Abstract
A major controversy persists within the field of glial biology concerning whether or not, under physiological conditions, neuronal activity leads to Ca2+-dependent release of neurotransmitters from astrocytes, a phenomenon known as gliotransmission. Our perspective is that, while we and others can apply techniques to cause gliotransmission, there is considerable evidence gathered using astrocyte-specific and more physiological approaches which suggests that gliotransmission is a pharmacological phenomenon rather than a physiological process. Approaches providing evidence against gliotransmission include stimulation of Gq-GPCRs expressed only in astrocytes, as well as removal of the primary proposed source of astrocyte Ca2+ responsible for gliotransmission. These approaches contrast with those supportive of gliotransmission, which include mechanical stimulation, strong astrocytic depolarization using whole-cell patch-clamp or optogenetics, uncaging Ca2+ or IP3, chelating Ca2+ using BAPTA, and nonspecific bath application of agonists to receptors expressed by a multitude of cell types. These techniques are not subtle and therefore are not supportive of recent suggestions that gliotransmission requires very specific and delicate temporal and spatial requirements. Other evidence, including lack of propagating Ca2+ waves between astrocytes in healthy tissue, lack of expression of vesicular release machinery, and the demise of the d-serine gliotransmission hypothesis, provides additional evidence against gliotransmission. Overall, the data suggest that Ca2+-dependent release of neurotransmitters is the province of neurons, not astrocytes, in the intact brain under physiological conditions.
Dual Perspectives Companion Paper: Gliotransmission: Beyond Black-and-White, by Iaroslav Savtchouk and Andrea Volterra
Keywords: astrocyte, calcium, d-serine, glutamate, GPCR, IP3R
Introduction
The goal of this portion of the Dual Perspectives feature is to share information and evidence that have led us to reject the hypothesis that astrocytes participate in gliotransmission under physiological conditions. As such, this evidence has been built on “negative” data, which by definition is more difficult to defend, requires a higher level of scrutiny, and more often than not goes unpublished. However, we would like to emphasize at the outset the difference between “absence of evidence” versus “evidence of absence.” “Absence of evidence” suggests that something has not been observed but has never been objectively and carefully tested. In the case of gliotransmission, there is strong and ample evidence from carefully designed and controlled studies leading to the conclusion that gliotransmission does not occur under physiological conditions; that is, there is substantial evidence of absence. The reader is encouraged to read through both viewpoints and, with the information provided, form their own perspectives and conclusions regarding the existence of gliotransmission. We invite the reader to consider the following questions as they read through both perspectives: In weighing the evidence for and against gliotransmission, what methods or conditions were used in each case? Did one set of methods or conditions approach astrocyte physiology more closely than another? Are sufficient controls in place to eliminate potential involvement of astrocytic pumps, ion channels, and transporters that could also be modulated by ion fluxes, changes in membrane potential, G protein-coupled receptors (GPCRs), and/or Ca2+? Finally, is gliotransmission necessary or important for brain function and behavior? Here we concisely highlight findings from a wide spectrum of laboratories that argue against gliotransmission being a physiological process.
Discovery of gliotransmission and methods used to stimulate astrocytes
Cultured astroglia in vitro directly signal to neurons and other astroglia through the Ca2+-dependent exocytosis of neurotransmitters (Parpura et al., 1994, 1995; Araque et al., 1998a, b, 1999a, 2000, 2001; Parpura and Haydon, 2000); the term “gliotransmission” was created to describe this phenomenon (Bezzi and Volterra, 2001). The discovery of gliotransmission in vitro was very exciting as it challenged the classical view of nervous system function and quickly led to study of this process in acute brain sections. Pharmacological approaches were the easiest to use and were therefore the first used to stimulate astroglial Ca2+ elevations. Many of the early techniques used in vitro, including mechanical stimulation of astrocytes with a pipette tip, stimulating endogenous GPCRs with bath-applied agonists, uncaging Ca2+ within the cell, and chelating astrocyte Ca2+ by whole-cell dialysis of BAPTA, were carried over to work in intact tissue (for review, see Araque et al., 1999b; Bezzi and Volterra, 2001). While these techniques resulted in changes in neuronal synaptic activity interpreted as gliotransmission, they were limited by a lack of specificity or physiological relevance. For example, strong evidence from work in vitro indicated that stimulation of astrocytic Gq-GPCRs was sufficient to drive gliotransmission through release of Ca2+ from IP3 receptor-dependent internal stores (Parpura et al., 1994; Jeftinija et al., 1996; Pasti et al., 2001; Montana et al., 2006). However, in intact brain tissue, the application of agonists with the intent to stimulate astrocytic receptors directly stimulates these same receptors on neurons and other glia. Continual dialysis of astrocytes with BAPTA dilutes basal Ca2+ levels as well as other signaling molecules within the cell, making it nonspecific while also potentially buffering extracellular Ca2+ required for synaptic transmission. Overall, the methods used to study gliotransmission have either been nonselective (e.g., activation of endogenous Gq-GPCRs) or nonphysiological and wrought with potential artifacts (e.g., uncaging or chelating Ca2+). Development and use of genetic approaches to study gliotransmission were transformative by ensuring selectivity of astrocyte stimulation in intact tissue while more closely replicating astrocyte Ca2+ release mechanisms.
Transgenic expression of a Gq-GPCR only in astrocytes and the concept of astrocytic “calcium codes” necessary for gliotransmission
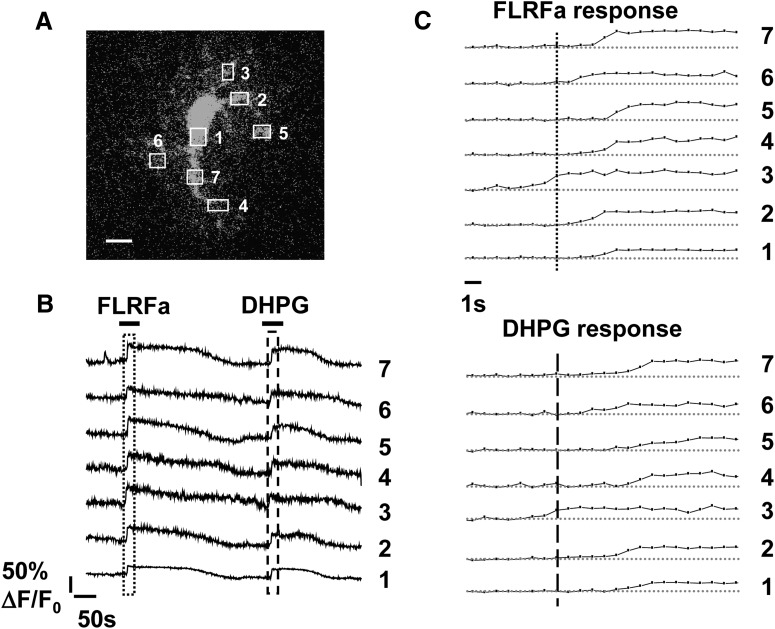
Out of concern for limitations imposed by the available techniques used to stimulate astrocytes, a transgenic line of mice was developed to stimulate Ca2+ elevations only in astrocytes (to provide selectivity) and in a GPCR-dependent manner (to replicate endogenous astrocyte Ca2+ release mechanisms). This was achieved by driving expression of an endogenous Gq-GPCR to astrocytes that is not normally expressed in forebrain, and whose ligand cannot activate other forebrain GPCRs (Fiacco et al., 2007). The Gq-GPCR, Mas-related gene receptor A1 (MrgA1R), is endogenously expressed by nociceptive sensory terminals in the spinal cord but is not expressed in brain. Mice expressing the MrgA1R in astrocytes are phenotypically normal, breed well, and have normal lifespans, presumably because the receptor is expressed but never activated by endogenously released neurotransmitters. Approximately 90% of astrocytes express MrgA1R (Fiacco et al., 2007). The spatial and temporal pattern of Ca2+ activity evoked by MrgA1R stimulation was found to closely resemble the pattern of Ca2+ evoked by mGluR agonists in the same astrocytes (Fiacco et al., 2007), suggesting that the MrgA1R uses the same intracellular machinery as native astrocytic Gq-GPCRs, and does not interfere with Ca2+ elevations mediated by native astrocytic Gq-GPCRs (Fiacco et al., 2007). Importantly, MrgA1R Ca2+ elevations universally initiated in astrocyte processes (as opposed to the soma) and propagated into the entire visible extent of the astrocyte, including the fine processes (Fig. 1; Movie 1) (Fiacco et al., 2007). Among the hippocampal astrocyte population, MrgA1R Ca2+ elevations were highly synchronous, making it easy to correlate astrocyte Ca2+ to any possible changes in neuronal activity. In contrast to findings observed in earlier studies of gliotransmission, which recorded Ca2+ elevations in the astrocyte soma only (Parri et al., 2001; Bowser and Khakh, 2004; Fellin et al., 2004; Perea and Araque, 2005; Serrano et al., 2006; D'Ascenzo et al., 2007), MrgA1R-evoked astrocyte Ca2+ elevations did not alter in any way neuronal excitatory synaptic activity or short- or long-term synaptic plasticity (Fiacco et al., 2007; Agulhon et al., 2010).
