdf mutant female showed an excess of immature follicles (stage-I and -II), a few early vitellogenic follicles (stage-III), but no late vitellogenic follicles (stage-IV). Follicle cells surround oocytes (red arrow heads, panel R) in mutants as in wild types. (M, Q). Cross section of an old (18mpf) female wild type with many immature follicles, a few normal vitellogenic follicles (O, S), and an 18mpf female gsdf mutant female with many young follicles (stage-I and -II) but no mature stages (P, T). U-Y. Cross sections of 8mpf wild-type (U, V) and gsdf mutant (X, Y) males at low (U, X) and high magnification (V, Y). Although male gsdf mutants had larger testes than wild types, both genotypes formed all spermatogenic stages (Abbreviations: O, ovary; s, sertoli cells; sc, spermatocytes; sg, spermatogonia; st, spermatids; sz, spermatozoa; T, testis). Black scale bar in H for A-H; black scale bar in J for I, J; white scale bar in L for K, L; black scale bar in P for M-P; Black scale bar in T for Q-T; black scale bar in X for U, X; white scale bar in Y for V, Y. All scale bars: 100μm.
At 4.5 months post fertilization (mpf), gsdf mutant ovaries contained nearly normal follicles from stage-I to stage-IV as in wild-type siblings (Fig. 3C, Fig. 4I–L) (for staging, see (Selman et al., 1993)). Mutant ovaries, however, had a greater number of younger and fewer older stage follicles than wild types (Fig. 3C). At 8mpf, the trend continued (Fig. 3C, Fig. 4M, N, Q, R). At 18mpf, mutant females contained only pre-vitellogenic follicles but no stage-IV follicles (Fig. 3C, Fig. 4P, T), compared to wild types, which had many mature oocytes (Fig. 3C, Fig. 4O, S). We conclude that in fully adult zebrafish, gsdf activity is required for follicles to mature beyond stage-III and to prevent the accumulation of young ovarian follicles; the second phenotype might be a consequence of the first.
In contrast to gsdf mutant females, gsdf mutant males were fertile but developed larger testes than wild types. Histological sections showed that, like wild-type males (Fig. 4U, V), homozygous 8mpf gsdf mutant males contained all stages of sperm development, including mature sperm (Fig. 4X, Y), but had about three times more mass than wild-type testes (see also Fig. 6N), due to an increase of lobules of all stages. We conclude that gsdf is not required for zebrafish male sex determination or sperm development and maturation, but helps to regulate testis size.
Figure 6.
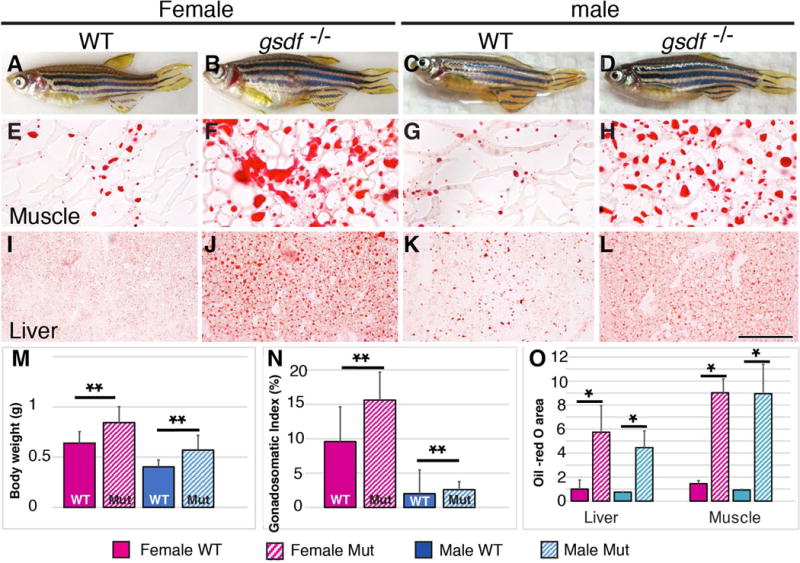
Body size and lipid homeostasis. (A-D) Adult 8mpf zebrafish: wild types (A, female; C, male) and gsdf mutants (B, female; D, male), showing enlarged abdomens in mutants. (E-T) Histological sections showing lipid stained with Oil Red O. (E-H) trunk muscle); (I-L) hepatopancreas (liver). Mutants accumulated more and larger lipid droplets than wild types. (M) Body weight (g); (N) Gonadosomatic Index (%); (O) Oil-red O area. Solid boxes, wild types; striped boxes, mutants; red boxes, females; blue boxes, males. *, 0.05<p<0.01; **, 0.01<p<0.001. Scale bar in L: 100μm.
Gene mis-expression in gsdf mutant gonads
To investigate the molecular genetic basis for the observed histological phenotypes, we studied gene expression patterns. At eight months post fertilization, gsdf mutant females showed less intense gsdf expression in somatic cells surrounding oocytes, but greater amounts in oocytes compared to histological sections of wild type ovaries stained under the same conditions (Fig. 5A, A′). To determine whether the lack of gsdf expression surrounding oocytes was due to the absence of follicle cells or to their failure to mature, we examined expression of the granulosa cell markers amh cyp19a1a, and gata4 (Rodriguez-Mari et al., 2005; von Hofsten et al., 2005); (Chiang et al., 2001c; Efimenko et al., 2013). Amh inhibits the FSH-induced recruitment of ovarian follicles (Weenen et al., 2004); cyp19a1 encodes aromatase, the enzyme that converts testosterone to estrogen (Rouiller-Fabre et al., 1998); and Gata4 encourages granulosa cell proliferation and theca cell recruitment (Anttonen et al., 2003). Expresson of none of these markers was detected surrounding oocytes in 8mpf gsdf mutant females (Fig. 5B, B′, D, D′, E, E′), despite the clear presence of gonadal somatic cells (Fig. 4R). Although cyp19a1a expression was not detected in 8mpf follicle cells, it must have been produced in younger mutant fish at 4.5mpf that laid eggs because cyp19a1a is necessary for gonadal estrogen production. We conclude that in zebrafish, gsdf is required for the maintenance or maturation of granulosa cells acting either as an autocrine signal or a paracrine signal to the oocyte, which then might reciprocally support granulosa cells.
Figure 5.
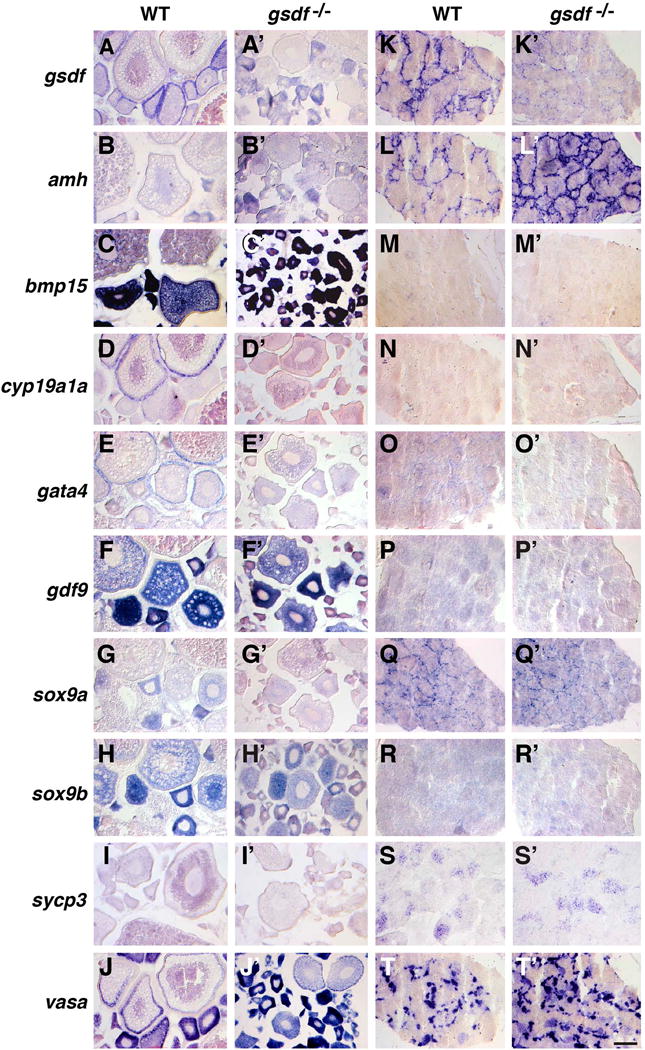
Gene expression patterns in adult gonads at 8mpf. (A-J) Wild-type ovaries. (A′-J′) gsdf mutant ovaries. (K-T) Wild-type testis. (K′-T′) gsdf mutant testis. In situ hybridization for gsdf (A, A′, K, K′), amh (B, B′, L, L′), bmp15 (C, C′, M, M′), cyp19a1a (D, D′, N, N′), gata4 (E, E′, O, O′), gdf9 (F, F′, P, P′), sox9a (G, G′, Q, Q′), sox9b (H, H′, R, R′), sycp3 (I, I′, S, S′), vasa (J, J′, T, T′). Little gsdf transcript was apparent in mutant gonads. Transcript of cyp19a1a and gata4 was less abundant in mutant ovarian follicles and amh and vasa was more abundant in mutant testis. Scale bar in T′: 100μm.
In contrast to gsdf, which wild types expressed in ovarian follicle cells (Fig. 5A), the gsdf paralog bmp15 was expressed in wild-type oocytes, granulosa cells, and theca cells (Clelland et al., 2006; Dranow et al., 2016), but its expression gradually decreased as oocytes matured (Fig. 5C). In mutant ovaries, bmp15 expression was strong only in oocytes younger than stage-III (Fig. 5C′). Adult wild-type females at 8mpf expressed the gsdf paralog gdf9 mainly in young oocytes, stronger in stage-I follicles and weaker or diluted as follicles matured (Fig. 5F, F′), confirming previous qPCR evidence (Wang and Ge, 2003; Liu and Ge, 2007; Poon et al., 2009). Expression of gdf9 was not greatly changed in gsdf mutant ovaries (Fig. 5F,F′ and Fig. 7.B3). Expression of other markers, including sox9b (Chiang et al., 2001a), sycp3 (synaptonemal complex protein 3) (Rodriguez-Mari and Postlethwait, 2011), and vasa were not dramatically different between wild types and gsdf mutants (Fig. 5H–T′). We conclude that expression of gonadal soma genes is greatly disrupted in gsdf mutants, but expression of early oocyte genes is nearly normal.
Figure 7.
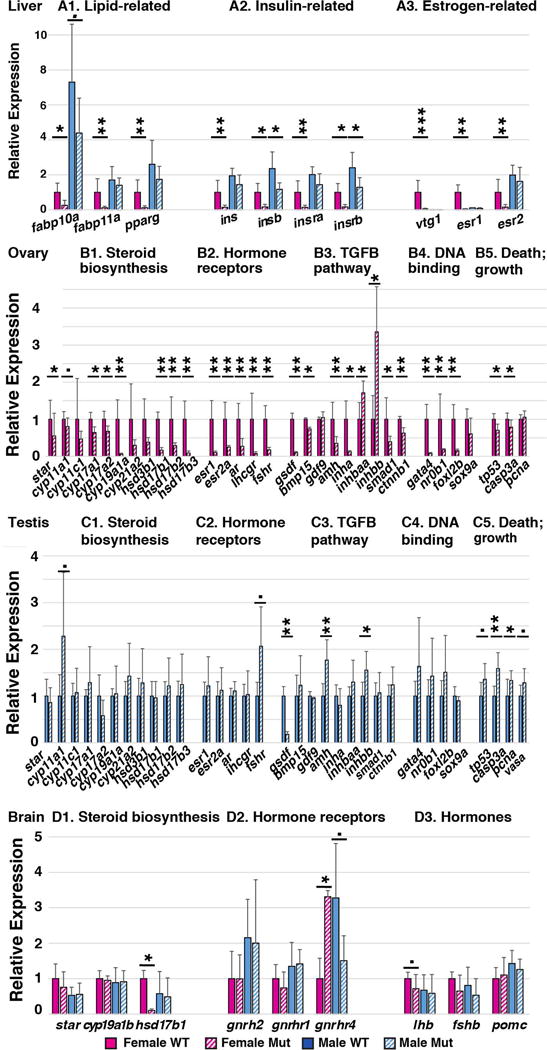
Loss of gsdf function alters gene expression in several organs. mRNA was extracted from gsdf mutant and wild-type sibling tissues and assayed by qPCR for (A) hepatopancreas (liver), (B) ovary, (C) testis, and (D) brain. Expression of each gene was compared to expression of beta-actin. The ratio of expression of each gene relative to beta-actin in wild-type females was set to 1.0. Statistical significance: •, 0.05<p<0.1; *, 0.05<p<0.01; **, 0.01<p<0.001; and ***, p< 0.001.
