Abstract
Context:
Albright hereditary osteodystrophy (AHO) is caused by heterozygous inactivating mutations in GNAS. Depending on the parental origin of the mutated allele, patients develop either pseudohypoparathyroidism type 1A (PHP1A), with multihormone resistance and severe obesity, or pseudopseudohypoparathyroidism (PPHP), without hormonal abnormalities or marked obesity. Subcutaneous ossifications (SCOs) are a source of substantial morbidity in both PHP1A and PPHP.
Objective:
This study investigated the previously undetermined prevalence of SCO formation in PHP1A vs PPHP as well as possible correlations with genotype, sex, age, hormonal resistance, and body mass index (BMI).
Design:
This study evaluated patients with AHO for SCOs by physical examination performed by one consistent physician over 16 years.
Setting:
Albright Clinic, Kennedy Krieger Institute; Institute for Clinical and Translational Research, Johns Hopkins Hospital; Albright Center, Connecticut Children’s Medical Center.
Patients:
We evaluated 67 patients with AHO (49 with PHP1A, 18 with PPHP) with documented mutations in GNAS.
Main Outcome Measures:
Relationships of SCOs to genotype, sex, age, hormonal resistance, and BMI.
Results:
Forty-seven of 67 participants (70.1%) had SCOs. Patients with PHP1A and PPHP had similar prevalences and degrees of ossification formation. Patients with frameshift and nonsense mutations had much more extensive SCOs than those with missense mutations. Males were affected more than females. There was no correlation with hormonal status or BMI.
Conclusions:
There is a similar prevalence of SCOs in PHP1A and PPHP, and the extent of SCO formation correlates with the severity of the mutation. Males are affected more extensively than females, and the SCOs tend to worsen with age.
Subcutaneous ossifications in Albright hereditary osteodystrophy correlate with genotype and sex but not parent of origin of the mutant allele, hormonal status, or BMI.
Albright hereditary osteodystrophy (AHO) is a disorder caused by heterozygous inactivating mutations affecting exons 1 to 13 of GNAS, the gene encoding the α-subunit of the stimulatory G protein (Gαs), which couples heptahelical receptors to the stimulation of adenylyl cyclase [for review (1, 2)]. AHO is associated with a constellation of features, including short stature, brachydactyly, subcutaneous ossifications (SCOs), and dental abnormalities. Patients with GNAS mutations on the maternally inherited allele are obese (3) and often manifest resistance to several hormones [such as parathyroid hormone (PTH), thyrotropin (TSH), luteinizing hormone, follicle-stimulating hormone, and growth hormone releasing hormone (GHRH)] that use Gαs-coupled receptors, a condition termed pseudohypoparathyroidism type 1A (PHP1A). In contrast, patients who inherit mutations from the paternal allele have the AHO phenotype alone and do not exhibit any of the previously mentioned hormonal resistances or severe obesity, a condition termed pseudopseudohypoparathyroidism (PPHP) (2, 4–15). Moreover, there is a subgroup of patients with paternally inherited GNAS mutations who have progressive osseous heteroplasia (POH), a separate disorder characterized by severe heterotopic ossifications penetrating into the deep connective tissue and skeletal muscle without the AHO phenotype or hormonal abnormalities (16–19). For the current study, we excluded any patients with concomitant POH, as previously described in patients with PHP1A (20, 21). [Please note that PHP1A, PPHP, and POH are all classified as iPPSD2 (inactivating PTH/PTHrP signaling disorder 2) according to a new proposed classification nomenclature (22), which does not include the parental origin of the mutation.]
Some of the variable phenotypes that arise from identical GNAS gene defects can be attributed to paternal imprinting. For example, the presence or absence of hormonal resistance in association with AHO was first defined through the clinical analysis of numerous pedigrees (23). Subsequent to this, murine models with targeted disruption of the Gnas gene were studied and found to display evidence for tissue-specific silencing of Gαs expression from the paternal allele (24–26). This tissue-specific imprinting has been found to be partial in most tissues examined with preferential expression of the maternal allele in the renal cortex, pituitary, thyroid, and gonad (6, 8, 12, 13, 24–26). In addition to the partial tissue-specific imprinting in certain hormonally responsive tissues, there is biallelic expression in other tissues such as skin (27), bone (14), chondrocytes (28, 29), adipocytes (14, 25), and heart (25).
The AHO phenotype is extremely variable in its severity, even among members of the same family and generation (9, 14, 30). SCOs have long been described as part of the phenotype of AHO and are a very distinct and specific characteristic of this disorder. SCOs can cause substantial pain and morbidity that can affect the activities of daily life, and they can occur spontaneously or secondary to trauma. Even when surgically removed, they can often recur, thereby leading to unnecessary medical procedures, risks, and expenses. Understanding the prevalence and etiology of these SCOs could potentially lead to the discovery of treatments not only for SCOs in AHO but also for heterotopic bone formation that occurs commonly in the general population, such as secondary to hip surgery, spinal cord and brain injury, disc replacement, burns, blunt trauma, and amputations (31–34). These heterotopic ossifications can lead to substantial morbidity, including decreased joint mobility, functional impairment, pain, and possible additional surgeries. Finally, determining the mechanism of ectopic bone formation could potentially help with conditions in which it would be advantageous for bone to form, such as fracture repair (35). In addition, a better comprehension of the etiology of ossification formation could lead to a better understanding of the mechanisms of osteogenesis in general, with potential implications for therapies in osteoporosis. In this regard, recent studies have suggested that a key role for Gαs in bone is to regulate osteoblast differentiation by maintaining the proper balance between Hedgehog and Wnt signaling pathways (36–38).
Our study examined the presence and progression of SCOs in the largest cohort of mutation-confirmed patients with AHO to date. Of note is that the physical examinations within this study were all performed by a single examiner over a period of 16 years. In addition, this is the first study, to our knowledge, to evaluate the relationship of SCO in AHO to genotype, sex, age, hormonal status, and body mass index (BMI). Prior examination of clinical data from multiple centers reported the presence of various components of the AHO phenotype, including SCOs in 55 children with AHO (45 with PHP1A and 10 with PPHP), although these patients were not mutation confirmed, and no correlations were made regarding the SCOs (39). Adegbite et al. (40) examined specific genotype-phenotype correlations in 111 individuals with cutaneous and SCOs with confirmed mutations in GNAS. Most of the patients examined had POH, although 10 had PPHP, and 12 had PHP1A/1C. No genotype correlation could predict the progressive forms of heterotopic ossifications (e.g., POH) from the nonprogressive forms (osteoma cutis, PPHP, and PHP1A/1C). Most recently, de Sanctis et al. (41) analyzed a group of patients with PHP1A for the presence of SCOs. In general, they reported similar findings to those described later in our study, although the prevalence of SCOs was higher in our study, perhaps reflecting the longer time period over which individual patients were evaluated in our study. The de Sanctis et al. study also did not include patients with PPHP.