Figure 1.
Stimulation of astrocytic MrgA1 receptors produces a widespread Ca2+ elevation that propagates throughout the cell, including the fine processes and with a pattern similar to a native Gq-GPCR. A, Numbered regions of interest over astrocytic compartments correspond to the fluorescence over time measurements recorded in the same regions (B, C). Increases in fluorescence indicate astrocyte Ca2+ elevations. B, Application of the MrgA1 receptor agonist FLRFa produced a Ca2+ response very similar to the one produced by application of the endogenous Group I mGluR agonist DHPG. Hatched boxes represent regions of expanded timescale shown in C. Stimulation of astrocytic MrgA1Rs evoked a Ca2+ elevation that initiated in a process and then propagated throughout the visible extent of the astrocyte, including the fine processes (see Movie 1 for this cell, where it is clearly evident that the Ca2+ elevation enters the fine astrocyte compartments, even though fluorescence intensity was recorded only in a small number of regions of interest). C, The Ca2+ elevation evoked by MrgA1R stimulation (top) produced a pattern nearly identical to that produced by DHPG application (bottom). Scale bars, 10 μm. Reprinted with permission from Elsevier Limited, copyright 2007.
MrgA1-evoked astrocyte Ca2+ elevation propagates throughout all visible astrocytic compartments, including the fine processes with a similar pattern as DHPG. This movie shows the same astrocyte as in Figure 1. The first Ca2+ elevation occurs in response to application of the MrgA1 agonist FLRFa to activate astrocytic MrgA1 receptors, followed by application of 20 μm DHPG to stimulate Group I mGluRs. The Ca2+ elevation in response to stimulation of MrgA1Rs propagates throughout the entire visible astrocyte, including the fine processes. The pattern of the response, including initiation in an astrocyte process and propagation into the small compartments, is very similar to the pattern evoked by stimulation of Group I mGluRs. The movie shows 30 frames per second, which is sped up ∼40×. Actual acquisition speed is one frame per 1.3 s.
The MrgA1R agonist concentration used by Fiacco et al. (2007) (10 μm) produced very strong and sustained astrocytic Ca2+ elevations. It has since been suggested that these Ca2+ elevations are the wrong “code” for gliotransmission (Araque et al., 2014). This assertion seems unlikely for a number of reasons. First, as has already been pointed out, the Ca2+ elevations evoked by MrgA1R stimulation propagated into all visible astrocytic compartments where the putative diffuse, more scattered astrocytic vesicles have been suggested to reside (Bezzi et al., 2004; Crippa et al., 2006; Santello et al., 2011). Second, vesicular exocytosis of neurotransmitter increases as a function of Ca2+ concentration. In fundamental work, Llinás (1977) demonstrated that the amplitude of the postsynaptic potential increases stepwise with the amount of presynaptic Ca2+ influx. The strong Ca2+ elevations evoked by MrgA1R stimulation seem well suited to propagate into the small astrocytic compartments to induce exocytosis. Santello et al. (2011) discussed the importance of stronger and spatially larger synchronous astrocyte Ca2+ elevations for exocytosis of astrocytic glutamate in sufficient quantity to be detected by adjacent neurons. Third, approaches used to elevate astrocyte Ca2+ in reports of gliotransmission, including bath application of agonists to native astrocytic GPCRs, uncaging Ca2+ or IP3, optogenetic stimulation, mechanical stimulation, and strong astrocytic depolarization via whole-cell voltage-clamp (Araque et al., 1999b; Bezzi and Volterra, 2001; Fiacco and McCarthy, 2004; Perea and Araque, 2007; Perea et al., 2014) do not produce universally local or subtle Ca2+ responses, suggesting that gliotransmission is not a finicky process dependent on delicate temporal or spatial requirements as is now being suggested (Araque et al., 2014; Sherwood et al., 2017). Fourth, Araque et al. (2014) referred to previous work in cultured astroglia where long-lasting astrocyte Ca2+ increases (similar to those generated by MrgA1R stimulation) were observed to trigger a solitary episode of gliotransmitter release at the onset of the Ca2+ increase (Pasti et al., 2001). However, this finding in itself refutes the idea that subtle and discrete changes in Ca2+ are required for gliotransmission: The long-lasting Ca2+ increases evoked by Pasti et al. (2001) did indeed result in gliotransmission (in vitro). Indeed, the sustained astroglial Ca2+ elevations evoked in those experiments released enough glutamate from astroglia to produce massive, 30 s duration Ca2+ elevations in cocultured sniffer cells. Such responses would not have been missed by Fiacco et al. (2007) who, in addition to performing continuous recordings of EPSCs in CA1 pyramidal neurons, also recorded neuronal Ca2+ activity. At no time during those recordings, including the onset and rising phase of the fast, widely synchronous astrocyte Ca2+ elevations, was there any change in neuronal activity or any effect on neuronal Ca2+ (Fiacco et al., 2007). It is important to note that, in the same study where MrgA1 stimulation failed to elicit gliotransmission, the nonphysiological approach of uncaging IP3 in astrocytes increased EPSC frequency in line with a previous study (Fiacco and McCarthy, 2004); similar findings have also been reported by Nedergaard and colleagues (Wang et al., 2013). Overall, the findings using the MrgA1 transgenic mice demonstrate that when astrocytic Gq-GPCR signaling cascades are selectively stimulated, the resultant fast and synchronous astrocytic Ca2+ elevations that propagate throughout the fine processes of astrocytes do not produce gliotransmission. Importantly, stimulation of native astrocytic Gq-GPCRs that are enriched in astrocytes (the endothelin receptors) also increases astrocyte Ca2+ but produces no effect on neuronal synaptic transmission or short- or long-term plasticity (Fiacco et al., 2007; Agulhon et al., 2010). The data argue against a subtle Ca2+ code required to evoke gliotransmission, but rather just the opposite: A variety of very nonphysiological methods that evoke increases in astrocyte Ca2+ led to gliotransmission.
