Abstract
The incidence of metabolic disorders like type 2 diabetes and obesity continues to increase. In addition to the well-known contributors to these disorders, such as food intake and sedentary lifestyle, recent research in the exposure science discipline provides evidence that exposure to endocrine-disrupting chemicals like bisphenol A and phthalates via multiple routes (e.g., food, drink, skin contact) also contribute to the increased risk of metabolic disorders. Endocrine-disrupting chemicals (EDCs) can disrupt any aspect of hormone action. It is becoming increasingly clear that EDCs not only affect endocrine function but also adversely affect immune system function. In this review, we focus on human, animal, and in vitro studies that demonstrate EDC exposure induces dysfunction of the immune system, which, in turn, has detrimental effects on metabolic health. These findings highlight how the immune system is emerging as a novel player by which EDCs may mediate their effects on metabolic health. We also discuss studies highlighting mechanisms by which EDCs affect the immune system. Finally, we consider that a better understanding of the immunomodulatory roles of EDCs will provide clues to enhance metabolic function and contribute toward the long-term goal of reducing the burden of environmentally induced diabetes and obesity.
In this review, we focus on studies that demonstrate endocrine-disruptor exposure induces dysfunction of the immune system, which, in turn, has detrimental effects on metabolic health.
The worldwide incidence of metabolic diseases, including type 2 diabetes and obesity, has dramatically increased (1, 2). In the United States alone, the linear time-trend forecasts predict an increase of 33% in prevalence of obesity (3) and 54% in prevalence of diabetes (type 1 and type 2) (4) within the next two decades. The World Health Organization envisages that these metabolic disorders will be a major cause of death by 2030, placing a substantial economic burden on the health care system globally (1, 2). The factors underlying the explosion in the incidence of obesity and diabetes are multifactorial. Although we know overnutrition and sedentary lifestyle are among the top contributors, environmental exposure to a variety of synthetic chemicals may also play a substantial role (5–9).
We are ubiquitously exposed to environmental chemicals through food, drink, and skin contact, and exposure to these chemicals is associated with an increased risk of endocrine-related disorders in humans and animal studies (9). The US Environmental Protection Agency and the Endocrine Society classify these exogenous chemicals as endocrine-disrupting chemicals (EDCs) because they can disrupt any aspect of hormone action (10, 11). In particular, these chemicals have been shown to be associated with increased risk of diabetes and obesity; bisphenol A (BPA) (12–19), and phthalates (17, 20–25) are two widely studied types of EDCs in this context. Although several mechanisms have been elucidated linking EDC exposure and later development of metabolic disease (26, 27), there is relatively little known about how EDCs affect immune function and or about the interplay between EDCs, immune function, and metabolic health. This is a critically important question to address, because cross-talk between the immune and the metabolic systems is pivotal in promoting metabolic health throughout life (28–32).
Exposure to EDCs has been associated with altered immune function, typically by either suppressing immunity, thereby increasing susceptibility to infections, or by enhancing the immune response leading to inflammation, allergies, or autoimmune diseases (33–36). In this review, we provide an overview of the role of the immune system in mediating metabolic health and then review studies that provide evidence of the effects of EDCs (BPA and phthalates, specifically) on the immune system, focusing on how these effects are associated with altered metabolic health.
Immune System and Its Role in Mediating Metabolic Health
Immune modulation of metabolic health has recently gained widespread attention. Both innate (e.g., natural killer cells, mast cells, eosinophils, basophils, and phagocytic cells like macrophages, dendritic cells, and neutrophils) and adaptive (e.g., CD8+ and CD4+ T lymphocytes, B lymphocytes) immune systems play a critical role in metabolic disease progression. The predominant cell types that have been implicated are effector CD8+ cytotoxic T cells [these recognize major histocompatibility class (MHC) I antigen presentation], CD4+ T-helper (Th) cells (Th1 and Th2 recognize MHC II antigen presentation), Th17 cells, T regulatory (Treg) cells, and natural killer (NK) T cells (which possess both innate and adaptive properties).
The cross-talk between the innate and adaptive immune systems has been explored in greater detail in adipose tissue (28). Briefly, in obesity, the enlarged adipocytes produce chemotactic adipokines and chemokines such as monocyte chemotactic protein-1, leukotriene B4, which attract monocytes to adipocytes, where they become adipose tissue macrophages (37). Additionally, adipocytes also secrete chemokines like C-C motif chemokine ligand 5, C-C motif chemokine ligand 20, and C-X-C motif chemokine ligand 12 that contribute to the recruitment of proinflammatory invariant NK T cells, CD8+ cytotoxic T cells, and Th17 cells of the adaptive immune system to adipose tissue (31, 38). These cells release proinflammatory cytokines such as tumor necrosis factor α (TNF-α; from invariant NK T cells cells), interferon-γ (IFN-γ; from cytotoxic T cells) and interleukin (IL) 17 (from Th17 cells) that promote polarization of adipose tissue macrophages to proinflammatory M1 macrophages (28, 31). M1 macrophages with MHC II antigen presentation polarize naïve CD4+ cells to Th1 cells. Th1 cell polarization is also activated by leptin secreted by adipocytes (38). Th1 cells, in turn, produce TNF-α and IFN-γ, and further activate M1 macrophages, establishing a vicious cycle (28, 31). In obese mice, the number of anti-inflammatory Th2 and Treg cells, which produce IL-4, IL-10, or IL-13 to promote an anti-inflammatory M2 macrophage polarization, is reduced compared with Th1 cells (i.e., there is a shift toward an increased Th1:Th2 and Th1:Treg response in adipocytes (28, 31). The cross-talk between adipocytes and adaptive and innate immune cells progressively creates a proinflammatory environment in adipose tissue. The proinflammatory cytokines inhibit insulin signaling by direct serine phosphorylation of insulin receptor substrate (IRS)-1/2, thereby inducing insulin resistance (37). Similarly, in insulin resistance and type 2 diabetes, cross-talk between different innate and adaptive immune cells is triggered in metabolically sensitive tissues like pancreas, liver, skeletal muscle, gut, and blood vessels. This disrupts the pro- and anti-inflammatory balance, eventually contributing to perturbed metabolic health as summarized in Fig. 1.
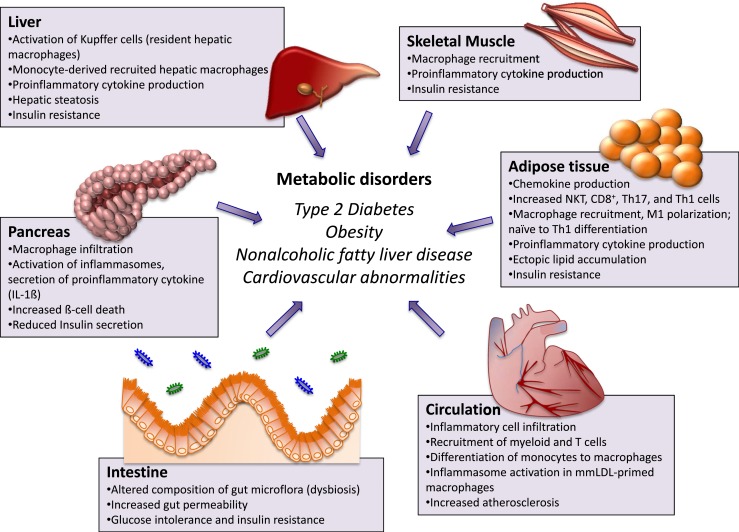
Figure 1.