Our results confirm gsdf expression in Sertoli cells of wild-type zebrafish males (Gautier et al., 2011a), but show greatly reduced gsdf expression in gsdf mutant testes (Fig. 5K, K′), as would be expected from nonsense-mediated transcript decay. Expression of the Sertoli cell marker amh (Rodriguez-Mari et al., 2005; von Hofsten et al., 2005) appeared to increase in gsdf mutant males (Fig. 5L, L′, see also qPCR results in Fig. 7C3). Ovarian follicle cell markers bmp15, cyp19a1a, and gata4, as well as oocyte markers gdf9 and sox9b, were not strongly expressed in wild-type or gsdf mutant testis (Fig. 5M–O′, R, R′); the Sertoli cell marker sox9a (Chiang et al., 2001a) and the early meiotic spermatocyte marker sycp3, were expressed at about the same level in mutant and wild-type testes (Fig. 5Q, Q′ and S, S′); and the germ cell marker vasa appeared to be expressed more extensively in gsdf mutant testes than in wild-type testes (Fig. 5T, T′ and Fig. 6C5). These experiments suggest that some activities of Sertoli cells may be up-regulated in zebrafish gsdf mutant testes, a conclusion verified below by qPCR experiments.
Pathophysiology of gsdf mutants
In PCOS, ovaries accumulate young ovarian follicles as in gsdf mutants, but in addition, PCOS often includes obesity, lipid accumulation, type II diabetes, hypertension, and ovarian cancers (Franks, 1995; Daniilidis and Dinas, 2009; van Houten and Visser, 2014). The series of experiments reported in this section test two questions: Do zebrafish gsdf mutants show changes in genes relevant to the pathophysiology of PCOS patients? And, how does the knockdown of gsdf, which is expressed only in ovarian follicle cells, alter the physiology of non-gonadal organs, including liver and brain?
We found that the body mass of 8mpf zebrafish gsdf mutant females was 32% greater than wild-type siblings and the mass of mutant males was 42% greater mass than wild-type siblings (Fig. 6A–D and M), and that only about half of the excess mass was due to gonad hypertrophy (Fig. 6N). If increased body mass is related to obesity, then lipid metabolism in mutants might be disturbed in gsdf mutants as in PCOS (Macut et al., 2006; Wild et al., 2011). At 8mpf, gsdf mutant muscle and hepatopancreas (liver) (Fig. 6E–L, O) (also gonads and intestine, not shown) all accumulated substantially more and larger lipid droplets than these organs in wild types when stained with Oil Red O for neutral lipids. Female gsdf mutants had 6.2 times more Oil Red area than wild-type females, and mutant males had 9.6 times more. We used qPCR to examine expression of fabp10a and fabp11a, which encode fatty acid binding proteins (Her et al., 2003; Furuhashi and Hotamisligil, 2008), and pparg, which encodes a transcription factor for adipocyte differentiation and insulin sensitivity (Lowell, 1999; Ferre, 2004). At 8mpf, mutant females had greatly reduced expression of all three genes and mutant males tended to have reduced expression of fabp10a (Fig. 7A1). We conclude that lipid metabolism is dysregulated in zebrafish gsdf mutant livers, likely a secondary effect because gsdf is expressed only in gonads.
To test insulin signaling (Dunaif et al., 2001; Vigouroux, 2010), we examined the hepatopancreas for expression of both zebrafish insulin genes (ins and insb) (Papasani et al., 2006) and both insulin receptor genes (insra and insrb) (Irwin, 2004; Toyoshima et al., 2008). Results showed that in females, expression of all four genes was dramatically reduced as in human PCOS (Vigouroux, 2010; Carreau and Baillargeon, 2015), and that in males, expression was statistically reduced for insb and insrb (Fig. 7A2). We conclude that insulin signaling is altered in gsdf mutant zebrafish.
PCOS patients have increased levels of testosterone and decreased titers of estrogen (Laven et al., 2002; Escobar-Morreale et al., 2005; Pellatt et al., 2010). A convenient assay for estrogen activity in egg-laying vertebrates is the synthesis of yolk protein (vitellogenin, Vtg) because estrogen binds to hormone receptors encoded by esr1 and esr2a in the hepatopancreas (Tingaud-Sequeira et al., 2004), stimulating synthesis of Vtg, which is secreted into the blood and sequestered into oocytes (Arukwe and Goksoyr, 2003; Hutchinson et al., 2006; Meng et al., 2010). Expression of vtg1 and the estrogen receptors esr1 and esr2a was significantly reduced to 3.6%, 2.9%, and 14.9% of wild-type levels in gsdf mutant females, but was normal in mutant males (Fig. 7A3). These results are consistent with decreased estrogen signaling from mutant ovaries (Heldring et al., 2007).
We used ELISAs to measure estradiol and the primary fish androgen, 11-ketotestosterone (11KT). Female mutants at 4.5mpf had normal amounts of estradiol compared to WT control (Fig. 8A), consistent with their ability to ovulate. In contrast, when fish became older, for example at 11mpf and 20mpf, females had significantly less estradiol than controls (Fig. 8A). Estradiol levels in mutant males did not appear to differ from normal (Fig. 8A). Levels of 11KT in gsdf mutant females at 4.5mpf and 20mpf were significantly higher than in wild-type females of the same age, and overall, disregarding age, genotype was highly significant for elevated 11KT in mutant females (p=0.005) (Fig. 8B), mimicking high testosterone in PCOS patients. Male gsdf mutants also tended to have higher levels of 11KT, as expected from their larger testes, but differences were significant only at 4.5mpf (Fig. 8C).
Figure 8.
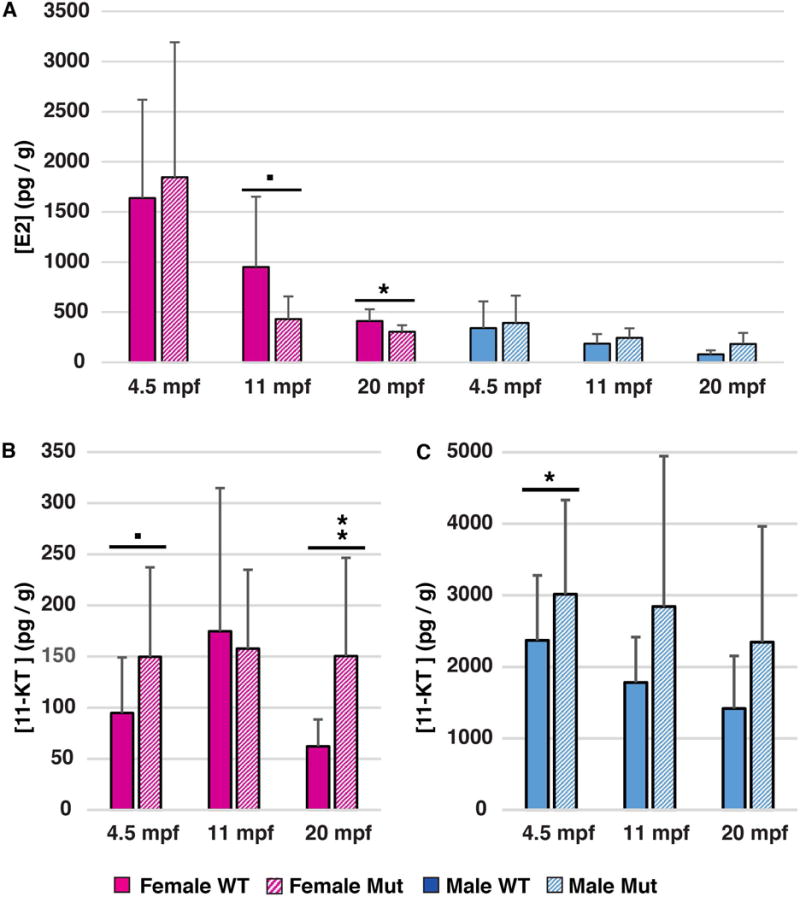
Assays for estradiol and 11-ketotestosterone (ELISAs). A. Estradiol (E2) levels (pg/g) in female and male gsdf mutants and wild-type siblings at 4.5mpf, 11mpf and 20mpf. B, C. 11 KT levels (pg/g) in female (B) and male (C) gsdf mutants and wild-type siblings at 4.5mpf, 11mpf, and 20mpf. Statistical significance: •, 0.05<p<0.1; *, 0.05<p<0.01; **, 0.01<p<0.001; and ***, p< 0.001. Solid boxes, wild types; striped boxes, mutants; red boxes, females; blue boxes, males.
Mutant ovaries at 8mpf had a significant overall reduction in expression of many steroid production genes including star, cyp11a1, cyp17a1, cyp17a2, cyp19a1a, hsd17b1, hsd17b2, hsd17b3 (Fig. 7B1). Expression of hsd17b3, which encodes the enzyme that converts androstenedione to testosterone, was reduced to 9% of normal and cyp19a1a, which encodes aromatase, the enzyme that converts testosterone to estrogen, was reduced to about 6% of normal in mutant ovaries (Fig. 7B1 and Fig. 5D, D′). We conclude that in 8mpf mutant females, enzymes that provide estrogen are dramatically reduced, low enough to greatly diminish Vtg production (Fig. 7A3) but not so low that gsdf mutants switched sex to become males like nanos3 and bmp15 mutants do (Dranow et al., 2013).
Regarding hormone receptors, gsdf mutant ovaries down-regulated estrogen receptor genes (Liu et al., 2011; She and Yang, 2014) esr1 to 10% and esr2a to 25% of normal (Fig. 7B2). The lhcgr and fshr genes (encoding receptors for brain-derived hormones LH and FSH) were both significantly reduced in mutant ovaries (Fig. 7B2). In zebrafish ovaries, fhsr and lhcgr both increase dramatically when follicles enter vitellogenesis (Kwok et al., 2005). Because gsdf mutant ovaries are deficient in vitellogenic stages, follicle cell development must derail in gsdf mutants before the stage appropriate for gonadotropin receptor expression.
