Abstract
Functionally distinct actin filament arrays cluster organelles and define cellular scale flow patterns for secretion.
INTRODUCTION TO PLANT CELL SHAPE CONTROL AND THE ACTIN CYTOSKELETON
Plant cell shape is defined and constrained by a tough outer cell wall. Morphogenesis is the output of complex interactions among turgor pressure, cytoplasmic control of cell wall material properties, and the regulated rheological properties responses of the cell wall (Baskin, 2005; Szymanski and Cosgrove, 2009; Cosgrove, 2016). The vacuole and cytosol are osmotically balanced with a high concentration of solutes that creates a lower cytoplasmic water potential compared with the extracellular space. As a result, water moves across the plasma membrane into the cytoplasm until the hydrostatic pressure on the wall is equal to the water potential difference between the cytoplasm and the extracellular space. Changes in cell volume occur when the hydrostatic pressure on the wall exceeds the yielding threshold (Lockhart, 1965). Growth proceeds gradually as secretion and cell wall synthesis maintain cell wall thickness and the cell volume increases.
Although turgor pressure is an isotropic force pushing outward on the plasma membrane and the cell wall equally at all locations, the resulting stresses in the cell wall are heterogeneous and are affected by cell geometry (Jordan and Dumais, 2010) and the material properties of the wall (Yanagisawa et al., 2015). Cell wall stress is strongly reduced by adjacent cells that are joined by a middle lamella (Kutschera and Briggs, 1988). Cell wall stress is also influenced by subcellular gradients in cell wall thickness, modulus, or cellulose fiber alignment (Fayant et al., 2010; Yanagisawa et al., 2015). Therefore, a detailed mechanistic understanding of growth control requires knowledge about how the cell wall is patterned, and how cytoskeletal and cell wall systems feedback on one another over time.
A dynamic network of filamentous elements, the cytoskeleton, holds the key to patterning of the cell wall during growth. This review focuses on the actin cytoskeleton during cell morphogenesis. The field is progressing rapidly, enabled by forward- and reverse-genetic analyses in an ever-increasing number of plant species. The characterization and function of plant actin cytoskeletal proteins have been reviewed previously in several comprehensive reviews (Ren and Xiang, 2007; Li et al., 2015). The field has been further empowered by broad adoption of quantitative phenotyping and high spatiotemporal resolution live-cell imaging. The dynamics of single actin filaments (Staiger et al., 2009; Smertenko et al., 2010; Augustine et al., 2011), actin network remodeling (Vidali et al., 2010; Breuer et al., 2017), organelle/cargo motility (Ueda et al., 2010), and genes of interest are being analyzed from a broader perspective that often includes cell wall heterogeneity, intracellular flow patterns, and growth. This Update focuses on the integration of cytoskeletal, endomembrane, and cell wall functions during morphogenesis and highlights recent studies illuminating how plant cells use the actin cytoskeleton to control patterns of cell growth.
A SYSTEMS-LEVEL ANALYSIS OF ACTIN-BASED CELL MORPHOGENESIS
The first important point alluded to above is that the actin cytoskeleton functions as part of a cellular system in which vesicle trafficking, organization of actin networks, and cell wall assembly are integrated and feedback on one another over space and time. For example, Brefeldin A (BFA) is a fungal toxin that inhibits Arf family guanine nucleotide exchange factors and vesicle trafficking in the endomembrane system (Ritzenthaler et al., 2002). In tip-growing cells, treatment with BFA leads to disassembly of dynamic actin networks near the pollen tube tip (Hörmanseder et al., 2005), and application of the actin polymerization inhibitor Latrunculin B (LatB) causes mislocalization and eventual dispersal of secretory vesicles that are normally clustered at the cell apex (Preuss et al., 2004; Bibeau et al., 2018). The interdependence of the actin, endomembrane, and cell wall systems has been confirmed in subsequent genetic and pharmacological studies (Szumlanski and Nielsen, 2009; Y. Zhang et al., 2010).
The actin and endomembrane components are part of broader network of spatially and temporally controlled oscillators that pattern growth, ultimately by defining the local mechanical properties of the wall (Zerzour et al., 2009). In cells that use a diffuse growth mechanism, the microtubule and actin cytoskeletons work cooperatively to maintain cell wall mechanical properties and spatial heterogeneity that support long-term, irreversible cell expansion (Yanagisawa et al., 2015). The output of the process is an altered cell shape that persists or the generation of a growth axis that can be reoriented in response to a directional cue. How the cytoskeleton senses and responds to the geometry of the cell wall to generate or maintain a predictable growth pattern is an important unanswered question.
Actin-based cell morphogenesis is also a multidimensional problem. Actin-binding proteins (ABPs) and filament nucleators that influence growth patterns exist at the approximately 1- to 100-nm spatial scale. Plant cells are quite large, and individual cells and specialized subregions of cells exist at the approximately 10- to 100-μm scale. Actin filaments polymerize and reorganize rapidly to span these disparate spatial scales, and the cytoplasm is partitioned such that immediately adjacent regions contain distinct cytoskeletal structures. On a temporal scale, individual actin filaments are remarkably dynamic with half-lives on the order of tens of seconds (Staiger et al., 2009; Smertenko et al., 2010; Augustine et al., 2011; Qu et al., 2013; Zheng et al., 2013). In hypocotyl epidermal cells, for example, growth at filament plus ends approaches 2 μm s−1, and disassembly occurs through prolific severing activity (Staiger et al., 2009). In pollen tubes, however, comparatively modest filament growth rates of <1 μm s−1 are balanced by nearly equal rates of depolymerization at filament minus ends (Qu et al., 2013; Zheng et al., 2013). These dynamic single actin filaments coexist with longer-lived, more stable actin filament bundles in the same regions of cytoplasm. Moreover, myosin-driven filament translocation also plays a prominent role in shaping individual filaments and modulating network architecture (Staiger et al., 2009; Smertenko et al., 2010; Augustine et al., 2011; Cai et al., 2014). Plant cell expansion tends to be slow in comparison to these rates of actin turnover. Tip-growing cells elongate at rates that vary from approximately 1 μm min−1 to a few μm h−1 (Stephan, 2017), and the strain rates of cells that use a diffuse growth mechanism vary from a few percent h−1 to 30% h−1 (Rahman et al., 2007; Zhang et al., 2011). How are different actin networks assembled and disassembled at the extended time scales necessary to pattern the cell wall, and how are specific actin networks constructed locally to carry out specific tasks?
Rapid progress is being made in the actin field because many labs are taking an interdisciplinary approach to quantitatively analyze the actin cytoskeleton and morphogenesis. Genetic screens provide the necessary collections of mutants and molecular genetic tools to create increasingly realistic models for cell shape control. Sophisticated imaging-based assays generate real-time data on how cytoskeletal proteins affect individual actin filaments in vitro (Henty-Ridilla et al., 2013) and how actin networks change over time in vivo (Li et al., 2015; Breuer et al., 2017). Last and perhaps most importantly, the research community is more broadly using multivariate live-cell imaging to analyze cytoskeletal, endomembrane, and cell wall mechanical properties as a function of growth. This approach was pioneered for pollen tube cell biology (Cárdenas et al., 2006), and is now being used to analyze other tip-growing cells (Furt et al., 2013) as well as polarized epidermal cell types that use a diffuse-growth mechanism (Yanagisawa et al., 2015). This review will focus on actin-based morphogenesis at the systems level and on recent work that helps to place specific actin assemblies into a functional context during tip growth and polarized diffuse growth.
ACTIN-BASED CONTROL OF TIP GROWTH
Tip growth is a morphogenetic strategy adopted by pollen tubes, root hairs, and the filamentous growth stage of moss (Physcomitrella patens) protonemata. A genetic characterization of tip growth in rhizoids of a nonvascular plant revealed broadly conserved mechanisms for tip growth (Honkanen et al., 2016). Tip growth has been the topic of numerous comprehensive reviews (Ren and Xiang, 2007; Rounds and Bezanilla, 2013; Stephan, 2017). Briefly, tip growth is a strategy by which an axially symmetric cell establishes a tube-like protuberance that maintains a roughly hemispherical geometry at the cell apex as the cell elongates. New cell wall materials are selectively delivered to the apical domain (Shaw et al., 2000; Menand et al., 2007; Rojas et al., 2011), and, during growth, secreted material at the apex is displaced toward the cell flank. Although the cell geometry at the apex is more or less maintained during elongation, the direction of growth can change in response to a guidance cue, and during this change the cytoplasm and the cell wall are reorganized to generate a different growth axis.
