Abstract
In response to an acute threat to homeostasis or well-being, the hypothalamic-pituitary-adrenocortical (HPA) axis is engaged. A major outcome of this HPA axis activation is the mobilization of stored energy, to fuel an appropriate behavioral and/or physiological response to the perceived threat. Importantly, the extent of HPA axis activity is thought to be modulated by an individual’s nutritional environment. In this study, we report that nutritional manipulations signaling a relative depletion of dietary carbohydrates, thereby inducing nutritional ketosis, acutely and chronically activate the HPA axis. Male rats and mice maintained on a low-carbohydrate high-fat ketogenic diet (KD) exhibited canonical markers of chronic stress, including increased basal and stress-evoked plasma corticosterone, increased adrenal sensitivity to adrenocorticotropin hormone, increased stress-evoked c-Fos immunolabeling in the paraventricular nucleus of the hypothalamus, and thymic atrophy, an indicator of chronic glucocorticoid exposure. Moreover, acutely feeding medium-chain triglycerides (MCTs) to rapidly induce ketosis among chow-fed male rats and mice also acutely increased HPA axis activity. Lastly, and consistent with a growing literature that characterizes the hepatokine fibroblast growth factor-21 (FGF21) as both a marker of the ketotic state and as a key metabolic stress hormone, the HPA response to both KD and MCTs was significantly blunted among mice lacking FGF21. We conclude that dietary manipulations that induce ketosis lead to increased HPA axis tone, and that the hepatokine FGF21 may play an important role to facilitate this effect.
Inducing dietary ketosis by two distinct mechanisms (one acute and one chronic) results in HPA axis activation, and these HPA effects are attenuated in mice that lack FGF21.
In response to an acute threat to homeostasis or well-being, stress regulatory systems are engaged. One of the primary physiological responses to stress is activation of the hypothalamic-pituitary-adrenocortical (HPA) axis (1). In this system, information regarding the presence of a stressor is conveyed to hypophysiotropic neurons in the hypothalamic paraventricular nucleus (PVN). Activation of these neurons promotes the release of adrenocorticotropin hormone (ACTH) into systemic circulation, which acts on the adrenal cortex to release glucocorticoids (i.e., corticosterone in rodents and cortisol in humans). Glucocorticoids exert powerful effects throughout the body, including to enhance liver gluconeogenesis and mobilize stored energy, providing fuel for behavioral and physiological responses that promote survival during acute stress exposure. During chronic stress, excessive, prolonged, or repeated HPA axis activation results in marked changes in HPA axis tone (1). As a result, chronic stress typically leads to elevated basal/nonstress corticosterone secretion (which may occur despite normal nonstress ACTH secretion), habituated responses to homotypic stressors, facilitated responses to heterotypic stressors, overall increased adrenal responsivity to ACTH, and adrenal gland hypertrophy in rats (2–16) [Although the extent to which each of these effects occur can vary among species and/or rodent strains (10).] Importantly, chronic elevations in HPA tone, as occurs during chronic stress, are often associated with negative side effects, including depression and anxiety disorders (17).
The extent of HPA axis activity is thought to be influenced by an individual’s nutritional environment (18). For instance, when chow-fed rats were also provided free access to 30% sucrose drink for 10 days, both the ACTH and corticosterone response to an acute restraint stress were reduced (19). Similarly, free access to sucrose drink was sufficient to prevent the marked increase in central HPA axis tone that occurs following adrenalectomy. Whereas adrenalectomized control rats exhibited increased corticotropin-releasing hormone (CRH) messenger RNA expression in the PVN, resulting from lack of glucocorticoid negative feedback, this was abrogated among adrenalectomized counterparts consuming the 30% sucrose (19). Moreover, high dietary carbohydrates are associated with reduced plasma glucocorticoids in both people and experimental animals (20–22). Collectively, these findings indicate HPA axis tone may be inversely related to carbohydrate status, such that high glucose availability leads to decreased HPA activation.
Conversely, we reasoned that HPA axis tone may be increased during ketosis, a distinctive metabolic state that occurs when available glucose (the primary fuel of brain and body) is insufficient for metabolic needs. In response to this metabolic crisis, the liver converts fatty acids to ketone bodies, providing an alternate fuel source for the brain and other organs. Consistent with this idea, prolonged fasting induces ketosis and is accompanied by elevated glucocorticoids (23). Ketosis can be induced even in the absence of a fast (i.e., when excess calories are available), for example, by dramatically restricting carbohydrate consumption in favor of dietary fats. The implications of this “dietary” ketosis for stress system function are unknown, despite the fact that low-carbohydrate ketogenic diets (KDs) are popular and effective for weight loss and are thought to be useful for the treatment of various diseases (24, 25). In this study, we tested the hypothesis that dietary manipulations that acutely and chronically induce ketosis lead to acute and chronic HPA activation, respectively.
Fibroblast growth factor-21 (FGF21) is an important stress-regulatory hormone that is produced by the liver during metabolic stressors including ketosis (26–30) and that crosses the blood-brain barrier to act on its receptors in HPA-regulatory brain regions, including the PVN (31, 32). Recent evidence supports that FGF21 acts via the PVN to activate the HPA axis and increase corticosterone secretion in vivo (23). We therefore investigated the possibility that increased FGF21 signaling contributes to the HPA effects of dietary ketosis.
Materials and Methods
Animals
Adult male Long-Evans (∼225 to 275 g) rats were purchased from Harlan. Adult male C57BL/6J mice were bred in-house from breeders obtained from the Jackson Laboratories. Adult, male FGF21-deficient [knockout (KO)] and wild-type (WT) control mice were bred in-house as previously described (33) and maintained in our facilities on a C57Bl/6J background. All animals were singly housed on a 12-hour light, 12-hour dark cycle (lights typically on at 06:00 hours) in a temperature (22°C) and humidity-controlled vivarium with ad libitum access to food and water unless otherwise noted. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and/or the University of California- Davis, and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Diet treatments, food intake, body weight, and body composition
Normal chow (LM-485; Harlan Teklad) contained 5% fat, 19% protein, and 44% carbohydrate by weight. KD (F3666; Bio-Serv) contained 75% fat, 9% protein, and 3% carbohydrate by weight. To acutely induce ketosis in chow-fed animals (34), we delivered medium-chain triglycerides (MCTs; Neobee 895; Stepan Lipid Nutrition) by orogastric gavage. In these studies, gavage of long-chain triglycerides (LCTs; pure corn oil) was used to control for the thermogenic effect of the triglycerides.
Food intake and body weight were monitored regularly, as noted, and body composition was assessed by time domain nuclear magnetic resonance. Body length was measured along the dorsal body surface as the distance between the nose and the base of the tail.
Blood sample collection and plasma measures
For HPA axis assessments, tail blood samples (200 μL for rats; 20 μL for mice) were collected at the indicated time points into chilled tubes containing EDTA. For basal (prestress) measures, sample collection was completed in less than 3 minutes from first handling each rat's cage, thereby ensuring plasma ACTH and corticosterone levels that were reflective of the basal, unstressed state (35). Similarly, when we challenged rats and mice with 20 minutes of restraint in a well-ventilated Plexiglas (rats) or polyethylene (mice) tube, it took less than 3 minutes to collect each poststress blood sample. Note that in all cases, the total collected blood volume represents ≤5% of the total blood volume, an amount that is well below the threshold for HPA activation (17, 36). Blood samples were centrifuged (3000g, 15 minutes, 4°C) and plasma was stored at −20°C until measurement of immunoreactive ACTH and corticosterone by radioimmunoassay as described previously (37). For mice, the smaller blood collection volume precluded measurement of plasma ACTH. Integrated plasma corticosterone responses were calculated as the area under the curve (AUC) of the corticosterone time course data. Rats were also perfused at 1 hour after restraint stress onset, with collection of brains for cFos immunolabeling in the PVN (as described later in the Brain Immunolabeling section). cFos is an immediate-early gene induced following membrane depolarization and is often used as a general marker of neuronal activation (38). Elevated cFos immunolabeling is typically observed within 30 to 60 minutes after stress onset and remains elevated through at least 2 hours (38–40), and reportedly up to 4 hours after stress onset (41). Rat adrenal and thymus glands were removed and weighed as indirect indices of chronic ACTH and glucocorticoid tone, respectively.
