Abstract
As the sources of natural gas become more diverse, the trace constituents of the C6+ fraction are of increasing interest. Analysis of fuel gas (including natural gas) for compounds with more than 6 carbon atoms (the C6+ fraction) has historically been complex and expensive. Hence, this is a procedure that is used most often in troubleshooting rather than for day-to-day operations. The C6+ fraction affects gas quality issues and safety considerations such as anomalies associated with odorization. Recent advances in dynamic headspace vapor collection can be applied to this analysis and provide a faster, less complex alternative for compositional determination of the C6+ fraction of natural gas. Porous layer open tubular capillaries maintained at low temperatures (PLOT-cryo) form the basis of a dynamic headspace sampling method that was developed at NIST initially for explosives in 2009. This method has been recently advanced by the combining of multiple PLOT capillary traps into one “bundle,” or wafer, resulting in a device that allows the rapid trapping of relatively large amounts of analyte. In this study, natural gas analytes were collected by flowing natural gas from the laboratory (gas out of the wall) or a prepared surrogate gas flowing through a chilled wafer. The analytes were then removed from the PLOT-cryo wafer by thermal desorption and subsequent flushing of the wafer with helium. Gas chromatography (GC) with mass spectrometry (MS) was then used to identify the analytes.
Keywords: Headspace analysis, multi-capillary, PLOT-cryoadsorption, fuel gas, natural gas
Introduction
Natural gas is a complex mixture of organic and inorganic constituents. While predominately methane, natural gas also contains heavier hydrocarbons (constituents with higher carbon numbers), inert diluents, and other impurities whose concentrations and types vary considerably with geographical source, time of year, and treatments applied during production and transportation.1, 2 Additionally, an increasing number of unconventional (shale gas, coal seam gas, deep-water gas, ultradeep-water gas deposits, hydraulic fracturing gas, etc.) and bio-derived (landfill gas, digester gas, etc.) feedstocks add variability to the number and types of trace species in natural gas. Composition can influence many properties of natural gas, including its density, dew point, ignition properties, pollution profile, and odor properties.1, 3, 4 Detailed knowledge of the composition of natural gas is critical to ensuring quality and safety, as well as profitability.
The detailed composition of natural gas can affect the ability to odorize the final product. In order to provide for safe delivery and use, odorant (typically a mixture of sulfur compounds) is added to natural gas to enable detection (without instrumentation) by a person with a normal sense of smell at a concentration of one-fifth of the lower explosive limit in air. While natural gas odorization is a well-understood, mature technology that is usually trouble free in operation, odor fade and odor masking are problems that pose significant liability. Occasionally, the natural gas industry encounters periods in which previously odorized natural gas has no perceptible odor.5–10 Masking is suspected when the odorant that is added to natural gas can be detected by analytical instrumentation, but cannot be properly detected by an observer with a normal sense of smell. Note that this phenomenon is distinct from odor fade, which more properly describes a decrease in the concentration of an odorant (usually because of absorption, adsorption or a chemical reaction of the odorant) rather than a decrease, disappearance or qualitative change in the perception of the odor without a change in absolute concentration. Anecdotal descriptions of masking events in the natural gas industry have persisted for over a decade, with the frequency of such events on the rise.5, 6 These events make the detailed analysis of natural gas composition (especially the trace constituents) even more critical.
Detailed fuel gas compositional information (needed for determining phase equilibrium behavior, thermophysical properties, compliance with gas quality and safety-engineering specifications, and for assessing interchangeability) has traditionally been obtained by gas chromatography.3 In spite of the need for this information, gas chromatographic analysis of the C6+ fraction constituents of natural gas has been technically complex and expensive.3 Currently, the majority of natural gas producers, processors, pipeline companies, and distributors use basic GC analytical procedures that have been standardized in methods such as ASTM D-1945 or ASTM D783311 for custody transfer, heating value determination and quick analysis of composition.12 While significantly more complex, some local distribution companies (LDC) have the capability to carry out the “extended natural gas analysis” (or engage the services of a contract lab) and identify and quantitate components such as those listed in Table 1 (through methods similar to ASTM D1945). The extended natural gas analysis is a much more extensive analyses than ASTM D-1945, which concentrates only on the 16 most abundant components (Table 2), and this analysis is usually only used for problem solving and not for routine analysis.3 Beyond the basic procedures and the extended analysis are detailed hydrocarbon analyses that separate the C6, C7 C8, etc. components, resulting in a more comprehensive analysis used for dew point determination and troubleshooting.
Table 1.
Names and Molecular Mass of Components for Extended Natural Gas Analysis. Components are listed in order of retention times.
| Component Name | Molecular Mass |
|---|---|
| nitrogen | 28.013 |
| methane | 16.043 |
| carbon dioxide | 44.010 |
| ethane | 30.070 |
| propane | 44.097 |
| iso-butane | 58.123 |
| n-butane | 58.123 |
| iso-pentane | 72.150 |
| n-pentane | 72.150 |
| neopentane | 86.177 |
| cyclopentane + 2,3-dimethylbutane |
78.156 |
| 2-methylpentane | 86.177 |
| 3-methylpentane | 86.177 |
| n-hexane | 86.177 |
| methylcyclopentane | 84.162 |
| benzene | 78.114 |
| cyclohexane | 84.162 |
| dimethylpentanes | 100.204 |
| 2-methylhexane | 100.204 |
| dimethylcyclopentanes | 98.189 |
| 3-methylhexane | 100.204 |
| n-heptane | 100.204 |
| methylcyclohexane | 98.189 |
| ethylcyclopentane | 98.189 |
| dimethylhexanes | 114.231 |
| trimethylcyclopentanes | 112.215 |
| toluene | 92.141 |
| methylheptanes | 114.231 |
| Dimethylcyclohexanes | 112.215 |
| methylethylcyclopentanes | 112.215 |
| n-octane | 114.231 |
| C9 paraffins | 128.258 |
| C9 naphthenes | 126.243 |
| C8 benzenes | 106.168 |
| n-nonane | 128.258 |
| C9 benzenes | 120.195 |
| C10 and heavier | 142.285 |
Table 2.