Role of immune system in mediating metabolic phenotype. The liver contains Kupffer cells (resident hepatic macrophages derived from yolk sac) and monocyte-derived recruited hepatic macrophages (recruited in liver from circulation), which become activated under metabolic distress and induce a proinflammatory phenotype by secreting a variety of inflammatory factors. This proinflammatory phenotype then contributes to hepatic steatosis and hepatic insulin resistance. Increased numbers of cytokines induce expression of genes involved in ceramide synthesis, an excess of which disrupts insulin signaling by inhibiting activation of Akt. In skeletal muscle, during metabolic distress, macrophages are recruited in myocytes. These accumulate within the intermyocyte adipocyte depots and in the surrounding vasculature, and secrete proinflammatory cytokines such as TNF, leading to decreased insulin signaling. In adipose tissue, activated adipocytes secrete adipokines and chemokines, which recruit monocytes (once recruited they become adipose-tissue macrophages) and proinflammatory invariant NK T cells, cytotoxic T cells (CD8+), and Th17 cells to adipocytes. The proinflammatory cells secrete proinflammatory TNF-α, IFN-γ, and IL-17, which polarize adipose-tissue macrophages to proinflammatory M1 macrophages. M1 macrophages with MHC II antigen presentation polarize naïve CD4+ cells to Th1 cells. Th1 cells, in turn, produce TNF-α and IFN-γ and further activate M1 macrophages, establishing a vicious cycle. The proinflammatory cytokines inhibit insulin signaling by direct serine phosphorylation of IRS1, thereby inducing insulin resistance. In the pancreas, in rodent models of type 2 diabetes and insulin resistance, macrophage infiltration into pancreatic islets is increased. Various signals within pancreatic islets activate macrophage NLRP3 inflammasomes, which subsequently cleave the proinflammatory IL-1 family of cytokines into their bioactive forms like IL-1β and IL-18, which bind to IL-1R1 on pancreatic islets, triggering inflammation-induced cell death. This eventually reduces insulin secretion. Furthermore, increased saturated fatty acid levels can induce lipotoxicity and β-cell apoptosis. In the intestine, in obesity and diabetes, the dysbiotic microbiota produces many metabolites, all of which can enter the circulation and negatively influence energy metabolism and insulin sensitivity. Additionally, altered metabolic state gastrointestinal leakiness can result in microbial products such as lipopolysaccharide gaining access to the circulation. Lipopolysaccharide in the bloodstream can contribute to insulin resistance by promoting tissue inflammation. In the circulatory system, minute cholesterol crystals in early atherosclerotic lesions activate the NLRP3 inflammasome in mmLDL-primed macrophages, promoting inflammatory cell infiltration and increased atherosclerosis. mmLDL, minimally modified low-density lipoprotein.
Remarkable advances have been made in understanding how the host immune system senses metabolic stress at a cellular level. A distinct property of innate immune cells is the presence of receptors that recognize pathogen [e.g. lipopolysaccharide (LPS) and peptidoglycan of bacterial cells, or microbial nucleic acids], and host derived damage (e.g. increased glucose, free fatty acids, minimally modified low density lipoprotein, or endogenous stress induced ATP) associated molecular patterns (30, 39). Once the receptors are triggered by either pathogens or cellular stress, a downstream signaling cascade is activated, which initiates the production of cytokines and chemokines. This amplifies the immune response and induces recruitment of antigen-presenting cells that activates the adaptive immune cell population to promote inflammation to resolve and restore normal tissue function (30, 39).
There are three key interconnected, often overlapping, intracellular proinflammatory signaling pathways associated with metabolic disorders: nuclear factor-κB (NFκB)–inhibitor of κB kinase, c-Jun N-terminal kinase and activator protein-1 (JNK-AP1), and inflammasomes (29, 37) (Fig. 2). These signaling pathways can be activated by different receptors like membrane bound Toll-like receptors (TLRs), TNF-α receptor, or cytoplasmic NOD-like receptors (NLRs) (29, 37). In addition, mitochondrial dysfunction leading to production of reactive oxygen species, and increased endoplasmic reticulum stress leading to protein misfolding can also trigger these proinflammatory pathways (29, 37) (Fig. 2). Once activated, the proinflammatory pathways increase serine kinase phosphorylation of IRS1 or IRS2 (which prevents the downstream insulin signaling) and transcription of inflammatory genes (i.e., cytokines, chemokines, and components of the inflammasome) (29, 37) (Fig. 2). A combination of IRS1/2 phosphorylation and increased inflammatory gene transcription increases insulin resistance (29, 37) (Fig. 2). Additionally, upon assembly, the inflammasome, a multiprotein complex comprising scaffold, adaptor, and caspase proteins, activates caspase-1, which subsequently cleaves the proinflammatory IL-1 family of cytokines into their bioactive forms like IL-1β and IL-18, leading to inflammatory cell death (e.g., pancreatic β-cell death associated with reduced insulin secretion) (29, 39–41). IL-1β and IL-18 also further contribute to increased insulin resistance (29, 37) (Fig. 2). Inflammasomes can sense various signals of disrupted homeostatic cellular processes; therefore, they serve as a cytoplasmic surveillance system (30) (Fig. 3). Strowig et al. (30) and Osborn and Olefsky (37) provide in-depth reviews of cellular inflammatory signaling and the role of inflammasomes in mediating metabolic health.
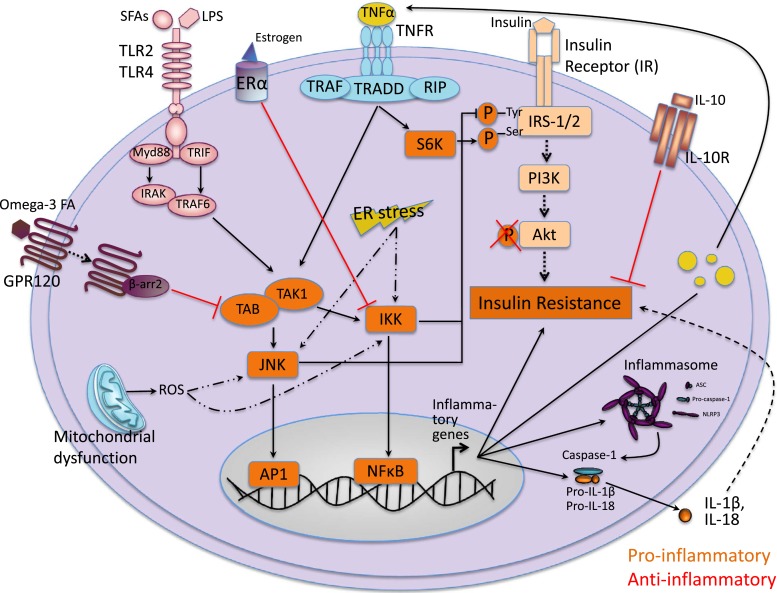
Figure 2.
Inflammation-associated signaling pathways involved in insulin resistance. Proinflammatory signaling induces insulin resistance: Activation of TLR2 or TLR4 and/or TNFR initiates association of TAK1 and TAK1-binding protein 1, which, in turn, activates the NFκB IKK, JNK, and activator protein 1 pathways. Mitochondrial dysfunction leading to production of reactive oxygen species can also trigger IKK and JNK pathways, as can ER stress. Once triggered, IKK and JNK pathways activate serine kinase phosphorylation of IRS 1 and IRS2 and transcription of inflammatory genes. Together, these increase insulin resistance. Once assembled, the inflammasome activate caspase-1 subsequently cleaves the proinflammatory IL-1 family of cytokines into their bioactive forms, which further contribute to increased insulin resistance. Anti-inflammatory signaling reduces insulin resistance: Binding of omega-3 FAs activates GPR120, which initiates anti-inflammatory signaling by blocking TAK activation. Bonding of estrogen to estrogen receptor α (ERα) initiates anti-inflammatory response by blocking IKK–NFκB signaling. IL-10 binding to IL-10R also initiates anti-inflammatory signaling. The anti-inflammatory signals reduce insulin resistance. Akt, serine threonine kinase; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; ER, endoplasmic reticulum; FA, fatty acid; GPR120, G-protein coupled receptor 120; IKK, inhibitor of κB kinase; IL-10R, IL-10 receptor; IRAK, interleukin 1 receptor associated kinase 1; Myd88, myeloid differentiation primary response gene 88; NLRP3, NOD-like receptor protein 3; PI3K, phosphoinositide 3-kinase; RIP, receptor interacting protein; SFA, saturated fatty acid; TAK1, transforming growth factor-β-activated kinase 1; TNFR, tumor necrosis factor receptor; TRADD, TNF receptor associated death domain; TRAF6, TNF receptor associated factor 6; TRIF, TIR domain containing adaptor protein inducing IFN-β; β-arr 2, β-arrestin 2.