The purpose of our study was to examine mutation-confirmed patients with AHO to investigate possible genotype-phenotype correlations and further elucidate the role of GNAS in the formation of SCOs. Based on our observations of a high prevalence of SCOs in patients with mutations in GNAS, we hypothesized that the severity of the mutation may correlate with the severity and/or extent of formation of SCOs. In addition, we wished to examine whether imprinting as well as sex, age, hormonal resistance, and BMI could be involved with this unique manifestation of heterotopic bone formation.
Materials and Methods
All clinical histories, physical examinations, and diagnostic workups were performed by one consistent physician over a 16-year time span (E.L.G-L.). GNAS mutation analysis was performed in all patients (see later). Of the 67 patients with documented GNAS mutations, 49 had PHP1A, and 18 had PPHP. It is important to note that it can be difficult to distinguish cases with severe PPHP from those with mild POH. The age at presentation is the age at which the patient presented either to the Albright Clinic at the Kennedy Krieger Institute or to the Johns Hopkins Institute for Clinical and Translational Research (ICTR). (Only follow-up occurred at the Connecticut sites.) The current age refers to the age at the last clinic visit. For this 16-year study, multiple physical examinations were performed by one consistent examiner (E.L.G-L.). The longest continual monitoring of the SCOs in a given patient was approximately 14.7 years with a mean ± standard error of the mean (SEM) of 5.4 ± 0.5 years. (Three adult patients without ossifications were the only patients who were not monitored over time, and they are not included in this calculation.) We excluded three patients who presented with an overlap syndrome of PHP1A and POH, in whom ossifications progressed much more rapidly and in whom the ossifications extended beyond the subcutaneous layer based on imaging studies. Anthropometric data were obtained as part of our evaluation. The most recent height and weight measurements were used to determine BMI. All heights were performed using a stadiometer (Harpenden; Holtain Limited, Crosswell, Crymych, Pembrokeshire, UK), with concomitant accurate weights [Scaletronix, White Plains, NY (Kennedy Krieger Institute, Connecticut Children’s Medical Center); Detecto scale, Webb City, MO (Johns Hopkins ICTR)].
All patients had a combination of physical features consistent with AHO such as SCOs, brachydactyly/brachymetacarpia/brachymetatarsia, obesity, round face, cognitive impairment, and, in the adults, short stature [children are usually not short (7)]. The diagnosis of PHP1A was differentiated from PPHP by the presence of multihormone resistance, including two or more of the following: PTH resistance (elevated serum intact PTH sometimes associated with hypocalcemia and hyperphosphatemia), TSH resistance (elevated TSH with low or normal free T4), clinical manifestations of GnRH resistance (oligomenorrhea, amenorrhea, and/or incomplete pubertal progression plateauing at late Tanner stage III to Tanner stage IV), and GHRH resistance as assessed by standard growth hormone (GH) stimulation testing. All patients were treated appropriately for their hormonal resistances, and all were documented to be euthyroid, had PTH values in the normal range (or intermittently within 30 pg/mL above the top normal range) while on calcitriol therapy, and were normal biochemically at the time of their physical examinations and anthropometric measurements. Except for one child who had not yet manifested PTH resistance, the remainder were on calcitriol treatment throughout the study. In any patients in whom vitamin D deficiency was determined, vitamin D levels were corrected initially and then maintained in the normal range with a few exceptions in which the insufficiency was extremely minimal intermittently. All patients were evaluated in the Albright Clinic at the Kennedy Krieger Institute (Baltimore, MD), ICTR at the Johns Hopkins Hospital (Baltimore, MD), or the Albright Center at the Endocrinology Clinic at Connecticut Children’s Medical Center (Farmington, CT). All studies were approved by the Johns Hopkins Medicine Institutional Review Board (includes Kennedy Krieger Institute), Connecticut Children’s Medical Center Institutional Review Board, and the University of Connecticut School of Medicine Institutional Review Board. Informed consent was obtained from all patients, or a parent of each patient, before participation. Assent was obtained when appropriate based on age and emotional/cognitive maturity.
Ossification determination
Physical examinations were performed as described previously by one consistent examiner. SCOs were documented by palpation in all but one of the patients for whom the ossifications were confirmed incidentally upon a radiograph analysis (examples of SCOs are shown in Fig. 1). For all ossifications palpated, they were localized solely to the subcutaneous tissue. If the patient presented with ossifications that were deeper than the subcutaneous tissue, the patient was excluded. This was confirmed by imaging studies (such as magnetic resonance imaging). We were able to palpate ossifications as small as 1 mm on physical examination. We classified the degree of ossification formation in each patient by assigning a number (1 to 4) reflecting the extent of ossification formation as follows: 1 = <4 ossifications, 2 = 4 to 15, 3 = 16 to 25, and 4 = >25. When examining the severity of ossification formation across all patient groups, the numbers presented previoiusly were used to calculate totals for statistical significance calculations. The number of ossifications did not necessarily correlate with the size of the ossifications. The size was measured during the physical examination at each visit to follow the progression of the heterotopic bone formation and varied considerably even within a given patient. In a small subset of cases, the early ossifications started with a bluish discoloration. The usual progression with respect to our classification was that approximately half of the patients who presented with SCO severity at level 1 or level 2 progressed one level (i.e., to level 2 or level 3, respectively). Of the 8 patients with SCOs at level 4, three males had initially presented at level 3. As stated previously, over the 16 years of monitoring by one consistent examiner, the longest continual follow-up of the SCOs in a given patient was 14.7 years, with a mean ± SEM of 5.4 ± 0.5 years.
Figure 1.
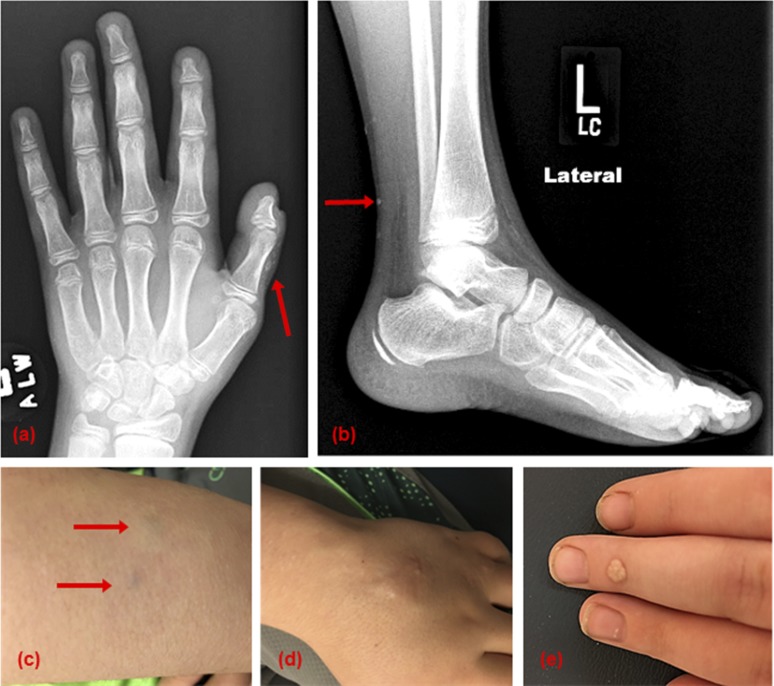
SCOs detected by X-ray and physical examination. (a) Small SCOs are noted incidentally on a bone age X-ray. (b) Small SCOs are noted incidentally on a left ankle X-ray superficially along the posterior aspect of the left calf. (c) Blue-tinged lesions on the dorsum of the forearm are early SCOs; these often can evolve into the ossifications shown in panel (d). (d) Cluster of SCOs over the dorsum of the left hand of varying sizes. Note the small punctate white lesions that are part of the ossification. (e) Very superficial ossification on the dorsum of the middle phalange. An almost identical area on the patient’s thigh was thought to be a wart, but biopsy proved it to be bone.