Natural gas components and range of composition covered by ASTM D 1945-14. The ranges listed are for the method and not the acceptable ranges for commercial natural gas. Components are listed in order of retention times.
| Component | Mol % |
|---|---|
| helium | 0.01 to 10 |
| hydrogen | 0.01 to 10 |
| oxygen | 0.01 to 20 |
| nitrogen | 0.01 to 100 |
| carbon dioxide | 0.01 to 20 |
| methane | 0.01 to 100 |
| ethane | 0.01 to 100 |
| hydrogen sulfide | 0.3 to 30 |
| propane | 0.01 to 100 |
| isobutane | 0.01 to 10 |
| n-butane | 0.01 to 10 |
| neopentane | 0.01 to 2 |
| isopentane | 0.01 to 2 |
| n-pentane | 0.01 to 2 |
| hexane isomers | 0.01 to 2 |
| heptanes+ | 0.01 to 1 |
Although the extended natural gas analysis is practiced throughout segments of the gas industry, the method is difficult since each analysis must be tailored to the specific chromatographic retention properties of the compounds that are unique to the C6+ fraction of each individual gas sample.3 Twenty years ago, NIST developed a database of physical properties (needed for separations design) for known C6+ fraction constituents that was used to facilitate the design of more efficient and universally applicable gas chromatographic analysis procedures on a variety of stationary phases.3 This database compiled and critically reviewed physical properties data for 132 hydrocarbons compounds identified as being native constituents of natural gas, 21 gas treatment compounds, and 23 gas odorant compounds. The treatment and odorant compounds that were addressed in the database were not simply the commercially used mixtures, but also their expected reaction and degradation products that might be found downstream. Later, a computer program based on this database was released (consisting of a hydrocarbon edition and a sulfur compound edition) that facilitated automated chromatographic peak identification on the basis of retention indices.13 This database and computer program have been extremely helpful to the industry; however, with an ever-increasing number of natural and fuel gas feed stocks, this database will need to be significantly revised and expanded in the future to fully cover the types and number of emerging compounds in the C6+ fraction of natural gas. Moreover, this expansion must be an ongoing effort, considering the increasing feed stock variability discussed earlier. Of course, the expansion of this compilation cannot occur in a vacuum; it must be accompanied by enhanced analytical methods, especially methods geared to the identification of trace constituents.
The literature contains many studies and reports on the composition of natural gas,14–42 but relatively few have concentrated in detail on the C6+ fraction.43–50 While there are some studies dealing with the effects of C6+ fraction on gas performance,1, 4 the large majority of analysis methods that are used on a daily basis deal with the most abundant C1-C6 components.3
In order to meet the demand for critical information about the C6+ fraction of natural gas, with specific interest in the prediction of odorization anomalies such as masking events, we introduce the application of PLOT-cryoadsorption (sampling by use of short, 0.5 m to 3 m, porous layer open tubular (PLOT) capillaries maintained at low temperatures). PLOT-cryoadsorption (or PLOT-cryo) is a dynamic headspace sampling method developed at NIST in 2009,51 initially for the study of the vapor signature above explosives.52 This approach has proven to be sensitive and quantitative, with a sampling limit of detection below 1 ppb (mass/mass) of solute in the analyte matrix. Moreover, it provides results that are of low enough uncertainty to permit thermodynamic interpretation (by way of the equilibrium constant and associated enthalpy) of recovered concentrations through the van’t Hoff equation. The method has other important advantages in addition to sensitivity. The low temperature that is used to improve efficiency and facilitate collection is usually generated with a vortex tube.53, 54 This aspect makes the approach attractive for environments with explosive or flammable materials.55 The same vortex tube that is used to generate the low-temperature air stream (which can be as low as − 40 °C) can be used to generate a high-temperature stream of air (which can be as high as 160 °C) to thermally desorb solutes from the PLOT capillary (or to assist with solvent desorption by use of more gentle heating).
The PLOT capillaries are robust and inexpensive, and unlike other headspace collection methods, PLOT-cryo is especially applicable for relatively involatile solutes because it has a large temperature operability range. Moreover, a particularly attractive feature of PLOT-cryo is the ability to simultaneously sample headspace with multiple, different sorbent phases (selected for their specific functionalities), including the clay and organoclay phases developed that are especially suited for aromatic compounds.56 In the past as many as eight separate phases have been used, simultaneously, to collect vapor from a single sample. Alternatively, the approach allows sampling with multiple PLOT capillaries of the same phase, for repeatability and quality assurance. These features have been successful with applications to explosives, pyrolysis products, food safety, cadaver detection, fire debris analysis, and a field portable unit was recently described.52, 57–62
In this paper, we describe an application of the advanced PLOT-cryo method, which combines multiple PLOT capillary traps into one “wafer,” and then builds the bundle into a polymeric wafer for stability. This method has been described in detail previously.59 The wafer itself was directly connected to the natural gas sample flow. This multi PLOT-cryo technique allows for the rapid trapping of relatively large quantities of a light and medium volatile analyte. In this study, natural gas analytes were collected by flowing commercial product or surrogate gas (from a cylinder) through a chilled wafer. The analytes were subsequently eluted from the wafer by thermal desorption and flushing the wafer with nitrogen or helium. The eluted analytes were then analyzed by gas chromatography-mass spectroscopy (GC-MS).