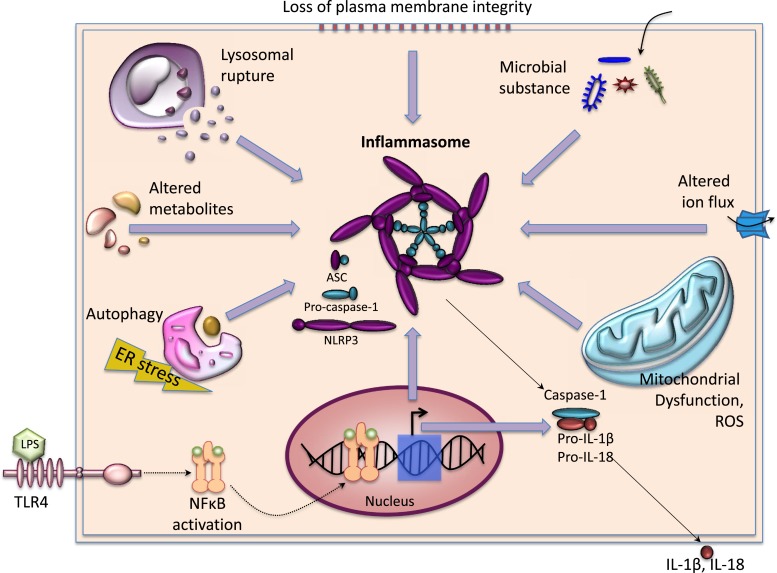
Figure 3.
Activation signals of inflammasomes. Signal 1 is provided by ligands of other pattern recognition receptors like TLR4, which, upon binding to exogenous pathogen-associated molecular patterns like LPSs, activates the downstream NFκB signaling pathway. NFκB translocates to the nucleus and induces expression of NLRP3 as well as other proinflammatory cytokines. Signal 2 includes organelle dysfunction (e.g., loss of plasma membrane integrity, lysosome rupture, mitochondrial dysfunction, ROS production, or autophagy induced by endoplasmic reticulum stress), invasion of microbial products (e.g., microbial protein, DNA), and perturbed homeostatic set points of cellular processes (e.g., aberrations in metabolites or influx of ions). Once assembled, the inflammasomes activate caspase-1, which subsequently cleaves the proinflammatory IL-1 family of cytokines into their bioactive forms, leading to pyroptosis. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; ER, endoplasmic reticulum; NLRP3, NOD-like receptor protein 3; ROS, reactive oxygen species.
In addition to the proinflammatory signaling pathways, anti-inflammatory signaling is triggered by G-protein coupled receptor 120, estrogen receptor α (ERα), and interleukin receptor 10, which attenuates insulin resistance (29, 37, 42) (Fig. 2). Although proinflammatory signaling usually triggers insulin resistance and glucose intolerance, a fine balance between pro- and anti-inflammatory cytokine levels determines the magnitude of inflammation and is essential in maintaining metabolic homeostasis.
EDCs and Effects on Immune Cells and Cytokine Levels
The effect of EDCs on the immune system has been studied largely in the context of allergy and autoimmune disorders (33–36). Human epidemiological studies have shown an association between increased EDC exposure and development of allergic disease; this is reviewed elsewhere (33–36). Emerging evidence from animal studies also suggests that EDC exposure during the critical developmental stages of pregnancy and lactation could adversely affect the developing immune system in the offspring, leading to health defects later in life. For example, exposure of pregnant female rodents to varying relevant human-exposure levels of BPA (ranging from the lowest observed adverse effect level dose of 50 mg/kg/d to 50 µg/kg/d, at which there is no observed adverse effect) has been shown to adversely affect the immune system of the offspring (43–46). Most of these studies observed an imbalance in proinflammatory and anti-inflammatory immune responses with consequent detrimental effects (e.g., development of food intolerance or antigen-specific antibody production) in young rats (43), 12-week-old BALB/c mice (44), 6-month-old isogenic mice (45), and 8-week-old C57BL/6 mice (46). Although there was some discrepancy in results between these studies with regard to the effect of exposure to lower-dose BPA (<50 µg/kg/d) on the immune response, the differences are likely due to differences in route (oral gavage vs feed) and timing (preconception through lactation vs pregnancy only) of BPA exposure, measurement of different end points (allergy vs asthma), or the use of different rodent species or strains. Interestingly, all four studies observed an increase in a proinflammatory Th1 response in the offspring.
Although the effect of EDC exposure on increasing the risk of allergies and autoimmunity has been widely studied, evidence of the effects of EDC exposure on the immune system and its role in mediating metabolic health is sparse. Many researchers have used in vitro models to determine the immunomodulatory metabolic effects of EDC exposure. Exposure of human umbilical vascular endothelial cells to environmentally relevant doses of 3.4 μmol/L, or 5 μmol/L of polychlorinated biphenyl, a common environmental pollutant, increased proinflammatory NFκB gene transcription, which was associated with increased IL-6, TNF-α protein expression, and reduced insulin induced Akt phosphorylation levels (47). Similarly, exposure of human subcutaneous adipose tissue explants in vitro to 10 nM of BPA resulted in increased IL-6 and TNF-α levels and reduced levels of adiponectin (48); adiponectin is anti-inflammatory and typically increases insulin sensitivity (49). There is additional evidence of BPA-exposure activation of the JNK and NFκB pathways, increased proinflammatory cytokine levels, and insulin resistance in 3T3-L1 cells (a human adipocyte cell line) (50, 51). Notably, cytokine-induced cell death was not observed in INS-1E cells (a pancreatic β-cell line) exposed to either between 1 and 500 nM BPA or phthalates for 72 hours (52). Nevertheless, the authors of this latter study observed reduced β-cell viability (at 5, 50, and 500 µM BPA), and reduced maximal glucose-stimulated (16.7 mM) insulin secretion in the INS-1E cell line exposed to 100 µM BPA for 72 hours relative to unexposed controls (52). However, typically, human levels of BPA range from 0.3 to 5 ng/mL (∼1 to 20 nM) (53). This last study used significantly higher doses of BPA in in vitro experiments. Therefore, these studies need to be repeated with lower doses of BPA.
Nonetheless, the findings from these in vitro studies indicate that exposure to EDCs is associated with impaired metabolic end points such as endothelial cell function, insulin sensitivity, and insulin secretion, with most studies indicating a potential correlation with pro- and anti-inflammatory cytokine imbalance. These studies need to be confirmed in in vivo models. Furthermore, it is highly likely that there is cross-talk between the immune effects of EDCs in different organs. For example, changes in adipose tissue are likely to affect muscle and, perhaps, the pancreas as well, as illustrated in Fig. 1. Therefore, the contribution of different immune cell populations in different organs to this cytokine imbalance requires further investigation.