To assess adrenal responsiveness to ACTH, rats were first given a maximal dose of dexamethasone [800 µg dexamethasone phosphate (Sigma-Aldrich) in 200 µL saline vehicle; subcutaneous] to block endogenous ACTH release. At 2 hours after dexamethasone treatment, rats were given exogenous rat ACTH [150 ng/kg body weight; vehicle was 0.5% bovine serum albumin in phosphate-buffered saline (PBS), subcutaneous; Bachem]. At 15 minutes after ACTH treatment, rats were euthanized by rapid decapitation with collection of trunk blood for measurement of plasma corticosterone, as described previously.
Plasma β-hydroxybutyrate (the primary circulating ketone body) was measured to indicate the presence and degree of ketosis. For rats, tail blood samples were collected into chilled heparin-coated tubes as described previously and plasma β-hydroxybutyrate was measured using KetoSite test cards in the STAT-Site analyzer system (Stanbio Laboratory). For mice, tail blood samples were directly measured using blood ketone test strips in the Precision Xtra meter (Abbott) because this approach requires significantly less blood volume. Among mice that received MCT gavage, some were not in ketosis at the time of β-hydroxybutyrate measurement (which was performed as a positive control for the presence of ketosis), possibly because the stomach contents of the nonfasted animals affected MCT dynamics. As such, mice that received MCT gavage with β-hydroxybutyrate levels below 1 mM were removed from the HPA assessment; this β-hydroxybutyrate threshold was set before measuring the corticosterone values, and these instances occurred regardless of genotype. Plasma FGF21 was measured by enzyme-linked immunosorbent assay (Rat/Mouse FGF21 ELISA kit; Millipore).
Behavioral assays
Rats were tested in a standard test of behavioral anxiety [elevated plus-maze (EPM)] as well as a standard test for depressive-like behavior [forced swim test (FST)]. For the EPM, rats were placed onto the maze apparatus for 5 minutes in a dimly lit room. Video-recordings of the ensuing behavior were scored for the number of open arm entries as an index of behavioral anxiety as well as for the total number of open plus closed arm entries as an index of locomotor activity (42). For the FST, rats were individually placed for 10 minutes into a cylinder filled with water sufficiently deep to prevent standing on the bottom. Video-recordings were scored for the amount of time spent immobile (defined as doing on the minimal movements necessary to prevent drowning) as an index of depressive-like behavioral despair (43, 44). In all cases, behavior was scored by an individual unaware of group assignments.
Intracerebroventricular cannula implantation and infusions
Rats were outfitted with stainless-steel cannulas as described previously (45), with the exception that they were directed at the lateral ventricle (stereotaxic coordinates: 1.4 mm lateral from midline, 0.8 mm posterior from bregma, and 3.6 mm ventral from dura). Rats were regularly handled for habituation to cannula insertion and removal. Human recombinant FGF21 (ProSpec) was diluted into PBS vehicle for intracerebroventricular (ICV) administration at a dose of 3 μg in 3 μL vehicle. The HPA axis response to acute ICV FGF21 (vs vehicle) was tested, with blood samples collected for measurement of plasma ACTH and corticosterone, as described previously, just before (0 minutes) and at 1, 2 and 3 hours after ICV infusion. Several weeks later (to allow abundant washout time), rats again received acute ICV FGF21 (vs vehicle); at 2 hours after ICV infusion, animals were perfused with collection of brains for PVN immunolabeling (as described in the following section). The 2-hour time point was selected to allow sufficient time for FGF21 to activate the PVN, and then initiate the expression of cFos protein which peaks at ∼1 to 2 hours after PVN activation (46, 47). Brains were postfixed for ∼16 hours at room temperature and then stored in sucrose (30% in PBS) at 4°C until microtome sectioning (see the following section). Cannula location was verified histologically; n = 4 rats with cannulas that missed the lateral ventricle were removed from all analyses. In addition, one rat had an abnormally short and stiff tail (possibly from a developmental defect) precluding collection of tail blood samples for HPA assessment; this rat could still be used for assessment of brain immunolabeling following ICV infusion.
Brain immunolabeling
Brains were sectioned (25 µm) on a microtome, and the sections were stored in cryoprotectant (0.1 M PBS, 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol) at −20°C. Sections were immunolabeled (Table 1) with rabbit primary antiserum directed against cFos (1:5000; catalog no. sc-52; Santa Cruz Biotechnology; Research Resource Identifier: AB_2106783) via a standard immunolabeling procedure (48). Immunolabeling was not present when the primary antibody was omitted. Positive immunolabeling was detected by use of biotin-conjugated goat antirabbit secondary antibody (Vector Laboratories) followed by incubation with avidin-biotin-peroxidase (Vectastain ABC solution; Vector Laboratories) and reaction with 3,3′-diaminobenzidine. Immunolabeling was imaged using brightfield light microscopy (Zeiss Imager.M2 microscope with Apotome, AxioCam camera, Zen 2011 software; Carl Zeiss). Positive cells were counted from all available, intact sections at the mid-PVN rostral-caudal level [between approximately −1.8 to −2.0 mm from bregma (49) using Image J (W. Rasband, National Institutes of Health)] software. This typically resulted in bilateral quantification of one to three sections of PVN. These values were then averaged to obtain the representative cell count for each rat. Analyses were performed by personnel unaware of group assignments.
Table 1.
Antibody Used for Immunolabeling
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| cFos | Anti-cFos | Santa Cruz Biotechnology, sc-52 | Rabbit; polyclonal | 1:5000 | AB_2106783 |
Abbreviation: RRID, Research Resource Identifier.
Statistical analyses
Data are shown as mean ± standard error of the mean. When comparing two treatment groups, data were analyzed by two-tailed t test (for parametric) or Mann-Whitney test (for nonparametric). For multiple group comparisons, data were analyzed by analysis of variance (ANOVA), with repeated measures when appropriate, followed by Neuman-Keuls or two-tailed Dunnett post hoc analysis. For ANOVA, if the variance between treatment groups was not homogenous, analyses were performed following square root transformation. Potential outliers were evaluated using two standard outlier tests and were removed only if they failed both tests, as described previously (46). Statistical significance was taken as P < 0.05.
Results
Low-carbohydrate KD activates the HPA axis
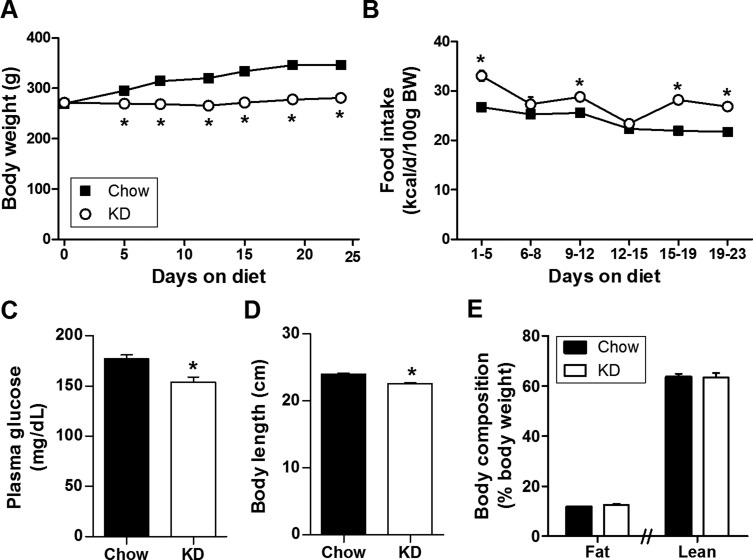
First, we maintained age-matched groups of adult male Long-Evans rats on a standard low-carbohydrate, high-fat KD or normal chow for 2 to 3 weeks. In agreement with a number of previous rodent studies (26, 50–52), and with the widespread popularity of KDs for weight loss, KD-fed rats gained less body weight despite consuming more daily calories (Fig. 1A and 1B). KD lowered morning, postprandial plasma glucose levels, consistent with the low-carbohydrate content of the diet (Fig. 1C), and also modestly (∼6%) reduced body length, consistent with the observation that KDs inhibit linear growth (Fig. 1D) (53–55). KD-fed rats were therefore smaller overall, but had equivalent total body composition (Fig. 1E).
Figure 1.
