Abstract
The trans-Golgi network in plants is a major sorting station of Golgi derived cargo while it also receives recycled material from endocytosis.
Transport networks may be defined as sets of connected nodes or hubs where cargo from different origins are sorted to their final destinations. The trans-Golgi network (TGN) is the most discussed and arguably busiest hub operating in the cell. The versatility of the plant TGN distinguishes it from its mammalian counterpart. It is essential for the assembly of cell walls, including the cell plate, and organizes traffic of cargoes not only to but also from the plasma membrane, two pathways that animal cells separately confine to TGN and endosomes, respectively. We stand far from models that integrate the multiple trafficking functions of the plant TGN in physiologically different cellular contexts; however, with current and recent studies, we are gaining insights into the molecular determinants, trafficking routes, and functions of TGN subcompartments.
PLANT TGN BIOGENESIS
The trans-Golgi network (TGN) is defined as the membrane compartment on the trans-side of Golgi stacks responsible for the sorting and packaging of cargo molecules for delivery to the plasma membrane and vacuoles (Roth et al., 1985; Griffiths and Simons, 1986; Kang et al., 2011). In plants, the TGN not only provides a final sorting station for Golgi-derived cargoes but also is involved in trafficking/recycling of endosomal material; therefore, the term TGN/early endosome (TGN/EE) better suits its function (Fig. 1; Tanchak et al., 1988; Dettmer et al., 2006; Viotti et al., 2010). The identity of the plant TGN as a distinct organelle and not just a tubular reticulum on the trans-side of the Golgi has been supported by results from a number of experimental approaches in recent years. Cell fractionation and electron microscopy/tomography studies allowed Kang et al. (2011) to propose a model for TGN biogenesis in which the trans-most Golgi cisterna is first transformed into a TGN-type compartment that is still tightly associated with the trans-Golgi stack. Transformation of a trans-most Golgi cisterna into a TGN cisterna is accompanied by cisternae peeling, proliferation of round, secretory-type vesicle (SV) buds, and a reduction of the cisternal membrane area (Kang et al., 2011). These authors elegantly showed that there are two forms of TGN: (1) the Golgi-associated TGN (GA-TGN) cisternae attached to the trans-side of the Golgi; and (2) the detached, free TGN cisternae. The amounts of associated clathrin-coated vesicles (CCVs) and SV buds distinguished the two fractions. Both CCVs and SV buds were more abundant in the free TGN cisternae (Kang et al., 2011).
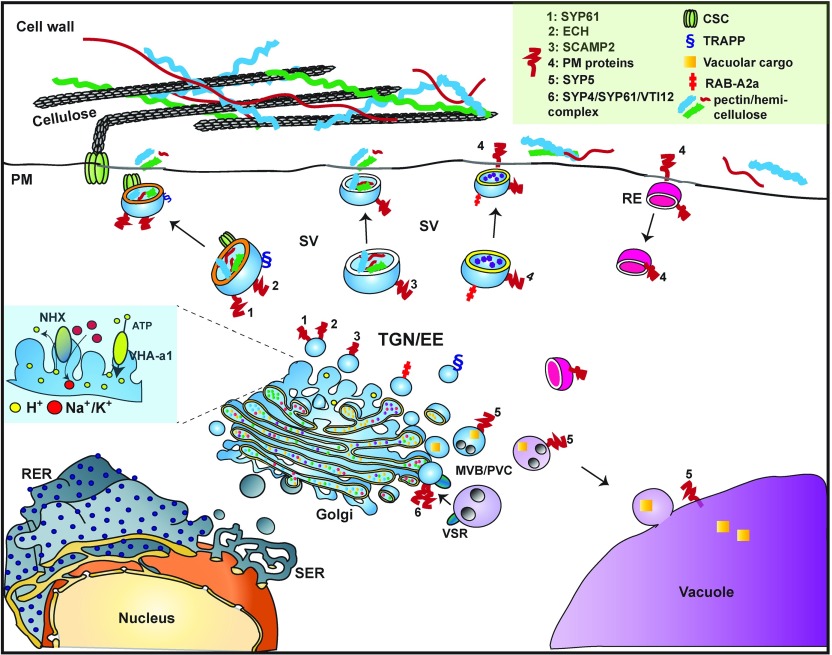
Figure 1.
Simplified illustration of TGN/EE-mediated trafficking. The plant TGN comprises a very dynamic and heterogenous network of vesicles. An essential function of plant TGN is the secretion of cell wall polysaccharides and plasma membrane (PM)-associated CSC. TGN compartments defined by SYP61/ECH and SCAMP2 have been implicated in the secretory transport of CSCs, pectin, and hemicellulose (light blue vesicles). TGN also functions in the delivery of cargo to the vacuole via the multivesicular body/prevacuolar compartment (MVB/PVC; purple vesicles). Vacuolar sorting receptors (VSR) are used in this cartoon to depict the recycling of vacuolar cargo back to the TGN. In plants, TGN operates as an EE in the recycling of PM proteins (pink vesicles). Different classes of proteins regulate the trafficking functions of TGN/EE and define specific subcompartments. These include SNAREs, RAB GTPases, and tethering factors, of which examples are provided in the cartoon. The physical properties of TGN/EE are crucial for its functions. A role for vesicle lipid composition in defining TGN subcompartmentalization is emerging, here exemplified by the different lipid profiles of the SYP61 and RABA2a SVs (orange versus yellow bilayer). V-ATPase VHA-a1 and NHX antiporters are provided as examples of ion transporters involved in the critical regulation of TGN pH (see light blue-shaded inset on the left, representing a magnification of a TGN membrane fragment). The dotted interior of the Golgi represents cargo. RE, Recycling endosome; RER, rough endoplasmic reticulum; SER, smooth endoplasmic reticulum.
Superresolution live imaging of the TGN-localized soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein SYP43 was used recently to characterize the dynamic behavior of the GA-TGN and Golgi-independent TGN (GI-TGN) populations (Uemura et al., 2014), corroborating the Golgi cisternal maturation model (Kang et al., 2011). The GA-TGNs localized on the trans-side of the Golgi apparatus, while the GI-TGNs (Golgi-released independent TGNs) were located away from the Golgi apparatus and behaved independently (Uemura et al., 2014). The authors proposed that segregation of GA-TGNs gives rise to the GI-TGNs with a core of the GA-TGN remaining after the process.
It is plausible that GA-TGN represents a population characterized by the TGN-localized COV1 and RAB GTPase-interacting YIP4A/B proteins, since both yip4a/b and cov1 mutants affect the TGN association with Golgi (Gendre et al., 2013; Shirakawa et al., 2014). Further characterization of these proteins could reveal important, unknown elements regarding the functional connections between Golgi and GA-TGN, which appear bidirectional.
Brefeldin A (BFA) is a fungal inhibitor that causes aggregation of TGN, endosomal, and Golgi material in large intracellular bodies (Langhans et al., 2011). Regeneration of Golgi and TGN populations after the removal of BFA from treated cells proceeded independently in tobacco (Nicotiana tabacum) BY2 cells (Ito et al., 2017), supporting the notion that plant TGN is an independent organelle. How GI-TGN and GA-TGN functionally connect and how TGN functions feed back Golgi functions remain poorly understood. Notably, GI-TGNs were found highly abundant in the differentiation zone of the root, in contrast with the meristematic region (Uemura et al., 2014). Thus, it would be interesting to explore the likely reorganization of TGN during physiological responses, such as those evoking a more active secretory function.
PROTON PUMPS AND LEAKS: TGN PH IS ESSENTIAL
The regulation of pH homeostasis within the secretory pathway is crucial (for review, see Schumacher, 2014). An increase of only 0.2 pH units markedly impairs terminal α(2,3)-sialylation of an N-glycosylated reporter protein and induces mislocalization of the corresponding sialyltransferase into the endosomal compartments of mammalian cells (Rivinoja et al., 2009). Genetically encoded pH sensors targeted to specific endomembrane organelles have been engineered in an effort to establish a pH map of the plant endomembrane system. A gradual acidification from pH 7.1 in the endoplasmic reticulum (ER) to pH 5.2 in the vacuole was observed recently, with the pH of TGN at 6.3 (Shen et al., 2013). However, lower TGN pH values of 5.6 and 6.1 have been reported by other groups (Martinière et al., 2013; Luo et al., 2015).