Few studies report that effects of BPA on T-cell immunomodulation may be dose specific. For instance, 1 mg/L BPA (∼160 μg/kg/d) exposure increased levels of splenic IFN-γ, TNF-α, and IL-6, whereas exposure to 10 mg/L BPA (1600 μg/kg/d) reduced levels of these cytokines in a streptozotocin-induced model of type 1 diabetes in male mice (54). These splenic changes were associated with the development of insulitis later in life (54). Our recent study showed that maternal exposure to 10 µg and 10 mg/kg/d of BPA from preconception through lactation was associated with increased numbers of T lymphocytes and macrophages, and increased proinflammatory cytokine levels in the pancreas of adult male mice offspring across two generations (55). This alteration in immune cell population and cytokine levels was associated with dose-specific effects of BPA on mitochondrial dysfunction and reduced β-cell mass in male mice. In both these studies, the effects were reported in male mice only. There is paucity of research examining the mechanisms underlying the sex-specific effects of EDCs on the immune system. A recent study demonstrated that BPA exposure sex-specifically altered cellular and microanatomical structures of the spleens in CD1 mice (56). It is possible that the differences in phenotypes observed in EDC exposure between sexes is related to sex-specific effects on immune function. This remains to be determined and is potentially an exciting area of research.
Taken together, these studies suggest it is possible that EDCs may be mediating their effect on the metabolic health via the immune system. Although most studies indicate that EDC exposure may induce a proinflammatory phenotype and increase cytokine-induced cell death, some findings are contradictory and it is possible that EDCs dampen the anti-inflammatory response, thereby upsetting the balance between pro- and anti-inflammatory responses. Additional research is required to clarify and substantiate these studies.
Potential Immunomodulatory Mediators of EDC Exposure
The effects of EDCs on the immune system are likely to be mediated via multiple mechanisms. In this section, we describe four possible mediators, which can be affected by EDCs and have been shown to be critical for maintaining metabolic homeostasis.
EDCs, receptors, and inflammation
It is well known that many EDCs mediate their effects on multiple organs by binding to various receptors. Similarly, EDCs may also mediate their effects on the immune system via these receptors. Some of the commonly studied receptors in this context are estrogen receptors, estrogen-related receptors (ERRs), peroxisome proliferator–activated receptor γ (PPARγ), TLRs, and NLRs. For example, BPA and phthalates alter cytokine levels, and this may be mediated through estrogen receptors (57). Interestingly Miao et al. (58) reported that ERα gene expression was reduced in male, but increased in female, first- and second-generation offspring of maternally BPA-exposed F344 rats. These changes were associated with reduced levels of IL-2, IL-12, IFN-γ, and TNF-α in spleen of all BPA-exposed groups across two generations (58). Consistent with these previous findings, we recently reported that first- and second-generation male offspring of maternally BPA-exposed mice had reduced expression of ERα gene expression in islets, and this was associated with increased proinflammatory cytokine levels in pancreatic lysates (55). Estrogen receptor signaling also has been shown to be altered by other EDCs like nonylphenol and 4-octylphenol, byproducts of domestic and industrial detergents, in human myeloid dendritic cells (59). It is known that ERα has anti-inflammatory action such that it can block NFκB signaling and reduce expression of inflammatory genes (42, 60, 61). Therefore, it is possible that altered levels of cytokines observed in EDC-exposed animals may be due to EDCs’ consequent effect on ERα expression; thus indicating that ERα may be one way via which EDCs can alter cytokine levels.
EDCs such as BPA have greater affinity for ERRs than they do for estrogen receptors. ERRs are involved in regulating multiple functions, including oxidative metabolism and activation of effector T cells (62, 63). BPA has been shown to alter the expression of estrogen receptors and ERRs in a sex and dose-specific manner (64). Recently, it has been shown that BPA may alter T-cell function by modulating ERRα expression (65). These results suggest that ERRs may also be potential targets of the immunomodulatory role of EDCs; however, these results need to be confirmed in other model systems.
Another receptor by which EDCs can mediate their effect is PPARγ, an adipocyte-specific nuclear hormone receptor essential for regulating adipogenesis (66). PPARγ typically has an anti-inflammatory effect (67). BPA analogs like tetrabromobisphenol A and bisphenol A diglycidyl ether have been shown to antagonize PPARγ agonists in vitro and in vivo, respectively (68, 69). Thus, by disrupting PPARγ’s anti-inflammatory action, EDCs could direct immune cells toward a proinflammatory phenotype.
As mentioned, TLRs play a key role in mediating an inflammatory phenotype. EDCs have been shown to act via TLRs, although the evidence is contradictory. For example, when zebrafish embryos were exposed to human-relevant doses of 0.1 µg/L, 1 µg/L, 10 µg/L, 100 µg/L, and 1000 µg/L BPA for 4 to 168 hours postfertilization, gene expression of the proinflammatory cytokines IL-1β and IFN-γ, and key components of the TLR pathway was increased relative to that of aqueous controls that were exposed to dechlorinated tap water (70). However, in humans, the levels of BPA in cord blood from women who had high occupational BPA exposure negatively correlated with TLR3, TLR4-induced TNF-α response, and TLR7-8–stimulated IL-6 response in neonatal mononuclear cells (71). These authors did not observe a BPA dose-response relationship and also found no increased risk of infection in 1-year-old infants (71). Therefore, it is unclear whether EDCs like BPA have stimulatory or inhibitory action via TLRs ,and more studies need to be done to elucidate these effects.
Finally, as discussed, inflammasome assembly leads to release of bioactive IL-1β and IL-18, and initiates an inflammation-mediated cell death (e.g., pancreatic β-cell death) and insulin resistance; therefore, it is critical in regulating metabolic health. Recent studies indicate EDC exposure can affect the assembly of inflammasomes. For example, the environmental chemical, perfluorodecanoic acid has been shown to stimulate NLRP3 inflammasome assembly in a gastric cell line and, in mice, is also associated with increased IL-1β and IL-18 levels in stomach tissue, leading to gastric inflammation (72). Perfluorodecanoic acid is found commonly in food and drinks because of its use in manufacturing nonstick cookware, fire-fighting foam, and many other industrial products (72). Similarly, other environmental chemicals like silica and asbestos. once phagocytosed by pulmonary macrophages. activate the assembly of inflammasomes through reactive oxygen species production and lysosomal rupture, leading to silicosis and asbestosis (73, 74). BPA exposure has been shown to activate NLPR3 inflammasome assembly by inducing IFN-γ signaling in murine bone marrow–derived cells (75). Thus, various environmental chemicals can activate inflammasome assembly and lead to abnormal pathological responses. In particular, a recent study demonstrated that phthalate exposure activated NLRP3 inflammasome assembly in a hepatic cell line, indicating a potential role in phthalate-exposure–related liver damage (76). We know the liver is central in maintaining insulin sensitivity and perturbed inflammasome-mediated liver function may increase metabolic imbalance in the body. Because the studies in this paragraph were carried out in cell lines, there is a critical need for research in in vivo animal models to substantiate the effects of EDCs on inflammasomes in mediating abnormal metabolic health.
Thus, the effects of EDCs on the aforementioned receptors with consequent effects on immune regulation appear to be an emerging mechanism by which EDCs may be triggering impaired metabolic health. However, additional work in this area will need to be done to clarify whether this is, indeed, the case and, if so, what are the principle modes of action (i.e., whether EDCs do so by degrading the target receptor, by disrupting the ligand receptor interaction, or, perhaps, indirectly by initiating coactivator and/or corepressor recruitment).