Materials and Methods
Two natural gas samples were chosen for the initial studies. First, we sampled a prepared natural gas surrogate from a cylinder. This gas surrogate was prepared to be representative of natural gas from Statoil ASA, a Norwegian multinational oil and gas company. The sample was prepared for a 1987 round-robin study of natural gas properties for custody transfer modeling, thus, the gravimetric preparation process was carefully performed. A 25 kg two-pan beam balance with a readability of 1 mg was used, with buoyancy correction, to arrive at a composition with mol % uncertainty of 0.0005. Despite the care taken with the preparation, it is acknowledged that the sample was nearly 30 years old at the time of this work. It had been stored in a sheltered, outdoor chemical and gas storage facility. Thus, the composition may be somewhat less certain than a freshly prepared gas surrogate but it was deemed a reliable approximation.
Second, we sampled the natural gas supplied to the laboratory (gas out of the wall). The gas is the commercial product supplied to the front range of Colorado, and is sourced from natural gas deposits, associated gas, coal seam gas and shale gas. This gas was transferred from a lab cock retrofitted with a compression fitting and a pressure of approximately 0.5 psi, as will be discussed in more detail below.
Detailed information on the development of multi PLOT-cryo instrumentation and associated applications can be found in previous papers;59, 63 hence, only a brief description will be provided here. Bundles of six individual capillaries (made from alumina (a highly retentive, polar adsorbent) porous layer open tubular column with a 0.32 mm inside diameter) were embedded in epoxy to form a wafer equipped with compression fittings (Figure 1). In order to condition the multi PLOT capillary wafer for the run, it was again placed in a surplus GC oven with a gentle flow of nitrogen (with a purity greater than 99.99%) and allowed to remain there at 90 °C for one hour. After conditioning, one end of the multi PLOT capillary bundle was fitted to either a natural gas line or cylinder containing natural gas surrogate by use of 0.3175 cm (0.125 in) Teflon tubing, and the other end of the bundle was fitted with a 400 series stainless steel syringe needle (28 gauge). The multi PLOT capillary wafer was placed inside of a dry ice/ethanol bath (with the end of the needle outside of the bath) and allowed to equilibrate for five minutes. The operating temperature of the wafer was approximately − 72 °C during collection,64 which is significantly colder than the temperature usually used for PLOT-cryo.
Figure 1.
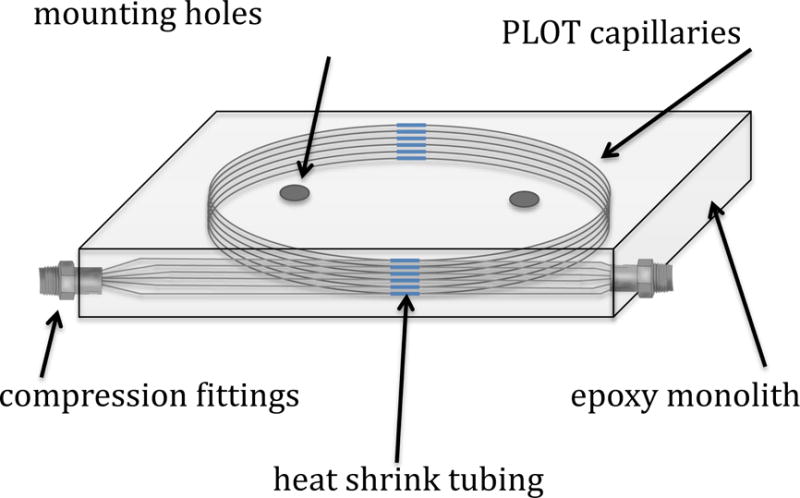
A schematic diagram of a six capillary multi PLOT-cryo wafer equipped with compression fittings for use as a headspace vapor collection device. A photograph of the wafer can be found in Supporting Information Figure S1.
After the five-minute thermal equilibration time, natural gas (or the natural gas surrogate mixture) was passed through the capillaries of the wafer at flow rates between (7 to 20) mL/min ± 1 mL/min. A range of collection times, which varied from one minute to one hour were examined. Short collection times are important for on-the-spot analyses in the field. Longer collection times are important to simulate the potential of a PLOT-cryo wafer being placed on a natural gas line as a fixture for constant, QA/QC sampling. After collection, the multi-PLOT wafer was then removed from the cooling bath, disconnected from the sample line, and placed, uncapped, into a surplus GC oven where it was slowly brought to 110 °C and held for 10 minutes for thermal desorption. Leakage of desorbed solute outside of the PLOT wafer was likely minimal because of the relatively long diffusion length provided by the fittings and transfer lines. At the end of the desorption time, the wafer was immediately brought to the GC-MS where the needle pierced the septum, extending downward into the GC-MS injection port. 10 mL of He was passed through the bundle (delivered with a gas-tight syringe) and a subambient temperature GC method (discussed below) was used to identify the analytes.53 This sweep into the GC injector required approximately 2 s.