Low-carbohydrate KD reduced body size despite adequate caloric intake. (A) Body weight (BW; two-way ANOVA), (B) caloric intake (two-way ANOVA), (C) morning postprandial plasma glucose (two-tailed t test), and (D) final body length (two-tailed t test), of rats given KD (vs normal chow) for 23 days. n = 12 to 13 per group. (E) Final percent body fat (left: two-tailed t test) and lean (right: two-tailed t test) of rats given KD (vs normal chow) for 14 days. n = 10 to 11 per group. *P < 0.05 vs chow.
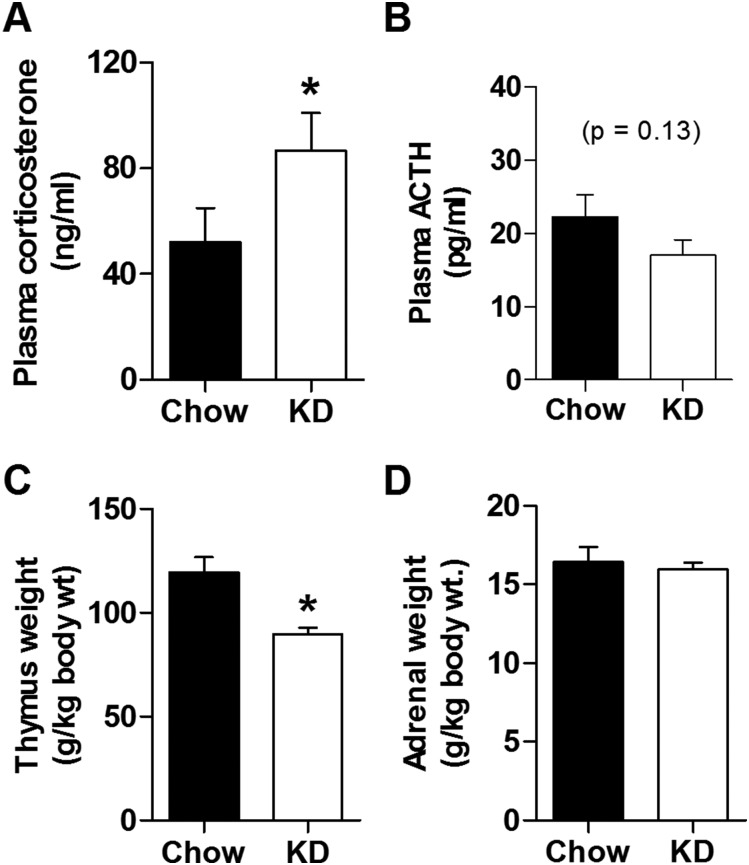
Next we explicitly tested the hypothesis that, as with other chronic stressors, KD facilitates HPA axis tone, resulting in elevated basal and stress-evoked HPA responses. We found KD-fed rats have increased morning basal (nonstress) plasma corticosterone compared with chow-fed controls, with unaltered plasma ACTH (Fig. 2A and 2B). KD-fed rats also exhibited thymic atrophy (Fig. 2C), consistent with chronic exposure to elevated glucocorticoids, with no change in adrenal gland weight (Fig. 2D). Notably, this pattern of elevated morning nonstress corticosterone, normal resting plasma ACTH, thymic involution, and normal adrenal weight is consistent with our previous studies in which Long-Evans rats were exposed to chronic variable stress (56–58), suggesting that chronic KD-feeding enhances basal HPA axis tone in a manner that resembles chronic stress exposure.
Figure 2.
KD increased resting plasma corticosterone in the morning. Basal, unstressed plasma (A) corticosterone (two-tailed Mann-Whitney test) and (B) ACTH (two-tailed Mann-Whitney test) sampled near the circadian nadir after 19 to 23 days of KD feeding (vs normal chow). n = 24 to 26 per group. (C) Thymus (two-tailed Mann-Whitney test) and (D) adrenal (two-tailed Mann-Whitney test) weights normalized to body weight for rats given KD for 23 days. n = 12 to 13 per group. *P < 0.05 vs chow.
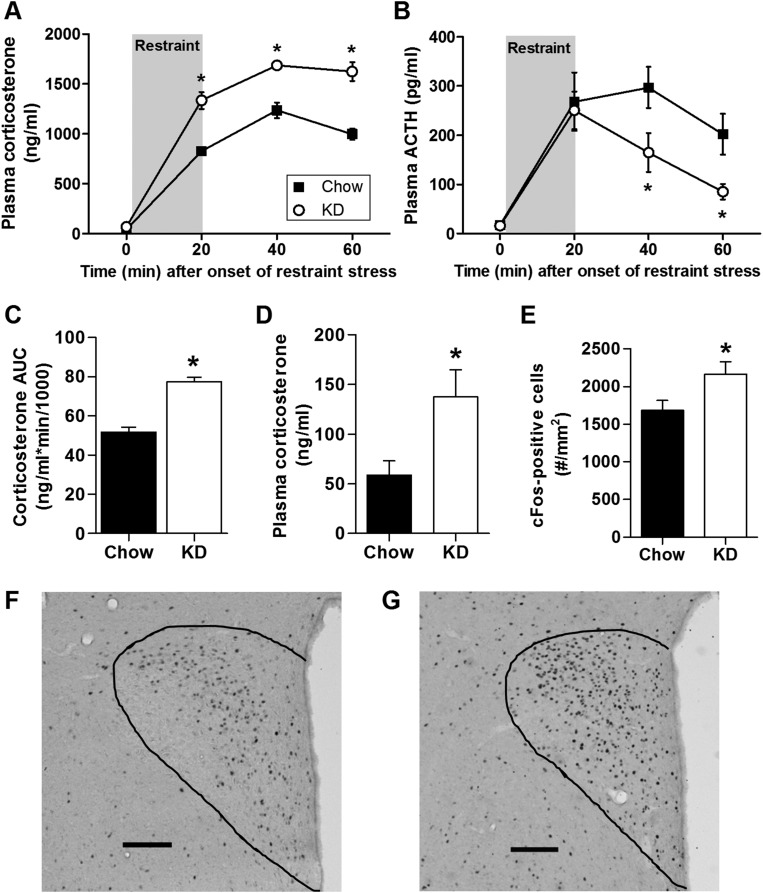
When challenged with a 20-minute acute restraint stress, KD-fed rats exhibited an exaggerated plasma corticosterone response (Fig. 3A and Fig. 3C). The initiation of the plasma ACTH response to restraint was unaffected by KD, and ACTH levels returned to baseline more rapidly after the onset of restraint (Fig. 3B), suggesting the possibility that the history of elevated basal (nonstress) plasma corticosterone exerted negative feedback on the HPA axis to limit the extent and duration of the ACTH response to acute restraint stress. This pattern of elevated basal and poststress corticosterone levels, despite normal basal and poststress ACTH, may implicate greater adrenal responsiveness to ACTH. We tested this directly, by first injecting rats with dexamethasone to block endogenous ACTH release, and then challenging them with exogenous ACTH. Rats maintained on a KD exhibited greater plasma corticosterone following the ACTH challenge, compared with chow-fed controls (Fig. 3D). Thus, we conclude that chronic consumption of KD is associated with greater adrenal responsivity to ACTH.
Figure 3.
KD increased the plasma corticosterone response to an acute psychological stressor. Time course of the plasma (A) corticosterone and (B) ACTH response to a 20-minute restraint stress in rats consuming KD (vs normal chow) for 23 days (two-way ANOVA). (C) The AUC of the corticosterone response shown in panel (A) (two-tailed t test). n = 12 to 13 per group. (D) Plasma corticosterone response to ACTH treatment (150 ng/kg body weight) in dexamethasone-blocked rats (two-tailed t test) after 15 days on KD. n = 5 to 6 per group. (E) Activation of cFos-positive cells in the paraventricular nucleus of the hypothalamus 1 hour after the onset of a 20-minute restraint stress in rats consuming KD for 23 days (two-tailed t test). n = 12 per group. *P < 0.05 vs chow. (F and G) Representative images of cFos-positive cells in the PVN of (F) chow-fed and (G) KD-fed rats 1 hour after the onset of a 20-minute restraint stress. The outline denotes the border of the PVN. Scale bar = 100 μm.