EDCs, microbiome, and inflammation
The gut microbiota vary substantially from person to person (77). Different EDCs can be differently metabolized by gut microbiota, which can alter the absorption, distribution, metabolism, and excretion of these chemicals. Depending on the mode of metabolism by gut microbiota, the ingested chemicals could be either more or less bioavailable, which would obviously affect their toxicity. Due to significant interhuman variation in the gut microbiota (77), individuals could metabolize EDCs differently, with potentially variable health outcomes. The effects of EDCs on the gut microbiota are reviewed elsewhere (78–80). Briefly, using 16S RNA sequencing, scientists have characterized the microbial population in rodents postnatally exposed to different EDCs, like BPA (81), diethyl phthalate, methylparaben, triclosan, a mixture of these chemicals (82), or polychlorinated biphenyls (83). Exposure to these EDCs either favored or reduced growth of specific gut microbial populations (81–83). These populations include high abundant population levels of Bacteroidetes [27.8% relative abundance (84)] and Firmicutes [38.8% relative abundance (84)], or relatively low abundant Proteobacteria [2.1% relative abundance; a marker of gut inflammation (84)] and Actinobacteria [8.2% relative abundance (84)]. Interestingly, in California mice, exposure from periconception through weaning to 50 mg/kg feed weight of BPA and 0.1 ppb of ethinyl estradiol favored growth of a beneficial gut bacterium (Bifidobacterium) in female offspring (85). In contrast, BPA exposure increased the percentage of detrimental gut bacterium (Akkermansia, and Methanobrevibacter) in male offspring (85). Detailed reviews are available (78–80). Taken together, these studies suggest exposure to EDCs can impair the normal gut microbiome and potentially adversely impact metabolic health.
The gut microbiome plays an essential role in regulating fat storage (86), regulating intestinal capability to extract energy from food (87), modulating levels of hormones that regulate appetite (88), and altering inflammatory pathways (89–91). A perturbed gut microbiota, termed dysbiosis, produces many metabolites, including acetate, bile-acid derivatives, and short-chain fatty acids, all of which can enter the circulation and negatively influence energy metabolism and insulin sensitivity. Additionally, in obese individuals, increased fasting glucose and insulin levels, and homeostatic model assessment index is associated with gastrointestinal “leakiness” in which microbial products such as LPS, or even bacteria, can gain access to the circulation (87). LPS in the bloodstream can contribute to insulin resistance by promoting tissue inflammation, which has consequent effects on metabolic health (Figs. 1 and 2). Unexpectedly, therefore, perturbations in the gut microbiome have been associated with obesity (87, 92) and diabetes (93). In fact, altering the gut microbiome has been shown to improve glycemic control or initiate weight loss in patients with type 2 diabetes and obesity independent of caloric intake, as evident in patients after bariatric surgery (94, 95).
Although it is evident from previous studies that EDCs can influence the gut microbiome, the mechanisms underlying these effects remain to be elucidated. Data from a recent study suggest exposure to EDCs may modify the gut microbiome and increase the risk of metabolic diseases, as observed in mice exposed to persistent organic pollutants (96). This interaction of persistent organic pollutants with the gut microbiome was shown to be mediated via aryl hydrocarbon receptor activation (96). However, these findings remain to be confirmed by other studies. Results of elegant knock-out studies in mice suggest components of the innate immune system like TLR5 (97) or inflammasomes (98) play a critical role in modulating the gut microbiome and contribute to the development of an abnormal metabolic phenotype. As discussed, EDCs can alter these innate immune system components; therefore, it is possible that EDCs may mediate their effects on the gut microbiome via the immune system. Alternatively, EDCs might affect the gut microbiome independent of the immune system, and a dysbiotic gut microbiome, itself, can trigger inflammatory response contributing to insulin resistance and a diabetic phenotype (89–93). Research needs to be done to provide a better understanding of the interaction of EDCs, the microbiome, and the immune system.
EDCs, oxidative stress, and inflammation
Many studies have demonstrated that exposure to EDCs impairs mitochondrial function in metabolic tissues, including liver (99, 100) and pancreas (101–103). Similarly, EDCs have been shown to increase endoplasmic reticulum stress in in vitro and in vivo studies involving kidney (104), pancreas (105, 106), and liver (107). Mitochondrial dysfunction and endoplasmic reticulum stress are associated with increased oxidative stress (108) and metabolic dysfunction (109). Increased oxidative stress can activate various inflammatory pathways and increases the risk of metabolic abnormalities such as insulin resistance, diabetes, and obesity (Fig. 3). Consistent with these observations is evidence suggesting that BPA exposure is associated with increased oxidative stress and increased proinflammatory cytokine levels in vivo (110) and in vitro (111) in the liver. BPA exposure has also been shown to activate NFκB signaling, increase cell death, and reduce insulin secretion in ex vivo cultures of primary murine pancreatic islets (112). As mentioned, we have shown that maternal BPA exposure was associated with mitochondrial dysfunction and proinflammatory cytokine levels in the pancreas of adult male mice offspring across two generations (55). In our study, we did not determine whether oxidative stress was a contributory factor. However, effects of EDC on oxidative stress in mediating metabolic health remain an interesting possibility to be confirmed by future studies.
EDCs, circadian disruption, and inflammation
It is now well accepted that disruption in circadian rhythm impairs metabolic homeostasis in humans and animals (113). Chronic inflammation is a key factor underlying the metabolic syndrome; in addition to considering the effects of circadian disruption on metabolic health, several studies have shown circadian disruption potentiates inflammation. Using a genetic manipulation approach, it has been demonstrated that circadian disruption such as deletion of the period 2 (Per 2) clock gene was associated with reduced function of NK cell function as characterized by reduced IFN-γ levels in NK cells of spleen of Per2−/− knockout mice (114) and reduced granzyme B and perforin levels in an RNA interference–mediated Per2 knockdown–derived NK cell line (115). Similarly, lifestyle factors such as sleep/wake pattern, shift work, and jet lag, which lead to circadian disruption, are associated with altered immune system function. For example, irregular sleep/wake patterns are associated with increased secretion of proinflammatory cytokines (116) and increased risk of infections (117, 118). Also, isolated peritoneal macrophages from mice exposed to four consecutive, weekly, 6-hour phase advances of the light/dark schedule produced increased proinflammatory cytokines in response to LPS, strongly suggesting that jet-lag–related circadian disruption affects the innate immune system (119). In fact, key metabolic hormones like glucocorticoids and melatonin are affected by circadian disruption and have been shown to influence immune function (120, 121).
Recent study findings suggest EDCs can disrupt circadian rhythmicity. For example, higher BPA urinary levels are associated with sleep deprivation in US adults in the National Health and Nutrition Examination Survey cohort, suggesting that BPA exposure can alter the normal circadian cycle (122). Moreover, 8-week-old C57BL/6 mice exposed for 12 weeks to tolylfluanid, a common fungicide found in European agricultural products, exhibited reduced respiratory exchange ratios in dark phase, increased total calorie consumption, decreased energy expenditure, and daily activity in light phase compared with unexposed controls (123). These findings suggest tolylfluanid exposure disrupts the normal circadian feeding and energy expenditure pattern in mice. Interestingly, it has also been shown that maternal EDC exposure is associated with offspring circadian disruption. For example, exposure of female mice from 5 weeks before mating throughout lactation to a low-dose mixture of EDCs including 2,3,7,8-tetrachlorodibenzo-p-dioxine [2 pg/kg body weight per day (bw/d)], polychlorinated biphenyl 153 (80 ng/kg bw/d), diethylhexyl-phthalate (50 μg/kg bw/d), and BPA (5 μg/kg bw/d) was associated with changes in expression of several genes (measured by microarray analysis) regulating circadian clock metabolic pathways in the liver of 12-week-old female offspring (124). These microarray findings were confirmed by quantitative polymerase chain reaction, which revealed increased mRNA expression levels of the clock genes Per1 and reduced ArntI (which encodes BMAL1) in the liver of female offspring (124). These changes were associated with genotypic changes indicating reduced steroid and fatty acid synthesis, and increased cholesterol and steroid transport and lipid accumulation, as well as phenotypic changes of increased hepatic triglyceride levels and mild insulin resistance in female offspring (124).