Laboratory evaluation
Biochemical and hormonal testing was performed in the Johns Hopkins Hospital Clinical Laboratory as described previously (3, 7).
GNAS mutation analyses
Peripheral blood from all patients was collected at the Albright Clinic at the Kennedy Krieger Institute or at the Johns Hopkins ICTR. DNA isolation and GNAS mutation analyses of the 13 coding exons and all intron/exon boundaries were performed in the Johns Hopkins DNA Diagnostic Laboratory (http://www.hopkinsmedicine.org/dnadiagnostic/), Center for Genetic Testing at St. Francis (http://www.sfh-lab.com/), or a research laboratory (E.L.G-L.) at the Johns Hopkins University School of Medicine (7, 42). The DNA diagnostic laboratories at both Johns Hopkins and St. Francis are approved by the Clinical Laboratory Improvement Amendments.
Radiograph analyses
Most children had radiographs for bone age analyses as part of routine clinical care. These included hand/wrist and at least one other site (knee, foot/ankle) because the brachydactyly and brachymetacarpia, as well as the GNAS mutation itself, typically lead to premature epiphyseal fusion with marked advancement of the hand/wrist bone age (42). These radiographs were performed at the Albright Clinic at the Kennedy Krieger Institute, in the Radiology Department at Johns Hopkins Hospital, or at Jefferson Radiology (Farmington, CT) as part of routine clinical evaluation in most cases, and informed consent was obtained prior to these if not part of the routine standard of care (radiographs at Kennedy Krieger and Johns Hopkins only).
Statistical analyses
SEM was calculated for each mean value. Differences in BMI z scores between patients were analyzed by unpaired two-tailed Student t tests; P values <0.05 were considered statistically significant. Comparison of proportions and severity between males and females were also calculated by unpaired two-tailed Student t tests.
Results
Prevalence
Over the past 16 years, 67 patients with AHO were carefully examined for the presence of SCOs. In total, 47 of 67 (70.1%) patients had ossifications. Of the patients with PHP1A, 71.4% (35/49) had ossifications compared with 66.7% (12/18) of patients with PPHP (P > 0.05). Therefore, the prevalence of SCOs is approximately the same in both PHP1A and PPHP.
Sex
Examination of the prevalence among sex revealed that 86.4% (19/22) of males had SCOs (16/19 with PHP1A and 3/3 with PPHP) compared with 62.2% (28/45) of females (19/30 with PHP1A and 9/15 with PPHP). The proportion of males with ossifications was statistically significantly different than for females (P = 0.043). Examination of the severity across sex revealed statistical significance for a higher severity of SCO in males vs females (P = 0.015) as described in the Materials and Methods section. Nineteen of the patients had ossifications of >5 cm in the longest dimension at the end of 16 years of monitoring. Of these, 14 were males (73.7%).
Hormonal resistance
Hormonal resistance was evaluated and is noted in Table 1. All patients were treated appropriately for their PTH and TSH resistances, and all were documented to be euthyroid, had PTH values in the normal range or within 30 pg/mL above the top normal range, and were normal biochemically at the time of their physical examinations. Specifically, GHRH resistance is important to note because bone has GH receptors (45, 46). Twenty-nine of 44 patients with PHP1A tested (65.9%) had GHRH resistance, and 20 of 29 (69.0%) of these patients had SCOs. There was no difference in the prevalence of SCOs regardless of whether the patients were GHRH resistant (69.0% vs 80.0%, respectively). In addition, many of these patients were treated with GH as part of a long-term study analyzing the effect of GH therapy in this patient population. The results of that study will be described elsewhere; however, we did not observe any effect of GH treatment on the development of SCOs.
Table 1.
Ossifications and Genotype-Phenotype Correlations
| Sex | Age at Presentation to Clinic, y | Current Age, y | Degree of Ossifications at Current Age | Mutation | Hormonal Resistances | Physical Characteristics | BMI z score at Current Age |
|---|---|---|---|---|---|---|---|
| PHP1A | |||||||
| Fa | 17.9 | 29.5 | y-2 | Exon 1: c.21insT | PTH, TSH, GHRH, LH/FSH | RBO | 2.10 |
| Ma | 21.1 | 32.4 | y-1 | Exon 1: c.21insT | PTH, TSH, GHRH | RBO | 2.24 |
| Fb | 1.8 | 5.5 | y-2 | Exon 1: c.34C>T (p.Gln12*) | PTH, TSH, GHRH | RO | 2.54 |
| Fc | 4.3 | 17.8 | y-4 | Exon 1: c.85C>T (p.Gln29*) | PTH, TSH, GHRH, LH/FSH | RBO | 1.16 |
| F§ | 10.3 | 20.8 | y-2 | Exon 1: c.85C>T (p.Gln29*) | PTH, TSH, GHRH, LH/FSH | RB | 1.43 |
| Fd | 6.3 | 12.6 | y-2 | Exon 1: c.85C>T (p.Gln29*) | PTH, TSH, GHRH | RBO | 2.64 |
| F | 19.2 | 30.0 | y-4 | Exon 1: c.103C>T (p.Gln35*) | PTH, TSH, GHRH, LH/FSH | RBO | 2.30 |
| M | 3.7 | 5.4 | y-4 | Exon 1: c.111C>A (p.Tyr37*)† | TSH | NA | −0.33 |
| M | 11.2 | 20.7 | y-3 | Exon 1: c.139insCTG† | PTH | O | 1.93 |