Because the brain plays a key role to initiate stress responses, and because chronic stress facilitates HPA responses to a novel stressor, we hypothesized that KD-fed rats would have greater restraint-induced neuronal activation in stress-regulatory brain regions compared with chow-fed controls. To test this, we euthanized rats 1 hour after the restraint stress challenge and collected their brains for cFos immunolabeling. Consistent with the increased corticosterone response, we observed a greater number of cell bodies expressing cFos in the PVN of KD-fed rats (Fig. 3E–3G).
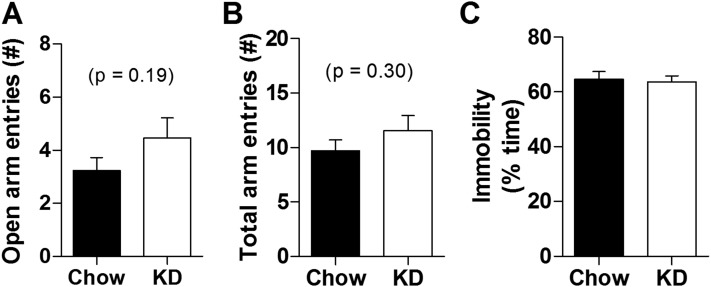
Because elevated HPA axis tone is often accompanied by increased depressive-like and anxiety-related behavior, we reasoned that KD may affect these types of behavior. To test this possibility, a new cohort of rats was maintained on either KD or normal chow and their behavior was assessed in the EPM and FST. The EPM test was performed on day 29 of diet treatment. KD did not alter the number of open arm entries (Fig. 4A) nor the total number of arm entries (i.e., open + closed; Fig. 4B), indicating that KD did not alter either anxiety-related behavior or total locomotion in this test. Moreover, KD did not alter the percent of time spent immobile on the FST that was performed on day 32 of diet exposure (Fig. 4C). Collectively these behavioral data suggest that anxiety- and depressive-like behaviors are largely unaffected by KD, although it is still possible that KD could affect behavior in other types of tests.
Figure 4.
KD had little impact on anxiety- and depression-related behaviors in EPM and FST, respectively. (A) Number of open arm (two-tailed t test) and (B) number of total (open plus closed) arm entries in the EPM on day 29 of KD (vs normal chow) consumption. (C) Percent of time spent immobile in the FST (two-tailed t test) on day 32 of KD (vs normal chow) treatment. n = 11 to 13 per group. *P < 0.05 vs chow.
Ketogenic MCTs activate the HPA axis
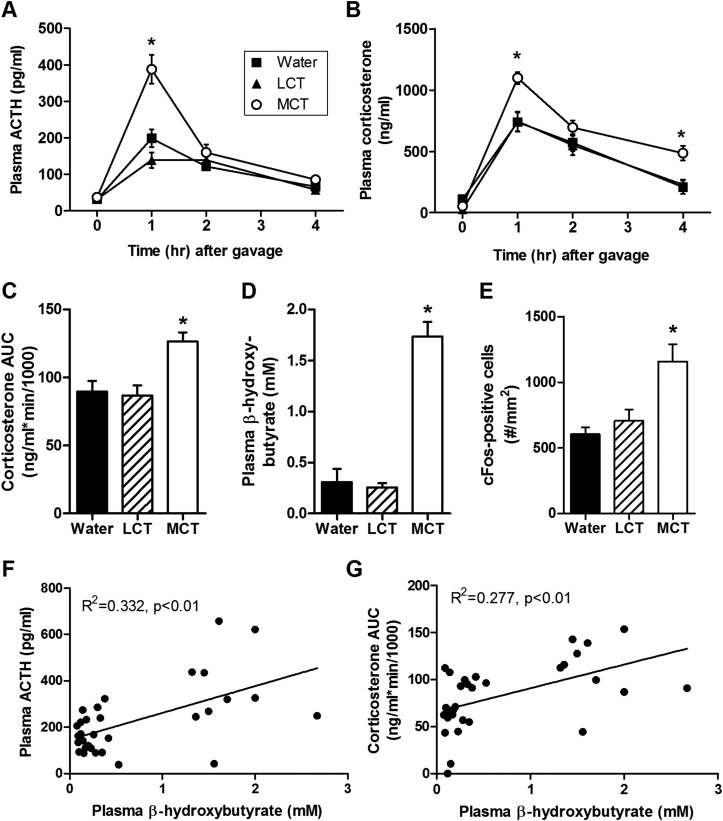
To acutely induce dietary ketosis without chronic alterations to macronutrient content of the diet, we administered MCTs by oral gavage to adult chow-fed rats. Unlike LCTs, dietary fatty acids from MCT (chain length of C6 to C12) are absorbed in the portal system and are carried directly to the liver, where they are rapidly oxidized to ketone bodies (34, 59, 60). Age- and weight-matched control groups were administered an equal volume of either LCT or water. As expected, rats receiving MCT gavage exhibited a rapid increase in the plasma ketone body β-hydroxybutyrate relative to both control groups (Fig. 5D). Concurrently, rats receiving MCT gavage exhibited robust activation of the HPA axis. In this case, both plasma ACTH (Fig. 5A) and corticosterone (Fig. 5B and 5C) were increased by the stress of handling and gavage, and this was significantly exaggerated among chow-fed MCT-treated rats relative to chow-fed LCT- and water-treated controls. Moreover, both the plasma ACTH (Fig. 5F) and corticosterone (Fig. 5G) responses were linearly related to the postgavage levels of plasma β-hydroxybutyrate.
Figure 5.
Ketogenic MCT activated the HPA axis. Time course of the plasma (A) ACTH and (B) corticosterone response to gavage of 3 mL MCT, LCT, or water in chow-fed rats (two-way ANOVA). (C) The AUC of the corticosterone response shown in panel A (one-way ANOVA). n = 8 to 12 per group. (D) Plasma β-hydroxybutyrate in rats 1 hour after gavage of MCT, LCT, or water in chow-fed rats (one-way ANOVA). (E) Activation of cFos-positive cells in the PVN of the hypothalamus 2 hours after gavage of MCT, LCT, or water in chow-fed rats (one-way ANOVA). n = 9 to 11 per group. *P < 0.05 vs both LCT and water. (F) Relationship between plasma ACTH vs plasma β-hydroxybutyrate 1 hour after gavage of MCT, LCT, or water in chow-fed rats (linear regression, P < 0.01). (G) Relationship between the AUC of the corticosterone response vs plasma β-hydroxybutyrate 1 hour after gavage of MCT, LCT, or water in chow-fed rats (linear regression, P < 0.01).
Again, because the brain plays a key role to initiate stress responses, we hypothesized that MCT-fed rats would have greater neuronal activation in stress-regulatory brain regions compared with LCT and water controls. To test this, we euthanized a new cohort of rats at 2 hours following MCT, LCT, or water gavage and collected their brains for cFos immunolabeling. Consistent with the increased corticosterone and ACTH response, we observed a greater number of cell bodies expressing cFos in the PVN (Fig. 5E). Thus, acute dietary (MCT) ketosis elicited an acute stress response, characterized by elevated ACTH and corticosterone. Chronic dietary (KD) ketosis, on the other hand, increased adrenal responsivity to ACTH, resulting in a larger corticosterone response to stress despite an equivalent ACTH response. Such a pattern of elevated adrenal responsivity and disproportionate glucocorticoid-to-ACTH responses resembles that which occurs during chronic stress (3, 61–66). Accordingly, inducing dietary ketosis by two distinct mechanisms (one acute and one chronic) resulted in typical patterns of HPA axis activation by acute and chronic stress, respectively (Table 2).
Table 2.
