Abstract
Ghrelin is a 28–amino acid polypeptide that regulates feeding, glucose metabolism, and emotionality (stress, anxiety, and depression). Plasma ghrelin circulates as desacyl ghrelin (DAG) or, in an acylated form, acyl ghrelin (AG), through the actions of ghrelin O-acyltransferase (GOAT), exhibiting low or high affinity, respectively, for the growth hormone secretagogue receptor (GHSR) 1a. We investigated the role of endogenous AG, DAG, and GHSR1a signaling on anxiety and stress responses using ghrelin knockout (Ghr KO), GOAT KO, and Ghsr stop-floxed (Ghsr null) mice. Behavioral and hormonal responses were tested in the elevated plus maze and light/dark (LD) box. Mice lacking both AG and DAG (Ghr KO) increased anxiety-like behaviors across tests, whereas anxiety reactions were attenuated in DAG-treated Ghr KO mice and in mice lacking AG (GOAT KO). Notably, loss of GHSR1a (Ghsr null) did not affect anxiety-like behavior in any test. Administration of AG and DAG to Ghr KO mice with lifelong ghrelin deficiency reduced anxiety-like behavior and decreased phospho–extracellular signal-regulated kinase phosphorylation in the Edinger-Westphal nucleus in wild-type mice, a site normally expressing GHSR1a and involved in stress- and anxiety-related behavior. Collectively, our data demonstrate distinct roles for endogenous AG and DAG in regulation of anxiety responses and suggest that the behavioral impact of ghrelin may be context dependent.
We investigated the role of acylated and desacyl ghrelin in regulation of anxiety-related behavior in mice. Desacyl ghrelin inhibits anxiety-like behavior in the central nervous system.
The orexigenic hormone ghrelin is primarily synthesized by the endocrine X/A-like cells in the oxyntic mucosa of the stomach (1–3). Ghrelin is encoded by the preproghrelin gene. During posttranslational processing, proghrelin is acylated at the serine 3 position by the integral membrane-bound enzyme ghrelin O-acyltransferase (GOAT) to form acyl ghrelin (AG) (4, 5). This fatty-acid modification is unique to ghrelin and required for optimal binding of the peptide to its receptor, the growth hormone secretagogue receptor (GHSR) 1a (5). Desacyl ghrelin (DAG) is thought to result from incomplete acylation of the peptide and/or deacylation of ghrelin and exists as the predominant isoform of ghrelin in the circulation (DAG and AG exist in a ratio of ∼4:1) (6). DAG also can activate GHSR1a in vitro and in vivo, although its potency is significantly reduced when compared with AG (7). However, DAG has been shown to inhibit cell death of primary adult and H9c2 cardiomyocytes and endothelial cells (8) and protect against ischemic neuronal injury of primary cultured rat cortical neurons (9). Some reports also suggest that DAG regulates glucose metabolism and food intake by GHSR-independent mechanisms (10–12). For example, DAG regulates genes involved in glucose and lipid metabolism in liver, fat, and muscle of Ghsr-deficient mice (13, 14). Despite these intriguing findings, the identification of a DAG-specific receptor remains elusive to date (1).
In addition to its well-known effects on appetite/food intake and glucose metabolism, ghrelin is also increasingly recognized for its role in regulating physiological and behavioral stress responses, anxiety, and depression (15). Both acute and chronic stress increase circulating ghrelin levels in rats and mice in parallel with hypothalamic-pituitary-adrenal (HPA) axis activation (16, 17). Central administration of AG by intracerebral injection injection or direct injection to the hypothalamus, amygdala, or hippocampus can elicit anxiety-like behaviors (18–20). Importantly, GHSR1a is highly expressed in brain regions that control feeding, anxiety, and stress such as the anterior pituitary, hypothalamic arcuate nucleus, amygdala, hippocampus, and Edinger-Westphal (EW) nucleus (20, 21). There is also evidence suggesting that effects of ghrelin on anxiety- and depressive-like behavior may be dependent on prior stress exposure (15, 22). Spencer et al. (15) reported that Ghr knockout (KO) mice lacking DAG and AG exhibit enhanced anxiety-related behaviors after acute restraint stress but exhibit less anxiety-related behaviors when tested under stress-naive conditions. Moreover, Ghr KO mice have lower corticosterone responses after acute stress despite increased neuronal activation in the paraventricular nucleus (15). These findings illustrate the complex relationship among ghrelin, anxiety, and stress.
In the current study, we investigated the role of endogenous AG and DAG in regulation of stress responses and anxiety-related behavior. Our data reveal that lifelong exposure to increased levels of endogenous DAG inhibits anxiety-like behavior. Consistent with this, exogenous DAG supplementation has anxiolytic properties in ghrelin-deficient mice. Collectively, our results are consistent with the hypothesis that GOAT activity plays a critical role in dictating the net impact of ghrelin action in the central nervous system (CNS). Furthermore, our results suggest that the role of ghrelin in the modulation of anxiety-like behavior appears to be context dependent.
Materials and Methods
Animals
All studies were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Cincinnati.
Ghsr null mice were obtained from Joel Elmquist and Jeff Zigman (University of Texas Southwestern, Dallas, TX) (23). The whole-body Ghsr null mice were created using ET cloning and related technologies within EL250 cells (24, 25), as described previously (23). The global Ghr KO mice were generated using the high-throughput homologous recombination Veloci Gene technology (26, 27). The whole-body GOAT KO mice were generated by Taconic Biosciences (New York, NY) as described (5, 28). All mouse lines mentioned above were maintained and backcrossed on the C57BL/6J background.
Male mice 2 to 4 months of age were used and single-housed with enrichment and huts at the Reading Road Campus, University of Cincinnati, under standard conditions in a temperature- and humidity-controlled room on a 12:12-hour light/dark (LD) cycle (lights on at 7:00 am). The mice were allowed ad libitum access to water and standard rodent chow (Teklad; Harlan Laboratories, Indianapolis, IN; 3.1 kcal/g; ∼5% fat).