In eukaryotic cells, acidification of the various endomembrane compartments has been proposed to depend largely on the proton pump activity of highly conserved vacuole-type H+-ATPases (V-ATPases; Hager and Helmle, 1981; Nishi and Forgac, 2002; Dettmer et al., 2006). V-ATPases are organized in two multisubunit domains. The peripheral V1 domain, responsible for ATP hydrolysis, is composed of eight different subunits (A–H), while the integral V0 domain forms the proton pore and includes a, c, c′, c′′, d, and e subunits (Nishi and Forgac, 2002; Toei et al., 2010). In plant cells, interfering with the TGN-resident VHA-a1 isoform causes the swelling of Golgi cisternae and inhibits cell expansion and hypocotyl growth (Dettmer et al., 2006; Brüx et al., 2008). Mutants with reduced activity of the cytosolic VHA-c subunit alter the steady-state pH in the TGN/EE, leading to reduced motility of both Golgi and TGN and defects in secretion of the brassinosteroid receptor BRI1 and Cellulose Synthase (Luo et al., 2015). Curiously, the TGN/EE-localized V-ATPase seems to contribute via an undefined mechanism to vacuolar pH (Kriegel et al., 2015). Such results suggest a rather complex interplay between different V-ATPase isoforms in the regulation of pH within each compartment of the plant endomembrane system.
Luminal pH initially determined by the activity of H+ pumps is thought to be fine-tuned by alkalization mechanisms (Orlowski and Grinstein, 2011; Bassil and Blumwald, 2014). Exchange of luminal H+ for Na+ or K+ by NHX antiporters counteracts the acidity generated by the H+ pumps (proton leaks; Orlowski and Grinstein, 2011; Reguera et al., 2015). Two Arabidopsis (Arabidopsis thaliana) NHX isoforms (NHX5 and NHX6) are expressed at the TGN, where they colocalize with the H+ pump VHA-a1 and likely counteract its activity (Bassil et al., 2011, 2012). nhx5 nhx6 double mutants have a more acidic TGN lumen, altered trafficking to the vacuole, and are hypersensitive to salt (Bassil et al., 2012). The latter highlights the relevance of luminal pH in the orchestration of crucial adaptive responses. The transporter AtCLC-d is yet another player thought to adjust TGN’s pH by mediating the transport of a counter anion such as Cl− or NO3− into the TGN lumen. As NHX5/6, it colocalizes with VHA-a1 at the TGN, and the deleterious effect of inhibiting VHA-a1 was exaggerated in the clcd-1 mutant, indicating synergistic activities between the two transporters (von der Fecht-Bartenbach et al., 2007).
LIPID COMPOSITION: AN INDEPENDENT SORTING MECHANISM?
A role for lipid rafts in the regulation of post-Golgi sorting events has received extensive attention. Lipid rafts are nanoassemblies of specific proteins and lipids that define highly dynamic membrane microdomains and influence the spatiotemporal organization of protein complexes, thereby allowing the regulation of cellular processes (Simons and Sampaio, 2011; Cacas et al., 2012; Malinsky et al., 2013). Rafts might sort proteins and lipids by clustering them together at the TGN into bigger patches, which would then pinch off as vesicles for delivery to the plasma membrane (Schuck and Simons, 2004). Using a shotgun lipidomics approach, Klemm and coworkers first demonstrated that yeast TGN selectively sorts ergosterol and sphingolipid-enriched SVs transporting plasma membrane cargo (Klemm et al., 2009; Surma et al., 2011). C-Laurdan spectrophotometry measurements revealed a higher membrane order in the immunoisolated vesicles, compared with TGN, supporting the hypothesis that lipid rafts play a role in the TGN sorting machinery (Klemm et al., 2009; Surma et al., 2011).
To our knowledge, lipid rafts have not been observed in the plant TGN, but evidence for lipid-based TGN sorting was obtained recently in Arabidopsis, where sphingolipids with α-hydroxylated acyl chains of at least 24 carbon atoms were enriched in SV subdomains of the TGN. Importantly, the authors established a novel link between α-hydroxylated acyl chain enrichment of TGN membranes and secretory trafficking, specifically in polarized transport to the apical membrane of epidermal cells (Wattelet-Boyer et al., 2016).
TGN-ASSOCIATED RABS, SNARES, TETHERS, AND ACCESSORY PROTEINS: ENSURING THE PRECISION OF DELIVERY
RAB GTPases
As a trafficking hub, one may conceive the TGN as organized into distinct domains, facilitating cargo sorting into specific vesicles to be dispatched to the next destination. In such a scenario, it is tempting to assume that different RAB GTPases (for review, see Zhen and Stenmark, 2015), a major class of cellular proteins determining membrane identity and trafficking specificity, define separate TGN sorting compartments (Grosshans et al., 2006; Woollard and Moore, 2008; Hutagalung and Novick, 2011; Bhuin and Roy, 2014). Several lines of evidence support such RAB-determined subcompartmentalization. Two subclasses within the large A group of plant RABs, RABA2 and RABA3, identify a distinct TGN/EE membrane domain that functions in cargo delivery to the cell plate during cytokinesis (Chow et al., 2008). Whereas RABA2a defines TGN domains distinct from SYP61 vesicles, evidenced by differing lipid composition, RABA4b preferentially localizes to TGN SV sites together with SYP61, where it is involved in the transport of cell wall components (Preuss et al., 2004; Kang et al., 2011; Wattelet-Boyer et al., 2016; Jonsson et al., 2017). Another TGN domain harboring the secretory SNAREs VAMP721/722 and defined by RABA1 also has been suggested (Asaoka et al., 2013).
As to members of the plant RABB and RABD clades, they have been postulated to operate in traffic between the ER and Golgi (Cheung et al., 2002; Rutherford and Moore, 2002; Zheng et al., 2005; Woollard and Moore, 2008). However, fluorescently tagged RABD1 and RABD2a localized to punctate structures associated with TGN, and both RABD2a and RABD2b were found abundantly in isolated SYP61 vesicles that define a pathway en route from TGN to the plasma membrane (Pinheiro et al., 2009; Drakakaki et al., 2012).
SNAREs
SNAREs are integral membrane proteins required for the fusion of vesicles with their target membrane (for review, see Bombardier and Munson, 2015). SNAREs can be classified based on their site of function: v (vesicle)-SNAREs are localized to the vesicle membrane, and t (target)-SNAREs are localized to the target membrane (Rothman, 1994; Søgaard et al., 1994). Syntaxins are a family of SNAREs involved in recognizing and complexing with other SNAREs on the target membrane to create a t-SNARE complex. The genome of Arabidopsis encodes 24 syntaxins or Syntaxin of Plants (SYPs; Sanderfoot et al., 2000; Bassham and Blatt, 2008).
Distinct types of Arabidopsis syntaxins reside on the various membranes of the secretory pathway. The SYP4-type syntaxins (SYP41–SYP43) and their orthologs from yeast (Tlg2p) and mammals (Syntaxin16) have all been localized to TGN (Holthuis et al., 1998; Simonsen et al., 1998; Bassham et al., 2000). SYP4 members are implicated in the transport of secretory and vacuolar cargo from the TGN. Whether SYP4s function redundantly and localize to the same TGN compartment is to be determined (Bassham et al., 2000; Uemura et al., 2012; Kim and Bassham, 2013). TNO1 (TGN-localized SYP41-interacting protein1) is a TGN-localized coiled-coil protein that interacts with the SYP41 SNARE machinery. TNO1 mutants affect TGN dynamics, vacuolar cargo sorting, and the response to salt stress (Kim and Bassham, 2011).
The Arabidopsis SYP5 family of SNAREs consists of two members, SYP51 and SYP52, which localize to tonoplast and TGN (Sanderfoot et al., 2001; Carter et al., 2004; De Benedictis et al., 2013). Both SYP5s mediate traffic to the vacuole, although they seem to differ in cargo selectivity. Intriguingly, SYP5s appear to inhibit homotypic fusion (SNARE interfering or i-SNARE) when accumulated at the tonoplast. Such an i-SNARE function could provide a control for cargo delivery to the vacuole (De Benedictis et al., 2013).
The SYP6 group is encoded by a single gene, SYP61 (Sanderfoot et al., 2000). SYP61 is proposed to form separate complexes with the SNARE VTI12 and either SYP41 or SYP42 at the TGN (Sanderfoot et al., 2001); however, some lines of evidence suggest that other members of the SYP and VTI families also could take part in these complexes. In the absence of VTI12, prevacuolar compartment-localized VTI11 is able to interact with SYP4s, and proteoliposome fusion assays demonstrated that SYP42 and SYP43 can substitute for SYP41 while VTI11 can substitute for VTI12 in driving lipid mixing (Surpin et al., 2003; Kim and Bassham, 2013). Finally, the ability of SYP41 and SYP61 to independently mediate the fusion of liposomes was shown by Chen et al. (2005), who also identified YKT61 and YKT62, two functionally interchangeable components of the SNARE complexes, required for both SYP41- and SYP61-mediated vesicle fusion
A role for the SYP4/SYP61/VTI12 complex in the recycling of vacuolar sorting receptors to the TGN has been suggested. Vacuolar Protein Sorting45, an interactor of the complex, was proposed to positively regulate this function (Zouhar et al., 2009). The role of the SYP61 compartment in post-Golgi trafficking is discussed later in this review.