Summary of the HPA Effects of Acute (MCT) and Chronic KD Dietary Ketosis and Their Resemblance to Typical Acute vs Chronic Stress Responses, Respectively
| HPA axis response to acute stress (46, 70) | HPA axis response to acute ketosis | HPA axis response to chronic stress | HPA axis response to chronic ketosis |
|---|---|---|---|
| Increased PVN cFos | Increased PVN cFos | Increased morning basal plasma corticosterone which may occur despite normal ACTH (7, 8, 10, 12–15, 57) | Increased morning basal plasma corticosterone despite normal ACTH |
| Increased plasma ACTH | Increased plasma ACTH | Reduced thymus weight (3, 11, 12, 57) | Reduced thymus weight |
| Increased plasma corticosterone | Increased plasma corticosterone | Increased adrenal weight (2, 3, 10–16) | No change in adrenal weight |
| Increased adrenal responsivity to ACTH (2, 16) | Increased adrenal responsivity to ACTH | ||
| Facilitated corticosterone responses to a heterotypic (e.g., restraint) stress (4, 8, 16, 57) | Facilitated corticosterone responses to a heterotypic (e.g., restraint) stress |
FGF21 contributes to HPA activation by low-carbohydrate KD and by ketogenic MCTs
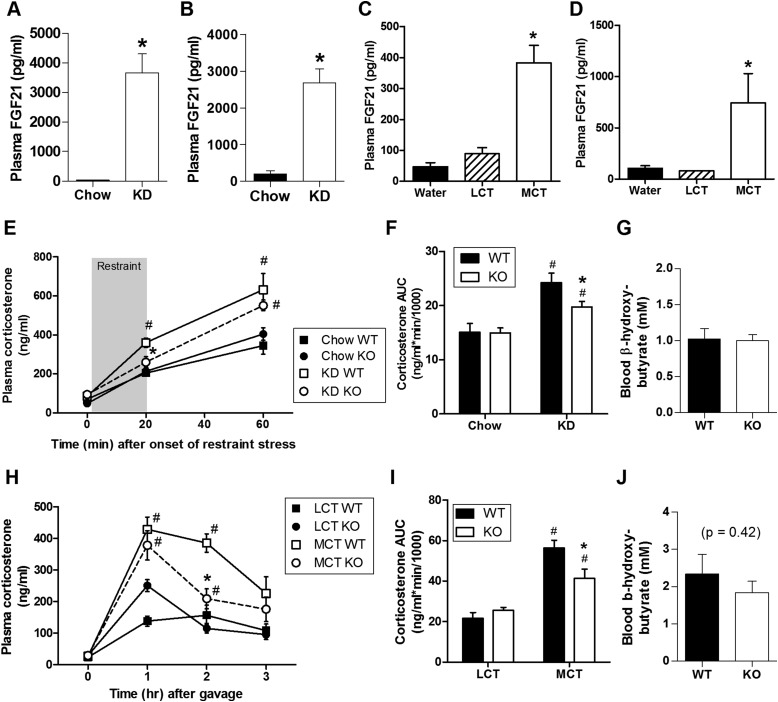
During ketosis, the liver produces the hormone FGF21, an endocrine member of the FGF superfamily (67). Importantly, FGF21 crosses the blood-brain barrier (31) and is thought to activate the HPA axis by stimulating CRH neurons in the PVN (23, 68, 69). Consistent with this, when we delivered either FGF21 (3 μg) or its vehicle acutely to the lateral ventricle of male rats, we observed significantly increased immunolabeling for cFos (Fig. 6A) in the PVN, together with greater circulating ACTH and corticosterone (Fig. 6B–6D). In light of this, we hypothesized that FGF21 contributes to HPA activation following dietary ketosis. Consistent with this possibility, plasma FGF21 was greater among both rats (Fig. 7A) and mice (Fig. 7B) maintained on KD compared with chow-fed controls. Likewise, plasma FGF21 was greater among both rats (Fig. 7C) and mice (Fig. 7D) receiving MCT gavage compared with LCT and water control groups.
Figure 6.
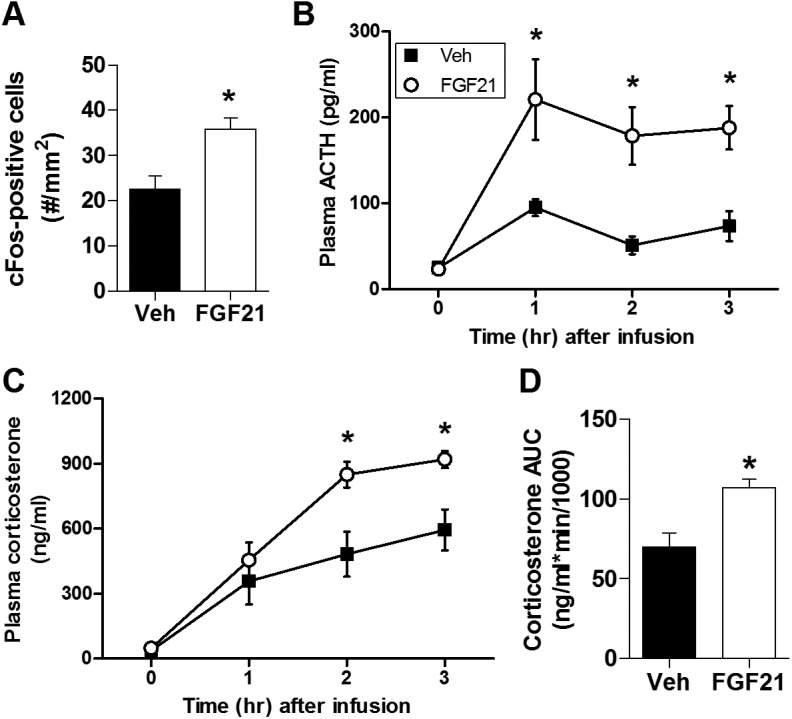
Central FGF21 administration activated the HPA axis. (A) cFos-positive (two-tailed t test) cells in the PVN 2 hours after ICV infusion of 3 μg FGF21 [vs vehicle (Veh)-treated controls]. n = 8 to 10 per group. Time course of the plasma (B) ACTH and (C) corticosterone response to ICV infusion of 3 μg FGF21 or vehicle (two-way ANOVA). (D) The AUC of the corticosterone response shown in panel C (two-tailed t test). n = 7 to 10 per group. *P < 0.05 vs Veh.
Figure 7.
FGF21 contributed to HPA activation by low-carbohydrate KD and by ketogenic MCT. Plasma FGF21 in (A) rats after 7 days on KD vs chow (two-tailed Mann-Whitney test, n = 7 to 8 per group, *P < 0.05 vs chow), (B) mice after 7 days on KD vs chow (two-tailed Mann Whitney test, n = 11 per group, *P < 0.05 vs chow), (C) rats 1 hour after gavage of 3 mL MCT, LCT, or water (one-way ANOVA, n = 9 to 11 per group, *P < 0.05 vs both LCT and water) and (D) mice 2 hours after gavage (200 μL) of MCT, LCT, or water (one-way ANOVA, n = 4 to 6 per group, *P < 0.05 vs both LCT and water). (E) Time course (three-way ANOVA) and (F) AUC (two-way ANOVA) of the plasma corticosterone response to a 20-minute restraint stress in WT and FGF21-deficient (KO) mice consuming KD (vs normal chow) for 20 days prior (*P < 0.05 vs KD WT, #P < 0.05 vs respective chow group). (G) Blood β-hydroxybutyrate after 3 weeks of KD feeding in KO and WT mice (two-tailed t test, P = 0.86). n = 6 to 11 per group. (H) Time course (three-way ANOVA), and (I) AUC (two-way ANOVA) of the plasma corticosterone response to gavage of 200 μL MCT or LCT in chow-fed WT and KO mice (*P < 0.05 vs MCT WT, #P < 0.05 vs respective LCT-treated group). (J) Blood β-hydroxybutyrate 1 hour after gavage of MCT in chow-fed WT and KO mice (two-tailed t test, P = 0.42). n = 6 to 12 per group.
To test the potential contribution of FGF21 to mediate the HPA response to dietary ketosis, we compared the effects of KD on HPA activity in FGF21-deficient (KO) mice and WT controls. Three weeks of KD feeding resulted in equivalent levels of blood β-hydroxybutyrate in KO and WT mice (Fig. 7G). Nonetheless, although the plasma corticosterone response to restraint stress was clearly enhanced by KD in both genotypes, this was significantly attenuated in FGF21-deficient mice (Fig. 7E and 7F). Similarly, we compared the effects of MCT gavage in FGF21-KO and WT control mice. Plasma corticosterone was increased after MCT (vs LCT) in both genotypes, and this was significantly reduced in FGF21-deficient mice (Fig. 7H–7I) despite equivalent levels of ketosis (Fig. 7J). These data suggest that FGF21 contributes to a portion of the robust increase in glucocorticoids that occur during ketosis.