Significant attenuation of both local and large scale spontaneous and evoked astrocyte calcium elevations in IP3R2-deficient mice produces no effect on synaptic transmission, plasticity, or behavior
Stimulation of astrocytic Gq-GPCRs activates a phospholipase C signaling pathway leading to cytosolic Ca2+ elevations resulting from Ca2+ release from IP3 receptor-sensitive intracellular stores (Falkenburger et al., 2010; Gresset et al., 2012). Pharmacological inhibition of IP3 receptors or emptying Ca2+ stores was found to block exocytotic release of glutamate from cultured astroglia and prevent gliotransmission (Araque et al., 1998a, b; Montana et al., 2006). Based on strong immunohistochemical evidence suggesting that astrocytes exclusively express the IP3 receptor Type 2 (IP3R2) isoform (Sharp et al., 1999; Holtzclaw et al., 2002; Hertle and Yeckel, 2007), IP3R2 knock-out (IP3R2−/− KO) mice were tested to determine whether removal of physiological sources of astrocyte Ca2+ impaired gliotransmission (Petravicz et al., 2008). Petravicz et al. (2008) observed the following: (1) removal of IP3R2 prevented spontaneous and evoked Gq-GPCR-mediated astrocyte Ca2+ elevations; and (2) there was no effect of abolishing IP3R-sensitive sources of astrocyte Ca2+ on neuronal synaptic activity. A caveat of the work is that Ca2+ activity was not examined in the fine processes of IP3R2−/− astrocytes, leaving open the possibility that not all Ca2+ activity was completely abolished.
A comprehensive evaluation of evoked and spontaneous astrocyte Ca2+ activity in IP3R2−/− mice has since been provided by Srinivasan et al. (2015) and Agarwal et al. (2017) using genetically encoded Ca2+ indicators expressed in astrocytes in brain slices and in vivo. These studies have revealed that, although not completely abolished, a highly significant amount of spontaneous and evoked astrocyte Ca2+ activity is absent in IP3R2−/− mice, including the fast, local microdomain Ca2+ activity suggested to be essential for gliotransmission (Araque et al., 2014). Srinivasan et al. (2015) observed that all spontaneous Ca2+ activity, and Ca2+ elevations evoked by agonist application or startle are absent in the astrocyte soma in IP3R2−/− mice, along with ∼60% of the local, spontaneous Ca2+ transients occurring in the fine processes. Fast, startle-evoked astrocyte Ca2+ elevations were also abolished in the fine processes of IP3R2−/− mice, leaving behind a very slow, nondecaying shift in baseline Ca2+. Agarwal et al. (2017) obtained similar results as Srinivasan et al. (2015) with regard to Ca2+ activity abolished in the IP3R2−/− mice. The number of spontaneously active microdomains was reduced by 65% (in slices) and 64% (in vivo), and the number of events remaining in each domain was significantly attenuated in both preparations. Use of a membrane-tethered GCaMP3 in astrocytes biased reporting of astrocyte Ca2+ activity to the finest compartments, indicating that IP3R2 KO significantly abolished Ca2+ activity exhibiting the spatiotemporal dynamics recently suggested to be important for gliotransmission (Araque et al., 2014). As to evoked responses, application of the purinergic receptor agonist ATP, considered a key neurotransmitter/gliotransmitter regulating astrocyte Ca2+ activity and gliotransmission, did not evoke any astrocyte Ca2+ increases, consistent with loss of IP3 receptor-dependent Ca2+ release mechanisms. Norepinephrine was still able to elicit a small increase in astrocyte microdomain activity. Locomotion-induced norepinephrine release in vivo evoked on average 125 microdomain Ca2+ events from a baseline of 25 events in mice expressing IP3R2, but only 9 microdomain Ca2+ events from a baseline of 4 in IP3R2−/− mice (Agarwal et al., 2017). The conclusion that can be made from these data is that, although Ca2+ activity persists in IP3R2−/− mice, it is markedly and significantly attenuated.
If Ca2+-dependent gliotransmission was an essential physiological process, the dramatic loss of astrocyte Ca2+ in IP3R2−/− mice would be expected to produce an equally dramatic impairment of synaptic function and animal behavior compared with control animals producing >60% more local spontaneous Ca2+ transients and much faster, more synchronized Ca2+ elevations in response to locomotor activity, sensory input, or startle. However, IP3R2−/− mice are healthy, viable, breed well, and live normal lifespans with no overt behavioral abnormalities (Petravicz et al., 2008, 2014). The significant loss of IP3R2-dependent Ca2+ signaling in astrocytes throughout the brain failed to affect tests of learning and memory, motor or sensory control, or measurements of anxiety and depression (Petravicz et al., 2014). Moreover, no differences were observed in evoked excitatory synaptic activity, post-tetanic potentiation (PTP)/LTP, or neurovascular coupling compared with recordings in littermate control animals (Petravicz et al., 2008; Agulhon et al., 2010; Bonder and McCarthy, 2014). The fact that these mice do not exhibit a behavioral or electrophysiological phenotype is stunning given the large number of reports on astrocytic GPCR-dependent Ca2+ modulation of synaptic transmission and plasticity, as well as functional hyperemia (discussed further below). Overall, the data show that removal of IP3R2, the predominant source of physiological Ca2+ elevations in astrocytes, has no effect on neuronal activity and animal behavior. These findings provide strong evidence that astrocytes do not release gliotransmitters in a Ca2+-dependent manner to actively control neuronal activity, synaptic transmission, and animal behavior. The most logical conclusion that can be made from these data is that gliotransmission does not occur in intact brain tissue under physiological conditions.
Neuronal receptors or astrocytic receptors?
One advantage provided by the MrgA1 transgenic approach is that stimulation of the receptors by bath-applied agonists is astrocyte selective. Because stimulation of astrocytic MrgA1 Gq-GPCRs did not result in gliotransmission (Fiacco et al., 2007; Agulhon et al., 2010), questions were raised as to differences between this approach versus those in which endogenous astrocytic receptors are stimulated. There are two competing hypotheses: The first is that the MrgA1 Ca2+ elevations are incapable of inducing gliotransmission. This is very improbable as discussed in the previous section: just about every report on gliotransmission uses approaches that produce universally nonphysiological increases in astrocyte Ca2+, whereas MrgA1 stimulation induces physiologically relevant astrocyte Ca2+ increases. The competing hypothesis is that agonists applied with the intent to stimulate native astrocytic receptors directly stimulate receptors on excitatory neurons, inhibitory neurons, or other glia. The most commonly used agonists to stimulate astrocytic Ca2+ elevations, the mGluR agonists (±)-1-Aminocyclopentane-trans-1,3-dicarboxylic acid (t-ACPD) and 3,5-dihydroxyphenylglycine (DHPG), directly depolarize neurons, increase their firing rates, potentiate NMDA receptor currents, and depotentiate synaptic responses following LTP (Mannaioni et al., 2001; Heidinger et al., 2002; Zho et al., 2002; Rae and Irving, 2004). It would be difficult, if not impossible, to disentangle these direct effects from putative astrocyte-mediated ones (Fiacco et al., 2007; Agulhon et al., 2010).
In support of the hypothesis that direct stimulation of neurons has been misinterpreted as gliotransmission has come from observations made from stimulation of endogenous endothelin receptors. Evidence to date suggests that endothelin receptors are enriched in astrocytes and minimally expressed by neurons (Andersson et al., 2007; Zhang et al., 2014). Recent work has further indicated that stimulation of astrocytic endothelin receptors produces local Ca2+ elevations in the fine astrocyte processes in IP3R2−/− mice, even in the absence of soma responses (Srinivasan et al., 2015), thereby displaying the spatiotemporal dynamics recently suggested to be important for gliotransmission (Araque et al., 2014; Sherwood et al., 2017). However, stimulation of endogenous astrocytic endothelin receptors produces no effect on neuronal excitatory EPSCs, evoked EPSCs, protein tyrosine phosphatase, or LTP in the same recordings in which the Group I mGluR agonist DHPG affects these measurements (Fiacco et al., 2007; Agulhon et al., 2010). These observations lend further support to the idea that when GPCRs are selectively stimulated in astrocytes, whether the receptors are endogenously or transgenically expressed, the resultant Ca2+ elevations do not produce gliotransmission.