Tethers
Tethering factors are traffic facilitators that function upstream of SNARE proteins in the establishment of an initial connection between an intracellular trafficking vesicle and its target membrane (Barlowe, 1997; Cao et al., 1998; for review, see Dubuke and Munson, 2016). Mechanistically, they have been suggested to mediate vesicle capturing, in virtue of their larger size compared with SNAREs, to accelerate the assembly of SNARE complexes and to provide checkpoints for SNARE specificity (Yu and Hughson, 2010). Plant tethers (for review, see Vukašinović and Žárský, 2016; Ravikumar et al., 2017) relevant to TGN-mediated trafficking include several members of the TRAPP family and the Golgi-associated retrograde protein (GARP) complex. TRAPPs assemble into fairly well-characterized multisubunit complexes in yeasts and mammals (Sacher et al., 2008; Kim et al., 2016). Our current knowledge of the function and organization of plant TRAPP homologs is extremely limited, although some lines of evidence have started to emerge with TGN-associated TRAPP involved in cytokinesis (Ravikumar et al., 2017). Several members of putative Arabidopsis TRAPP complexes were found in the proteome of TGN-associated SYP61 vesicles, suggesting a role for yet uncharacterized plant TRAPPs in the transport and/or delivery of secretory cargo to the plasma membrane (Drakakaki et al., 2012). The role of TGN-associated TRAPPs in cell plate formation and plant development (for review, see Vukašinović and Žárský, 2016; Ravikumar et al., 2017) is discussed at length in the cell plate section below.
In mammals, TGN-localized tetrameric GARP is required for retrograde trafficking from endosomes to the Golgi (Schmitt-John et al., 2005; Pérez-Victoria and Bonifacino, 2009). Mutants of Arabidopsis GARP subunits have implicated the complex in pollen tube elongation, acclimation to heat stress, and vacuolar targeting of the auxin carrier PIN1, necessary for PIN1 polar localization during the establishment of leaf vein patterning (Lobstein et al., 2004; Wang et al., 2011; Pahari et al., 2014). Interestingly, GARP shares three subunits with the Endosome-Associated Recycling Protein (EARP), which resides on recycling endosomes and is required for bringing recycling proteins/cargo to the cell surface (Schindler et al., 2015; Ravikumar et al., 2017). This phenomenon, also characteristic in TRAPP complexes, has been referred to as modularity. Multisubunit tethering complexes can exist in a variety of modular forms as a result of subunit exchange (Desfougères et al., 2015; Ravikumar et al., 2017). The existence of an Arabidopsis homolog also for the EARP-specific subunit, syndetin, hints at GARP modularity conservation in plants.
Adaptins
While the role of CCVs in plant endocytosis is well documented (Bandmann et al., 2012; Bashline et al., 2013; Di Rubbo et al., 2013), their involvement in post-Golgi trafficking remains poorly understood. Adaptor protein complexes (AP1–AP5) consisting of four adaptin subunits select cargo proteins into CCVs (Boehm and Bonifacino, 2001; Nakatsu and Ohno, 2003; Pertl-Obermeyer et al., 2016). The Arabidopsis genome encodes putative subunits of all five AP complexes (Happel et al., 2004; Hirst et al., 2011; Park et al., 2013). Arabidopsis AP1 localizes to the TGN (Park et al., 2013; Wang et al., 2014), and mutants of the AP1M2 subunit are affected in the secretory and vacuolar transport pathways. In addition, trafficking of the essential SNARE KNOLLE from the TGN to the cell plate is impaired in dividing cells of ap1m2 mutants (Park et al., 2013). More recently, TGN-localized AP1 γ-adaptins were found to mediate the targeting of membrane proteins with di-Leu motifs to the tonoplast (Wang et al., 2014). Intriguingly, Arabidopsis AP3 has been implicated in a TGN-bypass pathway that transfers cargo directly from Golgi cisternae to the vacuole and is facilitated by the HOPS-tethering complex (Feraru et al., 2010; Zwiewka et al., 2011; Feng et al., 2017). Perhaps with the exception of AP2, which operates in clathrin-mediated endocytosis at the plasma membrane (Krauss et al., 2006; Di Rubbo et al., 2013), a function in vacuolar transport appears to be the norm among Arabidopsis AP complexes. AP4 was recently found to localize to a TGN subdomain different from that of AP1 and for which mutants of four AP4 adaptins showed defects in the vacuolar sorting of the major storage protein 12S globulin (Fuji et al., 2016).
TGN AND THE CELL WALL: A PLANT-SPECIFIC CONNECTION
The Golgi apparatus and the TGN fulfill a highly dynamic and distinguishing function in plant cells: to sort and assemble plasma membrane cell wall biosynthetic enzymes, structural proteins, and the cross-linking glycans, pectin, and hemicellulose (Cosgrove, 2005; for review, see Worden et al., 2012; Kim and Brandizzi, 2016; van de Meene et al., 2017). Although extensive studies have led to the identification of the key enzymes involved in the biosynthesis of hemicellulose and pectin (for review, see Atmodjo et al., 2013; Pauly and Keegstra, 2016), comparatively little is known about their transport, deposition, and integration into the cell wall. Polysaccharides originate from distinct locations. Cellulose and callose are synthesized at the plasma membrane, while the Golgi apparatus is the synthesis site of noncellulosic cell wall polysaccharides, hemicellulose, and pectin, which are then transported through the secretory pathway to the apoplast (Driouich et al., 2012; Worden et al., 2012; Kim and Brandizzi, 2016). Our current knowledge of polysaccharide transport in the endomembrane system is derived mainly from immunohistochemical electron microscopy studies (Lynch and Staehelin, 1992; Zhang and Staehelin, 1992).
Golgi and TGN Involvement in the Biosynthesis of Xyloglucans and Pectins
The use of antibodies that recognize a number of xyloglucan (XyG) polymer epitopes identified trans-Golgi cisternae as the exclusive site of synthesis of the XyG backbone in a study conducted in suspension-cultured sycamore (Acer pseudoplatanus) cells. Fucosylated XyG side chains were detected in the trans-cisternae and the TGN, forming the hypothesis of an ordered mechanism of backbone biosynthesis and subsequent substitution in Golgi subcompartments (Zhang and Staehelin, 1992). The TGN involvement in XyG biosynthesis was highlighted further in studies with Arabidopsis roots, which showed labeling of TGN by a fucosylated XyG antibody (Kang et al., 2011). Earlier observations in tobacco BY-2 cells reported a sequence of events during XyG biosynthesis in cis- and medial Golgi cisternae (Chevalier et al., 2010), suggesting differences between species.
The Golgi apparatus also is the site of pectin biosynthesis. Pectins are synthesized and secreted in their methyl esterified form into the cell wall, where they are deesterified by pectin methyl esterases (Caffall and Mohnen, 2009). Studies in sycamore suspension cells showed that the synthesis of the nonesterified Rhamnogalacturonan I (RGI)/Homogalacturonan backbone and the methylesterification of GalUA residues take place in the cis-medial Golgi with subsequent completion in the medial cisternae. More complex pectin oligosaccharides, such as RGI-containing arabinose side chains, are detected only in the TGN (Zhang and Staehelin, 1992). However, the labeling of pectin epitopes differs among cell types of the root tip, suggesting that Golgi function may be altered/reorganized during cell differentiation (Lynch and Staehelin, 1992).
Several enzymes involved in both pectin and XyG biosynthesis have been identified and characterized. Contrasting the pectin biosynthesis assembly line hypothesis is the observation that several glycosyl transferases involved in the biosynthesis of pectin are present in multiprotein complexes at the Golgi. Currently, two pectin biosynthesis models have been proposed: the classical model, which predicts the consecutive addition of sugar residues to the growing polysaccharide; and a recently developed model, in which a block transfer of pectin from one domain onto another domain occurs (Atmodjo et al., 2013). The isolation of TGN SVs that carry polysaccharide cargo will contribute substantially to a more comprehensive understanding of the transport and assembly of cell wall components.
Cellular Determinants of Cell Wall Component Transport
Post-Golgi routes deployed by the plant cell to transport Golgi/TGN-synthesized polysaccharides to the plasma membrane are not fully elucidated. A TGN-resident complex formed by ECHIDNA (ECH) and two members of the YIP family of RAB GTPase-interacting proteins has been implicated in the secretion of pectin and XyG. Mutant plants of YIP4A and YIP4B are defective in cell elongation and the secretion of XyG and RGI pectins, a defect also observed in ech mutants (Gendre et al., 2011, 2013). ECH also seems to be necessary for the proper TGN localization of the ADP-Ribosylation Factor1 (ARF1)-GTPase and the ARF-guanine-exchange factor BIG4. This pathway mediates secretion of the auxin carrier AUX1 from the TGN to the plasma membrane (PM) during hook development (Jonsson et al., 2017). In tobacco BY-2 cells, immunostaining of pectin showed that the Secretory Carrier Membrane Protein2 (SCAMP2) vesicles are involved in pectin transport (Toyooka et al., 2009). A potential interplay between the SCAMP2 and ECH machineries in pectin secretion has not been explored yet.