Among TGFB pathway genes, gsdf expression decreased to about 10% of normal in gsdf mutant ovaries (Fig. 7B3), either as a result of nonsense-mediated transcript decay (Lykke-Andersen and Jensen, 2015) or failure to maintain gsdf-producing cells. Expression of bmp15 diminished a small but significant amount, and gdf9 expression was unchanged in gsdf mutant ovaries (Fig. 7B3). Mammalian testes secrete Amh, which eliminates female sex ducts, and mammalian ovaries express Amh in secondary, preantral, and small antral follicles, which retards follicular atresia (Josso, 1971; Merhi et al., 2013; Seifer and Merhi, 2014). In zebrafish gsdf mutant ovaries, amh expression weakened significantly to about a third of normal (Fig. 7B3). Zebrafish granulosa cells express amh in Stage-II follicles but not in younger (late stage-IB) or older (early or late stage-III) follicles (Rodriguez-Mari et al., 2005). Thus, although gsdf mutants accumulate stage-II follicles, both qPCR (Fig. 7B3) and in situ hybridization experiments (Fig. 5B, B′) confirm that granulosa cells do not express amh normally, showing that Gsdf positively regulates Amh production in zebrafish ovaries. In many species, Amh acts by binding to the Amhr2 receptor (Morinaga et al. 2007), but zebrafish lacks an amhr2 gene (Kamiya et al., 2012). Like zebrafish gsdf mutants, however, medaka amhr2 mutants fail to mature late stage oocytes and presumably have greatly reduced Amh signaling, which would mimic the low levels of amh transcript in zebrafish gsdf mutants (Fig. 7B3). In addition, however, half of medaka amhr2 mutants that have an XX sex chromosome constitution reverse their sex phenotype and become males (Morinaga et al. 2007); we did not observe sex reversal in zebrafish gsdf mutants. Ovaries in gsdf mutants expressed 1.7 and 3.4 times as much of the inhibin genes inhbaa and inhbb as normal (Fig. 7B3), consistent with their high level of expression in normal early stage follicles (Poon et al., 2009), and about a tenth as much inha (Fig. 7B3), which is expressed strongly only in full grown follicles (Poon et al., 2009), consistent with accumulation of early follicles and depletion of late-stage follicles.
Smad1 is an intracellular mediator of some TGFB family proteins in mammalian granulosa cells (Massague and Wotton, 2000; Pangas et al., 2008; Richards and Pangas, 2010). In zebrafish gsdf mutant ovaries, smad1 expression was about 35% of normal (Fig. 7B3), as expected from perturbed TGFB signaling.
In mammals, Gata4 is expressed in the bipotential genital ridge, continues in Sertoli cells, but down-regulates in granulosa cells (Viger et al., 1998). In zebrafish gsdf mutant ovaries, gata4 further down-regulated to 8% of normal (Fig. 7B4, Fig. 5E, E′). Sox9 also acts in the mammalian male pathway (Koopman, 1999). Zebrafish express sox9a strongly in testes (Chiang et al., 2001a; Yan et al., 2005) and weaker in ovaries. Expression of sox9a in gsdf mutant ovaries seemed lower, but was not significantly different from, that in wild-type siblings.
Nr0b1(Dax1) in mammals acts as a dominant-negative anti-testis gene (Swain and Lovell-Badge, 1999; Meeks et al., 2003) and Foxl2 represses male differentiation (Ottolenghi et al., 2007). Both genes were strongly down-regulated in gsdf mutant ovaries, suggesting that gsdf promotes development of ovarian support cells in zebrafish (Fig. 7B4).
Wnt4 is a female-promoting signal expressed by granulosa cells in mammals (Rastetter et al., 2014) and is essential for ovary development in zebrafish (High, 2016). Wnt4 can act by altering the cytoplasmic stability of beta-catenin protein (Bernard et al., 2008), and our studies showed that ctnnb1 transcript was significantly down-regulated in gsdf mutant ovaries (Fig. 7B3).
Tp53 is a transcription factor that induces cell death (Haupt et al., 2003) and Casp3 is a death protease (Boatright and Salvesen, 2003; Kumar, 2007); both were slightly, though significantly reduced in gsdf mutant ovaries (Fig. 7B5), suggesting that the enormous accumulation of early stage follicles in gsdf mutant ovaries at 8mpf is not associated with enormous amounts of follicle death. Expression of pcna (proliferating cell nuclear antigen, a marker of cell proliferation (Korfsmeier, 2002; Leung et al., 2005), showed no statistical change in gsdf mutant ovaries (Fig. 7B5), suggesting that ovarian follicles originate at a normal rate in mutants and accumulate simply because they do not disappear by either extensive death or ovulation.
Most steroid biosynthesis genes (star, cyp11c1, cyp17a1, cyp17a2, cyp19a1a, cyp21a2, hsd3b1, hsd17b1, hsd17b2, hsd17b3) were expressed normally in gsdf mutant males (Fig. 7C1). Cyp11a1 is the first, and rate-limiting enzyme in sex steroid biosynthesis; its increased expression in gsdf mutant males (Fig. 7C1) suggests a greater capacity for testosterone synthesis in the larger testes of mutants compared to wild types.
Among several hormone receptor genes (esr1, esr2, ar, lhcgr, and fshr), only fshr tended to be upregulated (p= 0.072), about twice normal, in gsdf mutant testes (Fig. 7C2). This result may reflect the role of Fsh in stimulating spermatogenesis through Fshr on Sertoli cells, which stimulates germ cell proliferation and androgen synthesis (Dierich et al., 1998; O’Shaughnessy et al., 2010).
Among TGFB pathway genes, expression of gsdf was 18% of normal in mutant testes and bmp15 and gdf9 were unchanged, but amh expression was significantly up-regulated nearly two-fold (177%) (Fig. 7C3, Fig. 5L, L′). As in mutant ovaries, inhbb was upregulated in gsdf mutant testes. Consistent with normal differentiation of fertile sperm in gsdf mutants, neither of the developmental signaling pathways we checked (smad1 in the TGFB pathway, and ctnnb1 in the Wnt pathway) had altered expression levels in mutant testes (Fig. 7C3).
None of the four genes we checked that encode sex-related DNA-binding proteins (gata4, nr0b1, foxl2b, sox9a) had significantly altered gene expression in mutant testes (Fig. 7C4).
Expression of p53 trended upward (p=0.0502) and casp3a was significantly up-regulated in mutant testes (Fig. 7C5), suggesting that, while gsdf mutants are fertile, cell death may be increased in some testis lobules (Fig. 4U, X). In addition, pcna expression was significantly higher in gsdf mutant testes (Fig. 7C5), suggesting that increased testis size might be mediated by increased cell proliferation. Expression of vasa increased 129% in gsdf mutant testis compared to wild-type testes, which is likely related to the enlarged testes in gsdf mutants (Fig. 7C5).
The brain, regulated by feedback from gonads, makes hormones that control gonadal functions. Among brain-expressed steroid biosynthesis genes, neither star nor brain aromatase (cyp19a1b) (Chiang et al., 2001b) were altered in expression in female or male gsdf mutants (Fig. 7D1). In contrast, expression of hsd17b1, which encodes the enzyme that converts estrone to estradiol, was greatly diminished in gsdf female brains, but was not changed in male brains (Fig. 7D1). This result reflects reduced hsd17b1 expression in mutant ovaries (Fig. 7B1) and decreased vtg expression in livers of female gsdf mutants (Fig. 6A3).
Gonadotropin releasing hormone (Gnrh2) (Bhattacharya et al., 2011; Biran et al., 2012) is secreted by the hypothalamus, binds to Gnrh receptor (encoded in zebrafish by gnrhr1 and gnrhr4, which are expressed in brain, ovary, and testis (Tello et al., 2008)), and stimulates the pituitary to release LH and FSH, which induce estrogen synthesis in ovaries and testosterone synthesis in testes (Kenealy et al., 2013). Expression of neither gnrh2 nor gnrhr1 changed significantly in gsdf mutants, but expression of gnrhr4 increased 3.3-fold in mutant females and decreased to about half of wild-type levels in mutant males (Fig. 7D2). Despite increased gnrhr4 expression in female mutant brains, expression of lhb tended to be lower in female brains (p=0.072), and the lower level of gnrhr4 expression in male mutant brains did not result in diminished lhb expression (Fig. 7D3).
In mammals, Pomc polypeptide is cleaved to make endogenous opioids and peptides that regulate appetite, sexual behavior, and secretion of the stress hormone cortisol from the adrenal cortex. Because pomca expression in female or male gsdf mutant brains was not statistically different from wild types, loss of gsdf activity does not likely result in serious physiological stress.
In brief summary, although gsdf is expressed only in gonadal cells, the impact of its loss was apparent throughout the fish, including effects on the liver, brain, and on fat and insulin biology that mimic the effects of PCOS. Because mammals do not have an ortholog of gsdf (Sawatari et al., 2007), for connectivity, it is important to understand the evolutionary relationships of gsdf to mammalian TGFB genes.
Origin of gsdf
Because phylogenetic analyses of TGFB family genes disagree on the affinities of gsdf (Sawatari et al., 2007; Shibata et al., 2010; Gautier et al., 2011a; Myosho et al., 2012; Forconi et al., 2013; Horiguchi et al., 2013; Kaneko et al., 2015), we analyzed conserved syntenies to learn which tetrapod TGFB genes are most closely related to those of fishes. Results showed that zebrafish gsdf shares conserved syntenies with gsdf of spotted gar (Fig. 9A1, A2), whose lineage diverged from that of teleosts before the teleost genome duplication (TGD) (Hoegg et al., 2004; Crow et al., 2006; Amores et al., 2011; Braasch et al., 2016). Coelacanth also has gsdf (Gautier et al., 2011a; Forconi et al., 2013), providing evidence that Gsdf was present in the last common ancestor of human and zebrafish but was lost from the tetrapod lineage after it diverged from coelacanth.
Figure 9.
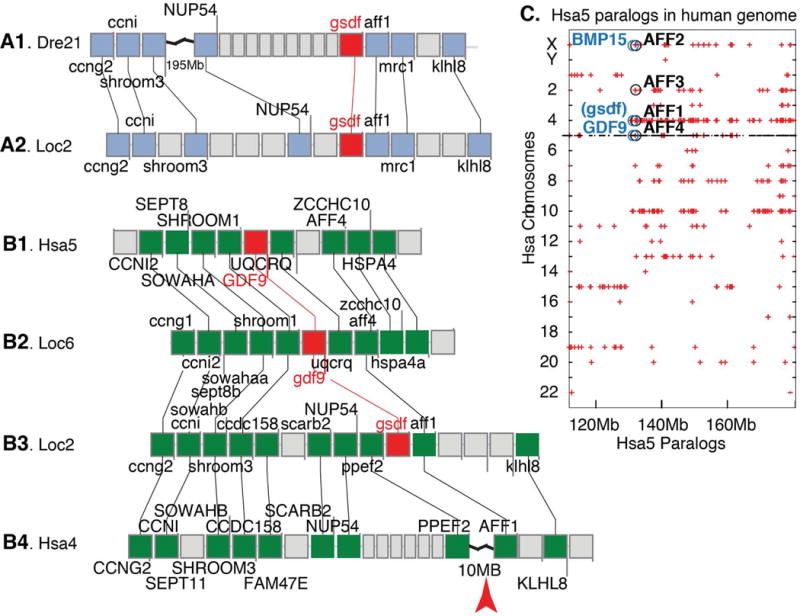
Conserved syntenies illuminate gsdf history. A. Part of zebrafish chromosome Dre21 (A1) compared to spotted gar chromosome Loc2 (A2) shows that genes to the right of gsdf in zebrafish and gar are conserved, but an indel to the left of gsdf in zebrafish disrupts syntenies. B. Comparing gar Loc2 (B3) to human Hsa4 (B4) shows a break that disrupts the expected location of a human gsdf ortholog. The gar gsdf region on Loc2 (B3) is paralogous to the gdf9 region on gar chromosome Loc6 (B2), which in turn is orthologous to the GDF9 region of human chromosome Hsa5 (B1), as predicted for paralogs. C. A dot plot identifying paralogs of Hsa5 genes on other human chromosomes shows that AFF gene family members, which are adjacent or nearly adjacent to gsdf, bmp15, and gdf9, occupy paralogous human chromosome segments, as expected by ohnologs from the vertebrate genome duplication VGD2.