Intercellularly propagating astrocyte Ca2+ waves that would provide compelling support for Ca2+-dependent gliotransmission are absent in healthy forebrain
One of the most convincing demonstrations of Ca2+-dependent gliotransmitter release was provided by the Ca2+ waves that propagate between cultured astroglia (Cornell-Bell et al., 1990; Cornell-Bell and Finkbeiner, 1991). Poking a single astroglial cell in culture initiates a Ca2+ wave that propagates among hundreds of astroglia. The mechanism behind propagating Ca2+ waves is well defined. It requires IP3 receptors (Boitano et al., 1992; Charles et al., 1993) and involves Ca2+-dependent release of ATP and stimulation of purinergic P2Y receptors on adjacent astroglia (Hassinger et al., 1996; Guthrie et al., 1999; Fam et al., 2000). Belonging to the family of Gq-GPCRs, P2YR stimulation results in activation of the PLC signaling cascade, production of IP3, and release of Ca2+ from internal stores. The store-liberated Ca2+ triggers release of the gliotransmitter ATP to stimulate P2YRs on adjacent astroglia to continue propagating the Ca2+ wave. Widespread astroglial Ca2+ waves were cited as strong evidence for gliotransmission (Araque et al., 1999b; Bezzi and Volterra, 2001; Gallagher and Salter, 2003).
While easily observed and well defined in culture, there is very little evidence for propagating Ca2+ waves between forebrain astrocytes in intact brain slices or in vivo. In response to afferent stimulation, sensory stimulation, or startle in vivo, are synchronous Ca2+ elevations involving many astrocytes simultaneously, not Ca2+ initiated in a single astrocyte that then propagates into adjacent astrocytes. This is an important distinction not always made clear in many studies, which often refer to these responses as “Ca2+ waves” due to their wave-like appearance. When stimuli are confined to a single hippocampal astrocyte (e.g., as opposed to puff application of a high concentration of agonist extracellularly), Ca2+ elevations do not propagate into adjacent astrocytes despite propagating throughout the entire stimulated cell (Fiacco and McCarthy, 2004). There is one noteworthy exception to lack of evidence for astrocyte Ca2+ waves, from studies in the hindbrain. In the intact cerebellum, Nimmerjahn et al. (2009) and Hoogland et al. (2009) observed radially expanding wave-like Ca2+ activity that propagated between Bergmann glia, a specialized astrocyte subtype. These Ca2+ waves depended on Ca2+ release from internal stores, and wave propagation was blocked by P2Y purinergic receptor antagonists (Hoogland et al., 2009). Similar Ca2+ waves, however, have not been reported in other brain areas.
Similar to observations made in cultured astroglia, propagating Ca2+ waves can also occur between reactive astrocytes in diseased tissue. Kuchibhotla et al. (2009) found Ca2+ waves propagating between astrocytes in Alzheimer's disease mice, but not in the tissue sections from control mice. Reactive astrocytes in diseased or damaged tissue are very different compared with healthy astrocytes, altering their expression profile of many genes (Zamanian et al., 2012). In an environment that includes secretion of inflammatory mediators, such as TNFα and prostaglandins, reactive astrocytes in many brain areas may become competent for gliotransmission (Bezzi et al., 1998; Domercq et al., 2006; Santello et al., 2011; Agulhon et al., 2012; Habbas et al., 2015). This may also explain why gliotransmission is clearly observed in vitro, as cultured astroglia represent an immature or reactive phenotype (Cahoy et al., 2008; Hamby et al., 2012) that express key vesicular proteins (Wilhelm et al., 2004). Unlike cultured astroglia, evidence suggests that astrocytes in healthy or intact tissue do not express Ca2+-sensitive vesicular release machinery for the commonly described gliotransmitters (Li et al., 2013; Zhang et al., 2014; Chai et al., 2017). This provides an explanation for why, in cultured astroglia, GPCR-linked Ca2+ elevations consistently result in gliotransmission, whereas those in astrocytes in intact tissue do not. In summary, the absence of propagating intercellular Ca2+ waves between forebrain astrocytes in situ and in vivo provides strong evidence that forebrain astrocytes do not release the gliotransmitter ATP or participate in gliotransmission.
Recent studies on functional hyperemia challenge the validity of the tools used to demonstrate gliotransmission
Functional hyperemia refers to the process whereby increases in neuronal activity lead to regionally restricted increases in blood flow (Vargová et al., 2001). Although functional hyperemia is not gliotransmission per se, the proposed involvement of astrocytes, astrocytic GPCRs, and Ca2+ is similar, and results vary greatly depending on use of pharmacological versus genetic approaches to stimulate astrocyte Ca2+ elevations. Investigators in this area have long thought that astrocytes play a role in functional hyperemia given that astrocyte processes cover >99% of brain vascular elements (Prokopová-Kubinová et al., 2001; Mathiisen et al., 2010). Findings from a large number of studies using acutely isolated brain slices demonstrated that manipulating astrocyte Ca2+ using a variety of pharmacological approaches affects arteriole diameter (Woerly et al., 1998; Syková et al., 2000; Zonta et al., 2003; Mulligan and MacVicar, 2004; Metea and Newman, 2006; Straub et al., 2006; Gordon et al., 2008; He et al., 2012). These findings led to the generally accepted hypothesis that neurovascular coupling results from neuronal activation of astrocytic GPCRs, IP3-mediated astrocyte Ca2+ elevations, and release of vasoactive molecules from the astrocyte to regulate arteriole diameter and blood flow.
While studies using pharmacological approaches in brain slices generally support this hypothesis, a number of in vivo studies do not (Prokopová et al., 1997; Nizar et al., 2013; Bonder and McCarthy, 2014). Bonder and McCarthy (2014) used in vivo imaging and selective genetic approaches to determine whether either increasing or inhibiting Ca2+ activity in astrocyte endfeet that wrap arterioles in the visual cortex affected visually stimulated functional hyperemia: the answer was no. In one set of experiments, IP3R2 KO mice were used to significantly reduce GPCR-mediated Ca2+ increases selectively in astrocytes; functional hyperemia was unaffected in these mice. In a second set of experiments, mice that express an engineered Gq-GPCR (Gq-designer receptor exclusively activated by designer drug [DREADD]) (Nichols and Roth, 2009) selectively in astrocytes were used to assess the role of GPCR-mediated Ca2+ increases in functional hyperemia. The activation of Gq-DREADD led to Ca2+ increases in astrocyte endfeet-wrapping arterioles but failed to affect arteriole diameter or blood flow (Bonder and McCarthy, 2014). It should be noted that increasing astrocyte Ca2+ through Gq-DREADD is far more physiological than methods typically used to study functional hyperemia, such as uncaging Ca2+ or IP3. A recent study out of Eric Newman's laboratory demonstrated that astrocytes modulate capillary, but not arteriole diameter, when using more physiological methods to stimulate astrocyte Ca2+ (Biesecker et al., 2016). Overall, similar to data on gliotransmission, studies in this area demonstrate that, although it is possible to use nonphysiological tools to drive astrocyte modulation of arteriole blood flow, this does not appear to occur when more physiological approaches are used.