Mouse-handling procedures
Mice were handled as described by Hurst and West (29) and modified by our group (30). This procedure has been documented to reduce the stress response to routine handling and specific interventions such as intraperitoneal (IP) injections. Briefly, adult mice were single-housed and retrieved by cupping (rather than capture by tail-picking), followed by a period of cupping with ∼2 minutes of massage on the dorsum (30). This was performed for 2 weeks prior to the behavioral testing. The control groups were retrieved by standard tail-picking but housed with like treatment groups (singly with enrichment and huts). To habituate the animals to injection procedures (experiment 4), mock injections were performed daily for 1 week prior to experimentation.
Drug preparation
Stock concentrations of synthetic octanoyl ghrelin/AG (1–28) and DAG (1–28) (CSBio, Menlo Park, CA) were prepared in sterile 0.9% normal saline at a concentration of 5 μg/μL, aliquoted, and stored at −20°C in a nonfreeze thaw freezer. On the day of experiment, stock was diluted 1 to 10 (0.5 μg/μL) in 0.9% saline, and 100 μL (50 μg) per mouse was injected IP.
Behavioral testing
To minimize background stress associated with negative energy balance, all animals were tested in the nonfasted state.
Elevated plus maze
The elevated plus maze (EPM) is a well-characterized assay of anxiety-like behavior and consists of an elevated platform with two open arms and two arms with walls. Mice were placed on an open arm, facing the center of the maze, and behavior was recorded for 5 minutes. Entries into open arm, time spent in open and closed arms, the number of end explorations, and total distance traveled during the EPM test were quantified (30, 31). Testing was performed under dim red light. After each trial, the apparatus was cleaned with 20% ethanol.
LD box
The apparatus used for the LD test was divided into two equal-size chambers by a partition with a door. One chamber was brightly illuminated by white light; the other was dark. Mice were placed within the light box, and behavior was recorded for 5 minutes. Latency to enter to the dark box, time spent in light box, and the number of entry times to light box were quantified. After each trial, the apparatus was cleaned with 20% ethanol (32).
Restraint stress and plasma corticosterone measurement
To assess corticosterone responses to an acute novel restraint challenge, KO mice were placed in well-ventilated 50-mL conical tubes for 30 minutes. The blood was collected and processed as described in the section on blood collection. For analyzing corticosterone responses, tail blood samples were taken at 0 (immediately after placement in restrainers) and 30, 60, and 120 minutes after placement. Animals were removed from the restrainers after the 30-minute bleed, and the 60- and 120-minute time points were restraint-free bleed post–stress measures after being returned to their home cages (33).
Blood collection for measuring plasma corticosterone concentrations
The distal millimeter of the tail was removed using a sterile scalpel blade and tail blood obtained as previously described (30, 34). Importantly, each blood sample, collected into EDTA tubes, was obtained within 2 minutes to minimize any increase in corticosterone concentration due to handling stress. Plasma corticosterone concentration was measured using a 125I RIA kit (MP Biomedicals, Solon, OH) as described previously (30, 34).
Immunohistochemistry
For assessment of phospho–extracellular signal-regulated kinase (pERK) immunohistochemistry following AG, DAG, or vehicle injection, animals were overdosed with pentobarbital 15 minutes following stress induction (experiment 4). Animals were then perfused transcardially with 1× phosphate-buffered saline (PBS) until blood was clear followed by perfusion with 4% paraformaldehyde/1× PBS for 15 minutes. The brains were postfixed overnight at 4°C in 4% paraformaldehyde/1× PBS, rinsed two to three times with 1× PBS, and cryoprotected in 1× PBS containing 30% sucrose at 4°C until the brains sank in the sucrose solution. Brains were frozen to −20°C on sliding microtome and sectioned at 30 μm, collected sequentially in a series of six, and placed in cryoprotectant consisting of 10% polyvinyl-pyrrolidone (molecular weight 40,000), 30% sucrose in 500 mL of 1× PBS, and 300 mL of ethylene glycol. Each tissue block for immunocytochemistry was serially cut as described above and labeled for pERK immunohistochemistry (catalog no. 9101; Cell Signaling Technology; Research Resource Identifier: AB_331646). Every sixth section over the full rostral to caudal extent of the nucleus or region of interest was incubated first in 1% sodium borohyhdride in 1× PBS for 30 minutes, followed by rinsing six times for 5 minutes in 1× PBS, incubated in 3% hydrogen peroxide in 1× PBS for 10 minutes, rinsing six times for 5 minutes in 1× PBS, followed by additional rinses in 1× PBS four times for 15 minutes. Sections were then incubated in 1× PBS containing 4% goat serum with 0.4% Triton X-100 and 0.2% bovine serum albumin (BSA) for 2 hours to block nonspecific binding, followed by rabbit anti-pERK antibody diluted 1:4000 in blocking solution consisting of 1× PBS containing 4% goat serum with 0.4% Triton X-100 and 0.2% BSA overnight at room temperature. After overnight antibody incubation, sections were rinsed in 1× PBS, three times for 5 minutes, incubated in biotinylated goat anti-rabbit IgG secondary antibody diluted 1:400 in in blocking solution consisting of 1× PBS containing 4% goat serum with 0.4% Triton X-100 and 0.2% BSA for 1 hour, incubated in avidin-biotin complex diluted 1:800 in 1× PBS Vectastain ABC Elite Standard for 1 hour (Vector Laboratories, Burlingame, CA), and rinsed three times for 5 minutes in 1× PBS, followed by color development using 0.05% diaminobenzidine tetrahydrochloride (catalog number D5905; Sigma-Aldrich) solution containing 0.002% H2O2 for 8 minutes.