Thus, emerging evidence indicates that EDC exposure is associated with circadian disruption. In light of multiple studies demonstrating that circadian disruption can modulate immune function, it is possible that EDC-induced circadian disruption may be a potential mechanism underlying EDC-induced immune dysfunction, which may exacerbate environment-induced metabolic disorders. This area of research requires further exploration.
Conclusion
The cross-talk between the immune system and metabolic tissues has been well explored and it is now accepted that immune dysfunction increases our risk of various metabolic disorders, including diabetes and obesity. Therapies have been developed to target different components of the immune system to improve metabolic health. Despite the complexity of the immune landscape, targeting a single immunologic factor in clinical trials has met with some success in treating type 2 diabetes and insulin resistance in obesity (125–128).
Our ubiquitous exposure to environmental chemicals further aggravates the risk of developing metabolic diseases. Dysregulation of the immune system has recently emerged as a novel mediator of this EDC–metabolic dysfunction cross-talk. The immunomodulatory role of EDCs could serve as a unifying mechanism underlying EDC-mediated metabolic dysfunction (Fig. 4). However, much research needs to be done to support this hypothesis. Moreover, in vitro and in vivo animal studies need to be verified in humans to establish clinical relevance. Research in the EDC field should consider the immune system as a major EDC target, thus allowing the development of novel treatment and prevention strategies against environment-induced metabolic diseases.
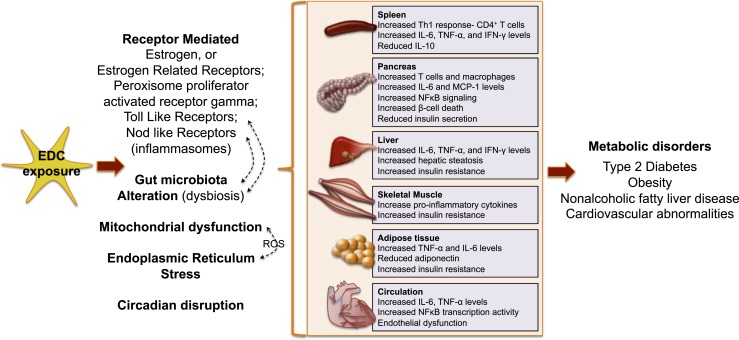
Figure 4.
Possible routes of EDC action on the immune system contributing to metabolic disorders. By interacting with various receptors, altering the gut microbiome, inducing oxidative stress via mitochondrial dysfunction and/or endoplasmic reticulum stress, or via circadian disruption, EDCs trigger immune dysfunction in various tissues. Together, this may contribute toward a perturbed metabolic health. See Fig. 3 legend for expansion of abbreviation.
Acknowledgments
Financial Support: This work was supported by National Institute of Environmental Health Sciences Grants ES023284 and ES013508 (to R.A.S.) and CEET-ES-013508-05 (to R.A.S. and A.B.); National Institutes of Health Grants R21AI128060, R21DK111755, and R01HL136572; and the Pew Charitable Trusts (to J.H.M.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- bw/d
- body weight per day
- EDC
- endocrine-disrupting chemical
- ERR
- estrogen-related receptor
- ERα
- estrogen receptor α
- IFN-γ
- interferon γ
- IL
- interleukin
- IRS
- insulin receptor substrate
- JNK
- c-Jun N-terminal kinase
- LPS
- lipopolysaccharide
- MHC
- major histocompatibility class
- NFκB
- nuclear factor-κB
- NK
- natural killer
- NLR
- NOD-like receptor
- PPARγ
- peroxisome proliferator–activated receptor γ
- Th
- T helper
- TLR
- Toll-like receptor
- TNF-α
- tumor necrosis factor
- Treg
- T regulatory.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2012 [published correction appears in Diabetes Care 2013;36(6):1797] Diabetes Care. 2013;36(4):1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. [DOI] [PubMed] [Google Scholar]
- 4.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31(2):201–208. [DOI] [PubMed] [Google Scholar]
- 6.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Magdalena P, Quesada I, Nadal Á. Prenatal exposure to BPA and offspring outcomes: the diabesogenic behavior of BPA. Dose Response. 2015;13(2):1559325815590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jašarević E, Sieli PT, Twellman EE, Welsh TH Jr, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci USA. 2011;108(28):11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996;104(Suppl 4):715–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153(9):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117(5):784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96(12):3822–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabanayagam C, Teppala S, Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013;50(4):625–631. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadkhaniha R, Mansouri M, Yunesian M, Omidfar K, Jeddi MZ, Larijani B, Mesdaghinia A, Rastkari N. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Health Sci Eng. 2014;12(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aekplakorn W, Chailurkit LO, Ongphiphadhanakul B. Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009. J Diabetes. 2015;7(2):240–249. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122(6):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista TM, Alonso-Magdalena P, Vieira E, Amaral ME, Cederroth CR, Nef S, Quesada I, Carneiro EM, Nadal A. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One. 2012;7(3):e33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114(1):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James-Todd T, Stahlhut R, Meeker JD, Powell SG, Hauser R, Huang T, Rich-Edwards J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001-2008. Environ Health Perspect. 2012;120(9):1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James-Todd TM, Huang T, Seely EW, Saxena AR. The association between phthalates and metabolic syndrome: the National Health and Nutrition Examination Survey 2001-2010. Environ Health. 2016;15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115(6):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smerieri A, Testa C, Lazzeroni P, Nuti F, Grossi E, Cesari S, Montanini L, Latini G, Bernasconi S, Papini AM, Street ME. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS One. 2015;10(2):e0117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lind PM, Zethelius B, Lind L. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care. 2012;35(7):1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH, Park HY, Bae S, Lim YH, Hong YC. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PLoS One. 2013;8(8):e71392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadal A, Quesada I, Tudurí E, Nogueiras R, Alonso-Magdalena P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat Rev Endocrinol. 2017;13(9):536–546. [DOI] [PubMed] [Google Scholar]
- 27.Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60(7):1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161(1):146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. [DOI] [PubMed] [Google Scholar]
- 30.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. [DOI] [PubMed] [Google Scholar]
- 31.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8(12):709–716. [DOI] [PubMed] [Google Scholar]
- 32.Shirakawa J, De Jesus DF, Kulkarni RN. Exploring inter-organ crosstalk to uncover mechanisms that regulate β-cell function and mass. Eur J Clin Nutr. 2017;71(7):896–903. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed SA. The immune system as a potential target for environmental estrogens (endocrine disrupters): a new emerging field. Toxicology. 2000;150(1-3):191–206. [DOI] [PubMed] [Google Scholar]
- 34.Chalubinski M, Kowalski ML. Endocrine disrupters--potential modulators of the immune system and allergic response. Allergy. 2006;61(11):1326–1335. [DOI] [PubMed] [Google Scholar]
- 35.Kuo CH, Yang SN, Kuo PL, Hung CH. Immunomodulatory effects of environmental endocrine disrupting chemicals. Kaohsiung J Med Sci. 2012;28(7, Suppl):S37–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson L, Miller R. The impact of bisphenol A and phthalates on allergy, asthma, and immune function: a review of latest findings. Curr Environ Health Rep. 2015;2(4):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. [DOI] [PubMed] [Google Scholar]