Discussion
Impact of dietary ketosis on the HPA axis and stress-related behavior
The present work tested the hypothesis that dietary manipulations that acutely and chronically induce ketosis lead to indices of acute and chronic HPA activation, respectively. Acute dietary ketosis, induced by MCT gavage in chow-fed rats, elevated plasma ACTH and corticosterone and increased cFos-positive cells in the PVN compared with both LCT and water gavage controls. The pattern and time course of these effects parallel those that occur following a variety of other acute stressors, including restraint, foot shock, and loud noise (46, 57, 70–72), consistent with the idea that acute dietary ketosis is a metabolic stressor that rapidly and transiently activates the HPA axis (Table 2).
When longer term feeding with a low-carbohydrate, high-fat diet was used to chronically induce dietary ketosis, body weight gain and final body length were attenuated despite greater caloric intake, consistent with prior findings using KDs (53, 54). KD feeding was then used to ask whether this alters HPA axis tone in a manner similar to chronic stress. Chronic stress enhances basal HPA tone and facilitates responses to a novel acute stressor (although the precise pattern can vary somewhat between species and strains) (10). More specifically, these effects of chronic stress include increased basal/nonstress plasma corticosterone near the nadir of the circadian rhythm (which may occur despite normal nonstress plasma ACTH), reduced thymus weight, increased adrenal weight, enhanced adrenal responsivity to ACTH, and elevated plasma corticosterone and PVN cFos responses to a novel stressor (2, 4, 7, 8, 12–16, 57). Importantly, the HPA effects of KD closely resemble this pattern (Table 2), with the only exception that KD did not affect adrenal weight, a finding that is not surprising because in our hands Long-Evans rats typically demonstrate little-to-no increase in adrenal weight following chronic stress (56–58). The ability of both chronic stress and KD to increase adrenal responsivity to ACTH is particularly noteworthy because it suggests that the adrenal secretes a greater amount of glucocorticoid for a given amount of ACTH hormone. This effect may explain, at least in part, KD-induced elevations in basal and postrestraint plasma corticosterone that occurred without concomitant KD-induced increases in plasma ACTH.
Because elevated HPA axis tone is often accompanied by anxiety- and depressive-like behaviors (17), the behavioral impact of longer term KD was tested in EPM and FST. Collectively, these data suggest that KD does not markedly alter anxiety- and depressive-like behaviors in either of these tests, indicating that KD does not uniformly alter all stress-related end points. The findings also underscore the fact that stress-related behaviors like anxiety and depression are controlled by complex and partially overlapping networks of brain circuitry whose function is not governed exclusively by glucocorticoid levels (73, 74).
The precise mechanisms by which chronic stressors facilitate basal and HPA axis tone are not fully known, but likely involve both central (brain) and peripheral (adrenal) mechanisms. Several stress-regulatory brain regions have been implicated in regulating the HPA response to chronic stress (1). These regions may influence HPA axis tone centrally, for example by directly or indirectly modulating PVN hypophysiotropic neuron activity. Stress-regulatory brain regions may also act in the periphery, for example by modulating adrenal responsivity to ACTH via the adrenal sympathetic innervation and/or via the trophic effects of prior ACTH secretion (3, 65, 67, 75). Moreover, a host of peripheral actors, such as circulating hormones and metabolites, could act directly on the adrenal gland to modulate its responsivity (65, 76, 77). Finally, signals from the periphery (either hormonal or neural sensory signals) could act directly or indirectly on brain stress-regulatory regions to alter HPA activity during chronic stress (18). Given this wide scope of potential mediators, the present work explored the potential role of hormonal FGF21 because it is elevated during ketosis in rodents and is linked with HPA axis activation (as detailed in the following section).
Contribution of FGF21 to ketosis-induced HPA axis activation
The present work investigated the possibility that increased FGF21 signaling contributes to the HPA effects of dietary ketosis. Both MCT gavage and KD increased plasma FGF21, consistent with prior observations that hormonal FGF21 is produced by the liver during a variety of metabolic stressors, including ketosis. Moreover, FGF21 can cross the blood-brain barrier and its receptors are expressed in the PVN, suggesting the FGF21 is well-positioned to alter PVN activity (23, 31). Consistent with this idea, previous reports convincingly demonstrate that direct ICV infusion of FGF21 activates the HPA axis in a CRH-dependent manner (23). The present data corroborate these findings; ICV administration of FGF21 increased HPA axis activation relative to vehicle controls and increased cFos immunolabeling in the PVN. It should be noted that the density of cFos-positive cells in the PVN varied markedly between experiments depending on the particulars of each experimental design. The highest cFos density was observed after restraint stress (Fig. 3E), more moderate levels occurred after gavage (Fig. 5E), and the lowest levels occurred after ICV drug administration in rats that were prehabituated to cannula manipulation (Fig. 6A). The differing extent of cFos responses likely reflect the relative intensity of stress associated with each experimental procedure (46). Moreover, whereas ICV FGF21 significantly increased the number of cFos-positive neurons in the PVN, the relatively low density of these neurons may indicate that central FGF21 signaling alone is a modest regulator of PVN activation. Alternatively, FGF21 receptors may act primarily via intracellular signaling pathways that are not strongly coupled to cFos expression.
To more directly test the potential role of FGF21 in mediating the HPA response to dietary ketosis, we compared the effects of KD and MCT gavage on HPA activity in FGF21-deficient (KO) mice and WT controls. Genetic FGF21 deficiency significantly blunted acute MCT-induced HPA activation, as well as longer term KD-induced facilitation of the HPA response to restraint stress. These results demonstrate that endogenous FGF21 contributes to ketosis-induced HPA activation. However, because FGF21 deficiency only partially abrogated the HPA effects of dietary ketosis, future work will be directed toward identifying additional factors that contribute to ketosis-induced HPA activation. This FGF21-independent regulation of corticosterone or other counterregulatory hormones (e.g., glucagon, epinephrine) is likely sufficient to maintain glycemia during nutritional ketosis because we previously reported that FGF21 KO mice are normoglycemic during KD feeding (55). Future studies can also determine the extent to which the HPA-modulatory effects of FGF21 during ketosis occur directly via FGF21 signaling in the PVN, indirectly via FGF21 signaling in other HPA-regulatory brain regions, or indirectly via the peripheral consequences of whole-body loss of FGF21 actions.
Of note, when maintained on a chow diet, FGF21 deficiency did not alter basal (prestress) plasma corticosterone nor the corticosterone response to an acute restraint stress (Fig. 7E and 7F). Likewise, neither basal nor post-LCT gavage corticosterone levels were affected by loss of FGF21 (Fig. 7H and 7I). These results suggest that endogenous FGF21 does not contribute to HPA responsivity when an individual is in a normal (e.g., nonketogenic) metabolic state. Moreover, consistent with our previous report (55), the ability of MCT gavage and KD to induce ketosis, as indicated by circulating β-hydroxybutyrate levels, was not affected by FGF21 ablation.
Potential clinical implications
Collectively, these findings may have important clinical implications. Chronic consumption of low-carbohydrate KDs have been popular and effective for weight loss (78, 79), and there is a growing interest in acutely boosting ketosis by consuming MCTs as a dietary supplement. Given the broad, systemic effects of chronic glucocorticoid exposure, it will be important to interrogate the translational value of these findings. Indeed, limited clinical evidence already links low-carbohydrate KDs with increased HPA axis activity in humans. Langfort and colleagues, for example, reported higher pre- and postexercise levels of circulating corticosterone among untrained volunteers consuming a KD compared with controls (80). Likewise, a recent study of the effects of dietary composition during weight loss maintenance observed higher urinary cortisol among participants consuming a very low-carbohydrate diet compared with those consuming low-glycemic index or low-fat diets (81, 82).
Low-carbohydrate KDs and consumption of MCTs are thought to be useful for the treatment of several diseases, including cancer, rheumatoid arthritis, various neurologic disorders, and pharmacotherapy-resistant epilepsy (83–86), but underlying physiological mechanisms have remained elusive. Several studies report increased plasma cortisol among patient populations receiving KDs as putative therapies. For example, when cortisol was measured in plasma from epileptic children collected before and after KD therapy, elevated cortisol was observed in all individuals (87). Likewise, plasma cortisol was increased with KD among adult patients with rheumatoid arthritis (88). To the extent that dietary ketosis increases HPA axis activity in humans, the present work supports the possibility that diet-induced increases in circulating glucocorticoids may contribute to these therapeutic effects.