A highly specialized and subfunctionalized cellular organization is associated with the cell apex, and this cytoplasmic organization has strict requirements for several different types of actin filament arrays (Stephan, 2017). At the spatial scale of the entire cell, one function of the actin cytoskeleton is to provide spatially organized filaments and bundles that define the overall intracellular flow patterns. Not all tip-growing cells have rapid and directional organelle transport that underlies cytoplasmic streaming; however, for those that do, the streaming often takes on a reverse-fountain pattern of particle movement (Shimmen and Yokota, 2004). This flow pattern is powered by myosin-dependent transport of multiple types of vesicles and/or organelles along actin filament tracks. For additional information on the function and regulation of plant myosins, please see the Ryan and Nebenführ Update in this issue (Ryan and Nebenführ, 2018). Actin filaments have an intrinsic polarity, as asymmetric G-actin subunits add preferentially to the growing plus end of the filament and ATP hydrolysis lags behind assembly. Plant genomes encode class VIII and XI myosins (Reddy and Day, 2001; Geitmann and Nebenführ, 2015). Myosins XI are actin-based motors that move exclusively in the direction of the filament plus end (Tominaga et al., 2003) and are implicated in long-distance intracellular transport and cell expansion (Geitmann and Nebenführ, 2015). Myosin mutants have strong phenotypes in tip-growing cells that stream (Ojangu et al., 2007; Peremyslov et al., 2008; Prokhnevsky et al., 2008) as well as those that don’t (Vidali et al., 2010). Actomyosin transport and the rate at which the cytoplasm moves in vacuolated cells appear to be rate limiting for plant growth, since plants expressing engineered chimeric myosins with increased velocity have faster rates of cytoplasmic streaming, enhanced axial cell expansion, and increased stature (Tominaga et al., 2013). On the other hand, several studies suggest that local actin architecture and finely tuned cytoplasmic organization are more important than general cytoplasmic stirring. Specifically, tip growth and a particular actin array in the apex are sensitive to nm concentrations of LatB, whereas bulk streaming and actin filament bundles are only perturbed at much higher concentrations (Gibbon et al., 1999; Vidali et al., 2001).
ASSEMBLY OF ACTIN NETWORKS THAT SPECIFY CYTOPLASMIC ZONATION AND CELLULAR FLOW PATTERNS
Given that most myosins are plus-end-directed motors, the directionality of cytoplasmic flow is defined by the orientation of actin filaments. Flow patterns in tip-growing pollen tubes appear to be choreographed by two actin filament arrays: a cortical collar or fringe of actin filaments oriented toward the cell apex and axial bundles of actin filaments in the shank (Fig. 1, left images). The cortical collar or fringe is an unstable filament array (Gibbon et al., 1999) that spans from the cell flank to the subapex proximal to the cell (Lovy-Wheeler et al., 2005). Actin polymer assembly and disassembly allow the array to maintain its position relative to the cell apex, and its importance during tip growth has been established in many tip-growing cell types (Stephan, 2017). The precise geometry of actin filaments in the cortical cytoplasm is not known, but based on the plus-end-directed movement of plant myosins (Tominaga et al., 2000) in cells with a reverse-fountain pattern of intracellular flow, the filament plus ends in the collar and cortical filaments are likely oriented toward the apex and those within core shank bundles are predicted to have their plus ends oriented away from the apex. Computational simulations of actomyosin flow in pollen tubes indicate that the orientation and localization of the collar and shank filaments are sufficient to generate observed flow patterns and an accumulation of vesicles in an inverted cone (Kroeger et al., 2009), and this is supported by experimental data in which the orientation of filaments in a tip-growing pollen tube were examined directly (Lenartowska and Michalska, 2008).
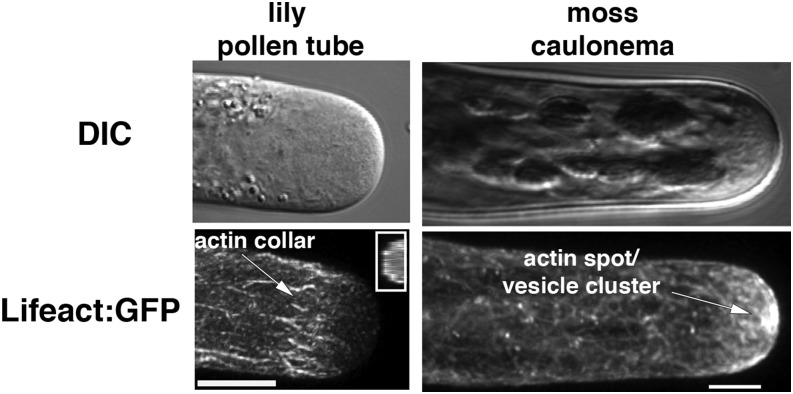
Figure 1.
Actin organization in living tip-growing lily pollen tubes (left) and moss caulonema (right) detected with the Lifeact:GFP actin-binding probe. Top images are differential interference contrast (DIC) images of the cell and the bottom images are the same cell imaged for Lifeact:GFP fluorescence. The cortical actin collar or fringe is indicated with an arrow; a resliced view showing its cortical location is shown in the inset. The filaments in the cortical actin collar are predicted to have their plus ends oriented toward the apex. Actin bundles in the core cytoplasm in the shank are oriented with their plus ends away from the cell tip. The dense meshwork of actin filaments focused into an actin spot at the apex of moss caulonema is labeled in the lower right image. Bars = 5 μm. Figure is adapted with permission from Vidali et al. (2009a).
The actin collar or fringe does more than just define local flow patterns. It establishes a sharp boundary for cytoplasmic organization, in which organelles such as ER, Golgi, and mitochondria are transported toward but diverted from the vesicle-rich clear zone at the extreme apex (Lovy-Wheeler et al., 2007). Using time-lapsed imaging and a flow cell system, the Hepler group showed that selective removal of the actin fringe with low concentrations of LatB disrupted Ca2+ oscillations and the positioning of the alkaline band that are needed for polarized growth (Cárdenas et al., 2008). Conversely, perturbing cellular pH alters the stability and location of the actin fringe, and this interdependence between actin organization and alkaline band is postulated to depend upon the pH-sensitive protein Actin-Depolymerizing Factor (or ADF; Lovy-Wheeler et al., 2006). The actin fringe may also define a distal diffusion boundary for plasma membrane specialization that confines ROP small GTPase signaling within the extreme apex (Kost, 2008).
An ongoing and important research challenge is to determine which cytoskeletal proteins are responsible for the assembly and maintenance of different subcellular arrays. Genetic approaches are providing important inroads. Assembly of actin filaments from monomeric actin, or profilin-actin complexes, is governed by actin filament nucleators such as formins and the actin-related protein 2/3 (ARP2/3) complex. Networks of actin filament are generated by ABPs that cross-link and bundle actin filaments, and still others that sever, destabilize, or protect actin filaments (for review, see Li et al., 2015). Forward- and reverse-genetic approaches have identified conserved cytoskeletal proteins and novel plant-specific proteins that are required for normal tip growth. Determining the precise function of these ABPs in general is not easy. First, the organization of the actin arrays and growth are interdependent, so it is a challenge to distinguish between direct and indirect effects of the mutations on the actin network. Second, cytoskeletal proteins frequently localize to multiple actin networks (Wu et al., 2010), different organelle surfaces (Vidali et al., 2009b; Cheung et al., 2010; van Gisbergen et al., 2012; Zhang et al., 2013), and traffic through the endomembrane system (Rounds et al., 2014). Time-lapsed analysis of actin and ABPs using purified components in vitro (Michelot et al., 2005; Vidali et al., 2009; Khurana et al., 2010; Henty et al., 2011) and two-color live-cell imaging of ABPs and actin in vivo is helping to identify active pools of ABPs and where they function (van Gisbergen et al., 2012; Furt et al., 2013; Yanagisawa et al., 2015; Li et al., 2017).
Formins are evolutionarily conserved filament nucleators and processive elongation factors, and are implicated in Arabidopsis (Arabidopsis thaliana), tobacco (Nicotiana tabacum; Cheung et al., 2010), moss (Vidali et al., 2009), and lily (Lilium longiflorum; Li et al., 2017) as central regulators of the cortical actin system at the plasma membrane in tip-growing cells. However, these proteins also colocalize with actin most prominently in a large vesicle-rich zone at the extreme apex (see below). The Arabidopsis class I formin AtFH5 was the first to be characterized in terms of localization and actin network formation in pollen tubes (Cheung et al., 2010). The lily pollen tube has distinct advantages for protein localization because the apical fringe is so prominent, with axially aligned filaments around the tube circumference that are precisely positioned relative to the apex (Lovy-Wheeler et al., 2005; Vidali et al., 2009). Knockdown of lily FORMIN1 (LlFH1), which is a class I formin with a signal sequence and a membrane-spanning domain, specifically reduced F-actin in the tube apex and the actin fringe was disassembled; however, there was no effect on the aligned bundles in the pollen tube shank (Li et al., 2017). LlFH1 possesses nucleation activity in vitro, and the GFP-tagged fusion protein colocalized with actin as well as the apical vesicle cluster and the plasma membrane. Interestingly, when the apical pool of LlFH1 was bleached in fluorescence recovery after photobleaching (FRAP) experiments, the first location to recover was at or near the plasma membrane at the flank of the subapex coincident with the leading edge of the actin fringe. The simplest explanation is that LlFH1 is a processive nucleator that generates filaments within the fringe with their plus ends oriented toward the apex. These results are generally consistent with those originally obtained with AtFH5 (Cheung et al., 2010).
Formins utilize profilin-actin complexes to processively assemble actin filaments at their plus ends. They also overcome the profilin-mediated suppression of spontaneous nucleation from the actin monomer pool (Michelot et al., 2005). In Physcomitrella, profilin is essential for tip growth, and cells lacking profilin have an altered apical actin organization (Vidali et al., 2007). Specifically, the cortical actin organization in the apex is disrupted, actin filaments appear disorganized, and polarized cortical patches become prominent. These findings suggest that formin and profilin cooperate to generate unique actin arrays that support tip growth.