To clarify the mechanism of gsdf loss, we compared gar and human genomes. Results revealed a chromosome rearrangement breakpoint at the location expected for a human ortholog of gsdf (Fig. 9B3, B4), as predicted if a chromosome break destroyed gsdf after the divergence of coelacanth and tetrapod lineages. Analysis of conserved syntenies showed that the region of gar chromosome 2 (Loc2) surrounding gsdf is paralogous to the region of Loc6 surrounding gdf9, with paralogs ccng2/ccng1, ccni/ccni2, wowahb/sowaha, shroom3/shroom1 and aff1/aff4 in addition to gsdf/gdf9 (Fig. 9B2, B3). In addition, the gar region around gdf9 shares almost exact conserved syntenies with the human GDF9 region (Fig. 9B1, B2). We performed sequence similarity searches of the Elephant shark genome (Venkatesh et al., 2014) and identified the previously unannotated shark sequence LOC103190318, which shares reciprocal best BLAST similarity to zebrafish Gsdf, as expected for orthologs. This result shows that the last common ancestor of all gnathostomes had a copy of gsdf. Our results also showed that a chromosome break in the tetrapod lineage occupies the ancestral position of gsdf; and that gsdf and gdf9 occupy paralogous segments and hence are likely paralogs from genome duplication events that preceded the vertebrate radiation (Holland, 1999; Dehal and Boore, 2005).
In phylogenies, GDF9 and BMP15 generally group as sister clades variably related to gsdf (Sawatari et al., 2007; Shibata et al., 2010; Gautier et al., 2011a; Myosho et al., 2012; Forconi et al., 2013; Horiguchi et al., 2013; Kaneko et al., 2015). Our comparative genomic investigations found that GDF9, BMP15, and gsdf; are all near Aff family genes, which occupy paralogous chromosome segments in the human genome (Fig. 9C). These data are as predicted by the hypothesis that GSDF, GDF9, and BMP15 arose as paralogs in the vertebrate genome duplication events; the fourth ohnolog disappeared, but should have been located near AFF3 on the ancestor of Hsa2 (Fig. 9C). According to the principle of subfunctionalization (Force et al., 1999), these considerations suggest that gsdf, gdf9, and bmp15 are likely to share some ancestral gene subfunctions.
DISCUSSION
Gsdf is not a sex determination gene in zebrafish
Gsdf is a male sex determinant in several species of fish. In O. luzonensis and sablefish, sex-specific gsdf variants are present in genetic males and females (Myosho et al., 2012; Rondeau et al., 2013), and in Nile tilapia, chromosomally XX fish that are homozygous gsdf mutants reverse their sex to develop as males (Jiang et al. 2016). In addition, in two fish (three-spot wrasse and rice field eel) that are sequential protogynous hermaphrodites, gsdf expression is up-regulated as ovaries transform to testes (Horiguchi et al. 2013; Zhu et al. 2016). Although gsdf is not the primary genetic sex determinant in O. latipes (Japanese medaka), forced expression of gsdf on an autosome causes XX females to become males (Zhang et al., 2016) and gsdf knockout causes XY O. latipes to become phenotypic females, demonstrating that gsdf is strongly male determining in this species (Imai et al., 2015; Zhang et al., 2016). In contrast to Oryzias and several other spiny-rayed fish (Acanthopterygii), we conclude that in zebrafish, gsdf is not a strong sex determinant because gsdf mutants are about as likely to become males or females as their wild-type siblings. It is a bit surprising that the function of gsdf in spiny-rayed fish and zebrafish are so different. These differences might be due to lineage-specific divergence of gene functions in the 290 million years since the spiny-ray fish and zebrafish lineages separated (Steinke et al., 2006). Without knowing the function of gsdf in the last common ancestor of zebrafish and spiny-rayed fish, it is not possible to know whether the lack of a strong male-determining role for gsdf in zebrafish is due to the loss of that role in the zebrafish lineage or the gain of that role in the spiny-rayed fish. In addition, even in zebrafish, individuals lacking gsdf activity attained their definitive sexual phenotype later than wild-type controls (Fig. 4A–H), showing that gsdf retains a weak male-determining function even in laboratory strains of zebrafish. Because gsdf is not located near any of the sex-linked loci found for a variety of zebrafish strains (Bradley et al., 2011; Anderson et al., 2012; Liew et al., 2012; Howe et al., 2013; Wilson et al., 2014), we conclude that gsdf does not play a direct role in zebrafish sex determination.
The role of Gsdf in zebrafish females
Although gsdf is not a strong sex determinant in zebrafish, it is nevertheless expressed early in the sex determination process. Somatic cells in zebrafish bipotential gonads already express gsdf in the gonadal soma by 8dpf, long before sex differentiation becomes apparent at about 21dpf (Takahashi, 1977; Uchida et al., 2002; Wang et al., 2007; Siegfried and Nusslein-Volhard, 2008; Orban et al., 2009; Rodriguez-Mari and Postlethwait, 2011; Tzung et al., 2015). This result suggests that Gsdf plays an early role in supporting cells of the somatic gonad.
Adult zebrafish express gsdf in Sertoli cells and granulosa cells (Gautier et al., 2011b) and our results confirmed that in juveniles, granulosa cells express gsdf only in pre-vitellogenic follicles. In contrast, female medaka stop expressing gsdf after 6dpf and male medaka up-regulate gsdf expression in cells that express dmrt1by, the male sex determinant (Shibata et al., 2010). Differences in gsdf expression in zebrafish and medaka suggest different functions.
In zebrafish, gsdf mutant females are briefly fertile, then become sterile as they accumulate non-vitellogenic follicles. qPCR showed that vtg expression in 8mpf gsdf mutant females was reduced to 4% of normal, so even if ovarian follicles in gsdf mutant females could sequester yolk, vitellogenesis would be greatly impaired. Estrogen induces vtg expression in the liver of zebrafish, other teleosts, and yolk-producing tetrapods (Wallace, 1985; Andersen et al., 2003; Tong et al., 2004; Reyhanian Caspillo et al., 2014). Our finding that the liver in zebrafish gsdf mutant females produces little vtg transcript would be expected if estrogen is low. Estrogen comes from testosterone that is converted to estrogen by aromatase in theca and granulosa cells (Nakamura et al., 2009; Dranow et al., 2016). These considerations suggest that normal Gsdf activity in granulosa cells is necessary for the maturation of theca and/or granulosa cells to the estrogen-producing stage, and with little estrogen, the liver produces little Vtg, leading to lack of yolky oocytes. Evidence that gsdf mutant ovaries have defective theca cells comes from our observation that expression of virtually all steroid-synthesizing enzyme genes is greatly reduced, and that ELISA assays detect reduced estradiol in gsdf mutant females.
Gsdf is required for normal granulosa cell maturation in zebrafish, according to our in situ hybridization and qPCR experiments that showed that granulosa cells in gsdf mutant females failed to express normal levels of amh, cyp19a1a, gata4, and foxl2b. Similarly, in mice lacking activity of the Gsdf paralog Gdf9, follicular development arrests at the primary follicle stage (Dong et al., 1996). The similarity of the follicle maturation defect in knockout mutations of gsdf in zebrafish and Gdf9 in mouse supports the notion that the original function of the progenitor of this gene family was to coordinate development of oocytes and support cells.
The failure of granulosa cells to mature in gsdf mutants could be due either to an autocrine mechanism that normally regulates interactions between granulosa cells, or a paracrine mechanism that normally regulates signalling between granulosa cells and oocytes or between granulosa cells and theca cells. Depressed expression of the TGFB signalling gene smad1 in gsdf mutant ovaries is consistent with either possibility.
In addition to the truncated maturation of granulosa cells and the subsequent reduction of estrogen, gsdf mutant ovaries abnormally accumulate hundreds of young ovarian follicles. Gonadotropin signaling from the brain regulates follicle recruitment and the brains of zebrafish gsdf mutants express several times more Gnrhr4 transcript than normal, but significantly less transcript for LH than normal. This result is consistent with dysregulation of feedback loops for oocyte maturation in zebrafish gsdf mutants, likely due to disrupted estrogen signaling.
The role of Gsdf in zebrafish males
Zebrafish males lacking gsdf activity were fertile with enlarged testes but nearly normal expression of almost all steroid biosynthesis genes tested. In addition, gsdf mutant males over-expressed fshr, amh, inhbb, tp53, casp3a, pcna, and vasa; this result is likely related to the enlarged testis phenotype.
Gsdf as a model for PCOS
The Rotterdam criteria for PCOS diagnosis rely on at least two of the following three features: infrequent or irregular ovulation, the accumulation of numerous immature antral follicles, and hyperandrogenism (Rotterdam, 2004b; Wei et al., 2014). PCOS usually also involves disrupted insulin profiles, altered gonadotrophin signaling (Diamanti-Kandarakis, 2008), and high levels of AMH (Pigny et al., 2003; Diamanti-Kandarakis, 2008). The phenotype of zebrafish gsdf mutant females mimics most of these criteria, and differ mainly in features related to differences in reproductive strategies of mammals and zebrafish.
People with PCOS experience oligo-ovulation or anovulation. In contrast to humans, where generally one oocyte matures each month, mature zebrafish continue to produce oocytes nearly daily. We found that zebrafish gsdf mutants produce and naturally ovulate when young, but then cease ovulation, mimicking the human oligo-ovulation phenotype.
The second key feature in PCOS is the accumulation of premature ovarian follicles that form ovarian cysts. Zebrafish oocytes do not form cysts, but this feature is related to the mammalian-specific relationship of follicle cells to oocytes with respect to mammalian ovulation rather than basic conserved features of vertebrate follicle development, including the absence of yolk sequestration in eutherian mammals. The accumulation of premature follicles in both syndromes suggests that in humans, proteins related to Gsdf may play a role in ovarian symptoms of PCOS. Candidates for the PCOS-relevant TGFB-family gene in humans include the two gsdf paralogs from the vertebrate genome duplications, BMP15, GDF9, and in addition, INHB, INHA, and AMH, all of which have altered expression in human PCOS patients (Eldar-Geva et al., 2001; Fleming et al., 2005; Glister et al., 2005; Welt et al., 2005; Kevenaar et al., 2008; Zhao et al., 2010; Wei et al., 2013; Wei et al., 2014), and all of which (except gdf9) are also mis-expressed in zebrafish gsdf mutant females.
Although human PCOS patients and zebrafish gsdf mutant females both accumulate follicles that fail to mature, the impeded stage of folliculogenesis appears to differ between the two species. In zebrafish, amh expression begins in Stage-II follicles, down-regulates in Stage-III, then disappears, and reciprocally, cyp19a1a expression up-regulates at Stage-III (Rodriguez-Mari et al., 2005; von Hofsten et al., 2005). Human oogenesis follows the same pattern, as primordial and primary follicles show little AMH expression, but secondary and small antral follicles express AMH strongly, followed by down-regulation in larger antral follicles (Weenen et al., 2004). While the developmental time course of amh/AMH expression is similar in zebrafish and human follicle development, zebrafish gsdf mutants had low levels of amh transcript in granulosa cells, but human PCOS patients have high levels of AMH expression (Durlinger et al., 2002; Pigny et al., 2003; Diamanti-Kandarakis, 2008; Pellatt et al., 2010; Seifer and Merhi, 2014), suggesting that follicle cell development arrests later in PCOS than in zebrafish gsdf mutants.
The third defining feature of PCOS is production of excess androgens (Legro et al., 1998; Nelson et al., 1999; Diamanti-Kandarakis, 2008). Likewise, our direct hormone assays showed that zebrafish gsdf mutants have low estrogen levels and high androgen levels. This key feature unites the zebrafish and human phenotypes, and may help explain the extra-gonadal phenotypes of PCOS and zebrafish gsdf mutants.
PCOS pathophysiology involves phenotypes outside the ovaries. Women with PCOS are often obese (van Houten and Visser, 2014). Accordingly, we found that zebrafish gsdf mutants were substantially heavier than normal and accumulated more lipid in their muscles, hepatopancreas, and other tissues. PCOS patients are also insulin resistant with low levels of insulin receptor (Diamanti-Kandarakis and Dunaif, 2012; van Houten and Visser, 2014; Dehghan et al., 2016). Likewise, we found that the liver in zebrafish gsdf mutants, especially females, had greatly reduced expression of genes encoding insulin receptor, which is a risk locus for PCOS in GWASs (Shi et al., 2012) and when mutated, causes insulin resistance in humans (Taylor et al., 1990). In addition, zebrafish gsdf mutants have greatly reduced expression of pparg, which encodes a nuclear receptor for adipocyte differentiation and diabetes, and highly suppressed expression of at least two fatty acid binding protein genes, especially in females. Because gsdf is expressed only in gonads, these extra-gonadal phenotypes in PCOS and in zebrafish gsdf mutants must arise secondarily due to altered gonadal signaling, either from altered levels of steroids, altered gonadotropins, abnormal levels of circulating Gsdf, or dysregulation of secreted proteins downstream of Gsdf signaling.