Results and Discussion
We first analyzed the natural gas surrogate using the multi PLOT-cryo method. The gas surrogate was passed through the wafer at a flow rate of 7 mL/min for 3 minutes and then thermally desorbed by use of the method described above. Low flow rates and collection times were used for these experiments in order to ensure analyte concentrations were within the working range of the GC-MS. The composition of analytes thermally desorbed from the bundle was investigated using a subambient temperature GC method. This method has been described previously and involves the use of vortex cooling to maintain the GC-MS at working temperatures below 0 °C.53, 54 In this study, the GC-MS was maintained at approximately (−15 ± 2) °C. Two columns were used to separate the analytes of the gas surrogate during independent separations. A packed porous polymer PLOT column was used to separate the methane and ethane (30 m capillary column packed with porous divinylbenzene homopolymer having a thickness of 320 μm, the column temperature was held at −15 °C for 15 minutes), and a 5 % phenyl-95 %-dimethyl polysiloxane (30 m capillary column having a thickness of 1.0 mm, the column temperature was held at −15 °C for 15 minutes) was used to separate propane and the heavier analytes in a second analysis. Both analyses used MS detection in scan mode and samples were injected directly from the bundle using a syringe into a split/splitless injector set with a 100:1 split ratio (this is a higher split ratio than used below with the natural gas in order to avoid overloading the detector). The inlet temperature was operated at ambient temperature with a constant head pressure of 41.4 kPa (6 psig). Mass spectra were collected for each peak from 15 to 550 relative molecular mass (RMM) units. Peaks were identified with guidance from the NIST/EPA/NIH Mass Spectral Database, and also on the basis of retention indices.64 All known components of the surrogate were easily captured and subsequently separated by this method (Figure 2). Quantitation requires calibration for each analyte; however, the integration of raw peak areas revealed analytes in approximately the correct ratios when compared with known composition of the natural gas surrogate. In future work, appropriate calibration procedures will be applied for quantitation (indeed, by use of gravimetric surrogate mixtures for the calibration), but for this initial report in which the sampling metrology is introduced, an approximate comparison is adequate. Previously, it was shown that PLOT-cryo could be used to detect and quantitate TNT in vapor above a substrate with 0.064 μg/g TNT (with a repeatability of 10%) on a glass bead matrix; similar repeatability is expected at this concentration level with multi PLOT-cryo with sensitivity commensurate with MS detection.52 Additionally, detection of water was observed in the sample, which is likely the result of storage conditions of the natural gas surrogate.
Figure 2.
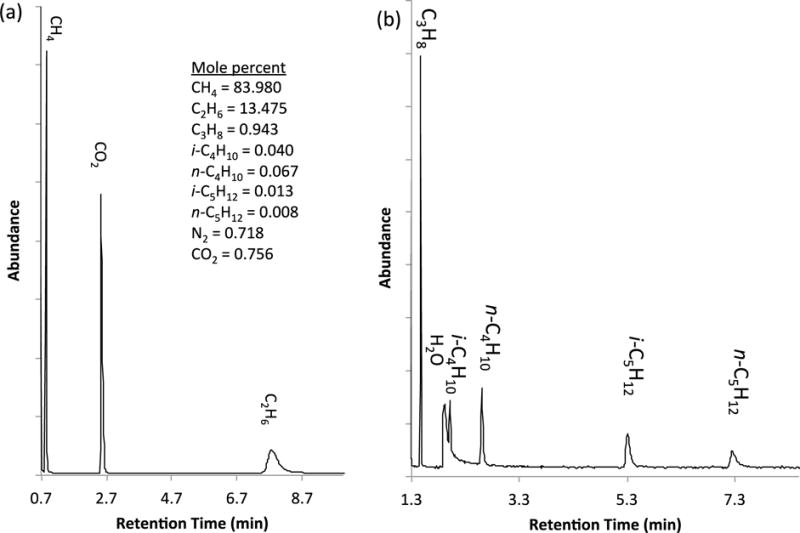
GC-MS spectra of the analytes of a natural gas surrogate (mole percent as prepared gravimetrically, uncertainty is discussed in the text) after collection by the multi-PLOT-cryo method. The light compounds, spectrum (a), were analyzed using GC-MS with a porous polymer PLOT column and the heavier compounds, spectrum (b), were analyzed using GC-MS with a 5 % phenyl-95 %-dimethyl polysiloxane column.
In addition to the gas surrogate, we analyzed natural gas supplied to the laboratory (gas out of the wall) sampled by use of the PLOT-cryo wafer method. It was noted that one typically employs a porous polymer PLOT column for the chromatographic analysis of natural gas samples. This is because it is usually desirable to separate the methane and ethane fractions from the remainder of the constituents. In this work, we seek not only to improve the workability of the extended natural gas analysis, but as stated in the introduction, we also seek to inform our work on odor masking. Since methane and ethane are not responsible for odor masking, a porous polymer phase was not selected in the chromatographic protocol when analyzing natural gas supplied to the laboratory from the commercial source.
One option for monitoring trace compounds in natural gas is to have multi PLOT-cryo wafers continually in place on gas lines, which could be removed and sampled during masking events or at desired sampling intervals. In order to simulate this, the multi-PLOT-cryo bundle was attached to laboratory gas supply lines, and placed into the −72 °C bath. The gas was allowed to pass through the wafer at a flow rate of 20 mL/min for one hour and then the analytes were thermally desorbed from the multi-PLOT capillary wafer by the method described above. The composition of thermally desorbed analytes was investigated using a subambient GC oven temperature program and a 5 % phenyl-95 %-dimethyl polysiloxane column (30 m capillary column having a thickness of 1.0 mm, the column temperature was held at −15 °C for 90 minutes). The analysis employed MS detection in scan mode. The samples were injected directly from the wafer with a syringe attachment into a split/splitless injector set with a 15:1 split ratio. The inlet temperature was operated at ambient temperature with a constant head pressure of 82.7 kPa (12 psig). Mass spectra were collected for each peak from 15 to 550 RMM units. Peaks were identified with guidance from the NIST/EPA/NIH Mass Spectral Database, and also on the basis of retention indices.64, 65 The repeatability of these assays is expected to be similar to that reported for single PLOT-cryo,52 with the most significant dissimilarities being caused by the variability in commercial product. The results from two of these experiments can be seen in Figure 3. These chromatograms can be compared to the direct injection of 10 mL of natural gas seen in Figure 4 (using the GC-MS parameters given above). These results demonstrate our ability to consistently identify a larger number of trace compounds using the multi-PLOT-cryo method than with the direct injection of similar volumes. In addition, changes in the daily composition of natural gas as delivered could be monitored. As can be seen in Figure 3, the composition and concentration of trace compounds may vary significantly in commercial products. This method provides a rapid, easy way to analyze trace compounds in natural gas and provides increased chromatographic signal strength (Figure 5) with sharper peak shapes compared to direct injection (Figure 4). This is due to the shorter spatial resolution of the injection, possible with the PLOT-cryo approach. Therefore, this method is an improvement over direct injection analysis. Clearly, for practical on-line natural gas analyses, alternative-cooling means would have to be implemented, such as a Peltier cooler or a pulse-tube cryocooler. For this initial demonstration as reported here, the dry ice/ethanol bath was considered to be adequate.