Gliotransmitter release and use of dn-SNARE transgenic mice
Early work suggested that the Ca2+-dependent glutamate release observed in cultured astroglia occurred via SNARE-dependent vesicular exocytosis (Araque et al., 2000; Montana et al., 2004; Zhang et al., 2004). These observations provided the rationale for the generation of a transgenic mouse line expressing a dominant-negative (dn) mutation of synaptobrevin 2 designed to block SNARE-dependent vesicular release from astrocytes (Pascual et al., 2005); these mice are referred to as dn-SNARE mice. In the making of dn-SNARE mice, the dn-SNARE was not directly tagged with a reporter construct. Therefore, to determine the cellular expression of dn-SNARE in vivo, two additional reporter constructs were coinjected with dn-SNARE into fertilized eggs before implantation into developing blasts. The advantage of this approach is that the binding of dn-SNARE to the SNARE complex would not be affected by directly tagging it with a reporter. The disadvantage is that it is not possible to directly visualize dn-SNARE. Although this method was not uncommon at the time, it is rarely used today due to difficulties in directly tracking the expression of the transgene. dn-SNARE mice have been used by a large number of investigators to demonstrate that expression of dn-SNARE in astrocytes interferes with synaptic transmission and plasticity.
Over the past several years, a number of issues have been raised that question the validity of using dn-SNARE mice to demonstrate gliotransmission. First, Maiken Nedergaard's group reported that the dn-SNARE peptide was widely expressed in neurons (although at low levels), thereby directly inhibiting neuronal vesicular exocytosis (Fujita et al., 2014). Second, in the case of dn-SNARE, there is an absence of published evidence (although unpublished data very likely exist) that expression of dn-SNARE blocks glutamate release from cultured astroglia. This is surprising because evidence suggests that cultured astroglia express the necessary SNARE components to perform vesicular-dependent gliotransmitter exocytosis (Wilhelm et al., 2004). Lack of evidence that dn-SNARE inhibits glutamate release from purified astroglia is somewhat alarming and raises the specter that actions of dn-SNARE in intact tissue are not due to its action in astrocytes, as recently reported (Fujita et al., 2014). Third, even if the dn-SNARE peptide were only expressed in astrocytes, it could be exerting its effects by interfering with trafficking of membrane proteins (such as glutamate transporters) to influence synaptic transmission (Ropert et al., 2016). This possibility is supported by a recent transcriptome analysis indicating that striatal and hippocampal astrocytes express membrane traffic-related genes but show little evidence for minimal requirements for Ca2+-dependent glutamate exocytosis (Chai et al., 2017).
Recent data using newer technologies indicate that d-serine is not a gliotransmitter
The three molecules most widely cited to be gliotransmitters are glutamate, ATP, and d-serine (Perea et al., 2009; Araque et al., 2014; Hollborn et al., 2015). It is difficult to selectively manipulate cellular levels of glutamate or ATP due to their role in metabolic pathways. However, this is not the case for d-serine, whose role is largely restricted to that of a required coagonist at NMDA receptors (Traynelis et al., 2010; Mothet et al., 2000). The synthesis of d-serine requires serine racemase (SR) to convert l-serine into d-serine. A large number of studies have reported that the release of d-serine as a gliotransmitter from astrocytes plays a necessary role in synaptic plasticity (Yang et al., 2003; Oliet and Mothet, 2006; Panatier et al., 2006; Oliet and Mothet, 2009; Henneberger et al., 2010; Berk et al., 2015; Pankratov and Lalo, 2015; Sherwood et al., 2017). The rationale for thinking that d-serine might serve as a gliotransmitter largely stemmed from early reports indicating that d-serine and SR were localized to astrocytes (Schell et al., 1995; Wolosker et al., 1999; Berk et al., 2015), and that d-serine was released from cultured astroglia via a Ca2+-dependent mechanism (Yang et al., 2003; Mothet et al., 2005; Zhuang et al., 2010). A series of recent studies using more advanced technologies clearly demonstrate that neurons, rather than astrocytes, synthesize and release d-serine and that it is the release of d-serine from neurons, not astrocytes, that acts as a coagonist with glutamate to regulate synaptic plasticity (Wolosker et al., 2016). The advance in this area has stemmed largely from the development of SR KO (Miya et al., 2008) and conditional KO (cKO) (Benneyworth et al., 2012) mice. These mice enable the development of highly specific immunocytochemical localization of SR and d-serine as well as electrophysiological and behavioral experiments where SR has been selectively deleted from either neurons or astrocytes. The primary findings clearly demonstrating that d-serine is not a gliotransmitter include the following: (1) using SR−/− mice as well as improved antibodies to define cellular specificity, SR protein (Kartvelishvily et al., 2006; Miya et al., 2008) and mRNA (Yoshikawa et al., 2007) are localized in vivo to neurons, not astrocytes; similar neuronal localization of d-serine and SR has been reported in human brain (Voigt et al., 2015); (2) using the SR transcriptional unit to drive GFP expression in mice leads to the exclusive expression of GFP in neurons, not astrocytes (Kartvelishvily et al., 2006); (3) a cKO of neuronal SR, but not astrocytic SR, significantly reduced LTP at the Schaffer collateral-CA1 synapse (Benneyworth et al., 2012); and (4) a cKO of neuronal SR, but not astrocytic SR, decreased dendritic spine complexity, an indicator of neuronal plasticity (Flo et al., 2004). These findings clearly demonstrate that neuronal d-serine is the NMDA receptor coagonist that participates in synaptic plasticity. It is worth noting that all of the experimental approaches being used to argue that glutamate and/or ATP are gliotransmitters are used to argue that d-serine is a gliotransmitter, including the following: (1) d/n SNARE mice; (2) toxins blocking vesicular release; (3) increasing astrocyte Ca2+; (4) buffering intracellular Ca2+; (5) decreasing extracellular Ca2+; (6) blocking release from intracellular stores; (7) blocking vesicular ATPase; and (8) isolation of synaptic vesicles containing gliotransmitters. Given the strong evidence that neurons, not astrocytes, make and release d-serine, it is again worth questioning the methods that continue to be used to stimulate Ca2+ and gliotransmission from astrocytes. The d-serine saga appears to be another example where glial biologists reported evidence for gliotransmission that was strongly invalidated with improved technology.
Gliotransmission, or regulation of astrocyte transporters, metabolic activity, gap junction proteins, or ion channels?
Astrocytes modulate neuronal function in a number of ways, including secretion of factors regulating synaptogenesis and pruning during development (Ullian et al., 2004; Chia et al., 2011; Chung et al., 2015), uptake and buffering of extracellular potassium (Djukic et al., 2007; Sanz et al., 2009), regulation of gap junctional coupling or metabolic activity (Hertz et al., 1999; Rouach et al., 2008; Wagnerova et al., 2009; Brown and Ransom, 2015), and intracellular transport of glutamate during synaptic transmission (Tanaka et al., 1997; Bergles et al., 1999). It is becoming increasingly evident that many of these astrocyte functions can be modified directly or through activation of astrocytic GPCR-signaling pathways in an activity-dependent manner. For example, astrocytic Gq-GPCRs, Ca2+, and protein kinase C acutely regulate glutamate and potassium uptake (Wang et al., 2012; Devaraju et al., 2013). Alterations in K+ uptake can modify neuronal excitability in an extracellular K+ concentration-dependent manner (Wang et al., 2012) and reduce synaptic responses to repetitive stimulation and post-tetanic potentiation (Sibille et al., 2014). Armbruster et al. (2016) found slowing of glutamate uptake following bursts of neuronal activity ≥30 Hz and that these changes affected the neuronal response to released glutamate on an acute timescale. Murphy-Royal et al. (2015) demonstrated that neuronal activity-dependent surface diffusion of the astrocyte glutamate transporter GLT-1 (EAAT2) can shape subsequent synaptic transmission. Activity-dependent regulation of astrocytic connexin 43 can allow intercellular trafficking of glucose and its metabolites through astroglial networks (Rouach et al., 2008) and modulate network “up” states and neuronal firing rates (Roux et al., 2015). Astrocyte Ca2+ microdomains driven by synergistic interaction between IP3R2 and mitochondria may facilitate ATP production by enhancing glycogenolysis in an activity-dependent manner (Agarwal et al., 2017).