Importantly, this work further identifies FGF21 signaling as a key downstream mechanism contributing to HPA axis activation during dietary ketosis. Plasma FGF21 was increased following both KD and MCT gavage in rats and mice. Moreover, and consistent with previous reports (23, 68), ICV administration of recombinant FGF21 acutely increased plasma ACTH and corticosterone (Fig. 5). Last, KD- and MCT-induced increases in circulating corticosterone were significantly attenuated among mice lacking FGF21 (Fig. 6). Taken together, these data are consistent with the hypothesis that this hepatokine acts as a metabolic stress hormone (89) and draw additional attention to potential stress-regulatory side effects of FGF21-based therapeutics currently in the pipeline for treatment of metabolic diseases (90). They also highlight the need for additional clinical research delineating the relationships among dietary ketosis, FGF21, and stress system function in human subjects.
Conclusions
The present data clearly demonstrate that two dietary manipulations that induce ketosis despite the availability of excess calories, one acute and one chronic, significantly increase activity of the HPA axis. This is mediated in part by associated increases in the hepatokine FGF21 because FGF21 KO mice exhibited a blunted HPA response to these dietary interventions relative to WT controls. These findings may have important clinical implications for individuals using KDs for weight loss and as nutritional therapy for the treatment of diseases.
Acknowledgments
The authors thank W.C. Engeland (University of Minnesota) for providing antiserum for the adrenocorticotropin hormone radioimmunoassay and Kristen Halcomb for her expert technical assistance. MCT (Neobee 895) was provided by Stepan Lipid Nutrition (Northfield, IL).
Acknowledgments
Financial Support: This work was funded by National Institutes of Health Grants DK091425 (Y.M.U.-L.), DK078906 (Y.M.U.-L.), DK093848 (R.J.S.), DK077975 (D.P.-T.), DK059803 and DK102334 (A.E.B.P.), and HL111319 (K.K.R.); the German Research Foundation (K.S.); and American Diabetes Association Grant 1-13-JF-21 (K.M.H.). The funding sources had no involvement in the study design; the collection, analysis, and interpretation of data; writing of the report; or the decision to submit the article for publication.
Author Contributions: K.K.R., A.E.B.P., J.S., S.M.F., A.M.K.T., K.L., K.M.H., K.S., D.P.-T., R.J.S., and Y.M.U.-L. planned experiments. K.K.R., A.E.B.P., K.R.L., J.S., S.M.F., A.M.K.T., K.L., K.M.H., K.S., and Y.M.U.-L. acquired data. K.K.R., K.M.H., N.I., D.P.-T., M.H.T., R.J.S., and Y.M.U.-L. interpreted data. K.K.R. and Y.M.U.-L. wrote the manuscript, which was read, edited, and approved by the other authors.
Disclosure Summary: R.J.S. is a consultant for Ethicon Endo-Surgery/Johnson & Johnson, Orexigen, Novo Nordisk, Daiichi Sankyo, Janssen/Johnson & Johnson, Novartis, Paul Hastings Law Firm, Zafgen, Takeda, and Boehringer-Ingelheim and receives research support from Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Janssen/Johnson & Johnson, MedImmune, Boehringer-Ingelheim, and Sanofi. The remaining authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropin hormone
- ANOVA
- analysis of variance
- AUC
- area under the curve
- CRH
- corticotropin-releasing hormone
- EPM
- elevated plus-maze
- FGF21
- fibroblast growth factor-21
- FST
- forced swim test
- HPA
- hypothalamic-pituitary-adrenocortical
- ICV
- intracerebroventricular
- KD
- ketogenic diet
- KO
- knockout
- LCT
- long-chain triglyceride
- MCT
- medium-chain triglyceride
- PBS
- phosphate-buffered saline
- PVN
- hypothalamic paraventricular nucleus
- WT
- wild-type.
References
- 1.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61(2):180–190. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291(5):E965–E973. [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. [DOI] [PubMed] [Google Scholar]
- 5.Odio M, Brodish A. Age-related adaptation of pituitary-adrenocortical responses to stress. Neuroendocrinology. 1989;49(4):382–388. [DOI] [PubMed] [Google Scholar]
- 6.Armario A, Hidalgo J, Giralt M. Evidence that the pituitary-adrenal axis does not cross-adapt to stressors: comparison to other physiological variables. Neuroendocrinology. 1988;47(3):263–267. [DOI] [PubMed] [Google Scholar]
- 7.Martí O, Gavaldà A, Gómez F, Armario A. Direct evidence for chronic stress-induced facilitation of the adrenocorticotropin response to a novel acute stressor. Neuroendocrinology. 1994;60(1):1–7. [DOI] [PubMed] [Google Scholar]
- 8.Kiss A, Aguilera G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993;630(1-2):262–270. [DOI] [PubMed] [Google Scholar]
- 9.Burchfield SR, Woods SC, Elich MS. Pituitary adrenocortical response to chronic intermittent stress. Physiol Behav. 1980;24(2):297–302. [DOI] [PubMed] [Google Scholar]
- 10.Gómez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63(4):327–337. [DOI] [PubMed] [Google Scholar]
- 11.Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res. 2000;80(2):142–152. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63(4):561–569. [DOI] [PubMed] [Google Scholar]
- 13.Martí O, Gavaldà A, Jolín T, Armario A. Effect of regularity of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology. 1993;18(1):67–77. [DOI] [PubMed] [Google Scholar]
- 14.Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001;13(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–134. [DOI] [PubMed] [Google Scholar]
- 16.Armario A, Restrepo C, Castellanos JM, Balasch J. Dissociation between adrenocorticotropin and corticosterone responses to restraint after previous chronic exposure to stress. Life Sci. 1985;36(22):2085–2092. [DOI] [PubMed] [Google Scholar]
- 17.Packard AEB, Egan AE, Ulrich-Lai YM, Packard AEB, Egan AE, Ulrich‐Lai YM. HPA axisinteractions with behavioral systems Comprehen Physiol. 2016;6(4):1897–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulrich-Lai YM, Ryan KK. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metab. 2014;19(6):910–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272(3 Pt 2):R840–R848. [DOI] [PubMed] [Google Scholar]
- 20.Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70(3-4):333–342. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36(10):1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. [DOI] [PubMed] [Google Scholar]
- 23.Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KSL, Xu A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63(12):4064–4075. [DOI] [PubMed] [Google Scholar]
- 24.Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140(10):769–777. [DOI] [PubMed] [Google Scholar]
- 25.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–506. [DOI] [PubMed] [Google Scholar]
- 26.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–437. [DOI] [PubMed] [Google Scholar]
- 27.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KH, Lee M-S. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J. 2014;38(4):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223–241. [DOI] [PubMed] [Google Scholar]
- 30.Larson KR, Russo KA, Fang Y, Mohajerani N, Goodson ML, Ryan KK. Sex differences in the hormonal and metabolic response to dietary protein dilution. Endocrinology. 2017;158(10):3477–3487. [DOI] [PubMed] [Google Scholar]
- 31.Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28(12):2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016;23(2):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150(10):4625–4633. [DOI] [PubMed] [Google Scholar]
- 34.Yeh YY, Zee P. Relation of ketosis to metabolic changes induced by acute medium-chain triglyceride feeding in rats. J Nutr. 1976;106(1):58–67. [DOI] [PubMed] [Google Scholar]
- 35.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289(5):E823–E828. [DOI] [PubMed] [Google Scholar]
- 36.Stricker EM, Vagnucci AH, McDonald RH Jr, Leenen FH. Renin and aldosterone secretions during hypovolemia in rats: relation to NaCl intake. Am J Physiol. 1979;237(1):R45–R51. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich-Lai YM, Engeland WC. Hyperinnervation during adrenal regeneration influences the rate of functional recovery. Neuroendocrinology. 2000;71(2):107–123. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14(3):173–213. [DOI] [PubMed] [Google Scholar]