Figure 3.
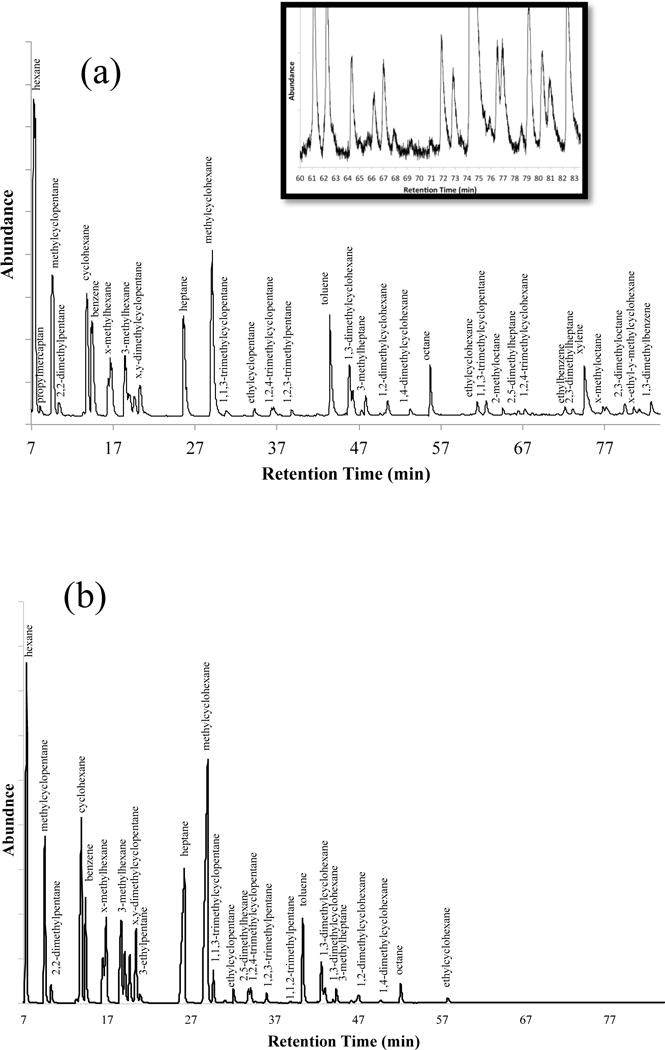
(a) and (b) are the GC-MS total ion chromatogram of the trace constituents of a commercial natural gas after collection by the multi PLOT-cryo method two weeks apart. Additional peaks could be identified (see insert in (a)), but for the sake of brevity only the major peaks have been labeled.
Figure 4.
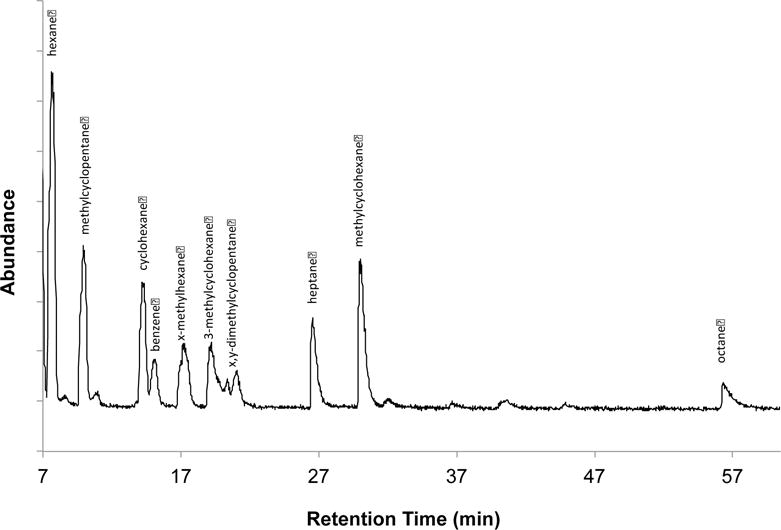
GC-MS spectrum of the analytes of a commercial natural gas by direct injection of 10 mL with a gas-tight syringe.
Figure 5.