| F | 2.0 | 4.9 | y-2 | Exon 2: c.140G>A (p.Gly47Asp) † | PTH, TSH (GHRH not tested) | RO | 3.55 |
| Me | 8.9 | 14.8 | y-1 | Intron 4: c.312+5G>A | PTH, TSH | NA | 1.61 |
| Me | 24.4 | 30.2 | y-1 | Intron 4: c.312+5G>A | PTH, TSH | B | 1.79 |
| Mf | 2.4 | 4.4 | y-4 | Exon 5: c.352 G>T (p.Glu118*)† | PTH, TSH | RBO | 3.53 |
| Fg | 9.4 | 11.2 | y-1 | Exon 6: c.468T>G (p.Asp156Glu)† | PTH, TSH, GHRH | RBO | 2.40 |
| F | 2.3 | 11.6 | y-1 | Exon 6: c.470-473del† | PTH, TSH, GHRH | RBO | 2.67 |
| M | 3.1 | 3.4 | y-4 | Exon 6: c.489 C>G (p.Tyr163*) | PTH, TSH | RO | 2.34 |
| F | 5.3 | 6.5 | y-2 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | RBO | 3.03 |
| M | 9.3 | 19.7 | y-3 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | RBO | 2.33 |
| Fh | 9.8 | 18.3 | y-3 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH, LH/FSH | RBO | 2.64 |
| M | 10.1 | 21.7 | y-2 | Exon 7: c.565-568delGACT | PTH, TSH | B | −0.02 |
| F | 11.0 | 19.3 | y-3 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | RBO | 2.39 |
| M | 11.3 | 17.9 | y-3 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | B | 1.05 |
| M | 9.3 | 24.0 | y-2 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | RB | 1.17 |
| F | 11.8 | 14.5 | y-3 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | RBO | 2.00 |
| Fi | 2.3 | 2.8 | y-3 | Exon 7: c.565-568delGACT | PTH, TSH (GHRH not tested) | RBO | 1.07 |
| Mi | 4.3 | 4.9 | y-3 | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | BO | 1.98 |
| M | 3.3 | 4.4 | y-4 | Exon 7: c.565-568delGACT | PTH, TSH | R | 1.64 |
| Mj | 2.1 | 5.9 | y-4 | Exon 9: c.701G>A (p.Trp234*)† | PTH, TSH, GHRH | RBO | 4.94 |
| F | 6.8 | 12.8 | y-3 | Exon 10: c.814C>T (p.Leu272Phe)† | PTH, TSH (GHRH not tested) | RBO | 3.21 |
| Fk | 33.0 | 36.5 | y-2 | Exon 12: c.1024C>T (p.Arg342*) | PTH, TSH | RBO | 2.78 |
| Mk | 8.6 | 12.1 | y-4 | Exon 12: c.1024C>T (p.Arg342*) | PTH, TSH | NA | 0.25 |
| Fk | 5.4 | 8.8 | y-3 | Exon 12: c.1024C>T (p.Arg342*) | PTH, TSH | NA | 0.15 |
| F | 6.8 | 18.4 | y-3 | Exon 13: c.1083insC | PTH, TSH, GHRH, LH/FSH | RB | 1.02 |
| F | 37.9 | 42.7 | y-1 | Exon 13: c.1096G>A (p.Ala366Thr)† | PTH, TSH, LH/FSH | RB | 1.90 |
| MI | 3.4 | 6.0 | y-2 | Exon 13: c.1100insA | PTH, TSH, GHRH | RO | 2.73 |
| Fm | 54.0 | 60.7 | n | Exon 1: c.77T>A (p.Ile26Asn) | PTH, GHRH | RB | 1.61 |
| Mm | 26.8 | 33.5 | n | Exon 1: c.77T>A (p.Ile26Asn) | PTH, TSH | B | 0.05 |
| F | 18.1 | 19.6 | n | Exon 1: c.124C>T (p.Arg42Cys) | PTH, TSH (GHRH not tested) | RBO | 2.00 |
| F | 5.9 | 9.8 | n | Exon 5: c.343C>T (p.Pro115Ser) | PTH, TSH | RB | 0.39 |
| Mg | 7.8 | 9.6 | n | Exon 6: c.468T>G (p.Asp156Glu)† | PTH, TSH, GHRH | RBO | 2.50 |
| F | 11.0 | 11.2 | n | Exon 7: c.565-568delGACT | PTH, TSH, GHRH | RBO | 2.25 |
| F | 7.9 | 8.2 | n | Exon 7: c.568-569delTA† | PTH, TSH, GHRH | RBO | 2.21 |
| F | 3.3 | 10.3 | n | Intron 7: c.586-1G>T | PTH, TSH (GHRH unclear) | RBO | 4.80 |
| Mn | 3.6 | 11.2 | n | Exon 10: c.730A>T (p.Ile244Phe) | PTH, TSH, GHRH | RBO | 3.94 |
| Fn | 9.8 | 17.5 | n | Exon 10: c.730A>T (p.Ile244Phe) | PTH, TSH, GHRH | RBO | 2.08 |
| F | 9.5 | 15.1 | n | Exon 11: c.885T>G (p.Asp295Glu)† | PTH, TSH, GHRH | RBO | 2.06 |
| F | 11.5 | 15.8 | n | Exon 12: c.1006C>T (p.Arg336Trp) | PTH, TSH, GHRH | RBO | 2.31 |
| F | 18.4 | 29.2 | n | Exon 13: c.1174G>A (p.Glu392Lys) | PTH, TSH, LH/FSH | B | 1.44 |
| Fo | 11.1 | 14.9 | n | Exon 13: c.1174G>A (p.Glu392Lys) | PTH, TSH, GHRH, LH/FSH | NA | 1.06 |
| PPHP | |||||||
| M | 11.0 | 17.4 | y-2 | Exon 1: c.2T>G (p.Met1Arg) | None | B | −1.45 |
| Fa | 54.1 | 60.7 | y-1 | Exon 1: c.21insT | None | B | 1.02 |
| Fb | 25.4 | 29.2 | y-3 | Exon 1: c.34C>T (p.Gln12*) | None | RB | 1.28 |
| Fd | 20.3 | 20.3 | y-1 | Exon 1: c.85C>T (p.Gln29*) | None | B | −0.74 |
| Fd | 37.3 | 43.5 | y-2 | Exon 1: c.85C>T (p.Gln29*) | None | B | 0.64 |
| Md | 49.8 | 49.8 | y-1 | Exon 1: c.85C>T (p.Gln29*) | None | B | 0.87 |
| Md | 88.3 | 88.3 | y-2 | Exon 1: c.85C>T (p.Gln29*) | None | B | 1.70 |
| Ff | 36.6 | 38.6 | y-2 | Exon 5: c.352 G>T (p.Glu118*)† | None | NA | 0.80 |
| Fh | 45.0 | 52.1 | y-2 | Exon 7: c.565-568delGACT | None | B | 1.00 |
| Fi | 40.0 | 40.5 | y-3 | Exon 7: c.565-568delGACT | None | B | 1.37 |
| Fj | 30.0 | 33.8 | y-1 | Exon 9: c.701G>A (p.Trp234*)† | None | B | 1.20 |
| Fl | 30.9 | 33.5 | y-2 | Exon 13: c.1100insA | None | B | 1.40 |
| Fc | 30.5 | 36.9 | n | Exon 1: c.85C>T (p.Gln29*) | None | RB | 1.92 |
| Fe | 45.9 | 51.7 | n | Intron 4: c.312+5G>A | None | B | 0.78 |
| F | 13.9 | 15.6 | n | Exon 5: c.325G>A (p.Ala109Thr)† | None | BO | 2.31 |
| Fg | 35.1 | 36.9 | n | Exon 6: c.468T>G (p.Asp156Glu)† | None | B | 1.36 |
| Fn | 30.3 | 37.9 | n | Exon 10: c.730A>T (p.Ile244Phe) | None | B | −1.03 |
| Fo | 38.2 | 42.0 | n | Exon 13: c.1174G>A (p.Glu392Lys) | None | RBO | 2.36 |
Presence of ossifications: n = no, y = yes (see text for number designation). Superscript letters (a to o) indicate patients from the same kindred. Based on den Dunnen and Antonarakis (44). All patients were tested for GH status except where otherwise noted.