A pioneering study of plant intracellular vesicle isolation and proteomic analysis revealed that the syntaxin SYP61 defines a TGN compartment carrying cell wall-relevant cargo. Several CSCs were identified in the SYP61 proteome, and electron microscopy immunostaining evidenced the colocalization of CESA6 and SYP61 in TGN vesicles and in close proximity to the PM (Drakakaki et al., 2012). The exogenous administration of CESTRIN, a small molecule that reduces the motility of CSCs at the plasma membrane, increases the association of CESAs with SYP61 vesicles, further implicating the SYP61 compartment in CESA trafficking (Worden et al., 2015). Both ECH and YIP proteins are cargo of SYP61 vesicles, favoring the hypothesis that SYP61-mediated trafficking is involved in the transport of cell wall components. Furthermore, antibodies for fucosylated XyG label a RABA4b TGN compartment, and colocalization of RABA4b and SYP61 was observed, supporting the role of SYP61 in the trafficking of cell wall components (Kang et al., 2011).
Despite the essential roles of glycoproteins in cell wall remodeling, development, and responses to biotic stress, their secretory routes remain poorly characterized. In addition to conventional secretion via TGN, exemplified by SYP61 and SCAMP2 vesicles, evidence pointing to the existence of TGN-independent, unconventional protein secretion pathways is accumulating, which may be implicated in cell wall protein transport (for review, see De Marchis et al., 2013; Davis et al., 2016a; Robinson et al., 2016; van de Meene et al., 2017).
TGN AND THE CELL PLATE: A TIMELY CONNECTION
The TGN’s key role in organizing and shipping cell wall material is never more obvious than during cytokinesis, in which an entirely new cell wall is built from inside the parent cell. Cytokinesis begins with the delivery of Golgi/TGN-derived vesicles to the plane of division, where they immediately start to fuse and tubulate. The vesicles continue to coalesce into a membrane network (the cell plate) following the tracks laid down by the radially expanding phragmoplast (Samuels et al., 1995; Seguí-Simarro et al., 2004; Smertenko et al., 2017). Deposition of polysaccharides leads to the maturation of the formed structure, which finally joins to the parental cell wall (Drakakaki, 2015).
Chemical inhibition of either endocytosis or secretion in dividing plant cells indicates that both pathways have a role during cell plate formation via the TGN. It is plausible that endocytosed proteins merge into the late secretory pathway and, thus, are delivered to the division plane instead of being recycled to the PM (Dhonukshe et al., 2006; Reichardt et al., 2007; Richter et al., 2014; Müller and Jürgens, 2016).
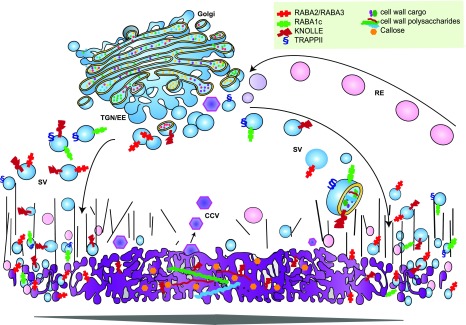
The TGN is intimately connected to the cell plate, as evidenced by the various proteins shared and shuttled between them, of which a few key components are mentioned below (Fig. 2). Excellent reviews describe in detail the cytoskeletal and membrane dynamics during cytokinesis (McMichael and Bednarek, 2013; Boruc and Van Damme, 2015; Müller and Jürgens, 2016).
Figure 2.
Illustration of TGN-cell plate trafficking. The TGN acts as a sorting hub for cell plate formation during cytokinesis. Representative components of the trafficking routes between the TGN and the cell plate are depicted. During early stages of cell plate formation, TGN compartments labeled by the GTPases RABA2/RABA3 and RABA1c relocate to the cell plate margins, where they are involved in vesicle targeting and the delivery of membrane to the expanding plate. The TRAPPII tethering complex is involved in cell plate biogenesis and expansion. The cytokinesis-specific SNARE protein, KNOLLE, which is found throughout the entire cell plate, mediates vesicle fusion. Cell plate maturation occurs radially from the interior directed outward toward the parental cell wall and involves the deposition of cell wall polysaccharides and the removal of excess membrane through clathrin-mediated recycling (purple hexagons). RE, Recycling endosome.
A key event in cell plate formation is vesicle fusion mediated by SNARE complexes (El Kasmi et al., 2013), with the complex formed by the Q-SNARE KNOLLE and the R-SNAREs Vesicle-Associated Membrane Protein721 (VAMP721) or VAMP722 playing a preponderant role (Lauber et al., 1997; Zhang et al., 2011). Additional proteins in this complex include the SEC1/Munc18 protein KEULLE, SNAP33, and NPSN11 (Assaad et al., 2001; Heese et al., 2001; Zheng et al., 2002). Interestingly, of the SNAREs involved in cell plate formation, only KNOLLE is specific to the process.
RABA2 and RABA3 preferentially localize to the leading edge of the cell plate, suggesting a function in the delivery and incorporation of new membrane material to the cell plate (Chow et al., 2008). Because overlapping localizations and complementary functions are common among RABAs, a complete roadmap for the multiple pathways and processes in which each is involved is still being drawn. RABA2a and RABA1e vesicles display a different spatiotemporal pattern during cytokinesis, which is exaggerated by the cytokinesis inhibitor Endosidin7 (ES7; Davis et al., 2016b). TGN/EE-resident RABA1d accumulates at early cell plate stages during cytokinesis, and its association with PM proteins suggests a role in the recycling of material from the PM to the growing plate (Berson et al., 2014). Given the fact that the RABA clade is highly elaborated in plants (26 of the 57 total RAB GTPases identified thus far; Woollard and Moore, 2008), it will be interesting to see how/if they are functionally connected to meet the myriad trafficking demands in a plant cell, dividing or otherwise. In addition to RABAs, RABE1s also localize to the cell plate, where they interact with the Stomatal Cytokinesis-Defective (SCD) complex. Inhibition of RABE1 causes cytokinesis defects, emphasizing its role in trafficking to the cell plate (Speth et al., 2009; Ahn et al., 2013; Mayers et al., 2017).
TRAPP tethering complexes are known to act as guanine exchange factors (GEFs) and, thus, activate RAB GTPases (Jones et al., 2000; Wang et al., 2000; Pinar et al., 2015). Colocalization at the TGN and functional studies suggest that TRAPPII acts as a GEF for RABA1c, facilitating RABA1c-mediated trafficking of material from the TGN to the cell plate (Qi et al., 2011). Mutants of the TRAPPII subunit, AtTRS130, exhibit severe cytokinesis defects and irregular aggregations of RABA1c around cell plate-like structures (Jaber et al., 2010; Qi et al., 2011). The expression of constitutively active RABA1c partially rescues attrs130 (Qi et al., 2011), supporting the role of AtTRS130 upstream of RABA1c. During cytokinesis, subunits of the exocyst-tethering complex interact with TRAPP subunits, suggesting synergistic activities of the two complexes (Rybak et al., 2014; for review, see Vukašinović and Žárský, 2016).
In addition to RAB GTPases and their regulators, other protein classes with vesicle formation/budding functions at the TGN participate in cell plate formation. For example, Clathrin Light Chain, Dynamin-Related Proteins, Epsin-like adaptors, and the adaptin-like T-PLATE all have been identified at the cell plate, providing evidence for clathrin-mediated endocytosis in the removal and/or recycling of excess membranes from the cell plate (Fujimoto et al., 2008; Konopka and Bednarek, 2008; Van Damme et al., 2011; Song et al., 2012; McMichael and Bednarek, 2013). Additionally, SCD1 and SCD2 act as a complex to mediate post-Golgi trafficking to the plasma membrane and the cell plate. Multiple lines of evidence point to the interaction between the SCD complex, the exocyst, and RABE1 GTPases in the trafficking of material to the cell plate (Mayers et al., 2017).
The aforementioned AP1, and specifically the µ-subunit (AP1M2), is found in the TGN and is essential for the trafficking of KNOLLE to the cell plate. In an ap1m2 mutant background, KNOLLE is mislocalized around the division plane and cell wall stubs are present, indicating a role of AP1M2 in targeting KNOLLE to the cell plate (Park et al., 2013; Teh et al., 2013).
Exciting discoveries in the nature and composition of cytokinesis vesicles are awaiting, which will form a better picture of membrane and polysaccharide delivery for the buildup of such an essential and dynamic structure.
TGN AS AN EE: TWO DIRECTIONS BUT HOW MANY LANES?
The plant TGN is unique in that it acts not only in the secretory but also in the endocytic pathway as an EE (Tanchak et al., 1988; Dettmer et al., 2006; Viotti et al., 2010). This distinct, additional function of plant TGN has been associated with the absence of the tubulated endosomes dedicated to recycling, normally found in animal cells (Paez Valencia et al., 2016). The function of TGN as an EE has been demonstrated by live imaging and electron microscopy studies (Dettmer et al., 2006; Lam et al., 2007; Viotti et al., 2010). Immunogold electron microscopy showed an accumulation of the PM-localized brassinosteroid receptor BRI1 to BFA bodies, not prevented by the protein synthesis inhibitor cycloheximide, lending evidence to the exclusively endocytic origin of the material accumulated in the BFA compartment (Viotti et al., 2010).