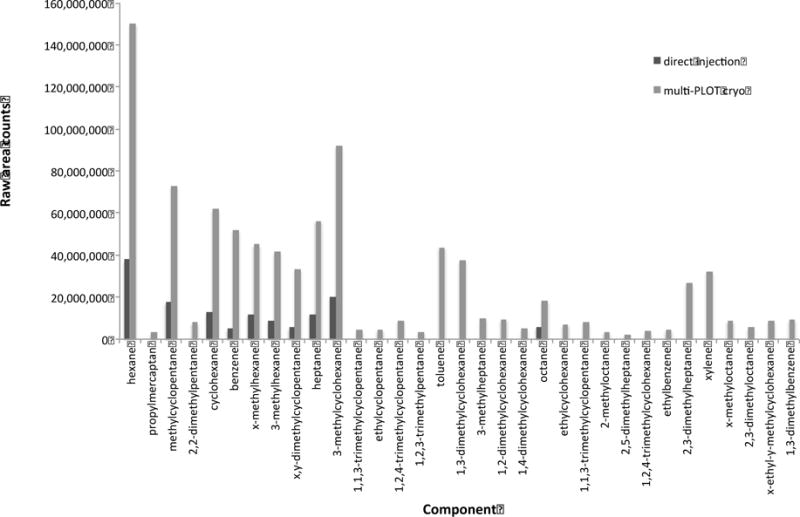
Comparison of GC-MS raw area counts for the C6+ fraction of natural gas as determined by direct injection and the multi PLOT-cryo method.
Conclusions
In this paper, a wafer formed from multiple PLOT capillaries maintained at low temperature was used to rapidly collect analytes from a natural gas surrogate and from commercial natural gas. Four points were demonstrated: (1) Collection may be performed while the multi-PLOT capillary wafer was maintained at low temperature (in this application approximately −72 °C) and the multi PLOT capillary wafer may undergo heating for activation or thermal desorption (in this application up to 110 °C) with no deleterious effects on the capillary, the polymer encapsulation, or the integrity of the stainless steel fittings integral to the wafer. (2) Vapor sampling periods of one minute to one hour could be used in order to simulate sampling both of wafers placed continually on gas lines and sampling at a single time point. (3) Flow rates of 7 mL/min to 20 mL/min were sufficient to collect trace compounds for detection by GC-MS. (4) This procedure enabled the identification of a large number of trace compounds in natural gas. Indeed, the use of multi-PLOT-cryo allowed for the identification of a significant number of compounds not detectable by direct injection into the GC-MS, as shown in Figure 5, and was shown to be overall superior to direct injection of similar volumes. (5) While preferential adsorption of analytes of differing polarities can occur on an individual PLOT surface, this was not observed or expected with the hydrocarbon stream of natural gas samples. If such preferential adsorption presents a problem, PLOT-cryo is the only headspace collection method that offers a solution by allowing multiple phases to be used for sampling, simultaneously.
This method complements existing extended natural gas analysis methods3 and will aid in the rapid identification of the native constituents of natural gas from non-traditional feedstocks. A multi-PLOT capillary wafer incorporating six individual PLOT capillaries was found to furnish a high enough flow rate to enable vapor sampling for the detection of trace compounds found in natural gas. The use of six capillaries is not a design limitation but is merely a convenience adopted in this initial work. Thus, wafers may be designed with more or fewer capillaries as needed. Analysis of the analyte recovered from natural gas was found to be readily accomplished by GC-MS in the scan mode; however, any applicable analytical technique could be applied. While all of the analysis discussed here was performed in the laboratory, development of both field portable chemical analysis instruments and in-line detection devices for use with natural gas sampling and in other fields of trace detection is underway.
Supplementary Material
Acknowledgments
JLB acknowledges Professional Research Experience Program (PREP) support for research performed at NIST-Boulder for this work.
Footnotes
Contribution of the United States government; not subject to copyright in the United States.
References
- 1.McTaggart-Cowan G, Rogak S, Munshi S, Hill P, Bushe W. The influence of fuel composition on a heavy-duty, natural-gas direct-injection engine. Fuel. 2010;89(3):752–759. [Google Scholar]
- 2.Kidnay AJ, Parrish WR, McCartney DG. Fundamentals of natural gas processing. Vol. 218 CRC Press; 2011. [Google Scholar]
- 3.Bruno TJ. Constituents and physical properties of the C6+ fraction of natural gas. Topical Report, Gas Research Institute April-June. 1994:40. ISTIR-5212, GRI-94/0274; Order No. PB95136644. [Google Scholar]
- 4.Atilhan M, Ejaz S, Zhou J, Cristancho D, Mantilla I, Holste J, Hall K. Characterization of deepwater natural gas samples. Part 1: 78% methane mixture with heavy components. Journal of Chemical & Engineering Data. 2010;55(11):4907–4911. [Google Scholar]
- 5.Halchuk R, P E. Gas Quality Engineer, Xcel Energy. Presented at the NIST Workshop on Odor Masking; March 21, 2011.Denver, CO: Odorization and Odor Masking in the Natural Gas Industry; [Google Scholar]
- 6.Louvat A, Polman E, Salati E, Favre P, Broomhall D, Strugnell B, Sleuyter K, Kvist T, Jorgensen B, Delahaye J. Impact of Biomethane on Odorisation in Gas Distribution Networks. 16th International Symposium on Olfaction and Electronic Noses; Dijon, Burgundy, France. 2015. [Google Scholar]
- 7.Halchuk R. Xcel Energy. 2001 personal communication. [Google Scholar]
- 8.Rawson N, Quraishi A, Bruno TJ. Findings and Recommendations From the Joint NIST-AGA Workshop on Odor Masking. Journal of Research of the National Institute of Standards and Technology. 2011;116(6):839. doi: 10.6028/jres.116.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Gas Association. Odorization Manual. Prepared by Transmission Measurement Committee and Former Chemical and Analytical Services Task Committee; 2000. [Google Scholar]