Image capture
The Paxinos and Franklin atlas (35) was used to define the paraventricular nucleus (−0.58 to −0.94 mm from bregma), arcuate nucleus (−1.46 to −1.94 from bregma), nucleus tractus solitarius (NTS; −7.32 to −7.92 from bregma), and EW (−3.16 to −3.64 from bregma). Sections of brains stained for pERK were imaged with a 10× objective on an Imager Z1 microscope (Carl Zeiss, Thornwood, NY). Quantitative analysis of the number of pERK-positive neurons within the brain regions specified were individually counted using the Zeiss Axiovision analysis software interactive measuring program (Carl Zeiss). Bilateral cell counts by a researcher blinded to the animals were conducted, group identification was obtained, and counts from each side combined for analysis. Immunopositive cells were counted from an average of three sections per animal, representing 540 μm in extent for each of the regions examined.
Experimental Design and Timeline
Experiment 1: effect of endogenous AG on anxiety-like behavior and stress
Cohorts of Ghr KO C57BL/6J mice and their wild-type (WT) littermate controls were divided into the nonhandled (n = 20) or handled (n = 20) groups and underwent behavioral testing (Fig. 1). As described in the Materials and Methods, mice received 2 weeks of handling prior to behavioral and stress testing. Mice received sequential exposure to the EMP, LD box, and restraint stress, with 1-week intervals between tests. Nonhandled mice underwent behavioral testing in the same sequence as the handled mice without the prior handling experience. All of the behavioral experiments were performed between 9:00 am and 1:00 pm. For the restraint challenge, tail blood was collected as noted previously.
Figure 1.
Timeline of the experimental design. (A) All genetically modified male mice of experiments 1, 2, and 3 [Ghr KO (n = 10), Ghsr null (n = 9), and GOAT KO (n = 9)] were handled for 2 weeks. Initially, a cohort of Ghr KO and their littermate WT controls were tested to compare handling vs nonhandling. As depicted, the mice underwent a sequence of tests for anxiety-like behavior: EPM and LD box test followed finally by restraint for 30 minutes and euthanasia at 120 minutes. Blood was collected every 15 minutes during restraint for 120 minutes to measure corticosterone level. (B) Ghr KO mice and their WT littermates were handled for 2 weeks followed by mock injections plus handling daily for 1 week prior to the experimental injection. Mice were injected with saline/DAG/AG (12 per group) and tested for anxiety-like behavior in the EPM followed by euthanasia at 15 minutes to study pERK expression, respectively.
Experiment 2: effect of ghrelin receptor on anxiety-like behavior and stress
Cohorts of Ghsr null C57BL/6J mice (n = 9) and WT littermate controls (n = 9) underwent training, behavioral testing, and stress challenge in the same sequence and time course described in experiment 1 (Fig. 1). All of the behavioral experiments were performed between 9:00 am and 1:00 pm.
Experiment 3: effect of endogenous DAG on anxiety-like behavior and stress
Cohorts of GOAT KO C57BL/6J mice (n = 9) and WT controls (n = 9) underwent training, behavioral testing, and stress challenge in the same sequence and time course described in experiment 1 (Fig. 1). All of the behavioral experiments were performed between 9:00 am and 1:00 pm.
All mutant models and their littermate controls in experiments 1, 2, and 3 were between 3 and 4 months of age at the start of behavioral testing.
Experiment 4: effects of exogenous AG and DAG on anxiety-like behavior and stress
To specifically assess the effects of exogenous AG and DAG, 8-week-old male (C57BL6/J) Ghr KO mice and their WT littermates (n = 12) naive to either drug treatment or behavior intervention were handled for 2 weeks and subsequently received mock injection training for 5 days. On the sixth day, mice received injections of synthetic mouse AG or DAG or vehicle (0.9% saline) at doses of 50 μg/mouse or saline at 8:30 am on experimental day by IP injection. This dose was chosen because it reliably increases food intake in C57BL6 mice in our laboratory (data not shown). At 15 minutes post–peptide/saline infusion, animals were placed in an EPM using the procedure described previously. Mice was euthanized by pentobarbital overdose 15 minutes after the stress test and perfused with saline followed by 4% paraformaldehyde to assess CNS pERK expression level (Fig. 1).
Mice were 8 weeks old when we began handling them for 2 weeks and 1-week mock injection. They were 11 weeks old when they were administered the drugs and exposed to the EPM test.
Statistical analysis
Data are shown as mean ± standard error of the mean (SEM). Behavioral data from EPM and LD were analyzed by unpaired Student t test for experiments 2 and 3. If the data did not pass equal variance test, the Mann-Whitney rank-sum test was used. Two-way analysis of variance with Fisher least significant difference (LSD) post hoc tests was used to determine statistical significance between treatment and genotype for experiments 1 and 4. Corticosterone data were analyzed with two-way repeated-measures analysis of variance followed by Fisher LSD post hoc test. All statistical analyses were performed using Sigma Plot version 13. Potential outliers were determined using two different tests: (1) outliers were values that differed from the mean by >1.96 times the standard deviation, and (2) outliers were values that were below the lower quartile or above the upper quartile by >1.5 times the interquartile range (36, 37). A positive identification by both outlier tests was required before a value was removed from the analysis. Statistical significance was taken as P ≤ 0.05.