The actin fringe dynamically tracks the growing tip, and its stability is likely influenced by ABPs that either stabilize or destabilize the network. Fimbrins are conserved filament cross-linking and bundling proteins (Thomas, 2012). Lily FIMBRIN1 (LlFIM1) localizes to the actin fringe, and disruption by antibody injection perturbs tip growth and fringe organization (Su et al., 2012). Fimbrins stabilize and bundle actin filaments and LlFIM1 appears to be conserved because a GFP fusion rescued the growth and actin organization defects associated with an Arabidopsis fim5 mutant (Su et al., 2012). A FIM5-EGFP fusion colocalized to actin bundles in the Arabidopsis pollen tube apex and shank (Zhang et al., 2016), which is consistent with latrunculin hypersensitivity, reduced actin bundling, and randomized cytoplasmic streaming patterns that had been reported previously (Wu et al., 2010). FIM5 appears to stabilize actin filaments, but given its localization to both long-lived actin bundles in the shank and less stable bundles in the fringe, it will be important to discover how FIM5 decoration of the network is coordinated and how it affects filament stability of different networks (Zhang et al., 2016). Individual filaments in the apex of fim5 pollen tubes have reduced elongation and depolymerization rates, are longer lived, but undergo more extensive buckling and waving than observed in wild-type tubes, all phenotypes consistent with a role in destabilizing cortical actin in the fringe region (Zhang et al., 2016). Actin filament turnover can also be promoted by actin-severing proteins. Villins are Ca2+-dependent bundling and/or severing proteins (Huang et al., 2005; Khurana et al., 2010). Arabidopsis VILLIN2 (VLN2) and VLN5 are required to maintain pollen tube diameter during elongation. The vln2 vln5 double mutants have an overproliferation of actin in the tubes with increased lifetimes of individual filaments (Qu et al., 2013).
ADF family members are best known as actin filament severing and destabilizing proteins. One such example is Arabidopsis ADF7, whose disruption leads to perturbation of tip growth, excessive actin filament bundles, and long-lived individual filaments with reduced severing and depolymerization (Zheng et al., 2013). A functional ADF7-EGFP fusion protein localizes along all actin filaments, suggesting that it facilitates turnover of all pollen arrays (Zheng et al., 2013). In tobacco pollen, on the other hand, a GFP-ADF reporter appears to be quite efficient at revealing the subapical actin structures (Cheung et al., 2010). Interestingly, Arabidopsis has two ADF family members, AtADF5 and AtADF9, that have simple bundling function rather than severing activity (Tholl et al., 2011; Nan et al., 2017). The adf5 mutant has pollen with germination and tip growth defects as well as perturbed actin dynamics; specifically, filament bundling frequency is reduced by 50% (Zhu et al., 2017). Similarly, disruption of ADF in Physcomitrella inhibits tip growth and alters actin organization (Augustine et al., 2008).
Actin bundles in the pollen tube shank are long lived and in cells with a reverse-fountain streaming pattern. Bundles in the core cytoplasm consist of filaments with their plus ends facing away from the cell tip (Lenartowska and Michalska, 2008). The source of these organized bundles is not entirely clear. One proposed mechanism is that the actin filament nucleator AtFOR3 polymerizes actin bundles in the shank (Ye et al., 2009). Formins certainly have the potential to generate actin bundles as they can both nucleate and bundle actin filaments (Michelot et al., 2005). The localization of active FOR3 in pollen tubes or root hairs has not been determined. It is also possible that actin filaments from the apical domain are transported by cytoplasmic flow into the shank regions and incorporated into bundles. For example, severed actin filaments flow away from the cell apex, coalesce into a subapical basket, and move toward the pollen tube shank (Cheung et al., 2010). The question would then be, how are these filaments assembled into functional bundles with aligned filaments? This likely requires the cooperative activity of bundling proteins, like fimbrin and villin (Wu et al., 2010; Qu et al., 2013), and potentially the inhibition of ADF activity through competition with bundlers (Huang et al., 2005). Myosin XI molecular motors also influence filament dynamics and array organization in diverse Arabidopsis cell types (Ueda et al., 2010; Park and Nebenführ, 2013; Cai et al., 2014), and a myo11c1 myo11c2 double mutant has less organized actin filament bundles in the shank of pollen tube with significantly reduced parallelness (Madison et al., 2015). Clustering of myosin motors at the base of the clear zone in tobacco pollen tubes may locally organize and/or transport actin filaments toward the shank (Stephan et al., 2014).
The fringe and shank constitute key elements that organize the cytoplasm and dictate the organelle and hydrodynamic flow patterns in the cell (Stephan, 2017). However, the endgame of tip growth is to selectively deliver and recycle vesicles at the apex to support sustained directional cell expansion. The next section, drawing primarily from the moss system, will focus on the role of actin in clustering vesicles at the apex and local secretion.
ACTIN NETWORKS ASSOCIATED WITH VESICLE CLUSTERING AND SECRETION
The raw materials for tip growth, which have been shown to primarily consist of pectin polysaccharides in growing pollen tubes (Li et al., 1994; Bosch and Hepler, 2005; Fayant et al., 2010; Rounds et al., 2011), are delivered by secretory vesicles that accumulate in the apex of tip-growing cells. Cell types such as root hairs and pollen tubes have a distinct clear zone in the shape of an inverted cone that is enriched in secretory vesicles (Picton and Steer, 1983; Lancelle et al., 1987; Preuss et al., 2004). Tip-growing caulonema cells of the moss P. patens do not have an organized pattern of cytoplasmic streaming, but a dense cluster of secretory vesicles is maintained near the apex (Furt et al., 2013; Bibeau et al., 2018). This vesicle cluster colocalizes with a dense meshwork of apical actin, commonly referred to as r the “actin spot” (Fig. 1, right images). The apical actin cluster localizes to and predicts the direction of growth. The actin spot in caulonema is positive for a protein marker for secretory vesicles but may contain endocytic vesicles as well. In tip-growing pollen tubes, the inverted cone and clear zone contain both anterograde secretory vesicles and organelles that receive membrane from an endocytic retrograde pathway (Grebnev et al., 2017). In pollen tubes, the vesicles in the clear zone are not completely depleted and replaced by the Golgi during each growth oscillation. For example, FRAP analysis of vesicle turnover rate as a function of growth rate and cell wall demand indicate that, on average, vesicles make multiple rounds of transport into and out of the clear zone prior to plasma membrane fusion and secretion events (Bove et al., 2008).
Vesicle clustering at the apex is important because it creates a high local concentration of organelles that are poised, in terms of their location and biochemical composition, for secretion. As discussed above, depolymerization of actin leads to loss of normal cytoplasmic organization in the apex of tip-growing cells, often accompanied by an invasion of the vacuole into the space previously occupied by the clear zone. One mechanism to cluster vesicles is to decorate the membrane surface with actin filament nucleators and myosin motors (Schuh, 2011). Myosin motors on one vesicle may move processively on an actin filament with its plus end anchored at the surface of an adjacent vesicle to promote vesicle clustering (Furt et al., 2013; Bibeau et al., 2018). The formin class of actin filament nucleators fit well within this model because they could processively nucleate actin filaments with the plus end of the actin filament oriented toward the membrane. Indeed, examples of class II formin-mediated actin polymerization in the apical cytoplasm of Physcomitrella tip-growing cells appear to propel a subset of vesicle movements at rates of nearly 2 μm s−1 (van Gisbergen et al., 2012). Class I formins from Arabidopsis (Cheung et al., 2010) and lily (Li et al., 2017) with a transmembrane domain and a class II formin from moss (Vidali et al., 2009; van Gisbergen et al., 2012) localize to the vesicle-rich zone in the apex. Lily formins appear to nucleate actin filaments in the clear zone because they overlap with actin at that location (Li et al., 2017). Blocking secretion with BFA in growing lily pollen tubes generates an intense apical spot of actin polymerization in the tube apex (Rounds et al., 2014), and this may reflect the activity of class I formins that are not delivered to the plasma membrane. Similarly, following washout of LatB, formation of an apical cluster of LlFH1 vesicles precedes accumulation of an actin focal site in the tip (Li et al., 2017).
Myosin decorates secretory vesicles at the apex in moss caulonema (Furt et al., 2013) and in Arabidopsis root hairs (Peremyslov et al., 2012). Myosin mutants have severely impaired tip growth (Vidali et al., 2010; Park and Nebenfuhr, 2013; Madison et al., 2015). In moss caulonema cells, myosin accumulates on secretory vesicles in an actin-independent manner, and cross-correlation analyses indicate that myosin accumulation precedes maximal actin polymerization in the vesicle cluster by about 18 s (Furt et al., 2013). A recent quantitative FRAP analysis of vesicle dynamics in moss caulonema defined the geometry of the apical target zone for secretion and threshold requirements for vesicle density that are needed to support cell wall matrix secretion during growth (Bibeau et al., 2018). This study pointed to the importance of the actin cytoskeleton in maintaining local high concentrations of vesicles in order to overcome diffusion barriers to vesicle movement during tip growth.
The coupling of myosin to vesicle and organelle cargo through adapter proteins (Li and Nebenführ, 2007; Peremyslov et al., 2013; Stephan et al., 2014) is an important but poorly understood early step in the tip growth process. Once myosins are clustered on vesicle surfaces, they have the potential to bind filaments that emanate from adjacent organelles and promote clustering as they move processively toward the filament plus end. This is a robust method to organize vesicles at µm-spatial scale in the clear zone. In moss, the ARP2/3 complex is also clustered at the apex in a pattern that resembles the apical vesicle cluster (Harries et al., 2005; Perroud and Quatrano, 2006, 2008). Perhaps ARP2/3 generates branched actin networks within the vesicle cluster to provide a mechanism to reversibly stabilize the vesicle cluster or define functionally specialized subdomains within it. Nonetheless, since actively growing pollen tubes and root hairs don’t have an equivalent apical actin spot, generalizing these mechanisms should be approached with caution.