Methods used with the intent of stimulating astrocyte Ca2+ elevations can also affect astrocyte functions. Strong astrocytic depolarization using a patch pipette or optogenetic stimulation of astrocytic ion channels can lead to changes in ionic driving forces with subsequent reduction or even reversal of transporter activity, producing effects on excitability of adjacent neurons independent of gliotransmission. Intracellular Na+ accumulation in astrocytes generated by glutamate uptake (or possibly as a result of stimulation of channelrhodopsins) has been proposed as an energy currency and mediator of metabolic signals in neuron-glia interactions (Chatton et al., 2016). In summary, there are many mechanisms through which astrocytes modulate neurons in an activity-dependent manner through regulation of astrocyte transporters, metabolic activity, gap junction proteins, or ion channels. It will be important in future studies to carefully consider alternative possibilities, such as these, before settling on gliotransmission as the mechanism by which astrocytes modulate neuronal activity. Discovery of valuable new information about the role of astrocytes in brain function may otherwise be overlooked.
A recent study on astrocyte heterogeneity finds no evidence to support Ca2+-dependent glutamate exocytosis from astrocytes
Recently, the Bal Khakh laboratory at the University of California–Los Angeles comprehensively evaluated the transcriptomic, proteomic, morphological, and functional profiles of striatal and hippocampal astrocytes (Chai et al., 2017). Neither striatal nor hippocampal astrocytes expressed significant RNA for vesicular glutamate transporters or Ca2+-sensitive synaptotagmins. Furthermore, although vesicles were readily observed in 138 striatal and 139 hippocampal synapses, no astrocyte processes contained structures akin to neurotransmitter vesicles at the same synapses. Stimulation of the Gq-GPCR hM3D DREADD always increased astrocyte Ca2+ levels but resulted in no change in signal of the coexpressed glutamate sensor iGluSnFR, whereas the positive controls of exogenous glutamate, electrical field stimulation, and inhibition of astrocyte glutamate uptake all resulted in significant glutamate detection by iGluSnFR. Stimulation of hM3D DREADD also failed to evoke NMDA receptor-dependent slow inward currents in striatal medium spiny neurons or hipoocampal pyramidal neurons. In summary, although considerable differences between the two astrocyte subtypes were found, neither subtype was capable of GPCR- or Ca2+-dependent release of glutamate (Chai et al., 2017). This recent study provides further strong evidence that astrocytes do not participate in gliotransmission.
In conclusion, there is little doubt that neuronal activity and the consequent activation of astrocytic GPCRs affect processes important for maintaining and modulating normal brain function. However, evidence that neuronal activity leads to Ca2+-dependent gliotransmitter release in intact brain tissue to actively control neuronal plasticity and synaptic transmission is being challenged by the findings from many laboratories using advanced technologies. Absence of propagating Ca2+ waves between forebrain astrocytes provides evidence against astrocytic release of the gliotransmitter ATP in sufficient quantity to affect activity of adjacent neurons or astrocytes. Recent evidence convincingly demonstrating neuronal synthesis and release of d-serine has led to the demise of d-serine as a gliotransmitter (Wolosker et al., 2016). Use of complementary genetic approaches that are specific to astrocytes and that recapitulate or inhibit endogenous activity-driven astrocyte Ca2+ release mechanisms provide strong evidence against gliotransmission. Specifically, stimulation of receptors selectively expressed or enriched in astrocytes results in IP3 receptor-dependent Ca2+ elevations exhibiting the spatiotemporal dynamics suggested to be important for gliotransmission, while producing no effect on neuronal synaptic transmission or plasticity. Removal of the predominant source of local microdomain and sensory- or startle-evoked astrocyte Ca2+ responses exhibiting the spatiotemporal dynamics recently suggested to be important for gliotransmission has no effect on synaptic transmission or plasticity, modulation of arteriole diameter, or behavior. Use of traditional pharmacological approaches to stimulate astrocyte Ca2+ elevations, most often recorded in the astrocyte soma even in recent publications supporting gliotransmission (e.g., Pankratov and Lalo, 2015; Martín et al., 2015), do not universally evoke astrocyte Ca2+ elevations displaying the delicate temporal or spatial requirements recently proposed to be essential for gliotransmission. On the contrary, gliotransmission can be caused to occur using a variety of very nonspecific, nonphysiological approaches to elicit astrocyte Ca2+ elevations. Together, the weight of the evidence strongly argues that, under physiological conditions, Ca2+-dependent release of neurotransmitters is the function of neurons, not astrocytes.
Footnotes
The authors declare no competing financial interests.
References
- Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles DE (2017) Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93:587–605.e7. 10.1016/j.neuron.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Fiacco TA, McCarthy KD (2010) Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327:1250–1254. 10.1126/science.1184821 [DOI] [PubMed] [Google Scholar]
- Agulhon C, Sun MY, Murphy T, Myers T, Lauderdale K, Fiacco TA (2012) Calcium signaling and gliotransmission in normal vs reactive astrocytes. Front Pharmacol 3:139. 10.3389/fphar.2012.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Blomstrand F, Hanse E (2007) Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol 585:843–852. 10.1113/jphysiol.2007.142737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1998a) Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci 10:2129–2142. 10.1046/j.1460-9568.1998.00221.x [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG (1998b) Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci 18:6822–6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG (1999a) Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol 77:699–706. 10.1139/y99-076 [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999b) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215. 10.1016/S0166-2236(98)01349-6 [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG (2000) SNARE protein-dependent glutamate release from astrocytes. J Neurosci 20:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG (2001) Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63:795–813. 10.1146/annurev.physiol.63.1.795 [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81:728–739. 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster M, Hanson E, Dulla CG (2016) Glutamate clearance is locally modulated by presynaptic neuronal activity in the cerebral cortex. J Neurosci 36:10404–10415. 10.1523/jneurosci.2066-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT (2012) Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol 32:613–624. 10.1007/s10571-012-9808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE (1999) Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol 9:293–298. 10.1016/S0959-4388(99)80043-9 [DOI] [PubMed] [Google Scholar]
- Berk BA, Vogler S, Pannicke T, Kuhrt H, Garcia TB, Wiedemann P, Reichenbach A, Seeger J, Bringmann A (2015) Brain-derived neurotrophic factor inhibits osmotic swelling of rat retinal glial (Muller) and bipolar cells by activation of basic fibroblast growth factor signaling. Neuroscience 295:175–186. 10.1016/j.neuroscience.2015.03.037 [DOI] [PubMed] [Google Scholar]
- Bezzi P, Volterra A (2001) A neuron-glia signalling network in the active brain. Curr Opin Neurobiol 11:387–394. 10.1016/S0959-4388(00)00223-3 [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A (1998) Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391:281–285. 10.1038/34651 [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinháuser C, Pilati E, Volterra A (2004) Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7:613–620. 10.1038/nn1246 [DOI] [PubMed] [Google Scholar]
- Biesecker KR, Srienc AI, Shimoda AM, Agarwal A, Bergles DE, Kofuji P, Newman EA (2016) Glial cell calcium signaling mediates capillary regulation of blood flow in the retina. J Neurosci 36:9435–9445. 10.1523/JNEUROSCI.1782-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano S, Dirksen ER, Sanderson MJ (1992) Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 258:292–295. 10.1126/science.1411526 [DOI] [PubMed] [Google Scholar]
- Bonder DE, McCarthy KD (2014) Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 34:13139–13150. 10.1523/JNEUROSCI.2591-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS (2004) ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci 24:8606–8620. 10.1523/JNEUROSCI.2660-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Ransom BR (2015) Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis 30:233–239. 10.1007/s11011-014-9588-2 [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, Coppola G, Khakh BS (2017) Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95:531–549.e9. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Dirksen ER, Merrill JE, Sanderson MJ (1993) Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia 7:134–145. 10.1002/glia.440070203 [DOI] [PubMed] [Google Scholar]