- 38.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li HB, Jin C, Chen Y, Flavell RA. Inflammasome activation and metabolic disease progression. Cytokine Growth Factor Rev. 2014;25(6):699–706. [DOI] [PubMed] [Google Scholar]
- 40.Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol. 2012;13(4):321–324. [DOI] [PubMed] [Google Scholar]
- 41.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller CN, Brown LM, Rayalam S, Della-Fera MA, Baile CA. Estrogens, inflammation and obesity: an overview. Front Biol. 2012;7(1):40–47. [Google Scholar]
- 43.Menard S, Guzylack-Piriou L, Leveque M, Braniste V, Lencina C, Naturel M, Moussa L, Sekkal S, Harkat C, Gaultier E, Theodorou V, Houdeau E. Food intolerance at adulthood after perinatal exposure to the endocrine disruptor bisphenol A. FASEB J. 2014;28(11):4893–4900. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien E, Bergin IL, Dolinoy DC, Zaslona Z, Little RJ, Tao Y, Peters-Golden M, Mancuso P. Perinatal bisphenol A exposure beginning before gestation enhances allergen sensitization, but not pulmonary inflammation, in adult mice. J Dev Orig Health Dis. 2014;5(2):121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien E, Dolinoy DC, Mancuso P. Perinatal bisphenol A exposures increase production of pro-inflammatory mediators in bone marrow-derived mast cells of adult mice. J Immunotoxicol. 2014;11(3):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshino S, Yamaki K, Li X, Sai T, Yanagisawa R, Takano H, Taneda S, Hayashi H, Mori Y. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology. 2004;112(3):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Lv X, Du Y. Inflammatory response and insulin signaling alteration induced by PCB77. J Environ Sci (China). 2010;22(7):1086–1090. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304(1-2):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valentino R, D’Esposito V, Passaretti F, Liotti A, Cabaro S, Longo M, Perruolo G, Oriente F, Beguinot F, Formisano P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS One. 2013;8(12):e82099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ariemma F, D’Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, Cimmino I, Longo M, Beguinot F, Formisano P, Valentino R. Low-dose bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS One. 2016;11(3):e0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weldingh NM, Jørgensen-Kaur L, Becher R, Holme JA, Bodin J, Nygaard UC, Bølling AK. Bisphenol A is more potent than phthalate metabolites in reducing pancreatic β-cell function. BioMed Res Int. 2017;2017:4614379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6, Suppl):S56–S69. [DOI] [PubMed] [Google Scholar]
- 54.Cetkovic-Cvrlje M, Thinamany S, Bruner KA. Bisphenol A (BPA) aggravates multiple low-dose streptozotocin-induced type 1 diabetes in C57BL/6 mice. J Immunotoxicol. 2017;14(1):160–168. [DOI] [PubMed] [Google Scholar]
- 55.Bansal A, et al. Sex- and dose-specific effects of maternal bisphenol A exposure on pancreatic islets of first and second generation adult mice offspring. Environ Health Perspect. 2017;125(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gear RB, Belcher SM. Impacts of bisphenol A and ethinyl estradiol on male and female CD-1 mouse spleen. Sci Rep. 2017;7(1):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Couleau N, Falla J, Beillerot A, Battaglia E, D’Innocenzo M, Plançon S, Laval-Gilly P, Bennasroune A. Effects of endocrine disruptor compounds, alone or in combination, on human macrophage-like THP-1 cell response. PLoS One. 2015;10(7):e0131428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miao S, Gao Z, Kou Z, Xu G, Su C, Liu N. Influence of bisphenol a on developing rat estrogen receptors and some cytokines in rats: a two-generational study. J Toxicol Environ Health A. 2008;71(15):1000–1008. [DOI] [PubMed] [Google Scholar]
- 59.Hung CH, Yang SN, Kuo PL, Chu YT, Chang HW, Wei WJ, Huang SK, Jong YJ. Modulation of cytokine expression in human myeloid dendritic cells by environmental endocrine-disrupting chemicals involves epigenetic regulation. Environ Health Perspect. 2010;118(1):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25(8):2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Chaudry IH. Salutary effects of 17beta-estradiol on T-cell signaling and cytokine production after trauma-hemorrhage are mediated primarily via estrogen receptor-alpha. Am J Physiol Cell Physiol. 2007;292(6):C2103–C2111. [DOI] [PubMed] [Google Scholar]
- 62.Ranhotra HS. The estrogen-related receptors: orphans orchestrating myriad functions. J Recept Signal Transduct Res. 2012;32(2):47–56. [DOI] [PubMed] [Google Scholar]
- 63.Michalek RD, Gerriets VA, Nichols AG, Inoue M, Kazmin D, Chang CY, Dwyer MA, Nelson ER, Pollizzi KN, Ilkayeva O, Giguere V, Zuercher WJ, Powell JD, Shinohara ML, McDonnell DP, Rathmell JC. Estrogen-related receptor-α is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci USA. 2011;108(45):18348–18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110(24):9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cipelli R, Harries L, Okuda K, Yoshihara S, Melzer D, Galloway T. Bisphenol A modulates the metabolic regulator oestrogen-related receptor-α in T-cells. Reproduction. 2014;147(4):419–426. [DOI] [PubMed] [Google Scholar]
- 66.Janesick A, Blumberg B. Minireview: PPARγ as the target of obesogens. J Steroid Biochem Mol Biol. 2011;127(1-2):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat Res. 2010;690(1-2):57–63. [DOI] [PubMed] [Google Scholar]
- 68.Raikwar HP, Muthian G, Rajasingh J, Johnson C, Bright JJ. PPARgamma antagonists exacerbate neural antigen-specific Th1 response and experimental allergic encephalomyelitis. J Neuroimmunol. 2005;167(1-2):99–107. [DOI] [PubMed] [Google Scholar]
- 69.Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect. 2011;119(9):1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu H, Yang M, Qiu W, Pan C, Wu M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ Toxicol Chem. 2013;32(8):1793–1799. [DOI] [PubMed] [Google Scholar]
- 71.Liao SL, Tsai MH, Lai SH, Yao TC, Hua MC, Yeh KW, Chiang CH, Huang SY, Huang JL. Prenatal exposure to bisphenol-A is associated with Toll-like receptor-induced cytokine suppression in neonates. Pediatr Res. 2016;79(3):438–444. [DOI] [PubMed] [Google Scholar]
- 72.Zhou X, Dong T, Fan Z, Peng Y, Zhou R, Wang X, Song N, Han M, Fan B, Jia J, Liu S. Perfluorodecanoic acid stimulates NLRP3 inflammasome assembly in gastric cells. Sci Rep. 2017;7:45468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panchanathan R, Liu H, Leung YK, Ho SM, Choubey D. Bisphenol A (BPA) stimulates the interferon signaling and activates the inflammasome activity in myeloid cells. Mol Cell Endocrinol. 2015;415:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ni J, Zhang Z, Luo X, Xiao L, Wang N. Plasticizer DBP activates NLRP3 inflammasome through the P2X7 receptor in HepG2 and L02 cells. J Biochem Mol Toxicol. 2016;30(4):178–185. [DOI] [PubMed] [Google Scholar]
- 77.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P; MetaHIT Consortium . Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin Y, Wu S, Zeng Z, Fu Z. Effects of environmental pollutants on gut microbiota. Environ Pollut. 2017;222:1–9. [DOI] [PubMed] [Google Scholar]
- 79.Snedeker SM, Hay AG. Do interactions between gut ecology and environmental chemicals contribute to obesity and diabetes? Environ Health Perspect. 2012;120(3):332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velmurugan G, Ramprasath T, Gilles M, Swaminathan K, Ramasamy S. Gut microbiota, endocrine-disrupting chemicals, and the diabetes epidemic. Trends Endocrinol Metab. 2017;28(8):612–625. [DOI] [PubMed] [Google Scholar]
- 81.Lai KP, Chung YT, Li R, Wan HT, Wong CK. Bisphenol A alters gut microbiome: comparative metagenomics analysis. Environ Pollut. 2016;218:923–930. [DOI] [PubMed] [Google Scholar]