There is evidence that dynamic actin filaments in the extreme apex determine the local pattern of pectin secretion (Rounds et al., 2014); however, this population is the most difficult to analyze because it appears to consist of loosely organized, short-lived individual actin filaments (Cheung et al., 2010; Zhu et al., 2013; Zhang et al., 2016). It is possible that relatively few apical actin filaments are needed to locally increase the concentration of a cluster of vesicles so that other short-range control mechanisms for vesicle fusion can orchestrate the final steps of vesicle fusion. For example, cells possess a diverse collection of vesicle tethering complexes (Vukašinović and Žárský, 2016) that may operate in concert with small GTPase signaling cascades at the plasma membrane to more precisely control the timing and location of secretion.
Class I formins are present at the plasma membrane of the growing apex, and, in moss, a class II formin uses a novel PTEN lipid-binding domain to localize FORMIN2A to the plasma membrane of protonemal cells (van Gisbergen et al., 2012). Actin dynamics in the apex are likely to be strongly affected by calcium and pH gradients. For a more detailed analysis of the importance of microtubules and the effects of ions on the actin network during tip growth please, see the Bascom et al. Update in this issue (Bascom et al., 2018). A new class of dual-function cytoskeletal proteins have been characterized recently during tip growth. Microtubule-associated protein 18 (MAP18)/PCAP2 (Wang et al., 2007) and MAP25/PCAP1 (Li et al., 2011) were first identified as microtubule-destabilizing proteins and were subsequently shown to sever actin filaments in a Ca2+-dependent manner. MAP18 mutant pollen tubes had a meandering phenotype and an overabundance of actin filaments in the apex (Zhu et al., 2013). MAP18 also binds selectively to inactive (GDP-bound) forms of ROP GTPases, and appears to compete with ROP GDP-dissociation-inhibitor (ROP-GDI) for ROP binding and increase the pool of plasma-membrane localized ROP that is in play to pattern tip growth (Kang et al., 2017). MAP18 may provide a way to learn how ROP signaling and actin reorganization are integrated during growth.
ACTIN FILAMENT NUCLEATION AND THE PATTERNING OF DIFFUSE GROWTH
In the final section of this Update, our discussion shifts to a brief analysis of the functions of actin during diffuse growth, focusing on recent developments in the Arabidopsis leaf hair system. Diffuse growth is the mechanism for cell expansion for the vast majority of plant cell types. Like tip growth, turgor pressure and the resulting cell wall tension forces drive cell expansion. Unlike tip growth, increases in cell area are broadly distributed across the cell surface. However, diffuse growth is not isotropic: Mechanical heterogeneities in the cell wall and the geometry of the cell generate anisotropic stress-strain behaviors that produce a variety of cell shapes and sizes during plant development. See the Cosgrove Update in this issue for a detailed update of the mechanisms of diffuse growth (Cosgrove, 2018).
During diffuse growth, cell wall synthesis and vesicle secretion are coupled with growth to maintain cell wall thickness within a factor of about 2 as cell volume increases. Therefore, the microtubule and actin cytoskeletons function as part of a system in which the spatial and temporal dynamics of the cytoskeleton, endomembrane, and cell wall are coordinated during growth. The organization and control of the cortical microtubule cytoskeleton is the topic of a separate Update in this issue (Elliott and Shaw, 2018). The importance of actin during diffuse growth is indisputable based on the detrimental effects of actin polymerization inhibitors and mutations in ABPs on all aspects of plant growth and development. The possible functions of actin during diffuse growth have been reviewed previously with respect to actin serving as a track for localized secretion (Smith and Oppenheimer, 2005; Hussey et al., 2006) and the possibility that polarized diffuse growth is a composite of diffuse growth and tip growth (Wasteneys and Galway, 2003). Similar to tip-growing cells, actin arrays in diffusely expanding cells typically comprise both dynamic single filaments and filament bundles (Staiger et al., 2009; Smertenko et al., 2010); however, these arrays are often comingled and often do not display specific orientations with respect to sites of growth. Thus, it has proven to be extremely difficult to ascribe specific functions to particular actin filament arrays during diffuse growth.
There are several additional factors that complicate functional analyses of actin networks. First, microtubules clearly have the ability to pattern cellulose microfibrils through coordinating the dynamic behavior of plasma membrane cellulose synthase complexes (Paredez et al., 2006) and thereby influence cell wall anisotropy. The actin system can influence microtubule behaviors (Sampathkumar et al., 2011) and the delivery of cellulose synthase to the plasma membrane (Sampathkumar et al., 2013); however, the exact mechanisms remain poorly understood. Actin may play a more direct role in secretion of noncellulosic polysaccharides, as it affects the secretion of noncellulosic polysaccharides that form part of the cell wall matrix and the middle lamella (Leucci et al., 2007). In leaf hairs, ARP2/3-generated actin filaments are needed for broadly distributed and balanced cell wall assembly during growth so that cell wall thickness gradients are maintained along the length of the elongating trichome branch (Yanagisawa et al., 2015). It is often accepted that fine networks of cortical actin filaments mediate the localized delivery of secretory vesicles (Fu et al., 2002). It may prove true that individual actin filaments guide vesicles to precise locations in the cortex in response to some localized cue. However, in living cells that use a diffuse growth mechanism, the cortical actin cytoskeleton is highly unstable, and in general its arrangement is poorly correlated with cell shape. Even in the case of leaf trichomes, which have a very specific requirement for ARP2/3-generated actin filaments (Le et al., 2003; Mathur et al., 2003), it has been very difficult to determine which actin arrays are affected in the mutant and what is their cellular function. Furthermore, the patterns of secretion during polarized diffuse growth are often inferred based on static images of growing cells. In reality, during diffuse growth, the material delivery budget for the wall will vary depending on cell geometry, growth rate, growth variability within the cell, and cell wall thickness (which also varies within the cell). Therefore, there is a strong need to more broadly analyze the spatial and temporal behaviors of the actin cytoskeleton and secretion as they relate to measured patterns of growth.
ARP2/3 IS ACTIVE IN A MICROTUBULE-DEPLETED ZONE IN THE TRICHOME BRANCH APEX
A major unanswered question is how information from the plasma membrane and the cell wall is relayed to the cytoplasmic actin bundle roadways so that they can rearrange in response to the mechanical requirement for more cell wall materials. No such wall-sensing protein that controls morphogenesis has been identified in plants. During trichome branch morphogenesis, a microtubule-depletion zone at the cell apex scales with the constricting radius of curvature of the cell wall at the apex (Yanagisawa et al., 2015). This also corresponds to the location where ARP2/3 is activated and reflects a specialized cellular domain in which information about the geometry of the cell wall is somehow sensed by cytoskeletal systems.
The evolutionarily WAVE-SCAR regulatory complex (W/SRC)-ARP2/3 conserved actin filament nucleation pathway is comprised of the heteromeric W/SRC and ARP2/3 complexes. W/SRC converts activating ROP-GTPase signals into an ARP2/3-dependent actin polymerization response (Szymanski, 2005; Stradal and Scita, 2006). In Arabidopsis, the DOCK family guanine nucleotide exchange factor SPIKE1 transmits activating ROP-GTP signals to the W/SRC (Qiu et al., 2002; C. Zhang et al., 2010). Although the pathway was elucidated in plants primarily based on the leaf trichome phenotype of the “distorted group” (W/SRC and ARP2/3) mutants, these protein complexes seem to be present in all plant species and are broadly used during root and shoot development (Frank and Smith, 2002; Mathur et al., 2003; Harries et al., 2005; Miyahara et al., 2010; Bai et al., 2015; Facette et al., 2015).
Trichome morphogenesis is an example of highly polarized diffuse growth based on the geometry of the shape change (Szymanski et al., 1999) and the measured strain behavior of the cell wall using cell wall bound particles as fiducial marks (Schwab et al., 2003; Yanagisawa et al., 2015). The distorted group mutants resemble wild-type trichomes that have been treated with actin-disrupting drugs (Mathur et al., 1999; Szymanski et al., 1999): Branch elongation and tapering are reduced, and the cell swells and twists in an unpredictable manner. Surprisingly, the actin phenotype of ARP2/3 null mutants is quite subtle and has only recently been clearly defined (Yanagisawa et al., 2015). For more than 10 years, the most reliable actin phenotype was a quantitative actin bundle mispositioning defect in the core cytoplasm of trichomes at the early stage of branch elongation (Le et al., 2003; Deeks et al., 2004; El-Assal et al., 2004; Zhang et al., 2005; Le et al., 2006; Sambade et al., 2014). Subsequently, live-cell probes for W/SRC subunits were shown to localize to the extreme apex of developing branches (Dyachok et al., 2008); however, the existence of tip-localized actin and its possible function were not addressed.
The existence and organization of tip-localized actin has been difficult to nail down. Our group published scattered evidence for a dense cortical actin meshwork at the extreme apex of fixed, detergent-extracted cells labeled with antibodies (Le et al., 2003; see Fig. 2, A and B) and phalloidin (Basu et al., 2004; Fig. 2C). However, this meshwork was not always present, and we did not analyze this structure or comment on its existence. Transverse actin filaments have been highlighted near the trichome apex (Zhang et al., 2005; Djakovic et al., 2006; Tian et al., 2015); however, previous studies showed that they can be detected in both wild-type and ARP2/3 mutant backgrounds (Zhang et al., 2005; Djakovic et al., 2006). The transverse actin filaments have been proposed to be of functional significance because the kinesin class of microtubule motor ZWICHEL/KCBP is required for branch tapering, and its N-terminal FERM domain can associate with actin filaments in vitro (Tian et al., 2015). However, the FERM domain is dispensable for ZWICHEL/KCBP function (Tian et al., 2015).