- 39.Ryan KK, Grayson BE, Jones KR, Schneider AL, Woods SC, Seeley RJ, Herman JP, Ulrich-Lai YM. Physiological responses to acute psychological stress are reduced by the PPARγ agonist rosiglitazone. Endocrinology. 2012;153(3):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan KK, Mul JD, Clemmensen C, Egan AE, Begg DP, Halcomb K, Seeley RJ, Herman JP, Ulrich-Lai YM. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5(1):3–13. [DOI] [PubMed] [Google Scholar]
- 42.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 44.Pollak DD, Rey CE, Monje FJ. Rodent models in depression research: classical strategies and new directions. Ann Med. 2010;42(4):252–264. [DOI] [PubMed] [Google Scholar]
- 45.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33(4):287–297. [DOI] [PubMed] [Google Scholar]
- 47.Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16(1):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci USA. 2010;107(47):20529–20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 50.Bielohuby M, Sisley S, Sandoval D, Herbach N, Zengin A, Fischereder M, Menhofer D, Stoehr BJM, Stemmer K, Wanke R, Tschöp MH, Seeley RJ, Bidlingmaier M. Impaired glucose tolerance in rats fed low-carbohydrate, high-fat diets. Am J Physiol Endocrinol Metab. 2013;305(9):E1059–E1070. [DOI] [PubMed] [Google Scholar]
- 51.Jornayvaz FR, Jurczak MJ, Lee H-Y, Birkenfeld AL, Frederick DW, Zhang D, Zhang X-M, Samuel VT, Shulman GI. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 2010;299(5):E808–E815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douris N, Desai BN, Fisher FM, Cisu T, Fowler AJ, Zarebidaki E, Nguyen NLT, Morgan DA, Bartness TJ, Rahmouni K, Flier JS, Maratos-Flier E. Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice. Mol Metab. 2017;6(8):854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vining EPG, Pyzik P, McGrogan J, Hladky H, Anand A, Kriegler S, Freeman JM. Growth of children on the ketogenic diet. Dev Med Child Neurol. 2002;44(12):796–802. [DOI] [PubMed] [Google Scholar]
- 54.Bielohuby M, Sawitzky M, Stoehr BJM, Stock P, Menhofer D, Ebensing S, Bjerre M, Frystyk J, Binder G, Strasburger C, Wu Z, Christ B, Hoeflich A, Bidlingmaier M. Lack of dietary carbohydrates induces hepatic growth hormone (GH) resistance in rats. Endocrinology. 2011;152(5):1948–1960. [DOI] [PubMed] [Google Scholar]
- 55.Stemmer K, Zani F, Habegger KM, Neff C, Kotzbeck P, Bauer M, Yalamanchilli S, Azad A, Lehti M, Martins PJF, Müller TD, Pfluger PT, Seeley RJ. FGF21 is not required for glucose homeostasis, ketosis or tumour suppression associated with ketogenic diets in mice. Diabetologia. 2015;58(10):2414–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodson ML, Packard AEB, Buesing DR, Maney M, Myers B, Fang Y, Basford JE, Hui DY, Ulrich-Lai YM, Herman JP, Ryan KK. Chronic stress and Rosiglitazone increase indices of vascular stiffness in male rats. Physiol Behav. 2017;172:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148(4):1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solomon MB, Jankord R, Flak JN, Herman JP. Chronic stress, energy balance and adiposity in female rats. Physiol Behav. 2011;102(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiley JH, Leveille GA. Metabolic consequences of dietary medium-chain triglycerides in the rat. J Nutr. 1973;103(6):829–835. [DOI] [PubMed] [Google Scholar]
- 60.Greenberger NJ, Skillman TG. Medium-chain triglycerides. N Engl J Med. 1969;280(19):1045–1058. [DOI] [PubMed] [Google Scholar]
- 61.Meerlo P, Koehl M, van der Borght K, Turek FW. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14(5):397–402. [DOI] [PubMed] [Google Scholar]
- 62.Machado TD, Dalle Molle R, Laureano DP, Portella AK, Werlang ICR, Benetti CS, Noschang C, Silveira PP. Early life stress is associated with anxiety, increased stress responsivity and preference for “comfort foods” in adult female rats. Stress. 2013;16(5):549–556. [DOI] [PubMed] [Google Scholar]
- 63.Golier JA, Caramanica K, Makotkine I, Sher L, Yehuda R. Cortisol response to cosyntropin administration in military veterans with or without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:151–158. [DOI] [PubMed] [Google Scholar]
- 64.Naito Y, Fukata J, Tamai S, Seo N, Nakai Y, Mori K, Imura H. Biphasic changes in hypothalamo-pituitary-adrenal function during the early recovery period after major abdominal surgery. J Clin Endocrinol Metab. 1991;73(1):111–117. [DOI] [PubMed] [Google Scholar]
- 65.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19(5):175–180. [DOI] [PubMed] [Google Scholar]
- 66.Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology. 2002;76(2):79–92. [DOI] [PubMed] [Google Scholar]
- 67.Domouzoglou EM, Maratos-Flier E. Fibroblast growth factor 21 is a metabolic regulator that plays a role in the adaptation to ketosis. Am J Clin Nutr. 2011;93(4):901S–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bookout AL, de Groot MHM, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camporez JPG, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154(9):3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spencer RL, Deak T. A users guide to HPA axis research. Physiol Behav. 2017;178:43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rivest S, Rivier C. Stress and interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J Neuroendocrinol. 1994;6(1):101–117. [DOI] [PubMed] [Google Scholar]
- 72.Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104(2):266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Canteras NS, Resstel LB, Bertoglio LJ, Carobrez A de P, Guimarães FS. Neuroanatomy of anxiety. Curr Top Behav Neurosci. 2010;2:77–96. [DOI] [PubMed] [Google Scholar]
- 74.Oakes P, Loukas M, Oskouian RJ, Tubbs RS. The neuroanatomy of depression: a review. Clin Anat. 2017;30(1):44–49. [DOI] [PubMed] [Google Scholar]
- 75.Engeland WC, Gann DS. Splanchnic nerve stimulation modulates steroid secretion in hypophysectomized dogs. Neuroendocrinology. 1989;50(2):124–131. [DOI] [PubMed] [Google Scholar]
- 76.Kagerer SM, Jöhren O. Interactions of orexins/hypocretins with adrenocortical functions. Acta Physiol (Oxf). 2010;198(3):361–371. [DOI] [PubMed] [Google Scholar]
- 77.Harmer SC, Bicknell AB. Role of gamma-MSH peptides in the regulation of adrenal steroidogenesis. Peptides. 2005;26(10):1944–1951. [DOI] [PubMed] [Google Scholar]
- 78.Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364(9437):897–899. [DOI] [PubMed] [Google Scholar]
- 79.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88(4):1617–1623. [DOI] [PubMed] [Google Scholar]
- 80.Langfort J, Pilis W, Zarzeczny R, Nazar K, Kaciuba-Uściłko H. Effect of low-carbohydrate-ketogenic diet on metabolic and hormonal responses to graded exercise in men. J Physiol Pharmacol. 1996;47(2):361–371. [PubMed] [Google Scholar]
- 81.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307(24):2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17(5-6):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paoli A, Bianco A, Damiani E, Bosco G.. Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int. 2014;2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tedeschi SK, Costenbader KH. Is there a role for diet in the therapy of rheumatoid arthritis? Curr Rheumatol Rep. 2016;18(5):23. [DOI] [PubMed] [Google Scholar]
- 85.Freeman JM, Vining EPG, Pillas DJ, Pyzik PL, Casey JC, Kelly LM. The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102(6):1358–1363. [DOI] [PubMed] [Google Scholar]
- 86.Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fraser DD, Whiting S, Andrew RD, Macdonald EA, Musa-Veloso K, Cunnane SC. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology. 2003;60(6):1026–1029. [DOI] [PubMed] [Google Scholar]
- 88.Fraser DA, Thoen J, Bondhus S, Haugen M, Reseland JE, Djøseland O, Førre O, Kjeldsen-Kragh J. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18(2):209–214. [PubMed] [Google Scholar]
- 89.Luo Y, McKeehan WL. Stressed liver and muscle call on adipocytes with FGF21. Front Endocrinol (Lausanne). 2013;4:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kharitonenkov A, DiMarchi R. FGF21 revolutions: recent advances illuminating FGF21 biology and medicinal properties. Trends Endocrinol Metab. 2015;26(11):608–617. [DOI] [PubMed] [Google Scholar]