Results
Ghr KO mice exhibit increased anxiety-like behavior
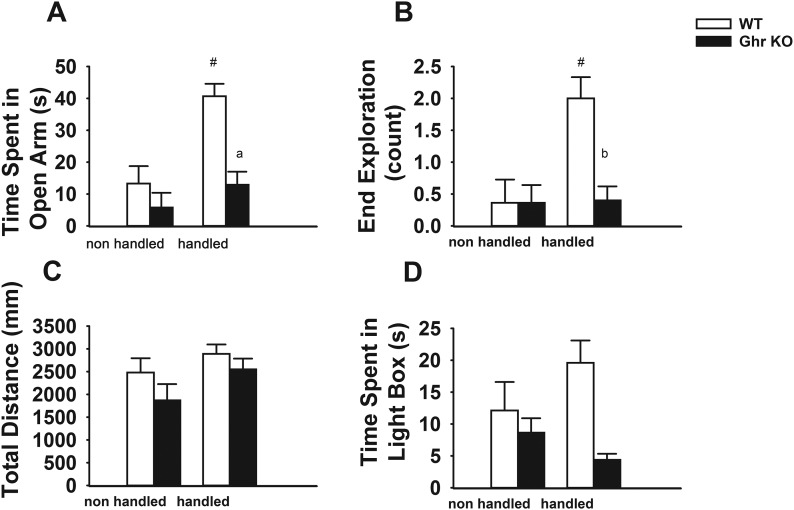
Observations in prior experiments by animal handlers in our laboratory suggested that Ghr KO mice did not habituate to handling, showing persistent struggling, urination, and defecation despite repeated handling (relative to WT controls). This led us to hypothesize that these animals would have impaired habituation to handling procedures, manifested as increased anxiety-like behavior. Therefore, behavioral responses to EPM (Fig. 2A–2C) and LD (Fig. 2D) were assessed in both handled and nonhandled Ghr KO and WT male mice. Ghr KO and WT mice that were nonhandled showed no difference in the time spent in the open arm (Fig. 2A), open-arm end exploration times (Fig. 2B), total distance traveled during EPM test (Fig. 2C), or time spent in the light box in the LD test (Fig. 2D). Consistent with the effect of handling that we and others previously reported (29, 30, 38), the open-arm time and the end exploration counts were higher in the handled than the nonhandled WT mice (P < 0.001 for both), whereas no difference was seen in the Ghr KO mice (P = 0.28 for open-arm time and P = 0.93 for end exploration) (Fig. 2A and 2B). These data showed that handling had an anxiolytic-like effect in the WT mice but not in the Ghr KO mice. Consistent with this finding, Ghr KO mice that were handled demonstrated decreased time spent in the open arm (P < 0.01) and end exploration times (P < 0.01) in the EPM test as compared with the handled WT mice (Fig. 2A and 2B). As for the LD box test, we observed a trend toward a significant difference in time spent in the light box between the handled and nonhandled WT mice (P = 0.09), but no difference was seen in the Ghr KO mice (P = 0.34). When the genotypes were compared, the handled Ghr KO mice spent significantly less time in the light box than the handled WT mice (P < 0.001) (Fig. 2D). No differences were seen between the Ghr KO and WT mice in the total distance traveled in EPM (Fig. 2C), indicating that overall exploratory/food-seeking behavior and general activity level were not affected. Because handling relieved floor effects seen in WT groups exposed to anxiogenic stimuli, only handled mice were used in subsequent behavioral experiments described later.
Figure 2.
Handling mice elicits anxiety-like behavior in Ghr KO mice. (A) Time [seconds (s)] in open arm. (B) Open-arm end exploration time (count). (C) Total distance moved (millimeters) during the 5-minute EPM in Ghr KO vs WT in two handled and nonhandled groups of mice. (D) Total time in light box during the 5-minute LD box test in Ghr KO vs WT in two handled and nonhandled groups of mice. Data are presented as mean ± SEM. #Significant difference between nonhandled and handled mice; a,bsignificant differences between KO and WT mice that were handled (P < 0.01).
Reduced anxiety-like behavior in GOAT KO mice
Next, we tested GOAT KO mice for behavioral stress responses. GOAT KOs lack AG, but have elevated DAG in the circulation (39). We found that deletion of GOAT caused a decrease in anxiety-like behavior as compared with WT mice, reflected as increased time spent in the open arm [t(16) = −2.90; P < 0.01 vs WT mice] (Fig. 3A), increased total distance traveled in the EPM [t(16) = −2.30; P < 0.01 vs WT mice] (Fig. 3C), and increased time spent in the light chamber of the LD box [t(16) = 2.16; P < 0.01 vs WT mice] (Fig. 3D). No significant difference was observed in open-arm end exploration times [t(16) = −2.00; P = 0.057]. Together, these findings are consistent with a decreased anxiety/stress phenotype.
Figure 3.
GOAT KO mice display reduced anxiety-like behavior. (A) Time [seconds (s)] in open arm. (B) Open-arm end exploration time (count). (C) Total distances moved (millimeters) in 5 minutes during EPM test in GOAT KO vs WT in a handled cohort of mice. (D) Total time in light box during 5-minute LD box test in GOAT KO vs WT handled cohort of mice. Data are presented as mean ± SEM. There was no significant difference vs WT in open-arm end exploration time (P = 0.057). a,b,cSignificant differences vs WT (P < 0.01), respectively.
Lack of distinct anxiety-like behavioral changes in Ghsr null mice
Unlike the Ghr KO mice, handled animals having impaired ghrelin signaling (Ghsr null) did not exhibit enhanced anxiety-like behavior in the open field test (Fig. 4A and 4B) or the LD box test (Fig. 4D). A modest decreases in total distance traveled in EPM was noted in the Ghsr null mice as compared with the WT [t(15) = 2.10; P < 0.01] (Fig. 4C). Perhaps this suggests a decrease in general activity level.
Figure 4.
Anxiety-like behavior similar to WT in Ghsr null μice. (A) Time [seconds (s)] in open arm. (B) Open-arm end exploration time (count). (C) Total distance moved (millimeters) during 5 minutes in EPM test in Ghsr null vs WT handled mice. (D) Total time in light box during 5-minute LD box test in Ghsr null vs WT trained group. Data are presented as mean ± SEM. aSignificant difference vs WT (P < 0.01).