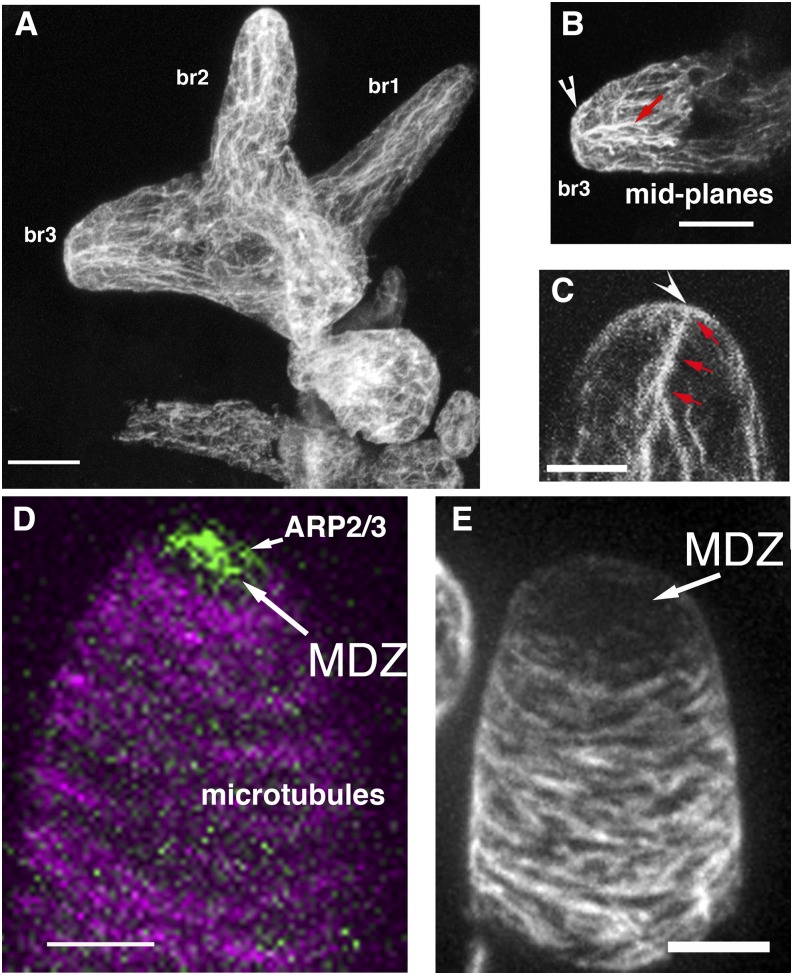
Figure 2.
Actin and microtubule organization in Arabidopsis leaf hairs and cotton fibers during the process of cell elongation and tapering. A, Whole mounted trichome with branches that are becoming progressively tapered (defined as stage 4). The cell is labeled with an anti-actin antibody using the freeze-shattering technique. Note prominent tip actin in branches 2 (br2) and 3 (br3). B, Midplanes of br3 in A showing cortical actin at the apex and cytoplasmic bundles that are oriented toward the apical meshwork. C, Whole mounted trichome labeled with phalloidin. Apical actin meshwork and cytoplasmic bundles are labeled as in B. D, Live-cell image of GFP-tagged ARP2/3 complex (green) and microtubules (magenta). ARP2/3 localizes within the apical microtubule-depletion zone (MDZ) and is required to polymerize the apical actin meshwork. E, Whole mounted 1-DPA cotton fiber in the process of cell tapering labeled with an anti-α-Tubulin antibody. Bars = 5 μm.
A detailed genetic and cytological analysis of the microtubule and actin systems at the early stages of trichome development described an “actin cap” in 50% of the emerging branches that was reduced to 20% in an arp2/3 mutant (Sambade et al., 2014). The actin cap was a broad apical domain extending 20 μm distal to the branch apex. The cap was defined based on increased cytoplasmic and cortical signal intensity of GFP-fABD2 along a longitudinal projection of the branch (Sambade et al., 2014). These signals could reflect differences in cytoplasmic density among branches rather than specific actin localization patterns. In our hands, we cannot reliably detect tip actin with GFP-fABD2 or GFP-Lifeact. One explanation is that this actin array is not accessible to the actin-binding live-cell probes. Direct fusions of GFP to actin could solve this technical issue. However, in fixed cells, we consistently detect cortical tip actin at the extreme apex (extending only a few microns back from the branch tip) in approximately 60% of the young branches (Yanagisawa et al., 2015). Although these localization data were obtained with fixed cells, they appear to be reliable because the tip actin meshwork colocalizes with active ARP2/3 and is undetectable in an arp2/3 null background (Yanagisawa et al., 2015). Interestingly, ARP2/3 and its associated actin meshwork are contained entirely within an apical microtubule-depletion zone that becomes progressively constricted as the branch tapers (Yanagisawa et al., 2015; Fig. 2D). A cartoon representing the location and activity of ARP2/3 in a young trichome branch that is becoming tapered is shown in Figure 3.
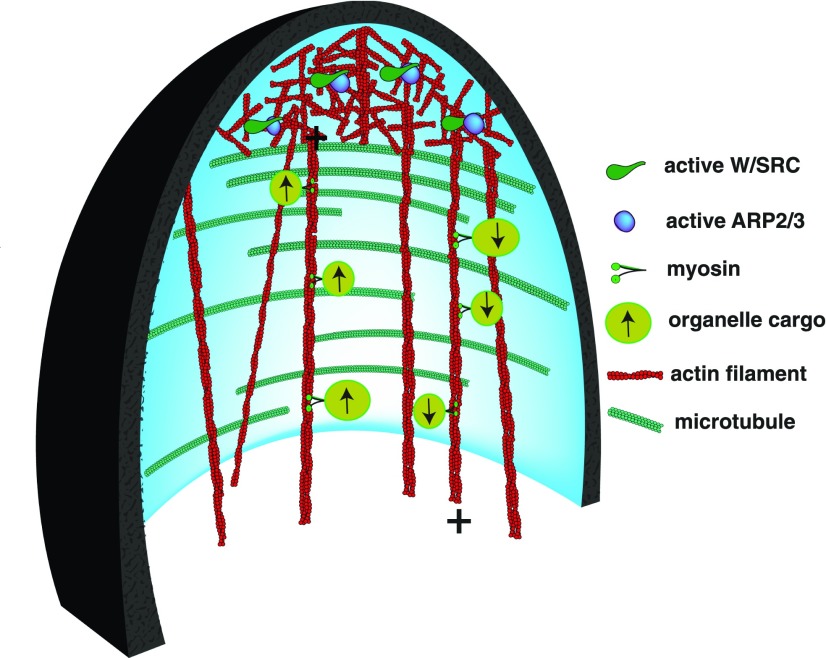
Figure 3.
ARP2/3-dependent cytoplasmic organization in a developing trichome branch. The cartoon is a view of the cytoplasm from a medial longitudinal section through the young branch. In response to ROP-GTP signals, the W/SRC complex (green) physically interacts with and activates ARP2/3 (blue ellipse). ARP2/3 generates branched networks of actin filaments (red). ARP2/3 activation is restricted to an apical zone that has a reduced density of cortical microtubules. Aligned microtubules (cyan) along the cell flanks promote highly anisotropic cell elongation. Actin bundles orient longitudinally in the core cytoplasm and terminate at or near the apical actin meshwork. A subset of the actin bundles is composed of parallel actin filaments with their plus ends (marked with +) oriented either toward or away from the cell apex. These bundles act as roadways for directional actomyosin transport. The symbols representing the myosin motors, the organelle cargo, and their direction of movement are defined in the figure. The plasma membrane is shaded light blue, and the cell wall thickness and its longitudinal thickness gradient are represented in black.
ARP2/3-GENERATED APICAL ACTIN MESHWORKS ORGANIZE INTRACELLULAR TRANSPORT AT A CELLULAR SCALE
These live-cell imaging and genetic approaches in Arabidopsis made it possible to analyze the functional importance of ARP2/3-generated actin filaments during highly anisotropic diffuse growth. A top-down finite element (FE) computational modeling approach and multivariate live-cell imaging were used to determine the functional importance of the apical microtubule-depletion zone and the ARP2/3-generated actin meshwork (Yanagisawa et al., 2015). In this approach, long-term time-lapsed analyses of trichome shape change and subcellular strain patterns were used to create a realistic FE model of the growing trichome branch. The FE model treats the cell as a thin-walled pressurized shell, the material properties of which can be varied in order to identify parameters that might define the growth behaviors of the cell. The model made specific predictions of how three cell wall parameters—fiber alignment along the branch flank, a longitudinal cell wall thickness gradient, and an isotropic patch within the microtubule-depletion zone—could generate the observed pattern of branch elongation coupled with cell tapering. Similar morphological transitions occur in early cotton (Gossypium hirsutum) fiber development (Butterworth et al., 2009) and in conical cells of the flower petal epidermis (Kramer and Irish, 1999). A similar microtubule-depletion zone is easily detected at the apex of young-stage cotton fibers that are in the process of tapering (Fig. 2E).