- 82.Hu J, Raikhel V, Gopalakrishnan K, Fernandez-Hernandez H, Lambertini L, Manservisi F, Falcioni L, Bua L, Belpoggi F, L Teitelbaum S, Chen J. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome. 2016;4(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121(6):725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D'Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451(Pt A):97–102. [DOI] [PubMed] [Google Scholar]
- 85.Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA, Rosenfeld CS. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7(6):471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teixeira TF, Souza NC, Chiarello PG, Franceschini SC, Bressan J, Ferreira CL, Peluzio MdoC. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31(5):735–740. [DOI] [PubMed] [Google Scholar]
- 88.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236–1243. [DOI] [PubMed] [Google Scholar]
- 89.Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, Corthesy I, Macé K, Chou CJ. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22(7):2416–2426. [DOI] [PubMed] [Google Scholar]
- 90.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. [DOI] [PubMed] [Google Scholar]
- 91.Kelly D, Mulder IE. Microbiome and immunological interactions. Nutr Rev. 2012;70(Suppl 1):S18–S30. [DOI] [PubMed] [Google Scholar]
- 92.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meijer RI, van Wagensveld BA, Siegert CE, Eringa EC, Serné EH, Smulders YM. Bariatric surgery as a novel treatment for type 2 diabetes mellitus: a systematic review. Arch Surg. 2011;146(6):744–750. [DOI] [PubMed] [Google Scholar]
- 95.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, Rizkalla S, Clément K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L, Nichols RG, Correll J, Murray IA, Tanaka N, Smith PB, Hubbard TD, Sebastian A, Albert I, Hatzakis E, Gonzalez FJ, Perdew GH, Patterson AD. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ Health Perspect. 2015;123(7):679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan S, Beigh S, Chaudhari BP, Sharma S, Aliul Hasan Abdi S, Ahmad S, Ahmad F, Parvez S, Raisuddin S. Mitochondrial dysfunction induced by bisphenol A is a factor of its hepatotoxicity in rats. Environ Toxicol. 2016;31(12):1922–1934. [DOI] [PubMed] [Google Scholar]
- 100.Jiang Y, Xia W, Zhu Y, Li X, Wang D, Liu J, Chang H, Li G, Xu B, Chen X, Li Y, Xu S. Mitochondrial dysfunction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicol Lett. 2014;228(2):85–92. [DOI] [PubMed] [Google Scholar]
- 101.Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, Zhou Z, Lv Z, Xia W, Chen X, Xu S. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152(8):3049–3061. [DOI] [PubMed] [Google Scholar]
- 102.Song L, Xia W, Zhou Z, Li Y, Lin Y, Wei J, Wei Z, Xu B, Shen J, Li W, Xu S. Low-level phenolic estrogen pollutants impair islet morphology and β-cell function in isolated rat islets. J Endocrinol. 2012;215(2):303–311. [DOI] [PubMed] [Google Scholar]
- 103.Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, Ye T, Kang M, Shen H, Dong S. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis. 2013;4(1):e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peropadre A, Fernández Freire P, Pérez Martín JM, Herrero Ó, Hazen MJ. Endoplasmic reticulum stress as a novel cellular response to di (2-ethylhexyl) phthalate exposure. Toxicol In Vitro. 2015;30(1 Pt B):281–287. [DOI] [PubMed] [Google Scholar]
- 105.Lu TH, Su CC, Chen YW, Yang CY, Wu CC, Hung DZ, Chen CH, Cheng PW, Liu SH, Huang CF. Arsenic induces pancreatic β-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol Lett. 2011;201(1):15–26. [DOI] [PubMed] [Google Scholar]
- 106.Sun X, Lin Y, Huang Q, Shi J, Qiu L, Kang M, Chen Y, Fang C, Ye T, Dong S. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015;19(3):581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asahi J, Kamo H, Baba R, Doi Y, Yamashita A, Murakami D, Hanada A, Hirano T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010;87(13-14):431–438. [DOI] [PubMed] [Google Scholar]
- 108.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21(3):396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rocha M, Diaz-Morales N, Rovira-Llopis S, Escribano-Lopez I, Bañuls C, Hernandez-Mijares A, Diamanti-Kandarakis E, Victor VM. Mitochondrial dysfunction and endoplasmic reticulum stress in diabetes. Curr Pharm Des. 2016;22(18):2640–2649. [DOI] [PubMed] [Google Scholar]
- 110.Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, Kim SH, Yoon YC, Cho BJ, Park KS, Jang HC, Park YJ. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. 2012;27(6):644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huc L, Lemarié A, Guéraud F, Héliès-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol In Vitro. 2012;26(5):709–717. [DOI] [PubMed] [Google Scholar]
- 112.Carchia E, Porreca I, Almeida PJ, D’Angelo F, Cuomo D, Ceccarelli M, De Felice M, Mallardo M, Ambrosino C. Evaluation of low doses BPA-induced perturbation of glycemia by toxicogenomics points to a primary role of pancreatic islets and to the mechanism of toxicity. Cell Death Dis. 2015;6(10):e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock [published correction appears in Infect Immun. 2007;75(8):4186] Infect Immun. 2006;74(8):4750–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20(5):469–476. [DOI] [PubMed] [Google Scholar]
- 116.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. [DOI] [PubMed] [Google Scholar]
- 117.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol. 1993;265(5 Pt 2):R1148–R1154. [DOI] [PubMed] [Google Scholar]
- 118.Mohren DC, Jansen NW, Kant IJ, Galama J, van den Brandt PA, Swaen GM. Prevalence of common infections among employees in different work schedules. J Occup Environ Med. 2002;44(11):1003–1011. [DOI] [PubMed] [Google Scholar]
- 119.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185(10):5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dimitrov S, Lange T, Fehm HL, Born J. A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain Behav Immun. 2004;18(4):368–374. [DOI] [PubMed] [Google Scholar]
- 121.Srinivasan V, Maestroni GJ, Cardinali DP, Esquifino AI, Perumal SR, Miller SC. Melatonin, immune function and aging. Immun Ageing. 2005;2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beydoun HA, Beydoun MA, Jeng HA, Zonderman AB, Eid SM. Bisphenol-A and sleep adequacy among adults in the National Health and Nutrition Examination Surveys. Sleep. 2016;39(2):467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Regnier SM, Kirkley AG, Ye H, El-Hashani E, Zhang X, Neel BA, Kamau W, Thomas CC, Williams AK, Hayes ET, Massad NL, Johnson DN, Huang L, Zhang C, Sargis RM. Dietary exposure to the endocrine disruptor tolylfluanid promotes global metabolic dysfunction in male mice. Endocrinology. 2015;156(3):896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Labaronne E, Pinteur C, Vega N, Pesenti S, Julien B, Meugnier-Fouilloux E, Vidal H, Naville D, Le Magueresse-Battistoni B. Low-dose pollutant mixture triggers metabolic disturbances in female mice leading to common and specific features as compared to a high-fat diet. J Nutr Biochem. 2017;45:83–93. [DOI] [PubMed] [Google Scholar]
- 125.Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(1):E146–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rizos CV, Elisaf MS, Mikhailidis DP, Liberopoulos EN. How safe is the use of thiazolidinediones in clinical practice? Expert Opin Drug Saf. 2009;8(1):15–32. [DOI] [PubMed] [Google Scholar]
- 127.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526. [DOI] [PubMed] [Google Scholar]
- 128.de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, Potarca A, Tesar V, Heerspink HJ, Schall TJ; CCX140-B Diabetic Nephropathy Study Group . The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3(9):687–696. [DOI] [PubMed] [Google Scholar]