- 10.Burger J, Jeerage K, Bruno T. Direct NMR Observation of Odorant Binding to Mouse Odorant Receptor MOR244-3. Analytical Biochemistry. doi: 10.1016/j.ab.2016.03.006. in press. [DOI] [PubMed] [Google Scholar]
- 11.ASTM D7833 Standard Test Method for Determination of Hydrocarbons and Non-Hydrocarbon Gases is Gaseous Mixtures by Gas Chromatography. ASTM International; West Conshohocken, PA: 2012. [Google Scholar]
- 12.ASTM Standard D 1945 - 14, Standard Test Method for Analysis of Natural Gas by Gas Chromatography. ASTM International; West Conshohocken, PA: 2014. [Google Scholar]
- 13.Bruno TJ. Gas chromatographic retention parameters: the basis of chromatographic peak identification for the extended natural gas analysis. Proc. Institute of Gas Technology Conference on Gas Quality and Energy Measurement; Orlando, FL. 1996; Orlando, FL, 1996. [Google Scholar]
- 14.Beggs HD. Gas production operations. OGCI publications; 1984. [Google Scholar]
- 15.Elliot T, Ballentine C, O’nions R, Ricchiuto T. Carbon, helium, neon and argon isotopes in a Po Basin (northern Italy) natural gas field. Chemical Geology. 1993;106(3):429–440. [Google Scholar]
- 16.Anon. US Geological Survey Professional Paper 1570. Howell, DG: 1993. The Future of Energy Gases. [Google Scholar]
- 17.McCarthy K, Rojas K, Niemann M, Palmowski D, Peters K, Stankiewicz A. Basic petroleum geochemistry for source rock evaluation. Oilfield Review. 2011;23(2):32–43. [Google Scholar]
- 18.Petersen H, Tru V, Nielsen L, Duc NA, Nytoft H. Source rock properties of lacustrine mudstones and coals (Oligocene Dong Ho Formation), onshore Song Hong Basin, northern Vietnam. Journal of Petroleum Geology. 2005;28(1):19–38. [Google Scholar]
- 19.Ahrens F. What’s in a flame. AGA Monthly;(United States) 1992;74(4) [Google Scholar]
- 20.Barker C, Takach NE. Prediction of Natural Gas Composition in Ultradeep Sandstone Reservoirs (1) AAPG Bulletin. 1992;76(12):1859–1873. [Google Scholar]
- 21.Pedersen KS, Blilie AL, Meisingset KK. PVT calculations on petroleum reservoir fluids using measured and estimated compositional data for the plus fraction. Industrial & Engineering Chemistry Research. 1992;31(5):1378–1384. [Google Scholar]
- 22.Liss W, Thrasher W, Steinmetz G, Chowdiah P, Attari A. Final report, August 1990-February 1992. American Gas Association Labs; Cleveland, OH (United States): 1992. Variability of natural gas composition in select major metropolitan areas of the United States. [Google Scholar]
- 23.Manning FS, Thompson RE. Oilfield Processing of Petroleum, Volume One: Natural Gas. Penn-Well Publishing Company; Tulsa, Oklahoma: 1991. [Google Scholar]
- 24.Moore BJ, Sigler S. Analyses of natural gases, 1917–85. 1987 [Google Scholar]
- 25.Igari S-I, Sakata S. Origin of natural gas of dissolved-in-water type in Japan inferred from chemical and isotopic compositions: Occurrence of dissolved gas of thermogenic origin. Geochemical Journal. 1989;23(3):139–142. [Google Scholar]
- 26.Hiltner J, Agama R, Mauss F, Johansson B, Christensen M. Homogeneous charge compression ignition operation with natural gas: fuel composition implications. Journal of engineering for gas turbines and power. 2003;125(3):837–844. [Google Scholar]
- 27.Jenden P, Kaplan I. Origin of natural gas in Sacramento Basin, California. AAPG Bulletin. 1989;73(4):431–453. [Google Scholar]
- 28.Snowdon LR. Natural gas composition in a geological environment and the implications for the processes of generation and preservation. Organic Geochemistry. 2001;32(7):913–931. [Google Scholar]
- 29.Tassi F, Vaselli O, Capaccioni B, Montegrossi G, Barahona F, Caprai A. Scrubbing process and chemical equilibria controlling the composition of light hydrocarbons in natural gas discharges: an example from the geothermal fields of El Salvador. Geochemistry, Geophysics, Geosystems. 2007;8(5) [Google Scholar]
- 30.Bordenave ML, Espitalie J, Lopat P, Oudin JL. Appl Pet Geochem. Technip; Paris: 1993. Screening techniques for source rock evaluations. [Google Scholar]
- 31.Hamak JE, Sigler S. Analyses of Natural Gas, 1986-90. 1991 [Google Scholar]
- 32.Gammon BR. Table of physical properties of hydrocarbons for extended anlysis of natural gases. Gas Processors Association; Tulsa: 1988. [Google Scholar]
- 33.Lake LW, Carroll HB., Jr . Reservoir Characterization. Academic Press; Orlanda, Florida: 1986. [Google Scholar]
- 34.Wilbur LC. Handbook of energy systems engineering: Production and utilization. 1985 [Google Scholar]
- 35.Meyers RA. Handbook of petroleum refining processes. Vol. 548 McGraw-Hill; New York: 2004. [Google Scholar]
- 36.The Petroleum Handbook. Elsevier Science Publishers; Amsterdam: 1983. [Google Scholar]
- 37.Miller RD, Hertweck FD., Jr . Analysis of natural gases. Amarillo, TX: 1982. [Google Scholar]
- 38.Bailey RL. Sampling Procedures and Techniques. CenrefLabs, Natural Gas Ampling Workshop. 1982 Sep 27; [Google Scholar]
- 39.McGraw-Hill Encyclopedia of Energy. McGraw Hill Book Co; New York: 1981. [Google Scholar]
- 40.Sloan ED. Fundamental principles and applications of natural gas hydrates. Nature. 2003;426(6964):353–363. doi: 10.1038/nature02135. [DOI] [PubMed] [Google Scholar]