HPA response to acute stress in ghrelin mutant mice
HPA axis activation during and following acute restraint stress was assessed in the Ghr KO, GOAT KO, and Ghsr null mouse models. First, in nonhandled animals, there is a significant genotype-by-time interaction [F(3, 67) = 5.11; P = 0.004]. The corticosterone response to restraint was greater at 30 minutes poststress in the Ghr KO mice as compared with the WT (P < 0.05, Fisher LSD) (Fig. 5A). This difference was not observed in the handled animals (Fig. 5B). When we assessed the Ghsr null group, we found a significant effect of genotypes [F(1, 67) = 9.05; P = 0.008] and significant effect of time [F(3, 67) = 53.58; P < 0.001] on corticosterone secretion. Handled Ghsr null mice had higher plasma corticosterone at 60 and 120 minutes compared with the WT (P < 0.05, Fisher LSD) (Fig. 5C). We did not see any marked difference between the corticosterone levels in GOAT KO and WT handled (Fig. 5D).
Figure 5.
HPA response to acute stress in disrupted Ghrelin axis mice. (A) Corticosterone (CORT) level after 30-minute restraint in Ghr KO vs WT in nonhandled mice. (B) There was no considerable difference between handled Ghr KO and WT mice. (C) Ghsr null vs WT handled mice in 60 and 120 minutes. (D) GOAT KO vs WT handled mice after 30-minute restraint. aSignificant difference in 30 minutes vs WT (P < 0.01); bsignificant difference vs WT (P < 0.01).
Exogenous DAG/AG decreases anxiety-like behavior in Ghr KO mice
To test the effect of DAG and AG on anxiety-like behavior in naive animals, we injected DAG, AG, or saline IP to groups of Ghr KO mice and littermate WT control mice (N = 12 per group) 15 minutes before the 5-minute EPM testing. There was a significant genotype-by-treatment interaction effect on open-arm time [F(2, 72) = 5.72; P = 0.005] (Fig. 6A). Saline-treated Ghr KO showed reduced open-arm times relative to controls (P < 0.05, Fisher LSD post hoc) (Fig. 6A). However, anxiety-like behavior (open-arm avoidance) was reduced in Ghr KO mice following either DAG or AG injection, respectively (P < 0.05, Fisher LSD post hoc) (Fig. 6A).
Figure 6.
Exogenous DAG decreased anxiety-like behavior in Ghr KO mice. Effect on a 5-minute EPM test, 15 minutes after IP injection of saline, DAG, and AG in Ghr KO mice or their WT littermate controls. (A) Ghr KO mice that were treated with saline show less time in open arm in comparison with their littermate WT control mice. Ghr KO mice injected with DAG or AG spent greater time in open-arm than saline-treated Ghr KO control. aSignificant difference between saline-treated Ghr KO vs saline-treated WT (P < 0.05); b,csignificant differences between ghrelin-treated Ghr KO vs saline-treated Ghr KO (P < 0.05). (B) There were no noteworthy differences in end exploration time between either genotype or treatment. (C) DAG-treated Ghr KO mice traveled longer distances than saline-treated Ghr KO mice. aDifference between DAG-treated Ghr KO vs Ghr KO mice receiving saline (P < 0.05).
Total distance traveled in EPM revealed a significant effect of treatment [F(2, 73) = 3.43; P = 0.038). DAG-treated Ghr KO mice also showed a slight increase in distance traveled in the maze, consistent with either decreased inhibition of exploratory behavior or a drug-induced increase in locomotion (P < 0.05, Fisher LSD post hoc) (Fig. 6C).
Differential effects of AG and DAG on pERK expression following EPM
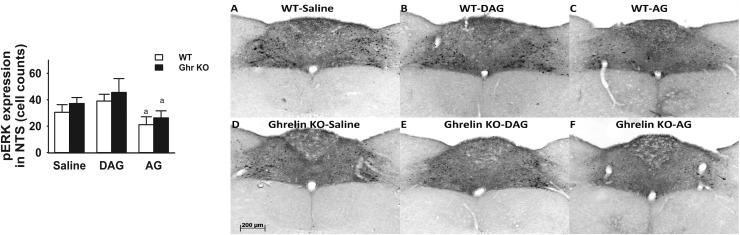
We assessed pERK expression to examine the effect of ghrelin treatment on neuronal activity in the CNS. Quantitative analysis of the number of pERK-positive neurons revealed that there was a significant treatment-by-genotype interaction [F(2, 30) = 3.59; P = 0.042] (Fig. 7). pERK expression was decreased in WT mice that received DAG in the EW nucleus relative to saline controls (P = 0.05, Fisher LSD post hoc) (Fig. 7). There was a significant treatment effect on pERK expression in the NTS [F(2, 31) = 6.00; P = 0.01]. pERK expression was decreased in the NTS region of AG-treated animals compared with those receiving DAG in both WT and Ghr KO mice (P = 0.05, Fisher LSD post hoc) (Fig. 8).
Figure 7.
pERK immunohistochemistry after EPM test in EW nucleus after IP injection of saline, DAG, or AG in WT and Ghr KO mice. Ghr KO mice and their littermate WT controls receiving saline, DAG, or AG were perfused 15 minutes after EPM to study pERK expression in EW nucleus. pERK expression level was lower in WT animals that were treated with DAG in comparison with the group of WT mice that received saline injection in the EW nucleus. (A–C) pERK expression in EW of WT mice receiving saline, DAG, and AG, respectively. (D–F) pERK expression in EW of Ghr KO mice receiving saline, DAG, and AG, respectively. aSignificant difference vs WT (P ≤ 0.05).
Figure 8.
pERK immunohistochemistry after EPM test in NTS after IP injection of saline, DAG, or AG in WT and Ghr KO mice. Ghr KO mice and their littermate WT controls receiving saline, DAG, or AG were perfused 15 minutes after EPM to study pERK expression in NTS. Both WT and Ghr KO mice injected with AG showed lower pERK expression in the NTS in comparison with DAG injection. (A–C) pERK expression in NTS of WT mice receiving saline, DAG, and AG, respectively. (D–F) pERK expression in NTS of Grh KO mice receiving saline, DAG, and AG, respectively. aSignificant difference between AG and DAG (P < 0.05).