The striking phenotypic parallels of zebrafish gsdf mutants and human PCOS patients suggest mechanistically related etiologies. Although humans lack an ortholog of gsdf, the last common ancestor of humans and zebrafish had a gsdf gene (Sawatari et al., 2007; Forconi et al., 2013) and here we show that the ancestor of all jawed vertebrates had a gsdf ortholog. In addition, our comparative genomics showed that gsdf, gdf9, and bmp15 all originated as paralogs (ohnologs) in the second vertebrate genome duplication event, the fourth paralog now missing from all surviving jawed vertebrates. Evolutionary principles (Force et al., 1999) suggest that the pre-duplication ortholog of these three genes would have had some functions that are now distributed among gsdf, gdf9, and bmp15. Expression of all three genes to varying degrees in oocytes and follicle cells in fish and of Bmp15 and Gdf9 in mammals supports this conclusion (Sidis et al., 1998; Duffy, 2003; Silva et al., 2005; Clelland et al., 2006; Liu and Ge, 2007). In addition, Gdf9 and Bmp15 retain partially overlapping functions because the phenotype of homozygous Bmp15 mutant mice is stronger with one rather than two functional Gdf9 alleles (Yan et al., 2001; Su et al., 2004). Likewise, sheep with large litters are heterozygous for mutations in BMP15 or GDF9, but homozygous single and double mutants are sterile like Gdf9 mutant mice (Galloway et al., 2000; Hanrahan et al., 2004). Experiments also show that the GDF9:BMP15 heterodimer is far more potent than either homodimer in stimulating cumulus expansion (Peng et al., 2013). Finally, supporting this hypothesis is the decreased levels of GDF9 and BMP15 in PCOS (Goodarzi et al., 2011; McAllister et al., 2015).
PCOS has a strong familial component (Legro et al., 1998), but genome-wide association studies (GWASs) have not identified GDF9, BMP15, or other TGFB-pathway genes as significant candidates (Kevenaar et al., 2008; Chen et al., 2011; Shi et al., 2012). GWASs, however, did associate PCOS with variants in Fibrillin-3, an extracellular matrix protein belonging to a small protein family that can bind TGFB-pathway proteins and regulate their signaling (Urbanek et al., 1999; Urbanek et al., 2005; Stewart et al., 2006; Jordan et al., 2010; Raja-Khan et al., 2014). This consideration raises the hypothesis that in human PCOS, altered signaling by a Gsdf paralog might contribute to the PCOS phenotype via protein-protein interactions with genetic variations in Fibrillin-3 structure and function.
PCOS patients with larger ovaries have more severe phenotypes (Legro et al., 2005), and surgical reduction of ovary size can lead to successful reproduction (Stein and Leventhal, 1935; Donesky and Adashi, 1995). This result would be expected if a diffusible factor secreted by immature follicles contributed to the phenotype and could help explain the extra-ovarian phenotypes shared by PCOS patients and zebrafish gsdf mutants. Candidates for the deleterious factor include Gsdf-related molecules, perhaps especially Gdf9 and/or Bmp15, or a steroid that depends on properly functioning theca and granulosa cells, especially relative levels of estrogen and testosterone.
Conclusions
PCOS is defined by polycystic ovaries, ovulation failure, and hyperandrogenism often associated with obesity, infertility, and insulin resistance (Rotterdam, 2004a; van Houten and Visser, 2014). Zebrafish gsdf mutants show many of these phenotypes, including gradual loss of ovulation, accumulation of immature follicles, hyperandrogenism, obesity, and dysregulation of insulin-related genes, but differ in aspects related to lineage-specific reproductive physiology. In zebrafish gsdf mutants, follicles arrest before granulosa cells begin to express large quantities of amh, whereas PCOS patients accumulate follicles at a later stage that expresses substantial levels of AMH. In contrast to PCOS, where aromatase is the most strongly reduced steroidogenic gene and CYP17, CYP11A, and HSD3B are up-regulated (Tamura et al., 1993), zebrafish gsdf mutant females depress not only aromatase, but most other sex-steroid biosynthesis genes as well. As in PCOS, zebrafish gsdf mutants are obese, accumulate fat, abnormally express lipid genes, and lose fertility, and although we did not experimentally check insulin resistance, the great depression of insulin receptor gene expression suggests that insulin resistance would be part of the zebrafish phenotype. Our results suggest that zebrafish gsdf mutants could serve as a model for human PCOS and that supplying a related TGFB molecule to human granulosa cells might provide a therapy for major aspects of the disease.
EXPERIMENTAL PROCEDURES
TALEN-mutagenesis generated gsdf mutations based on zebrafish gsdf sequence (ENSDARG00000075301, http://ensembl.org) verified by sequencing the target site in AB-strain fish. TALEN sites were designed by “TAL Effector Nucleotide Targeter 2.0” (Doyle et al., 2012) to target the first exon. The TALEN sequence is TCGTCCTGCTGCTGCTGGCcttccctttgggggagatGTTTGTGCTCCATCCGTCA (LEFT TALEN ARM–cleavage site—RIGHT TALEN ARM). A BslI restriction enzyme recognition site in the target identified wild-type alleles. TALEN arms were built using the Golden Gate method (Sanjana et al., 2012). Constructs were linearized with SmaI and TALEN mRNAs were synthesized with T7 mMESSAGE mMACHINE Kit (Ambion, Naugatuck, CT, USA). TALEN assembly vectors were from Addgene (Cambridge, MA, USA). 250 pl TALEN mRNA for each arm were co-microinjected into one-cell zebrafish embryos. Genomic DNA extracted from injected embryos at 24 hours post-fertilization provided template to amplify a 251bp PCR fragment including the target using primers: (F): GCCAAGCCTGGCCAGCGTAGATAA and (R1): ACCCACACGATGAACACCTGAGGC. For cDNA sequencing from gsdf and WT sibling, primer F above and R2 (CAGCTGGGACAGGGAGTTGCTCGG) were used for PCR to amplify a 275 bp fragment (Fig. 2A, D). Oligonucleotides were purchased from Integrated DNA Technologies (IDT) Coralville, Iowa USA). Sequencing the 251 bp DNA genomic PCR fragment and the 275 bp cDNA fragment was performed by Sanger sequencing (GENEWIZ, Inc. NJ, USA). We established stable lines for a 1-nucleotide deletion, an 8-nucleotide deletion, and a 14-nucleotide deletion (see Fig. 2) and showed that these alleles failed to complement. To verify the absence of Gsdf, we purchased the synthesis of one anti-gsdf antibody from Alpha Diagnostic Intl. Inc. (ADI) and two anti-gsdf antibodies from Genscript and performed Western blots and histology with all three antibodies under various conditions. None of these antibodies actually recognized Gsdf. Work was performed under IACUC protocol #14-08R.
Histology and in situ hybridization
In situ hybridization was performed as described (Rodriguez-Mari et al., 2005). Probes were: a 328 bp gsdf fragment including part of exon-5 and the 3′UTR, amplified by primers: F-GACACACTCGACCCCGCAGC and R-CTGCCAGAGCCAAACCCGCA), amh (ENSDARG00000014357) (Rodriguez-Mari et al., 2005), bmp15 (ENSDARG00000037491) (Dranow et al., 2016), cyp19a1a (ENSDARG00000041348) (Chiang et al., 2001b), gata4 (ENSDARG00000098952) using a 763 bp fragment including exon-1 to exon-6 amplified by primers: F-AGCACCGGGCACCATCATTCTCCG and R-GAGCTGGAGGATCCGCTTGGAGGC), gdf9 (ENSDARG00000003229) using a 979 bp fragment including most of the coding region amplified by primers: F-TGTTGAACCCGACGTGCCCC and R-TGGTGTGCATTGGCGACCCG, sox9a (ENSDARG00000003293) (Chiang et al., 2001a), sox9b (ENSDARG00000043923) (Chiang et al., 2001a), sycp3 (ENSDARG00000013438) (Rodriguez-Mari and Postlethwait, 2011) and vasa (ENSDARG00000014373) (Yoon et al., 1997). Two color in situ hybridization was as described (Yan et al., 2011). Gonad histology used paraffin-embedded Bouin’s fixed tissue sectioned at 10μm stained with hematoxylin and eosin (H&E).
Oil red O staining
Adult fish trunks were embedded in OCT, frozen in liquid nitrogen, cut into 10 μm-thick cryosections, and stained with Oil Red O (Mehlem et al., 2013). Images were quantified from 1–7 images per tissue per individual, converted to 8-bit grayscale in ImageJ, analyzed with the Threshold tool to select Oil Red O stain, and quantified with the Measure tool. Significant differences were identified by the non-parametric Wilcoxon rank sum test under the superiority or inferiority alternatives hypothesis using R v.3.1.2.
qPCR
RNAs were extracted and cDNAs were synthesized as described (Desvignes et al., 2014). Briefly, total RNA was isolated from 3–5 fish using Tri-Reagent® (Molecular Research Center, Cincinnati, OH, USA). Contaminating DNA was removed using the DNA-free RNA kit (Zymo Research, Irvine, CA, USA), and reverse transcription (RT) was performed using 1 μg of RNA for each sample with SuperScript III reverse transcriptase and OligoDT20 primers (Thermo Fisher Scientific, Danvers, MA, USA). Control reactions were run without reverse transcriptase. cDNAs were treated with RNaseH before PCR. qPCR was performed using a StepOnePlus q-PCR machine (Applied Biosystems, Waltham, MA, USA) as described (Desvignes et al., 2011). Products of reverse transcription, including control reactions, were diluted 1/25, and a 2 μl aliquot was used for each assay. All qPCR reactions were performed in triplicate using a real-time PCR kit with Fast-SYBR® Green fluorophore (Kappa Biosystems (Pty) Ltd. Wilmington, MA, USA) and 200 nM of each primer. To avoid genomic DNA, primers occupied exon junctions. Supplementary Table 1 lists primer sequences. The relative abundance of target cDNA within sample sets was calculated from serially diluted cDNA pools (a 7-point standard curve with 1:2 dilution steps) using Applied Biosystem StepOne™ V.2.0 software. Primer concentrations and reaction temperatures were adjusted to obtain efficiencies between 97.5 and 102.5%. A fusion curve validated amplification of each PCR product and verified single transcripts. Control reactions informed background expression and identified levels significantly above background at p<0.05 and within the range of the standard curve. Beta-actin actb1 (ENSDARG00000037746) provided normalization. Significant differences were tested using the non-parametric Wilcoxon rank sum test under the superiority or inferiority alternatives hypothesis using R v.3.1.2.
Hormone assays
Sex steroids were extracted from zebrafish homogenates as described (Newman et al., 2008). Briefly, individual fish were homogenized and aliquots transferred to glass test tubes. Water and HPLC-grade methanol were added and tissues homogenized and sonicated. Tubes were shaken at 500 rpm for 1 h at room temperature and stored overnight at 4C. Tubes were shaken and centrifuged at 1500 g for 15 minutes at 4C. Supernatant (1 mL) was combined with 10 mL water and extracted by solid phase extraction. Eluates were dried under N2 gas at 38C and stored at 20C. One day prior to assay, samples were resuspended with assay buffer and shaken at 500 rpm for 1h at room temperature. After storage at 4C overnight, samples were shaken and assayed. Estradiol and 11-ketotestosterone were measured using commercially available ELISA (Cayman Chemical, Ann Arbor, MI). Kits were validated for zebrafish using tests of parallelism and standard addition. Intra- and inter-assay variation for E2 were 5.9% and 13.9% respectively and for 11-KT were 5.5% and 11.4%, respectively. Hormone concentrations are expressed as pg/g (pg hormone/g whole body homogenate). Significant differences were identified using the non-parametric Wilcoxon rank sum test under the superiority or inferiority alternatives hypothesis using R v.3.1.2.