Recycling of PM proteins through the TGN/EE has proven crucial for the polar distribution of plasma membrane proteins, including auxin carriers like PIN1 (Geldner et al., 2003; Kleine-Vehn et al., 2008, 2011; Luschnig and Vert, 2014). Endosomal recycling in plants largely depends on the activity of small GTPases of the ARF family and their associated GTPase-activating proteins and GEFs (Casanova, 2007; Kleine-Vehn et al., 2008; Paez Valencia et al., 2016). Twelve ARF genes are present in the genome of Arabidopsis (Robinson et al., 2007). The best-characterized ARF1 localizes to endocytic organelles and is implicated in the establishment of apical-basal polarity in epidermal cells (Xu and Scheres, 2005).
Among Arabidopsis ARF-GEFs, GNOM has been arguably the most studied in its function of restricting PM protein localization to basal domains (Geldner et al., 2003). BFA treatments inhibit GNOM, causing the accumulation of the internalized auxin effluxer PIN1 in aggregating GNOM-positive intracellular compartments (Geldner et al., 2003). However, superresolution/electron microscopy studies showed its exclusive localization to Golgi cisternae, thereby challenging its function solely in protein recycling (Naramoto et al., 2014). In support, GNOM was recently implicated in the ER-to-Golgi transport of PIN1 (Doyle et al., 2015). BEN1 is another, BFA-insensitive ARF-GEF that localizes to early endocytic compartments distinct from GNOM-positive endosomes and whose mutants display cell polarity defects (Tanaka et al., 2009). Finally, a role for the BIG subfamily in nonbasal trafficking of plasma membrane proteins was recently supported by pharmacological evidence (Li et al., 2017). Such functions place BIGs distinct from GNOM in the scheme of factors regulating the polarity of PM proteins. Interestingly, and in contrast with GNOM, BIGs do not seem to affect recycling but only protein secretion (Richter et al., 2014). Such functional divergence could lie at the core of the observed stage-dependent, mutually exclusive involvement of GNOM and BIGs in the regulation of the secretion of AUX1 influx carrier to the plasma membrane from the TGN during hook development (Jonsson et al., 2017).
Several lines of evidence support a model for the maturation of TGN/EE into late endosomes or multivesicular bodies (LE/MVB; Scheuring et al., 2011; Singh et al., 2014). Endocytosed plasma membrane proteins that are not recycled back are transferred from EE (TGN) to LE/MVB, where they are internalized into the intraluminal vesicles of the LE. Fusion of the LE with the lysosome/vacuole releases the vesicle cargoes, leading to their degradation. Scheuring et al. (2011) showed that the formation of intraluminal vesicles takes place already at the TGN/EE, while Singh et al. (2014) suggested that endosomal maturation in Arabidopsis originates in a subdomain of the TGN/EE that recruits Rab5-like ARA7 and subsequently transitions into an MVB. The topics of plant EE and LE are covered extensively by other reviews in this issue of Plant Physiology.
EXPERIMENTAL TOOLS
The plant TGN comprises an extremely dynamic and diverse vesicle population, which raises many questions for most of which we do not have an answer; meanwhile, technological advances are helping us on the road. Improved protocols for the immunoisolation of vesicles based on specific, vesicle membrane markers have been successfully established, an example of which is the characterization of the SYP61 compartment (Drakakaki et al., 2012; Groen et al., 2014; Heard et al., 2015). Organelle proteomics is allowing the identification of vesicle protein cargoes, and extending glycomic analysis to isolated vesicles will help us to better characterize the secretory routes of cell wall polysaccharides (Obel et al., 2009; Pattathil et al., 2010; Drakakaki et al., 2012; Parsons et al., 2013; Heard et al., 2015; Kračun et al., 2017; Wood et al., 2017). Chemical genomics is enabling the characterization of vesicle-trafficking pathways, recently evidenced by the use of the small molecules ES7 and ES16 to discern the contributions of two RAB GTPases to cell plate formation and to demonstrate the specificity of a trafficking pathway involved in cell polarity, respectively (Davis et al., 2016b; Li et al., 2017). Spatiotemporal image correlation spectroscopy has proven useful to characterize the dynamics of vesicle trafficking to the cell plate (Hebert et al., 2005; van Oostende-Triplet et al., 2017). Finally, lipid profiling, metabolic click labeling, and the use of oligosaccharide-based probes for high-resolution real-time imaging of glycans have recently added to the list of promising avenues to dissect the role of TGN in cargo transport (Anderson et al., 2012; Pattathil et al., 2012; Mravec et al., 2014; Wattelet-Boyer et al., 2016). Such a panoply of tools promises new exciting discoveries about the multiple functions of the plant TGN.
Acknowledgments
We apologize to our colleagues whose work we were not able to include in this review due to length limitations.
Footnotes
This work was supported by NSF IOS 1258135 to G.D.
Articles can be viewed without a subscription.
References
- Ahn CS, Han JA, Pai HS (2013) Characterization of in vivo functions of Nicotiana benthamiana RabE1. Planta 237: 161–172 [DOI] [PubMed] [Google Scholar]
- Anderson CT, Wallace IS, Somerville CR (2012) Metabolic click-labeling with a fucose analog reveals pectin delivery, architecture, and dynamics in Arabidopsis cell walls. Proc Natl Acad Sci USA 109: 1329–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka R, Uemura T, Ito J, Fujimoto M, Ito E, Ueda T, Nakano A (2013) Arabidopsis RABA1 GTPases are involved in transport between the trans-Golgi network and the plasma membrane, and are required for salinity stress tolerance. Plant J 73: 240–249 [DOI] [PubMed] [Google Scholar]
- Assaad FF, Huet Y, Mayer U, Jürgens G (2001) The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE. J Cell Biol 152: 531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev Plant Biol 64: 747–779 [DOI] [PubMed] [Google Scholar]
- Bandmann V, Müller JD, Köhler T, Homann U (2012) Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett 586: 3626–3632 [DOI] [PubMed] [Google Scholar]
- Barlowe C. (1997) Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J Cell Biol 139: 1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashline L, Li S, Anderson CT, Lei L, Gu Y (2013) The endocytosis of cellulose synthase in Arabidopsis is dependent on μ2, a clathrin-mediated endocytosis adaptin. Plant Physiol 163: 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Blatt MR (2008) SNAREs: cogs and coordinators in signaling and development. Plant Physiol 147: 1504–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV (2000) AtVPS45 complex formation at the trans-Golgi network. Mol Biol Cell 11: 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol 22: 1–6 [DOI] [PubMed] [Google Scholar]
- Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63: 5727–5740 [DOI] [PubMed] [Google Scholar]
- Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23: 224–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson T, von Wangenheim D, Takáč T, Šamajová O, Rosero A, Ovečka M, Komis G, Stelzer EH, Šamaj J (2014) Trans-Golgi network localized small GTPase RabA1d is involved in cell plate formation and oscillatory root hair growth. BMC Plant Biol 14: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuin T, Roy JK (2014) Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res 328: 1–19 [DOI] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS (2001) Adaptins: the final recount. Mol Biol Cell 12: 2907–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier JP, Munson M (2015) Three steps forward, two steps back: mechanistic insights into the assembly and disassembly of the SNARE complex. Curr Opin Chem Biol 29: 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruc J, Van Damme D (2015) Endomembrane trafficking overarching cell plate formation. Curr Opin Plant Biol 28: 92–98 [DOI] [PubMed] [Google Scholar]
- Brüx A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, Wasternack C, Schumacher K (2008) Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis. Plant Cell 20: 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacas JL, Furt F, Le Guédard M, Schmitter JM, Buré C, Gerbeau-Pissot P, Moreau P, Bessoule JJ, Simon-Plas F, Mongrand S (2012) Lipids of plant membrane rafts. Prog Lipid Res 51: 272–299 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J 17: 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CJ, Bednarek SY, Raikhel NV (2004) Membrane trafficking in plants: new discoveries and approaches. Curr Opin Plant Biol 7: 701–707 [DOI] [PubMed] [Google Scholar]
- Casanova JE. (2007) Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic 8: 1476–1485 [DOI] [PubMed] [Google Scholar]
- Chen Y, Shin YK, Bassham DC (2005) YKT6 is a core constituent of membrane fusion machineries at the Arabidopsis trans-Golgi network. J Mol Biol 350: 92–101 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Chen CY, Glaven RH, de Graaf BHJ, Vidali L, Hepler PK, Wu HM (2002) Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14: 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier L, Bernard S, Ramdani Y, Lamour R, Bardor M, Lerouge P, Follet-Gueye ML, Driouich A (2010) Subcompartment localization of the side chain xyloglucan-synthesizing enzymes within Golgi stacks of tobacco suspension-cultured cells. Plant J 64: 977–989 [DOI] [PubMed] [Google Scholar]