Discussion
Our results provide evidence for a role of endogenous DAG in limiting anxiety-related behavior in mice. Removal of all forms of ghrelin (Ghr KO) prevented the anxiolytic effect of handling on anxiety-related behaviors, whereas deletion of GOAT (high concentrations of DAG from acylation block) had an anxiety behavior–reducing impact. Moreover, acute injections with DAG reduced anxiety-related behavior in Ghr KO mice, indicating that DAG plays an anxiolytic role in these animals. Mice that lack Ghsr had no clear anxiety-related phenotype, further suggesting that DAG effects are not mediated by the canonical AG signaling pathway. Consistent with these findings, peripheral administration of DAG decreased pERK phosphorylation in the EW nucleus in WT mice, a region both high in GHSR1a expression and involved in the control of anxiety-related behavior. Together, our data support a role for DAG in the processing of emotional behaviors.
Our results indicate that handling of animals prior to testing (habituating the response to being physically manipulated by the investigator) is important in dictating the net impact of AG/DAG on physiology and behavior. As we previously reported, handled animals have lower basal and stimulated plasma corticosterone levels than the nonhandled animals (30). In the current study, the proanxiety phenotype of Ghr KO mice was only revealed after handling, a maneuver that preferentially reduces anxiety-like behavior in the handled control group. It is also important to note that the handling procedure served to establish not only a consistent behavioral phenotype across controls in the various testing paradigms, but also stabilized corticosterone responses to restraint (Fig. 5B–5D). Indeed, the cupping method pioneered by Hurst and West (29), if applied routinely, could improve interexperimental and intraexperimental reproducibility (40). Our data suggest that training both reduces variability of the data and unmasks behavioral phenotypes. We therefore elected to study the role of endogenous ghrelin and GHSR in the regulation of anxiety-related behavior under the stress-minimizing conditions such as handling.
Although both AG and DAG are mainly produced in the stomach, once in the circulation, they can cross the blood-brain barrier to exert effects in the CNS, including regions that are involved in the regulation of anxiety and behavior (41). Although AG is considered the active isoform, DAG is the predominant ghrelin isoform in plasma (2, 42). In vitro studies demonstrate markedly reduced potency of DAG to activate the GHSR1a, estimated to be ∼103 less than for AG (40). This has led to the assumption that the biological effects of DAG are independent of GHSR (43, 44) and, therefore, likely via a unique DAG-specific receptor yet to be identified. However, recent in vitro and in vivo evidence demonstrates that DAG can recapitulate the well-known AG effects on energy balance by acting on brain GHSR1a (7). Moreover, DAG can exert its physiological function via GOAT and GHSR1a, as demonstrated by Hopkins et al. in bone marrow adipocytes (45). It is also possible that DAG could act through GHSR1a in a cell-specific manner (e.g., certain cells may increase the affinity of DAG for GHSR1a by interacting with an intermembrane docking protein or an intracellular protein) (i.e., the receptor activity-modifying proteins). This speculation and the premise of the existence of a DAG receptor will require rigorous scientific validation.
Future experiments using exogenous DAG administration in Ghsr null may clarify whether DAG has an effect on behavioral responses that are independent of GHSR. Relevant to this question, GHSR can not only be constitutively active in the absence of ghrelin (46), but also can dimerize with the dopamine 1 and 2 receptors (47, 48), the melanocortin-3 receptor (49), the serotonin 2C receptor (50), and others, resulting in a modulation of function of both dimerized receptors. Although, in some cases, the ghrelin peptide is not required for these modifications to occur, in others, the presence is necessary for these changes to take effect (51). In both the GOAT KO and Ghr KO models, the only known endogenous ligand for GHSR is absent. Thus, we cannot rule out the possibility that unliganded GHSR may contribute to our behavioral phenotype.
A recent report by Stark et al. (52) showed that chronic administration of DAG decreases anxiety-related behavior following stress exposure. However, DAG was anxiogenic under unstressed conditions, suggesting that the effects of DAG are context specific. It is likely that these differences are related to long-term exposure to DAG in the study by Stark et al. (52), which may have had lasting actions on brain circuitry regulating anxiety behavior. Alternatively, differential effects of DAG in our two studies may be due to our handling procedure, which may have reduced reactivity to the novel environment in our control groups. In addition, our study suggests that Ghr KO is anxiogenic in handled animals. Prior studies suggest that Ghr KO only reduces anxiety-related behaviors in the context of an acute stressor (53). Moreover, Stark et al. (52) indicate that GOAT KO produces a stress-dependent increase in anxiety related-behaviors, whereas our data indicate anxiolytic actions of GOAT depletion under stress-minimized conditions. Another example of the context-dependent behavioral phenotype in the literature can be found in the study by Lutter et al. (22), in which ad libitum–fed Ghsr null mice and WT littermates performed similarly in the EPM. It was only after calorie restriction that the WT mice demonstrated a relatively anxiolytic-like behavior, whereas the Ghsr null mice did not. Calorie restriction in the study by Lutter et al. (22) unmasked differences in anxiety-related behavior in the Ghsr null mice vs WT littermates, just as the long-term handling regimen in the current study unmasked differences in anxiety-related behaviors in Ghr KO vs WT groups. Collectively, these results suggest that DAG acts to modulate behavior and HPA axis function in a context-dependent fashion. Stress appears to flip the impact of DAG from anxiolytic (our study) to anxiogenic (52). Moreover, our data suggest that a stress-reduction regimen reveals an anxiolytic action of DAG not observed in (what we assume to be) conventionally handled mice. These context-dependent actions of DAG indicate that the peptide plays a modulatory rather than primary causal role in control of behavioral reactivity to anxiogenic stimuli.