Supplementary Material
Bullet points.
In zebrafish, the TGFB factor gsdf is expressed in gonadal somatic cells before the time of sex determination, and expression continues in granulosa and Sertoli cells.
Zebrafish gsdf mutant females ovulate only a few oocytes, then become sterile as they accumulate hundreds of immature follicles.
Mutant fish achieve greater mass and accumulate more lipid than wild types.
Female mutants down-regulate genes for granulosa cells, lipid and insulin pathways, brain-derived reproductive hormones, and up-regulate androgen.
Male gsdf mutants have normal fertility.
Gsdf, Gdf9, and Bmp15 arose as paralogs in the vertebrate genome duplication events.
Zebrafish gsdf mutants mimic the ovulation failure, young oocyte accumulation, hyperandrogenism, obesity, and insulin-related phenotypes of human polycystic ovarian syndrome (PCOS).
Zebrafish gsdf mutants provide a model for human PCOS disease.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM085318 and by the National Institute of Child Health and Human Development Award Numbers P01HD22486 (JHP) and 1R01HD081551 (BD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Jeremy Wegner for helping with TALEN assembly, Sam Peterson for help with initial qPCR analysis, and Trevor Enright for animal care.
Grant Sponsors: NIH grants: R01GM085318 (JHP) P01HD22486 (J. Eisen, and JHP). 1R01HD081551 (BD)
Literature cited
- Amores A, Catchen J, Ferrara A, Fontenot Q, Postlethwait JH. Genome evolution and meiotic maps by massively parallel DNA sequencing: spotted gar, an outgroup for the teleost genome duplication. Genetics. 2011;188:799–808. doi: 10.1534/genetics.111.127324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen L, Holbech H, Gessbo A, Norrgren L, Petersen GI. Effects of exposure to 17alpha-ethinylestradiol during early development on sexual differentiation and induction of vitellogenin in zebrafish (Danio rerio) Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:365–374. doi: 10.1016/s1532-0456(03)00006-1. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Rodriguez Mari A, Braasch I, Amores A, Hohenlohe P, Batzel P, Postlethwait JH. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One. 2012;7:e40701. doi: 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. FOG-2 and GATA-4 Are coexpressed in the mouse ovary and can modulate mullerian-inhibiting substance expression. Biol Reprod. 2003;68:1333–1340. doi: 10.1095/biolreprod.102.008599. [DOI] [PubMed] [Google Scholar]
- Arukwe A, Goksoyr A. Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: oogenetic, population, and evolutionary implications of endocrine disruption. Comp Hepatol. 2003;2:4. doi: 10.1186/1476-5926-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J, Davy A, Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–1494. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Fleming A, Lacombe A, Harley VR, Vilain E. Wnt4 inhibits beta-catenin/TCF signalling by redirecting beta-catenin to the cell membrane. Biol Cell. 2008;100:167–177. doi: 10.1042/BC20070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Yan YL, Postlethwait J, Rubin DA. Evolution of the vertebrate pth2 (tip39) gene family and the regulation of PTH type 2 receptor (pth2r) and its endogenous ligand pth2 by hedgehog signaling in zebrafish development. J Endocrinol. 2011;211:187–200. doi: 10.1530/JOE-10-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran J, Palevitch O, Ben-Dor S, Levavi-Sivan B. Neurokinin Bs and neurokinin B receptors in zebrafish-potential role in controlling fish reproduction. Proc Natl Acad Sci U S A. 2012;109:10269–10274. doi: 10.1073/pnas.1119165109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Bottner M, Krieglstein K, Unsicker K. The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75:2227–2240. doi: 10.1046/j.1471-4159.2000.0752227.x. [DOI] [PubMed] [Google Scholar]
- Braasch I, Gehrke AR, Smith JJ, Kawasaki K, Manousaki T, Pasquier J, Amores A, Desvignes T, Batzel P, Catchen J, Berlin AM, Campbell MS, Barrell D, Martin KJ, Mulley JF, Ravi V, Lee AP, Nakamura T, Chalopin D, Fan S, Wcisel D, Canestro C, Sydes J, Beaudry FE, Sun Y, Hertel J, Beam MJ, Fasold M, Ishiyama M, Johnson J, Kehr S, Lara M, Letaw JH, Litman GW, Litman RT, Mikami M, Ota T, Saha NR, Williams L, Stadler PF, Wang H, Taylor JS, Fontenot Q, Ferrara A, Searle SM, Aken B, Yandell M, Schneider I, Yoder JA, Volff JN, Meyer A, Amemiya CT, Venkatesh B, Holland PW, Guiguen Y, Bobe J, Shubin NH, Di Palma F, Alfoldi J, Lindblad-Toh K, Postlethwait JH. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet. 2016;48:427–437. doi: 10.1038/ng.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, Smith JR. An SNP-Based Linkage Map for Zebrafish Reveals Sex Determination Loci. G3 (Bethesda) 2011;1:3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau AM, Baillargeon JP. PCOS in adolescence and type 2 diabetes. Curr Diab Rep. 2015;15:564. doi: 10.1007/s11892-014-0564-3. [DOI] [PubMed] [Google Scholar]
- Chakraborty T, Zhou LY, Chaudhari A, Iguchi T, Nagahama Y. Dmy initiates masculinity by altering Gsdf/Sox9a2/Rspo1 expression in medaka (Oryzias latipes) Sci Rep. 2016;6:19480. doi: 10.1038/srep19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Matzuk MM. Smad5 is required for mouse primordial germ cell development. Mech Dev. 2001;104:61–67. doi: 10.1016/s0925-4773(01)00367-7. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC. Genetics of ovarian differentiation: Rspo1, a major player. Sex Dev. 2008;2:219–227. doi: 10.1159/000152038. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hong WS, Wang Q, Chen SX. Cloning and expression pattern of gsdf during the first maleness reproductive phase in the protandrous Acanthopagrus latus. Gen Comp Endocrinol. 2015;217–218:71–80. doi: 10.1016/j.ygcen.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55–59. doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- Chiang EF, Pai CI, Wyatt M, Yan YL, Postlethwait J, Chung B. Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev Biol. 2001a;231:149–163. doi: 10.1006/dbio.2000.0129. [DOI] [PubMed] [Google Scholar]
- Chiang EF, Yan YL, Guiguen Y, Postlethwait J, Chung B. Two Cyp19 (P450 aromatase) genes on duplicated zebrafish chromosomes are expressed in ovary or brain. Mol Biol Evol. 2001b;18:542–550. doi: 10.1093/oxfordjournals.molbev.a003833. [DOI] [PubMed] [Google Scholar]
- Chiang EF, Yan YL, Tong SK, Hsiao PH, Guiguen Y, Postlethwait J, Chung BC. Characterization of duplicated zebrafish cyp19 genes. J Exp Zool. 2001c;290:709–714. doi: 10.1002/jez.1121. [DOI] [PubMed] [Google Scholar]
- Clelland E, Kohli G, Campbell RK, Sharma S, Shimasaki S, Peng C. Bone morphogenetic protein-15 in the zebrafish ovary: complementary deoxyribonucleic acid cloning, genomic organization, tissue distribution, and role in oocyte maturation. Endocrinology. 2006;147:201–209. doi: 10.1210/en.2005-1017. [DOI] [PubMed] [Google Scholar]
- Clelland ES, Tan Q, Balofsky A, Lacivita R, Peng C. Inhibition of premature oocyte maturation: a role for bone morphogenetic protein 15 in zebrafish ovarian follicles. Endocrinology. 2007;148:5451–5458. doi: 10.1210/en.2007-0674. [DOI] [PubMed] [Google Scholar]
- Crespo B, Gomez A, Mazon MJ, Carrillo M, Zanuy S. Isolation and characterization of Ff1 and Gsdf family genes in European sea bass and identification of early gonadal markers of precocious puberty in males. Gen Comp Endocrinol. 2013;191:155–167. doi: 10.1016/j.ygcen.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Crow KD, Stadler PF, Lynch VJ, Amemiya C, Wagner GP. The “fish-specific” Hox cluster duplication is coincident with the origin of teleosts. Mol Biol Evol. 2006;23:121–136. doi: 10.1093/molbev/msj020. [DOI] [PubMed] [Google Scholar]
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia. 2009;13:90–92. [PMC free article] [PubMed] [Google Scholar]
- Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan R, Saidijam M, Mehdizade M, Shabab N, Yavangi M, Artimani T. Evidence for decreased expression of APPL1 associated with reduced insulin and adiponectin receptors expression in PCOS patients. J Endocrinol Invest. 2016 doi: 10.1007/s40618-016-0468-y. [DOI] [PubMed] [Google Scholar]
- Desvignes T, Contreras A, Postlethwait JH. Evolution of the miR199-214 cluster and vertebrate skeletal development. RNA Biol. 2014;11:281–294. doi: 10.4161/rna.28141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes T, Fauvel C, Bobe J. The NME gene family in zebrafish oogenesis and early development. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:439–449. doi: 10.1007/s00210-011-0619-9. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e3. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donesky BW, Adashi EY. Surgically induced ovulation in the polycystic ovary syndrome: wedge resection revisited in the age of laparoscopy. Fertil Steril. 1995;63:439–463. doi: 10.1016/s0015-0282(16)57408-1. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, Vandyk JK, Bogdanove AJ. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40:W117–122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranow DB, Hu K, Bird AM, Lawry ST, Adams MT, Sanchez A, Amatruda JF, Draper BW. Bmp15 Is an Oocyte-Produced Signal Required for Maintenance of the Adult Female Sexual Phenotype in Zebrafish. PLoS Genet. 2016;12:e1006323. doi: 10.1371/journal.pgen.1006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranow DB, Tucker RP, Draper BW. Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev Biol. 2013;376:43–50. doi: 10.1016/j.ydbio.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Duffy DM. Growth differentiation factor-9 is expressed by the primate follicle throughout the periovulatory interval. Biol Reprod. 2003;69:725–732. doi: 10.1095/biolreprod.103.015891. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS) Am J Physiol Endocrinol Metab. 2001;281:E392–399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- Efimenko E, Padua MB, Manuylov NL, Fox SC, Morse DA, Tevosian SG. The transcription factor GATA4 is required for follicular development and normal ovarian function. Dev Biol. 2013;381:144–158. doi: 10.1016/j.ydbio.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homburg R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552–2556. doi: 10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Luque-Ramirez M, San Millan JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005;26:251–282. doi: 10.1210/er.2004-0004. [DOI] [PubMed] [Google Scholar]
- Ferre P. The adipocyte and insulin resistance. Ann Endocrinol (Paris) 2004;65:61–62. doi: 10.1016/s0003-4266(04)95631-4. [DOI] [PubMed] [Google Scholar]
- Fleming R, Harborne L, MacLaughlin DT, Ling D, Norman J, Sattar N, Seifer DB. Metformin reduces serum mullerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fertil Steril. 2005;83:130–136. doi: 10.1016/j.fertnstert.2004.05.098. [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forconi M, Canapa A, Barucca M, Biscotti MA, Capriglione T, Buonocore F, Fausto AM, Makapedua DM, Pallavicini A, Gerdol M, De Moro G, Scapigliati G, Olmo E, Schartl M. Characterization of sex determination and sex differentiation genes in Latimeria. PLoS One. 2013;8:e56006. doi: 10.1371/journal.pone.0056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- Gautier A, Le Gac F, Lareyre JJ. The gsdf gene locus harbors evolutionary conserved and clustered genes preferentially expressed in fish previtellogenic oocytes. Gene. 2011a;472:7–17. doi: 10.1016/j.gene.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Gautier A, Sohm F, Joly JS, Le Gac F, Lareyre JJ. The proximal promoter region of the zebrafish gsdf gene is sufficient to mimic the spatio-temporal expression pattern of the endogenous gene in Sertoli and granulosa cells. Biol Reprod. 2011b;85:1240–1251. doi: 10.1095/biolreprod.111.091892. [DOI] [PubMed] [Google Scholar]
- Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146:1883–1892. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol Reprod. 2004;70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, Fernandino JI, Somoza GM, Yokota M, Strussmann CA. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci U S A. 2012;109:2955–2959. doi: 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt S, Louria-Hayon I, Haupt Y. P53 licensed to kill? Operating the assassin. J Cell Biochem. 2003;88:76–82. doi: 10.1002/jcb.10311. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Her GM, Chiang CC, Chen WY, Wu JL. In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio) FEBS Lett. 2003;538:125–133. doi: 10.1016/s0014-5793(03)00157-1. [DOI] [PubMed] [Google Scholar]
- High SK. In: Sex determination in zebrafish: genetics of sex and wnt4a. Department of Biology, editor. Eugene, Oregon, USA: University of Oregon; 2016. p. 180. [Google Scholar]
- Hoegg S, Brinkmann H, Taylor JS, Meyer A. Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J Mol Evol. 2004;59:190–203. doi: 10.1007/s00239-004-2613-z. [DOI] [PubMed] [Google Scholar]
- Holland PW. Gene duplication: past, present and future. Semin Cell Dev Biol. 1999;10:541–547. doi: 10.1006/scdb.1999.0335. [DOI] [PubMed] [Google Scholar]
- Horiguchi R, Nozu R, Hirai T, Kobayashi Y, Nagahama Y, Nakamura M. Characterization of gonadal soma-derived factor expression during sex change in the protogynous wrasse, Halichoeres trimaculatus. Dev Dyn. 2013;242:388–399. doi: 10.1002/dvdy.23929. [DOI] [PubMed] [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, Plasterk RH, Hannon GJ, Draper BW, Ketting RF. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson TH, Ankley GT, Segner H, Tyler CR. Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ Health Perspect. 2006;114(Suppl 1):106–114. doi: 10.1289/ehp.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Saino K, Matsuda M. Mutation of Gonadal soma-derived factor induces medaka XY gonads to undergo ovarian development. Biochem Biophys Res Commun. 2015;467:109–114. doi: 10.1016/j.bbrc.2015.09.112. [DOI] [PubMed] [Google Scholar]
- Irwin DM. A second insulin gene in fish genomes. Gen Comp Endocrinol. 2004;135:150–158. doi: 10.1016/j.ygcen.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jiang DN, Yang HH, Li MH, Shi HJ, Zhang XB, Wang DS. gsdf is a downstream gene of dmrt1 that functions in the male sex determination pathway of the Nile tilapia. Mol Reprod Dev. 2016 doi: 10.1002/mrd.22642. [DOI] [PubMed] [Google Scholar]
- Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010;58:903–915. doi: 10.1369/jhc.2010.956615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josso N. Interspecific character of the Mullerian-inhibiting substance: action of the human fetal testis, ovary and adrenal of the fetal rat Mullerian duct in organ culture. J Clin Endocrinol Metab. 1971;32:404–409. doi: 10.1210/jcem-32-3-404. [DOI] [PubMed] [Google Scholar]
- Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu) PLoS Genet. 2012;8:e1002798. doi: 10.1371/journal.pgen.1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Ijiri S, Kobayashi T, Izumi H, Kuramochi Y, Wang DS, Mizuno S, Nagahama Y. Gonadal soma-derived factor (gsdf), a TGF-beta superfamily gene, induces testis differentiation in the teleost fish Oreochromis niloticus. Mol Cell Endocrinol. 2015;415:87–99. doi: 10.1016/j.mce.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. doi: 10.1523/JNEUROSCI.3878-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevenaar ME, Laven JS, Fong SL, Uitterlinden AG, de Jong FH, Themmen AP, Visser JA. A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2008;93:1310–1316. doi: 10.1210/jc.2007-2205. [DOI] [PubMed] [Google Scholar]
- Koopman P. Sry and Sox9: mammalian testis-determining genes. Cell Mol Life Sci. 1999;55:839–856. doi: 10.1007/PL00013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfsmeier KH. PCNA in the ovary of zebrafish (Brachydanio rerio, Ham.-Buch.) Acta Histochem. 2002;104:73–76. doi: 10.1078/0065-1281-00632. [DOI] [PubMed] [Google Scholar]
- Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Saito D, Nakamura S, Katoh-Fukui Y, Ohta K, Baba T, Morohashi K, Tanaka M. Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl Acad Sci U S A. 2007;104:16958–16963. doi: 10.1073/pnas.0609932104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok HF, So WK, Wang Y, Ge W. Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors–evidence for their distinct functions in follicle development. Biol Reprod. 2005;72:1370–1381. doi: 10.1095/biolreprod.104.038190. [DOI] [PubMed] [Google Scholar]
- Laven JS, Imani B, Eijkemans MJ, Fauser BC. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv. 2002;57:755–767. doi: 10.1097/00006254-200211000-00022. [DOI] [PubMed] [Google Scholar]
- Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab. 2005;90:2571–2579. doi: 10.1210/jc.2004-0219. [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AY, Leung JC, Chan LY, Ma ES, Kwan TT, Lai KN, Meng A, Liang R. Proliferating cell nuclear antigen (PCNA) as a proliferative marker during embryonic and adult zebrafish hematopoiesis. Histochem Cell Biol. 2005;124:105–111. doi: 10.1007/s00418-005-0003-2. [DOI] [PubMed] [Google Scholar]
- Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, Orban L. Polygenic sex determination system in zebrafish. PLoS One. 2012;7:e34397. doi: 10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KC, Lin SW, Ge W. Differential regulation of gonadotropin receptors (fshr and lhcgr) by estradiol in the zebrafish ovary involves nuclear estrogen receptors that are likely located on the plasma membrane. Endocrinology. 2011;152:4418–4430. doi: 10.1210/en.2011-1065. [DOI] [PubMed] [Google Scholar]
- Liu L, Ge W. Growth differentiation factor 9 and its spatiotemporal expression and regulation in the zebrafish ovary. Biol Reprod. 2007;76:294–302. doi: 10.1095/biolreprod.106.054668. [DOI] [PubMed] [Google Scholar]
- Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–242. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- Luckenbach JA, Iliev DB, Goetz FW, Swanson P. Identification of differentially expressed ovarian genes during primary and early secondary oocyte growth in coho salmon, Oncorhynchus kisutch. Reprod Biol Endocrinol. 2008;6:2. doi: 10.1186/1477-7827-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen S, Jensen TH. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat Rev Mol Cell Biol. 2015;16:665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- Macut D, Damjanovic S, Panidis D, Spanos N, Glisic B, Petakov M, Rousso D, Kourtis A, Bjekic J, Milic N. Oxidised low-density lipoprotein concentration - early marker of an altered lipid metabolism in young women with PCOS. Eur J Endocrinol. 2006;155:131–136. doi: 10.1530/eje.1.02187. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- McAllister JM, Legro RS, Modi BP, Strauss JF., 3rd Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26:118–124. doi: 10.1016/j.tem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JJ, Weiss J, Jameson JL. Dax1 is required for testis determination. Nat Genet. 2003;34:32–33. doi: 10.1038/ng1141. [DOI] [PubMed] [Google Scholar]
- Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8:1149–1154. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- Mendis SH, Meachem SJ, Sarraj MA, Loveland KL. Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol Reprod. 2011;84:379–391. doi: 10.1095/biolreprod.110.086231. [DOI] [PubMed] [Google Scholar]
- Meng X, Bartholomew C, Craft JA. Differential expression of vitellogenin and oestrogen receptor genes in the liver of zebrafish, Danio rerio. Anal Bioanal Chem. 2010;396:625–630. doi: 10.1007/s00216-009-3112-2. [DOI] [PubMed] [Google Scholar]
- Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S, Jr, Jindal S. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28:1661–1669. doi: 10.1093/humrep/det072. [DOI] [PubMed] [Google Scholar]
- Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, Shimizu N, Mitani H, Furutani-Seiki M, Tanaka M, Kondoh H. The hotei mutation of medaka in the anti-Mullerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc Natl Acad Sci U S A. 2007;104:9691–9696. doi: 10.1073/pnas.0611379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, Naruse K, Hamaguchi S, Sakaizumi M. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics. 2012;191:163–170. doi: 10.1534/genetics.111.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K, Presslauer C, Kirtiklis L, Babiak I, Fernandes JM. Sexually dimorphic transcription of estrogen receptors in cod gonads throughout a reproductive cycle. J Mol Endocrinol. 2014;52:357–371. doi: 10.1530/JME-13-0187. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kurokawa H, Asakawa S, Shimizu N, Tanaka M. Two Distinct Types of Theca Cells in the Medaka Gonad: Germ Cell-Dependent Maintenance of cyp19a1-Expressing Theca Cells. Developmental Dynamics. 2009;238:2652–2657. doi: 10.1002/dvdy.22068. [DOI] [PubMed] [Google Scholar]
- Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci U S A. 2002;99:11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- Newman AE, Chin EH, Schmidt KL, Bond L, Wynne-Edwards KE, Soma KK. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen Comp Endocrinol. 2008;155:503–510. doi: 10.1016/j.ygcen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction. 2010;139:177–184. doi: 10.1530/REP-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban L, Sreenivasan R, Olsson PE. Long and winding roads: testis differentiation in zebrafish. Mol Cell Endocrinol. 2009;312:35–41. doi: 10.1016/j.mce.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Uda M, Crisponi L, Omari S, Cao A, Forabosco A, Schlessinger D. Determination and stability of sex. Bioessays. 2007;29:15–25. doi: 10.1002/bies.20515. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasani MR, Robison BD, Hardy RW, Hill RA. Early developmental expression of two insulins in zebrafish (Danio rerio) Physiol Genomics. 2006;27:79–85. doi: 10.1152/physiolgenomics.00012.2006. [DOI] [PubMed] [Google Scholar]
- Pellatt L, Rice S, Mason HD. Anti-Mullerian hormone and polycystic ovary syndrome: a mountain too high? Reproduction. 2010;139:825–833. doi: 10.1530/REP-09-0415. [DOI] [PubMed] [Google Scholar]
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110:E776–785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- Poon SK, So WK, Yu X, Liu L, Ge W. Characterization of inhibin alpha subunit (inha) in the zebrafish: evidence for a potential feedback loop between the pituitary and ovary. Reproduction. 2009;138:709–719. doi: 10.1530/REP-09-0198. [DOI] [PubMed] [Google Scholar]
- Presslauer C, Nagasawa K, Dahle D, Babiak J, Fernandes JM, Babiak I. Induced autoimmunity against gonadal proteins affects gonadal development in juvenile zebrafish. PLoS One. 2014;9:e114209. doi: 10.1371/journal.pone.0114209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja-Khan N, Urbanek M, Rodgers RJ, Legro RS. The role of TGF-beta in polycystic ovary syndrome. Reprod Sci. 2014;21:20–31. doi: 10.1177/1933719113485294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastetter RH, Bernard P, Palmer JS, Chassot AA, Chen H, Western PS, Ramsay RG, Chaboissier MC, Wilhelm D. Marker genes identify three somatic cell types in the fetal mouse ovary. Dev Biol. 2014;394:242–252. doi: 10.1016/j.ydbio.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Reyhanian Caspillo N, Volkova K, Hallgren S, Olsson PE, Porsch-Hallstrom I. Short-term treatment of adult male zebrafish (Danio Rerio) with 17alpha-ethinyl estradiol affects the transcription of genes involved in development and male sex differentiation. Comp Biochem Physiol C Toxicol Pharmacol. 2014;164:35–42. doi: 10.1016/j.cbpc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo D, Ribas L, Cal R, Sanchez L, Piferrer F, Martinez P, Vinas A. Gene expression analysis at the onset of sex differentiation in turbot (Scophthalmus maximus) BMC Genomics. 2015;16:973. doi: 10.1186/s12864-015-2142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mari A, Postlethwait JH. The role of Fanconi anemia/BRCA genes in zebrafish sex determination. Methods Cell Biol. 2011;105:461–490. doi: 10.1016/B978-0-12-381320-6.00020-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mari A, Yan YL, Bremiller RA, Wilson C, Canestro C, Postlethwait JH. Characterization and expression pattern of zebrafish Anti-Mullerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr Patterns. 2005;5:655–667. doi: 10.1016/j.modgep.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Rondeau EB, Messmer AM, Sanderson DS, Jantzen SG, von Schalburg KR, Minkley DR, Leong JS, Macdonald GM, Davidsen AE, Parker WA, Mazzola RS, Campbell B, Koop BF. Genomics of sablefish (Anoplopoma fimbria): expressed genes, mitochondrial phylogeny, linkage map and identification of a putative sex gene. BMC Genomics. 2013;14:452. doi: 10.1186/1471-2164-14-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A, Munger S, Capel B. Bmp7 regulates germ cell proliferation in mouse fetal gonads. Sex Dev. 2007;1:127–137. doi: 10.1159/000100034. [DOI] [PubMed] [Google Scholar]
- Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004a;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004b;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Rouiller-Fabre V, Carmona S, Merhi RA, Cate R, Habert R, Vigier B. Effect of anti-Mullerian hormone on Sertoli and Leydig cell functions in fetal and immature rats. Endocrinology. 1998;139:1213–1220. doi: 10.1210/endo.139.3.5785. [DOI] [PubMed] [Google Scholar]
- Sanjana NE, Cong L, Zhou Y, Cunniff MM, Feng G, Zhang F. A transcription activator-like effector toolbox for genome engineering. Nat Protoc. 2012;7:171–192. doi: 10.1038/nprot.2011.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatari E, Shikina S, Takeuchi T, Yoshizaki G. A novel transforming growth factor-beta superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss) Dev Biol. 2007;301:266–275. doi: 10.1016/j.ydbio.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Merhi Z. Is AMH a regulator of follicular atresia? J Assist Reprod Genet. 2014;31:1403–1407. doi: 10.1007/s10815-014-0328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman K, Wallace RA, Sarka A, Qi X. Stages of Oocyte Development in the Zebrafish, Brachydanio rerio. Stages of Oocyte Development in the Zebrafish, Brachydanio rerio. 1993;218:203–224. doi: 10.1002/jmor.1052180209. [DOI] [PubMed] [Google Scholar]
- She ZY, Yang WX. Molecular mechanisms involved in mammalian primary sex determination. J Mol Endocrinol. 2014;53:R21–37. doi: 10.1530/JME-14-0018. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Zhao J, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li W, Li B, Wan C, Qin Y, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang Y, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44:1020–1025. doi: 10.1038/ng.2384. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Paul-Prasanth B, Suzuki A, Usami T, Nakamoto M, Matsuda M, Nagahama Y. Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expr Patterns. 2010;10:283–289. doi: 10.1016/j.gep.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Sidis Y, Fujiwara T, Leykin L, Isaacson K, Toth T, Schneyer AL. Characterization of inhibin/activin subunit, activin receptor, and follistatin messenger ribonucleic acid in human and mouse oocytes: evidence for activin’s paracrine signaling from granulosa cells to oocytes. Biol Reprod. 1998;59:807–812. doi: 10.1095/biolreprod59.4.807. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Nusslein-Volhard C. Germ line control of female sex determination in zebrafish. Dev Biol. 2008;324:277–287. doi: 10.1016/j.ydbio.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Silva JR, van den Hurk R, van Tol HT, Roelen BA, Figueiredo JR. Expression of growth differentiation factor 9 (GDF9), bone morphogenetic protein 15 (BMP15), and BMP receptors in the ovaries of goats. Mol Reprod Dev. 2005;70:11–19. doi: 10.1002/mrd.20127. [DOI] [PubMed] [Google Scholar]
- Slanchev K, Stebler J, de la Cueva-Mendez G, Raz E. Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci U S A. 2005;102:4074–4079. doi: 10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IF, Leventhal ML. Amenorhea associated with bilateral polcystic ovaries. American Journal of Obstetrics and Gynaecology. 1935;29:181–191. [Google Scholar]
- Steinke D, Salzburger W, Meyer A. Novel relationships among ten fish model species revealed based on a phylogenomic analysis using ESTs. J Mol Evol. 2006;62:772–784. doi: 10.1007/s00239-005-0170-8. [DOI] [PubMed] [Google Scholar]
- Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss JF, 3rd, Dunaif A, Spielman RS. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91:4112–4117. doi: 10.1210/jc.2006-0951. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wu X, O’Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276:64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Swain A, Lovell-Badge R. Mammalian sex determination: a molecular drama. Genes Dev. 1999;13:755–767. doi: 10.1101/gad.13.7.755. [DOI] [PubMed] [Google Scholar]
- Takahashi H. Juvenile Hermaphroditism in the Zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ. 1977;28:57–65. [Google Scholar]
- Tamura T, Kitawaki J, Yamamoto T, Osawa Y, Kominami S, Takemori S, Okada H. Immunohistochemical localization of 17 alpha-hydroxylase/C17-20 lyase and aromatase cytochrome P-450 in polycystic human ovaries. J Endocrinol. 1993;139:503–509. doi: 10.1677/joe.0.1390503. [DOI] [PubMed] [Google Scholar]
- Taylor SI, Kadowaki T, Accili D, Cama A, Kadowaki H, McKeon C, Moncada V, Marcus-Samuels B, Bevins C, Ojamaa K, et al. Mutations in the insulin receptor gene in genetic forms of insulin resistance. Recent Prog Horm Res. 1990;46:185–213. doi: 10.1016/b978-0-12-571146-3.50011-0. discussion 213-187. [DOI] [PubMed] [Google Scholar]
- Tello JA, Wu S, Rivier JE, Sherwood NM. Four functional GnRH receptors in zebrafish: analysis of structure, signaling, synteny and phylogeny. Integr Comp Biol. 2008;48:570–587. doi: 10.1093/icb/icn070. [DOI] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Andre M, Forgue J, Barthe C, Babin PJ. Expression patterns of three estrogen receptor genes during zebrafish (Danio rerio) development: evidence for high expression in neuromasts. Gene Expr Patterns. 2004;4:561–568. doi: 10.1016/j.modgep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Tong Y, Shan T, Poh YK, Yan T, Wang H, Lam SH, Gong Z. Molecular cloning of zebrafish and medaka vitellogenin genes and comparison of their expression in response to 17beta-estradiol. Gene. 2004;328:25–36. doi: 10.1016/j.gene.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y, Monson C, Duan C, Wu Y, Gao C, Yakar S, Sadler KC, LeRoith D. The role of insulin receptor signaling in zebrafish embryogenesis. Endocrinology. 2008;149:5996–6005. doi: 10.1210/en.2008-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzung KW, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, Yamaha E, Hossain MS, Calvert ME, Orban L. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports. 2015;4:61–73. doi: 10.1016/j.stemcr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002;205:711–718. doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss JF, 3rd, Spielman RS, Dunaif A. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96:8573–8578. doi: 10.1073/pnas.96.15.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek M, Woodroffe A, Ewens KG, Diamanti-Kandarakis E, Legro RS, Strauss JF, 3rd, Dunaif A, Spielman RS. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90:6623–6629. doi: 10.1210/jc.2005-0622. [DOI] [PubMed] [Google Scholar]
- van Houten EL, Visser JA. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reprod Biol. 2014;14:32–43. doi: 10.1016/j.repbio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, Hoon S, Gangu V, Roy SW, Irimia M, Korzh V, Kondrychyn I, Lim ZW, Tay BH, Tohari S, Kong KW, Ho S, Lorente-Galdos B, Quilez J, Marques-Bonet T, Raney BJ, Ingham PW, Tay A, Hillier LW, Minx P, Boehm T, Wilson RK, Brenner S, Warren WC. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505:174–179. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development. 1998;125:2665–2675. doi: 10.1242/dev.125.14.2665. [DOI] [PubMed] [Google Scholar]
- Vigouroux C. What have we learned form monogenic forms of severe insulin resistance associated with PCOS/HAIRAN? Ann Endocrinol (Paris) 2010;71:222–224. doi: 10.1016/j.ando.2010.02.017. [DOI] [PubMed] [Google Scholar]
- von Hofsten J, Larsson A, Olsson PE. Novel steroidogenic factor-1 homolog (ff1d) is coexpressed with anti-Mullerian hormone (AMH) in zebrafish. Dev Dyn. 2005;233:595–604. doi: 10.1002/dvdy.20335. [DOI] [PubMed] [Google Scholar]
- Wallace RA. A comprehensive synthesis. In: Browder LW, editor. Developmental Biology. New York: Plenum Press; 1985. pp. 127–177. [Google Scholar]
- Walton KL, Makanji Y, Chen J, Wilce MC, Chan KL, Robertson DM, Harrison CA. Two distinct regions of latency-associated peptide coordinate stability of the latent transforming growth factor-beta1 complex. J Biol Chem. 2010;285:17029–17037. doi: 10.1074/jbc.M110.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XG, Bartfai R, Sleptsova-Freidrich I, Orban L. The timing and extent of ‘juvenile ovary’ phase are highly variable during zebrafish testis differentiation. J Fish Biol. 2007;70:33–44. [Google Scholar]
- Wang Y, Ge W. Gonadotropin regulation of follistatin expression in the cultured ovarian follicle cells of zebrafish, Danio rerio. Gen Comp Endocrinol. 2003;134:308–315. doi: 10.1016/s0016-6480(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Wei LN, Huang R, Li LL, Fang C, Li Y, Liang XY. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31:1483–1490. doi: 10.1007/s10815-014-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LN, Li LL, Fang C, Huang R, Liang XY. Inhibitory effects of controlled ovarian stimulation on the expression of GDF9 and BMP15 in oocytes from women with PCOS. J Assist Reprod Genet. 2013;30:1313–1318. doi: 10.1007/s10815-013-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab. 2005;90:5582–5587. doi: 10.1210/jc.2005-0695. [DOI] [PubMed] [Google Scholar]
- Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073–1079. e1071–1011. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Wilson CA, High SK, McCluskey BM, Amores A, Yan YL, Titus TA, Anderson JL, Batzel P, Carvan MJ, 3rd, Schartl M, Postlethwait JH. Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics. 2014;198:1291–1308. doi: 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Zhang Y, Sarida M, Hattori RS, Strussmann CA. Coexistence of genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. PLoS One. 2014;9:e102574. doi: 10.1371/journal.pone.0102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yan Y, Talbot JC, BreMiller R. Tyr-Fluor/Cy3/Cy5 triple fluorescent in situ protocol on cryostat sections of zebrafish. ZFIN Protocol Wiki/In Situ Hybridization Techniques 2011 [Google Scholar]
- Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, Miller CT, Singer A, Kimmel C, Westerfield M, Postlethwait JH. A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development. 2005;132:1069–1083. doi: 10.1242/dev.01674. [DOI] [PubMed] [Google Scholar]
- Yazawa R, Takeuchi Y, Higuchi K, Yatabe T, Kabeya N, Yoshizaki G. Chub mackerel gonads support colonization, survival, and proliferation of intraperitoneally transplanted xenogenic germ cells. Biol Reprod. 2010;82:896–904. doi: 10.1095/biolreprod.109.081281. [DOI] [PubMed] [Google Scholar]
- Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- Zhang X, Guan G, Li M, Zhu F, Liu Q, Naruse K, Herpin A, Nagahama Y, Li J, Hong Y. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci Rep. 2016;6:19738. doi: 10.1038/srep19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SY, Qiao J, Chen YJ, Liu P, Li J, Yan J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril. 2010;94:261–267. doi: 10.1016/j.fertnstert.2009.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