- Chow CM, Neto H, Foucart C, Moore I (2008) Rab-A2 and Rab-A3 GTPases define a trans-Golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 20: 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Davis DJ, Kang BH, Heringer AS, Wilkop TE, Drakakaki G (2016a) Unconventional protein secretion in plants. Methods Mol Biol 1459: 47–63 [DOI] [PubMed] [Google Scholar]
- Davis DJ, McDowell SC, Park E, Hicks G, Wilkop TE, Drakakaki G (2016b) The RAB GTPase RABA1e localizes to the cell plate and shows distinct subcellular behavior from RABA2a under Endosidin 7 treatment. Plant Signal Behav 11: e984520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis M, Bleve G, Faraco M, Stigliano E, Grieco F, Piro G, Dalessandro G, Di Sansebastiano GP (2013) AtSYP51/52 functions diverge in the post-Golgi traffic and differently affect vacuolar sorting. Mol Plant 6: 916–930 [DOI] [PubMed] [Google Scholar]
- De Marchis F, Bellucci M, Pompa A (2013) Unconventional pathways of secretory plant proteins from the endoplasmic reticulum to the vacuole bypassing the Golgi complex. Plant Signal Behav 8: e25129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desfougères Y, D’Agostino M, Mayer A (2015) A modular tethering complex for endosomal recycling. Nat Cell Biol 17: 540–541 [DOI] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K (2006) Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, Gadella TW Jr (2006) Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell 10: 137–150 [DOI] [PubMed] [Google Scholar]
- Di Rubbo S, Irani NG, Kim SY, Xu ZY, Gadeyne A, Dejonghe W, Vanhoutte I, Persiau G, Eeckhout D, Simon S, et al. (2013) The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell 25: 2986–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SM, Haeger A, Vain T, Rigal A, Viotti C, Łangowska M, Ma Q, Friml J, Raikhel NV, Hicks GR, et al. (2015) An early secretory pathway mediated by GNOM-LIKE 1 and GNOM is essential for basal polarity establishment in Arabidopsis thaliana. Proc Natl Acad Sci USA 112: E806–E815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G. (2015) Polysaccharide deposition during cytokinesis: challenges and future perspectives. Plant Sci 236: 177–184 [DOI] [PubMed] [Google Scholar]
- Drakakaki G, van de Ven W, Pan S, Miao Y, Wang J, Keinath NF, Weatherly B, Jiang L, Schumacher K, Hicks G, et al. (2012) Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res 22: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A, Follet-Gueye ML, Bernard S, Kousar S, Chevalier L, Vicré-Gibouin M, Lerouxel O (2012) Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants. Front Plant Sci 3: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuke ML, Munson M (2016) The secret life of tethers: the role of tethering factors in snare complex regulation. Front Cell Dev Biol 4: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi F, Krause C, Hiller U, Stierhof YD, Mayer U, Conner L, Kong L, Reichardt I, Sanderfoot AA, Jürgens G (2013) SNARE complexes of different composition jointly mediate membrane fusion in Arabidopsis cytokinesis. Mol Biol Cell 24: 1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng QN, Song SJ, Yu SX, Wang JG, Li S, Zhang Y (2017) Adaptor protein-3-dependent vacuolar trafficking involves a subpopulation of COPII and HOPS tethering proteins. Plant Physiol 174: 1609–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Paciorek T, Feraru MI, Zwiewka M, De Groodt R, De Rycke R, Kleine-Vehn J, Friml J (2010) The AP-3 β adaptin mediates the biogenesis and function of lytic vacuoles in Arabidopsis. Plant Cell 22: 2812–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji K, Shirakawa M, Shimono Y, Kunieda T, Fukao Y, Koumoto Y, Takahashi H, Hara-Nishimura I, Shimada T (2016) The adaptor complex AP-4 regulates vacuolar protein sorting at the trans-Golgi network by interacting with VACUOLAR SORTING RECEPTOR1. Plant Physiol 170: 211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Arimura S, Nakazono M, Tsutsumi N (2008) Arabidopsis dynamin-related protein DRP2B is co-localized with DRP1A on the leading edge of the forming cell plate. Plant Cell Rep 27: 1581–1586 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Gendre D, McFarlane HE, Johnson E, Mouille G, Sjödin A, Oh J, Levesque-Tremblay G, Watanabe Y, Samuels L, Bhalerao RP (2013) Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell 25: 2633–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre D, Oh J, Boutté Y, Best JG, Samuels L, Nilsson R, Uemura T, Marchant A, Bennett MJ, Grebe M, et al. (2011) Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc Natl Acad Sci USA 108: 8048–8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Simons K (1986) The trans Golgi network: sorting at the exit site of the Golgi complex. Science 234: 438–443 [DOI] [PubMed] [Google Scholar]
- Groen AJ, Sancho-Andrés G, Breckels LM, Gatto L, Aniento F, Lilley KS (2014) Identification of trans-Golgi network proteins in Arabidopsis thaliana root tissue. J Proteome Res 13: 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Helmle MH (1981) Properties of an ATP-fueled, Cl-dependent proton pump localized in membranes of microsomal vesicles from maize coleoptiles. Z Naturforsch C 36: 997–1008 [Google Scholar]
- Happel N, Höning S, Neuhaus JM, Paris N, Robinson DG, Holstein SEH (2004) Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J 37: 678–693 [DOI] [PubMed] [Google Scholar]
- Heard W, Sklenář J, Tomé DFA, Robatzek S, Jones AME (2015) Identification of regulatory and cargo proteins of endosomal and secretory pathways in Arabidopsis thaliana by proteomic dissection. Mol Cell Proteomics 14: 1796–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert B, Costantino S, Wiseman PW (2005) Spatiotemporal image correlation spectroscopy (STICS) theory, verification, and application to protein velocity mapping in living CHO cells. Biophys J 88: 3601–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jürgens G (2001) Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol 155: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MN, Dacks JB, Robinson MS (2011) The fifth adaptor protein complex. PLoS Biol 9: e1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HR (1998) Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J 17: 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91: 119–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Toyooka K, Fujimoto M, Ueda T, Uemura T, Nakano A (2017) The trans-Golgi network and the Golgi stacks behave independently during regeneration after brefeldin A treatment in tobacco BY-2 cells. Plant Cell Physiol 58: 811–821 [DOI] [PubMed] [Google Scholar]
- Jaber E, Thiele K, Kindzierski V, Loderer C, Rybak K, Jürgens G, Mayer U, Söllner R, Wanner G, Assaad FF (2010) A putative TRAPPII tethering factor is required for cell plate assembly during cytokinesis in Arabidopsis. New Phytol 187: 751–763 [DOI] [PubMed] [Google Scholar]
- Jones S, Newman C, Liu F, Segev N (2000) The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol Biol Cell 11: 4403–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson K, Boutté Y, Singh RK, Gendre D, Bhalerao RP (2017) Ethylene regulates differential growth via BIG ARF-GEF-dependent post-Golgi secretory trafficking in Arabidopsis. Plant Cell 29: 1039–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA (2011) Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lipatova Z, Segev N (2016) TRAPP complexes in secretion and autophagy. Front Cell Dev Biol 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Bassham DC (2011) TNO1 is involved in salt tolerance and vacuolar trafficking in Arabidopsis. Plant Physiol 156: 514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Bassham DC (2013) Functional redundancy between trans-Golgi network SNARE family members in Arabidopsis thaliana. BMC Biochem 14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Brandizzi F (2016) The plant secretory pathway for the trafficking of cell wall polysaccharides and glycoproteins. Glycobiology 26: 940–949 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wiśniewska J, Paciorek T, Benková E, Friml J (2008) ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol 18: 526–531 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Wabnik K, Martinière A, Łangowski Ł, Willig K, Naramoto S, Leitner J, Tanaka H, Jakobs S, Robert S, et al. (2011) Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol Syst Biol 7: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, et al. (2009) Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol 185: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka CA, Bednarek SY (2008) Comparison of the dynamics and functional redundancy of the Arabidopsis dynamin-related isoforms DRP1A and DRP1C during plant development. Plant Physiol 147: 1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kračun SK, Fangel JU, Rydahl MG, Pedersen HL, Vidal-Melgosa S, Willats WGT (2017) Carbohydrate microarray technology applied to high-throughput mapping of plant cell wall glycans using comprehensive microarray polymer profiling (CoMPP). Methods Mol Biol 1503: 147–165 [DOI] [PubMed] [Google Scholar]