- Chatton JY, Magistretti PJ, Barros LF (2016) Sodium signaling and astrocyte energy metabolism. Glia 64:1667–1676. 10.1002/glia.22971 [DOI] [PubMed] [Google Scholar]
- Chia WJ, Dawe GS, Ong WY (2011) Expression and localization of the iron-siderophore binding protein lipocalin 2 in the normal rat brain and after kainate-induced excitotoxicity. Neurochem Int 59:591–599. 10.1016/j.neuint.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Chung WS, Allen NJ, Eroglu C (2015) Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7:a020370. 10.1101/cshperspect.a020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM (1991) Ca2+ waves in astrocytes. Cell Calcium 12:185–204. 10.1016/0143-4160(91)90020-F [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247:470–473. 10.1126/science.1967852 [DOI] [PubMed] [Google Scholar]
- Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmignoto G (2006) Synaptobrevin2-expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J Physiol 570:567–582. 10.1113/jphysiol.2005.094052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG (2007) mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A 104:1995–2000. 10.1073/pnas.0609408104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraju P, Sun MY, Myers TL, Lauderdale K, Fiacco TA (2013) Astrocytic group I mGluR-dependent potentiation of astrocytic glutamate and potassium uptake. J Neurophysiol 109:2404–2414. 10.1152/jn.00517.2012 [DOI] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD (2007) Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27:11354–11365. 10.1523/JNEUROSCI.0723-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P (2006) P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem 281:30684–30696. 10.1074/jbc.M606429200 [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Jensen JB, Dickson EJ, Suh BC, Hille B (2010) Phosphoinositides: lipid regulators of membrane proteins. J Physiol 588:3179–3185. 10.1113/jphysiol.2010.192153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW (2000) P2Y(1) purinoceptor-mediated Ca(2+) signaling and Ca(2+) wave propagation in dorsal spinal cord astrocytes. J Neurosci 20:2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G (2004) Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43:729–743. 10.1016/j.neuron.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD (2004) Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci 24:722–732. 10.1523/JNEUROSCI.2859-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD (2007) Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54:611–626. 10.1016/j.neuron.2007.04.032 [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921. 10.1038/nature03104 [DOI] [PubMed] [Google Scholar]
- Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, Takano T, Wang S, Nedergaard M (2014) Neuronal transgene expression in dominant-negative SNARE mice. J Neurosci 34:16594–16604. 10.1523/JNEUROSCI.2585-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CJ, Salter MW (2003) Differential properties of astrocyte calcium waves mediated by P2Y1 and P2Y2 receptors. J Neurosci 23:6728–6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA (2008) Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456:745–749. 10.1038/nature07525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresset A, Sondek J, Harden TK (2012) The phospholipase C isozymes and their regulation. Subcell Biochem 58:61–94. 10.1007/978-94-007-3012-0_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB (1999) ATP released from astrocytes mediates glial calcium waves. J Neurosci 19:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, Klaus FR, Kollias G, Fontana A, Pryce CR, Suter T, Volterra A (2015) Neuroinflammatory TNFalpha impairs memory via astrocyte signaling. Cell 163:1730–1741. 10.1016/j.cell.2015.11.023 [DOI] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV (2012) Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci 32:14489–14510. 10.1523/JNEUROSCI.1256-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB (1996) An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci U S A 93:13268–13273. 10.1073/pnas.93.23.13268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Linden DJ, Sapirstein A (2012) Astrocyte inositol triphosphate receptor type 2 and cytosolic phospholipase A2 alpha regulate arteriole responses in mouse neocortical brain slices. PLoS One 7:e42194. 10.1371/journal.pone.0042194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, Behrens MM (2002) Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J Neurosci 22:5452–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA (2010) Long-term potentiation depends on release of d-serine from astrocytes. Nature 463:232–236. 10.1038/nature08673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle DN, Yeckel MF (2007) Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience 150:625–638. 10.1016/j.neuroscience.2007.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR (1999) Astrocytes: glutamate producers for neurons. J Neurosci Res 57:417–428. [DOI] [PubMed] [Google Scholar]
- Hollborn M, Vogler S, Reichenbach A, Wiedemann P, Bringmann A, Kohen L (2015) Regulation of the hyperosmotic induction of aquaporin 5 and VEGF in retinal pigment epithelial cells: involvement of NFAT5. Mol Vis 21:360–377. [PMC free article] [PubMed] [Google Scholar]
- Holtzclaw LA, Pandhit S, Bare DJ, Mignery GA, Russell JT (2002) Astrocytes in adult rat brain express type 2 inositol 1,4,5-trisphosphate receptors. Glia 39:69–84. 10.1002/glia.10085 [DOI] [PubMed] [Google Scholar]
- Hoogland TM, Kuhn B, Göbel W, Huang W, Nakai J, Helmchen F, Flint J, Wang SS (2009) Radially expanding transglial calcium waves in the intact cerebellum. Proc Natl Acad Sci U S A 106:3496–3501. 10.1073/pnas.0809269106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV, Stefanovic G, Liu F (1996) Neuroligand-evoked calcium-dependent release of excitatory amino acids from cultured astrocytes. J Neurochem 66:676–684. 10.1046/j.1471-4159.1996.66020676.x [DOI] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H (2006) Neuron-derived d-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem 281:14151–14162. 10.1074/jbc.M512927200 [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ (2009) Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323:1211–1215. 10.1126/science.1169096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hérault K, Silm K, Evrard A, Wojcik S, Oheim M, Herzog E, Ropert N (2013) Lack of evidence for vesicular glutamate transporter expression in mouse astrocytes. J Neurosci 33:4434–4455. 10.1523/JNEUROSCI.3667-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR. (1977) Depolarization-release coupling systems in neurons. Neurosci Res Program Bull 15:555–687. [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ (2001) Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci 21:5925–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R, Bajo-Grañeras R, Moratalla R, Perea G, Araque A (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–734. 10.1126/science.aaa7945 [DOI] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103. 10.1002/glia.20990 [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA (2006) Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26:2862–2870. 10.1523/JNEUROSCI.4048-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H (2008) Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol 510:641–654. 10.1002/cne.21822 [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V (2004) Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci 24:2633–2642. 10.1523/JNEUROSCI.3770-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V (2006) Vesicular transmitter release from astrocytes. Glia 54:700–715. 10.1002/glia.20367 [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH (2000) d-Serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A 97:4926–4931. 10.1073/pnas.97.9.4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G (2005) Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci U S A 102:5606–5611. 10.1073/pnas.0408483102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA (2004) Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431:195–199. 10.1038/nature02827 [DOI] [PubMed] [Google Scholar]
- Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH (2015) Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci 18:219–226. 10.1038/nn.3901 [DOI] [PubMed] [Google Scholar]
- Nichols CD, Roth BL (2009) Engineered G-protein coupled receptors are powerful tools to investigate biological processes and behaviors. Front Mol Neurosci 2:16. 10.3389/neuro.02.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Mukamel EA, Schnitzer MJ (2009) Motor behavior activates Bergmann glial networks. Neuron 62:400–412. 10.1016/j.neuron.2009.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, Cui J, Gratiy SL, Sakadzić S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, Silva GA, et al. (2013) In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci 33:8411–8422. 10.1523/JNEUROSCI.3285-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Mothet JP (2006) Molecular determinants of d-serine-mediated gliotransmission: from release to function. Glia 54:726–737. 10.1002/glia.20356 [DOI] [PubMed] [Google Scholar]