A direct effect of exogenous AG in modulating anxiety-like behavior in rodents has been proposed by several investigators, but both anxiolytic and anxiogenic effects have been reported (15, 18, 53). Acute intracerebral injection of AG elicits increased anxiety-like behavior when tested minutes following the injection (18), whereas anxiolytic actions are observed upon IP injection (53). Lutter et al. (22) also showed that subcutaneous ghrelin injection in ad libitum–fed mice reduced anxiety-like behavior. These effects were not seen in the Ghsr null mice. In our study, under basal, stress-minimized conditions, we did not observe any change in anxiety-like behavior 15 minutes following IP injection of AG in WT mice during EPM testing. Taken together, the evidence suggests that the effects of DAG and AG to influence stress and anxiety-like behaviors may be similarly dependent on the testing conditions.
Our data are consistent with a dissociation between effects of DAG and AG on behavioral and physiological stress responses under stress-minimized conditions. In handled animals, there was no effect of Ghr or GOAT deletion on corticosterone responses following 30 minutes of restraint, suggesting the loss of AG did not affect endocrine stress reactivity in parallel with behavioral anxiety after handling. It is important to note that unhandled Ghr KO mice had higher HPA axis responses to restraint relative to WT mice, suggesting that unlike the floor effect in open-arm time seen for anxiety responses, hormonal responses are not maximal in the absence of handling. Moreover, corticosterone responses to stress are reduced to equivalent levels by handling in both Ghr KO and control mice, indicating that the Ghr KO mice can reduce HPA reactivity because of handling. Interestingly, Ghsr null mice have an exaggerated HPA axis response to stress with no accompanying anxiety-related phenotype, suggesting a role for AG in modulation of stress responsivity. Taken together, our data suggest that ghrelin effects on behavior and HPA axis responsiveness are differentially regulated.
We also assessed pERK phosphorylation, a downstream indicator of GHSR activation, in different brain regions in WT and Ghr KO animals receiving DAG and AG IP injections. We found that administration of DAG reduced stress-induced pERK only in the EW nucleus in the WT but not in the Ghr KO mice (Fig. 7), whereas the anxiolytic effect of DAG treatment on behavior was seen only in the Ghr KO animals (Fig. 6A). We speculate that these discrepancies could be due to Ghr KO mice exhibiting a phenotype that reflects a primordial, lifelong absence of ghrelin. This lack of ghrelin presence in the system could lead to altered sensitivity of the receptor to the ligand or the neurons to the stimuli (of DAG) and other compensatory changes.
In summary, we undertook a comprehensive approach to evaluate the role of endogenous ghrelin in the regulation of anxiety-like behavior and stress response using three genetic mutant models and pharmacological manipulations. Our data suggest that AG and DAG play important and dissociable roles in control of stress and anxiety. Given the need to inhibit anxiety reactions and promote foraging behavior, it is possible that the stress/anxiety-buffering effects of DAG (and perhaps AG) are related to the well-characterized effects of ghrelin on hunger to maximize energy intake and survival. Additional research will be required to decipher the molecular mechanisms that differentiate AG from DAG effects in vivo and to determine whether these mechanisms interface with other more classical hormonal actions of ghrelin (51).
Acknowledgments
We thank Amanda Nunley for providing animal care and Brittany Smith and Ana Franco for recording data and assisting with behavioral experiments.
Financial Support: J.T. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK097550.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| pERK | Residues surrounding Thr202/Tyr204 of human p44 mitogen-activated protein kinase | Rabbit anti-pERK antibody | Cell Signaling Technology, 9101 | Rabbit | 1:4000 | AB_331646 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- AG
- acyl ghrelin
- BSA
- bovine serum albumin
- CNS
- central nervous system
- DAG
- desacyl ghrelin
- EPM
- elevated plus maze
- EW
- Edinger-Westphal
- GHSR
- growth hormone secretagogue receptor
- GOAT
- ghrelin O-acyltransferase
- HPA
- hypothalamic-pituitary-adrenal
- IP
- intraperitoneal
- KO
- knockout
- LD
- light/dark
- LSD
- least significant difference
- NTS
- nucleus tractus solitarius
- PBS
- phosphate-buffered saline
- pERK
- phospho–extracellular signal-regulated kinase
- SEM
- standard error of the mean
- WT
- wild-type.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 2.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279(3):909–913. [DOI] [PubMed] [Google Scholar]
- 3.Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LHT, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab. 2015;4(6):437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Zhao T-J, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci USA. 2008;105(31):10750–10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105(17):6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93(5):1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heppner KM, Piechowski CL, Müller A, Ottaway N, Sisley S, Smiley DL, Habegger KM, Pfluger PT, Dimarchi R, Biebermann H, Tschöp MH, Sandoval DA, Perez-Tilve D. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes. 2014;63(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159(6):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008;198(3):511–521. [DOI] [PubMed] [Google Scholar]
- 10.Delhanty PJ, Neggers SJ, Van Der Lely AJ. Des-acyl ghrelin: A metabolically active peptide In: Benso A, Casanueva FF, Ghigo E, Granata R, eds. The Ghrelin System. Vol 25 Basel, Switzerland: Karger Publishers; 2013:112–121. [DOI] [PubMed] [Google Scholar]
- 11.Delhanty PJD, Huisman M, Baldeon-Rojas LY, van den Berge I, Grefhorst A, Abribat T, Leenen PJM, Themmen APN, van der Lely A-J. Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J. 2013;27(4):1690–1700. [DOI] [PubMed] [Google Scholar]
- 12.Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen C-CC-Y, Ueno N, Fujimiya M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129(1):8–25. [DOI] [PubMed] [Google Scholar]