Abbreviations: B, brachydactyly and/or brachymetacarpia; del, deletion; F, female; FSH, follicle-stimulating hormone; ins, insertion; LH, luteinizing hormone; M, male; NA, none apparent; O, obesity; R, round face.
Stop codon.
Previously unreported mutations based on Lemos and Thakker RV (43).
Ossifications detected on X-ray.
Age
There was no effect of age on the prevalence of ossifications, although the size of a small subset of the SCOs did increase with age as described previously (∼15% of patients). We found that 22 of 33 (66.7%) children and 25 of 34 (73.5%) adults had SCOs. Of the children aged birth to 18 years with PHP1A, 67.7% (21/31) had SCOs, whereas 77.8% (14/18) of the adults with PHP1A had SCOs. Of the two children aged birth to 18 years with PPHP, one had SCO (50%), whereas 68.8% (11/16) of the adults with PPHP had SCOs.
Puberty
We were interested in determining whether the hormonal effect of puberty has any relationship to SCO formation. Interestingly, one boy had no SCOs at presentation at 8.9 years of age but developed SCOs at 11.3 years just after the onset of puberty. Aside from this one patient, however, there did not appear to be any worsening directly associated with puberty. One adult had worsening with pregnancy. The others seemed to have SCOs that gradually worsened with time, with the range of progression being mild in general (advancement of only one level in severity).
BMI
Anthropometric data, including BMI, are included in Table 1. Even in the presence of obesity, we were able to detect ossifications on examination. We did not observe any relationship between BMI and ossification formation in our patients. The mean ± SEM for the BMI z score for the PHP1A group was 2.02 ± 0.16 (total of 49 patients), whereas the average for the PPHP group was 0.93 ± 0.25 (total of 18 patients) (P = 0.0007). This is consistent with our prior findings (3). Although the average BMI z score was statistically significantly higher in the PHP1A group, we did not observe any statistically significant difference in the prevalence of SCO formation between the two groups, as stated previously.
Genotype
Patients with missense mutations in GNAS had fewer SCOs than patients with GNAS frameshift or nonsense mutations, as shown in Table 1. Patients are shown as two groups, PHP1A and PPHP, and within each group, the mutations in GNAS that lead to a premature stop codon (i.e., nonsense and frameshift mutations) are listed first, followed by the missense mutations. Mutations are identified using standard nomenclature (44). Any previously unreported mutations [based on the review by Lemos and Thakker (43)] are denoted in Table 1 by a cross (†). For a listing of references for each of the reported mutations, please refer to Supplemental Table 1 (31.9KB, docx) .
Of the patients with PHP1A, 12 had nonsense mutations, all with ossifications (100%). Of the patients with PPHP, 8 had nonsense mutations, of whom 7 had SCOs (87.5%). Of the 18 patients with PHP1A who had frameshift insertions or deletions, 16 (88.9%) had SCOs. Of the 4 patients with PPHP who had frameshift mutations, all had SCOs (100%). One patient with PHP1A had a mutation resulting in a single amino acid insertion, and this patient also had SCOs. In contrast, the patients with missense mutations in GNAS had a much lower frequency of SCOs. Of the 18 patients with PHP1A who had missense mutations (three within introns), 6 had SCOs (33.3%), and of the 6 patients with PPHP who had missense mutations (one mutation in intron), only 1 had SCOs (16.7%).
We found that 13 of the 14 patients with the common exon 7 four base pair deletion (47) had SCOs with a wide variation in presentation. All but 1 of 8 patients with exon 1 c.85>T (p.Gln29*) had ossifications, again with a wide variation in presentation, as shown in Table 1. Because of the paucity of patients with the other mutations, further associations cannot be made.
Table 1 lists kindred data for the patients (denoted as a to o superscripts). We analyzed whether members of the same kindred had the same degree of SCOs. Here we observed that parents with SCOs usually had children with ossifications to the same degree. Similarly, those parents without SCOs usually had children without ossifications. Although many of the patients analyzed were the sole members of their family examined in this study, for 15 patients, multiple family members of those patients were involved in this study. Nine of the families had both a parent and a child with SCOs.
Phenotype
The physical features of AHO found in each patient, including round facies, obesity, and brachydactyly and/or brachymetacarpia, are noted in Table 1. We have not found that the presence or degree of brachydactyly/brachymetacarpia correlates with the findings of SCOs. Of our 38 patients with PHP1A who had brachydactyly, 25 had SCOs (65.8%). Seventeen patients with PPHP had brachydactyly, and 11 had SCOs (64.7%). The total of those with both brachydactyly and SCOs was 36 of 67 (53.7%).
Discussion
Our results (summarized in Table 2) demonstrate that SCOs have an equal prevalence in PHP1A vs PPHP (71.4% vs 66.7%). Studies examining SCOs in large populations in these two groups have not been previously reported. Specifically, this is the first large study, to our knowledge, of SCOs in mutation-confirmed patients with AHO.
Table 2.