- Oliet SH, Mothet JP (2009) Regulation of N-methyl-D-aspartate receptors by astrocytic d-serine. Neuroscience 158:275–283. 10.1016/j.neuroscience.2008.01.071 [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH (2006) Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell 125:775–784. 10.1016/j.cell.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U (2015) Role for astroglial alpha1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front Cell Neurosci 9:230. 10.3389/fncel.2015.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Haydon PG (2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A 97:8629–8634. 10.1073/pnas.97.15.8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369:744–747. 10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- Parpura V, Liu F, Brethorst S, Jeftinija K, Jeftinija S, Haydon PG (1995) Alpha-latrotoxin stimulates glutamate release from cortical astrocytes in cell culture. FEBS Lett 360:266–270. 10.1016/0014-5793(95)00121-O [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V (2001) Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4:803–812. 10.1038/90507 [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG (2005) Astrocytic purinergic signaling coordinates synaptic networks. Science 310:113–116. 10.1126/science.1116916 [DOI] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G (2001) Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci 21:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A (2005) Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci 25:2192–2203. 10.1523/JNEUROSCI.3965-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A (2007) Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317:1083–1086. 10.1126/science.1144640 [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. 10.1016/j.tins.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Perea G, Yang A, Boyden ES, Sur M (2014) Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun 5:3262. 10.1038/ncomms4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD (2008) Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 28:4967–4973. 10.1523/JNEUROSCI.5572-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Boyt KM, McCarthy KD (2014) Astrocyte IP3R2-dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8:384. 10.3389/fnbeh.2014.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopová S, Vargová L, Syková E (1997) Heterogeneous and anisotropic diffusion in the developing rat spinal cord. Neuroreport 8:3527–3532. 10.1097/00001756-199711100-00022 [DOI] [PubMed] [Google Scholar]
- Prokopová-Kubinová S, Vargová L, Tao L, Ulbrich K, Subr V, Syková E, Nicholson C (2001) Poly[N-(2-hydroxypropyl)methacrylamide] polymers diffuse in brain extracellular space with same tortuosity as small molecules. Biophys J 80:542–548. 10.1016/S0006-3495(01)76036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae MG, Irving AJ (2004) Both mGluR1 and mGluR5 mediate Ca2+ release and inward currents in hippocampal CA1 pyramidal neurons. Neuropharmacology 46:1057–1069. 10.1016/j.neuropharm.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Ropert N, Jalil A, Li D (2016) Expression and cellular function of vSNARE proteins in brain astrocytes. Neuroscience 323:76–83. 10.1016/j.neuroscience.2015.10.036 [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C (2008) Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322:1551–1555. 10.1126/science.1164022 [DOI] [PubMed] [Google Scholar]
- Roux L, Madar A, Lacroix MM, Yi C, Benchenane K, Giaume C (2015) Astroglial connexin 43 hemichannels modulate olfactory bulb slow oscillations. J Neurosci 35:15339–15352. 10.1523/JNEUROSCI.0861-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A (2011) TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69:988–1001. 10.1016/j.neuron.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS (2009) Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A 106:13939–13944. 10.1073/pnas.0907143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH (1995) d-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A 92:3948–3952. 10.1073/pnas.92.9.3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R (2006) GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci 26:5370–5382. 10.1523/JNEUROSCI.5255-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AH, Nucifora FC Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA (1999) Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol 406:207–220. [DOI] [PubMed] [Google Scholar]
- Sherwood MW, Arizono M, Hisatsune C, Bannai H, Ebisui E, Sherwood JL, Panatier A, Oliet SH, Mikoshiba K (2017) Astrocytic IP3 Rs: contribution to Ca2+ signalling and hippocampal LTP. Glia 65:502–513. 10.1002/glia.23107 [DOI] [PubMed] [Google Scholar]
- Sibille J, Pannasch U, Rouach N (2014) Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J Physiol 592:87–102. 10.1113/jphysiol.2013.261735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS (2015) Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat Neurosci 18:708–717. 10.1038/nn.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub SV, Bonev AD, Wilkerson MK, Nelson MT (2006) Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol 128:659–669. 10.1085/jgp.200609650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syková E, Mazel T, Vargová L, Vorísek I, Prokopová-Kubinová S (2000) Extracellular space diffusion and pathological states. Prog Brain Res 125:155–178. 10.1016/S0079-6123(00)25008-5 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702. 10.1126/science.276.5319.1699 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496. 10.1124/pr.109.002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA (2004) Role for glia in synaptogenesis. Glia 47:209–216. 10.1002/glia.20082 [DOI] [PubMed] [Google Scholar]
- Vargová L, Jendelová P, Chvátal A, Syková E (2001) Glutamate, NMDA, and AMPA induced changes in extracellular space volume and tortuosity in the rat spinal cord. J Cereb Blood Flow Metab 21:1077–1089. 10.1097/00004647-200109000-00005 [DOI] [PubMed] [Google Scholar]
- Voigt J, Grosche A, Vogler S, Pannicke T, Hollborn M, Kohen L, Wiedemann P, Reichenbach A, Bringmann A (2015) Nonvesicular release of ATP from rat retinal glial (Muller) cells is differentially mediated in response to osmotic stress and glutamate. Neurochem Res 40:651–660. 10.1007/s11064-014-1511-z [DOI] [PubMed] [Google Scholar]
- Wagnerova D, Jiru F, Dezortova M, Vargová L, Syková E, Hajek M (2009) The correlation between 1H MRS choline concentrations and MR diffusion trace values in human brain tumors. Magma 22:19–31. 10.1007/s10334-008-0150-2 [DOI] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M (2012) Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+. Sci Signal 5:ra26. 10.1126/scisignal.2002334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Goldman S, Peng W, Huang JH, Takano T, Nedergaard M (2013) Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci 33:17404–17412. 10.1523/JNEUROSCI.2178-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm A, Volknandt W, Langer D, Nolte C, Kettenmann H, Zimmermann H (2004) Localization of SNARE proteins and secretory organelle proteins in astrocytes in vitro and in situ. Neurosci Res 48:249–257. 10.1016/j.neures.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Woerly S, Pinet E, De Robertis L, Bousmina M, Laroche G, Roitback T, Vargová L, Syková E (1998) Heterogeneous PHPMA hydrogels for tissue repair and axonal regeneration in the injured spinal cord. J Biomater Sci Polym Ed 9:681–711. 10.1163/156856298X00091 [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH (1999) Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A 96:13409–13414. 10.1073/pnas.96.23.13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Balu DT, Coyle JT (2016) The rise and fall of the d-serine-mediated gliotransmission hypothesis. Trends Neurosci 39:712–721. 10.1016/j.tins.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S (2003) Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. Proc Natl Acad Sci U S A 100:15194–15199. 10.1073/pnas.2431073100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Takayasu N, Hashimoto A, Sato Y, Tamaki R, Tsukamoto H, Kobayashi H, Noda S (2007) The serine racemase mRNA is predominantly expressed in rat brain neurons. Arch Histol Cytol 70:127–134. 10.1679/aohc.70.127 [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG (2004) Fusion-related release of glutamate from astrocytes. J Biol Chem 279:12724–12733. 10.1074/jbc.M312845200 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zho WM, You JL, Huang CC, Hsu KS (2002) The group I metabotropic glutamate receptor agonist (S)-3,5-dihydroxyphenylglycine induces a novel form of depotentiation in the CA1 region of the hippocampus. J Neurosci 22:8838–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang B, Theus MH, Sick JT, Bethea JR, Sick TJ, Liebl DJ (2010) EphrinBs regulate d-serine synthesis and release in astrocytes. J Neurosci 30:16015–16024. 10.1523/JNEUROSCI.0481-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G (2003) Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6:43–50. 10.1038/nn980 [DOI] [PubMed] [Google Scholar]