The FE model predictions were validated using multivariate live-cell imaging in which the localization and dynamics of microtubules, actin, ARP2/3, cell wall thickness, and cytoplasmic flow patterns were analyzed as a function of shape change (Yanagisawa et al., 2015). The ARP2/3 system did not affect the transverse organization of microtubules or cellulose microfibrils at early stages, as shown previously (Basu et al., 2005). However, ARP2/3 can associate with microtubules (Havelková et al., 2015) and influence microtubule-dependent cell twisting during the later stages of cell elongation (Zhang et al., 2005; Sambade et al., 2014). The ARP2/3 mutants failed to generate or maintain a wall thickness gradient (Yanagisawa et al., 2015). This does not simply reflect a failure to deliver material to the cell apex. The calculated wall synthesis budget for the trichome branch, based on its geometry, strain distribution, and a decreasing cell wall thickness from base to tip, is a broad pattern of delivery with a very shallow tip-biased gradient. Although there may be specialized trafficking to the extreme apex, the intracellular organization must support nearly uniform vesicle supply to the cortex. Two-color live-cell imaging of actin bundles and Golgi cargo indicated that the apical actin meshwork is required to position actin roadways that support bidirectional transport of organelles along the cell axis and expose the entire cortex to actomyosin-dependent organelle transport (Yanagisawa et al., 2015). This model is consistent with the distorted trichome phenotype of MYOSIN XI mutants (Ojangu et al., 2012). The mechanism by which the apical meshwork organizes bundles that are both tip- and base-directed is not known. However, these results clearly showed that the clustering and activation of ARP2/3 within a discrete microtubule-depleted apical domain has the ability to influence cytoplasmic organization at extended spatial scales that influence whole-cell behaviors (Fig. 3).
In expanding trichome branches, the microtubule-depletion zone is predicted to generate an apical patch of isotropic cell wall, the size of which is progressively constricted during branch elongation to control the degree of cell tapering (Yanagisawa et al., 2015). The strong correlation between cell tip radius of curvature, the geometry of the ARP2/3 activation domain, and the presence of the microtubule-depletion zone indicates that there is bidirectional signaling between the cell wall and the cytoskeleton during trichome morphogenesis. Perhaps cell geometry and material gradients in the cell wall generate stress patterns that are decoded by cytoskeletal systems so that cell wall properties can be modulated at the extended temporal and spatial time scales of plant cell morphogenesis. It may be that modulation of cortical microtubule-depletion zones is a conserved strategy to control local cell wall assembly (Oda and Fukuda, 2012) and the stress-strain behaviors highly polarized cell types. Given that the leaf hairs defend the plant against herbivores and the diameter of cotton fibers is an important agricultural trait, the knowledge from Arabidopsis has practical importance. There will be many future opportunities to use basic cell biology knowledge to engineer the mechanical properties of cells, tissue, and organs that are important for plant productivity. The combined use of FE modeling and multivariate live-cell imaging in trichomes (Yanagisawa et al., 2015) and other cell types (Fayant et al., 2010; Sampathkumar et al., 2014) has the potential to accelerate systems-level analyses of morphogenesis across wide spatial scales.
Footnotes
Articles can be viewed without a subscription.
References
- Augustine RC, Pattavina KA, Tüzel E, Vidali L, Bezanilla M (2011) Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell 23: 3696–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine RC, Vidali L, Kleinman KP, Bezanilla M (2008) Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J 54: 863–875 [DOI] [PubMed] [Google Scholar]
- Bai J, Zhu X, Wang Q, Zhang J, Chen H, Dong G, Zhu L, Zheng H, Xie Q, Nian J, et al. (2015) Rice TUTOU1 encodes a suppressor of cAMP receptor-like protein that is important for actin organization and panicle development. Plant Physiol 169: 1179–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom CS Jr, Hepler PK, Bezanilla M (2018) Interplay between ions, the cytoskeleton, and cell wall properties during tip growth. Plant Physiol 176: 28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21: 203–222 [DOI] [PubMed] [Google Scholar]
- Basu D, El-Assal Sel-D, Le J, Mallery EL, Szymanski DB (2004) Interchangeable functions of Arabidopsis PIROGI and the human WAVE complex subunit SRA1 during leaf epidermal development. Development 131: 4345–4355 [DOI] [PubMed] [Google Scholar]
- Basu D, Le J, El-Essal Sel-D, Huang S, Zhang C, Mallery EL, Koliantz G, Staiger CJ, Szymanski DB (2005) DISTORTED3/SCAR2 is a putative Arabidopsis WAVE complex subunit that activates the Arp2/3 complex and is required for epidermal morphogenesis. Plant Cell 17: 502–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibeau JP, Kingsley JL, Furt F, Tüzel E, Vidali L (2018) F-actin mediated focusing of vesicles at the cell tip is essential for polarized growth. Plant Physiol 176: 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hepler PK (2005) Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17: 3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J, Vaillancourt B, Kroeger J, Hepler PK, Wiseman PW, Geitmann A (2008) Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol 147: 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer D, Nowak J, Ivakov A, Somssich M, Persson S, Nikoloski Z (2017) System-wide organization of actin cytoskeleton determines organelle transport in hypocotyl plant cells. Proc Natl Acad Sci USA 114: E5741–E5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth KM, Adams DC, Horner HT, Wendel JF (2009) Initiation and early development of fiber in wild and cultivated cotton. Int J Plant Sci 170: 561–574 [Google Scholar]
- Cai C, Henty-Ridilla JL, Szymanski DB, Staiger CJ (2014) Arabidopsis myosin XI: a motor rules the tracks. Plant Physiol 166: 1359–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146: 1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, McKenna ST, Kunkel JG, Hepler PK (2006) NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol 142: 1460–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Niroomand S, Zou Y, Wu HM (2010) A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc Natl Acad Sci USA 107: 16390–16395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2016) Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J Exp Bot 67: 463–476 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2018) Diffuse growth of plant cell walls. Plant Physiol 176: 16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks MJ, Kaloriti D, Davies B, Malhó R, Hussey PJ (2004) Arabidopsis NAP1 is essential for Arp2/3-dependent trichome morphogenesis. Curr Biol 14: 1410–1414 [DOI] [PubMed] [Google Scholar]
- Djakovic S, Dyachok J, Burke M, Frank MJ, Smith LG (2006) BRICK1/HSPC300 functions with SCAR and the ARP2/3 complex to regulate epidermal cell shape in Arabidopsis. Development 133: 1091–1100 [DOI] [PubMed] [Google Scholar]
- Dyachok J, Shao M-R, Vaughn K, Bowling A, Facette M, Djakovic S, Clark L, Smith L (2008) Plasma membrane-associated SCAR complex subunits promote cortical F-actin accumulation and normal growth characteristics in Arabidopsis roots. Mol Plant 1: 990–1006 [DOI] [PubMed] [Google Scholar]
- El-Assal Sel-D, Le J, Basu D, Mallery EL, Szymanski DB (2004) Arabidopsis GNARLED encodes a NAP125 homolog that positively regulates ARP2/3. Curr Biol 14: 1405–1409 [DOI] [PubMed] [Google Scholar]
- Elliott A, Shaw SL (2018) Update: plant cortical microtubule arrays. Plant Physiol 176: 94–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facette MR, Park Y, Sutimantanapi D, Luo A, Cartwright HN, Yang B, Bennett EJ, Sylvester AW, Smith LG (2015) The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat Plants 1: 14024. [DOI] [PubMed] [Google Scholar]
- Fayant P, Girlanda O, Chebli Y, Aubin CE, Villemure I, Geitmann A (2010) Finite element model of polar growth in pollen tubes. Plant Cell 22: 2579–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Smith LG (2002) A small, novel protein highly conserved in plants and animals promotes the polarized growth and division of maize leaf epidermal cells. Curr Biol 12: 849–853 [DOI] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt F, Liu YC, Bibeau JP, Tüzel E, Vidali L (2013) Apical myosin XI anticipates F-actin during polarized growth of Physcomitrella patens cells. Plant J 73: 417–428 [DOI] [PubMed] [Google Scholar]
- Geitmann A, Nebenführ A (2015) Navigating the plant cell: intracellular transport logistics in the green kingdom. Mol Biol Cell 26: 3373–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon BC, Kovar DR, Staiger CJ (1999) Latrunculin B has different effects on pollen germination and tube growth. Plant Cell 11: 2349–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebnev G, Ntefidou M, Kost B (2017) Secretion and endocytosis in pollen tubes: models of tip growth in the spot light. Front Plant Sci 8: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries PA, Pan A, Quatrano RS (2005) Actin-related protein2/3 complex component ARPC1 is required for proper cell morphogenesis and polarized cell growth in Physcomitrella patens. Plant Cell 17: 2327–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelková L, Nanda G, Martinek J, Bellinvia E, Sikorová L, Šlajcherová K, Seifertová D, Fischer L, Fišerová J, Petrášek J, et al. (2015) Arp2/3 complex subunit ARPC2 binds to microtubules. Plant Sci 241: 96–108 [DOI] [PubMed] [Google Scholar]
- Henty JL, Bledsoe SW, Khurana P, Meagher RB, Day B, Blanchoin L, Staiger CJ (2011) Arabidopsis actin depolymerizing factor4 modulates the stochastic dynamic behavior of actin filaments in the cortical array of epidermal cells. Plant Cell 23: 3711–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty-Ridilla JL, Li J, Blanchoin L, Staiger CJ (2013) Actin dynamics in the cortical array of plant cells. Curr Opin Plant Biol 16: 678–687 [DOI] [PubMed] [Google Scholar]