- Krauss M, Kukhtina V, Pechstein A, Haucke V (2006) Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci USA 103: 11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel A, Andrés Z, Medzihradszky A, Krüger F, Scholl S, Delang S, Patir-Nebioglu MG, Gute G, Yang H, Murphy AS, et al. (2015) Job sharing in the endomembrane system: vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 27: 3383–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Robinson DG, Jiang L (2007) Tracking down the elusive early endosome. Trends Plant Sci 12: 497–505 [DOI] [PubMed] [Google Scholar]
- Langhans M, Förster S, Helmchen G, Robinson DG (2011) Differential effects of the brefeldin A analogue (6R)-hydroxy-BFA in tobacco and Arabidopsis. J Exp Bot 62: 2949–2957 [DOI] [PubMed] [Google Scholar]
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jürgens G (1997) The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol 139: 1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Rodriguez-Furlan C, Wang J, van de Ven W, Gao T, Raikhel NV, Hicks GR (2017) Different endomembrane trafficking pathways establish apical and basal polarities. Plant Cell 29: 90–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobstein E, Guyon A, Férault M, Twell D, Pelletier G, Bonhomme S (2004) The putative Arabidopsis homolog of yeast vps52p is required for pollen tube elongation, localizes to Golgi, and might be involved in vesicle trafficking. Plant Physiol 135: 1480–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Scholl S, Doering A, Zhang Y, Irani NG, Rubbo SD, Neumetzler L, Krishnamoorthy P, Van Houtte I, Mylle E, et al. (2015) V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat Plants 1: 15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Vert G (2014) The dynamics of plant plasma membrane proteins: PINs and beyond. Development 141: 2924–2938 [DOI] [PubMed] [Google Scholar]
- Lynch MA, Staehelin LA (1992) Domain-specific and cell type-specific localization of two types of cell wall matrix polysaccharides in the clover root tip. J Cell Biol 118: 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinsky J, Opekarová M, Grossmann G, Tanner W (2013) Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annu Rev Plant Biol 64: 501–529 [DOI] [PubMed] [Google Scholar]
- Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N (2013) In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 25: 4028–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers JR, Hu T, Wang C, Cárdenas JJ, Tan Y, Pan J, Bednarek SY (2017) SCD1 and SCD2 form a complex that functions with the exocyst and RabE1 in exocytosis and cytokinesis. Plant Cell 29: 2610–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael CM, Bednarek SY (2013) Cytoskeletal and membrane dynamics during higher plant cytokinesis. New Phytol 197: 1039–1057 [DOI] [PubMed] [Google Scholar]
- Mravec J, Kračun SK, Rydahl MG, Westereng B, Miart F, Clausen MH, Fangel JU, Daugaard M, Van Cutsem P, De Fine Licht HH, et al. (2014) Tracking developmentally regulated post-synthetic processing of homogalacturonan and chitin using reciprocal oligosaccharide probes. Development 141: 4841–4850 [DOI] [PubMed] [Google Scholar]
- Müller S, Jürgens G (2016) Plant cytokinesis: no ring, no constriction but centrifugal construction of the partitioning membrane. Semin Cell Dev Biol 53: 10–18 [DOI] [PubMed] [Google Scholar]
- Nakatsu F, Ohno H (2003) Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct 28: 419–429 [DOI] [PubMed] [Google Scholar]
- Naramoto S, Otegui MS, Kutsuna N, de Rycke R, Dainobu T, Karampelias M, Fujimoto M, Feraru E, Miki D, Fukuda H, et al. (2014) Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell 26: 3062–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T, Forgac M (2002) The vacuolar (H+)-ATPases: nature’s most versatile proton pumps. Nat Rev Mol Cell Biol 3: 94–103 [DOI] [PubMed] [Google Scholar]
- Obel N, Erben V, Schwarz T, Kühnel S, Fodor A, Pauly M (2009) Microanalysis of plant cell wall polysaccharides. Mol Plant 2: 922–932 [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S (2011) Na+/H+ exchangers. Compr Physiol 1: 2083–2100 [DOI] [PubMed] [Google Scholar]
- Paez Valencia J, Goodman K, Otegui MS (2016) Endocytosis and endosomal trafficking in plants. Annu Rev Plant Biol 67: 309–335 [DOI] [PubMed] [Google Scholar]
- Pahari S, Cormark RD, Blackshaw MT, Liu C, Erickson JL, Schultz EA (2014) Arabidopsis UNHINGED encodes a VPS51 homolog and reveals a role for the GARP complex in leaf shape and vein patterning. Development 141: 1894–1905 [DOI] [PubMed] [Google Scholar]
- Park M, Song K, Reichardt I, Kim H, Mayer U, Stierhof YD, Hwang I, Jürgens G (2013) Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc Natl Acad Sci USA 110: 10318–10323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons HT, Drakakaki G, Heazlewood JL (2013) Proteomic dissection of the Arabidopsis Golgi and trans-Golgi network. Front Plant Sci 3: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, et al. (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Miller JS, Hahn MG (2012) Immunological approaches to plant cell wall and biomass characterization: glycome profiling. Methods Mol Biol 908: 61–72 [DOI] [PubMed] [Google Scholar]
- Pauly M, Keegstra K (2016) Biosynthesis of the plant cell wall matrix polysaccharide xyloglucan. Annu Rev Plant Biol 67: 235–259 [DOI] [PubMed] [Google Scholar]
- Pérez-Victoria FJ, Bonifacino JS (2009) Dual roles of the mammalian GARP complex in tethering and SNARE complex assembly at the trans-Golgi network. Mol Cell Biol 29: 5251–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertl-Obermeyer H, Wu XN, Schrodt J, Müdsam C, Obermeyer G, Schulze WX (2016) Identification of cargo for Adaptor Protein (AP) complexes 3 and 4 by sucrose gradient profiling. Mol Cell Proteomics 15: 2877–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinar M, Arst HN Jr, Pantazopoulou A, Tagua VG, de los Ríos V, Rodríguez-Salarichs J, Díaz JF, Peñalva MA (2015) TRAPPII regulates exocytic Golgi exit by mediating nucleotide exchange on the Ypt31 ortholog RabERAB11. Proc Natl Acad Sci USA 112: 4346–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro H, Samalova M, Geldner N, Chory J, Martinez A, Moore I (2009) Genetic evidence that the higher plant Rab-D1 and Rab-D2 GTPases exhibit distinct but overlapping interactions in the early secretory pathway. J Cell Sci 122: 3749–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E (2004) The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16: 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Kaneda M, Chen J, Geitmann A, Zheng H (2011) A specific role for Arabidopsis TRAPPII in post-Golgi trafficking that is crucial for cytokinesis and cell polarity. Plant J 68: 234–248 [DOI] [PubMed] [Google Scholar]
- Ravikumar R, Steiner A, Assaad FF (2017) Multisubunit tethering complexes in higher plants. Curr Opin Plant Biol 40: 97–105 [DOI] [PubMed] [Google Scholar]
- Reguera M, Bassil E, Tajima H, Wimmer M, Chanoca A, Otegui MS, Paris N, Blumwald E (2015) pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. Plant Cell 27: 1200–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt I, Stierhof YD, Mayer U, Richter S, Schwarz H, Schumacher K, Jürgens G (2007) Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr Biol 17: 2047–2053 [DOI] [PubMed] [Google Scholar]
- Richter S, Kientz M, Brumm S, Nielsen ME, Park M, Gavidia R, Krause C, Voss U, Beckmann H, Mayer U, et al. (2014) Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion. eLife 3: e02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivinoja A, Hassinen A, Kokkonen N, Kauppila A, Kellokumpu S (2009) Elevated Golgi pH impairs terminal N-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J Cell Physiol 220: 144–154 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Ding Y, Jiang L (2016) Unconventional protein secretion in plants: a critical assessment. Protoplasma 253: 31–43 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Herranz MC, Bubeck J, Pepperkok R, Ritzenthaler C (2007) Membrane dynamics in the early secretory pathway. CRC Crit Rev Plant Sci 26: 199–225 [Google Scholar]
- Roth J, Taatjes DJ, Lucocq JM, Weinstein J, Paulson JC (1985) Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell 43: 287–295 [DOI] [PubMed] [Google Scholar]
- Rothman JE. (1994) Mechanisms of intracellular protein transport. Nature 372: 55–63 [DOI] [PubMed] [Google Scholar]
- Rutherford S, Moore I (2002) The Arabidopsis Rab GTPase family: another enigma variation. Curr Opin Plant Biol 5: 518–528 [DOI] [PubMed] [Google Scholar]
- Rybak K, Steiner A, Synek L, Klaeger S, Kulich I, Facher E, Wanner G, Kuster B, Zarsky V, Persson S, et al. (2014) Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev Cell 29: 607–620 [DOI] [PubMed] [Google Scholar]