Ossifications vs Patient Characteristics
| Characteristic | Total No. of Patients | No. of Patients With SCOs | % of Patients With SCOs |
|---|---|---|---|
| PHP1A | 49 | 35 | 71.4 |
| Males | 19 | 16 | 84.2 |
| Females | 30 | 19 | 63.3 |
| Children | 31 | 21 | 67.7 |
| Adults | 18 | 14 | 77.8 |
| Nonsense | 12 | 12 | 100.0 |
| Frameshift | 18 | 16 | 88.9 |
| AA insertion | 1 | 1 | 100.0 |
| Missense | 18 | 6 | 33.3 |
| Brachydactyly | 38 | 25 | 65.8 |
| GHRH | |||
| Resistant | 29 | 20 | 69.0 |
| Not resistant | 15 | 12 | 80.0 |
| PPHP | 18 | 12 | 66.7 |
| Males | 3 | 3 | 100.0 |
| Females | 15 | 9 | 60.0 |
| Children | 2 | 1 | 50.0 |
| Adults | 16 | 11 | 68.8 |
| Nonsense | 8 | 7 | 87.5 |
| Frameshift | 4 | 4 | 100.0 |
| Missense | 6 | 1 | 16.7 |
| Brachydactyly | 17 | 11 | 64.7 |
| PHP1A + PPHP | 67 | 47 | 70.1 |
| Males | 22 | 19 | 86.4 |
| Females | 45 | 28 | 62.2 |
| Children | 33 | 22 | 66.7 |
| Adults | 34 | 25 | 73.5 |
| Nonsense | 20 | 19 | 95.0 |
| Frameshift | 22 | 20 | 90.9 |
| AA insertion | 1 | 1 | 100.0 |
| Missense | 24 | 7 | 29.2 |
| Brachydactyly | 55 | 36 | 65.5 |
Abbreviation: AA, amino acid.
Upon our analysis of data reported by Marguet et al. (39), we noticed similar findings in that there was an equal prevalence of SCOs in PHP1A and PPHP; however, we found a greater prevalence of SCOs in both conditions. Marguet et al. (39) reported SCOs in 42.2% (19/45) of patients with PHP1A compared with our 71.4% (35/49); they found SCOs in 50% (5/10) of patients with PPHP compared with our 66.7% (12/18). Of note, however, in the Marguet et al. (39) study, the patients were not reported as having been examined by a single, consistent physician, and GNAS mutations were not confirmed. Our human data regarding the prevalence of SCO formation were found to be similar to our mice with targeted disruption of exon 1 whether the mutated allele was inherited from the mother (analogous to PHP1A) or from the father (analogous to PPHP) (48, 49). There is no evidence of paternal imprinting in skin or bone (14, 27), and therefore, we postulate that the similar rates of SCO formation in PHP1A and PPHP are due to the fact that Gαs is normally biallelically expressed in these tissues. These data indicate that Gαs haploinsufficiency is directly related to the development of SCOs, irrespective of whether the GNAS mutation resides on the maternal or the paternal allele in both mice (48, 49) and humans. These findings also suggest that hormonal resistance does not appear to play a role in SCO formation because patients with PPHP do not have hormonal resistance.
In our mouse model of AHO (25), SCO formation in male mice was much more extensive than in female mice (48, 49). This finding prompted us to look at sex differences as a factor, which also has not been previously examined. The occurrence of SCOs in males was 86.4% (19/22) (16/19 with PHP1A and 3/3 with PPHP) compared with 62.2% (28/45) in females (19/30 with PHP1A and 9/15 with PPHP; P = 0.043). The severity of SCOs in males vs females was also found to be statistically significant (P = 0.015), as described in the Materials and Methods section. Interestingly, our results parallel those that have been reported by several patient case series of heterotopic ossification formation in other conditions. Males are also at higher risk than females to develop heterotopic ossifications following hip surgery (50–54); the predominance in males was found to be two to three times that in females in one report (55).
The severity of ossifications and the severity of the GNAS mutations had a direct relationship (i.e., patients with missense mutations in GNAS had fewer SCOs than patients with GNAS frameshift or nonsense mutations), implying that a genotype-phenotype correlation exists and that persistent basal or agonist-stimulated cyclic adenosine monophosphate signaling is preventative in the development of SCOs. A similar correlation between the presence of SCOs and severity of GNAS mutations was also observed by de Sanctis et al. (41). Based on these considerations, it will be important to assess expression and signaling of the mutant Gαs proteins using murine fibroblast-like cell lines lacking Gαs (56), as well as assess quantitatively Gαs levels in the ossified lesions and to correlate these with the degree of ossifications. In addition, parents with SCOs usually have children with ossifications to the same degree. Similarly, those parents without SCOs usually have children without ossifications, adding further evidence to the genotype-phenotype correlation.
We also examined the age at which SCO formation occurred to determine whether these are evident from birth or if they develop progressively. This was difficult information to ascertain because patients often present to us later than the newborn period. Many times, these ossifications go undetected for some time.
In addition, we were interested in determining whether the hormonal effect of puberty has any relationship to SCO formation. As described in the Results section, we did not observe any worsening of SCOs that seemed to correlate with puberty except for one patient in our study who developed SCOs just after the onset of puberty. Rather, SCOs gradually worsened with time, and progression was generally mild. This is consistent with our data in mice (49).
A limitation of our study is that not all SCOs are palpable. For example, in one of the patients we examined, we were unable to palpate any SCOs; however, we were able to see the SCOs as an incidental finding on X-ray (performed for purposes of a bone age determination). This makes us question whether all patients would have some evidence of SCO formation if they were to undergo full-body radiographs. In addition, it is not possible to state that if ossifications do not develop by a specific age, then they do not develop. We saw a varying range of ages at which ossifications formed.
In summary, we have found that the prevalence of SCOs in PHP1A and PPHP is similar, as in our mouse model (48, 49), which in and of itself implicates that paternal imprinting, hormonal resistance, and BMI are not involved. The degree of SCO formation correlates with the severity of the GNAS mutation, and the SCOs are more frequent and extensive in males than in females, again correlating with our findings in mice (48, 49). The severity of the SCOs seems to increase with age (57, 58).
SCOs are a great source of morbidity for patients with AHO, as well as for patients with other conditions. They can form spontaneously or secondary to trauma (49, 57). Patients often have these SCOs surgically removed, but even then, they often recur. There are no successful drug therapies to date for the SCOs in AHO. Understanding the etiology for SCO formation could lead to new treatment modalities for this problem in AHO, as well as therapies for conditions involving more invasive heterotopic ossifications. In addition, understanding the factors that limit the heterotopic bone formation specifically to the subcutaneous layer in AHO could lead to treatments aimed at hindering ossification formation that is more invasive, such as with POH or the common heterotopic ossifications following surgery and trauma. Finally, these findings may be of future relevance in helping to understand the etiology of osteogenesis in general.
Acknowledgments
We thank the patients and their families who made this work possible.
Financial Support: This work was supported in part by US Food and Drug Administration Orphan Products Development Grants R01 FD-R-002568 and R01 FD-R-003409 (to E.L.G-L.), Thrasher Research Foundation Grant 02818-8 (to E.L.G-L.), and National Institutes of Health Grants R21 HD078864 (to E.L.G-L.) and M01 RR00052 (to the Johns Hopkins University School of Medicine ICTR).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHO
- Albright hereditary osteodystrophy
- BMI
- body mass index
- Gαs
- α-subunit of the stimulatory G protein
- GH
- growth hormone
- GHRH
- growth hormone releasing hormone
- ICTR
- Institute for Clinical and Translational Research
- PHP1A
- pseudohypoparathyroidism type 1A
- POH
- progressive osseous heteroplasia
- PPHP
- pseudopseudohypoparathyroidism
- PTH
- parathyroid hormone
- SCO
- subcutaneous ossification
- SEM
- standard error of the mean
- TSH
- thyrotropin.