- Honkanen S, Jones VAS, Morieri G, Champion C, Hetherington AJ, Kelly S, Proust H, Saint-Marcoux D, Prescott H, Dolan L (2016) The mechanism forming the cell surface of tip-growing rooting cells is conserved among land plants. Curr Biol 26: 3238–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörmanseder K, Obermeyer G, Foissner I (2005) Disturbance of endomembrane trafficking by brefeldin A and calyculin A reorganizes the actin cytoskeleton of Lilium longiflorum pollen tubes. Protoplasma 227: 25–36 [DOI] [PubMed] [Google Scholar]
- Huang S, Robinson RC, Gao LY, Matsumoto T, Brunet A, Blanchoin L, Staiger CJ (2005) Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization. Plant Cell 17: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey PJ, Ketelaar T, Deeks MJ (2006) Control of the actin cytoskeleton in plant cell growth. Annu Rev Plant Biol 57: 109–125 [DOI] [PubMed] [Google Scholar]
- Jordan BM, Dumais J (2010) Biomechanics of plant cell growth. In Encyclopedia of Life Sciences. John Wiley & Sons, Chichester, UK [Google Scholar]
- Kang E, Zheng M, Zhang Y, Yuan M, Yalovsky S, Zhu L, Fu Y (2017) The microtubule-associated protein MAP18 affects ROP2 GTPase activity during root hair growth. Plant Physiol 174: 202–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana P, Henty JL, Huang S, Staiger AM, Blanchoin L, Staiger CJ (2010) Arabidopsis VILLIN1 and VILLIN3 have overlapping and distinct activities in actin bundle formation and turnover. Plant Cell 22: 2727–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B. (2008) Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends Cell Biol 18: 119–127 [DOI] [PubMed] [Google Scholar]
- Kramer EM, Irish VF (1999) Evolution of genetic mechanisms controlling petal development. Nature 399: 144–148 [DOI] [PubMed] [Google Scholar]
- Kroeger JH, Daher FB, Grant M, Geitmann A (2009) Microfilament orientation constrains vesicle flow and spatial distribution in growing pollen tubes. Biophys J 97: 1822–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U, Briggs WR (1988) Growth, in vivo extensibility, and tissue tension in developing pea internodes. Plant Physiol 86: 306–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK (1987) Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma 140: 141–150 [Google Scholar]
- Le J, El-Assal Sel-D, Basu D, Saad ME, Szymanski DB (2003) Requirements for Arabidopsis ATARP2 and ATARP3 during epidermal development. Curr Biol 13: 1341–1347 [DOI] [PubMed] [Google Scholar]
- Le J, Mallery EL, Zhang C, Brankle S, Szymanski DB (2006) Arabidopsis BRICK1/HSPC300 is an essential WAVE-complex subunit that selectively stabilizes the Arp2/3 activator SCAR2. Curr Biol 16: 895–901 [DOI] [PubMed] [Google Scholar]
- Lenartowska M, Michalska A (2008) Actin filament organization and polarity in pollen tubes revealed by myosin II subfragment 1 decoration. Planta 228: 891–896 [DOI] [PubMed] [Google Scholar]
- Leucci MR, Di Sansebastiano G-P, Gigante M, Dalessandro G, Piro G (2007) Secretion marker proteins and cell-wall polysaccharides move through different secretory pathways. Planta 225: 1001–1017 [DOI] [PubMed] [Google Scholar]
- Li J, Blanchoin L, Staiger CJ (2015) Signaling to actin stochastic dynamics. Annu Rev Plant Biol 66: 415–440 [DOI] [PubMed] [Google Scholar]
- Li J, Wang X, Qin T, Zhang Y, Liu X, Sun J, Zhou Y, Zhu L, Zhang Z, Yuan M, et al. (2011) MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell 23: 4411–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Nebenführ A (2007) Organelle targeting of myosin XI is mediated by two globular tail subdomains with separate cargo binding sites. J Biol Chem 282: 20593–20602 [DOI] [PubMed] [Google Scholar]
- Li S, Dong H, Pei W, Liu C, Zhang S, Sun T, Xue X, Ren H (2017) LlFH1-mediated interaction between actin fringe and exocytic vesicles is involved in pollen tube tip growth. New Phytol 214: 745–761 [DOI] [PubMed] [Google Scholar]
- Li Y, Chen F, Linskens HF, Cresti M (1994) Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex Plant Reprod 7: 145–152 [Google Scholar]
- Lockhart JA. (1965) An analysis of irreversible plant cell elongation. J Theor Biol 8: 264–275 [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Cárdenas L, Kunkel JG, Hepler PK (2007) Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motil Cytoskeleton 64: 217–232 [DOI] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Kunkel JG, Allwood EG, Hussey PJ, Hepler PK (2006) Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell 18: 2182–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK (2005) Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta 221: 95–104 [DOI] [PubMed] [Google Scholar]
- Madison SL, Buchanan ML, Glass JD, McClain TF, Park E, Nebenführ A (2015) Class XI myosins move specific organelles in pollen tubes and are required for normal fertility and pollen tube growth in Arabidopsis. Plant Physiol 169: 1946–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Kirik V, Kernebeck B, Srinivas BP, Hülskamp M (2003) Arabidopsis CROOKED encodes for the smallest subunit of the ARP2/3 complex and controls cell shape by region specific fine F-actin formation. Development 130: 3137–3146 [DOI] [PubMed] [Google Scholar]
- Mathur J, Spielhofer P, Kost B, Chua N (1999) The actin cytoskeleton is required to elaborate and maintain spatial patterning during trichome cell morphogenesis in Arabidopsis thaliana. Development 126: 5559–5568 [DOI] [PubMed] [Google Scholar]
- Menand B, Calder G, Dolan L (2007) Both chloronemal and caulonemal cells expand by tip growth in the moss Physcomitrella patens. J Exp Bot 58: 1843–1849 [DOI] [PubMed] [Google Scholar]
- Michelot A, Guérin C, Huang S, Ingouff M, Richard S, Rodiuc N, Staiger CJ, Blanchoin L (2005) The formin homology 1 domain modulates the actin nucleation and bundling activity of Arabidopsis FORMIN1. Plant Cell 17: 2296–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara A, Richens J, Starker C, Morieri G, Smith L, Long S, Downie JA, Oldroyd GE (2010) Conservation in function of a SCAR/WAVE component during infection thread and root hair growth in Medicago truncatula. Mol Plant Microbe Interact 23: 1553–1562 [DOI] [PubMed] [Google Scholar]
- Nan Q, Qian D, Niu Y, He Y, Tong S, Niu Z, Ma J, Yang Y, An L, Wan D, et al. (2017) Plant actin-depolymerizing factors possess opposing biochemical properties arising from key amino acid changes throughout evolution. Plant Cell 29: 395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2012) Initiation of cell wall pattern by a Rho- and microtubule-driven symmetry breaking. Science 337: 1333–1336 [DOI] [PubMed] [Google Scholar]
- Ojangu EL, Järve K, Paves H, Truve E (2007) Arabidopsis thaliana myosin XIK is involved in root hair as well as trichome morphogenesis on stems and leaves. Protoplasma 230: 193–202 [DOI] [PubMed] [Google Scholar]
- Ojangu EL, Tanner K, Pata P, Järve K, Holweg CL, Truve E, Paves H (2012) Myosins XI-K, XI-1, and XI-2 are required for development of pavement cells, trichomes, and stigmatic papillae in Arabidopsis. BMC Plant Biol 12: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Park E, Nebenführ A (2013) Myosin XIK of Arabidopsis thaliana accumulates at the root hair tip and is required for fast root hair growth. PLoS One 8: e76745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Klocko AL, Fowler JE, Dolja VV (2012) Arabidopsis myosin XI-K localizes to the motile endomembrane vesicles associated with F-actin. Front Plant Sci 3: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Morgun EA, Kurth EG, Makarova KS, Koonin EV, Dolja VV (2013) Identification of myosin XI receptors in Arabidopsis defines a distinct class of transport vesicles. Plant Cell 25: 3022–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV (2008) Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol 146: 1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud P-F, Quatrano RS (2006) The role of ARPC4 in tip growth and alignment of the polar axis in filaments of Physcomitrella patens. Cell Motil Cytoskeleton 63: 162–171 [DOI] [PubMed] [Google Scholar]
- Perroud P-F, Quatrano RS (2008) BRICK1 is required for apical cell growth in filaments of the moss Physcomitrella patens but not for gametophore morphology. Plant Cell 20: 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton JM, Steer MW (1983) Membrane recycling and the control of secretory activity in pollen tubes. J Cell Sci 63: 303–310 [DOI] [PubMed] [Google Scholar]
- Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E (2004) The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16: 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky AI, Peremyslov VV, Dolja VV (2008) Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc Natl Acad Sci USA 105: 19744–19749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu JL, Jilk R, Marks MD, Szymanski DB (2002) The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell 14: 101–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zhang H, Xie Y, Wang J, Chen N, Huang S (2013) Arabidopsis villins promote actin turnover at pollen tube tips and facilitate the construction of actin collars. Plant Cell 25: 1803–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50: 514–528 [DOI] [PubMed] [Google Scholar]
- Reddy ASN, Day IS (2001) Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol 2: research0024.1–research0024.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Xiang Y (2007) The function of actin-binding proteins in pollen tube growth. Protoplasma 230: 171–182 [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenführ A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14: 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas ER, Hotton S, Dumais J (2011) Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys J 101: 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds CM, Bezanilla M (2013) Growth mechanisms in tip-growing plant cells. Annu Rev Plant Biol 64: 243–265 [DOI] [PubMed] [Google Scholar]