- Sacher M, Kim YG, Lavie A, Oh BH, Segev N (2008) The TRAPP complex: insights into its architecture and function. Traffic 9: 2032–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH Jr, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Assaad FF, Raikhel NV (2000) The Arabidopsis genome: an abundance of soluble N-ethylmaleimide-sensitive factor adaptor protein receptors. Plant Physiol 124: 1558–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV (2001) Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell 12: 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuring D, Viotti C, Krüger F, Künzl F, Sturm S, Bubeck J, Hillmer S, Frigerio L, Robinson DG, Pimpl P, et al. (2011) Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23: 3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Chen Y, Pu J, Guo X, Bonifacino JS (2015) EARP is a multisubunit tethering complex involved in endocytic recycling. Nat Cell Biol 17: 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-John T, Drepper C, Mussmann A, Hahn P, Kuhlmann M, Thiel C, Hafner M, Lengeling A, Heimann P, Jones JM, et al. (2005) Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat Genet 37: 1213–1215 [DOI] [PubMed] [Google Scholar]
- Schuck S, Simons K (2004) Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci 117: 5955–5964 [DOI] [PubMed] [Google Scholar]
- Schumacher K. (2014) pH in the plant endomembrane system: an import and export business. Curr Opin Plant Biol 22: 71–76 [DOI] [PubMed] [Google Scholar]
- Seguí-Simarro JM, Austin JR, White EA, Staehelin LA (2004) Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16: 836–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L (2013) Organelle pH in the Arabidopsis endomembrane system. Mol Plant 6: 1419–1437 [DOI] [PubMed] [Google Scholar]
- Shirakawa M, Ueda H, Koumoto Y, Fuji K, Nishiyama C, Kohchi T, Hara-Nishimura I, Shimada T (2014) CONTINUOUS VASCULAR RING (COV1) is a trans-Golgi network-localized membrane protein required for Golgi morphology and vacuolar protein sorting. Plant Cell Physiol 55: 764–772 [DOI] [PubMed] [Google Scholar]
- Simons K, Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3: a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Bremnes B, Rønning E, Aasland R, Stenmark H (1998) Syntaxin-16, a putative Golgi t-SNARE. Eur J Cell Biol 75: 223–231 [DOI] [PubMed] [Google Scholar]
- Singh MK, Krüger F, Beckmann H, Brumm S, Vermeer JEM, Munnik T, Mayer U, Stierhof YD, Grefen C, Schumacher K, et al. (2014) Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr Biol 24: 1383–1389 [DOI] [PubMed] [Google Scholar]
- Smertenko A, Assaad F, Baluška F, Bezanilla M, Buschmann H, Drakakaki G, Hauser MT, Janson M, Mineyuki Y, Moore I, et al. (2017) Plant cytokinesis: terminology for structures and processes. Trends Cell Biol 27: 885–894 [DOI] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T (1994) A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78: 937–948 [DOI] [PubMed] [Google Scholar]
- Song K, Jang M, Kim SY, Lee G, Lee GJ, Kim DH, Lee Y, Cho W, Hwang I (2012) An A/ENTH domain-containing protein functions as an adaptor for clathrin-coated vesicles on the growing cell plate in Arabidopsis root cells. Plant Physiol 159: 1013–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth EB, Imboden L, Hauck P, He SY (2009) Subcellular localization and functional analysis of the Arabidopsis GTPase RabE. Plant Physiol 149: 1824–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surma MA, Klose C, Klemm RW, Ejsing CS, Simons K (2011) Generic sorting of raft lipids into secretory vesicles in yeast. Traffic 12: 1139–1147 [DOI] [PubMed] [Google Scholar]
- Surpin M, Zheng H, Morita MT, Saito C, Avila E, Blakeslee JJ, Bandyopadhyay A, Kovaleva V, Carter D, Murphy A, et al. (2003) The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15: 2885–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Kitakura S, De Rycke R, De Groodt R, Friml J (2009) Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Curr Biol 19: 391–397 [DOI] [PubMed] [Google Scholar]
- Tanchak MA, Rennie PJ, Fowke LC (1988) Ultrastructure of the partially coated reticulum and dictyosomes during endocytosis by soybean protoplasts. Planta 175: 433–441 [DOI] [PubMed] [Google Scholar]
- Teh OK, Shimono Y, Shirakawa M, Fukao Y, Tamura K, Shimada T, Hara-Nishimura I (2013) The AP-1 μ adaptin is required for KNOLLE localization at the cell plate to mediate cytokinesis in Arabidopsis. Plant Cell Physiol 54: 838–847 [DOI] [PubMed] [Google Scholar]
- Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49: 4715–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Goto Y, Asatsuma S, Koizumi M, Mitsui T, Matsuoka K (2009) A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 21: 1212–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Kim H, Saito C, Ebine K, Ueda T, Schulze-Lefert P, Nakano A (2012) Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc Natl Acad Sci USA 109: 1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Suda Y, Ueda T, Nakano A (2014) Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol 55: 694–703 [DOI] [PubMed] [Google Scholar]
- Van Damme D, Gadeyne A, Vanstraelen M, Inzé D, Van Montagu MCE, De Jaeger G, Russinova E, Geelen D (2011) Adaptin-like protein TPLATE and clathrin recruitment during plant somatic cytokinesis occurs via two distinct pathways. Proc Natl Acad Sci USA 108: 615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Meene AML, Doblin MS, Bacic A (2017) The plant secretory pathway seen through the lens of the cell wall. Protoplasma 254: 75–94 [DOI] [PubMed] [Google Scholar]
- van Oostende-Triplet C, Guillet D, Triplet T, Pandzic E, Wiseman PW, Geitmann A (2017) Vesicle dynamics during plant cytokinesis reveals distinct developmental phases. Plant Physiol 174: 1544–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, et al. (2010) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U (2007) Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J 50: 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukašinović N, Žárský V (2016) Tethering complexes in the Arabidopsis endomembrane system. Front Cell Dev Biol 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Tsai MC, Chang KY, Fan YS, Yeh CH, Wu SJ (2011) Involvement of the Arabidopsis HIT1/AtVPS53 tethering protein homologue in the acclimation of the plasma membrane to heat stress. J Exp Bot 62: 3609–3620 [DOI] [PubMed] [Google Scholar]
- Wang W, Sacher M, Ferro-Novick S (2000) TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol 151: 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cai Y, Wang H, Zeng Y, Zhuang X, Li B, Jiang L (2014) Trans-Golgi network-located AP1 gamma adaptins mediate dileucine motif-directed vacuolar targeting in Arabidopsis. Plant Cell 26: 4102–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattelet-Boyer V, Brocard L, Jonsson K, Esnay N, Joubès J, Domergue F, Mongrand S, Raikhel N, Bhalerao RP, Moreau P, et al. (2016) Enrichment of hydroxylated C24- and C26-acyl-chain sphingolipids mediates PIN2 apical sorting at trans-Golgi network subdomains. Nat Commun 7: 12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood IP, Pearson BM, Garcia-Gutierrez E, Havlickova L, He Z, Harper AL, Bancroft I, Waldron KW (2017) Carbohydrate microarrays and their use for the identification of molecular markers for plant cell wall composition. Proc Natl Acad Sci USA 114: 6860–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard AA, Moore I (2008) The functions of Rab GTPases in plant membrane traffic. Curr Opin Plant Biol 11: 610–619 [DOI] [PubMed] [Google Scholar]
- Worden N, Park E, Drakakaki G (2012) Trans-Golgi network: an intersection of trafficking cell wall components. J Integr Plant Biol 54: 875–886 [DOI] [PubMed] [Google Scholar]
- Worden N, Wilkop TE, Esteve VE, Jeannotte R, Lathe R, Vernhettes S, Weimer B, Hicks G, Alonso J, Labavitch J, et al. (2015) CESA TRAFFICKING INHIBITOR inhibits cellulose deposition and interferes with the trafficking of cellulose synthase complexes and their associated proteins KORRIGAN1 and POM2/CELLULOSE SYNTHASE INTERACTIVE PROTEIN1. Plant Physiol 167: 381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IM, Hughson FM (2010) Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol 26: 137–156 [DOI] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells: immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99: 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang H, Liu P, Hao H, Jin JB, Lin J (2011) Arabidopsis R-SNARE proteins VAMP721 and VAMP722 are required for cell plate formation. PLoS ONE 6: e26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Stenmark H (2015) Cellular functions of Rab GTPases at a glance. J Cell Sci 128: 3171–3176 [DOI] [PubMed] [Google Scholar]
- Zheng H, Bednarek SY, Sanderfoot AA, Alonso J, Ecker JR, Raikhel NV (2002) NPSN11 is a cell plate-associated SNARE protein that interacts with the syntaxin KNOLLE. Plant Physiol 129: 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Camacho L, Wee E, Batoko H, Legen J, Leaver CJ, Malhó R, Hussey PJ, Moore I (2005) A Rab-E GTPase mutant acts downstream of the Rab-D subclass in biosynthetic membrane traffic to the plasma membrane in tobacco leaf epidermis. Plant Cell 17: 2020–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhar J, Rojo E, Bassham DC (2009) AtVPS45 is a positive regulator of the SYP41/SYP61/VTI12 SNARE complex involved in trafficking of vacuolar cargo. Plant Physiol 149: 1668–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiewka M, Feraru E, Möller B, Hwang I, Feraru MI, Kleine-Vehn J, Weijers D, Friml J (2011) The AP-3 adaptor complex is required for vacuolar function in Arabidopsis. Cell Res 21: 1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]