References
- 1.Plagge A, Kelsey G, Germain-Lee EL. Physiological functions of the imprinted Gnas locus and its protein variants Galpha(s) and XLalpha(s) in human and mouse. J Endocrinol. 2008;196(2):193–214. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22(5):675–705. [DOI] [PubMed] [Google Scholar]
- 3.Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Galpha(s) in the development of human obesity. J Clin Endocrinol Metab. 2007;92(3):1073–1079. [DOI] [PubMed] [Google Scholar]
- 4.Albright F, Burnett CH, Smith PH. Pseudohypoparathyroidism: an example of “Seabright-Bantam syndrome.” Endocrinology. 1942;30:922–932. [Google Scholar]
- 5.Albright F, Forbes AP, Henneman PH. Pseudo-pseudohypoparathyroidism. Trans Assoc Am Physicians. 1952;65:337–350. [PubMed] [Google Scholar]
- 6.Germain-Lee EL, Ding CL, Deng Z, Crane JL, Saji M, Ringel MD, Levine MA. Paternal imprinting of Galpha(s) in the human thyroid as the basis of TSH resistance in pseudohypoparathyroidism type 1a. Biochem Biophys Res Commun. 2002;296(1):67–72. [DOI] [PubMed] [Google Scholar]
- 7.Germain-Lee EL, Groman J, Crane JL, Jan de Beur SM, Levine MA. Growth hormone deficiency in pseudohypoparathyroidism type 1a: another manifestation of multihormone resistance. J Clin Endocrinol Metab. 2003;88(9):4059–4069. [DOI] [PubMed] [Google Scholar]
- 8.Hayward BE, Barlier A, Korbonits M, Grossman AB, Jacquet P, Enjalbert A, Bonthron DT. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107(6):R31–R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine MA. Pseudohypoparathyroidism. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. San Diego, CA: Academic Press; 1996:853–876. [Google Scholar]
- 10.Levine MA. Pseudohypoparathyroidism: from bedside to bench and back. J Bone Miner Res. 1999;14(8):1255–1260. [DOI] [PubMed] [Google Scholar]
- 11.Levine MA, Germain-Lee E, Jan de Beur S. Genetic basis for resistance to parathyroid hormone. Horm Res. 2003;60(Suppl 3):87–95. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Erlichman B, Weinstein LS. The stimulatory G protein alpha-subunit Gs alpha is imprinted in human thyroid glands: implications for thyroid function in pseudohypoparathyroidism types 1A and 1B. J Clin Endocrinol Metab. 2003;88(9):4336–4341. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A. The gsalpha gene: predominant maternal origin of transcription in human thyroid gland and gonads. J Clin Endocrinol Metab. 2002;87(10):4736–4740. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani G, Bondioni S, Locatelli M, Pedroni C, Lania AG, Ferrante E, Filopanti M, Beck-Peccoz P, Spada A. Biallelic expression of the Gsalpha gene in human bone and adipose tissue. J Clin Endocrinol Metab. 2004;89(12):6316–6319. [DOI] [PubMed] [Google Scholar]
- 15.Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G. The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat Genet. 2004;36(8):818–826. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed SF, Barr DGD, Bonthron DT. GNAS1 mutations and progressive osseous heteroplasia. N Engl J Med. 2002;346(21):1669–1671. [DOI] [PubMed] [Google Scholar]
- 17.Shore EM, Ahn J, Jan de Beur S, Li M, Xu M, Gardner RJM, Zasloff MA, Whyte MP, Levine MA, Kaplan FS. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346(2):99–106. [DOI] [PubMed] [Google Scholar]
- 18.Lebrun M, Richard N, Abeguilé G, David A, Coëslier Dieux A, Journel H, Lacombe D, Pinto G, Odent S, Salles JP, Taieb A, Gandon-Laloum S, Kottler ML. Progressive osseous heteroplasia: a model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab. 2010;95(6):3028–3038. [DOI] [PubMed] [Google Scholar]
- 19.Yeh GL, Mathur S, Wivel A, Li M, Gannon FH, Ulied A, Audi L, Olmstead EA, Kaplan FS, Shore EM. GNAS1 mutation and Cbfa1 misexpression in a child with severe congenital platelike osteoma cutis. J Bone Miner Res. 2000;15(11):2063–2073. [DOI] [PubMed] [Google Scholar]
- 20.Lin MH, Numbenjapon N, Germain-Lee EL, Pitukcheewanont P. Progressive osseous heteroplasia, as an isolated entity or overlapping with Albright hereditary osteodystrophy. J Pediatr Endocrinol Metab. 2015;28(7–8):911–918. [DOI] [PubMed] [Google Scholar]
- 21.Eddy MC, Jan De Beur SM, Yandow SM, McAlister WH, Shore EM, Kaplan FS, Whyte MP, Levine MA. Deficiency of the alpha-subunit of the stimulatory G protein and severe extraskeletal ossification. J Bone Miner Res. 2000;15(11):2074–2083. [DOI] [PubMed] [Google Scholar]
- 22.Thiele S, Mantovani G, Barlier A, Boldrin V, Bordogna P, De Sanctis L, Elli FM, Freson K, Garin I, Grybek V, Hanna P, Izzi B, Hiort O, Lecumberri B, Pereda A, Saraff V, Silve C, Turan S, Usardi A, Werner R, de Nanclares GP, Linglart A. From pseudohypoparathyroidism to inactivating PTH/PTHrP signalling disorder (iPPSD), a novel classification proposed by the EuroPHP network. Eur J Endocrinol. 2016;175(6):P1–P17. [DOI] [PubMed] [Google Scholar]
- 23.Davies SJ, Hughes HE. Imprinting in Albright’s hereditary osteodystrophy. J Med Genet. 1993;30(2):101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu S, Yu D, Lee E, Eckhaus M, Lee R, Corria Z, Accili D, Westphal H, Weinstein LS. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsalpha) knockout mice is due to tissue-specific imprinting of the gsalpha gene. Proc Natl Acad Sci USA. 1998;95(15):8715–8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Germain-Lee EL, Schwindinger W, Crane JL, Zewdu R, Zweifel LS, Wand G, Huso DL, Saji M, Ringel MD, Levine MA. A mouse model of Albright hereditary osteodystrophy generated by targeted disruption of exon 1 of the Gnas gene. Endocrinology. 2005;146(11):4697–4709. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Gavrilova O, Liu J, Xie T, Deng C, Nguyen AT, Nackers LM, Lorenzo J, Shen L, Weinstein LS. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci USA. 2005;102(20):7386–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine MA, Eil C, Downs RW Jr, Spiegel AM. Deficient guanine nucleotide regulatory unit activity in cultured fibroblast membranes from patients with pseudohypoparathyroidism type I. a cause of impaired synthesis of 3′,5′-cyclic AMP by intact and broken cells. J Clin Invest. 1983;72(1):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastepe M, Weinstein LS, Ogata N, Kawaguchi H, Jüppner H, Kronenberg HM, Chung UI. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci USA. 2004;101(41):14794–14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS. Chondrocyte-specific knockout of the G protein G(s)alpha leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J Bone Miner Res. 2004;20(4):663–671. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein LS, Chen M, Liu J. Gs(alpha) mutations and imprinting defects in human disease. Ann N Y Acad Sci. 2002;968(1):173–197. [DOI] [PubMed] [Google Scholar]