- 41.Neumann HJ, Paczynska-Lahme B, Severin D. Composition and Properties of Petroleum Volume 5. Halstead Press; New York: 1981. [Google Scholar]
- 42.Atkinson G, Zuckerman JJ. Origin and Chemistry of Petroeum. Pergamon Press; 1981. [Google Scholar]
- 43.Robertson CR. Characterization of heavy components in NGL and natural gas (extended analysis) Proceedings of the International School of Hydrocarbon Measurements. 1992;67(537) [Google Scholar]
- 44.Batten R. Chromatographic analysis of natural gas liquids. Proceedings of the International School of Hydrocarbon Measurements. 1992;67(261) [Google Scholar]
- 45.Wegener DC. Linkage of Extended Natural Gas Analysis Data from a Thermal Conductivity Detector and a Flame Ionization Detector With Use of a Spreadsheet Template. Proceedings of the 70th Gas Processors Association Annual Convention. 1989 Mar 11–12;:32–42. [Google Scholar]
- 46.David F, Nikolai A, Sandra P. Analysis of C10-20 hydrocarbons in natrual gas by solid phase extraction and CGC. J High Res Chromatogr & Chromatogr Comm. 1989;12:657–660. [Google Scholar]
- 47.Johnson SJ, Hines WJ. Extended Analysis of Natural Gas Liquids - Precision, Accuracy, and Limitations of Method 2186 and Results From Specific Applications. Proceedings of the 68th Gas Processors Association Annual Convention; March 13-14, 1989; pp. 75–88. [Google Scholar]
- 48.Scripsick M. Chromatographic analysis of natural gas liquids. Proceedings of the International School of Hydrocarbon Measurements. 1988;63(176) [Google Scholar]
- 49.Wilkins CM. Chromatographic analysis of natural gas liquids. Proceedings of International School of Hydrocarbon Measurements. 1982;62(334) [Google Scholar]
- 50.Schmidt T, Rennemann D, Schulz T. Natural gas treatment: simultaneous water and hydrocarbon-dew point-control, part1: process design, absorbent selection and performance. Erdölnund Kohle - Erdgas - Petrochemie vereinigt mit Brennstoff-Chemie. 1993;46(10):366–374. [Google Scholar]
- 51.Bruno TJ. Simple, quantitative headspace analysis by cryoadsorption on a short alumina PLOT column. J Chromatogr Sci. 2009 Aug;47:1. doi: 10.1093/chromsci/47.7.569. [DOI] [PubMed] [Google Scholar]
- 52.Lovestead TM, Bruno TJ. Trace headspace sampling for quantitative analysis of explosives with cryoadsorption on short alumina porous layer open tubular columns. Anal Chem. 2010;82:5621–5627. doi: 10.1021/ac1005926. [DOI] [PubMed] [Google Scholar]
- 53.Bruno TJ. Vortex cooling for subambient temperature gas chromatography. Anal Chem. 1986;58(7):1595–6. [Google Scholar]
- 54.Bruno TJ. Laboratory applications of the vortex tube. Journal of Chemical Education. 1987;64(11):987–8. [Google Scholar]
- 55.National Electrical Code 2008. National Fire Protection Association; 2007. [Google Scholar]
- 56.Bruno TJ, Lewandowska A, Tsvetkov F, Miller KE, Hanley HJM. Wall-coated open-tubular column chromatography on an organo-clay stationary phase. J Chromatogr A. 2002;973(1–2):143–149. doi: 10.1016/s0021-9673(02)01124-x. [DOI] [PubMed] [Google Scholar]
- 57.Bruno TJ, Nichols JE. Method and apparatus for pyrolysis-PLOT-cryoadsorption headspace sampling and analysis. J Chromatog A. 2013;1286:192–199. doi: 10.1016/j.chroma.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 58.Bruno TJ. Method and apparatus for field portable PLOT-cryoadsorption headspace sampling and analysis, NIST docket 13-031, patent applied for [Google Scholar]
- 59.Bruno TJ. Field portable low temperature porous layer open tubular cryoadsorption headspace sampling and analysis part I: instrumentation. Journal of Chromatography A. doi: 10.1016/j.chroma.2015.12.013. in press. [DOI] [PubMed] [Google Scholar]
- 60.Nichols JE, Harries ME, Lovestead TM, Bruno TJ. Analysis of arson fire debris by low temperature dynamic headspace adsorption porous layer open tubular columns. Journal of Chromatography A. 2014;1334:126–138. doi: 10.1016/j.chroma.2014.01.080. [DOI] [PubMed] [Google Scholar]
- 61.Lovestead TM, Bruno TJ. Detecting gravesoil with headspace analysis with adsorption on short porous layer open tubular (PLOT) columns. Forensic Science International. 2011;204:156–161. doi: 10.1016/j.forsciint.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 62.Lovestead TM, Bruno TJ. Detection of poultry spoilage markers from headspace analysis with cryoadsorption on a short alumina PLOT column. Food Chemistry. 2010;121:1274–1282. [Google Scholar]
- 63.Harries M, Bukovsky-Reyes S, Bruno TJ. Field portable low temperature porous layer open tubular cryoadsorption headspace sampling and analysis part II: applications. Journal of Chromatography A. doi: 10.1016/j.chroma.2015.12.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bruno TJ, Svoronos PDN. CRC Handbook of Basic Tables for Chemical Analysis. 3rd. Taylor and Francis CRC Press; Boca Raton: 2011. [Google Scholar]
- 65.NIST/EPA/NIH Mass Spectral Database, S. R. D. SRD Program. National Institute of Standards and Technology; Gaithersburg, MD: 2010. 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