- 13.Delhanty PJD, Sun Y, Visser JA, van Kerkwijk A, Huisman M, van IJcken WFJ, Swagemakers S, Smith RG, Themmen APN, van der Lely A-J. Unacylated ghrelin rapidly modulates lipogenic and insulin signaling pathway gene expression in metabolically active tissues of GHSR deleted mice. PLoS One.2010;5(7):e11749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delhanty PJD, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides. 2011;32(11):2309–2318. [DOI] [PubMed] [Google Scholar]
- 15.Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: Implications for mood disorders. Biol Psychiatry. 2015;78(1):19–27. [DOI] [PubMed] [Google Scholar]
- 16.Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1358–R1365. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MV, Levine S, Alam S, Harbich D, Sterlemann V, Ganea K, de Kloet ER, Holsboer F, Müller MB. Metabolic signals modulate hypothalamic-pituitary-adrenal axis activation during maternal separation of the neonatal mouse. J Neuroendocrinol. 2006;18(11):865–874. [DOI] [PubMed] [Google Scholar]
- 18.Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299(5):739–743. [DOI] [PubMed] [Google Scholar]
- 19.Currie PJ, Khelemsky R, Rigsbee EM, Dono LM, Coiro CD, Chapman CD, Hinchcliff K. Ghrelin is an orexigenic peptide and elicits anxiety-like behaviors following administration into discrete regions of the hypothalamus. Behav Brain Res. 2012;226(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313(3):635–641. [DOI] [PubMed] [Google Scholar]
- 21.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muyrers JP, Zhang Y, Stewart AF. Techniques: Recombinogenic engineering--new options for cloning and manipulating DNA. Trends Biochem Sci. 2001;26(5):325–331. [DOI] [PubMed] [Google Scholar]
- 25.Lee E-C, Yu D, Martinez De Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73(1):56–65. [DOI] [PubMed] [Google Scholar]
- 26.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA. 2004;101(21):8227–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, Chernomorsky R, Boucher M, Elsasser AL, Esau L, Zheng J, Griffiths JA, Wang X, Su H, Xue Y, Dominguez MG, Noguera I, Torres R, Macdonald LE, Stewart AF, DeChiara TM, Yancopoulos GD. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21(6):652–659. [DOI] [PubMed] [Google Scholar]
- 28.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schürmann A, Joost H-G, Jandacek RJ, Hale JE, Heiman ML, Tschöp MH. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15(7):741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Methods. 2010;7(10):825–826. [DOI] [PubMed] [Google Scholar]
- 30.Ghosal S, Nunley A, Mahbod P, Lewis AG, Smith EP, Tong J, D’Alessio DA, Herman JP. Mouse handling limits the impact of stress on metabolic endpoints. Physiol Behav. 2015;150:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorhees CV, Johnson HL, Burns LN, Williams MT. Developmental treatment with the dopamine D2/3 agonist quinpirole selectively impairs spatial learning in the Morris water maze. Neurotoxicol Teratol. 2009;31(1):1–10. [DOI] [PubMed] [Google Scholar]
- 33.Ghosal S, Packard AEB, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, Herman JP. Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J Neurosci. 2017;37(1):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149(11):5482–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2004. [Google Scholar]
- 36.Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav. 2011;103(1):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClave J, Dietrich F. Statistics. 6th ed Englewood Cliffs, NJ: Macmillan College Publishing Company, Inc.; 1994. [Google Scholar]
- 38.Gouveia K, Hurst JL. Reducing mouse anxiety during handling: effect of experience with handling tunnels. PLoS One. 2013;8(6):e66401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirchner H, Heppner KM, Holland J, Kabra D, Tschöp MH, Pfluger PT. Ablation of ghrelin O-acyltransferase does not improve glucose intolerance or body adiposity in mice on a leptin-deficient ob/ob background. PLoS One. 2013;8(4):e61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailoo JD, Reichlin TS, Würbel H. Refinement of experimental design and conduct in laboratory animal research. ILAR J. 2014;55(3):383–391. [DOI] [PubMed] [Google Scholar]
- 41.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302(2):822–827. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93(5):1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callaghan B, Furness JB. Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacol Rev. 2014;66(4):984–1001. [DOI] [PubMed] [Google Scholar]
- 44.Thompson NM, Gill DAS, Davies R, Loveridge N, Houston PA, Robinson ICAF, Wells T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145(1):234–242. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins AL, Nelson TAS, Guschina IA, Parsons LC, Lewis CL, Brown RC, Christian HC, Davies JS, Wells T. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R 1a activity: evidence for target cell-induced acylation. Sci Rep. 2017;7:45541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Mol Endocrinol. 2003;17(11):2201–2210. [DOI] [PubMed] [Google Scholar]
- 47.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20(8):1772–1785. [DOI] [PubMed] [Google Scholar]
- 48.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73(2):317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rediger A, Piechowski CL, Yi C-X, Tarnow P, Strotmann R, Grüters A, Krude H, Schöneberg T, Tschöp MH, Kleinau G, Biebermann H. Mutually opposite signal modulation by hypothalamic heterodimerization of ghrelin and melanocortin-3 receptors. J Biol Chem. 2011;286(45):39623–39631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schellekens H, van Oeffelen WEPA, Dinan TG, Cryan JF. Promiscuous dimerization of the growth hormone secretagogue receptor (GHS-R1a) attenuates ghrelin-mediated signaling. J Biol Chem. 2013;288(1):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wellman M, Abizaid A. Growth hormone secretagogue receptor dimers: A new pharmacological target(1,2,3). eNeuro. 2015;2(2):ENEURO.0053-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stark R, Santos VV, Geenen B, Cabral A, Dinan T, Bayliss JA, Lockie SH, Reichenbach A, Lemus MB, Perello M, Spencer SJ, Kozicz T, Andrews ZB. Des-acyl ghrelin and ghrelin O-acyltransferase regulate hypothalamic-pituitary-adrenal axis activation and anxiety in response to acute stress. Endocrinology. 2016;157(10):3946–3957. [DOI] [PubMed] [Google Scholar]
- 53.Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, Kozicz T, Andrews ZB. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry. 2012;72(6):457–465. [DOI] [PubMed] [Google Scholar]