- 31.Pignolo RJ, Foley KL. Nonhereditary heterotopic ossification: implications for injury, arthropathy, and aging. Clin Rev Bone Miner Metab. 2005;3(3–4):261–266. [Google Scholar]
- 32.Pape HC, Lehmann U, van Griensven M, Gänsslen A, von Glinski S, Krettek C. Heterotopic ossifications in patients after severe blunt trauma with and without head trauma: incidence and patterns of distribution. J Orthop Trauma. 2001;15(4):229–237. [DOI] [PubMed] [Google Scholar]
- 33.Tortolani PJ, Cunningham BW, Eng M, McAfee PC, Holsapple GA, Adams KA. Prevalence of heterotopic ossification following total disc replacement: a prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg Am. 2007;89(1):82–88. [DOI] [PubMed] [Google Scholar]
- 34.Evans EB. Heterotopic bone formation in thermal burns. Clin Orthop Relat Res. 1991;(263):94–101. [PubMed]
- 35.DiGirolamo DJ, Germain-Lee EL. Effects of aging on bone. In: Kauffman TL, Scott R, Barr JO, Moran ML, eds. A Comprehensive Guide to Geriatric Rehabilitation. 3rd ed London, UK: Elsevier; 2014:14–18. [Google Scholar]
- 36.Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM. Gsα enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121(9):3492–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regard JB, Cherman N, Palmer D, Kuznetsov SA, Celi FS, Guettier JM, Chen M, Bhattacharyya N, Wess J, Coughlin SR, Weinstein LS, Collins MT, Robey PG, Yang Y. Wnt/β-catenin signaling is differentially regulated by Gα proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci USA. 2011;108(50):20101–20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19(11):1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marguet C, Mallet E, Basuyau JP, Martin D, Leroy M, Brunelle P. Clinical and biological heterogeneity in pseudohypoparathyroidism syndrome: results of a multicenter study. Horm Res. 1997;48(3):120–130. [DOI] [PubMed] [Google Scholar]
- 40.Adegbite NS, Xu M, Kaplan FS, Shore EM, Pignolo RJ. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A. 2008;146A(14):1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Sanctis L, Giachero F, Mantovani G, Weber G, Salerno M, Baroncelli GI, Elli MF, Matarazzo P, Wasniewska M, Mazzanti L, Scirè G, Tessaris D; Study Group Endocrine diseases due to altered function of Gsα protein of the Italian Society of Pediatric Endocrinology and Diabetology (ISPED) . Genetic and epigenetic alterations in the GNAS locus and clinical consequences in pseudohypoparathyroidism: Italian common healthcare pathways adoption. Ital J Pediatr. 2016;42(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Germain-Lee EL. Short stature, obesity, and growth hormone deficiency in pseudohypoparathyroidism type 1a. Pediatr Endocrinol Rev. 2006;3(Suppl 2):318–327. [PubMed] [Google Scholar]
- 43.Lemos MC, Thakker RV. GNAS mutations in pseudohypoparathyroidism type 1a and related disorders. Hum Mutat. 2014;36(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15(1):7–12. [DOI] [PubMed] [Google Scholar]
- 45.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40(1):46–62. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64(4):157–165. [DOI] [PubMed] [Google Scholar]
- 47.Weinstein LS, Gejman PV, de Mazancourt P, American N, Spiegel AM. A heterozygous 4-bp deletion mutation in the Gs α gene (GNAS1) in a patient with Albright hereditary osteodystrophy. Genomics. 1992;13(4):1319–1321. [DOI] [PubMed] [Google Scholar]
- 48.Huso D, McGuire S, Germain-Lee E. 2007 Heterotopic subcutaneous ossifications in a mouse model of Albright hereditary osteodystropy. Paper presented at The Endocrine Society 89th Annual Meeting; June 2, 2007; Toronto, Canada. [Google Scholar]
- 49.Huso DL, Edie S, Levine MA, Schwindinger W, Wang Y, Jüppner H, Germain-Lee EL. Heterotopic ossifications in a mouse model of Albright hereditary osteodystrophy. PLoS One. 2011;6(6):e21755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazansky MG. Complications revisited: the debit side of total hip replacement. Clin Orthop Relat Res. 1973;(95):96–103. [PubMed]
- 51.Morrey BF, Adams RA, Cabanela ME. Comparison of heterotopic bone after anterolateral, transtrochanteric, and posterior approaches for total hip arthroplasty. Clin Orthop Relat Res. 1984;(188):160–167. [PubMed]
- 52.Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty: predisposing factors, frequency, and effect on results. J Bone Joint Surg Am. 1977;59(3):345–351. [PubMed] [Google Scholar]
- 53.Søballe K, Christensen F, Kristensen SS. Ectopic bone formation after total hip arthroplasty. Clin Orthop Relat Res. 1988;(228):57–62. [PubMed]
- 54.Hu HP, Slooff TJ, van Horn JR. Heterotopic ossification following total hip arthroplasty: a review. Acta Orthop Belg. 1991;57(2):169–182. [PubMed] [Google Scholar]
- 55.DeLee J, Ferrari A, Charnley J. Ectopic bone formation following low friction arthroplasty of the hip. Clin Orthop Relat Res. 1976;(121):53–59. [PubMed]
- 56.Bastepe M, Gunes Y, Perez-Villamil B, Hunzelman J, Weinstein LS, Jüppner H. Receptor-mediated adenylyl cyclase activation through XLalpha(s), the extra-large variant of the stimulatory G protein alpha-subunit. Mol Endocrinol. 2002;16(8):1912–1919. [DOI] [PubMed] [Google Scholar]
- 57.Pignolo RJ, Xu M, Russell E, Richardson A, Kaplan J, Billings PC, Kaplan FS, Shore EM. Heterozygous inactivation of Gnas in adipose-derived mesenchymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J Bone Miner Res. 2011;26(11):2647–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Convente MR, Wang H, Pignolo RJ, Kaplan FS, Shore EM. The immunological contribution to heterotopic ossification disorders. Curr Osteoporos Rep. 2015;13(2):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]