- Rounds CM, Hepler PK, Winship LJ (2014) The apical actin fringe contributes to localized cell wall deposition and polarized growth in the lily pollen tube. Plant Physiol 166: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds CM, Lubeck E, Hepler PK, Winship LJ (2011) Propidium iodide competes with Ca2+ to label pectin in pollen tubes and Arabidopsis root hairs. Plant Physiol 157: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JM, Nebenführ A (2018) Update on myosin motors: molecular mechanisms and physiological functions. Plant Physiol 176: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambade A, Findlay K, Schäffner AR, Lloyd CW, Buschmann H (2014) Actin-dependent and -independent functions of cortical microtubules in the differentiation of Arabidopsis leaf trichomes. Plant Cell 26: 1629–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Gutierrez R, McFarlane HE, Bringmann M, Lindeboom J, Emons AM, Samuels L, Ketelaar T, Ehrhardt DW, Persson S (2013) Patterning and lifetime of plasma membrane-localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol 162: 675–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jönsson H, Meyerowitz EM (2014) Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 3: e01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Lindeboom JJ, Debolt S, Gutierrez R, Ehrhardt DW, Ketelaar T, Persson S (2011) Live cell imaging reveals structural associations between the actin and microtubule cytoskeleton in Arabidopsis. Plant Cell 23: 2302–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M. (2011) An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol 13: 1431–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab B, Mathur J, Saedler R, Schwarz H, Frey B, Scheidegger C, Hülskamp M (2003) Regulation of cell expansion by the DISTORTED genes in Arabidopsis thaliana: actin controls the spatial organization of microtubules. Mol Genet Genomics 269: 350–360 [DOI] [PubMed] [Google Scholar]
- Shaw SL, Dumais J, Long SR (2000) Cell surface expansion in polarly growing root hairs of Medicago truncatula. Plant Physiol 124: 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmen T, Yokota E (2004) Cytoplasmic streaming in plants. Curr Opin Cell Biol 16: 68–72 [DOI] [PubMed] [Google Scholar]
- Smertenko AP, Deeks MJ, Hussey PJ (2010) Strategies of actin reorganisation in plant cells. J Cell Sci 123: 3019–3028 [DOI] [PubMed] [Google Scholar]
- Smith LG, Oppenheimer DG (2005) Spatial control of cell expansion by the plant cytoskeleton. Annu Rev Cell Dev Biol 21: 271–295 [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Sheahan MB, Khurana P, Wang X, McCurdy DW, Blanchoin L (2009) Actin filament dynamics are dominated by rapid growth and severing activity in the Arabidopsis cortical array. J Cell Biol 184: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan O, Cottier S, Fahlén S, Montes-Rodriguez A, Sun J, Eklund DM, Klahre U, Kost B (2014) RISAP is a TGN-associated RAC5 effector regulating membrane traffic during polar cell growth in tobacco. Plant Cell 26: 4426–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan OOH. (2017) Actin fringes of polar cell growth. J Exp Bot 68: 3303–3320 [DOI] [PubMed] [Google Scholar]
- Stradal TE, Scita G (2006) Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol 18: 4–10 [DOI] [PubMed] [Google Scholar]
- Su H, Zhu J, Cai C, Pei W, Wang J, Dong H, Ren H (2012) FIMBRIN1 is involved in lily pollen tube growth by stabilizing the actin fringe. Plant Cell 24: 4539–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlanski AL, Nielsen E (2009) The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell 21: 526–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB. (2005) Breaking the WAVE complex: the point of Arabidopsis trichomes. Curr Opin Plant Biol 8: 103–112 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Cosgrove DJ (2009) Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol 19: R800–R811 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Marks MD, Wick SM (1999) Organized F-actin is essential for normal trichome morphogenesis in Arabidopsis. Plant Cell 11: 2331–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl S, Moreau F, Hoffmann C, Arumugam K, Dieterle M, Moes D, Neumann K, Steinmetz A, Thomas C (2011) Arabidopsis actin-depolymerizing factors (ADFs) 1 and 9 display antagonist activities. FEBS Lett 585: 1821–1827 [DOI] [PubMed] [Google Scholar]
- Thomas C. (2012) Bundling actin filaments from membranes: some novel players. Front Plant Sci 3: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Han L, Feng Z, Wang G, Liu W, Ma Y, Yu Y, Kong Z (2015) Orchestration of microtubules and the actin cytoskeleton in trichome cell shape determination by a plant-unique kinesin. eLife 4: e09351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Kimura A, Yokota E, Haraguchi T, Shimmen T, Yamamoto K, Nakano A, Ito K (2013) Cytoplasmic streaming velocity as a plant size determinant. Dev Cell 27: 345–352 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kojima H, Yokota E, Orii H, Nakamori R, Katayama E, Anson M, Shimmen T, Oiwa K (2003) Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J 22: 1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Yokota E, Vidali L, Sonobe S, Hepler PK, Shimmen T (2000) The role of plant villin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 210: 836–843 [DOI] [PubMed] [Google Scholar]
- Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I (2010) Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci USA 107: 6894–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen PA, Li M, Wu SZ, Bezanilla M (2012) Class II formin targeting to the cell cortex by binding PI(3,5)P(2) is essential for polarized growth. J Cell Biol 198: 235–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Augustine RC, Kleinman KP, Bezanilla M (2007) Profilin is essential for tip growth in the moss Physcomitrella patens. Plant Cell 19: 3705–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Burkart GM, Augustine RC, Kerdavid E, Tüzel E, Bezanilla M (2010) Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell 22: 1868–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, McKenna ST, Hepler PK (2001) Actin polymerization is essential for pollen tube growth. Mol Biol Cell 12: 2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Rounds CM, Hepler PK, Bezanilla M (2009a) Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS One 4: e5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, van Gisbergen PA, Guérin C, Franco P, Li M, Burkart GM, Augustine RC, Blanchoin L, Bezanilla M (2009b) Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc Natl Acad Sci USA 106: 13341–13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukašinović N, Žárský V (2016) Tethering complexes in the Arabidopsis endomembrane system. Front Cell Dev Biol 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu L, Liu B, Wang C, Jin L, Zhao Q, Yuan M (2007) Arabidopsis MICROTUBULE-ASSOCIATED PROTEIN18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell 19: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteneys GO, Galway ME (2003) Remodeling the cytoskeleton for growth and form: an overview with some new views. Annu Rev Plant Biol 54: 691–722 [DOI] [PubMed] [Google Scholar]
- Wu Y, Yan J, Zhang R, Qu X, Ren S, Chen N, Huang S (2010) Arabidopsis FIMBRIN5, an actin bundling factor, is required for pollen germination and pollen tube growth. Plant Cell 22: 3745–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Desyatova AS, Belteton SA, Mallery EL, Turner JA, Szymanski DB (2015) Patterning mechanisms of cytoskeletal and cell wall systems during leaf trichome morphogenesis. Nat Plants 1: 15014. [DOI] [PubMed] [Google Scholar]
- Ye J, Zheng Y, Yan A, Chen N, Wang Z, Huang S, Yang Z (2009) Arabidopsis formin3 directs the formation of actin cables and polarized growth in pollen tubes. Plant Cell 21: 3868–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerzour R, Kroeger J, Geitmann A (2009) Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev Biol 334: 437–446 [DOI] [PubMed] [Google Scholar]
- Zhang C, Halsey LE, Szymanski DB (2011) The development and geometry of shape change in Arabidopsis thaliana cotyledon pavement cells. BMC Plant Biol 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Kotchoni SO, Samuels AL, Szymanski DB (2010) SPIKE1 signals originate from and assemble specialized domains of the endoplasmic reticulum. Curr Biol 20: 2144–2149 [DOI] [PubMed] [Google Scholar]
- Zhang C, Mallery EL, Szymanski DB (2013) ARP2/3 localization in Arabidopsis leaf pavement cells: a diversity of intracellular pools and cytoskeletal interactions. Front Plant Sci 4: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang R, Qu X, Huang S (2016) Arabidopsis FIM5 decorates apical actin filaments and regulates their organization in the pollen tube. J Exp Bot 67: 3407–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dyachok J, Krishnakumar S, Smith LG, Oppenheimer DG (2005) IRREGULAR TRICHOME BRANCH1 in Arabidopsis encodes a plant homolog of the actin-related protein2/3 complex activator Scar/WAVE that regulates actin and microtubule organization. Plant Cell 17: 2314–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, He J, Lee D, McCormick S (2010) Interdependence of endomembrane trafficking and actin dynamics during polarized growth of Arabidopsis pollen tubes. Plant Physiol 152: 2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Xie Y, Jiang Y, Qu X, Huang S (2013) Arabidopsis actin-depolymerizing factor7 severs actin filaments and regulates actin cable turnover to promote normal pollen tube growth. Plant Cell 25: 3405–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nan Q, Qin T, Qian D, Mao T, Yuan S, Wu X, Niu Y, Bai Q, An L, et al. (2017) Higher-ordered actin structures remodeled by Arabidopsis ACTIN-DEPOLYMERIZING FACTOR5 are important for pollen germination and pollen tube growth. Mol Plant 10: 1065–1081 [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhang Y, Kang E, Xu Q, Wang M, Rui Y, Liu B, Yuan M, Fu Y (2013) MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell 25: 851–867 [DOI] [PMC free article] [PubMed] [Google Scholar]